Abstract
Background
The role of tumor necrosis factor alpha (TNF-α) in targeted therapy for hepatocellular carcinoma (HCC) remains largely unknown. The current study aimed to clarify the mechanistic effects of targeting TNF-α to overcome sorafenib resistance in HCC.
Methods
A correlation of TNF-α expression with the prognosis was analyzed in 62 HCC patients who underwent surgical resection and subsequent received adjuvant sorafenib treatment. The relation of TNF-α expression and sorafenib sensitivity was determined in different HCC cell lines. The combined therapeutic effects of sorafenib and ulinastatin, which could inhibit TNF-α expression, on HCC were examined in vitro and in vivo.
Findings
High TNF-α expression was correlated with poor outcomes in HCC patients who received adjuvant sorafenib after surgery. In vitro experiments showed that TNF-α promotes HCC cell resistant to sorafenib through inducing epithelial-mesenchymal transition (EMT). Notably, the current study revealed that sorafenib has no significant influence on the expression and secretion of TNF-α, and sorafenib had limited effectiveness on reversing EMT in HCC cells with high TNF-α expression. Inhibiting the expression of TNF-α with ulinastatin significantly enhanced the anti-tumor effect of sorafenib on HCC cells with high expression of TNF-α in vitro and in vivo.
Interpretation: Our findings indicate that TNF-α may serve as a novel predictor of sorafenib sensitivity in HCC patients. Sorafenib combined with ulinastatin may improve the effectiveness of treatment of HCC in patients with high expression of TNF-α.
Fund
This work was supported by grants from the National Natural Science Foundation of China (no.81572398; no.81672419), the Science and Technology Planning Project of Guangdong Province (no. 2017A010105003; no.2015A050502023; no.2016A020216010), and the Natural Science Foundation of Guangdong Province (no.2014A030313061; no. 2013B021800101).
Keywords: Hepatocellular carcinoma, Sorafenib resistance, TNF-α, Ulinastatin, Combination treatment
Research in context.
Evidence before this study
Clinical trials have proven that sorafenib improves the prognosis of patients with advanced HCC. However, drug resistance has led to a low response rate of sorafenib treatment. Thus, it is necessary to seek novel methods to improve the therapeutic effects of sorafenib. It is known that inflammatory factors play important roles in oncogenesis. TNF-α is a central mediator of inflammation, and has been shown to provide a molecular link between chronic inflammation and tumor development. However, the effect of TNF-α expression on sorafenib sensitivity in HCC patients is unknown.
Added value of this study
We demonstrated that TNF-α expression is positively correlated with sorafenib response in HCC patients. The results also indicated that sorafenib did not reduce the expression and secretion of TNF-α in HCC cells. Importantly, inhibiting the expression of TNF-α with ulinastatin, a urinary protease inhibitor widely used in the treatment of inflammatory diseases, markedly enhance the effectiveness of sorafenib on suppressing the progression of HCC in vitro and in vivo.
Implications of all the available evidence
The current study suggested that inhibiting the expression of TNF-α may improve the response to sorafenib in patients with HCC.
Alt-text: Unlabelled Box
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the digestive system [1]. In recent years, radical treatments such as surgical resection, ablation, and liver transplantation have improved the prognosis of patients with HCC. However, since it is not easy to diagnosis mild damage of liver function [2], most HCC patients are diagnosed at an advanced stage, and they are not candidates for radical treatment. The overall prognosis for HCC is still not optimistic. Sorafenib, a multikinase inhibitor, was the first targeted drug approved for the treatment of advanced HCC [3,4]. Although sorafenib has been proven to improve the prognosis of patients with advanced HCC, drug resistance has resulted in limited survival benefits because of low response rates [5]. As such, it is necessary to clarify the underlying mechanisms associated with sorafenib resistance and identify ways to overcome resistance to improve the anti-tumor effects of sorafenib in HCC.
The concept of inflammation-induced cancer has been established during the past decades, and studies have found that inflammatory factors affect nearly all the stages of tumor development, as well as the effectiveness of therapy [6]. Inflammatory cytokines like interleukin (IL)-1α are considered tumorigenic cytokines, and IL-1α is a promising therapeutic target in advanced colorectal cancer [7]. Elevated levels of IL-6 are associated with poor survival outcomes in many cancers, and recent studies have shown that IL-6 antibody can enhance the anti-tumor effects of chemotherapy or gefitinib in multiple tumor cells [8,9]. Transforming growth factor (TGF)-β, which promotes cell migration and vascular invasion and induces angiogenesis, is markedly elevated in a number of different cancers, including HCC. Recent study has also shown that down-regulation of TGF-β enhances the anti-tumor efficacy of epidermal growth factor receptor (EGFR)-targeted therapy [10,11]. Taken together, the results of the aforementioned studies suggest that inflammatory factors may be potential anti-tumor therapeutic targets.
Tumor necrosis factor (TNF)-α is one of the most important inflammatory cytokines, and it was first identified as an anti-tumor cytokine that induced tumor necrosis. Recent evidence has indicated that TNF-α is a central mediator of inflammation, and thus provides a molecular link between chronic inflammation and the development of malignancies [12]. TNF-α is predominantly produced by macrophages, but it is also produced by a variety of tumor cells including gallbladder and kidney cancer and facilitates tumor invasion and metastasis [13,14]. Recent study has also demonstrated that high expression of TNF-α in clear cell renal cell carcinomas was associated with resistance to sunitinib [15]. Study has also shown that TNF-α expression in HCC is significantly higher than that in normal hepatic tissue [16]. However, the role of TNF-α expression with respect to the sensitivity of HCC to sorafenib is unknown. Taken together, these prompt us to explore the exact role of TNF-α in HCC progression and further to investigate its potential role as a therapy target against HCC.
This study examined the association of TNF-α expression and sorafenib resistance in HCC patients. The results indicated that high TNF-α expression was associated with a poorer response to sorafenib. We also demonstrated that sorafenib has no significant influence on TNF-α expression. In addition, our results also showed that inhibiting TNF-α with ulinastatin (UTI) enhanced the effect of sorafenib in HCC cells with high expression of TNF-α through the NF-κB/EMT signaling pathway. Therefore, our study suggests that TNF-α overexpression is a novel mechanism of sorafenib resistance, and inhibition of TNF-α may increase the effectiveness of sorafenib in patients with HCC.
2. Materials and methods
2.1. Cell lines and culture
The human HCC cell lines HepG2, SK-HEP-1, and Huh-7 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Hep3B and PLC/PRF/5 HCC cells were obtained from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco BRL), 100 U/mL penicillin, and 100 U/mL streptomycin, and incubated at 37 °C under an atmosphere containing 5% CO2.
TNF-α was knowdown by lentiviruses containing shot hairpin RNA (shRNA): TNF-α shRNA, 5′-GTAGCCCATGTTGTAGCAA-3′. A scrambled shRNA lentivirus containing a non-targeting sequence (5′-TTCTCCGAACGTGTCACGT-3′, named LV-shNon) was used as a control. The shRNA sequence was synthesized by Shanghai GeneChem Co. Ltd. (Shanghai, China). shRNA were cloned into pLKO.1 (GV248) lentiviral vectors. Culture supernatants containing sh-RNA were added to HCC cells in the presence of polybrene. The cells were selected using 2 μg/mL puromycin after 24 h.
