Abstract
Objective
The main purpose of the present study was to investigate the possible somatosensory-related brain functional reorganization after traumatic spinal cord injury (SCI).
Methods
Thirteen patients with subacute incomplete cervical cord injury (ICCI) and thirteen age- and sex-matched healthy controls (HCs) were recruited. Eleven patients and all the HCs underwent both sensory task-related brain functional scanning and whole brain structural scanning on a 3.0 Tesla MRI system, and two patients underwent only structural scanning; the process of structural scanning was completed on thirteen patients, while functional scanning was only applied to eleven patients. We performed sensory task-related functional MRI (fMRI) to investigate the functional changes in the brain. In addition, voxel-based morphometry (VBM) was applied to explore whether any sensory-related brain structural changes occur in the whole brain after SCI.
Results
Compared with HCs, ICCI patients exhibited decreased activation in the left postcentral gyrus (postCG), the brainstem (midbrain and right pons) and the right cerebellar lobules IV-VI. Moreover, a significant positive association was found between the activation in the left PostCG and the activation in both the brainstem and the right cerebellar lobules IV-VI. Additionally, the decrease in gray matter volume (GMV) was detected in the left superior parietal lobule (SPL). The decrease of white matter volume (WMV) was observed in the right temporal lobe, the right occipital lobe, and the right calcarine gyrus. No structural change in the primary sensory cortex (S1), the secondary somatosensory cortex (S2) or the thalamus was detected.
Conclusion
These functional and structural findings may demonstrate the existence of an alternative pathway in the impairment of somatosensory function after SCI, which consists of the ipsilateral cerebellum, the brainstem and the contralateral postCG. It provides a new theoretical basis for the mechanism of sensory-related brain alteration in SCI patients and the rehabilitation therapy based on this pathway in the future.
Keywords: Somatosensory pathway, Task-related, Reorganization, fMRI, Incomplete cervical cord injury
Highlights
-
•
We found that sensory-related brain reorganization may not occur in the thalamus in patients with ICCI.
-
•
We found that brain structural reorganization did not occur in the S1 or the S2 in patients with ICCI.
-
•
We observed that SCI can cause brain structural reorganization in non-sensory-related areas.
-
•
We observed that an alternative pathway may exist in the impairment of somatosensory function after SCI.
Non-standard abbreviations
| ASIA | American Spinal Injury Association |
| GM | gray matter |
| GMV | gray matter volume |
| WM | white matter |
| WMV | white matter volume |
| CSF | cerebrospinal fluid |
| HC | healthy control |
| SCI | spinal cord injury |
| ICCI | incomplete cervical cord injury |
| VBM | voxel-based morphometry |
| ROI | region of interest |
| PostCG | postcentral gyrus |
| SPL | superior parietal lobule |
| TBI | traumatic brain injury |
| MNI | Montreal Neurological Institute |
1. Introduction
In addition to motor dysfunction, the loss of sensory function after traumatic spinal cord injury (SCI) also seriously affects the patients' quality of life and functional independence (Siddall and Loeser, 2001; Siddall et al., 2003; Wrigley et al., 2018). Therefore, understanding the mechanism of sensory impairment and the potential recovery of sensory function can help us to find a better method of rehabilitation for patients with sensory dysfunction. To date, many studies have shown that the brain reorganization after SCI is the key factor affecting the motor function and rehabilitation of patients (Grabher et al., 2017; Ilvesmäki et al., 2017; Villiger et al., 2015; Wrigley et al., 2018), while the number of studies on somatosensory functions is still relatively small (Grabher et al., 2015; Höller et al., 2017; Wrigley et al., 2018).
Prior studies have shown that sensory-related brain alterations occurred in SCI patients with somatosensory impairment (Aguilar et al., 2010; Chen et al., 2012; Freund et al., 2013; Freund et al., 2011; Ghosh et al., 2009; Grabher et al., 2015; Höller et al., 2017; Humanes-Valera et al., 2013; Jurkiewicz et al., 2006; Jutzeler et al., 2015, Jutzeler et al., 2016; Qi et al., 2011; Wrigley et al., 2009b), such as the reorganization of gray matter (GM) in the primary sensory cortex (S1) or secondary somatosensory cortex (S2) (Grabher et al., 2015; Höller et al., 2017; Hou et al., 2014; Jurkiewicz et al., 2006; Jutzeler et al., 2016) and the changes of white matter (WM) in the corticospinal or spinothalamic tracts (Cohen-Adad et al., 2011; Freund et al., 2012a, Freund et al., 2012b). The reorganization of GM resulted from the atrophy of cell size or apoptotic cell death in axotomized sensory fibers, while the alteration of WM was caused by demyelination and degeneration along the sensory pathway after SCI. To date, task-related research is still the main method to investigate somatosensory-related brain functional changes (Aguilar et al., 2010; Chen et al., 2012; Ghosh et al., 2009; Henderson et al., 2011; Jutzeler et al., 2015; Qi et al., 2011; Stroman et al., 2016; Wrigley et al., 2018). Sensory-related task studies on SCI animal models (Aguilar et al., 2010; Chen et al., 2012; Ghosh et al., 2009; Qi et al., 2011) and patients (Gustin et al., 2014; Stroman et al., 2016; Wrigley et al., 2009b, Wrigley et al., 2018) have shown the changes of brain activation in the sensory-related cortex and thalamus. It has been proved that brain remodeling occurs along the classical somatosensory pathway after SCI, which consists of the spinothalamic tract or dorsal columns, which that transmit the sensory signal to the thalamus, and then project to the S1 after transformation (Gustin et al., 2014; Lilja et al., 2006; Mtui et al., 2016; Wrigley et al., 2018).
However, contrary to the studies mentioned above, some scholars have shown that there were no structural changes in the thalamus, S1 or any other sensory-related areas after SCI (Chen et al., 2017; Florence et al., 1998; Henderson et al., 2011). At the same time, in a series of sensory task-related MRI studies (Henderson et al., 2011; Jutzeler et al., 2015; Yoon et al., 2013), no abnormal activations were found in the thalamus or the S1. These studies suggested that apart from the classical somatosensory pathway, there may exist another pathway in the process of sensory-related brain changes after SCI. This provides new insight into the mechanism of brain reorganization in SCI patients.
Therefore, the aim of this study is to identify whether any alternative sensory pathways replaced the classical pathway to play an important role in the sensory impairment after SCI using sensory task-related fMRI. Many SCI patients would develop neuropathic pain (Gustin et al., 2014; Jutzeler et al., 2015, Jutzeler et al., 2016; Wrigley et al., 2009b; Jutzeler et al., 2016; Warner et al., 2018), and a recent meta-analysis even showed the presence of neuropathic pain at 1-month post injury (Warner et al., 2018), thus, in order to avoid its interference in the study of sensory function, we chose subacute patients without neuropathic pain as subjects. To ensure the stability of the frequency and intensity of stimulation, we chose electrical stimulation as a sensory-related task. Additionally, we applied voxel-based morphometry (VBM) to further investigate possible brain structural changes along the sensory pathway.
