Abstract
Background: We performed a network meta-analysis to compare the diagnostic accuracy of contrast-enhanced ultrasound (CEUS) and shear wave elastography (SWE) in differentiating benign and malignant lesions in different body sites.
Methods: A computerized literature search of Medline, Embase, SCOPUS, and Web of Science was performed using relevant keywords. Following data extraction, we calculated sensitivity, specificity, positive likelihood ratio (LR), negative LR, and diagnostic odds ratio (DOR) for CEUS, and SWE compared to histopathology as a reference standard. Statistical analyses were conducted by MetaDiSc (version 1.4) and R software (version 3.4.3).
Results: One hundred and fourteen studies (15,926 patients) were pooled in the final analyses. Network meta-analysis showed that CEUS had significantly higher DOR than SWE (DOR = 27.14, 95%CI [2.30, 51.97]) in breast cancer detection. However, there were no significant differences between CEUS and SWE in hepatic (DOR = −6.67, 95%CI [−15.08, 1.74]) and thyroid cancer detection (DOR = 3.79, 95%CI [−3.10, 10.68]). Interestingly, ranking analysis showed that CEUS achieved higher DOR in detecting breast and thyroid cancer, while SWE achieved higher DOR in detecting hepatic cancer. The overall DOR for CEUS in detecting renal cancer was 53.44, 95%CI [29.89, 95.56] with an AUROC of 0.95, while the overall DOR for SWE in detecting prostate cancer was 25.35, 95%CI [7.15, 89.89] with an AUROC of 0.89.
Conclusion: Both diagnostic tests showed relatively high sensitivity and specificity in detecting malignant tumors in different organs. Network meta-analysis showed that CEUS had higher diagnostic accuracy than SWE in detecting breast and thyroid cancer, while SWE had higher accuracy in detecting hepatic cancer. However, the results were not statistically significant in hepatic and thyroid malignancies. Further head-to-head comparisons are needed to confirm the optimal imaging technique to differentiate each cancer type.
Keywords: contract enhanced ultrasonography, malignant lesions benign lesions, network meta analysis, shear wave elastography, lesions
Introduction
Ultrasound (US) has been used for decades in differentiating benign and malignant lesions because of its low cost, ease of access, and non-invasiveness. For example, it belongs to the triad (physical examination, mammography and US), commonly used to assess the risk of breast cancer (1). Moreover, it can detect thyroid nodules as small as 2 mm in size and predicts malignancy based on features like irregular border, hypo-echogenicity, and calcification (2, 3). However, none of these features can individually predict malignancy and conventional US alone has shown moderate accuracy in detecting malignant lesions (4). Therefore, improvements to US technique have been sought.
The introduction of contrast agents (contrast-enhanced US/CEUS) allows for visibility of blood flow within the lesion, which improves its characterization (5). The current in-use contrast media are second-generation agents as SonoVue. These agents remain within the intravascular space, which increases their safety and allows for continuous imaging over the enhancement period (6). Several studies have reported high sensitivity and specificity for CEUS in differentiating malignant lesions with the breast, thyroid, liver and kidneys (5, 7–9). A recent meta-analysis showed no significant difference between CEUS and contrast-enhanced computed tomography (CECT) and magnetic resonance imaging (CEMRI) in terms of the diagnostic accuracy in characterizing focal liver lesions (FLLs) (8).
Shear wave elastography (SWE) relies on the degree of lesion stiffness when subjected to external pressure. Malignant nodules have harder consistency (less elasticity) than benign ones due to the uncontrolled proliferation of cancer cells (10). Therefore, SWE has been investigated for differentiating benign and malignant nodules. Compared to conventional US, SWE is more quantitative and is less operator-dependent, allowing more effective detection of malignant tumors (11). Recent diagnostic test accuracy (DTA) studies and meta-analyses showed high sensitivity and specificity for SWE in detecting malignant lesions within the breast and hepatic tissues (11–13).
According to our knowledge, data are lacking on the direct comparison between CEUS and SWE; therefore, we performed a meta-analysis to evaluate the diagnostic accuracy of CEUS and SWE in differentiating malignant tumors in the breast, liver, thyroid, kidneys, and prostate tissues in comparison to histopathology as a reference test. Moreover, we used network meta-analysis (NMA) to compare the diagnostic accuracy of both tests in malignant tumor differentiation.
Materials and Methods
This meta-analysis has been conducted and reported in accordance with the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (The PRISMA-DTA Statement) (14); Supplementary File I.
Literature Search
We searched Medline (via PubMed), Embase, SCOPUS and Web of Science for diagnostic accuracy studies that evaluated the use of CEUS and SWE in the differentiation of malignant tumors in different body organs. The following search terms were used with different combinations in different databases: Contrast-enhanced Ultrasound OR CEUS OR Ultrasound OR SonoVue OR Shear Wave Elastography OR SWE OR Sonoelastography OR Elastosonography AND Malignant OR Cancer OR Tumor OR Benign OR Adenoma OR Adenocarcinoma OR Carcinoma OR Nodule. No search filters of any sort were used during the search. All retrieved search results from database search (including bibliographic data and abstracts) were imported into EndNote (X7) for duplicate removal and then were transferred to a Microsoft Excel Sheet for screening.
Study Screening
For a study to be eligible for inclusion, it must have matched all the following criteria: (1) Population: Patients, suspected or diagnosed with malignancy in any body organ, (2) Intervention: CEUS or SWE [no specifications by US system or probe type], (3) Comparator: Histopathology, (4) Outcomes: Sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV], and (5) Study type: Diagnostic accuracy study. Two independent authors reviewed the title and abstract of retrieved records against our eligibility criteria and classified them into: eligible, non-eligible, or requires further screening (seems to fit the inclusion criteria, but further confirmation is required). The full-text articles of the latter type were retrieved and underwent a second wave of screening. Any discrepancy between the two reviewers' decisions was solved by a senior reviewer (with a 15-year experience in secondary analysis and evidence synthesis methods) after reviewing the debated studies in reference to the pre-specified PICO criteria.
Data Extraction and Quality Assessment
An extraction sheet (in Microsoft Excel) was formatted and pilot-tested before final extraction. The sheet was customized to extract the baseline data of the imaging device, enrolled patients, as well as the raw diagnostic data of each included study. For pilot testing, two reviewers extracted these data from 5 included studies and the datasets were matched and compared with the original studies by a third reviewer. Each set of data was extracted by two reviewers and discordant decisions were resolved by discussion. These discussions included re-examining the studies, inspecting their available additional data sources and re-evaluating the former decisions. When the discrepancies remained, a senior reviewer examined the studies and settled the differences. The extracted data included (I) baseline characteristics of enrolled participants, (II) study design, (III) diagnostic test parameters: Parameters, cutoff value and US system for SWE and contrast agent, US technique, probe and mechanical index for CEUS, and (IV) Outcome data: true positive (TP), true negative (TN), false positive (FP), and false negative (FN) values. When these values were not directly given, they were calculated from the processed data as sensitivity, specificity, PPV, and NPV, using the statistical calculator on RevMan software (Version 5.3 for Windows). We used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) score to assess the quality of included studies. It consists of 14 (yes/no/unclear) questions to assess different forms of bias within DTA studies (15).
Data Analysis
Pairwise meta-analyses were done under the random-effects model when two or more studies investigated the same predefined research question with the same laboratory test. We extracted the sensitivity, specificity, positive likelihood ratio (LR), negative LR, and diagnostic odds ratio (DOR) values for CEUS and SWE compared to histopathology as a reference standard. The DOR is calculated as (TP X TN)/ (FP X FN) and defined as the odds of having a positive test result in a patient with disease compared with the odds of a positive test result in a patient without disease. Moreover, summary receiver operating characteristic (SROC) curves were constructed to examine diagnostic accuracy. All statistics were reported as absolute values with their 95% confidence interval (95% CI). A p-value < 0.05 was considered statistically significant. The Chi-square and I-square statistics were calculated in order to assess heterogeneity. Significant heterogeneity was considered to be present if the chi-square p-value was < 0.1 (as per the Cochrane Handbook for Systematic Reviews of Intervention). Data were presented into five subgroups according to cancer site: breast, liver, thyroid, kidneys, and prostate. Network meta-analyses were conducted to compare the diagnostic accuracy of CEUS vs. SWE in malignancy detection. Heterogeneity and inconsistency were checked by the I2 and the corresponding p-value. All statistical analyses were conducted on MetaDiSc (version 1.4) and R software (version 3.4.3).
Results
Literature Search and Study Characteristics
Database search retrieved 5896 unique citations. Following title and abstract screening, 422 full-text articles were retrieved for further scrutiny. Finally, 114 diagnostic accuracy studies (65 on SWE and 50 on CEUS; one study by 4 assessed both modalities), reporting data from 15926 patients (5680 for CEUS and 10392 for SWE) were included in our network meta-analysis (Figure 1, Bibliographic details in Supplementary File II). According to the QUADAS score, 25 (21.5%), 30 (25.8%), 22 (18.9%), 23 (19.8%), and 16 (13.8%) studies scored 10, 11, 12, 13, and 14, respectively. The baseline data of enrolled participants, as well as the characteristics of the used US systems for SWE and CEUS tests are illustrated in Tables 1, 2, respectively.
Figure 1.
PRISMA flow diagram of literature search and study selection.
Table 1.
Baseline characteristics of enrolled patients and criteria of the used SWE system.
