Introduction
It is estimated that there are 17.6 million alcoholic individuals in the US and 140 million worldwide; while not all alcoholics develop symptomatic liver disease, about 12,109 deaths/year are attributed to alcoholic liver disease in the US [1,2]. The clinical spectrum of ALD includes alcoholic fatty liver, alcoholic steatohepatitis with or without fibrosis and cirrhosis over time [2]. Acute alcoholic hepatitis is the most severe form of the disease with high mortality and limited treatment options. Over-activation of the innate immune system is one of the major characteristics of acute alcoholic hepatitis and it plays a central role in disease pathogenesis. Even in subclinical steatohepatitis, the presence of inflammation in the liver contributes to diseases progression and development of fibrosis.
Innate Immunity and Inflammation
Inflammation is a response of the innate immune system to danger signals derived from pathogens or from damaged self [3–6]. These pathogen-associated molecular patterns (PAMPs) or damage associated molecules (DAMPs) are recognized by cells of the innate immune system via various pattern recognition receptors. In recent years, several major families of pattern recognition receptors have been identified including Toll-like Receptors, Nod-like receptors and helicase receptors (RIG-I, Mda5). There are 13 different mammalian TLRs of which TLR1,2,4,5,6 are expressed on the cell surface and TLR3,7,8,9 expressed in endosomes [4,7]. Each of these TLRs have specific ligand recognition profiles. Immune recognition of danger signals occurs with the help of pattern recognitions receptors (PRRs). In “sterile” inflammation associated with tissue injury, damaged host cells release damage-associated molecular patterns “DAMPs” that are recognized by the same repertoire of TLRs and PPRs as exogenous danger signals [6]. In certain pathologies, PAMPs and DAMPs can be both involved and amplify inflammatory responses.
Toll-like receptors are evolutionarily conserved sensors of PAMPs. Of the 13 TLRs, most are functionally active in humans. TLRs expressed on the cell surface (TLR1,2,4,5,6) recognize extracellular PAMPs while intracellularly localized TLRs (TLR3, &, 8, 9) sense nucleic acid sequences [4,7]. The cytoplasmic TIR domain of TLRs interact with the TIIR domain of adapter molecules such as the My88 common adapter utilized by all TLRS except for TLR3. MyD88 recruitment triggers downstream signaling via IRK1/4 kinases and IKK kinase activation to culminate in NK-ϰB activation and induction of pro-inflammatory cytokine genes. TLR3 and TLR4 utilize the TRIF adapter that activates IKK-ε/TBK-1 leading to IRF3 or IRF7 phoshorylation and after their nuclear translocation, induction of Type I IFNs [4,5,7].
Nod-like receptors (NLRs) are sensors of the inflammasome complex that upon activation, leads to caspase-1 cleavage and the activated caspase-1 cleaves pro-IL-1ß to become biologically active and secreted (17 kD IL-1ß) [6]. Secreted IL-1ß has autocrine effects through the IL-1 receptor to increase its own production and it also amplifies inflammation via induction of other pro-inflammatory cytokines [8].
Inflammation and Innate Immunity in Alcoholic Steatohepatitis
The pathomechanism of ALD involves complex interactions between the effects of alcohol and its toxic metabolites on various cell types in the liver, induction of reactive oxygen species, and up-regulation of the inflammatory cascade [9–13]. Studies using antibiotics to “sterilize” the gut and experiments with elimination of Kupffer cells (KC) identified both gut- derived factors, such as lipopolysaccharide (LPS), and Kupffer cell activation as central components in the development of ALD (Figure 1) [14–18]. Studies demonstrated that alcohol use results in impairment of the gut barrier function contributing to increased gut-derived LPS delivery to the liver via the portal blood [19–22]. LPS, also known as endotoxin, activates immune cells including Kupffer cells (liver resident macrophages), and modulates the function of hepatocytes, sinusoidal endothelial cells and stellate cells [3,23]. At the cellular and molecular level, LPS is recognized by the Toll-like receptor (TLR) 4 complex and induces specific intracellular activation pathways. Chronic alcohol exposure sensitizes macrophages to LPS-induced inflammatory cytokine production, however the molecular mechanisms underlying this effect are yet to be fully explored [24–26].
Figure 1.