2. Materials and methods
2.1. Subjects
Thirteen right-handed patients with subacute (within 2 months postinjury; duration: 11.69 ± 15.28d) incomplete cervical cord injury (ICCI) (10 male and 3 female patients, with a mean age of 51.3 ± 6.4 years and an age range of 22–70 years) were enrolled in this study. Nine patients were classified as grade D, two as grade B and the rest as grade C according to the American Spinal Injury Association (ASIA) Impairment Scale 2012 (www.asiaspinalinjury.org). All the subjects met the inclusion criteria: subacute (within 2 months postinjury) traumatic ICCI, without history of associated brain diseases confirmed by conventional MRI, no pre-existing mental illness or cognitive disorders affecting the functional outcome, no neuropathic pain (all of them with musculoskeletal pain at the injury site) and no contradictions to MRI. To eliminate the possibility of traumatic brain injury (TBI), all the patients underwent a comprehensive clinical assessment by two experienced physicians when they were admitted to the hospital, including the Glasgow Coma Scale, posttraumatic amnesia scale and loss of consciousness assessment, no positive findings were reported. All patients suffered from various forms of bilateral motor dysfunction, except one patient who exhibited only left side motor dysfunction. In addition, 9 of 13 patients had only upper limb sensory dysfunction and 4 of 13 the patients had sensory dysfunction in both the upper and lower extremities. For all patients, no other dysfunction was found except sensorimotor disorders. All patients underwent a comprehensive clinical assessment prior to the MR scan, including the light touch sensory score, the pinprick sensory score, and the motor score. These parameters were assessed by two qualified clinicians using the ASIA classification scale (Marino et al., 2003, Marino et al., 2008). The visual analog scale (VAS) was also administered to evaluate the severity of pain at the time of MRI acquisition. Sensory levels were assessed by testing two aspects of sensation, light touch and pinprick sensation (sharp/dull discrimination), at key points in each dermatome (C4-S4–5, bilateral). Motor function assessment comprised key muscle functions testing of 10 paired myotomes (C5-T1 and L2-S1; Jutzeler et al., 2016). Thirteen age-, sex- and education-matched right-handed healthy volunteers (9 male and 4 female controls with a mean age of 48.6 ± 5.6 years and a range of 24–62 years) were recruited as healthy controls (HCs). In our study, all the participants underwent sensory task-related brain functional scanning and whole brain structural scanning except for two patients who could not complete the remaining functional scanning due to the long data acquisition time. Thus, eleven SCI patients (8 male and 3 female, mean age: 50 ± 6.4 years, age range: 22–70 years) and thirteen HCs were included in the sensory-related task fMRI analysis. Table 1 provides detailed information of the patients with ICCI.
Table 1.
Clinical data of 13 patients with subacute traumatic incomplete cervical cord injury.
| ID | Age at Injury (years) | Sex | Etiology of the injury | Time since injury | Level of lesioana | Side of the injury | ASIA score | Motor (0−100) | Pinprick | Light touch | Sensory (0–224) | VAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | Vehicle accident | 60d | C4–6 | Bilateral | D | 80 | 112 | 112 | 224 | 0 |
| 2 | 22 | M | Vehicle accident | 7d | C6 | Bilateral | D | 100 | 108 | 108 | 216 | 0 |
| 3 | 70 | M | Vehicle accident | 2d | C3–7 | Bilateral | D | 94 | 107 | 107 | 214 | 0 |
| 4 | 53 | M | Hit by weight | 17d | C5–6 | Bilateral | D | 90 | 102 | 102 | 204 | 0 |
| 5b | 47 | M | Vehicle accident | 6d | C5 | Bilateral | D | 94 | 104 | 104 | 208 | 0 |
| 6 | 48 | M | Fall injury | 11d | C3–7 | Bilateral | B | 33 | 64 | 64 | 128 | 0 |
| 7 | 53 | M | Vehicle accident | 4d | C5–8 | Bilateral | D | 86 | 104 | 104 | 208 | 0 |
| 8 | 53 | F | Fall injury | 4d | C5–8 | Bilateral | C | 68 | 106 | 106 | 212 | 0 |
| 9 | 36 | M | Fall injury | 17d | C6–7 | Left | C | 40 | 93 | 93 | 186 | 0 |
| 10 | 58 | M | Fall injury | 6d | C3–5 | Bilateral | D | 62 | 108 | 108 | 216 | 0 |
| 11 | 46 | F | Vehicle accident | 5d | C3–4 | Bilateral | D | 76 | 100 | 100 | 200 | 0 |
| 12b | 60 | M | Fall injury | 4d | C3–4 | Bilateral | B | 0 | 44 | 44 | 88 | 0 |
| 13 | 56 | M | Fall injury | 9d | C4–5 | Bilateral | D | 80 | 112 | 112 | 224 | 0 |
The level of lesion refers to the neurological level. ASIA impairment scale: B: incomplete-sensory but not motor function is preserved below the neurological level and extends through sacral segments S4-S5; C: incomplete-motor function is preserved below the neurological level, and more than half of the key muscles below the neurological level have a muscle grade of <3; D: incomplete-motor function is preserved below the neurological level, and at least half of the key muscles below the neurological level have a muscle grade of >3. Sensory score: sum of segmental light touch and pinprick classifications. ASIA = American Spinal Injury Association. VAS = visual analogue scale. d = day.
Only brain structural data were acquired in these two patients.
All the participants were informed of the purpose of the study and gave written consent. The current study was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University (Beijing, China) and was in accordance with the Declaration of Helsinki.
2.2. Image acquisition
Images were obtained using a 3.0 T MRI system (Trio Tim, Siemens, Erlangen, Germany) with a 12-channel phase-array head coil. A conventional brain axial fluid-attenuated inverse recovery sequence was scanned and used to exclude visible brain abnormalities. The sensory task-related functional images were acquired with an echo-planar imaging sequence with the following parameters: 35 axial slices with a slice thickness = 3 mm and inter-slice gap = 1 mm, repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; readout bandwidth, 2004 Hz/pixel; field of view (FOV) = 220 × 220 mm2; matrix = 64 × 64; resulting in an isotropic voxel size of 3.4 × 3.4 × 3.0 mm3. The total acquisition time of task-state functional images was 3.08 min with 90 volumes. High-resolution three-dimensional (3D) structural T1-weighted images were acquired in sagittal orientation using a 3D magnetization-prepared rapid gradient-echo sequence (MP-RAGE) with the following parameters: TR = 1800 ms; TE = 2.13 ms; inversion time (TI) = 1100 ms; FA = 9°; number of slices = 192; slice thickness = 1 mm; FOV = 256 × 256 mm2; and matrix = 256 × 256; resulting in an isotropic voxel size of 1 × 1 × 1 mm3.
