Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by a slow heterogeneous progression. Therefore, improved biomarkers that can accurately identify patients with the highest likelihood of progression and therefore the ability to benefit from a given treatment, are needed. Elastin is an essential structural protein of the lungs. In this study, we investigated whether elastin degradation products generated by the enzymes proteinase 3, cathepsin G, neutrophil elastase, MMP7 or MMP9/12 were prognostic biomarkers for COPD-related outcomes. The elastin degradome was assessed in a subpopulation (n = 1307) of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort with 3 years of clinical follow-up. Elastin degraded by proteinase 3 could distinguish between COPD participants and non-smoking controls (p = 0.0006). A total of 30 participants (3%) died over the 3 years of observation. After adjusting for confounders, plasma levels of elastin degraded by proteinase 3 and cathepsin G were independently associated with mortality outcome with a hazard ratio per 1 SD of 1.49 (95%CI 1.24–1.80, p < 0.0001) and 1.31 (95%CI 1.10–1.57, p = 0.0029), respectively. Assessing the elastin degradome demonstrated that specific elastin degradation fragments have potential utility as biomarkers identifying subtypes of COPD patients at risk of poor prognosis and supports further exploration in confirmatory studies.
Introduction
Disease progression of chronic obstructive pulmonary disease (COPD) is slow and very heterogeneous, this is most likely consequent to different phenotypes with different disease trajectories that should be treated individually1,2. Phase III studies in COPD are long and costly, and consequently, there is a medical need to develop new and improved biomarkers that accurately identify COPD patients who progress within a short time period, consequent to a given disease phenotype which may be pharmaceutically attenuated. This is essential for the execution of improved phase II clinical studies that will allow confident phase III decision based on actual effects on forced expiratory volume in the first second (FEV1)3,4.
Elastin is an essential structural protein of the lungs and is responsible for tissue elasticity5,6. Loss of the elasticity and elastin content during pathological situations is reported in inflammatory diseases including COPD with co-existing emphysema7–11. Tropoelastin, the monomeric form of elastin, has a unique structure that is composed of highly cross-linked and extremely hydrophobic domains, which renders it resistant to proteolytic degradation in healthy conditions12,13. Under pathological conditions such as COPD increased numbers of inflammatory cells and fibroblasts leads to an up-regulation of proteases including serine proteinases and matrix metalloproteinases (MMPs)14. Both excessive serine proteinase and MMP activity are associated with the destruction of elastin, resulting in specific pathological protein fragments and loss of lung elasticity11,15. These proteolytically processed fragments also referred to as neoepitopes are released into the circulation and may be assessed as simple non-invasive biomarkers. These neoepitopes represent a unique fingerprint of proteolytic cleavage of the protein and may be used to identify whether the tissue is pathologically affected16,17. Neoepitopes have been proven to be more accurate predictors of disease than their unmodified intact mature protein18,19, since measurement of different fragments from the same protein has yielded different information19–21. For example, such a fragment is produced when elastin is degraded by neutrophils elastase which may be assessed as a biomarker (EL-NE) associated with chronic inflammation22 and emphysema23. Such a fragment can also be produced by MMP-7 (ELM7) associated with lung remodeling in IPF10, or by MMP9/12 (ELM12) elevated during acute myocardial infarction24. In direct alignment, markers of elastin degraded predominantly by the serine proteinases, proteinase 3 (ELP-3) and cathepsin G (EL-CG), are also the result of specific elastin degradation providing relations to other pathological events in lung diseases25.
We evaluated aspects of degraded elastin by five different proteinases in a subpopulation in the Evaluation of COPD Longitudinal to Identify Predictive Surrogate End-points (ECLIPSE) cohort. We hypothesized that different elastin fragments would provide complementary pathophysiological information with the hypothesis that MMP, neutrophil elastase, proteinase 3 and cathepsin G activity may play different roles in lung tissue damage in COPD. We also tested the hypothesis that these fragments were prognostic of poor clinical outcomes: a decline in lung function and mortality.
Results
Elastin fragments have different pathological specificity
The five unique elastin neoepitope biomarkers investigated in this study were generated from either cleavage of human elastin by proteinase 3 (ELP-3), cathepsin G (EL-CG), neutrophil elastase (EL-NE), MMP7 (ELM7) or MMP9/12 (ELM12). ELP-3 correlated positively with EL-CG, EL-NE and ELM7 (0.6; 0.36; 0.33), respectively. No significant correlation was observed for any of the other neoepitope biomarkers (Fig. 1).
Figure 1.
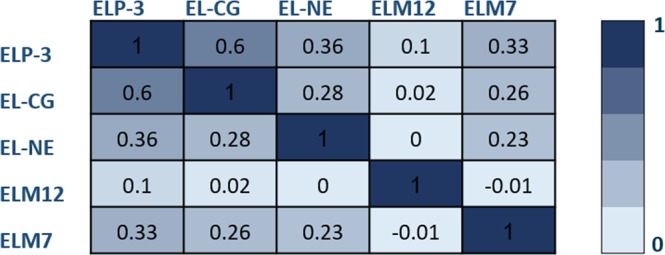
Spearman’s coefficient of rank correlations is listed in the table between the elastin biomarkers. The darkest color represents the most correlated biomarkers.