2.2. Reagents and antibodies
Sorafenib (Nexavar®) was purchased from Bayer Pharmaceuticals, and ulinastatin was purchased from Techpool Bio-Pharma (Guangzhou, China). Primary antibody for Snail, Vimentin, E-cadherin, Bcl-2, Bax, and NF-κB (pathway sample kit #9936) were purchased from Cell Signaling Technology (Danvers, MA, USA). The anti-TNF-α antibody was purchased from Novus (Littleton, CO, USA), and anti-PCNA antibody was purchased from Abcam (Cambridge, UK). Antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin, and the horseradish peroxidase (HRP)-labeled anti-rabbit secondary antibodies were from CWBIO (Beijing, China). The human TNF-α ELISA kit was purchased from Abcam.
2.3. Patients and specimens
The clinical data of 62 HCC patients who underwent surgical resection and subsequent received adjuvant sorafenib treatment at Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University (Guangzhou, China) from January 2007 to June 2014 were collected. Patients were treated with oral sorafenib at a dose of 400 mg twice a day beginning within 30 days after surgery. Patients eligible to receive adjuvant sorafenib after liver resection were those defined as having a high risk of recurrence, including macroscopic vascular invasion (portal vein or branches), microvascular invasion in pathology, or those who were only receiving palliative surgical and locoregional therapies [4]. Tissue specimens were fixed in formalin, and paraffin embedded for immunohistochemical study. The clinical characteristics of the patients included in the study are shown in Supplemental Table S1. The study was approved by the Ethical Committee of Sun Yat-Sen Memorial Hospital. All patients provided written informed consent for all treatment performed and to have their data used for research purposes.
2.4. Immunohistochemical (IHC) analysis
Serial sections of HCC tissue were stained with a 3-step immunoperoxidase method. Sections were deparaffinized with xylene, ethanol, and demineralized water. Antigens were then retrieved by boiling in 1 mM EDTA (pH 8.0) for 10 to 15 min. After the sections were washed in demineralized water and phosphate buffered saline (PBS), the primary antibodies were added and sections were incubated overnight at 4 °C. After washing with PBS, the sections were incubated with EnVision Mouse or Rabbit conjugate (Dako Corporation, Carpinteria, CA, USA) for 60 min at 37 °C. The color reaction was completed with the DAB-positive substrate. Sections were counterstained with hematoxylin.
The staining results were scored independently by two pathologists in the following manner. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The percentage of positive tumor cells was divided into five grades (percentage scores): 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The overall score of each sample was determined using the following formula: overall score = intensity score × percentage score. A total score of 0–2, 3–6, 7–9, and 10–12 was defined as negative, weak positive, moderate positive, and strong positive, respectively.
2.5. Western blot
The total protein was extracted from cells lysed with RIPA Lysis Buffer (CWBIO) containing protease inhibitors and phosphatase inhibitors (Roche). The protein concentrations of the cell lysates were measured using a bicinchoninic acid assay (Beyotime, Jiangsu, China) and equalized before loading. A total of 30 μg of protein were separated by SDS–PAGE, and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Immunoblot analyses were carried out using the appropriate antibodies, and the bands were visualized using an enhanced chemiluminescence detection kit (Millipore).
2.6. Cell viability, colony formation, ELISA, apoptosis analyses and cell invasion assays
Cell viability, colony formation, ELISA, apoptosis analyses and cell invasion assays were performed as described in the Supplementary Materials and Methods.
2.7. Immunofluorescence assay
HepG2 and SK-HEP-1 cells (2 × 103/well) were seeded in 24-well plates, incubated for 24 h, then treated with TNF-α (40 ng/mL) or ulinastatin (1600 U/mL). Next, HCC cells were washed and fixed with 4% paraformaldehyde (PFA) for 20 min, and permeabilized in 0.3% Triton X-100. Incubation with monoclonal rabbit anti-P65 antibody (CST) overnight at 4 °C was followed by incubation with fluorescein isothiocyanate (Alexa Fluor® 555)-labeled goat anti-rabbit IgG secondary antibody for 60 min in a dark wet box. Following triplicate washes with PBS-T, the cells were counterstained with DAPI (CWBIO) for 5 min. The results were photographed under an inverted fluorescence microscope (×400).
2.8. Animal experiments
Female BALB/c athymic nude mice, 4–6 weeks old, were purchased from the Laboratory Animal Center of Sun Yat-Sen University (Guangzhou, China). The mice were maintained in the laboratory for animal experimentation in a specific pathogen-free environment with laminar air-flow conditions, a 12-h light-dark cycle, and at a temperature of 22 °C to 25 °C. All animals had free access to standard laboratory mouse food and water. Animal experiments were approved by the Bioethics Committee of Sun Yat-Sen University and were performed according to the established guidelines.
2.9. Xenograft tumor growth
A total of 5 × 106 SK-HEP-1 cells were suspended in 200 μL serum-free DMEM and injected into the right flanks of the mice. The mice were then randomized into three groups (n = 6/group): vehicle control (ddH2O, orally), sorafenib (30 mg/kg/d, orally), and sorafenib (30 mg/kg/d, orally) combined with UTI (20,000 U twice a week, IP). Once palpable tumors were observed, measurements of tumor volume were taken every 3 days using calipers. The tumor volume was calculated using the following formula: V = (l × w2) × 0.5, where l and w refer to the larger and smaller dimensions collected at each measurement. The mice were killed after 2 weeks' treatment, and the solid tumors were excised, weighed, and fixed in formaldehyde.
2.10. In vivo metastasis analysis
SK-HEP-1 cells (1 × 106/0.2 mL) were injected into 4–6 weeks old female nude mice by way of tail vein to imitate tumor metastasis. Experimental animals (n = 6/group) were divided into four groups: vehicle control (ddH2O, orally), sorafenib (30 mg/kg/d, orally), UTI (20,000 U twice a week, IP), and sorafenib (30 mg/kg/d, orally) combined with UTI (20,000 U twice a week, IP). The mice were sacrificed after 4 weeks of treatment, and their livers and lungs were weighed and sampled for tissue sectioning.
2.11. Statistical analysis
Data were reported as mean ± standard deviations, and compared using Student's t-test and two-way analysis of variance (ANOVA) with Bonferroni correction. A value of p (p < .05) was considered to indicate statistical significance.