| References | Country | Study design | Patients/Lesions (N) | Age (Years) | Male: Female | Organ | Condition | Reference test/Gold standard | SWE parameters | Cutoff value (Kpa) | US system |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al. (16) | China | Prospective cohort | 276 (296 lesions) | 45.4 ± 14.7 | 100% F | Breast | Benign vs. malignant breast masses | Histopathology | SWS | 4.39 m/sec | S3000 US scanner (Siemens) |
| Yang et al. (17, 18) | China | Retrospective cohort | 218 (225 lesions) | 45.3 ± 14.6 | 100% F | Breast | Benign vs. malignant breast masses | Histopathology | Emean | 36.1 Kpa | Aplio500 US machine (Toshiba) |
| Elmoneam et al. (13) | Egypt | Prospective cohort | 63 (63 lesions) | 34.7 ± 5.9 | 100% F | Breast | Benign vs. malignant breast masses | Histopathology | Emax | 106.55 Kpa | N/A |
| Kim et al. (19) | Korea | Retrospective cohort | 171 (177 lesions) | 45.17 ± 9.37 | 100% F | Breast | Small breast lesions < 2 cm | Histopathology | Emax | 87.5 Kpa | Aixplorer system (Supersonic Imagine |
| Youk et al. (20) | Korea | Prospective cohort | 123 (130 lesions) | 46.7 ± 11.2 | 100% F | Breast | Breast cancer | Histopathology | Emean | 82.2 Kpa | Aixplorer ultrasound system |
| Tang et al. (21) | China | Prospective cohort | 98 (133 lesion) | N/A | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Mean SWV | 3.68 m/s | Siemens S3000 US scanner |
| Choi et al. (22) | Korea | Retrospective cohort | 54 (56 lesions) | 40.76 + 68.07 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Emean | 44.3 Kpa | Aixplorer US system (SuperSonic Imagine |
| Liu et al. (12) | China | Prospective cohort | 130 (139 lesions) | 44.74 ± 14.77 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Max SWV | 5.37 m/s | Siemens Acuson S3000 ultra-sound machine |
| Golatta et al. (23) | Germany | Prospective cohort | 103 (104 lesions) | 51 ± 18.56 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Mean SWV | 5.18 m/s | Siemens Medical Solutions |
| Youk et al. (24) | Korea | Retrospective cohort | 324 (389 lesions) | 46.0 ± 11.4 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Eratio | 5.14 | Aixplorer US system (SuperSonic Imagine |
| Ko et al. (25) | Korea | Retrospective cohort | 33 (34 lesions) | 46.4 ± 7.5 | 100% F | Breast | Breast Non-mass lesions | Histopathology | Emean | 41.6 Kpa | Aixplorer US system (SuperSonic Imagine |
| Lee et al. (26) | Korea | Prospective cohort | 134 (144 lesions) | 49.1 ± 12.8 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Emax | 147.2 Kpa | Aixplorer US system (SuperSonic Imagine |
| Ng et al. (27) | Malaysia | Prospective cohort | 152 (159 lesions) | 52 + 20.5 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Emax | 56.0 Kpa | Aixplorer ultrasound system (SuperSonic Imagine |
| Tian et al. (28) | China | Retrospective cohort | 210 (210 lesions) | 43.12 ± 13.34 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Emax | 80.8 Kpa | Aixplorer ultrasound system (SuperSonic Imagine |
| Olgun et al. (29) | Turkey | Prospective cohort | 109 (115 lesions) | 51 + 17.5 | 0.02:1 | Breast | Benign vs. malignant breast lesion | Histopathology | Eratio | 4.7 | Aixplorer ultrasound system (SuperSonic Imagine |
| Chang et al. (30) | Korea | Prospective cohort | 115 (133 lesions) | 51.4 + 11.75 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Emean | 60.7 Kpa | IU-22 (Phillips) OR HDI 5000 sonography unit |
| Yao et al. (31) | China | Prospective cohort | 206 (206 lesions) | 44.6 + 13.3 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Mean SWV | 4.22 m/s | Acuson S2000 ultrasound system (Siemens |
| Lee et al. (26) | Korea | Retrospective cohort | 139 (156 lesions) | 43.54 ± 9.94 | 100% F | Breast | Solid breast masses | Histopathology | Emax | 82.3 Kpa | Aixplorer ultrasound system (SuperSonic Imagine |
| Seo et al. (32) | Korea | Prospective cohort | 37 (45 lesions) | 47.4 +14.75 | 100% F | Breast | Benign vs. malignant breast lesion | Histopathology | Emean | 67.8 Kpa | Aplio 500; Toshiba |
| Au et al. (33) | Canada | Prospective cohort | 112 (123 lesions) | 49.2+10.7 | 100% F | Breast | Solid breast masses | Histopathology | Eratio | 3.56 | Aixplorer Multiwave V3, Supersonic Imagine |
| Chang et al. (34) | Korea | Prospective cohort | 129 (150 lesions) | 47.8+8.83 | 100% F | Breast | Benign vs. malignant solid breast lesions | Histopathology | Emean | 80 Kpa | Aixplorer, SuperSonic Imagine |
| Choi et al. (35) | Korea | Retrospective cohort | 113 (116 lesions) | 48.4+10 | 100% F | Breast | Breast non-mass lesions | Histopathology | Emean | 85.1 Kpa | Aixplorer, SuperSonic Imagine |
| Chung et al. (36) | Korea | Retrospective cohort | 71 (79 lesions) | 48+10.67 | 100% F | Breast | Breast papillary lesions | Histopathology | Emax | 62.1 Kpa | Aixplorer, SuperSonic Imagine |
| Choi et al. (22) | Korea | Retrospective cohort | 199 (205 lesions) | 51.7 ± 13.3 | 100% F | Breast | Benign vs. malignant solid breast lesions | Histopathology | Emean | 85.8 Kpa | Aixplorer, SuperSonic Imagine |
| Dobruch-Sobczak et al. (37) | Poland | Retrospective cohort | 76 (84 lesions) | 59.9+13 | 100% F | Breast | Focal breast lesions | Histopathology | Eav.adj. | 68.5 Kpa | Aixplorer, SuperSonic Imagine |
| Guo et al. (38) | China | Prospective cohort | 379 (404 lesions) | N/A | 100% F | Breast | Focal breast lesions | Histopathology | SWS | 3.015 m/s | Siemens ACUSON S2000 |
| Hong et al. (39) | Korea | Prospective cohort | 218 (264 lesions) | 46.4+10.5 | 100% F | Breast | Solid breast masses | Histopathology | Emax | 44.1 Kpa | N/A |
| Kim et al. (40) | China | Retrospective cohort | 67 (67 lesions) | 41.5+2.29 | 100% F | Breast | Fibroadenoma vs. phylloids tumor | Histopathology | Emean | 43.9 Kpa | Aixplorer, SuperSonic Imagine |
| Klotz et al. (41) | France | Retrospective cohort | 142 (167 lesions) | 57.7 +11 | 100% F | Breast | Benign vs. malignant solid breast lesions | Histopathology | Emax | 106 Kpa | Aixplorer, SuperSonic Imagine |
| Lee et al. (42) | Korea | Retrospective cohort | 139 (140 lesions) | 45.5 + 10.33 | 100% F | Breast | Complex cystic and solid breast lesions | Histopathology | Emax | 108.5 Kpa | Aixplorer, SuperSonic Imagine |
| Li et al. (16) | China | Retrospective cohort | 116 (116 lesions) | 48.56+ 14.4 | 100% F | Breast | Breast lesions BIRADS IV | Histopathology | SWS | 3.49 m/s | Siemens S3000 US machine |
| Shi et al. (43) | China | Prospective cohort | 251 (279 lesions) | 45.3 6 11.8 | 100% F | Breast | Benign vs. malignant solid breast lesions | Histopathology | SD | 8.05 Kpa | Aixplorer, SuperSonic Imagine |
| Sim et al. (44) | UK | Retrospective cohort | 52 (52 lesions) | 67 | 100% F | Breast | IDC | Histopathology | Emean | 50 Kpa | Aixplorer, SuperSonic Imagine |
| Sim et al. (44) | UK | Retrospective cohort | 52 (52 lesions) | 67 | 100% F | Breast | ILC | Histopathology | Emean | 50 Kpa | Aixplorer, SuperSonic Imagine |
| Wu et al. (45) | China | Retrospective cohort | 192 (209 lesions) | N/A | 100% F | Breast | Benign vs. malignant solid breast lesions | Histopathology | N/A | N/A | Siemens ACUSON S2000 |
| Youk et al. (20) | Korea | Retrospective | 78 (79 lesions) | 45.5 + 11.6 | 100% F | Breast | Benign vs. malignant solid breast lesions | Histopathology | Eratio | 3.7 | Aixplorer, SuperSonic Imagine |
| Zhang et al. (46) | China | Prospective cohort | 97 (98 lesions) | 44.74 ± 14.77 | 100% F | Breast | Small breast lesions < 10 cm | Histopathology | SWV | 3.27 m/s | Siemens ACUSON S2000 |
| Cong et al. (47) | China | Prospective cohort | 315 (326 lesions) | 44.51 + 11.81 | 100% F | Breast | Breast masses | Histopathology | SD | 13.75 | Aixplorer, SuperSonic Imagine |
| Park et al. (48, 49) | Korea | Retrospective cohort | 133 (156 lesions) | 47.8 ± 12.7 | 100% F | Breast | Palpable breast masses | Histopathology or periodic imaging surveillance | Emax | 45.1 Kpa | Aixplorer, SuperSonic Imagine |
| Wang et al. (50) | China | Retrospective cohort | 63 (67 lesions) | 40.1 + 21.2 | 100% F | Breast | Non-mass breast lesions | Histopathology | Emax | 81.07 Kpa | iU22 Philips |
| Kasai et al. (51) | Japan | Prospective cohort | 273 patients with chronic liver disease | 59.64 ± 14.40 70.98 ± 9.33 | 1:01 | Liver | HCC | Histopathology | Young's Modulus | N/A | Aixplorer US system (SuperSonic Imagine S.A.) |
| Gerber et al. (52) | Germany | Prospective cohort | 106 (106 lesions) | 55.5+16.74 | 3.8:1 | Liver | Characterization of solid HFLs | Histopathology and CE imaging for benign lesions | Emedian | 37.6 Kpa | Aixplorer ultrasound system (SuperSonic Imagine) |
| Özmen et al. (53) | Turkey | Prospective cohort | 20 (20 lesions) | 4.74+4 | 2.3:1 | Liver | Heamangioma vs. malignant liver lesions | Histopathology | Emean | 23.62 Kpa | Aixplorer ultrasound system (SuperSonic Imagine) |
| Tian et al. (54) | China | Prospective cohort | 221 (229 lesions) | 48.9 + 13.2 | 2.4:1 | Liver | Benign vs. malignant HFLs | Histopathology | Emax | 39.6 Kpa | Aixplorer, SuperSonic Imagine |
| Ahmad et al. (55) | UK | Prospective cohort | 50 (11 with PSA> 20) | 69 | 100% M | Prostate | Prostate cancer | Histopathology | Shear wave velocity and Young's modulus | N/A | SuperSonic Imagine |
| Boehm et al. (56) | Germany | Prospective cohort | 60 patients with suspected prostate cancer | N/A | 100% M | Prostate | Prostate cancer | histopathology | Young's Modulus | 50 Kpa | TRUS Aixplorer |
| Porsch et al. (57) | Germany | Prospective cohort | 69 (794 samples) | 65+8 | 100% M | Prostate | Prostate cancer | Histopathology | Young's Modulus | 48 Kpa | SuperSonic Imagine Ultrasound System AIXPLORER |
| Woo et al. (58) | Korea | Prospective cohort | 87 (87 lesions) | 66 ± 9.0 | 100% M | Prostate | Prostate cancer | Histopathology | Young's Modulus | 43.9 Kpa | SuperSonic Imagine |
| Correas et al. (59) | France | Prospective cohort | 184 (1040 samples) | 65.1 6 7.6 | 100% M | Prostate | Prostate cancer | Histopathology | Young's Modulus | 35 Kpa | SuperSonic Imagine |
| Glybochko et al. (60) | Russia | Prospective cohort | 302 (134 with suspected PC, 120 with confirmed PC and 48 healthy men) | N/A | 100% M | Prostate | Prostate cancer | Histopathology | Young's Modulus | 50 Kpa | Super Sonic Imagine |
| Zhang et al. (61, 62) | China | Prospective cohort | 59 (71 lesions) | 50.5 ± 9.1 | 0.4:1 | Thyroid | Benign vs. malignant thyroid nodules < 10 mm | Histopathology | Shear wave velocity | 2.910 m/s | Acuson S2000 Seimens VTTQ |
| Azizi et al. (63) | USA | Prospective cohort | 676 (707 lesions) | 51.2+15 | 0.2:1 | Thyroid | Thyroid cancer | Histopathology | Shear wave velocity | 3.54 m/s | Virtual Touch IQ Software on the Siemens ACU-SON S3000 US |
| Liu et al. (12) | China | Prospective cohort | 271 (331 lesions) | 45.9 ± 13.4 | 0.3:2 | Thyroid | Malignant thyroid nodule | Histopathology | SWE mean | 39.3 Kpa | SuperSonic Imagine |
| Wang et al. (64) | China | Prospective cohort | 322 (322 nodules) | 50.5 ± 12.6 | 0.3:1 | Thyroid | Malignant thyroid nodule | Histopathology | Elastic modulous and SWS | 3.52 m/s | Aplio500, Toshiba Medical Systems |
| Duan et al. (65) | China | Prospective cohort | 118 (137 lesions) | 45.9 ± 13.4 | 0.6:1 | Thyroid | Malignant thyroid nodule | Histopathology | SWE mean | 34.5 | Aixplorer; Supersonic Imagine |
| Liu et al. (66) | China | Prospective cohort | 238 (254 lesions) | 50.9 ± 11.9 | 0.3:1 | Thyroid | Malignant thyroid nodule | Histopathology | SWS | 2.78 m/s | N/A |
| Liu et al. (67) | China | Retrospective cohort | 227 (313 lesions) | 46.14 ± 9.70 | 0.2:1 | Thyroid | Malignant thyroid nodule | Histopathology | Emax | 51.95 Kpa | N/A |
| Kim et al. (68) | Korea | Retrospective cohort | 99 (99 lesions) | 45.7+13 | N/A | Thyroid | Malignant thyroid nodule | Histopathology | Emean | 62 Kpa | Aixplorer US system (SuperSonic Imagine) |
| Deng et al. (69) | China | Prospective cohort | 146 (175 nodules) | 46.36 ± 12.5 | 0.4:1 | Thyroid | Malignant thyroid nodule | Histopathology | SWS | 2.59 m/s. | Siemens Acuson S2000 US machine |
| Baig et al. (70) | China | Prospective cohort | 122 (163 nodules) | 53 ± 13.7 | 0.2:1 | Thyroid | Malignant thyroid nodule | Histopathology | Emax | 67.3 Kpa | Aixplorer, Supersonic Imagine |
| Dobruch-Sobczak et al. (71) | Poland | Prospective cohort | 119 (169 lesions) | 49.2+14 | 0.3:1 | Thyroid | Characterization of thyroid nodules | Histopathology | Emean | 30.5 Kpa | Aixplorer, SuperSonic Imagine |
| Liu et al. (72) | China | Prospective cohort | 49 (64 lesions) | 45.3 ± 13.1 | 0.4:1 | Thyroid | benign vs. malignant solid Thyroid lesions | Histopathology | Emean | 38.3 Kpa | Q-box TM; Super Sonic Imagine |
| Park et al. (73) | Korea | Retrospective cohort | 453 (476 nodules) | 45.7+10.33 | 0.2:1 | Thyroid | Benign vs. malignant solid Thyroid lesions | Histopathology | Emean | 85.2 Kpa | Aixplorer, SuperSonic Imagine |
| Samir et al. (74) | USA | Prospective cohort | 35 (35 lesions) | 55 + 16.1 | 0.5:1 | Thyroid | Benign vs. malignant thyroid follicular lesions | Histopathology | Young's Modulus | 22.3 Kpa | Aixplorer, SuperSonic Imagine |
| Yang et al. (75) | China | Prospective cohort | 107 (107 lesions) | 54.0 ± 9.4 | 0.26:1 | Thyroid | Benign vs. malignant solid Thyroid lesions | Histopathology | Mean SWS | 3.01 m/s | Acuson S3000 (Siemens) |
| Zhou et al. (76) | China | Prospective cohort | 290 (302 lesions) | 49.80+12.34 | 0.4:1 | Thyroid | Benign vs. malignant solid Thyroid lesions | Histopathology | Mean SWS | 2.6 m/s | Acuson S3000 (Siemens) |
Table 2.