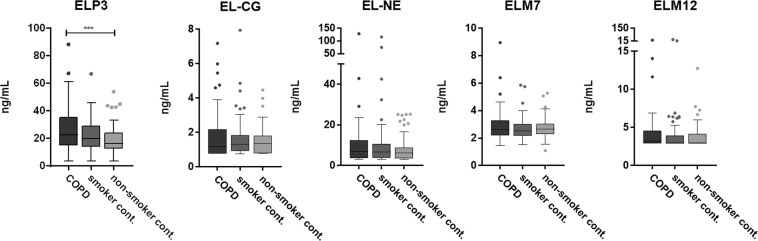
Baseline characteristics of smoking controls, non-smoking controls and a subgroup of age, BMI and gender-matched COPD participants are listed in Table 1. As expected, COPD subjects had lower FEV1 and FEV1/FVC ratio compared to both control groups. Plasma ELP-3 level was significantly up-regulated in COPD when compared to levels of non-smoking controls (p = 0.0004), but not when compared to smoking controls (Fig. 2). No significant difference was observed for EL-CG, EL-NE, ELM7 and ELM12. Furthermore, no correlation between the elastin fragments and FEV1 or degree of emphysema defined as more than 10% of lung volume with a density of −950 Hounsfield units on inspiratory computed tomography was observed (data not shown).
Table 1.
Population demographics and characteristics at baseline.
| Characteristic | COPD (n = 100) | Smoker controls (n = 99) | non-smoker controls (n = 98) | P-value |
|---|---|---|---|---|
| Age (yr) | 60 ± 7 | 60 ± 7 | 59 ± 7 | 0.68 |
| Female sex | 50 (50) | 49 (49) | 49 (50) | 0.99 |
| Body-mass-index | 27 ± 5.7 | 27 ± 4.3 | 28 ± 4.5 | 0.14 |
| Smoking status | ||||
| Current smoker | 50 (50) | 99 (100) | 0 (0) | 0.002 |
| Smoking history (pack-yr) | 40 ± 18 | 32 ± 17 | 0 ± 0 | <0.0001 |
| Clinical variables | ||||
| FEV1 (L) | 1.46 ± 0.6 | 3.08 ± 0.8 | 3.18 ± 0.8 | <0.0001 |
| FEV1 (% predicted) | 48 ± 16 | 103 ± 15 | 110 ± 15 | <0.0001 |
| FEV1/FVC | 0.47 ± 0.12 | 0.75 ± 0.06 | 0.78 ± 0.05 | <0.0001 |
| ELP-3 | 26.34 ± 15.96 | 21.39 ± 11.78 | 18.14 ± 10.30 | 0.0009 |
| EL-CG | 1.76 ± 1.46 | 2.25 ± 6.60 | 1.45 ± 0.71 | 0.83 |
| EL-NE | 10.31 ± 14.11 | 10.22 ± 14.72 | 7.79 ± 5.64 | 0.36 |
| ELM7 | 2.80 ± 1.01 | 2.65 ± 0.76 | 2.72 ± 0.67 | 0.46 |
| ELM12 | 4.21 ± 2.83 | 4.01 ± 3.37 | 3.70 ± 1.46 | 0.20 |
Data are shown as mean ± SD, median (25th; 75th) or number (%). FEV1, post- bronchodilator forced expiratory volume in 1 second; FVC; forced vital capacity. Statistical significance was determined using Kruskal-Wallis test or chi-squared test.
Figure 2.