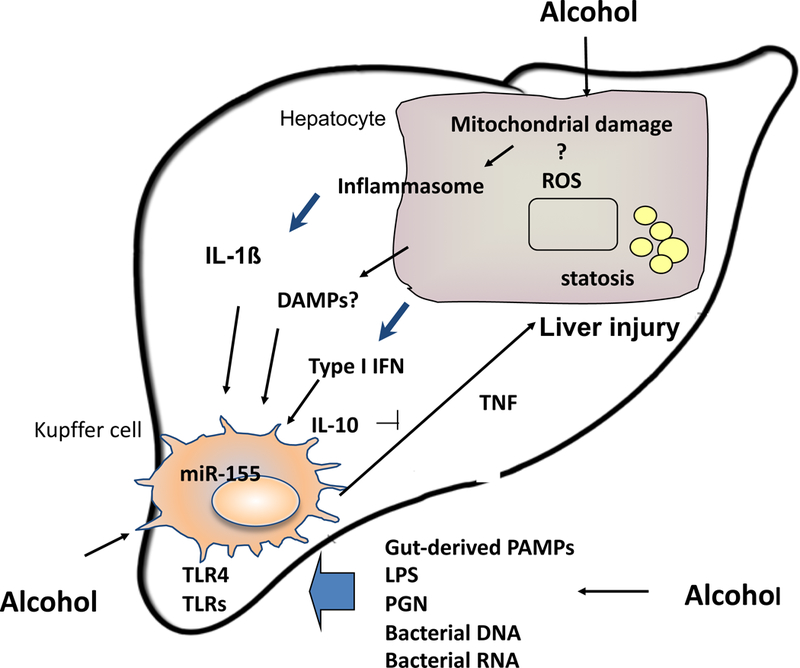
Innate immune activation in ALD
Acute alcoholic hepatitis is a state of systemic pro-inflammatory cascade activation where not only intrahepatic but also systemic signs of inflammation are present together with hepatocyte/liver dysfunction. Previous studies identified TNFα as a central mediator of ALD [27,28]. TNFα was increased in human alcoholic hepatitis in the serum and in the liver on immunohistochemical staining [29,30]. However, human clinical trials with TNFα blockade using anti-TNF antibodies were discontinued due to infectious complications [31,32]. The currently used treatment for alcoholic steatohepatitis, corticosteroids or pentoxiphylline, both have anti-inflammatory properties but limited benefits [33,34]. It has been shown that pro-inflammatory cytokines, other than TNFα, are also increased including IL-6, IL-8 and IL-1 [26,30].
TNFα and IL-1 are both induced by pathogen-associated and/or damage-associated danger signals (PAMPs and DAMPs) via pattern recognition receptors (TLRs and/or inflammasomes) [3–6]. Furthermore, TNFα and IL-1 amplify one and another’s production and inflammation [4–6,8]. While many cells produce pro-inflammatory cytokines, monocytes and macrophages the most robust source of TNFα and IL-1. In liver inflammation, monocytes are recruited via the circulation from the bone marrow to the liver where they become macrophages [7]. Indeed, in AH the phenotype of circulating monocytes already indicates over-activation of the inflammatory cascade as these cells produce TNFα and showed increased NF-κB activation. Thus, it is very likely that in alcoholic hepatitis, cells of the innate immune system including blood monocytes are over-activated and fail to activate homeostatic regulatory mechanisms that limit inflammation. This hypothesis is supported by the lack of IL-10 production in monocytes/macrophages in ALD [35]. While in normal monocytes repeated LPS challenge results in attenuated TNFα production as a result of TLR tolerance, in monocytes exposed to chronic alcohol TLR tolerance is lost [36]. The loss of TLR tolerance is associated with a hyper-responsiveness to LPS in monocytes after chronic alcohol exposure as well as in KCs of alcohol-fed mice [36].
The Role of IL-1 in ALD
Inflammatory cytokines, including TNF-α, IL-6 and IL-1 are increased in the serum of patients with alcoholic liver disease [27,30,37,38]. We evaluated the effects of a 4 week alcohol feeding with a Lieber-deCarli or pair-fed control diet in C57Bl/6 mice and found significant induction of liver steatosis and evidence of pro-inflammatory cytokine induction in the liver. In addition, we found increased concentration of IL-1β, but not IL-1α, in the serum and in the liver of alcohol-fed mice. Pro-IL-1β is cleaved by Caspase-1 which is activated by the multiprotein cytoplasmic complex, inflammasome [39]. Inflammasomes are composed of different NOD-like receptors, intracellular sensors of danger signals, adapter molecules, such as ASC and cleave pro-Caspase-1 to induce Caspase-1 activation [6]. We found that Caspase-1 activity was increased in livers after alcohol administration and there was also an incresase in the mRNA expression of some of the key components of the inflammasome including pro-Caspase 1, ASC and NALP3 (Figure 2). These results suggested that IL-1 as well as upregulation of the inflammasome are major components of the pathogenesis of alcoholic liver disease.