Baseline characteristics of enrolled patients and criteria of the used CEUS system.
| References | Country | Study design | Organ | Condition | Patients/ Lesions (N) | Age (Years) | Male: Female | Contrast agent | Reference test | US technique | Mechanical index | Probe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bertolotto et al. (5) | Italy | Retrospective | Kidney | Indeterminate renal masses with equivocal enhancement on CT | 47 (30 HP) | 65 ± 13 | 4.75:1 | 2.4 mL SonoVue | Histopathology | Pulse inversion harmonic imaging Cadence contrast pulse sequencing | 0.05–0.21 | Convex array (C5–1) & (4C1) &(C5–2 HDI) & (CA430E) |
| Cai et al. (77) | China | Prospective cohort | Kidney | Benign vs. malignant renal masses | 73 (73 lesions) | 56.36 ± 12.2 | 1.6:1 | 1.0–1.8 mL SonoVue | Histopathology and follow up data | Acuson Sequoia 512, Siemens, | 0.21–0.23 | 4C1-S convex probe 1–4 MHz |
| Chang et al. (30) | USA | Prospective cohort | Kidney | Renal solid and cystic lesions | 44 (23 HP lesions) | 56 ± 14 | 0.7:1 | Sonazoid | Histopathology and follow up data | Siemens Acuson Sequoia 512 | 0.19 | 4C1 abdominal transducer |
| Chen et al. (78, 79) | China | Prospective cohort | Kidney | RCC vs. AML | 99 (102 lesions) | 56.6 ± 16.5 | 2:01 | 1.2 ml of SonoVue | Histopathology | Acuson S2000 (contrast pulse sequencing) | N/A | N/A |
| Chen et al. (80) | China | Prospective cohort | Kidney | Complex cystic renal masses | 59 (71 lesions) | 49.6 + 14.25 | 2.9:1 | 2.4 mL of SonoVue | Histopathology and follow up data | Coded phase inversion harmonic imaging (Logiq 9 scanner GE Healthcare) | 0.07–0.10 | 3.5C (2.5–5.0 MHz) and 4C (1.0–4.0 MHz) convex transducers |
| Defortescu et al. (81) | France | Prospective cohort | Kidney | Complex renal cysts | 47 (47 lesions) | 46 + 9.75 | 1.8:1 | 1.2 mL SonoVue | Histopathology and follow up data | ACUSON S2000-Siemens−10 | 0.06–0.1 | Convex probe 3–4.5 mHz |
| Li et al. (16) | China | Retrospective | Kidney | RCC vs. AML | 411 (429 lesions) | 54.12 ± 12.57 | 1.9:1 | 1.2 mL SonoVue | Histopathology | E9 system (GE Healthcare | 0.11 | C1-5, 1–5 MHz |
| Li et al. (82) | China | Retrospective | Kidney | Solid Renal Masses | 91 (100 lesions) | 62.0 ± 15.6 | 2.6:1 | 1.0–1.2 ml SonoVue | Histopathology | Acuson Sequoia 512 scanner | < 0.2 | 4V1 vector transducer, 1–4 MHz |
| Lu et al. (83) | China | Retrospective | Kidney | RCC vs. AML | 189 (189 lesions) | 47.3 ± 20.7 | 1.6:1 | 1.2 ml SonoVue | Histopathology | LOGIC E9 | < 0.1 | C1–5, 1.5 MHz |
| Nicolau et al. (84) | Spain | Prospective cohort | Kidney | Indeterminate renal masses by CT | 72 (83 nodules) | 64.9 + 14.5 | 1.9:1 | 2.4 mL of SonoVue | Histopathology and follow up data | Cadence contrast pulse sequencing technology (CPS) | < 0.2 at Sequoia 512, < 0.009 at S2000) | 4C1 convex array probe |
| Oh et al. (85) | Korea | Retrospective | Kidney | RCC vs. AML (small masses) | 49 lesions | 61+11.5 | 2.5:1 | SonoVue | Histopathology | N/A | N/A | N/A |
| Sanz et al. (86) | Spain | Prospective cohort | Kidney | Complex cystic renal masses | 66 (67 lesions) | 67.8+ 1.83 | 2.7:1 | 2.4 mL SonoVue | Histopathology | Hitachi Preirus | N/A | EUP-C715 probe (5–1 MHz |
| Tamas-Szora et al. (87) | Romania | Prospective cohort | Kidney | RCC | 32 (33 lesions) | 60.9 ± 10.43 | 1:01 | 1.6 mL of SonoVue | Histopathology | General Electric Logiq 7 system | 0.09–0.11 | Convex wide-band transducer (2–5.5 MHz) |
| Tian et al. (28) | China | Prospective cohort | Kidney | Renal SOL | 367 (378 lesions) | N/A | N/A | 1.2 mL SonoVue | Histopathology | ACUSON S2000 Ultrasound System | Probe 4C1, 2.5–5 MHz | |
| Wei et al. (88) | China | Retrospective | Kidney | Benign vs. malignant solid renal masses | 118 (118 lesions) | 53.5 ± 12.6 | 1.6:1 | 1.6–2.4 mL SonoVue | Histopathology | Contrast pulse sequence, Sequoia 512 ultrasound system (Siemens | 0.18−0.20 | 4C1, 3–4 MHz |
| Yong et al. (89) | Singapore | Retrospective | Kidney | Undetermined renal masses | 63 (74 nodules) | 62.4 ± 14.5 | 1.6:1 | 1.5 ml of SonoVue | Histopathology | Aplio 500, Toshiba Medical Systems AND iU22, Philips Healthcare | N/A | N/A |
| Zhang et al. (90) | China | Prospective cohort | Kidney | Benign vs. malignant thyroid nodules | 148 (157 lesions) | 45.4 ± 10.5 | N/A | 2.4 ml SonoVue | Histopathology | Contrast pulse sequence (CPS) imaging. Acuson, Sequoia 512 Encompass | 0.20–0.23 | 15L8w probe (8–14 MHz) |
| Miyamoto et al. (91) | Japan | Prospective cohort | Breast | Focal breast lesions | 127 (127 lesions) | 48.5 ± 12.3 | :1 | 0.015 mL/kg Sonazoid | Histopathology | AplioXG, Toshiba AND, Hitachi-Aloka AND Logiq E9, GE | 0.1–0.4 | Broadband linear phased-array transducer |
| Xia et al. (92) | China | Retrospective | Breast | Papillary breast lesions | 50 (52 lesions) | 51 +13.57 | :1 | 2.4 mL SonoVue | Histopathology | Pulse-inverse harmonic imaging technique [Philips iU22] | 0.05–0.08 | 3–9-MHz linear transducer |
| Xiao et al. (93) | China | Prospective cohort | Breast | Subcentimetric breast lesions | 203 (209 lesions) | 47+15.25 | :1 | 4.8 mL of SonoVue | Histopathology | Pulse inversion harmonic technique w iU22 (Philips) | 0.06 | 9–3-MHz linear transducer |
| Yuan et al. (94) | China | Prospective cohort | Breast | Breast tumors | 216 (216 lesions) | 46 ± 12 | :1 | 2.5 mL SonoVue | Histopathology | Sequoia; Siemens Medical Solutions | N/A | 10 MHz transducer |
| Aubé et al. (95) | France | Prospective cohort | Liver | Diagnosis of HCC (< 3 cm) | 381 (544 lesions) | 62 ± 9.69 | 4.6:1 | SonoVue | Histopathology, CT and MRI according to EASL-AASLD | N/A | N/A | N/A |
| Beyer et al. (96) | Germany | Retrospective | Liver | Benign vs. malignant liver nodules | 83 (83 lesions) | 59.8 +10 | 2.6:1 | 1–2.4 ml SonoVue | Histopathology | LOGIQ E9, GE | N/A | 1–6 MHz curved probe |
| Corvino et al. (97) | Italy | Prospective cohort | Liver | Cystic and cyst like liver lesions | 48 (50 lesions) | 65+15 | 0.9:1 | 2.4 or 4.8 mL SonoVue | Histopathology | MyLab 70 Twice scanner (Esaote) | N/A | D multifrequency (2.5–5 MHz) convex probes |
| Feng et al. (98) | China | Retrospective | Liver | HCC differentiation | 271 (374 lesions) | 49.25 + 17 | 3.9:1.0 | 2.4 mL SonoVue | Histopathology | iU22 system (Philips) | < 0.1 | (5–2 MHz) convex transducer (C5-2). |
| Iwamoto et al. (99) | Japan | Retrospective | Liver | Macroscopic HCC | 77 (79 lesions) | 70 ± 9 | 2.7:1 | 0.015 ml/kg Sonazoid | Histopathology | (tissue harmonic grayscale imaging) LOGIQ 7 or E9 US | 0.2–0.3 | Convex or linear probes with a frequency of 2–5 or 4–9 MHz |
| Kobayashi et al. (100) | Japan | Retrospective | Liver | NS-HCC | 85 (85 lesions) | 66 + 13.75 | 2.9:1 | 0.015 ml/kg Sonazoid | Histopathology | Wide-band pulse-inversion harmonic imaging (HI VISION Ascendus (Hitachi)) | 0.16–0.2 | Microconvex probe (EUP- C715, 3.5 MHz |
| Kobayashi et al. (101) | Japan | Retrospective | Liver | Liver metastasis | 98 (148 lesions) | 66.46 ± 11.2 | 1.7:1 | 0.0075 mL/kg Sonazoid | Histopathology | SSA 770 A or 790 A US system (Toshiba) | 0.17–0.27 | 3.75-MHz convex probe |