During the scanning process, tight but comfortable foam padding was used to minimize head motion, and earplugs were used to reduce imaging noise. The participants were asked to close their eyes, stay awake, breathe evenly, and try to avoid specific thoughts.
2.3. Peripheral sensory stimulation task
Electrical stimuli were applied using bipolar electrodes located on the skin surface of the wrist (radial nerve distribution area) and medial malleolus (saphenous nerve distribution area) of the right extremities separately. The rationale for this stimulation was to activate all the somatosensory fibers originating within the extremities under the appropriate intensity threshold by using a special medical electrical stimulator (MyoNet-COW, NCC Inc., Shanghai, China) placed outside the magnet room, including light touch and pinprick sensory stimulation, except for the nociceptive fibers. The whole stimulation was conducted during the scanning process.
The experimental design was as follows: The task state sequence had 4 repetitions, which were alternated by a 10-scan task block and a 10-scan rest block (beginning with a task block and ending with a rest block) and the first 10 scans were not given any stimulation in order to help the participants adjust and adapt themselves. To reduce unintended increases in somatosensory cortical activity from awareness, subjects were prevented from seeing cutaneous stimulation during the fMRI by asking them to lie down in the supine position and relax with their eyes closed. They were also asked to keep still during the scan and to stop mentally rehearsing the next stimulation process during the rest blocks. An electrical impulse was applied to the peripheral stimulation process, a unidirectional waveform with a duration of stimulation of 2 s, rest of 1 s, frequency of 50 Hz, and pulse width of 200 us.
To ensure the accuracy of the intensity of peripheral sensory stimulation during the entire scanning process, the stimulation task was practiced for every participant prior to the scanning procedure in a quiet and comfortable room. Beginning from 0 mA, the current amplitude was increased slowly at a rate of approximately 1 mA/s, and subjects were asked to say “yes” when they first perceived the electrical current. That amplitude was recorded as the sensory threshold (mA). Then, the amplitude was increased in the same manner up to the highest level that the subjects could tolerate without pain. That level was recorded as tolerance (mA). We chose the tolerance level current as the optimum intensity stimulation for the participants applied on the right upper or lower extremities during the scanning in the study, respectively. The method of measuring sensory threshold and tolerance was consistent with previous studies (Citak et al., 2014; Manganotti et al., 2012). This procedure was conducted by the same researcher using the same stimulator.
2.4. Image analysis
2.4.1. Task-related functional data processing
Statistical Parametric Mapping (SPM) software (version 12; http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB 2013a (Math Works, Natick, MA, USA) was used to perform the preprocessing of task functional images. First, the original images were slice-time corrected. Then, they were spatially realigned in order to correct the inter-volume motion displacement. The realignment parameters were checked, and all data were within the lower threshold of 2 mm of translation and 2° of rotation. Next, normalization was performed to register the realigned fMRI data into the standard Montreal Neurological Institute (MNI) space and convert them into 3 × 3 × 3 mm3 voxel sizes. Finally, the normalized data were spatially smoothed using a full-width half-maximum Gaussian kernel of 8 mm3.
2.4.2. Structural data processing and whole brain voxel-based morphometry analysis
We performed the preprocessing of structural data using SPM12 as well, the steps were consistent with our previous study (Chen et al., 2017). First, all the structural images were checked to make sure there were no apparent artifacts caused by factors such as head motion, susceptibility artifacts, and instrument malfunction. Then, the images were manually reoriented to place the anterior commissure at the origin and the anterior-posterior commissure in the horizontal plane. Next, the structural images were segmented into GM, white matter, and cerebrospinal fluid (CSF) areas using the unified standard segmentation option in SPM12. The individual WM and GM components were then normalized into the standard MNI space using the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) algorithm (Ashburner, 2007) after segmentation. The normalized GM component was modulated to generate the relative GMV multiplied by the nonlinear part of the deformation field at the DARTEL step. The resulting GMV images were then smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
2.5. Statistical analysis
The task-related fMRI data were first subjected to first-level analyses in SPM 12 to obtain the individual activation maps of the right hand and right foot. Then, a random-effect statistical analysis was performed within each group using the individual activation maps to generate group-wise activation maps. The threshold for significant activation was q < 0.01, with a cluster-level family-wise (FWE) method corrected for multiple comparisons at the cluster level and a minimum cluster size of 100 voxels. Using the general linear model (GLM) in SPM12, two sample t-tests were performed to test the intergroup differences in the activation intensity between the ICCI and HCs, with age and gender as nuisance covariates (uncorrected voxel-wise P < 0.01, cluster-level FWE correction with P < 0.05, with a minimum cluster size of 100 voxels). Then, a voxel-wise two-sample t-test was applied to compare the GMV and WMV differences in the whole brain between the subacute ICCI group and the HC group (voxel-level uncorrected P < 0.001, non-stationary cluster-level FWE correction with P < 0.05), and age and sex served as nuisance covariates. After that, region of interest (ROI)-based analysis was applied to verify the accuracy and reliability of the results of the whole brain method. Based on AAL Atlas, we used REST software (http://restfmri.net/forum) to obtain the bilateral S1, M1 and thalamus as ROIs for the subsequent analyses. Next, a voxel-wise two-sample t-test in SPM12 was applied to compare the GMV and WMV differences between the SCI group and the HC group (uncorrected voxel-level P < 0.01, cluster-level correction with P < 0.05), with these ROIs as the explicit mask, respectively, and age and sex as nuisance covariates. Then, the activation intensity and the mean GMV and WMV of each cluster with statistical significance were extracted for subsequent analyses. After that, a correlation analysis (two-tailed Spearman correlation) was performed to explore any potential associations among the brain activation changes (Bonferroni correction for multiple comparisons with P < 0.05). Finally, partial correlation analysis was performed to explore any possible associations between the activation intensity and the mean GMV and WMV of each cluster with statistical significance and clinical variables, such as sensory scores and injury duration, in patients with ICCI after removing age and sex effects. The last step was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). All data in this study were assessed by the Kolmogorov–Smirnov Test for normality. Data identified as not normally distributed were analyzed using nonparametric tests.