Figure 2. Inflammasome components are upregulated in alcoholic liver disease in mice.
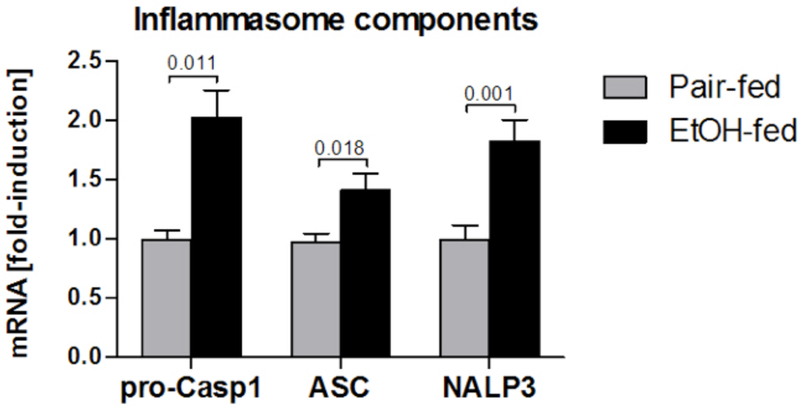
Wild-type mice were fed with control (pair-fed) or alcohol (EtOH-fed) diet and sacrificed 4 week later. The expression of inflammasome components pro-Caspase-1, ASC and NLRP3 in the liver was measured using qPCR. N=5–10 mice per group. Numbers in graph denote p values.
MicroRNAs in Regulation of Inflammation
Regulation of TNFα production involves multiple levels including microRNAs that contribute to fine-tuning of cellular responses. MicroRNAs(miRNAs) are a class of evolutionarily conserved, single-stranded non-coding RNAs of 19–24 nucleotides that control gene expression at the post-transcriptional levels by imperfectly base pairing with the 3’ untranslated regions (3’-UTR) of target RNAs, preventing protein accumulation by repressing their translation or by accelerating mRNA degradation [40,41]. Transcripts from genes encoding miRNA called primary miRNA (pri-miRNA) are processed to precursor miRNA (pre-miRA) and upon translocation to the cytosol, they are cleaved by Dicer into 21–23 nucleotide miRNA duplexes [41]. Then miRNA strands are loaded into RNA-induced silencing (RISC) complexes [41].
Innate immune responses are fine tuned by miR-155, miR-125b and miR-146a where these miRNAs act to positively or negatively regulate target genes/proteins. MiR-155 exerts positive regulation on TNF-α through enhancing its translation [42]. In contrast, miR146a acts as a negative regulator of inflammatory mediator production, involved in TLR tolerance and it inhibits IRAK-1 and TRAF6 [43]. MiR-125b functions as a post-transcriptional repressor of TNF-α [42]. MicroRNA-155 is a multifunctional miRNA involved in numerous biological processes including inflammation, immunity and hematopoesis [43]. MiR-155 has been characterized as a component of a macrophage inflammatory response and it is induced by TLR ligands LPS (TLR4) or poly I:C (TLR3), cytokines including TNF-α and IL-1B, or IFNB [43]. All of these cytokines are increased in alcoholic liver damage [27,30,37,38]. In contrast, the anti-inflammatory cytokine, IL-10 has negative regulatory effect on miR-155 induction [5], however, this cytokine is decreased after chronic alcohol administration in the liver [35]. We recently demonstrated that miR-155 induction involved NF-kB activation in macrophages and in Kupffer cells [44]. TNF-α, the most studied target gene of miR-155, is up-regulated by miR-155 via transcriptional regulation [45]. We also found that miR-155-mediated augmentation of TNFα production involved increases in the mRNA stability of TNFα in macrophages [44].