| Liu et al. (12) | China | Prospective cohort | Liver | Hyperechoic HFL | 102 (135 lesions) | 51.4 ± 12.5 | 2.8:1 | 1.5 mL of SonoVue | Histopathology | GE Logiq9 color Doppler ultrasonography | 0.11 | convex array probe (frequency: 3.5–5 MHz) |
| Quaia et al. (102) | Italy | Retrospective | Liver | Benign vs. malignant liver lesions in cirrhotic patients | 46 (55 lesions) | 55 ± 10 | 0.8:1 | 2.4 mL SonoVue | Histopathology | Sequoia, Acuson-Siemens AND iU22 (iU22; Philip) | 0.09–0.14 | Convex array 2–4 MHz 4C1 transducer AND 2–5-MHz broadband curvilinear probe |
| Sandrose et al. (103) | USA | Retrospective | Liver | CT undetermined HFL | 78 (163 lesions) | 61.8 + 15.25 | 1.1:1 | 1.2 ml bolus of SonoVue | Histopathology and PET/CT follow up | Pulse inversion harmonic imaging (GE LOGIQ 9E) | N/A | N/A |
| Schellhaas et al. (104) | Germany | Prospective cohort | Liver | HCC by CEUS and ESCULAP | 100 (100 lesions) | 66.1 + 7.17 | 5.7:1 | 1.5 ml SonoVue | Histology and imaging | GE Logiq E9 AND Siemens Acuson S2000 AND Toshiba Aplio 500 | N/A | N/A |
| Tada et al. (105) | Japan | Prospective cohort | Liver | Macroscopic HCC | 99 (99 lesions) | 67.8 ± 10.4 | 2.7:1 | 0.015 ml/kg of Sonazoid | Histopathology | Wideband harmonic imaging (Aplio XG system, Toshiba) | (0.18–0.28) | 5-MHz convex transducer 1.4 and 5.3 MHz |
| Thakur et al. (106) | India | Prospective cohort | Liver | HCC | 50 (50 lesions) | 52 + 14.25 | 1.4:1 | 2.4 ml SonoVue | Histopathology, CT and MRI | Xario XG (Toshiba) | < 0.2 | |
| Wang et al. (64) | Germany | Prospective cohort | Liver | Superficial HFL | 27 (27 lesions) | N/A | 2.4:1 | 2.4 ml SonoVue | Histopathology, one patient by MRI | Philips iU22, LOGIQ E9, Aplio 500 | N/A | High frequency transducer (7.5–12 MHz) |
| Wu et al. (107) | China | Prospective cohort | Liver | Focal hepatic lesions | 46 (55 lesions) | 46.5 + 15.2 | 1.2:1 | 2.4-mL dose of SonoVue | Histopathology, CECT and MRI | Philips iU22 US system | 0.06 | 5C2 multi- frequency convex probe |
| Yin et al. (108) | China | Prospective cohort | Liver | Cholangiocarcinoma vs. inflammatory lesions | 40 (40 lesions) | 58.7 + 9.701 | 1.4:1 | 1.5 mL of SonoVue | Histopathology | LOGIQ E9 (GE Healthcare) | < 0.1 | C5-1, 2.0–4.0 MHz |
| Zhang et al. (109) | China | Prospective cohort | Liver | Benign vs. malignant liver lesions | 156 (176 lesions) | 50.7 + 16.25 | 1.9:1 | 2.4 mL of SonoVue | Histopathology | Acuson S2000 ultrasound system Seimens | N/A | 4C1 convex array probe; frequency 2.0–4.0 MHz |
| Takahashi et al. (110) | Japan | Prospective cohort | Liver | HFL < 30 mm | 56 (67 lesions) | 65.8 ± 10.1 | 2.5:1 | 0.0075 mL/kg Sonazoid | Histopathology | SSA-790A ultrasound system (Aplio | (0.20–0.25) | 3.75 MHz convex probe |
| Taimr et al. (111) | Canada | Prospective cohort | Liver | Liver metastasis | 89 (89 lesions) | 31–87 | 1.6:1 | 1.5–2.4 mL SonoVue | Histopathology | Contrast-tuned imaging Hitachi 900 and Hitachi Preirus | N/A | 2.5–5.0 MHz probe |
| Cantisani et al. (9) | Italy | Prospective cohort | Thyroid | Thyroid nodules | 48 (53 lesions) | 49.4 + 8.75 | 2.7:1 | 4.8 mL SonoVue | Histopathology | MyLab 70XvG, Esaote | N/A | Linear probe (7–12 MHz) (N:36) |
| Deng et al. (69) | China | Prospective cohort | Thyroid | Malignant thyroid nodule | 146 (175 nodules) | 46.36 ± 12.5 | 0.4:1 | 2.4 mL of the SonoVue | Histopathology | Siemens Acuson S2000 US machine | 0.1 | 9L4, 5.0 MHz to 14.0 MHz |
| Diao et al. (112) | China | Prospective cohort | Thyroid | Benign vs. malignant thyroid nodules | 77 (87 lesions) | 52.4 ± 17.2 | N/A | 1.5 mL SonoVue | Histopathology | Siemens Acuson S2000 US | 0.06–0.1 | 5- to 14-MHz linear array transducer (9L4) |
| Giusti et al. (113) | Italy | Prospective cohort | Thyroid | Benign vs. malignant thyroid nodules | 63 (HP in 38 lesions) | 55.9 ± 14.7 | 0.2:1 | 4.8 ml of SonoVue | Histopathology | MyLab 70 US scanner | N/A | 7.5-MHz linear probe |
| Jiang et al. (114) | China | Prospective cohort | Thyroid | Benign vs. malignant calcified thyroid nodules | 122 (122 nodules) | 46 + 12 | 0.4:1 | 2.4 mL of the SonoVue | Histopathology | Contrast pulse sequencing (CPS) (ACUSON Sequoia 512 (Siemens Healthcare) | 0.32 | 15L8w high- frequency linear transducer |
| Wu et al. (107) | China | Retrospective | Thyroid | Benign vs. malignant thyroid nodules | 133 lesions | 46.3 + 10 | 0.5:1 | 1.2 mL SonoVue | Histopathology | ESAOTE MyLab 90 X-vision | 0.05–0.07) | L522 (3–9 MHz) linear-array probe |
| Zhang et al. (46) | China | Prospective cohort | Thyroid | Benign vs. malignant thyroid nodules | 70 (200 lesions) | 49.6 + 12.8 | 0.3:1 | 2.0 mL SonoVue | Histopathology | Acuson S2000 | < 0.10 | 9-MHztransducer |
| Zhang et al. (90) | China | Prospective cohort | Thyroid | Benign vs. malignant thyroid nodules | 246 (319 patients) | 46.1 ± 15.2 | 0.5:1 | 2.4 ml SonoVue | Histopathology | Contrast pulsed sequencing (CPS) Siemens Acuson S2000 | N/A | 9 L4 transducer |
| Zhang et al. (90) | China | Prospective cohort | Thyroid | Benign vs. malignant thyroid nodules | 111 (145 nodules) | 48 + 13.45 | 0.2:1 | 1.6 mL SonoVue | Histopathology | Contrast tuned imaging Mylab Twice Esaote | N/A | LA522 transducer (3–9 MHz) |
| Zhou et al. (115) | China | Prospective cohort | Thyroid | Benign vs. malignant thyroid nodules | 161 (167 lesions) | 44.14 + 12.01 | 0.4:1 | 2.4 ml SonoVue | Histopathology | DC-8EXP; Mindray | 0.15 | L12-3E transducer |
Outcomes of Pair-Wise Meta-Analysis
Breast Cancer
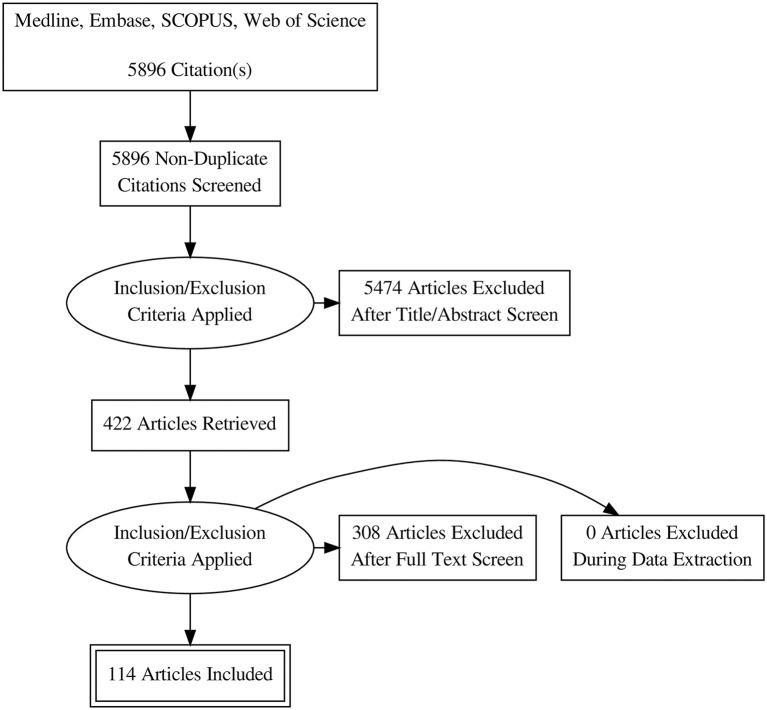
Detailed figures for pairwise meta-analysis in all five organs are illustrated in Supplementary File III. The pooled sensitivity, specificity, positive LR, and negative LR for CEUS in detection of breast malignant lesions were 0.89 (95% CI, 0.85, 0.92), 0.85 (95% CI, 0.81, 0.89), 6.13 (95% CI, 4.70, 8.01), and 0.12 (95% CI, 0.07, 0.21), respectively. The pooled DOR was 49.66 (95% CI, 29.42, 83.82) and the area under the receiving-operating characteristic (AUROC) curve was 0.92, Figure 2A. No heterogeneity was observed for sensitivity (p = 0.15) or specificity (p = 0.95).
Figure 2.
Summary receiver operating characteristic curve of (A) Contrast Enhanced Ultrasound, and (B) Shear Weight Elastography in breast cancer diagnosis.
For SWE, the pooled sensitivity, specificity, positive LR, and negative LR were 0.84 (95% CI, 0.83, 0.86), 0.86 (95% CI, 0.85, 0.87), 7.12 (95% CI, 5.54, 9.15), and 0.18 (95% CI, 0.15, 0.22), respectively. The pooled DOR was 46.22 (95% CI, 31.33, 68.18) with an AUROC of 0.93, Figure 2B. Significant heterogeneity was observed for sensitivity (p < 0.0001) and specificity (p < 0.0001).
Hepatic Cancer
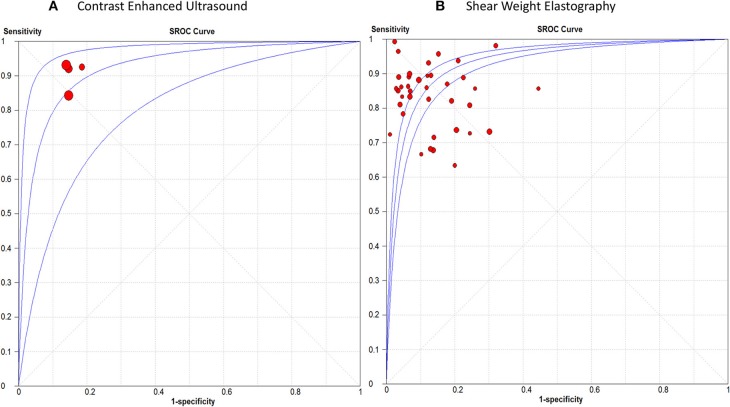
The pooled sensitivity, specificity, positive LR, and negative LR for CEUS in differentiating malignant hepatic lesions were 0.78 (95% CI, 0.76, 0.81), 0.89 (95% CI, 0.87, 0.91), 6.51 (95% CI, 3.90, 10.85), and 0.13 (95% CI, 0.06, 0.25), respectively. The overall DOR was 57.94 (95% CI, 24.78, 135.45) with an AUROC of 0.95, Figure 3A. The included studies were heterogeneous in the estimates of sensitivity (p < 0.0001) and specificity (p < 0.0001).