3. Results
3.1. High expression of TNF-α is correlated with poor prognosis in HCC
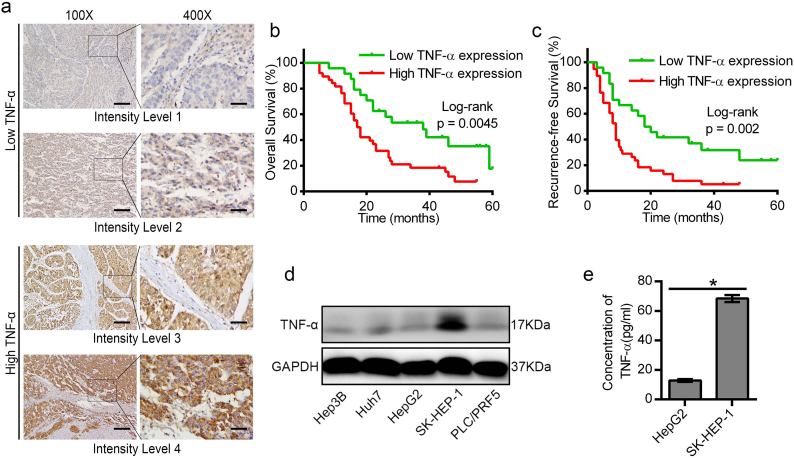
In order to test the expression of TNF-α and explore its expression with the prognosis in HCC patients, we collected the tissues of 62 HCC patients who received adjuvant sorafenib treatment after surgical resection. In our cohort, 61.3% (38/62) had a high level of TNF-α expression (Supplemental Table S1). Statistical analysis showed that high expression of TNF-α in HCC tissue was positively associated with multiple clinical pathological features including TNM stage (p < .05), BCLC stage (p < .05), and histological grade (p < .05) (Supplemental Table S2). The multivariate Cox regression analysis indicated that TNF-α was not an independent risk factor of overall survival (OS) after surgical resection (hazard ratio [HR] 0.898; 95% confidence interval [CI]: 0.413–1.955; P = .787), but the results demonstrated that TNF-α was an independent risk factor of recurrence free survival (RFS) after surgical resection (hazard ratio [HR] 2.487; 95% confidence interval [CI]: 1.146–5.396; P = .021) (Table S3 and S4). Furthermore, the Kaplan-Meier analysis showed that high expression of TNF-α was correlated with poorer overall survival and higher recurrence rates (Fig. 1b, c). Taken together, the results suggested that overexpression of TNF-α was correlated with poor prognosis in HCC. We also detected the expression level of TNF-α in five HCC cell lines by western blot and ELISA. The results revealed that SK-HEP-1 exhibited higher expression of TNF-α, while the TNF-α expression in HepG2 cells was much lower (Fig. 1d, e).
Fig. 1.
High expression of TNF-α was associated with bad prognosis in HCC patients. (a) Representative IHC images showing different levels of TNF-α expression in HCC patients. (b, c) Kaplan–Meier analysis indicated that HCC patients with lower TNF-α expression have better overall survival and recurrence-free survival (n = 62). (d) Analysis of the protein levels of TNF-α in HCC cells by western blot. (e) ELISA assay for the detection of TNF-α secretion in the cultured supernatants of HepG2 and SK-HEP-1 cells. Scale bars: 400 μm (100×); 100 μm (400×). Data represents the mean ± SD of three independent experiments. (*p < .05).
3.2. TNF-α promotes sorafenib resistance by inducing epithelial-mesenchymal transition (EMT) in HCC cells
To investigate the biological characteristics of TNF-α in HCC cells, we used a specific shRNA to knock down TNF-α expression in SK-HEP-1 (named SK-HEP-1-shTNF-α; supplemental Fig. 1a, b), and we added 40 ng/mL of TNF-α to stimulate HepG2 cell. The results showed that TNF-α stimulation could promote HCC cells proliferative and migratory ability, whereas down-regulated TNF-α in SK-HEP-1 cells significantly reduced the proliferation and migration of HCC cells. Moreover, its proliferation and migration abilities were rescued when we added extrinsic TNF-α to the SK-HEP-1-shTNF-α (Supplemental Fig. 1c-g). To determine the role of TNF-α in mediating sorafenib resistance in HCC, we treated HCC cells with different doses of sorafenib for 48 h. The IC50 values of the HepG2 and SK-HEP-1 cells were 6.20 μmol/L and 12.83 μmol/L, respectively. However, the IC50 values of the HepG2 cells pretreated with TNF-α was 8.12 μmol/L, and the IC50 values for the SK-HEP-1-shTNF-α cells were 7.32 μmol/L (Fig. 2a, b). Furthermore, the proliferation assay showed that sorafenib at the dose of 6 μmol/L, could dramatically inhibite the growth of HepG2 cells, but the inhibition efficiency decreased a lot when HepG2 cells pretreated with TNF-α stimulation. However, sorafenib (6 μmol/L) only showed mild inhibition on the growth of SK-HEP-1 cells, whereas it markedly suppressed the proliferation ability in SK-HEP-1-shTNF-α cells (Fig. 2c).
Fig. 2.
TNF-α promoted HCC cell proliferation, invasion, and resistant to sorafenib by inducing EMT. (a) Dose-dependent effects of sorafenib on the viability of HepG2 with or without TNF-α stimulation. (b) Dose-dependent effects of sorafenib on the viability of SK-HEP-1 and SK-HEP-1-sh-TNF-α cells. (c) MTS assays to detect the growth inhibition of sorafenib on HepG2 and SK-HEP-1 cells by the condition of TNF-α stimulation or down-regulation of TNF-α with the shRNA. (d) TNF-α markedly induced EMT in HepG2 cells, while knockdown of TNF-α reversed EMT in SK-HEP-1 cells. (e) The effect of sorafenib on EMT-related markers in HCC cells with or without exogenous TNF-α stimulation. (f) Western blot results showed that sorafenib had no effect on TNF-α expression. Data represents the mean ± SD of three independent experiments. IC50 was calculated by nonlinear regression analysis using Graphpad Prism software. (*p < .05).
Previous studies have implied an association between EMT and sorafenib resistance [17], and a wide range of evidence has suggested that TNF-α can induce EMT in different cancer cells [18,19]. Our results also demonstrated that TNF-α induced a typical change of EMT markers (up-regulation of snail and vimentin, and down-regulation of E-cadherin) in HCC cells (Fig. 2d). We explored the effect of sorafenib on EMT, and we found that sorafenib decreased snail and vimentin, and elevated E-cadherin in HepG2 cells. However, when TNF-α was pre-added to HepG2 cells, the effect of sorafenib on reversing EMT was decreased. Notably, our results showed that sorafenib almost had no effect on EMT in SK-HEP-1 cells with or without the presence of TNF-α (Fig. 2e). Furthermore, when HCC cells were treated with 3, 6, and 12 μmol/L concentrations of sorafenib, we found that sorafenib nearly had no influence on TNF-α expression in both the HCC cell lines (Fig. 2f).