Serological elastin neo-epitope biomarker levels in age, gender and BMI matched COPD (n = 100), smoker controls (n = 99) and non-smoker controls (n = 98). ELP-3 was significantly up-regulated in COPD patient compared to non-smoker controls. Data were analyzed using Kruskal-Wallis test and presented as a Tukey box plot. Asterisks indicate statistical significance *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Elastin degradation by proteinase 3 and cathepsin G is related to mortality
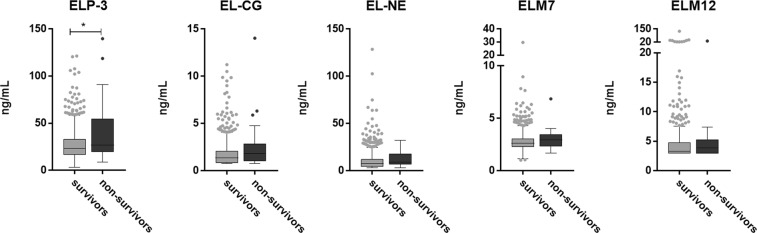
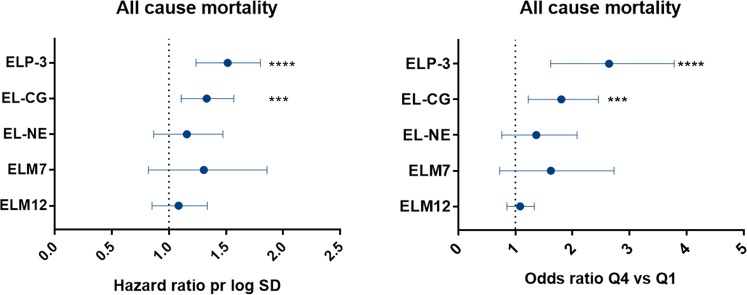
Baseline characteristics for survivors and non-survivors are listed in Table 2. Non-survivors were significantly older, had increased mMRC dyspnea score, a higher number of previous exacerbations, a lower use of inhaled corticosteroids, and fewer subjects were current smokers (Table 2). A total of 30 (3%) out of the 1000 COPD participants assessed died over the three years of observation. ELP-3 was significantly increased in non-survivors compared to survivors (p = 0.0202). No significant increase in EL-CG, EL-NE, ELM7 and ELM12 was observed (Fig. 3). After adjusting for relevant covariates, plasma levels of ELP-3 and EL-CG were independently associated with mortality with a hazard ratio per 1 SD increase in biomarker level of 1.49 [95%CI 1.24–1.80, p < 0.0001] and 1.31 [95%CI 1.10–1.57, p = 0.0029], respectively. Moreover, in adjusted analysis, the odds ratio for belonging to the highest quartile as compared to the lowest quartile was significantly associated with all-cause mortality for ELP-3 and EL-CG (2.52 [95%CI 1.62–3.79, p < 0.0001] and 1.74 [95%CI 1.22–2.46, p = 0.0019], respectively) (Fig. 4).
Table 2.
Population demographics and characteristics at baseline.
| Characteristic | Survivors (n = 970) | Non-survivors (n = 30) | P-value |
|---|---|---|---|
| Age (yr) | 63 ± 7 | 68 ± 6 | P < 0.0001 |
| Female sex | 351 (36) | 11 (37) | P = 0.14 |
| Body-mass-index | 27 ± 5.8 | 28 ± 7.4 | P = 0.80 |
| Smoking status | |||
| Current smoker | 364 (38) | 3 (10) | P = 0.002 |
| Smoking history (pack-yr) | 47 ± 25 | 56 ± 42 | P = 0.92 |
| Clinical variables | |||
| FEV1 (L) | 1.42 ± 0.5 | 1.35 ± 0.5 | P = 0.41 |
| FEV1 (% predicted) | 46 ± 15 | 46 ± 13 | P = 0.99 |
| GOLD stage | P = 0.708 | ||
| II | 480 (50) | 14 (47) | |
| III | 390 (40) | 14 (47) | |
| IV | 98 (10) | 2 (6.7) | |
| Number of previous exacerbations | P = 0.0170 | ||
| 0 | 432 (45) | 10 (33) | |
| 1 | 244 (25) | 12 (40) | |
| 2 | 144 (15) | 0 (0) | |
| >2 | 148 (15) | 8 (27) | |
| mMRC dyspnea score | 1 (1;2) | 2 (1;3) | P = 0.023 |
| %LLA | 16 ± 11 | 17 ± 11 | P = 0.45 |
| BODE index | 3 (1;4) | 3.5 (2;5) | P = 0.063 |
| Treatments | |||
| Inhaled corticosteroids | 139 (14) | 3 (10) | P = 0.048 |
| Systemic corticosteroids | 8 (0.8) | 0 | P = 0.77 |
| Statins | 237 (24) | 6 (20) | P = 0.37 |
| Biomarkers | |||
| ELP-3 | 26.8 ± 15.7 | 41.7 ± 32.7 | P = 0.020 |
| EL-CG | 1.7 ± 1.3 | 2.6 ± 2.6 | P = 0.066 |
| EL-NE | 10.1 ± 9.7 | 11.8 ± 8.8 | P = 0.16 |
| ELM7 | 2.8 ± 0.9 | 3.0 ± 1.0 | P = 0.16 |
| ELM12 | 4.5 ± 5.5 | 5.1 ± 5.4 | P = 0.22 |
Data are shown as mean ± SD, median (25th; 75th) or number (%). FEV1, post-bronchodilator forced expiratory volume in 1 second; GOLD, global initiative for chronic obstructive lung disease; mMRC, modified Medical Research Council dyspnea scale; %LLA, percent low areas of attenuation below 950 Hounsfield unit; BODE, BMI, airflow obstruction, dyspnea and exercise capacity index. Statistical significance was determined using Mann-Whitney U test or chi-squared test.
Figure 3.
Serological elastin neo-epitope biomarker levels in survivors (n = 970) and non-survivors (n = 30). ELP-3 was significantly elevated in non-survivors compared to survivors. Data were analyzed using Mann-Whitney test and presented as a Tukey box plot. Asterisks indicate statistical significance *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Figure 4.