3. Results
3.1. Brain activation amplitudes changes in subacute ICCI patients
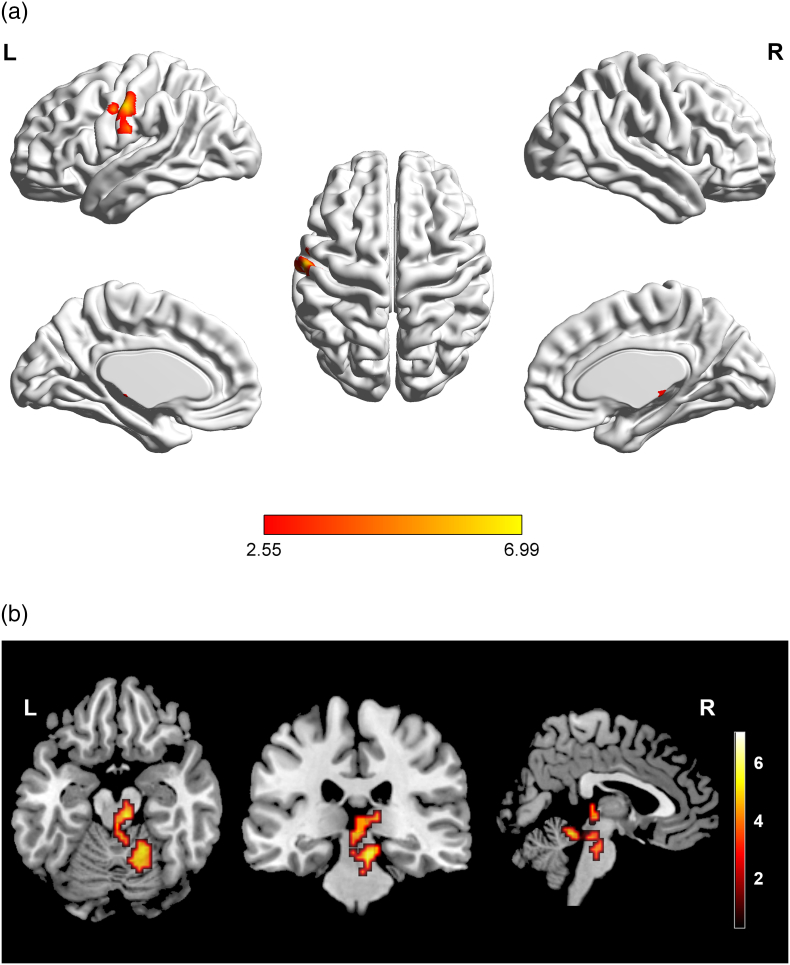
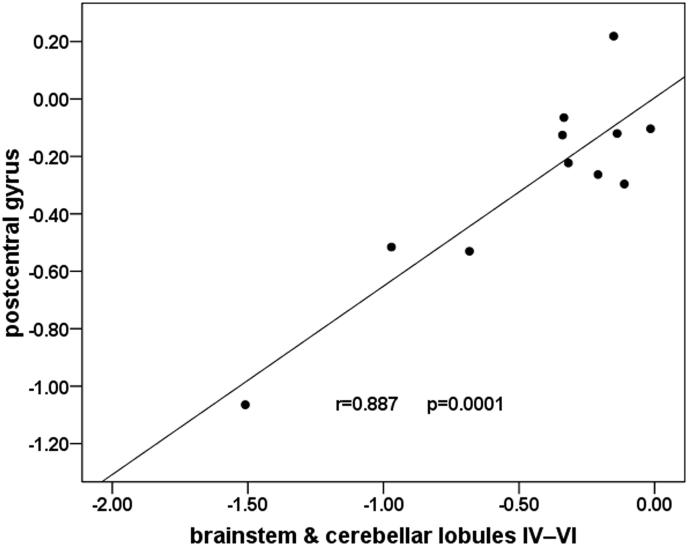
Stimulating the right lower extremity using the optimum current obtained by multiple tests (that result in significant superficial sensation, but do not cause pain) in all the participants, ICCI subjects showed significant signal intensity decreases in the left postcentral gyrus (PostCG) (Fig. 1A), the brainstem (midbrain and right pons) and the right cerebellar lobules IV-VI (Fig. 1B) when compared with HCs (cluster-level FWE correction with P < 0.05). Moreover, a significant positive association was found between the activation amplitude in the left PostCG and the activation amplitude in both the brainstem and right cerebellar lobules IV-VI (r = 0.887, P = 0.0001) in subacute ICCI patients (Bonferroni correction for multiple comparisons with P < 0.05) when compared with HCs (Fig. 2). When the electrical stimulation was performed on the right upper limb, we failed to find any significant differences in brain activation intensity between the ICCI patients and healthy volunteers. No activation intensity increases were observed in the whole brain of ICCI patients during the stimulation of the right upper and lower extremities.
Fig. 1.
Intergroup activation differences between the subacute ICCI subjects and HC subjects in the sensory task.
Compared to the healthy controls, the ICCI subjects showed decreased activation in the left PostCG (A), the brainstem (midbrain and right pons) and the right cerebellar lobules IV-VI (B) (corrected at cluster level with family-wise error P < 0.05 and cluster >100). PostCG = postcentral gyrus, ICCI = incomplete cervical spinal cord injury.
Fig. 2.
Correlation between the activation amplitude in the left PostCG and the activation amplitude in both the brainstem and right cerebellar lobules IV-VI in subacute ICCI patients.
Pearson's correlation revealed a significantly positive correlation between the activation amplitude in the left PostCG and the activation amplitude in both the brainstem and the right cerebellar lobules IV-VI in subacute ICCI patients (r = 0.887, P = 0.0001) (Bonferroni correction for multiple comparisons with P < 0.05). PostCG = postcentral gyrus, ICCI = incomplete cervical spinal cord injury.