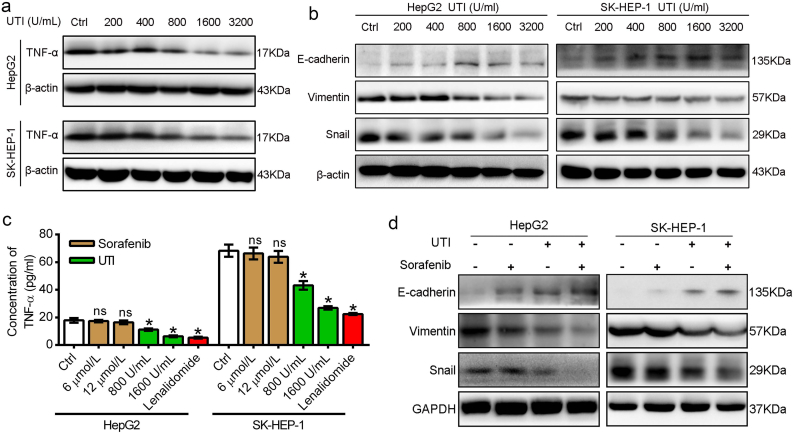
3.3. Ulinastatin suppresses EMT of HCC cells through inhibiting TNF-α expression and secretion
Ulinastatin is widely used for the treatment of inflammatory-related diseases. Previous studies have showed that ulinastatin inhibits the production of TNF-α in macrophages and breast cancer cells [20,21]. In the current study, HCC cells were cultivated with ulinastatin at various concentrations (200, 400, 800, 1600, and 3200 U/mL) for 48 h. Western blot results showed that ulinastatin down-regulated TNF-α expression in a dose-dependent manner (Fig. 3a). Detection of TNF-α by ELISA showed that 800 and 1600 U/mL of ulinastatin markedly decreased the TNF-α level in HCC cell supernatants, while sorafenib at the concentrations of 6 and 12 μmol/L almost had no effect on TNF-α secretion (Fig. 3c). We further examined the effect of ulinastatin on reversing the EMT of HCC cells. The results showed that when the concentration of ulinastatin exceeded 1600 U/mL, the epithelial marker E-cadherin was significantly up-regulated, and the mesenchymal markers (snail and vimentin) were markedly down-regulated in both the two HCC cell lines (Fig. 3b). Furthermore, when we used a concentration of 1600 U/mL of ulinastatin to down-regulated TNF-α in HepG2 and SK-HEP-1 cells, western blot results indicated that sorafenib markedly inhibited the EMT in both the two HCC cell lines (Fig. 3d).
Fig. 3.
Ulinastatin inhibited TNF-α expression and enhanced the effect of sorafenib on reversing EMT. (a) Ulinastatin (UTI) suppressed TNF-α expression in a dose-dependent manner. (b) Ulinastatin up-regulated the expression of E-cadherin, and down-regulated the expression of vimentin and snail when the concentration was >1600 U/mL. (c) The effect of sorafenib, ulinastatin on TNF-α secretion in HepG2 and SK-HEP-1 cells as measured by ELISA assay. Lenalidmide (an inhibitor of TNF-α secretion) used as the positive control. (d) The effect of sorafenib on EMT-related markers in HCC cells with or without ulinastatin (1600 U/mL) in HepG2 and SK-HEP-1 cells. Data represents the mean ± SD of three independent experiments. (*p < .05).
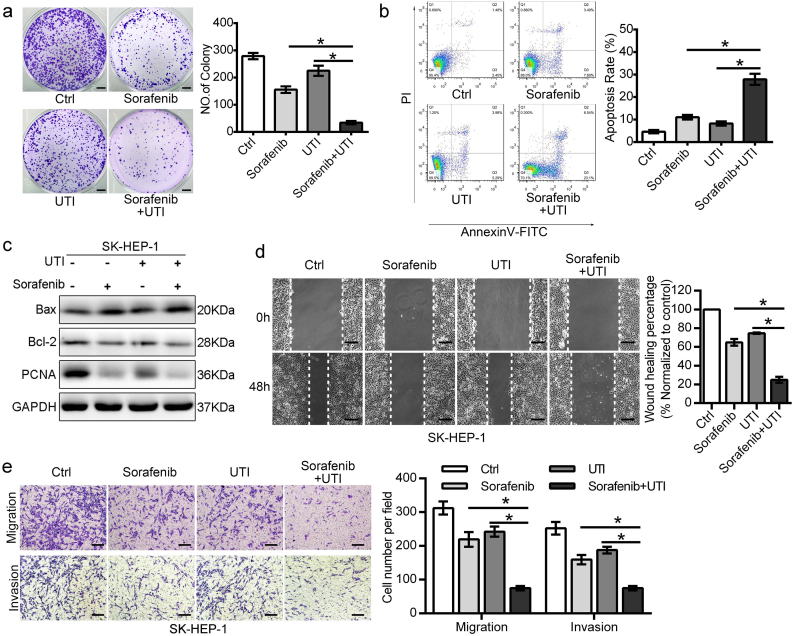
3.4. Ulinastatin enhances the anti-tumor effect of sorafenib in HCC cells
Since we showed that down-regulation of TNF-α by ulinastatin promoted sorafenib inhibition of EMT in HCC cells, we speculated that down-regulating TNF-α by ulinastatin might improve the effect of sorafenib against HCC cells, especially for the HCC cells with high expression of TNF-α. In the colony formation assay, sorafenib (6 μmol/L) alone could inhibit the proliferation of SK-HEP-1 cells to 55.79% compared with the control, while ulinastatin (1600 U/mL) alone could only mildly inhibit the proliferation of SK-HEP-1 cells. The effect of the two drugs combined was superior to the effect of either drug alone (Fig. 4a). Similar results were also found in the cell apoptotic ratio measured by flow cytometry (Fig. 4b), and western blot to detect PCNA (a proliferation-related protein), Bcl-2 (an anti-apoptosis-related protein), and Bax (an apoptosis-related protein) expression (Fig. 4c), where sorafenib treatment alone was superior to ulinastatin, and combination treatment was superior to either drug alone. We demonstrated the combination of sorafenib and ulinastatin could more effectively inhibit HCC cell proliferation and induce cell apoptosis than sorafenib or ulinastatin alone. Results of the wound healing assay indicated that the combination of sorafenib and ulinastatin significantly suppressed cell migration compared to sorafenib or ulinastatin alone in SK-HEP-1 cells (Fig. 4d). Moreover, the Transwell assay also revealed the combination of sorafenib and ulinastatin more significantly inhibited cell migration and invasion (Fig. 4e). In addition, we also detected the effect of combined therapy on HepG2 cells, and the results showed that sorafenib markedly suppressed the progression of HepG2 cells, while co-treatment with sorafenib and ulinastatin had better efficacy (Supplemental Fig. 2).
Fig. 4.
Ulinastatin enhanced the anti-tumor effect of sorafenib in SK-HEP-1 cells. (a) Colony formation assay showed the anti-proliferation effect of combined sorafenib and ulinastatin in SK-HEP-1 cells. (b) Co-treatment of sorafenib and ulinastatin more strongly induced apoptosis in SK-HEP-1 cells. (c) Western blot assay demonstrated that sorafenib or ulinastatin alone could, to some extent, down-regulate the expression of PCNA (proliferating cell nuclear antigen, a protein to reflect cell proliferation ability) and Bcl-2 (an anti-apoptotic protein), and up-regulate the expression of Bax (a pro-apoptsis protein), while co-treatment of these two drugs displayed better effect for these changes. (d) Representative images showed that the effect of sorafenib, ulinastatin and combined therapy of sorafenib and ulinastatin on SK-HEP-1 cells mobility, and the statistical data of the wound-healing assay. (E) Transwell migration and invasion assays showed that ulinastatin and sorafenib synergistically inhibited cell mobility of SK-HEP-1 cells. (Data represents the mean ± SD of three independent experiments. (*p < .05). (Scale bars in 4a: 4 mm; scale bars in 4d-e: 100 μm).