Figure 3.
receiver operating characteristic curve of (A) Contrast Enhanced Ultrasound, and (B) Shear Weight Elastography in hepatic cancer diagnosis.
For SWE, the pooled sensitivity, specificity, positive LR, and negative LR were 0.82 (95% CI, 0.77, 0.87), 0.83 (95% CI, 0.76, 0.89), 4.30 (95% CI, 2.85, 6.48), and 0.29 (95% CI, 0.12, 0.71), respectively. The overall DOR was 14.46 (95% CI, 4.09, 51.04) with an AUROC of 0.90, Figure 3B. The included studies were heterogeneous in the estimates of sensitivity (p < 0.0009) and specificity (p < 0.0001).
Thyroid Cancer
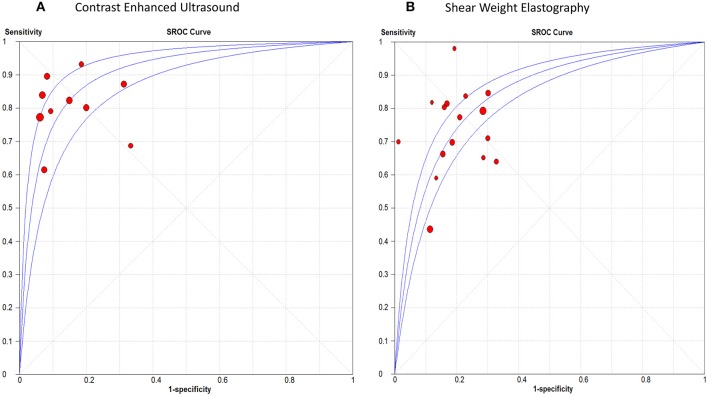
The pooled sensitivity, specificity, positive LR, and negative LR for CEUS in detecting malignant thyroid nodules were 0.81 (95% CI, 0.78, 0.84), 0.88 (95% CI, 0.86, 0.90), 6.01 (95% CI, 3.88, 9.31), and 0.23 (95% CI, 0.17, 0.31), respectively. The overall DOR was 28.54 (95% CI, 16.79, 48.51) with an AUROC of 0.91, Figure 4A. Significant heterogeneity was observed for sensitivity (p = 0.001) and for specificity (p < 0.0001).
Figure 4.
Summary receiver operating characteristic curve of (A) Contrast Enhanced Ultrasound, and (B) Shear Weight Elastography in thyroid cancer diagnosis.
For SWE, the pooled sensitivity, specificity, positive LR, and negative LR were 0.67 (95% CI, 0.64, 0.69), 0.77 (95% CI, 0.76, 0.79), 3.50 (95% CI, 2.93, 4.18), and 0.33 (95% CI, 0.25, 0.45), respectively. The overall DOR was 11.17 (95% CI, 8.04, 15.51) with an AUROC of 0.84, Figure 4B. Significant heterogeneity was observed for sensitivity (p < 0.0001) and specificity (p < 0.0001).
Renal Cancer
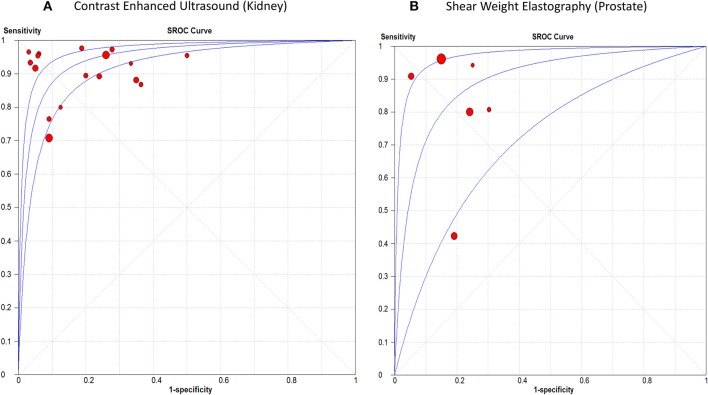
The sensitivity of CEUS ranged from 0.71 to 0.98 with a pooled sensitivity of 0.87 (95% CI, 0.85, 0.88). Specificity ranged from 0.50 to 0.97 with a pooled specificity of 0.84 (95% CI, 0.82, 0.87). The pooled positive and negative LRs were 5.55 (95% CI, 3.74, 8.22) and 0.12 (95% CI, 0.07, 0.19), respectively. The overall DOR was 53.44 (95% CI, 29.89, 95.56) with an AUROC of 0.95, Figure 5A. Significant heterogeneity was observed for sensitivity (p < 0.0001) and specificity (p < 0.0001).
Figure 5.
Summary receiver operating characteristic curve of (A) Contrast Enhanced Ultrasound in renal cancer diagnosis, and (B) Shear Weight Elastography in prostate cancer diagnosis.
Prostate Cancer
The sensitivity of SWE ranged from 0.42 to 0.96 with a pooled sensitivity of 84% (95% CI, 0.80, 0.87). Specificity ranged from 0.70 to 0.95 with a pooled specificity of 0.84 (95% CI, 0.82, 0.86). The pooled positive and negative LRs were 4.59 (95% CI, 2.68, 7.87) and 0.18 (95% CI, 0.07, 0.44), respectively. The overall DOR was 25.35 (95% CI, 7.15, 89.89) with an AUROC of 0.89 (Figure 5A). Significant heterogeneity was observed for sensitivity (p < 0.0001) and specificity (p < 0.0001) (Figure 5B). Table 3 summarizes the diagnostic results for both tests in different cancer sites.
Table 3.
Summary of the results of pooled sensitivity, specificity, positive, and negative likelihood ratios for SWE and CEUS in different cancers.
| Cancer | Test | Sensitivity | Specificity | + ve LR | -ve LR | DOR | AUROC |
|---|---|---|---|---|---|---|---|
| Breast cancer | SWE | 0.84 (95% CI, 0.83, 0.86) | 0.86 (95% CI, 0.85, 0.87) | 7.12 (95% CI, 5.54, 9.15) | 0.18 (95% CI, 0.15, 0.22) | 46.22 (95% CI, 31.33, 68.18) | 0.93 |
| CEUS | 0.89 (95% CI, 0.85, 0.92) | 0.85 (95% CI, 0.81, 0.89) | 6.13 (95% CI, 4.70, 8.01) | 0.12 (95% CI, 0.07, 0.21) | 49.66 (95% CI, 29.42, 83.82) | 0.92 | |
| Hepatic cancer | SWE | 0.82 (95% CI, 0.77, 0.87) | 0.83 (95% CI, 0.76, 0.89) | 4.30 (95% CI, 2.85, 6.48) | 0.29 (95% CI, 0.12, 0.71) | 14.46 (95% CI, 4.09, 51.04) | 0.90 |
| CEUS | 0.78 (95% CI, 0.76, 0.81) | 0.89 (95% CI, 0.87, 0.91) | 6.51 (95% CI, 3.90, 10.85) | 0.13 (95% CI, 0.06, 0.25) | 57.94 (95% CI, 24.78, 135.45) | 0.95 | |
| Thyroid cancer | SWE | 0.67 (95% CI, 0.64, 0.69) | 0.77 (95% CI, 0.76, 0.79) | 3.50 (95% CI, 2.93, 4.18) | 0.33 (95% CI, 0.25, 0.45) | 11.17 (95% CI, 8.04, 15.51) | 0.84 |
| CEUS | 0.81 (95% CI, 0.78, 0.84) | 0.88 (95% CI, 0.86, 0.90) | 6.01 (95% CI, 3.88, 9.31) | 0.23 (95% CI, 0.17, 0.31) | 28.54 (95% CI, 16.79, 48.51) | 0.91 | |
| Renal carcinoma | CEUS | 0.87 (95% CI, 0.85, 0.88) | 0.84 (95% CI, 0.82, 0.87) | 5.55 (95% CI, 3.74, 8.22) | 0.12 (95% CI, 0.07, 0.19) | 53.44 (95% CI, 29.89, 95.56) | 0.95 |
| Prostate cancer | SWE | 84% (95% CI, 0.80, 0.87) | 0.84 (95% CI, 0.82, 0.86) | 4.59 (95% CI, 2.68, 7.87) | 0.18 (95% CI, 0.07, 0.44) | 25.35 (95% CI, 7.15, 89.89) | 0.89 |
AUROC, Area under the receiving-operating curve; CEUS, contrast-enhanced ultrasound; DOR, Diagnostic odds ratio; LR, Likelihood ratio; SWE, Shear wave elastography.
Outcomes of Network Meta-Analysis
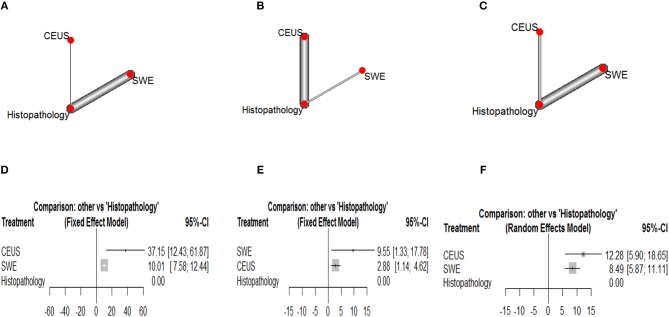
Corresponding network plots and forest plots of network meta-analysis between CEUS and SWE are shown in Figure 6. In breast cancer, NMA showed that CEUS was associated with significantly higher DOR than SWE (DOR = 27.14, 95% CI [2.30, 51.97], p = 0.011). While NMA showed no significant difference between CEUS and SWE in detecting hepatic (DOR = −6.67, 95% CI [-15.08, 1.74, p = 0.61]) and thyroid malignant lesions (DOR = 3.79, 95% CI [−3.10, 10.68], p = 0.58). No significant heterogeneity or inconsistency were observed between the pooled studies for breast (I2 = 10%, p = 0.30) and hepatic cancer (I2 = 20%, p = 0.21). While a p-value of 0.05 indicated significant heterogeneity among the studies of thyroid cancer; therefore, the random-effects model was employed.
Figure 6.
Network plots showing direct evidence between Contrast Enhanced Ultrasound and Shear Weight Elastography in (A) breast cancer, (B) hepatic caner, and (C) thyroid cancer. Also, forest plots of network meta-analysis between Contrast Enhanced Ultrasound and Shear Weight Elastography vs. histopathology in (A) breast cancer, (B) hepatic caner, and (C) thyroid cancer. (D) Forest plot CEUS vs. SWE of breast cancer. (E) Forest plot CEUS vs. SWE of hepatic cancer. (F) Forest plot CEUS vs. SWE of thyroid cancer.
Ranking Diagnostic Tests
According to Glas et al. (116), the DOR is considered as an indicator of ranking of competing diagnostic tests. According to our results, CEUS achieved the highest DOR in detecting breast and thyroid malignant lesions, while SWE achieved the highest DOR in detecting hepatic malignant lesions.
Discussion
This meta-analysis of DTA studies provides a comprehensive assessment and comparison of the diagnostic accuracy of two US modalities in differentiating malignant tumors in different body organs. It showed relatively high sensitivity (between 78 and 89%) and specificity (between 84 and 89%) for CEUS in identifying malignant lesions in the breast, liver, thyroid and kidneys. Moreover, it demonstrated relatively high sensitivity (between 82 and 84%) and specificity (between 83 and 86%) for SWE in differentiating malignant tumors within the breast, liver and prostate. However, it had relatively lower sensitivity (67%) and specificity (77%) in identifying malignant nodules within the thyroid gland.