3.2. Brain structural changes in the whole brain of subacute ICCI patients
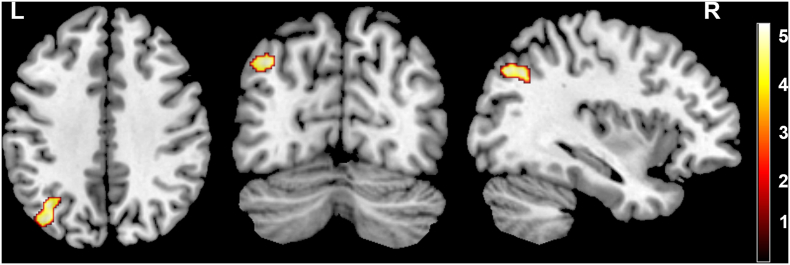
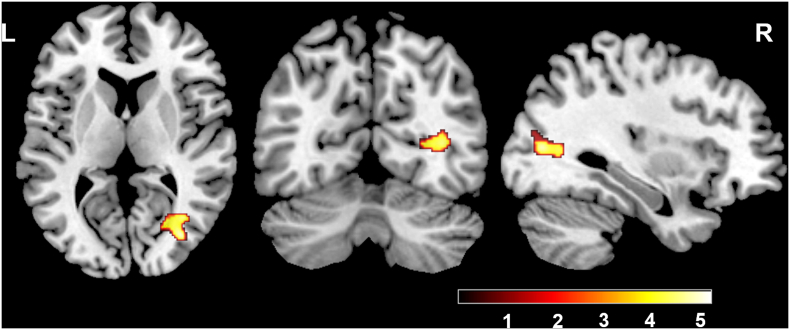
We found significantly decreased GMV in the left superior parietal lobule (Fig. 3) and WMV in the right temporal lobe, right occipital lobe and right calcarine gyrus (non-stationary cluster-level FWE correction with P < 0.05) (Fig. 4) of ICCI patients using the whole brain analysis. Except for these structural changes, patients with subacute ICCI exhibited no structural change in the S1, the S2, the thalamus, or along the spinothalamic tracts and the pathway from the thalamus project to the sensory-related cortex when compared with healthy controls. No significant difference was found in GMV or WMV between patients and HCs using ROI-based analysis (cluster-level P > 0.05, uncorrected). Moreover, no increased GMV and WMV were observed in the whole brain of ICCI patients.
Fig. 3.
Intergroup differences in GMV between the subacute ICCI patients and HCs.
Compared with healthy controls, subacute ICCI patients exhibited significantly decreased GMV in the left superior parietal lobule. Map threshold was set at P < 0.05 with non-stationary cluster-level FWE correction. GMV = gray matter volume, ICCI = incomplete cervical spinal cord injury.
Fig. 4.
Intergroup differences in WMV between the subacute ICCI patients and HCs.
Compared with healthy controls, subacute ICCI patients exhibited significantly decreased WMV in the right temporal lobe, the right occipital lobe and the right calcarine gyrus (non-stationary cluster-level FWE correction with P < 0.05). WMV = white matter volume, ICCI = incomplete cervical spinal cord injury.
3.3. Correlation analyses between clinical variables and the activation intensity and the mean GMV and WMV in subacute ICCI patients
In patients with subacute ICCI, partial correlation analyses showed that there were no correlations between the activation intensity and clinical variables and between the mean GMV and WMV and clinical variables (P > 0.05) (The clinical variables include sensory scores and injury duration).
4. Discussion
The present study showed significant brain functional reorganization in subacute ICCI patients when the right lower extremity was stimulated and all of these regions were closely related to somatosensory function (Grabher et al., 2017; Grodd et al., 2001; O'Reilly et al., 2010; Salmi et al., 2010; Sang et al., 2012; Stoodley and Schmahmann, 2009, Stoodley and Schmahmann, 2010). Moreover, a close association was found among the activation intensity of these activated areas. Additionally, GM atrophy was detected in the left SPL. The WMV decreased in the right temporal lobe, the right occipital lobe, and the right calcarine gyrus. No structural changes were found in the S1, the S2 or the thalamus. These brain functional and structural findings observed in this research may provide new insight into brain reorganization after spinal cord injury.
4.1. Another pathway of somatosensory-related brain functional reorganization in subacute ICCI patients
It has been widely accepted that the thalamus is the most important station to transform and integrate sensory afferent signals. Generally, the ventral posterior nucleus of the thalamus receives afferent fibers from two main somatosensory pathways: the spinothalamic tract and the dorsal columns. The spinothalamic tract originates from the paralemniscal pathway that carries mainly thermal and nociceptive signals (Wydenkeller et al., 2009). The dorsal columns originate from the lemniscal pathway that carries mainly tactile and proprioceptive signals from the periphery to the brain (Kaas et al., 2008). These two pathways interact with each other and show certain anatomical convergence at the thalamic level, then project to the primary sensory cortex after transformation (Mtui et al., 2016). Loss of sensory afferent information transmitted through this pathway would result in changes in the activation of the contralateral thalamus, the contralateral S1 (Curt et al., 2002; Jones and Pons, 1998; Mtui et al., 2016; Wrigley et al., 2018) and even the contralateral S2 (Mtui et al., 2016; Wrigley et al., 2018). However, in our study, significantly decreased activation was observed in the left S1, the brainstem (midbrain and right pons) and the right cerebellar lobules IV-VI of the ICCI patients when compared with HCs, while no activation change was found in the thalamus.
Reduced activation intensity of the ipsilateral cerebellar lobules would be expected given the loss of the ascending proprioceptive input received from the extremities projecting via the dorsal nucleus of the spinal cord and the dorsal spinocerebellar tract (Mtui et al., 2016; Wrigley et al., 2018). To date, many studies have demonstrated that the cerebellum plays an important role in the pathway of sensory function (Gao et al., 1996; O'Reilly et al., 2010; Sang et al., 2012; Stoodley and Schmahmann, 2009). Gao et al. (1996) showed the important role of the dentate nucleus of the human cerebellum in sensory acquisition and discrimination in a very early study. In recent decades, other researchers found that in the cerebellar hemispheres, the sensorimotor function is represented in lobules I—V (Grodd et al., 2001; Salmi et al., 2010) and occasionally in lobules VI and VIII (Stoodley and Schmahmann, 2009, Stoodley and Schmahmann, 2010). Some studies on the brain reorganization after SCI have also shown that the cerebellum is not only related to motor function (Villiger et al., 2015) but is also closely related to sensory function (Grabher et al., 2015), and even the motor function of the cerebellum is completed with the participation of sensory signals (Gao et al., 1996). Grabher et al. (2015) speculated that the decrease in the cerebellum likely shows a state of hypoactivity and the shrinkage of sensory neurons and their axons (Moxon et al., 2014). Moreover, some studies even indicated that close connections exist between the sensory-related subregions of the cerebellum and the sensory/sensorimotor network of the cerebrum (O'Reilly et al., 2010; Sang et al., 2012). O'Reilly et al. (2010) found that the primary sensorimotor region of the cerebellum (lobules V, VI, and VIII) is correlated with the somatosensory cortex of the cerebrum. In addition, Sang et al. (2012) showed that strong functional connectivity existed between lobules I-VI, VIII and the sensorimotor network of the cerebrum. Consistent with these findings, the cerebellar subregions of decreased activation we found in our study were closely related to sensory function. Apart from the decrease of activation in the cerebellum, we also found that the activation of the brainstem (the midbrain and right pons) decreased. The brainstem is phylogenetically highly conserved in mammals and plays a pivotal role in the process of sensory signal transmission (Benarroch, 2012; Brooks et al., 2017; Grabher et al., 2017; Hougaard et al., 2017; Liao et al., 2015). For instance, Brooks et al. (2017) and Grabher et al. (2017) found that the brainstem is an important structure for integrating and transmitting sensory signals, and the structural changes caused by SCI were directly related to impaired pinprick sensation (Grabher et al., 2017). As an important part of the brainstem, a few studies have proved that the midbrain is closely related to sensory function (Cuppini et al., 2018; Mylius et al., 2015) and is even involved in multisensory integration (Cuppini et al., 2018). Moreover, the pons was also found to be closely related to sensory function (Grabher et al., 2015; Insola et al., 2014), and SCI can lead to significant changes in the pons (Grabher et al., 2015). In conclusion, the brainstem, which includes the midbrain and pons, plays an important role in the transmission of sensory signals. All of these data demonstrated that, in addition to the primary somatosensory cortex, the brainstem and cerebellum lobules IV-VI are also closely related to sensory function. In addition, we found that the activation amplitude between them was significantly positively correlated, which means that the activation amplitude in the left postCG decreased, and the activation amplitude in both the brainstem and the cerebellar lobules IV-VI decreased accordingly. In other words, the activation changes in these regions were consistent with each other. Additionally, we performed correlation analyses between the brain activation and clinical sensory scores or injury duration after removing age and sex effects and observed no statistically significant differences. Thus, we believe the differences in sensory and motor impairments and the injury duration cannot be accountable for differences in sensory task-related activation.