The importance of miRNAs is not limited to regulation of intracellular processes, as they represent a novel approach in diagnostics and therapeutic interventions. Owed to their stability in the serum and plasma, miRNAs can serve as biomarkers in diseases including liver disease [46–49]. Mir-122 is liver-specific (represents 80% of the total liver miRNAs [50]) and it is abundantly expressed in hepatocytes where it regulates fat metabolism. Our preliminary data and recent reports suggest that circulating miR-122 is increased in liver injury and, thus, may be an attractive biomarker [49,51]. Our preclinical studies also indicate that circulating miR-155 levels correlate with inflammation in the liver warranting the need for further exploration of miRNAs as biomarkers of alcoholic liver disease (Figue 3).
Figure 3. Schematic of micro-RNAs in alcohol-related liver injury.
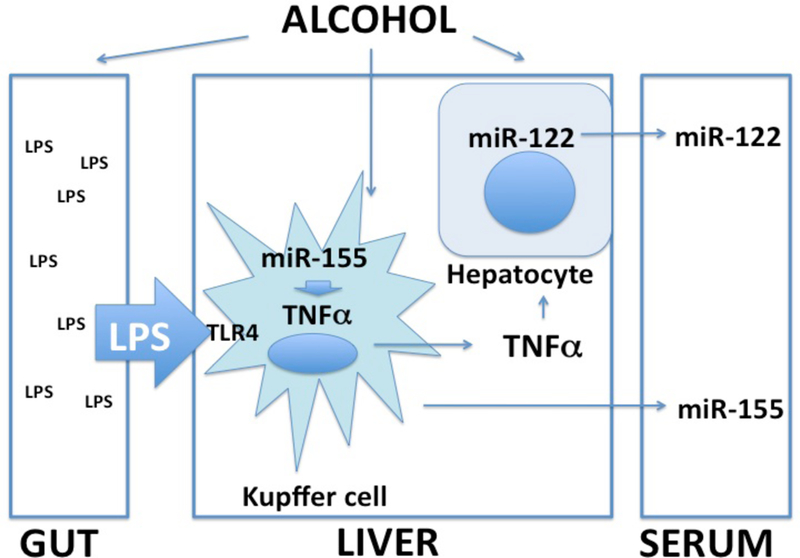
Alcohol-induced increase in gut permeability results in increased LPS exposure and sensitization of Kupffer cells to produce pro-inflammatory cytokines such as TNFα. Micro-RNA-155 is a key regulator of TNFα production, Alcohol increases miR-155 in KCs and this results in augmentation of LPS-induced KC TNFα production. Alcohol also sensitizes hepatocytes to TNFα-induced cell injury. Micro-RNA-122, a hepatocye-specific miRNA as well as miR-155 are increased in the serum in alcohol-induced liver damage in mice.
Alcohol-induced MiR-155 Sensitizes Kupffer cells to LPS-induced TNF-α Over-production
As detailed in our recent publication, prolonged alcohol exposure in RAW 264.7 cells, a macrophage type cell line resulted in a selective increase in the expression of miR-155 and not miR-125b or miR-146a [44,49]. Furthermore, alcohol exposure or miR-155 over-expression in normal macrophages sensitized to LPS and resulted in significantly higher TNFα production compared to untreated control cells. Using an anti-miR-155 inhibitor, we showed that the sensitization of macrophages by alcohol to LPS could be prevented by miR-155 inhibition. We demonstrated that alcohol alone increased the half-life of TNFa mRNA and miR-155 inhibition could prevent this effect [44]. We found that ex vivo LPS stimulation of Kupffer cells from C57/Bl6 mice increased miR-155 expression (Fig 4). Furthermore, chronic alcohol feeding in mice resulted in increased levels of miR-155 in isolated Kupffer cells and these KCs were in vivo sensitized for increased LPS-induced miR-155 expression compared ot KCs from pair-fed mice (Fig 4). Together these results suggest that chronic alcohol increases miR-155 in KCs and this likely contributes to the increased sensitivity of alcohol-exposed KCs to LPS stimulation which mechanisms amplifies inflammation.
Figure 4. Induction of miRNA-155 in Kupffer cells of alcohol-fed mice.