Our results support some recent practice guidelines that endorse the use of CEUS and SWE in differentiating malignant lesions within the liver and the breast (117, 118). Moreover, it provides new data on a comparison that can impact the clinical practice. Through NMA, we compared the diagnostic accuracy of CEUS and SWE in three organs (where data on both tests were available in the literature). Our network and ranking analysis showed that CEUS was more accurate than SWE in differentiating breast and thyroid lesions (although the difference was not significant in thyroid malignancy according to NMA). On the other hand, SWE ranked higher in terms of diagnostic accuracy in differentiating hepatic malignant lesions (although the difference was not significant according to NMA).
Our results are in agreement with a former meta-analysis by Sadigh et al. that showed high sensitivity and specificity for SWE in differentiating breast malignant lesions [88 and 83% in comparison to 84 and 86% in our analysis; (11)]. However, our sensitivity and specificity results are quite lower than those obtained by Liu et al. in a meta-analysis on SWE accuracy in differentiating thyroid malignancy [sensitivity 81% and specificity 84%; (12)]. Likewise, another meta-analysis reported high sensitivity and specificity (93 and 90%, respectively) for CEUS in identifying hepatic malignant lesions (119). The observed discrepancy between our findings and those of the aforementioned meta-analyses may be attributed to the different sample size (being larger in our analysis) or the lesional characteristics of enrolled patients (being easier to identify in the studies included in the other meta-analysis i.e., less depth and clear contrast from the surrounding tissue).
Interestingly, a meta-analysis by Guang et al. showed comparable diagnostic accuracy for SonoVue-enhanced US with contrast-enhanced computed tomography and magnetic resonance imaging (8). Moreover, CEUS has other advantages over these modalities as ease of access, lack of radiation exposure or nephrotoxic materials; limitations that affect the use of CT and MRI in several diagnostic applications (120, 121). It is also fair to recognize that both tests have limitations as well. For example, SWE suffers from operator-dependency and manual compression, while the adverse effects of the contrast agent is a concern with CEUS use. Further technical improvements with both modalities would further enhance their clinical potential.
Strength Points
This NMA directly compares the diagnostic accuracies of CEUS and SWE in different cancer sites and using different analytic approaches as pairwise, network and ranking pooled analyses. Therefore, it provides a holistic evaluation of the comparison of both techniques in different body organs. We performed a thorough literature search and retrieved a large number of studies (relatively large sample size), which adds to the validity and generalizability of our findings. Unlike former reviews that retrieved a small number of studies and focused on one test in one organ, we aimed to provide a comprehensive assessment of both tests in different organs and a high quality comparison whenever suitable data were provided.
Limitations and Future Research Implications
Our meta-analysis has some limitations. First, the observed heterogeneity in the majority of our outcomes may be due to differences in study design and patient characteristics. Second, we could not examine the effects of lesion characteristics, such as size and depth on the diagnostic accuracy of both tests due to lack of data. Third, many of the included studies did not mention whether the results of CEUS or SWE were interpreted with blinding to the findings of histopathology or not. Future studies should report diagnostic accuracy data based on the size and depth of the lesions to allow more detailed analysis. Moreover, they should adhere to the Standards for Reporting of Diagnostic Accuracy “STRAD” checklist in reporting their methods and findings to allow a more thorough critical appraisal.
Conclusion
Both diagnostic tests (CEUS and SWE) showed relatively high sensitivity and specificity in detecting malignant tumors in different organs; CEUS had higher diagnostic accuracy than SWE in detecting breast and thyroid cancer, while SWE had higher accuracy in detecting hepatic cancer (the differences in the latter two cancer types were not statistically significant). These results endorse the use of both tests for malignancy detection and rank their accuracy in different organs. Future studies should provide more data to allow characterization of both tests in lesions of different size or depth.
Author Contributions
YS developed the concept, designed the study, and prepared the manuscript. RH acquired the data, controlled quality of the work, analyzed the data, and prepared the manuscript. LJ acquired the data. YX analyzed the data. YG acquired the data. HR acquired the data and conducted the analysis. ZW analyzed the data and prepared the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are extremely thankful to authors of all the included papers for proving suitable data for analysis.
Footnotes
Funding. This work was supported by funding from National Natural Science Foundation of China. Award Number 31300137 received by RH.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00102/full#supplementary-material
PRISMA checklist for systematic reviews/meta-analysis.
Bibliographic Information of Included Studies.
Additional Pairwise Meta-analysis Figures.
References
- 1.Hatzung G, Grunwald S, Zygmunt M, Geaid AA, Behrndt PO, Isermann R, et al. Sonoelastography in the diagnosis of malignant and benign breast lesions: initial clinical experiences. Ultraschall Med. (2010) 31:596–603. 10.1055/s-0029-1245526 [DOI] [PubMed] [Google Scholar]
- 2.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. (2011) 260:892–9. 10.1148/radiol.11110206 [DOI] [PubMed] [Google Scholar]
- 3.Zhan J, Diao X-H, Chen L, Jin J-M, Chen Y. Role of contrast-enhanced ultrasound in diagnosis of thyroid nodules in acoustic radiation force impulse “Gray Zone.” Ultras Med Biol. (2017) 43:1179–86. 10.1016/j.ultrasmedbio.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 4.Cooper P. What can we learn from old wetlands? Lessons that have been learned and some that may have been forgotten over the past 20 years. Desalination. (2009) 246:11–26. 10.1016/j.desal.2008.03.040 [DOI] [Google Scholar]
- 5.Bertolotto M, Cicero C, Perrone R, Degrassi F, Cacciato F, Cova MA. Renal masses with equivocal enhancement at CT: characterization with contrast-enhanced ultrasound. Am J Roentgenol. (2015) 204:W557–65. 10.2214/AJR.14.13375 [DOI] [PubMed] [Google Scholar]
- 6.Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. (2007) 10:38–46. 10.1053/j.tvir.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Xu R, Ouyang Q, Chen L, Dong B, Huihua Y. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol. (2010) 73:288–93. 10.1016/j.ejrad.2009.05.043 [DOI] [PubMed] [Google Scholar]
- 8.Guang Y, Xie L, Ding H, Cai A, Huang Y. Diagnosis value of focal liver lesions with SonoVue®-enhanced ultrasound compared with contrast-enhanced computed tomography and contrast-enhanced MRI: a meta-analysis. J Cancer Res Clin Oncol. (2011) 137:1595. 10.1007/s00432-011-1035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantisani V, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, et al. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: preliminary experience. Eur J Radiol. (2013) 82:1892–8. 10.1016/j.ejrad.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Tranquart F, Bleuzen A, Pierre-Renoult P, Chabrolle C, Sam Giao M, Lecomte P. Elastosonography of thyroid lesions. J Radiol. (2008) 89:35–9. 10.1016/S0221-0363(08)70367-6 [DOI] [PubMed] [Google Scholar]
- 11.Sadigh G, Carlos RC, Neal CH, Dwamena BA. Accuracy of quantitative ultrasound elastography for differentiation of malignant and benign breast abnormalities: a meta-analysis. Breast Cancer Res Treat. (2012) 134:923–31. 10.1007/s10549-012-2020-x [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Zhao L-X, Xu G, Yao M-H, Zhang A-H, Xu HX, et al. Diagnostic value of virtual touch tissue imaging quantification for benign and malignant breast lesions with different sizes. Int J Clin Exp Med. (2015) 8:13118–26. [PMC free article] [PubMed] [Google Scholar]
- 13.Elmoneam GA, Almolla RM, Ahmed AF, Al Ekrashy MA. Supersonic shear waves quantitative elastography and kinetic magnetic resonance dynamic curve in discriminating BI-RADS 4 breast masses: a comparative study. Egypt J Radiol Nuclear Med. (2016) 47:1773–82. 10.1016/j.ejrnm.2016.08.004 [DOI] [Google Scholar]
- 14.McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 15.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. (2003) 3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D-D, Xu H-X, Guo L-H, Bo X-W, Li X-L, Wu R, et al. Combination of two-dimensional shear wave elastography with ultrasound breast imaging reporting and data system in the diagnosis of breast lesions: a new method to increase the diagnostic performance. Eur Radiol. (2016) 26:3290–300. 10.1007/s00330-015-4163-8 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y-P, Xu X-H, Guo L-H, He Y-P, Wang D, Liu B-J, et al. Qualitative and quantitative analysis with a novel shear wave speed imaging for differential diagnosis of breast lesions. Sci Rep. (2017) 7:40964. 10.1038/srep40964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y-P, Xu X-H, Bo X-W, Liu B-J, Guo L-H, Xu JM, et al. Comparison of virtual touch tissue imaging & quantification (VTIQ) and Virtual touch tissue quantification (VTQ) for diagnosis of thyroid nodules. Clin Hemorheol Microcirc. (2017) 65:137–49. 10.3233/CH-16142 [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Ko KH, Jung HK, Kim H. Shear wave elastography: is it a valuable additive method to conventional ultrasound for the diagnosis of small (≤ 2 cm) breast cancer? Medicine. (2015) 94:e1540. 10.1097/MD.0000000000001540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youk JH, Son EJ, Park AY, Kim JA. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography. (2014) 33:34–9. 10.14366/usg.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang L, Xu H-X, Bo X-W, Liu B-J, Li X-L, Wu R, et al. A novel two-dimensional quantitative shear wave elastography for differentiating malignant from benign breast lesions. Int J Clin Exp Med. (2015) 8:10920–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Choi H, Sohn Y-M, Seo M. Comparison of 3D and 2D shear-wave elastography for differentiating benign and malignant breast masses: focus on the diagnostic performance. Clin Radiol. (2017) 72:878–86. 10.1016/j.crad.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 23.Golatta M, Schweitzer-Martin M, Harcos A, Schott S, Gomez C, Stieber A, et al. Evaluation of virtual touch tissue imaging quantification, a new shear wave velocity imaging method, for breast lesion assessment by ultrasound. Biomed Res Int. (2014) 2014:960262. 10.1155/2014/960262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youk JH, Gweon HM, Son EJ, Han KH, Kim JA. Diagnostic value of commercially available shear-wave elastography for breast cancers: integration into BI-RADS classification with subcategories of category 4. Eur Radiol. (2013) 23:2695–704. 10.1007/s00330-013-2873-3 [DOI] [PubMed] [Google Scholar]
- 25.Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol. (2014) 24:305–11. 10.1007/s00330-013-3034-4 [DOI] [PubMed] [Google Scholar]