Hence, based on all the aspects mentioned above, we speculated that the somatosensory-related functional reorganization after SCI may not be through the classical somatosensory pathway, but through another pathway from the ipsilateral cerebellum lobule to the brainstem (the midbrain and right pons) and then directly projecting to the S1. The process is completed through the cortico-ponto-cerebellar pathway as some previous studies showed (Baradaran et al., 2016; Ferilli et al., 2018). This may explain why we did not find any functional or structural changes in the thalamus.
In the present study, we found significant changes in brain activation in SCI patients when stimulating the right lower extremity, while no difference in the activation between the patients and the HCs was found when the upper limb was stimulated, although most patients exhibited sensory dysfunction of the upper limb. We speculated that the sensory signal transmission pathway in the upper and lower extremities may be different, and further studies should be conducted on the difference between them by increasing the sample size.
4.2. The structural reorganization in the whole brain of subacute ICCI patients
In our study, no functional alterations were found in the thalamus after SCI, but did its structure change? In addition, were there any changes in the structure along the classical sensory pathway? Prior studies showed that SCI not only can result in brain activation changes but also can lead to the alteration of white matter along the sensory pathway (Freund et al., 2011) and the GM change in the S1 and the thalamus (Jutzeler et al., 2016). However, except for the reduced GMV in the left SPL, we did not find any structural change in the thalamus, the S1, or the S2 by choosing both whole brain- and ROI-based analyses, which was consistent with some previous studies (Chen et al., 2017; Crawley et al., 2004; Yoon et al., 2013). We believed that the GM atrophy may not result from Wallerian degeneration (Wrigley et al., 2009a), but another process such as disuse-induced transneuronal degeneration (Bose et al., 2005; Jurkiewicz et al., 2006). While the SPL, which is also called the somatosensory association cortex anatomically, is not related to the process of sensory directly. It is an important part of the somatosensory system by receiving the nearby primary somatosensory cortex fibers; and it is also related to the integration, analysis, processing, and storage of common sensation (Rao et al., 2014; Squatrito et al., 2001). Moreover, we also found the decrease of WMV in the right temporal lobe, the right occipital lobe and the right calcarine gyrus, which is along the visual pathway anatomically (Arrigo et al., 2017; Mtui et al., 2016). The mechanism underlying these changes is still unclear. Further studies will be conducted on SCI patients' white matter integrity in the future. Combined with the functional results, these structural findings further support our claim that the brain reorganization in ICCI patients of the present study may not occur through the classic sensory pathway but rather through an alternative pathway. The classical sensory pathway may be partially or completely destroyed or inhibited due to the deafferentation of the afferent signal after SCI; thus, it was compensated or even completely replaced by a new or previously suppressed sensory transduction pathway.
Moreover, we also did not find any structural alterations in the brainstem and cerebellum lobes IV-VI, which may be due to the relatively short duration (within 2 months postinjury) and/or the moderate injury degree of SCI patients (all the patients are incomplete SCI). We believe that the injury may not yet have caused any structural changes in these areas.
4.3. Limitations
Our study had a number of limitations. First, the sample size was relatively small. Second, as a cross-sectional study, we did not conduct this work as a longitudinal study. Future long-term follow-up studies are necessary to document the full-time course of somatosensory structural and functional reorganization after SCI. Third, due to the moderate injury, patients in this study all had incomplete SCI, we cannot simply apply the mechanism of sensory impairment to the complete patients, and further studies on complete SCI are needed.
5. Conclusion
Our study demonstrated that an alternative pathway in the impairment of the sensory function following SCI may exist, and this pathway consists of the ipsilateral cerebellum, the brainstem, and the contralateral primary somatosensory cortex. This study provides a new theoretical basis for the mechanism of sensory-related brain reorganization after SCI. Moreover, the findings of the present study may also provide valuable information for the development of new and effective rehabilitation therapies based on this pathway in the future.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81871339; No. 81271556), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201609), Beijing Municipal Natural Science Foundation (No. 7113155), and Science Foundation of Beijing Municipal Commission of Education (No. KM201210025013).
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
QC was responsible for 1. study conception; 2. the acquisition, analysis, and interpretation of data; 3. drafting of the manuscript; 4. final approval of the version of the manuscript to be published; and 5. agreement to be accountable for all aspects of the work.
WM Z/XC/XJ L/LW/WQ were responsible for 1. data analysis; 2. final approval of the version of the manuscript to be published; and 3. agreement to be accountable for all aspects of the work.
KL was responsible for 1. revising the manuscript; 2. final approval of the version of the manuscript to be published; and 3. agreement to be accountable for all aspects of the work.
NC* was responsible for 1. study design; 2. manuscript revision; 3. final approval of the version of the manuscript to be published; and 4. agreement to be accountable for all aspects of the work.
Acknowledgments
The authors thank the patients and healthy volunteers who participated in this study and generously gave their time.