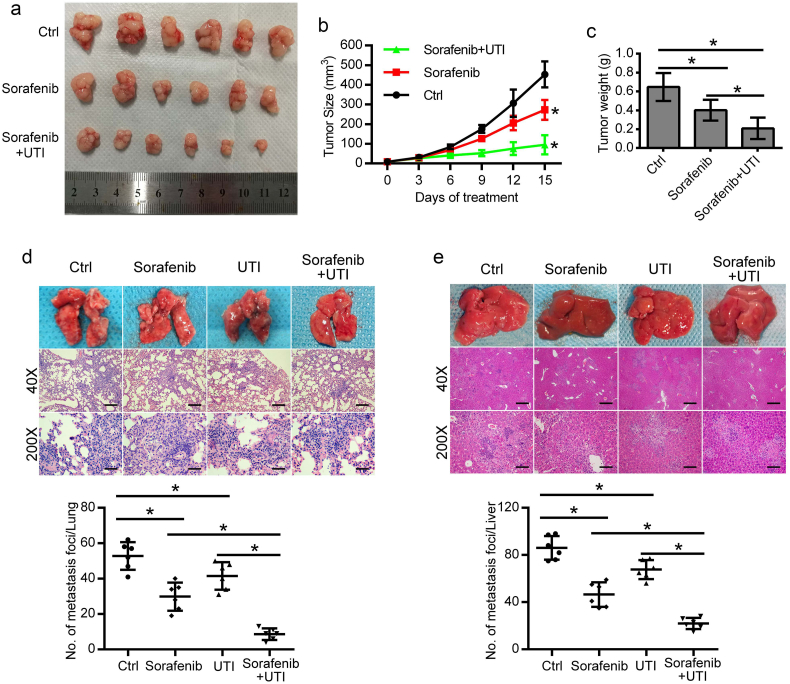
3.5. Combined treatment with sorafenib and ulinastatin exerts a more potent anti-tumor effect against HCC in vivo
To confirm the synergistic effect of sorafenib and ulinastatin in vivo, xenograft tumor models were prepared with SK-HEP-1 cells. Compared with the groups treated with sorafenib or control, tumor growth in the group treated with combined therapy of sorafenib and ulinastatin was significantly inhibited (Fig. 5a, b). Meanwhile, the tumor weight in sorafenib alone group was 61.94% to that of the control group, while tumor weight in the co-treatment group was 32.35% to that of the control group at the end of the study (Fig. 5c). To examine the effect of combined sorafenib and ulinastatin against metastasis in vivo, SK-HEP-1 cells were injected into nude mice by way of tail vein to imitate tumor metastasis. Metastases in the lung and liver reduced to 56.47% and 54.07%, respectively, in the sorafenib group. However, when using the co-treatment of ulinastatin with sorafenib, the metastases in the lung and liver reduced to 16.40% and 25.58%, respectively, compared with control group (Fig. 5d, e).
Fig. 5.
The anti-tumor effect of combined sorafenib and ulinastatin against HCC in vivo. (a) The mice were treated with intragastric administration of vehicle, sorafenib (30 mg/kg/d), or sorafenib and ulinastatin (20,000 U, twice a week, intraperitoneal injection). The photograph shows the dissected tumors from each group. (b) Tumor volumes were measured every 3 days, and tumor growth curves were created for each group. (c) The weight of dissected tumors from each group showed that sorafenib mildly suppressed proliferation of subcutaneous tumors, while combined ulinastatin and sorafenib had a better anti-tumor effect. (d, e) SK-HEP-1 cells (1 × 106/0.2 mL) were injected into the tail vein of the mice to imitate tumor metastasis. Representative hematoxylin and eosin (H&E) staining to assess pulmonary (d) and liver (e) metastasis at 4 weeks. The average number of foci in each group is presented as the mean ± SD. (*p < .05). (Scale bars: 500 μm (40×); 100 μm (200×).
3.6. Ulinastatin inhibits HCC cell invasion and proliferation via regulating the NF-κB/EMT signaling pathway
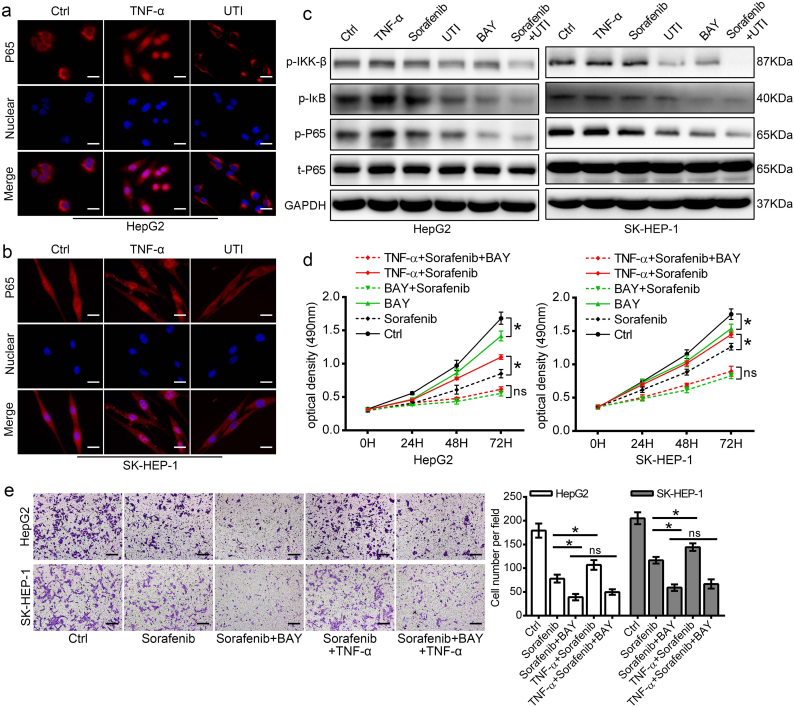
We further explored the signaling mechanisms of ulinastatin for its effect on TNF-α inhibition. Firstly, we observed P65 nuclear translocation using immunofluorescence staining, and we found that exogenous TNF-α markedly induced P65 nuclear translocation, while ulinastatin prevented P65 nuclear translocation (Fig. 6a, b). Then, we investigated the effect of sorafenib and ulinastatin on activation of the NF-κB signaling pathway. As shown in Fig. 6c, ulinastatin alone remarkably decreased the phosphorylation of IKK-β, IκB, and P65, while sorafenib had a limited inhibition effect on the NF-κB pathway in HCC cells. Moreover, co-treatment with the two drugs had a superior effect than that of either drug alone. Second, to determine whether inhibition of the NF-κB pathway in HCC cells has an anti-tumor effect, we treated the two HCC cell lines with BAY11–7082 (an inhibitor specifically inhibit P65 translating to the nuclear) to inhibit NF-κB activity. Western blot results showed that inhibiting the NF-κB pathway significantly reversed EMT (Supplemental Fig. 3b) and inhibited the mobility of HCC cells (Supplemental Fig. 3a). We also observed that co-treatment with sorafenib and BAY 11–7082 demonstrated superior effect than either drug alone for the growth inhibition of HCC cells. More importantly, our results revealed that the inhibition efficiency of the combined therapy nearly showed no diffidence with or without the presence of TNF-α (Fig. 6d). Similar results also found in the cell migration and invasion measured by Transwell migration assay (Fig. 6e and supplemental Fig. 3c).