Cox proportional hazard ratio on the left figure and odds ratio for patients belonging to biomarker quartile 4 vs 1 on the right figure. Data are shown as mean (95% CI) hazard ratio for 1 log SD increase in biomarker for all-cause mortality and adjusted for age, smoking status, BODE index, mMRC dyspnea score, inhaled corticosteroids and number of exacerbations the previous year of study start. Asterisks indicate statistical significance *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
There is a medical need for developing new and improved biomarkers to identify COPD subjects with a rapid disease progression, who potentially have a higher chance of benefitting from a symptom modifying COPD treatment. Here we investigated elastin degradation fragments, so-called neoepitopes, as blood-based biomarkers of disease progression for COPD in a non-invasive manner. We found that elastin degraded by protease 3 and cathepsin G, was associated with a higher risk of mortality in subjects with COPD.
Elastin is a unique signature protein of the lungs. Consequently, biomarkers generated from elastin could be associated with higher tissue accuracy and pathophysiological relevance; although these neoepitopes could potentially arrive from other organs than the lung. The importance of proteolytic elastin degradation in COPD is highlighted by the observation that exacerbations in COPD are associated with accelerated elastin turnover4,10. Desmosine and isodesmosine have long been proposed as biomarkers of lung tissue destruction as they are the crosslinking elements of elastin, released during lung tissue destruction26. It has been demonstrated that subjects with COPD have higher levels of plasma desmosine and that levels are able to predict mortality27 which is in concordance with our findings. Moreover, a study with 349 subjects with one-third suffering from COPD showed that urinary desmosine was significantly correlated with all lung function measures28. This underlines the importance of elastin degradation as a potential biomarker in COPD, however, the desmosine technology identifies non-specific elastin fragments that are being generated through many different processes, both physiological and pathophysiological. The technique and biomarkers used in the present study allowed for investigation of elastin degradation predominantly produced by specific proteinases, known to be up-regulated in respiratory diseases and therefore more carefully quantify the aspects of the elastin degradome10,22–24.
Plasma levels of ELP-3 and EL-CG were associated with all-cause mortality in COPD implying that proteinase 3 and cathepsin G play a significant role in COPD. During the progression of COPD, inflammatory cells infiltrate the lungs, which has been shown to release proteinase 3, cathepsin G and neutrophil elastase into the lungs29. These proteinases are known to efficiently degrade elastin30, which is in concordance with the results from this study where a higher degree of ELP-3 or EL-CG was significantly associated with a poor outcome. Moreover, proteinase 3 activity was present in the sputum of COPD subjects in a higher amount than the activity of neutrophil elastase implicating a bigger role for proteinase 3 in COPD than previously thought31. This may in part explain why ELP-3 demonstrated a better association with outcomes relative to EL-NE. Since plasma levels of ELP-3 and EL-CG were associated with all-cause mortality, a correlation with FEV1 might have been expected, as this was previously observed for desmosine28. In general, the FEV1 decline for the subpopulation of ECLIPSE studied here were associated with a considerable intra and inter-person variability which makes an evaluation of predictive biomarkers for lung function decline challenging, 31% improved or slightly decreased in FEV1 while only 38% participants in the entire cohort demonstrated significant FEV1 decline32.
The importance of unique elastin fragments has been emphasized by the fact that they can act as chemotactic peptide for different cell types, showing their ability to function as matrikines33. The hexapeptide VGVAPG within tropoelastin is well known for its chemotactic activity attracting monocytes and fibroblasts and its ability to regulate MMP expression and activity34,35. Likewise, other fragments of elastin corresponding to XGXXPG, where X is a generic hydrophobic residue, has also been shown to be active peptides35–37. In fact, the ELM12 fragment holds the sequence VGVAPG in its peptide, which indicates that it might be a matrikine, however, in the current study, no pathological relevance of this biomarker was observed.
The emphysema phenotype, such as the multi-organ loss of tissue (MOLT), is currently receiving increased attention, and high elastin turnover could be associated with it, which has been shown by others23,38,39. In accordance with our findings, another study measuring plasma desmosine in the ECLIPSE cohort was not able to show a relationship between emphysema and elastin degradation27. The lack of association could be explained by the fact that COPD participants from the ECLIPSE cohort had an established disease (GOLD II-IV) with a stable state during sampling. This could result in less elastin present in their lungs or a lower disease activity than during an exacerbation, which could explain why elastin degradation is not associated or increased in subjects with emphysema in this cohort.
The limitations of this study include the low number of deceased subjects, even though we were able to detect a significant association with all-cause mortality for the two biomarkers ELP-3 and EL-CG. To generalize these finding to a more general COPD population they have to be confirmed in secondary cohorts. Furthermore, using the subpopulation of the full ECLIPSE study comprising the study participant that progress the least and most in terms of FEV1 decline during the study period might explain the low number and make a less optimal subpopulation to study mortality. In addition, the ELP-3, EL-CG, ELM7 and ELM12 was measured in heparin plasma at year 1, whereas EL-NE was measured in 6-month serum. This could create some difficulties in directly comparing the results.