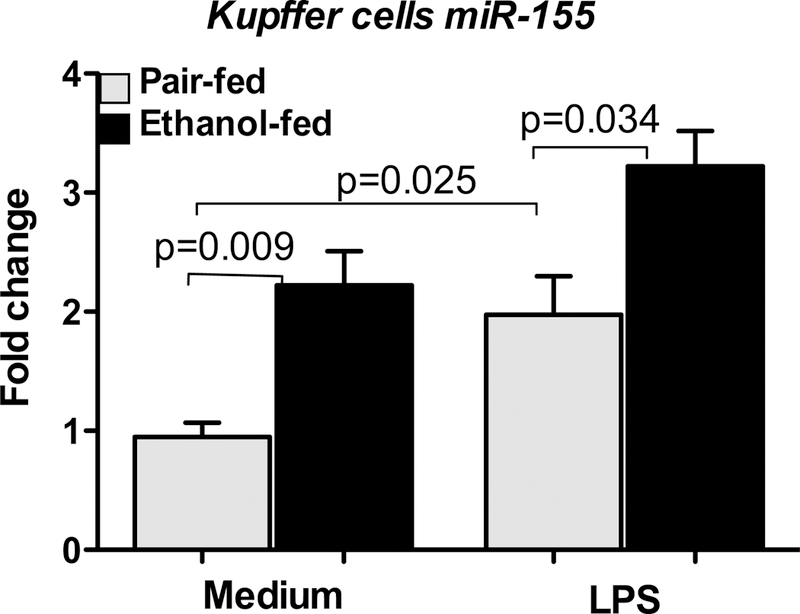
Kupffer cells isolated either from pair-fed or alcohol-fed mice (n=8–10/group, cells from two mice were pooled) after 5 weeks of Leiber DeCarli diet were plated onto 24 well plate. Nonadherent cells were removed after 2–3h of plating, fresh medium was added to adherent cells and rested overnight. Next day, cells were stimulated or not with 100ng/ml LPS for 6h, washed with PBS and lysed in Qiazole (Qiagen). Total RNA was isolated using miRNeasy kit (Qiagen) and used to quantify miRNA-155 expression by real time PCR using TaqMan miRNA Assays (Applied Biosystems). SnoRNA202 was used for normalization and fold increase over the pairfed control group is shown. Data represent mean values ± S.E.M. Non-parametric MannWhitney test was employed for statistical analysis.
Therapeutic implications
Evidence is emerging on evaluation of miRs as potential targets for new therapeutic interventions [49,52,53]. We have recently reported the regulatory role of alcohol-induced miR- 155 in sensitization of KCs to LPS that provides a basis for future investigation of anti-miR-155 strategies in alcoholic liver disease in this application. The clinical spectrum of alcoholic liver disease includes steatosis, inflammation and alcoholic steatohepatitis and the latter is associated with 50% mortality [54]. The currently available therapeutic modalities have limited benefit in alcoholic hepatitis. Administration of corticosteroids have limited benefits and carry the risk of infections [31,32]. Early clinical trials with anti-TNF-α blocking antibodies were based on the clinical observation of elevated serum and liver TNF-α levels and protection of TNFR1-deficient mice from alcoholic liver diseases. However, clinical trials using anti-TNFα blocking antibodies were discontinued due to increased frequency of infections. Pentoxyfillin, an inhibitor which minimally attenuates TNFα production, had modest benefit in clinical applications [33]. While TNFα and regulation of the inflammatory cascade remain attractive targets in regulation of innate immune system, instead of full inhibition of pro-inflammatory mediator production, are desirable to ameliorate the abnormal cytokine environment in ALD. Given the fine-tuning potential of miRNAs, which have modest regulatory effect on inflammatory processing, they might be desirable therapeutic targets in the future in ALD.
Acknowledgments
This work was supported by NIH grants AA020744, AA017729 and AA011576.