Fig. 6.
Ulinastatin enhanced the anti-tumor effect of sorafenib by suppressing the NF-κB signaling pathway. (a, b) Immunofluorescence staining to show the expression and nuclear translation of P65 in HCC cells. Representative images show that TNF-α markedly induced P65 nuclear translocation, while ulinastatin strongly prevented P65 nuclear translocation. (scale bars: 25 μm). (c) TNF-α was used as a positive control to activate the NF-κB signaling pathway, and BAY 11–7082, an inhibitor specifically inhibit P65 translating to the nuclear, was used as a control for suppressing the NF-κB signaling pathway. Western blot results showed that ulinastatin inhibited the phosphorylation of IKK-β, IκB, and P65, while sorafenib almost had no impact on the phosphorylation of IKK-β, IκB, and P65 in HCC cells. The effect of co-treatment with the two drugs was superior to that of either drug alone. (d) HCC cells were pretreated with or without TNF-α stimulation, and then the cells were treated with sorafenib, BAY 11–7082 or combined sorafenib with BAY 11–7082, MTS assay to detect the growth inhibition for each group. (e) Transwell migration assay to show the combined therapy of sorafenib and BAY 11–7082 on cell mobility with or without TNF-α stimulation. (Scale bars: 100 μm). (Data represents the mean ± SD of three independent experiments. (*p < .05).
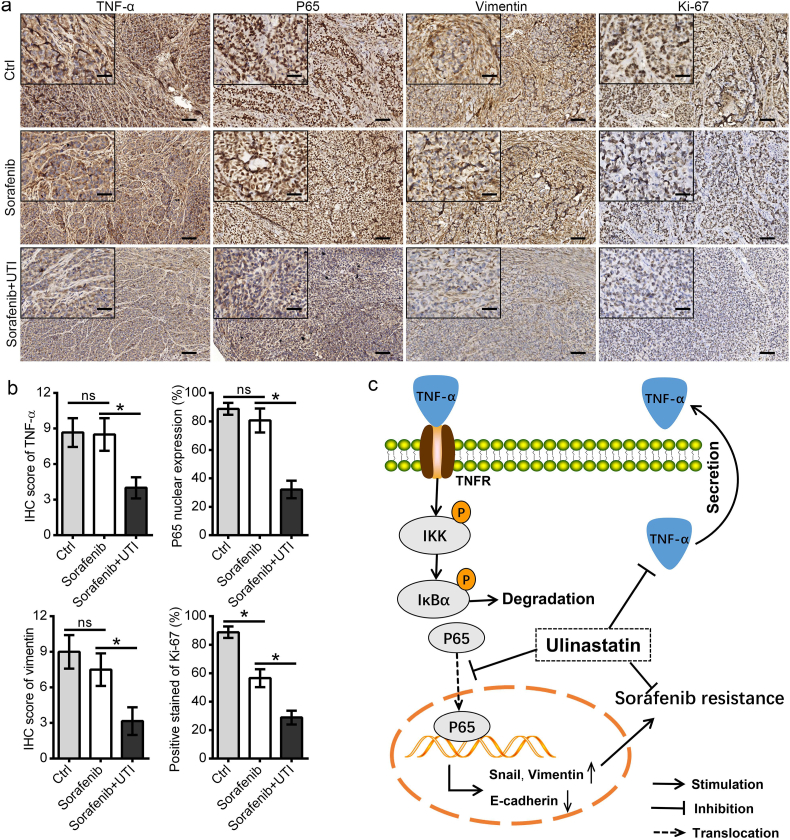
Tumor sections of the subcutaneous tumors in the SK-HEP-1 cell models were stained for TNF-α, P65, vimentin, and Ki67. The results showed that co-treatment with sorafenib and ulinastatin significantly suppressed the expression of TNF-α, vimentin, and Ki67, and the nuclear translocation of P65. Interestingly, the results demonstrated that sorafenib alone had a limited effect on TNF-α and vimentin expression, and it could not prevent the nuclear translocation of P65 (Fig. 7a, b). Taken together, these results indicated that ulinastatin could inhibit the TNF-α/NF-κB signaling pathway, and thus enhanced the anti-tumor effect of sorafenib in HCC.
Fig. 7.
The combination of sorafenib and ulinastatin suppressed the NF-κB signaling pathway. (a, b) Tumor sections from the subcutaneous tumor in the SK-HEP-1 cell models were stained for TNF-α, P65, vimentin, and Ki67. The results showed that sorafenib alone suppressed Ki67, but had limited effect on TNF-α, vimentin expression, and P65 nuclear translocation, while co-treatment of sorafenib and ulinastatin significantly suppressed the expression of TNF-α, vimentin, and Ki67, and inhibited nuclear translocation of P65. (c) Ulinastatin reverses sorafenib resistance through inhibiting the TNF-α/ NF-κB/EMT signaling pathway in HCC cells. (Data represents the mean ± SD of the IHC score of six independent animals for each group. (*p < .05). (Scale bars in 7a: 100 μm; inserts: 40 μm)
4. Discussion
In the present study, we demonstrated that the expression of TNF-α is positively correlated with the therapeutic response to sorafenib in HCC patients. For the first time we found that sorafenib could not reduce the expression and secretion of TNF-α. We also demonstrated that ulinastatin, a urinary protease inhibitor widely used in the treatment of inflammatory diseases, markedly enhanced the effect of sorafenib on suppressing the progression of HCC in vitro and in vivo.
Sorafenib is currently the only molecular targeted drug approved for advanced HCC patients. However, the response rate is unsatisfactory because of drug resistance. A recent examination of two phase 3 studies, the SHARP trial and ORIENTAL trial, found that inflammation is an indicator of a poor prognosis after sorafenib treatment in patients with HCC [22]. Other study has showed that a high baseline HBV load was an independent predictor of poor survival, and co-administration of antiviral therapy with sorafenib reduced the viral load and improved overall survival in HCC patients [23]. The above studies indicate that inhibiting the inflammatory reaction may be an effective adjuvant therapy in HCC patients.
Inflammatory factors affect nearly all the stages of tumor development, as well as the effectiveness of therapy. TNF-α is one of the most important inflammatory mediators of the cancer-associated inflammatory networks. Preclinical studies in breast cancer and pancreatic cancer have suggested that TNF-α promotes tumor growth in vivo and that anti-TNF-α treatments may suppress tumor progression [24,25]. Previous studies also showed that the serum TNF-α level in in patients with cirrhosis and HCC is significantly higher than that in healthy individuals, and high expression of TNF-α is positively associated with high-grade tumors and predicts poor survival in HCC patients [26,27]. Our results demonstrated that TNF-α was positively related with the proliferation and invasion ability of HCC cells, which is consistent with the results of previous studies of HCC and other tumors. However, the current study is the first to demonstrate that the expression of TNF-α is positively related to sorafenib resistance in HCC cells. We systematically showed that sorafenib could significantly inhibit the EMT in HepG2 cells (with low expression of TNF-α), but only slightly affect the EMT in HCC cells with high expression of TNF-α (SK-HEP-1 cells). Moreover, when exogenous TNF-α was added to HepG2 cells, the inhibition of EMT by sorafenib was dramatically impaired. Importantly, we found that sorafenib could not reduce the expression and secretion of TNF-α. Together, these results demonstrated that sorafenib has a limited effect on inhibiting the TNF-α-induced EMT in HCC cells.