Conclusion
In conclusion, we have demonstrated that five cleavage-specific fragments of elastin generated by five different proteinases reflect different pathological processes in COPD. ELP-3 and EL-CG demonstrated promise as prognostic biomarkers for all-cause mortality reflecting an increase of elastin remodeling by proteinase 3 and cathepsin G. This study demonstrated the importance of evaluating elastin turnover in the pathology and natural history of COPD.
Methods
Study design and participants
The analysis was based on the three-year observational longitudinal study ECLIPSE (ClinicalTrials.gov. number, NCT00292552), described previously32,40. The full ECLIPSE study included 2163 participants with COPD. The enrollment criteria included an FEV1 of less than 80% of the predicted value and a FEV1/forced vital capacity (FVC) ratio of 0.7 or less assessed after the use of bronchodilators. COPD participants had a smoking history of 10 or more pack-years. 343 smoking controls with a smoking history of 10 or more pack-years and 223 nonsmoking controls were also included in the study. The controls had an FEV1 of more than 85% of the predicted value and an FEV1/FVC ratio of 0.7 or more after the use of bronchodilators. Control participants had to be free of significant comorbidities, determined from screening investigation, physical examination and medical history. Eight study visits were conducted at baseline, month three, six and subsequently every six months over three years. The current analysis was performed on 1307 participants consisting of 1000 COPD, 207 smoking controls and 100 non-smoking controls. All-cause mortality was recorded until year 3. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by relevant ethics and review boards (Supplementary Table 1). Participants provided informed consent.
Quantification of serological biochemical biomarkers
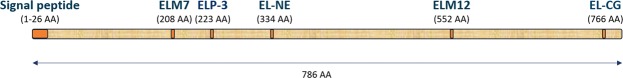
Whole blood was collected from fasting participants and transferred to vacutainers containing sodium heparin. Plasma was obtained by centrifugation of vacutainer tubes at 2,000 g for 10–15 minutes and stored at −80 °C until analysis. Elastin degradation by proteinase 3 (ELP-3), cathepsin G (EL-CG) and MMP9/12 (ELM12) was measured in heparin plasma obtained from 1307 study participants at the year 1 visit using well-validated ELISAs each utilizing monoclonal antibodies targeting a specific neoepitope (Nordic Bioscience, Herlev, Denmark), see specifications in Table 3. Measurements were performed in a blinded manner according to the instruction of the manufactures. Previously, elastin degradation by neutrophil elastase (EL-NE) was measured in month six serum samples whereas elastin degraded by MMP7 (ELM7) was measured in year 1 heparin plasma41. The five unique elastin neoepitope biomarkers investigated in this study ELP-3, EL-CG, EL-NE, ELM7 and ELM12 originates from locations throughout the tropoelastin (Fig. 5) and are very different in terms of activation, inhibition, substrate specificity and source (Table 4).
Table 3.
Biomarker specifications.
| Biomarker | Specification | Sequence | Location | Reference |
|---|---|---|---|---|
| ELP-3 | Elastin degraded by proteinase 3 | LPGGYGLPYT | 213–222 | 25 |
| EL-CG | Elastin degraded by cathepsin G | LGGVAARPGF | 756–765 | 25 |
| EL-NE | Elastin degraded by neutrophil elastase | GGPGFGPGVV | 325–334 | 22 |
| ELM7 | Elastin degraded by MMP7 | IKAPKLPGGY | 208–217 | 15 |
| ELM12 | Elastin degraded by MMP12 or MMP9 | GVAPGIGPGG | 543–552 | 42 |
Figure 5.
Schematic figure of tropoelastin and the location of the five unique elastin neoepitope biomarkers generated from either cleavage of human elastin by proteinase 3 (ELP-3), cathepsin G (EL-CG), neutrophil elastase (EL-NE), MMP7 (ELM7) or MMP9/12 (ELM12).
Table 4.
Proteinase characteristics.