References
- Aguilar J., Humanes-Valera D., Alonso-Calvino E., Yague J.G., Moxon K.A., Oliviero A., Foffani G. Spinal cord injury immediately changes the state of the brain. J. Neurosci. 2010;30:7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A., Calamuneri A., Milardi D., Mormina E., Rania L., Postorino E., Marino S., Di Lorenzo G., Anastasi G.P., Ghilardi M.F., Aragona P., Quartarone A., Gaeta M. Visual system involvement in patients with newly diagnosed Parkinson disease. Radiology. 2017;285:885–895. doi: 10.1148/radiol.2017161732. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baradaran H., Omran S., Chazen J.L. Teaching neuroImages: acute crossed cerebellar diaschisis. Neurology. 2016;86:e154–e155. doi: 10.1212/WNL.0000000000002544. [DOI] [PubMed] [Google Scholar]
- Benarroch E.E. Periaqueductal gray: an interface for behavioral control. Neurology. 2012;78:210. doi: 10.1212/WNL.0b013e31823fcdee. [DOI] [PubMed] [Google Scholar]
- Bose P., Parmer R., Reier P.J., Thompson F.J. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp. Neurol. 2005;191:13–23. doi: 10.1016/j.expneurol.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Brooks J.C.W., Davies W., Pickering A.E. Resolving the brainstem contributions to attentional analgesia. J. Neurosci. 2017;37:2279–2291. doi: 10.1523/JNEUROSCI.2193-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.M., Qi H.X., Kaas J.H. Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury. J. Neurosci. 2012;32:14649–14663. doi: 10.1523/JNEUROSCI.1841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Zheng W., Chen X., Wan L., Qin W., Qi Z., Chen N., Li K. Brain gray matter atrophy after spinal cord injury: a voxel-based morphometry study. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citak K.I., Guney O.F., Aydin Y., Karakaya M.G. Effects of thermal agents on electrical sensory threshold and current tolerance when applied prior to neuromuscular electrical stimulation. J. Back Musculoskelet. Rehabil. 2014;27:191–196. doi: 10.3233/BMR-130435. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J., El Mendili M., Lehéricy S., Pradat P., Blancho S., Rossignol S., Benali H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. NeuroImage. 2011;55:1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- Crawley A.P., Jurkiewicz M.T., Yim A., Heyn S., Verrier M.C., Fehlings M.G., Mikulis D.J. Absence of localized grey matter volume changes in the motor cortex following spinal cord injury. Brain Res. 2004;1028:19–25. doi: 10.1016/j.brainres.2004.08.060. [DOI] [PubMed] [Google Scholar]
- Cuppini C., Stein B.E., Rowland B.A. Development of the mechanisms governing midbrain multisensory integration. J. Neurosci. 2018;38:3453–3465. doi: 10.1523/JNEUROSCI.2631-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt A., Bruehlmeier M., Leenders K.L., Roelcke U., Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J. Neurotrauma. 2002;19:43–51. doi: 10.1089/089771502753460222. [DOI] [PubMed] [Google Scholar]
- Ferilli M., Brunetti V., Costantini E.M., Della M.G. Left hemispheric status epilepticus with crossed cerebellar diaschisis. J. Neurol. Neurosurg. Psychiatry. 2018;89:311–312. doi: 10.1136/jnnp-2017-315930. [DOI] [PubMed] [Google Scholar]
- Florence S.L., Taub H.B., Kaas J.H. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- Freund P., Weiskopf N., Ward N.S., Hutton C., Gall A., Ciccarelli O., Craggs M., Friston K., Thompson A.J. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P., Schneider T., Nagy Z., Hutton C., Weiskopf N., Friston K., Wheeler-Kingshott C.A., Thompson A.J. Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P., Wheeler-Kingshott C.A., Nagy Z., Gorgoraptis N., Weiskopf N., Friston K., Thompson A.J., Hutton C. Axonal integrity predicts cortical reorganisation following cervical injury. J. Neurol. Neurosurg. Psychiatry. 2012;83:629–637. doi: 10.1136/jnnp-2011-301875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P., Weiskopf N., Ashburner J., Wolf K., Sutter R., Altmann D.R., Friston K., Thompson A., Curt A. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 2013;12:873–881. doi: 10.1016/S1474-4422(13)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.H., Parsons L.M., Bower J.M., Xiong J., Li J., Fox P.T. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Sydekum E., Haiss F., Peduzzi S., Zorner B., Schneider R., Baltes C., Rudin M., Weber B., Schwab M.E. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabher P., Callaghan M.F., Ashburner J., Weiskopf N., Thompson A.J., Curt A., Freund P. Tracking sensory system atrophy and outcome prediction in spinal cord injury. Ann. Neurol. 2015;78:751–761. doi: 10.1002/ana.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabher P., Blaiotta C., Ashburner J., Freund P. Relationship between brainstem neurodegeneration and clinical impairment in traumatic spinal cord injury. NeuroImage. 2017;15:494–501. doi: 10.1016/j.nicl.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodd W., Hulsmann E., Lotze M., Wildgruber D., Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin S.M., Wrigley P.J., Youssef A.M., McIndoe L., Wilcox S.L., Rae C.D., Edden R.A., Siddall P.J., Henderson L.A. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain. 2014;155:1027–1036. doi: 10.1016/j.pain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.A., Gustin S.M., Macey P.M., Wrigley P.J., Siddall P.J. Functional reorganization of the brain in humans following spinal cord injury: evidence for underlying changes in cortical anatomy. J. Neurosci. 2011;31:2630–2637. doi: 10.1523/JNEUROSCI.2717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höller Y., Tadzic A., Thomschewski A.C., Höller P., Leis S., Tomasi S.O., Hofer C., Bathke A., Nardone R., Trinka E. Factors affecting volume changes of the somatosensory cortex in patients with spinal cord injury: to be considered for future neuroprosthetic design. Front. Neurol. 2017;8 doi: 10.3389/fneur.2017.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J.M., Yan R.B., Xiang Z.M., Zhang H., Liu J., Wu Y.T., Zhao M., Pan Q.Y., Song L.H., Zhang W., Li H.T., Liu H.L., Sun T.S. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience. 2014;266:208–215. doi: 10.1016/j.neuroscience.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Hougaard A., Amin F.M., Larsson H.B.W., Rostrup E., Ashina M. Increased intrinsic brain connectivity between pons and somatosensory cortex during attacks of migraine with aura. Hum. Brain Mapp. 2017;38:2635–2642. doi: 10.1002/hbm.23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humanes-Valera D., Aguilar J., Foffani G. Reorganization of the intact somatosensory cortex immediately after spinal cord injury. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilvesmäki T., Koskinen E., Brander A., Luoto T., Öhman J., Eskola H. Spinal cord injury induces widespread chronic changes in cerebral white matter. Hum. Brain Mapp. 2017;38:3637–3647. doi: 10.1002/hbm.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insola A., Padua L., Mazzone P., Scarnati E., Valeriani M. Low and high-frequency somatosensory evoked potentials recorded from the human pedunculopontine nucleus. Clin. Neurophysiol. 2014;125:1859–1869. doi: 10.1016/j.clinph.2013.12.112. [DOI] [PubMed] [Google Scholar]