Compelling evidence from recent investigations has highlighted the notion that EMT is an important mechanism of sorafenib resistance in advanced HCC [28,29]. The effect of sorafenib on inhibiting EMT has been reported previously, and it has been proven that sorafenib exerts a potent inhibitory effect against HGF mediated-EMT and TGF-β mediated-EMT in HCC cells [30,31]. However, the effect of sorafenib on reversing EMT in HCC cells with different TNF-α expression levels has not been reported. Our results revealed that sorafenib does not inhibit the EMT of HCC cells through regulating TNF-α expression, but possibly via other mechanisms (HGF or TGF-β). The NF-κB signaling pathway has been shown to play an important role in promoting inflammatory reactions, and functions as an essential mediator of EMT [32]. However, the effect of sorafenib on the NF-κB signaling pathway in HCC cells remains controversial. It has been reported that sorafenib effectively inhibits the activation of the NF-κB signaling pathway induced by radiotherapy in HCC, thereby enhancing the effect of radiotherapy [33]. However, another study showed that sustained sorafenib stimulation could activate NF-κB activity, thus induced HCC cell resistance to sorafenib [34]. Our results demonstrated that sorafenib had limited effect on inhibiting the NF-κB pathway in HCC cells, and this finding is consistent with a study by Osama et al. [35]. It is known that TNF-α is an activator of the NF-κB signaling pathway, and our results showed that sorafenib did not reduce the expression and secretion of TNF-α in the HCC cells. This might be the reason for the limited effect of sorafenib on the NF-κB/EMT signaling pathway in HCC cells.
Our study also showed that TNF-α plays an important role in HCC resistance to sorafenib. This promoted us to explore whether inhibiting TNF-α could overcome sorafenib resistance in HCC cells. Previous research has demonstrated that lenalidomide, an immune modulatory drug that could inhibit TNF-α expression, enhances the anti-tumor effect of sorafenib in HCC cells [36]. However, a phase I clinical trial found that the combination of lenalidomide and sorafenib was poorly tolerated because of toxicity side effects [37]. Therefore, exploring an anti-TNF-α drug with low toxicity side effect might benefit those HCC patients with high expression of TNF-α. Here we demonstrated that ulinastatin, which was widely used in clinic for the treatment of inflammatory-related diseases, could suppress the expression of TNF-α in HCC cells. Recent studies have reported that ulinastatin has therapeutic effects against several cancer types, including breast cancer, malignant mesothelioma, and colorectal cancer [[38], [39], [40]], but its role in HCC was unclear now. In the current study, we found that ulinastatin combined with sorafenib exerted a more significant anti-tumor effect in HCC cells with high expression of TNF-α in vitro and in vivo. Its mechanism might through suppressing the TNF-α/NF-κB/EMT signaling pathway. Other study have demonstrated that ulinastatin could down-regulate the expression of TNF-α, IL-6 and IL8 in breast cancer [20]. In this study, we mainly focused on the correlation of TNF-α and sorafenib sensibility in HCC, and we demonstrated that ulinastatin could suppress the expression of TNF-α. Therefore, it was worthwhile to explore other inflammatory cytokines like IL-6 or IL-8 might also participate in the mechanism of ulinastatin against HCC.
Taken together, our study results suggest that targeting TNF-α may overcome HCC resistance to sorafenib. We demonstrated that ulinastatin enhanced the anti-tumor effect of sorafenib against HCC by inhibiting the TNF-α/NF-κB/EMT signaling pathway. Further studies examining the effect of TNF-α suppression therapy on the outcomes of sorafenib treatment for HCC are warranted.
Acknowledgements and funding sources
This work was supported by grants from the National Natural Science Foundation of China (no. 81572398; no. 81672419), the Science and Technology Planning Project of Guangdong Province (no. 2017A010105003; no. 2015A050502023; no. 2016A020216010), the Natural Science Foundation of Guangdong Province (no. 2014A030313061; no. 2013B021800101). These funding sources had no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript.
Declaration of interests
The authors declare that they have no competing interests.
Author contributions
Yajin Chen and Changzhen Shang designed the experiments; Wenliang Tan performed the experiments and wrote the manuscript; Xuan Luo and Jun Cao collected the clinical samples; Wenda Li and Jinyi Zhong analyzed and made interpretation of the data. Sicong Zhu made up pictures and graphs. Xianqing Chen and Rui Zhou revised the manuscript; All the authors read and approved the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.047.
Contributor Information
Changzhen Shang, Email: shangcz_sysu@163.com.
Yajin Chen, Email: cyj0509@126.com.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Qi X., Berzigotti A., Cardenas A., Sarin S. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3(10):708–719. doi: 10.1016/S2468-1253(18)30232-2. [DOI] [PubMed] [Google Scholar]
- 3.Liu L., Cao Y., Chen C., Zhang X., McNabola A., Wilkie D. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66(24):11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva A., Llovet J.M. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clinical Cancer Research. 2012;18(7):1824–1826. doi: 10.1158/1078-0432.CCR-12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crusz S.M., Balkwill F.R. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 7.Hickish T., Andre T., Wyrwicz L., Saunders M., Sarosiek T., Kocsis J. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology. 2017;18(2):192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 8.Korkaya H., Kim G.I., Davis A., Malik F., Henry N.L., Ithimakin S. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47(4):570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong H., Davis A., Ouzounova M., Carrasco R.A., Chen C., Breen S. A Novel IL6 Antibody Sensitizes Multiple Tumor Types to Chemotherapy Including Trastuzumab-Resistant Tumors. Cancer Res. 2016;76(2):480–490. doi: 10.1158/0008-5472.CAN-15-0883. [DOI] [PubMed] [Google Scholar]
- 10.Bedi A., Chang X., Noonan K., Pham V., Bedi R., Fertig E.J. Inhibition of TGF-beta enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Mol Cancer Ther. 2012;11(11):2429–2439. doi: 10.1158/1535-7163.MCT-12-0101-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arjaans M., Oude Munnink T.H., Timmer-Bosscha H., Reiss M., Walenkamp A.M., Lub-de Hooge M.N. Transforming growth factor (TGF)-beta expression and activation mechanisms as potential targets for anti-tumor therapy and tumor imaging. Pharmacol Ther. 2012;135(2):123–132. doi: 10.1016/j.pharmthera.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Brenner D., Blaser H., Mak T.W. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15(6):362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 13.Zhu G., Du Q., Wang X., Tang N., She F., Chen Y. TNF-alpha promotes gallbladder cancer cell growth and invasion through autocrine mechanisms. Int J Mol Med. 2014;33(6):1431–1440. doi: 10.3892/ijmm.2014.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang M.J., Sun K.H., Tang S.J., Deng M.W., Wu Y.H., Sung J.S. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci. 2008;99(5):905–913. doi: 10.1111/j.1349-7006.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikami S., Mizuno R., Kosaka T., Saya H., Oya M., Okada Y. Expression of TNF-alpha and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. Int J Cancer. 2015;136(7):1504–1514. doi: 10.1002/ijc.29137. [DOI] [PubMed] [Google Scholar]