| Proteinase | Substrate specificity | Optimal pH for activity | Source | Activation | Activation enzyme | Inhibitors |
|---|---|---|---|---|---|---|
| Proteinase 3 | Small hydrophobic residues at P1: Val, Cys, Ala, Met, Leu, Ser | ~8.0 | Neutrophils Monocytes Basophils |
Cleavage of N-terminal signal peptide and then cleavage of N-terminal prodipeptide = > enzymatic activity and the C-terminal | Dipeptidyl peptidase I | α2-macroglobulins, α1-PI, SerpinB1, α1-ACT, Elafin, Eglin c |
| Cathepsin G | Aromatic or positively charged residue at P1: Phe, Tyr, Lys, Arg | ~7.5 | Neutrophils Monocytes Mastocytes |
Cleavage of N-terminal signal peptide and then cleavage of N-terminal prodipeptide = > enzymatic activity and the C-terminal | Dipeptidyl peptidase I | β-ketophosphonic acids, aminoalkylphosphonic esters and boswellic acids |
| Neutrophil elastase | Small hydrophobic residues at P1: Val, Cys, Ala, Met, Ile, Leu, Ser | 8.0–8.5 | Neutrophils Monocytes |
Cleavage of N-terminal signal peptide and then cleavage of N-terminal prodipeptide = > enzymatic activity and the C-terminal | Dipeptidyl peptidase I | α2-macroglobulins, α1-PI, SerpinB1, α1-ACT, Elafin, Eglin c |
| MMP7 | Acidic residues P1: Leu, Ile, Val, Met | 7.0 | Macrophages lymphocytes | Proteolytic cleavage resulting in removal of the prodomain, final step is autolytic cleavage at Glu77↓Tyr78 | Trypsin, plasmin, MMp-3, MMP10 and others | TIMPs, 1,10-phenanthroline, DTT |
| MMP9 | Small residues P1: Gly, Ala, Ser | 7.5 | Macrophages lymphocytes | Proteolytic removal of the N-terminal propeptide involving a cysteine switch mechanism | Matrilysin, interstitial collagenase, tissue kallikrein, plasmin, macrophage elastase | TIMPs |
| MMP12 | P1: Leu, | 8.0 | Macrophages | Protelytic removal of the prodomain and the C-terminal | Trypsin, plasmin, neutrophil elastase, stromelysin-1 | TIMP-1, α2-macroglobulin, 1,10-phenanthroline |
Statistical analysis
Population demographics were compared using Kruskal-Wallis test, Mann-Whitney U test and chi-squared test as appropriate. Kruskal-Wallis test and Mann-Whitney test were used to compare biomarker levels between COPD subjects, controls, survivors and non-survivors. Cox proportional hazard regression was used to assess the prognostic value of each biomarker for all-cause mortality for one standard deviation (SD) increase in biomarker level. Logistic regression was used to find the odds ratio for all-cause mortality belonging to the upper quartile versus the lower quartile. The risk of death was assessed adjusted for confounders that were significantly different between survivors and non-survivors. The covariates adjusted for were age, smoking status, mMRC dyspnea score, use of inhaled corticosteroids and number of exacerbations in the year prior to blood sampling.
The software MedCalc (MedCalc version 14.8.1, MedCalc software bvba, Ostend, Belgium) was used to perform all statistical analysis.
Supplementary information
Acknowledgements
R.T.-S., B.E.M. and J.V. developed the current study design and concept in collaboration with representatives of Nordic Bioscience (J.M.B.S., S.R.R., L.L.L., D.J.L., T.M.J. and M.A.K.). All approved the plan for the current analyses, had full access to the data, and were responsible for the decision to publish. S.S.R., L.L.A. and J.T.U. did all the biomarker measurements. The authors acknowledge all participants, medical, nursing, and technical staff involved in the ECLIPSE study: the principal investigators and centers participating in ECLIPSE: Bulgaria: Y Ivanov, Pleven; K Kostov, Sofia. Canada: J Bourbeau, Montreal; M Fitzgerald, Vancouver; P Hernández, Halifax; K Killian, Hamilton; R Levy, Vancouver; F Maltais, Montreal; D O’Donnell, Kingston. Czech Republic: J Krepelka, Praha. Denmark: J Vestbo, Hvidovre. The Netherlands: E Wouters, Horn. New Zealand: D Quinn, Wellington. Norway: P Bakke, Bergen, Slovenia: M Kosnik, Golnik. Spain: A Agusti, Jaume Sauleda, Palma de Mallorca. Ukraine: Y Feschenko, Kiev; V Gavrisyuk, Kiev; L Yashina, W MacNee, Edinburgh; D Singh, Manchester; J Wedzicha, London. USA: A Anzueto, San Antonio, TX; S Braman, Providence. RI; R Casaburi, Torrance CA; B Celli, Boston, MA; G Giessel, Richmond, VA; M Gotfried, Phoenix, AZ; G Greenwald, Rancho Mirage, CA; N Hanania, Houston, TX; D Mahler, Lebanon, NH; B Make, Denver, CO; S Rennard, Omaha, NE; C Rochester, New Haven, CT; P Scanlon, Rochester, MN; D Schuller, Omaha, NE; F Sciurba, Pittsburg, PA; A Sharafkhaneh, Houston, TX; T Siler, St Charles, MO; E Silverman, Boston, MA; A Wanner, Miami, FL; R Wise, Baltimore, MD; R ZuWallack, Hartford, CT. The Steering Committee: H Coxson (Canada), C Crim (GlaxoSmithKline, USA), L Edwards (GlaxoSmithKline, USA), D Lomas (UK), W MacNee (UK), E Silverman (USA), R Tal-Singer (Co-chair, GlaxoSmithKline, USA), J Vestbo (Co-chair, Denmark), J Yates (GlaxoSmithKline, USA) and the scientific Committee: A Agusti (Spain), P Calverley (UK), B Celli (USA), C Crim (GlaxoSmithKline, USA), B Miller (GlaxoSmithKline, US), W MacNee (Chair, UK), S Rennard (USA), R Tal-Singer (GlaxoSmithKline, USA), E Wouters (The Netherlands), J Yates (GlaxoSmithKline, USA). The study was sponsored by GlaxoSmithKline (ECLIPSE; SCO104960, NCT00292552); Nordic Bioscience; the Danish Agency for Science, Technology and Innovation; and the Danish Research Foundation. Two representatives of GlaxoSmithKline (R.T.-S., B.E.M.) and one academic (J.V.), together representing the ECLIPSE study investigators. J.V. is supported by the National Institute of Health Research (NIHR) Manchester Biomedical Research Centre.