References:
- 1.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009;373:2223–2233. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, Sandler RS. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol 2006;101:2128–2138. [DOI] [PubMed] [Google Scholar]
- 3.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol 2009;50:1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. TLR signaling. Cell Death Differ 2006;13:816–825. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 2011;11:163–175. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 2007;14:10–22. [DOI] [PubMed] [Google Scholar]
- 7.Szabo G, Mandrekar P, Petrasek J, Catalano D. The Unfolding Web of Innate Immune Dysregulation in Alcoholic Liver Injury. Alcohol Clin Exp Res 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis 2005;23:255–263. [DOI] [PubMed] [Google Scholar]
- 10.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res 2009;33:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis 2009;29:141–154. [DOI] [PubMed] [Google Scholar]
- 12.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002;27:63–68. [DOI] [PubMed] [Google Scholar]
- 13.Nath B, Szabo G. Alcohol-induced modulation of signaling pathways in liver parenchymal and nonparenchymal cells: implications for immunity. Semin Liver Dis 2009;29:166–177. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 1994;20:453–460. [PubMed] [Google Scholar]
- 15.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 1995;108:218–224. [DOI] [PubMed] [Google Scholar]
- 16.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med 1994;205:243–247. [DOI] [PubMed] [Google Scholar]
- 17.Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol- dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol 1997;51:944–950. [DOI] [PubMed] [Google Scholar]
- 18.Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol 2007;22 Suppl 1:S53–6. [DOI] [PubMed] [Google Scholar]
- 19.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol?. Alcohol Clin Exp Res 2005;29:166S–71S. [DOI] [PubMed] [Google Scholar]
- 20.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol 2010;16:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 2009;50:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 2008;447:171–183. [DOI] [PubMed] [Google Scholar]
- 23.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract 2010;2010:710381 Epub 2010 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto N, Schemmer P, Ikejima K, Takei Y, Sato N, Brenner DA, Thurman RG. Long-term alcohol exposure changes sensitivity of rat Kupffer cells to lipopolysaccharide. Alcohol Clin Exp Res 2001;25:1360–1367. [PubMed] [Google Scholar]
- 25.Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration. Clin Biochem 1999;32:571–578. [DOI] [PubMed] [Google Scholar]
- 26.Diehl AM. Cytokines and the molecular mechanisms of alcoholic liver disease. Alcohol Clin Exp Res 1999;23:1419–1424. [PubMed] [Google Scholar]
- 27.McClain C, Hill D, Schmidt J, Diehl AM. Cytokines and alcoholic liver disease. Semin Liver Dis 1993;13:170–182. [DOI] [PubMed] [Google Scholar]
- 28.Thurman RG II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998;275:G605–11. [DOI] [PubMed] [Google Scholar]
- 29.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology 1997;26:1530–1537. [DOI] [PubMed] [Google Scholar]
- 30.McClain CJ, Barve S, Barve S, Deaciuc I, Hill DB. Tumor necrosis factor and alcoholic liver disease. Alcohol Clin Exp Res 1998;22:248S–252S. [DOI] [PubMed] [Google Scholar]
- 31.Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A, Mookerjee RP, Graziadei I, Datz C, Trauner M, Schuppan D, Obrist P, Vogel W, Williams R. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol 2003;38:419–425. [DOI] [PubMed] [Google Scholar]
- 32.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broet P, Emilie D, Foie-Alcool group of the Association Francaise pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004;39:1390–1397. [DOI] [PubMed] [Google Scholar]
- 33.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:1637–1648. [DOI] [PubMed] [Google Scholar]
- 34.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol 2010;105:14–32; quiz 33. [DOI] [PubMed] [Google Scholar]
- 35.Hill DB, D’Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res 2002;26:74–82. [PubMed] [Google Scholar]
- 36.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol 2009;183:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 1991;13:267–276. [PubMed] [Google Scholar]
- 38.Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res 2011;35:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroder K, Tschopp J. The inflammasomes. Cell 2010;140:821–832. [DOI] [PubMed] [Google Scholar]
- 40.Ambros V The functions of animal microRNAs. Nature 2004;431:350–355. [DOI] [PubMed] [Google Scholar]
- 41.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 2007;179:5082–5089. [DOI] [PubMed] [Google Scholar]
- 43.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006;103:12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem 2011;286:1436–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta 2009;1792:497–505. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A 2009;106:4402–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997–1006. [DOI] [PubMed] [Google Scholar]
- 48.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol 2009;15:5633–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R, Zaret KS, Taylor JM. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol 2004;1:106–113. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem 2010;56:1830–1838. [DOI] [PubMed] [Google Scholar]
- 52.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005;438:685–689. [DOI] [PubMed] [Google Scholar]
- 53.Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 2009;10:578–585. [DOI] [PubMed] [Google Scholar]
- 54.Ceccanti M, Attili A, Balducci G, Attilia F, Giacomelli S, Rotondo C, Sasso GF, Xirouchakis E, Attilia ML. Acute alcoholic hepatitis. J Clin Gastroenterol 2006;40:833–841. [DOI] [PubMed] [Google Scholar]