- 16.Liu X.L., Li F.Q., Liu L.X., Li B., Zhou Z.P. TNF-alpha, HGF and macrophage in peritumoural liver tissue relate to major risk factors of HCC Recurrence. Hepatogastroenterology. 2013;60(125):1121–1126. doi: 10.5754/hge12982. [DOI] [PubMed] [Google Scholar]
- 17.Mir N., Jayachandran A., Dhungel B., Shrestha R., Steel J.C. Epithelial-to-Mesenchymal transition: a Mediator of Sorafenib Resistance in Advanced Hepatocellular Carcinoma. Curr Cancer Drug Targets. 2017;17(8):698–706. doi: 10.2174/1568009617666170427104356. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Fang R., Wang X.F., Zhang F., Chen D.Y., Zhou B. Stabilization of Snail through AKT/GSK-3beta signaling pathway is required for TNF-alpha-induced epithelial-mesenchymal transition in prostate cancer PC3 cells. Eur J Pharmacol. 2013;714(1–3):48–55. doi: 10.1016/j.ejphar.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 19.Li C.W., Xia W., Huo L., Lim S.O., Wu Y., Hsu J.L. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72(5):1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X., Sun X., Gao F., Luo J., Sun Z. Effects of ulinastatin and docataxel on breast tumor growth and expression of IL-6, IL-8, and TNF-alpha. Journal of Exp. Clin. Cancer Res. 2011;30(1):22. doi: 10.1186/1756-9966-30-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molor-Erdene P., Okajima K., Isobe H., Uchiba M., Harada N., Okabe H. Urinary trypsin inhibitor reduces LPS-induced hypotension by suppressing tumor necrosis factor-alpha production through inhibition of Egr-1 expression. Am J Physiol Heart Circ Physiol. 2005;288(3):H1265–H1271. doi: 10.1152/ajpheart.00885.2004. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J., Cheng A.L., Meinhardt G., Nakajima K., De Sanctis Y., Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., Wen F., Li J., Zhang P., Yan W., Hao P. A high baseline HBV load and antiviral therapy affect the survival of patients with advanced HBV-related HCC treated with sorafenib. Liver Int. 2015;35(9):2147–2154. doi: 10.1111/liv.12805. [DOI] [PubMed] [Google Scholar]
- 24.Egberts J.H., Cloosters V., Noack A., Schniewind B., Thon L., Klose S. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68(5):1443–1450. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 25.Yu M., Zhou X., Niu L., Lin G., Huang J., Zhou W. Targeting transmembrane TNF-alpha suppresses breast cancer growth. Cancer Res. 2013;73(13):4061–4074. doi: 10.1158/0008-5472.CAN-12-3946. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z.C., Ning F., Wang H.F., Chen D.Y., Cai Y.N., Sheng H.Y. Epidermal growth factor and tumor necrosis factor alpha cooperatively promote the motility of hepatocellular carcinoma cell lines via synergistic induction of fibronectin by NF-kappaB/p65. Biochim Biophys Acta. 2017;1861(11 Pt A):2568–2582. doi: 10.1016/j.bbagen.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Liu J., Hu X., Liu S., He B. Prognostic and Therapeutic Values of Tumor Necrosis Factor-Alpha in Hepatocellular Carcinoma. Med Sci Monit. 2016;22:3694–3704. doi: 10.12659/MSM.899773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X.Y., Ke A.W., Shi G.M., Zhang X., Zhang C., Shi Y.H. alphaB-crystallin complexes with 14-3-3zeta to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology (Baltimore, Md) 2013;57(6):2235–2247. doi: 10.1002/hep.26255. [DOI] [PubMed] [Google Scholar]
- 29.Chen H.A., Kuo T.C., Tseng C.F., Ma J.T., Yang S.T., Yen C.J. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2016;64(5):1637–1651. doi: 10.1002/hep.28773. [DOI] [PubMed] [Google Scholar]
- 30.Wei X., Tang C., Lu X., Liu R., Zhou M., He D. MiR-101 targets DUSP1 to regulate the TGF-beta secretion in sorafenib inhibits macrophage-induced growth of hepatocarcinoma. Oncotarget. 2015;6(21):18389–18405. doi: 10.18632/oncotarget.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai T., Arao T., Furuta K., Sakai K., Kudo K., Kaneda H. Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther. 2011;10(1):169–177. doi: 10.1158/1535-7163.MCT-10-0544. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y., Zhou B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102(4):639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J.C., Chuang H.Y., Hsu F.T., Chen Y.C., Chien Y.C., Hwang J.J. Sorafenib pretreatment enhances radiotherapy through targeting MEK/ERK/NF-kappaB pathway in human hepatocellular carcinoma-bearing mouse model. Oncotarget. 2016;7(51):85450–85463. doi: 10.18632/oncotarget.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudgeon C., Peng R., Wang P., Sebastiani A., Yu J., Zhang L. Inhibiting oncogenic signaling by sorafenib activates PUMA via GSK3beta and NF-kappaB to suppress tumor cell growth. Oncogene. 2012;31(46):4848–4858. doi: 10.1038/onc.2011.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alsaied O.A., Sangwan V., Banerjee S., Krosch T.C., Chugh R., Saluja A. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery. 2014;156(2):270–279. doi: 10.1016/j.surg.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 36.Ou D.L., Chang C.J., Jeng Y.M., Lin Y.J., Lin Z.Z., Gandhi A.K. Potential synergistic anti-tumor activity between lenalidomide and sorafenib in hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(12):2021–2031. doi: 10.1111/jgh.12708. [DOI] [PubMed] [Google Scholar]
- 37.Shahda S., Loehrer P.J., Clark R.S., Spittler A.J., Althouse S.K., Chiorean E.G. Phase I Study of Lenalidomide and Sorafenib in patients with Advanced Hepatocellular Carcinoma. Oncologist. 2016;21(6):664–665. doi: 10.1634/theoncologist.2016-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen F., Cai W.S., Li J.L., Feng Z., Liu Q.C., Xiao H.Q. Synergism from the combination of ulinastatin and curcumin offers greater inhibition against colorectal cancer liver metastases via modulating matrix metalloproteinase-9 and E-cadherin expression. OncoTargets and Therapy. 2014;7:305–314. doi: 10.2147/OTT.S57126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaguchi T., Muramoto M., Nakano T., Nishizaki T. Urinary trypsin inhibitor suppresses migration of malignant mesothelioma. Cancer Lett. 2010;288(2):214–218. doi: 10.1016/j.canlet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Sun Z.J., Yu T., Chen J.S., Sun X., Gao F., Zhao X.L. Effects of ulinastatin and cyclophosphamide on the growth of xenograft breast cancer and expression of CXC chemokine receptor 4 and matrix metalloproteinase-9 in cancers. J Int Med Res. 2010;38(3):967–976. doi: 10.1177/147323001003800323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2