Author Contributions
Two representatives of GlaxoSmithKline (R.T.-S., B.E.M.) and one academic (J.V.), together representing the ECLIPSE study investigators, developed the current study design and concept in collaboration with representatives of Nordic Bioscience (J.M.B.S., S.R.R., L.L.L., J.T.U., T.M.J., D.J.L., M.A.K.), they approved the plan for the current analyses, had full access to the data, and were responsible for the decision to publish.
Data Availability
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
J.M.B.S., S.R.R., L.L.A., J.T.U., T.M.J., D.J.L. and M.A.K. are employees and D.J.L. and M.A.K. are shareholders of Nordic Bioscience. R.T.-S. and B.E.M. are employees and shareholders of G.S.K. J.V. has received honoraria for presenting and advising from Astra Zeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline and Novartis, outside the submitted work.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruth Tal-Singer and Jørgen Vestbo The Evaluation of COPD Longitudinally to Identify Surrogate Endpoints (ECLIPSE) study, investigators.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40785-2.
References
- 1.Vestbo J, Rennard S. Chronic Obstructive Pulmonary Disease Biomarker(s) for Disease Activity Needed—Urgently. Am. J. Respir. Crit. Care Med. 2010;182:863–864. doi: 10.1164/rccm.201004-0602ED. [DOI] [PubMed] [Google Scholar]
- 2.Bihlet, A. R. et al. Clinical drug development using dynamic biomarkers to enable personalized health care in COPD. Chest148 (2015). [DOI] [PubMed]
- 3.Shaw JG, et al. Biomarkers of progression of chronic obstructive pulmonary disease (COPD) Journal of Thoracic Disease. 2014;6:1532–1547. doi: 10.3978/j.issn.2072-1439.2014.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turino GM, Lin YY, He J, Cantor JO, Ma S. Elastin degradation: An effective biomarker in COPD. COPD. J. Chronic Obstr. Pulm. Dis. 2012;9:435–438. doi: 10.3109/15412555.2012.697753. [DOI] [PubMed] [Google Scholar]
- 5.Chrzanowski, P., Keller, S., Cerreta, J., Mandl, I. & Turino, G. M. Elastin content of normal and emphysematous lung parenchyma. Am J Med69 (1980). [DOI] [PubMed]
- 6.Vrhovski, B. & Weiss, A. S. Biochemistry of tropoelastin. Eur J Biochem258 (1998). [DOI] [PubMed]
- 7.Petersen, E., Gineitis, A., Wagberg, F. & Angquist, K. Serum levels of elastin-derived peptides in patients with ruptured and asymptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg22 (2001). [DOI] [PubMed]
- 8.Jacob, M., Wei, S., Ghuysen-Itard, A., Fulop, T. & Robert, L. Elastin and arteriosclerosis: determination and characterization of elastin peptides in blood. C R Seances Soc Biol Fil186 (1992). [PubMed]
- 9.Schriver, E., Davidson, J., Sutcliffe, M., Swindell, B. & Bernard, G. Comparison of elastin peptide concentrations in body fluids from healthy volunteers, smokers, and patients with chronic obstructive pulmonary disease. Am Rev Respir Dis145 (1992). [DOI] [PubMed]
- 10.Sand JMB, et al. Accelerated extracellular matrix turnover during exacerbations of COPD. Respir. Res. 2015;16:69. doi: 10.1186/s12931-015-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristensen JH, et al. The role of extracellular matrix quality in pulmonary fibrosis. Respiration. 2014;88:487–499. doi: 10.1159/000368163. [DOI] [PubMed] [Google Scholar]
- 12.Mariani TJ, Sandefur S, Pierce RA. Elastin in lung development. Experimental Lung Research. 1997;23:131–145. doi: 10.3109/01902149709074026. [DOI] [PubMed] [Google Scholar]
- 13.Fritze, O., Romero, B., Schleicher, M., Jacob, M. & Oh, D. Age-related changes in the elastic tissue of the human aorta. J Vasc Res49 (2012). [DOI] [PubMed]
- 14.Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:253–68. doi: 10.2147/COPD.S2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristensen JH, et al. Levels of circulating MMP-7 degraded elastin are elevated in pulmonary disorders. Clin. Biochem. 2015;48:1083–1088. doi: 10.1016/j.clinbiochem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Karsdal MA, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G807–30. doi: 10.1152/ajpgi.00447.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skjot-Arkil, H., Barascuk, N., Register, T. & Karsdal, M. Macrophage-Mediated Proteolytic Remodeling of the Extracellular Matrix in Atherosclerosis Results in Neoepitopes: A Potential New Class of Biochemical Markers. Assay Drug Dev Technol8 (2010). [DOI] [PubMed]
- 18.Karsdal MA, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev. Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsdal MA, Delvin E, Christiansen C. Protein fingerprints - relying on and understanding the information of serological protein measurements. Clin. Biochem. 2011;44:1278–9. doi: 10.1016/j.clinbiochem.2011.08.1135. [DOI] [PubMed] [Google Scholar]