- 26.Lee EJ, Jung HK, Ko KH, Lee JT, Yoon JH. Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur Radiol. (2013) 23:1803–11. 10.1007/s00330-013-2782-5 [DOI] [PubMed] [Google Scholar]
- 27.Ng WL, Rahmat K, Fadzli F, Rozalli FI, Mohd-Shah MN, Chandran PA, et al. Shearwave elastography increases diagnostic accuracy in characterization of breast lesions. Medicine. (2016) 95:e3146. 10.1097/MD.0000000000003146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J, Liu Q, Wang X, Xing P, Yang Z, Wu C. Application of 3D and 2D quantitative shear wave elastography (SWE) to differentiate between benign and malignant breast masses. Sci Rep. (2017) 7:41216. 10.1038/srep41216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olgun DÇ, Korkmazer B, Kiliç F, Dikici AS, Velidedeoglu M, Aydogan F, et al. Use of shear wave elastography to differentiate benign and malignant breast lesions. Diagn Intervent Radiol. (2014) 20:239 10.5152/dir.2014.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JY, Moon JH, Koh SH, Park SY, Lee KS. Clinical application of shear wave elastography in breast masses. Iran J Radiol. (2017) 14:e39585 10.5812/iranjradiol.39585 [DOI] [Google Scholar]
- 31.Yao M, Wu J, Zou L, Xu G, Xie J, Wu R, et al. Diagnostic value of virtual touch tissue quantification for breast lesions with different size. Biomed Res. Int. (2014) 2014:142504. 10.1155/2014/142504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo M, Ahn HS, Park SH, Lee JB, Choi BI, Sohn YM, et al. Comparison and combination of strain and shear wave elastography of breast masses for differentiation of benign and malignant lesions by quantitative assessment: preliminary study. J Ultras Med. (2018) 37:99–109. 10.1002/jum.14309 [DOI] [PubMed] [Google Scholar]
- 33.Au FWF, Ghai S, Moshonov H, Kahn H, Brennan C, Dua H, et al. Diagnostic performance of quantitative shear wave elastography in the evaluation of solid breast masses: determination of the most discriminatory parameter. Am J Roentgenol. (2014) 203:W328–36. 10.2214/AJR.13.11693 [DOI] [PubMed] [Google Scholar]
- 34.Chang JM, Won J-K, Lee K-B, Park IA, Yi A, Moon WK. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. Am J Roentgenol. (2013) 201:W347–56. 10.2214/AJR.12.10416 [DOI] [PubMed] [Google Scholar]
- 35.Choi JS, Han B-K, Ko EY, Ko ES, Shin JH, Kim GR. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol. (2016) 26:3542–9. 10.1007/s00330-015-4201-6 [DOI] [PubMed] [Google Scholar]
- 36.Chung J, Lee WK, Cha E-S, Lee JE, Kim JH, Ryu YH. Shear-wave elastography for the differential diagnosis of breast papillary lesions. PLoS ONE. (2016) 11:e0167118. 10.1371/journal.pone.0167118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobruch-Sobczak K, Nowicki A. Role of shear wave sonoelastography in differentiation between focal breast lesions. Ultras Med Biol. (2015) 41:366–74. 10.1016/j.ultrasmedbio.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 38.Guo X, Liu Y, Li W. Diagnostic accuracy of shear wave elastography for prediction of breast malignancy in patients with pathological nipple discharge. BMJ Open. (2016) 6:e008848. 10.1136/bmjopen-2015-008848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong S, Woo OH, Shin HS, Hwang S-Y, Cho KR, Seo BK. Reproducibility and diagnostic performance of shear wave elastography in evaluating breast solid mass. Clin Imaging. (2017) 44:42–5. 10.1016/j.clinimag.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 40.Kim GR, Choi JS, Han B-K, Ko EY, Ko ES, Hahn SY. Combination of shear-wave elastography and color Doppler: feasible method to avoid unnecessary breast excision of fibroepithelial lesions diagnosed by core needle biopsy. PLoS ONE. (2017) 12:e0175380. 10.1371/journal.pone.0175380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klotz T, Boussion V, Kwiatkowski F, Dieu-de Fraissinette V, Bailly-Glatre A, Lemery S, et al. Shear wave elastography contribution in ultrasound diagnosis management of breast lesions. Diagn Intervent Imag. (2014) 95:813–24. 10.1016/j.diii.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 42.Lee BE, Chung J, Cha ES, Lee JE, Kim JH. Role of shear-wave elastography (SWE) in complex cystic and solid breast lesions in comparison with conventional ultrasound. Eur J Radiol. (2015) 84:1236–41. 10.1016/j.ejrad.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 43.Shi XQ, Li JL, Wan WB, Huang Y. A set of shear wave elastography quantitative parameters combined with ultrasound BI-RADS to assess benign and malignant breast lesions. Ultras Med Biol. (2015) 41:960–6. 10.1016/j.ultrasmedbio.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 44.Sim Y, Vinnicombe S, Whelehan P, Thomson K, Evans A. Value of shear-wave elastography in the diagnosis of symptomatic invasive lobular breast cancer. Clin Radiol. (2015) 70:604–9. 10.1016/j.crad.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Cui X, Huang L, Bai X. Combining virtual touch tissue imaging and BI-RADS may improve solid breast lesion evaluation. Breast Care. (2017) 12:97–100. 10.1159/000456026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S-P, Zeng Z, Liu H, Yao M-H, Xu G, Wu R. Combination of conventional ultrasonography and virtual touch tissue imaging quantification for differential diagnosis of breast lesions smaller than 10 mm. Clin Hemorheol Microcirc. (2017) 67:59–68. 10.3233/CH-170249 [DOI] [PubMed] [Google Scholar]
- 47.Cong R, Li J, Wang X. Comparing performance of combinations of shear wave elastography and B-mode ultrasound in diagnosing breast masses: is it influenced by mass size? Ultras Med Biol. (2017) 43:2133–43. 10.1016/j.ultrasmedbio.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 48.Park J, Woo OH, Shin HS, Cho KR, Seo BK, Kang EY. Diagnostic performance and color overlay pattern in shear wave elastography (SWE) for palpable breast mass. Eur J Radiol. (2015) 84:1943–8. 10.1016/j.ejrad.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 49.Park AY, Son EJ, Han K, Youk JH, Kim J-A, Park CS. Shear wave elastography of thyroid nodules for the prediction of malignancy in a large scale study. Eur J Radiol. (2015) 84:407–12. 10.1016/j.ejrad.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen M, et al. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. (2013) 41:177–96. 10.1142/S0192415X13500134 [DOI] [PubMed] [Google Scholar]
- 51.Kasai Y, Moriyasu F, Saito K, Hara T, Kobayashi Y, Nakamura I, et al. Value of shear wave elastography for predicting hepatocellular carcinoma and esophagogastric varices in patients with chronic liver disease. J Med Ultras. (2015) 42:349–55. 10.1007/s10396-014-0603-3 [DOI] [PubMed] [Google Scholar]
- 52.Gerber L, Fitting D, Srikantharajah K, Weiler N, Kyriakidou G, Bojunga J, et al. Evaluation of 2D-shear wave elastography for characterisation of focal liver lesions. J Gastrointest Liver Dis. (2017) 26:283–90. 10.15403/jgld.2014.1121.263.dsh [DOI] [PubMed] [Google Scholar]
- 53.Özmen E, Adaletli I, Kayadibi Y, Emre S, Kiliç F, Dervişoglu S, et al. The impact of share wave elastography in differentiation of hepatic hemangioma from malignant liver tumors in pediatric population. Eur J Radiol. (2014) 83:1691–97. 10.1016/j.ejrad.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Tian W-S, Lin M-X, Zhou L-Y, Pan F-S, Huang G-L, Wang W, et al. Maximum value measured by 2-D shear wave elastography helps in differentiating malignancy from benign focal liver lesions. Ultras Med Biol. (2016) 42:2156–66. 10.1016/j.ultrasmedbio.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 55.Ahmad S, Cao R, Varghese T, Bidaut L, Nabi G. Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer. Surg Endosc. (2013) 27:3280–7. 10.1007/s00464-013-2906-7 [DOI] [PubMed] [Google Scholar]
- 56.Boehm K, Salomon G, Beyer B, Schiffmann J, Simonis K, Graefen M, et al. Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. J Urol. (2015) 193:794–800. 10.1016/j.juro.2014.09.100 [DOI] [PubMed] [Google Scholar]
- 57.Porsch M, Wendler JJ, Liehr U-B, Lux A, Schostak M, Pech M. New aspects in shear-wave elastography of prostate cancer. J Ultrasonogr. (2015) 15:5–14. 10.15557/JoU.2015.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo S, Kim SY, Cho JY, Kim SH. Shear wave elastography for detection of prostate cancer: a preliminary study. Korean J Radiol. (2014) 15:346–55. 10.3348/kjr.2014.15.3.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Correas JM, Tissier AM, Khairoune A, Vassiliu V, Mejean A, Helenon O, et al. Prostate cancer: diagnostic performance of real-time shear-wave elastography. Radiology. (2015) 275:280–89. 10.1148/radiol.14140567 [DOI] [PubMed] [Google Scholar]
- 60.Glybochko P, Alyaev Y, Amosov A, Krupinov G, Ganzha T, Vorobev A, et al. Prostate cancer detection by assessing stiffness of different tissues using shear wave ultrasound elastog-raphy. Urologiia. (2016) 2016:56–61. [PubMed] [Google Scholar]
- 61.Zhang H, Shi Q, Gu J, Jiang L, Bai M, Liu L, et al. Combined value of Virtual Touch tissue quantification and conventional sonographic features for differentiating benign and malignant thyroid nodules smaller than 10 mm. J Ultras Med. (2014) 33:257–64. 10.7863/ultra.33.2.257 [DOI] [PubMed] [Google Scholar]
- 62.Zhang P, Zhou P, Tian S-M, Qian Y, Li J-L, Li RZ. Diagnostic performance of contrast-enhanced sonography and acoustic radiation force impulse imaging in solid liver lesions. J Ultras Med. (2014) 33:205–14. 10.7863/ultra.33.2.205 [DOI] [PubMed] [Google Scholar]
- 63.Azizi G, Keller JM, Mayo ML, Piper K, Puett D, Earp KM, et al. Thyroid nodules and shear wave elastography: a new tool in thyroid cancer detection. Ultras Med Biol. (2015) 41:2855–65. 10.1016/j.ultrasmedbio.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 64.Wang W-P, Dong Y, Cao J, Mao F, Xu Y, Si Q, et al. Detection and characterization of small superficially located focal liver lesions by contrast-enhanced ultrasound with high frequency transducers. Med Ultrason. (2017) 19:349–56. 10.11152/mu-1276 [DOI] [PubMed] [Google Scholar]
- 65.Duan S-B, Yu J, Li X, Han Z-Y, Zhai H-Y, Liang P. Diagnostic value of two-dimensional shear wave elastography in papillary thyroid microcarcinoma. Onco Targets Ther. (2016) 9:1311–7. 10.2147/OTT.S98583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. (2016) 23:R143–55. 10.1530/ERC-15-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu B-J, Zhao C-K, Xu H-X, Zhang Y-F, Xu J-M, Li DD, et al. Quality measurement on shear wave speed imaging: diagnostic value in differentiation of thyroid malignancy and the associated factors. Oncotarget. (2017) 8:4948–59. 10.18632/oncotarget.13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H, Kim J-A, Son EJ, Youk JH. Quantitative assessment of shear-wave ultrasound elastography in thyroid nodules: diagnostic performance for predicting malignancy. Eur Radiol. (2013) 23:2532–7. 10.1007/s00330-013-2847-5 [DOI] [PubMed] [Google Scholar]
- 69.Deng J, Zhou P, Tian SM, Zhang L, Qian Y. Comparison of diagnostic efficacy of contrast-enhanced ultrasound, acoustic radiation force impulse imaging, and their combined use in differentiating focal solid thyroid nodules. PLoS ONE. (2014) 9:e90674. 10.1371/journal.pone.0090674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baig FN, Liu SY, Lam H-C, Yip S-P, Law HK, Ying M. Shear wave elastography combining with conventional grey scale ultrasound improves the diagnostic accuracy in differentiating benign and malignant thyroid nodules. Applied Sciences. 7:1103 10.3390/app7111103 [DOI] [Google Scholar]