- Jones E.G., Pons T.P. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz M.T., Crawley A.P., Verrier M.C., Fehlings M.G., Mikulis D.J. Somatosensory cortical atrophy after spinal cord injury: a voxel-based morphometry study. Neurology. 2006;66:762–764. doi: 10.1212/01.wnl.0000201276.28141.40. [DOI] [PubMed] [Google Scholar]
- Jutzeler C.R., Freund P., Huber E., Curt A., Kramer J.L.K. Neuropathic pain and functional reorganization in the primary sensorimotor cortex after spinal cord injury. J. Pain. 2015;16:1256–1267. doi: 10.1016/j.jpain.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Jutzeler C.R., Huber E., Callaghan M.F., Luechinger R., Curt A., Kramer J.L.K., Freund P. Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. UK. 2016;6 doi: 10.1038/srep18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H., Qi H., Burish M.J., Gharbawie O.A., Onifer S.M., Massey J.M. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp. Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., DiCarlo G.E., Gharbawie O.A., Qi H., Kaas J.H. Spinal cord neuron inputs to the cuneate nucleus that partially survive dorsal column lesions: a pathway that could contribute to recovery after spinal cord injury. J. Comp. Neurol. 2015;523:2138–2160. doi: 10.1002/cne.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja J., Endo T., Hofstetter C., Westman E., Young J., Olson L., Spenger C. Blood oxygenation level-dependent visualization of synaptic relay stations of sensory pathways along the neuroaxis in response to graded sensory stimulation of a limb. J. Neurosci. 2006;26:6330–6336. doi: 10.1523/JNEUROSCI.0626-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P., Storti S.F., Formaggio E., Acler M., Zoccatelli G., Pizzini F.B., Alessandrini F., Bertoldo A., Toffolo G.M., Bovi P., Beltramello A., Moretto G., Fiaschi A. Effect of median-nerve electrical stimulation on BOLD activity in acute ischemic stroke patients. Clin. Neurophysiol. 2012;123:142–153. doi: 10.1016/j.clinph.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Marino R.J., Barros T., Biering-Sorensen F., Burns S.P., Donovan W.H., Graves D.E., Haak M., Hudson L.M., Priebe M.M. International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2003;26(Suppl. 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Marino R.J., Jones L., Kirshblum S., Tal J., Dasgupta A. Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2008;31:166–170. doi: 10.1080/10790268.2008.11760707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon K.A., Oliviero A., Aguilar J., Foffani G. Cortical reorganization after spinal cord injury: always for good? Neuroscience. 2014;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtui E., Gruener G., Dockery P. 7th ed. Elsevier; Philadelphia, PA: 2016. Fitzgerald's Clinical Neuroanatomy and Neuroscience. [Electronic Resource] [Google Scholar]
- Mylius J., Happel M.F.K., Gorkin A.G., Huang Y., Scheich H., Brosch M. Fast transmission from the dopaminergic ventral midbrain to the sensory cortex of awake primates. Brain Struct. Funct. 2015;220:3273–3294. doi: 10.1007/s00429-014-0855-0. [DOI] [PubMed] [Google Scholar]
- O'Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H.X., Chen L.M., Kaas J.H. Reorganization of somatosensory cortical areas 3b and 1 after unilateral section of dorsal columns of the spinal cord in squirrel monkeys. J. Neurosci. 2011;31:13662–13675. doi: 10.1523/JNEUROSCI.2366-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J., Ma M., Zhao C., Zhang A., Yang Z., Liu Z., Li X. Fractional amplitude of low-frequency fluctuation changes in monkeys with spinal cord injury: a resting-state fMRI study. Magn. Reson. Imaging. 2014;32:482–486. doi: 10.1016/j.mri.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Salmi J., Pallesen K.J., Neuvonen T., Brattico E., Korvenoja A., Salonen O., Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 2010;22:2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Sang L., Qin W., Liu Y., Han W., Zhang Y., Jiang T., Yu C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage. 2012;61:1213–1225. doi: 10.1016/j.neuroimage.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Siddall P.J., Loeser J.D. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Squatrito S., Raffi M., Maioli M.G., Battaglia-Mayer A. Visual motion responses of neurons in the caudal area pe of macaque monkeys. J. Neurosci. 2001;21:C130. doi: 10.1523/JNEUROSCI.21-04-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroman P.W., Khan H.S., Bosma R.L., Cotoi A.I., Leung R., Cadotte D.W., Fehlings M.G. Changes in pain processing in the spinal cord and brainstem after spinal cord injury characterized by functional magnetic resonance imaging. J. Neurotrauma. 2016;33:1450–1460. doi: 10.1089/neu.2015.4257. [DOI] [PubMed] [Google Scholar]
- Villiger M., Grabher P., Hepp-Reymond M.C., Kiper D., Curt A., Bolliger M., Hotz-Boendermaker S., Kollias S., Eng K., Freund P. Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: a longitudinal pilot study. Front. Hum. Neurosci. 2015;9:254. doi: 10.3389/fnhum.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner F., Cragg J.J., Jutzeler C., Finnerup N., Werhagen L., Weidner N., Maier D., Kalke Y.B., Curt A., Kramer J. The progression of neuropathic pain after acute spinal cord injury: a meta-analysis and framework for clinical trials. J. Neurotrauma. 2018 doi: 10.1089/neu.2018.5960. [DOI] [PubMed] [Google Scholar]
- Wrigley P.J., Gustin S.M., Macey P.M., Nash P.G., Gandevia S.C., Macefield V.G., Siddall P.J., Henderson L.A. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb. Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Wrigley P.J., Press S.R., Gustin S.M., Macefield V.G., Gandevia S.C., Cousins M.J., Middleton J.W., Henderson L.A., Siddall P.J. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Wrigley P.J., Siddall P.J., Gustin S.M. New evidence for preserved somatosensory pathways in complete spinal cord injury: a fMRI study. Hum. Brain Mapp. 2018;39:588–598. doi: 10.1002/hbm.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydenkeller S., Maurizio S., Dietz V., Halder P. Neuropathic pain in spinal cord injury: significance of clinical and electrophysiological measures. Eur. J. Neurosci. 2009;30:91–99. doi: 10.1111/j.1460-9568.2009.06801.x. [DOI] [PubMed] [Google Scholar]
- Yoon E.J., Kim Y.K., Shin H.I., Lee Y., Kim S.E. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013;1540:64–73. doi: 10.1016/j.brainres.2013.10.007. [DOI] [PubMed] [Google Scholar]