- 20.Karsdal MA, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 21.Karsdal MA, et al. The good and the bad collagens of fibrosis - Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen JH, et al. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm. Med. 2015;15:53. doi: 10.1186/s12890-015-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bihlet AR, et al. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 2017;18:22. doi: 10.1186/s12931-017-0509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skjøt-Arkil H, et al. Acute Myocardial Infarction and Pulmonary Diseases Result in Two Different Degradation Profiles of Elastin as Quantified by Two Novel ELISAs. PLoS One. 2013;8:e60936. doi: 10.1371/journal.pone.0060936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudmann, N. S. et al. Lung tissue destruction by proteinase 3 and cathepsin G mediated elastin degradation is elevated in chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun., 10.1016/J.BBRC.2018.07.038 (2018). [DOI] [PubMed]
- 26.Luisetti M, et al. Desmosine as a biomarker of elastin degradation in COPD: Current status and future directions. European Respiratory Journal. 2008;32:1146–1157. doi: 10.1183/09031936.00174807. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovich RA, et al. Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur. Respir. J. 2016;47:1365–1373. doi: 10.1183/13993003.01824-2015. [DOI] [PubMed] [Google Scholar]
- 28.Lindberg CA, et al. Total desmosines in plasma and urine correlate with lung function. Eur. Respir. J. 2012;39:839–845. doi: 10.1183/09031936.00064611. [DOI] [PubMed] [Google Scholar]
- 29.Craciun I, Fenner AM, Kerns RJ. N-Arylacyl O-sulfonated aminoglycosides as novel inhibitors of human neutrophil elastase, cathepsin G and proteinase 3. Glycobiology. 2016;26:701–709. doi: 10.1093/glycob/cww011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyot N, et al. Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. Am. J. Pathol. 2014;184:2197–2210. doi: 10.1016/j.ajpath.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Sinden NJ, Stockley RA. Proteinase 3 activity in sputum from subjects with alpha-1-antitrypsin deficiency and COPD. Eur. Respir. J. 2013;41:1042–1050. doi: 10.1183/09031936.00089712. [DOI] [PubMed] [Google Scholar]
- 32.Vestbo J, et al. Changes in Forced Expiratory Volume in 1 Second over Time in COPD. N. Engl. J. Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 33.Desforges M, Harris LK, Aplin JD. Elastin-derived peptides stimulate trophoblast migration and invasion: A positive feedback loop to enhance spiral artery remodelling. Mol. Hum. Reprod. 2015;21:95–104. doi: 10.1093/molehr/gau089. [DOI] [PubMed] [Google Scholar]
- 34.Senior RM, et al. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J. Cell Biol. 1984;99:870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duca L, Floquet N, Alix AJP, Haye B, Debelle L. Elastin as a matrikine. Crit. Rev. Oncol. Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Brassart B, et al. Conformational Dependence of Collagenase (Matrix Metalloproteinase-1) Up-regulation by Elastin Peptides in Cultured Fibroblasts. J. Biol. Chem. 2001;276:5222–5227. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 37.Grosso LE, Scott M. PGAIPG, a Repeated Hexapeptide of Bovine and Human Tropoelastin, Is Chemotactic for Neutrophils and Lewis Lung Carcinoma Cells. Arch. Biochem. Biophys. 1993;305:401–404. doi: 10.1006/abbi.1993.1438. [DOI] [PubMed] [Google Scholar]
- 38.Celli, B. R. et al. Emphysema and extrapulmonary tissue loss in COPD: a multi-organ loss of tissue phenotype. Eur. Respir. J., 10.1183/13993003.02146-2017 (2018). [DOI] [PubMed]
- 39.Shapiro SD. The pathogenesis of emphysema: the elastase:antielastase hypothesis 30 years later. Proc. Assoc. Am. Physicians. 1995;107:346–52. [PubMed] [Google Scholar]
- 40.Vestbo J, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur. Respir. J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 41.Sand JMB, et al. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD - results from the ECLIPSE study. Respir. Res. 2016;17:125. doi: 10.1186/s12931-016-0440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorlacius-Ussing J, Kehlet SN, Rønnow SR, Karsdal MA, Willumsen N. Non-invasive profiling of protease-specific elastin turnover in lung cancer: biomarker potential. J. Cancer Res. Clin. Oncol. 2018;0:0. doi: 10.1007/s00432-018-2799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.