- 71.Dobruch-Sobczak K, Zalewska EB, Guminska A, Słapa RZ, Mlosek K, Wareluk P, et al. Diagnostic performance of shear wave elastography parameters alone and in combination with conventional B-mode ultrasound parameters for the characterization of thyroid nodules: a prospective, dual-center study. Ultras Med Biol. (2016) 42:2803–11. 10.1016/j.ultrasmedbio.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 72.Liu B-X, Xie X-Y, Liang J-Y, Zheng Y-L, Huang G-L, Zhou LY, et al. Shear wave elastography versus real-time elastography on evaluation thyroid nodules: a preliminary study. Eur J Radiol. (2014) 83:1135–43. 10.1016/j.ejrad.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 73.Park SY, Seo AN, Jung HY, Gwak JM, Jung N, Cho NY, et al. Alu and LINE-1 hypomethylation is associated with HER2 enriched subtype of breast cancer. PLoS ONE. (2014) 9:e100429. 10.1371/journal.pone.0100429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samir AE, Dhyani M, Anvari A, Prescott J, Halpern EF, Faquin WC, et al. Shear-wave elastography for the preoperative risk stratification of follicular-patterned lesions of the thyroid: diagnostic accuracy and optimal measurement plane. Radiology. (2015) 277:565–73. 10.1148/radiol.2015141627 [DOI] [PubMed] [Google Scholar]
- 75.Yang GC, Fried KO, Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: a review of 179 cases. Diagn Cytopathol. (2017) 45:533–41. 10.1002/dc.23709 [DOI] [PubMed] [Google Scholar]
- 76.Zhou H, Zhou XL, Xu HX, Li DD, Liu BJ, Zhang YF, et al. Virtual Touch tissue imaging and quantification in the evaluation of thyroid nodules. J Ultras Med. (2017) 36:251–60. 10.7863/ultra.15.12070 [DOI] [PubMed] [Google Scholar]
- 77.Cai Y, Du L, Li F, Gu J, Bai M. Quantification of enhancement of renal parenchymal masses with contrast-enhanced ultrasound. Ultras Med Biol. (2014) 40:1387–93. 10.1016/j.ultrasmedbio.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 78.Chen L, Wang L, Diao X, Qian W, Fang L, Pang Y, et al. The diagnostic value of contrast-enhanced ultrasound in differentiating small renal carcinoma and angiomyolipoma. Biosci Trends. (2015) 9:252–8. 10.5582/bst.2015.01080 [DOI] [PubMed] [Google Scholar]
- 79.Chen Y, Wu N, Xue T, Hao Y, Dai J. Comparison of contrast-enhanced sonography with MRI in the diagnosis of complex cystic renal masses. J Clin Ultras. (2015) 43:203–9. 10.1002/jcu.22232 [DOI] [PubMed] [Google Scholar]
- 80.Chen D, Ma Y, Wang X, Yu S, Li L, Dai B, et al. Clinical characteristics and disease predictors of a large Chinese cohort of patients with autosomal dominant polycystic kidney disease. PLoS ONE. (2014) 9:e92232. 10.1371/journal.pone.0092232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Defortescu G, Cornu JN, Béjar S, Giwerc A, Gobet F, Werquin C, et al. Diagnostic performance of contrast-enhanced ultrasonography and magnetic resonance imaging for the assessment of complex renal cysts: a prospective study. Int J Urol. (2017) 24:184–9. 10.1111/iju.13289 [DOI] [PubMed] [Google Scholar]
- 82.Li X, Liang P, Guo M, Yu J, Yu X, Cheng Z, et al. Real-time contrast-enhanced ultrasound in diagnosis of solid renal lesions. Discov Med. (2013) 16:15–25. Available online at: http://www.discoverymedicine.com/Xin-Li/2013/07/26/real-time-contrast-enhanced-ultrasound-in-diagnosis-of-solid-renal-lesions/ [PubMed] [Google Scholar]
- 83.Lu Q, Xue LY, Huang BJ, Wang WP, Li CX. Histotype differentiation of hypo-echoic renal tumors on CEUS: usefulness of enhancement homogeneity and intensity. Abdom Imaging. (2015) 40:1675–83. 10.1007/s00261-014-0340-5 [DOI] [PubMed] [Google Scholar]
- 84.Nicolau C, Buñesch L, Paño B, Salvador R, Ribal MJ, Mallofré C, et al. Prospective evaluation of CT indeterminate renal masses using US and contrast-enhanced ultrasound. Abdom Imaging. (2015) 40:542–51. 10.1007/s00261-014-0237-3 [DOI] [PubMed] [Google Scholar]
- 85.Oh TH, Lee YH, Seo IY. Diagnostic efficacy of contrast-enhanced ultrasound for small renal masses. Korean J Urol. (2014) 55:587–92. 10.4111/kju.2014.55.9.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanz E, Hevia V, Gómez V, Álvarez S, Fabuel JJ, Martínez L, et al. Renal complex cystic masses: usefulness of contrast-enhanced ultrasound (CEUS) in their assessment and its agreement with computed tomography. Curr Urol Rep. (2016) 17:89. 10.1007/s11934-016-0646-7 [DOI] [PubMed] [Google Scholar]
- 87.Tamas-Szora A, Socaciu M, Crisan N, Dobrota F, Prundus P, Bungardean C, et al. Investigation of renal cell carcinoma by contrast-enhanced ultrasound-predictive value of time intensity curve analysis in establishing local tumor invasion and stage: a pilot study. Urol J. (2015) 12:2173–81. 10.22037/uj.v12i3.2851 [DOI] [PubMed] [Google Scholar]
- 88.Wei S-P, Xu C-L, Zhang Q, Zhang Q-R, Zhao Y-E, Huang PF, et al. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: comparison with contrast-enhanced CT. Abdom Radiol. (2017) 42:2135–45. 10.1007/s00261-017-1111-x [DOI] [PubMed] [Google Scholar]
- 89.Yong C, Teo Y-M, Kapur J. Diagnostic performance of contrast-enhanced ultrasound in the evaluation of renal masses in patients with renal impairment. Med J Malaysia. (2016) 71:193–8. Available online at: http://www.e-mjm.org/2016/v71n4/contrast-enhanced-ultrasound.pdf [PubMed] [Google Scholar]
- 90.Zhang Y, Luo YK, Zhang MB, Li J, Li J, Tang J. Diagnostic accuracy of contrast-enhanced ultrasound enhancement patterns for thyroid nodules. Med Sci Monit. (2016) 22: 4755–64. 10.12659/MSM.899834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyamoto Y, Ito T, Takada E, Omoto K, Hirai T, Moriyasu F. Efficacy of sonazoid (perflubutane) for contrast-enhanced ultrasound in the differentiation of focal breast lesions: phase 3 multicenter clinical trial. Am J Roentgenol. (2014) 202:W400–7. 10.2214/AJR.12.10518 [DOI] [PubMed] [Google Scholar]
- 92.Xia H-S, Wang X, Ding H, Wen J-X, Fan P-L, Wang WP. Papillary breast lesions on contrast-enhanced ultrasound: morphological enhancement patterns and diagnostic strategy. Eur Radiol. (2014) 24:3178–90. 10.1007/s00330-014-3375-7 [DOI] [PubMed] [Google Scholar]
- 93.Xiao X, Jiang Q, Wu H, Guan X, Qin W, Luo B. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound—a preliminary study in China. Eur Radiol. (2017) 27:2443–50. 10.1007/s00330-016-4628-4 [DOI] [PubMed] [Google Scholar]
- 94.Yuan Z, Quan J, Yunxiao Z, Jian C, Zhu H, Liping G. Diagnostic value of contrast-enhanced ultrasound parametric imaging in breast tumors. J Breast Cancer. (2013) 16:208–13. 10.4048/jbc.2013.16.2.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N'kontchou G, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. (2017) 37:1515–25. 10.1111/liv.13429 [DOI] [PubMed] [Google Scholar]
- 96.Beyer LP, Wassermann F, Pregler B, Michalik K, Rennert J, Wiesinger I, et al. Characterization of focal liver lesions using CEUS and MRI with liver-specific contrast media: experience of a single radiologic center. Ultr Med Eur J Ultras. (2017) 38:619–25. 10.1055/s-0043-105264 [DOI] [PubMed] [Google Scholar]
- 97.Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic performance and confidence of contrast-enhanced ultrasound in the differential diagnosis of cystic and cysticlike liver lesions. Am J Roentgenol. (2017) 209:W119–27. 10.2214/AJR.16.17062 [DOI] [PubMed] [Google Scholar]
- 98.Feng Y, Qin X-C, Luo Y, Li Y-Z, Zhou X. Efficacy of contrast-enhanced ultrasound washout rate in predicting hepatocellular carcinoma differentiation. Ultras Med Biol. (2015) 41:1553–60. 10.1016/j.ultrasmedbio.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 99.Iwamoto T, Imai Y, Kogita S, Igura T, Sawai Y, Fukuda K, et al. Comparison of contrast-enhanced ultrasound and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI for the diagnosis of macroscopic type of hepatocellular carcinoma. Dig Dis. (2016) 34:679–86. 10.1159/000448855 [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi T, Aikata H, Hatooka M, Morio K, Morio R, Kan H, et al. Usefulness of combining gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for diagnosing the macroscopic classification of small hepatocellular carcinoma. Eur Radiol. (2015) 25:3272–81. 10.1007/s00330-015-3725-0 [DOI] [PubMed] [Google Scholar]
- 101.Kobayashi K, Maruyama H, Kiyono S, Yokosuka O, Ohtsuka M, Miyazaki M, et al. Histology-based assessment of sonazoid-enhanced ultrasonography for the diagnosis of liver metastasis. Ultras Med Biol. (2017) 43:2151–8. 10.1016/j.ultrasmedbio.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 102.Quaia E, De Paoli L, Angileri R, Cabibbo B, Cova MA. Indeterminate solid hepatic lesions identified on non-diagnostic contrast-enhanced computed tomography: assessment of the additional diagnostic value of contrast-enhanced ultrasound in the non-cirrhotic liver. Eur J Radiol. (2014) 83:456–62. 10.1016/j.ejrad.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 103.Sandrose S, Karstrup S, Gerke O, Rafaelsen S. Contrast enhanced ultrasound in CT-undetermined focal liver lesions. Ultras Int Open. (2016) 2:E129. 10.1055/s-0042-120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schellhaas B, Görtz RS, Pfeifer L, Kielisch C, Neurath MF, Strobel D. Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: ESCULAP versus CEUS-LI-RADS. Eur J Gastroenterol Hepatol. (2017) 29:1036–44. 10.1097/MEG.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 105.Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Kaneoka Y, et al. Utility of contrast-enhanced ultrasound with perflubutane for diagnosing the macroscopic type of small nodular hepatocellular carcinomas. Eur Radiol. (2014) 24:2157–66. 10.1007/s00330-014-3254-2 [DOI] [PubMed] [Google Scholar]
- 106.Thakur S, Jhobta A, Dhiman D, Sood R, Chauhan A, Thakur CS. Role of contrast enhanced ultrasound in characterization of focal liver lesions. Egypt J Radiol Nuclear Med. (2014) 45:7–17. 10.1016/j.ejrnm.2013.11.001 [DOI] [Google Scholar]
- 107.Wu JP, Shu R, Zhao YZ, Ma GL, Xue W, He QJ, et al. Comparison of contrast-enhanced ultrasonography with virtual touch tissue quantification in the evaluation of focal liver lesions. J Clin Ultras. (2016) 44:347–53. 10.1002/jcu.22335 [DOI] [PubMed] [Google Scholar]
- 108.Yin S, Cui Q, Yan K, Yang W, Wu W, Bao L, et al. Effect of contrast-enhanced ultrasound on differential diagnosis of intrahepatic cholangiocarcinoma and arterial phase enhanced hepatic inflammatory lesions. Chin J Cancer Res. (2017) 29:272. 10.21147/j.issn.1000-9604.2017.03.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang F, Zhu H, Wu Y, Dou Z, Zhang Y, Kleinman N, et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–12: a retrospective observational cohort study. Lancet Infect Dis. (2014) 14:1065–72. 10.1016/S1473-3099(14)70946-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takahashi M, Maruyama H, Shimada T, Kamezaki H, Sekimoto T, Kanai F, et al. Characterization of hepatic lesions (≤ 30 mm) with liver-specific contrast agents: a comparison between ultrasound and magnetic resonance imaging. Eur J Radiol. (2013) 82:75–84. 10.1016/j.ejrad.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 111.Taimr P, Jongerius VL, Pek CJ, Krak NC, Hansen BE, Janssen HL, et al. Liver contrast-enhanced ultrasound improves detection of liver metastases in patients with pancreatic or periampullary cancer. Ultras Med Biol. (2015) 41:3063–9. 10.1016/j.ultrasmedbio.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 112.Diao X, Zhan J, Chen L, Chen Y, Liu Y. Quantification of solid hypo-echoic thyroid nodule enhancement with contrast-enhanced ultrasound. Transl Cancer Res. (2017) 6:1078–87. 10.21037/tcr.2017.09.15 [DOI] [Google Scholar]
- 113.Giusti M, Orlandi D, Melle G, Massa B, Silvestri E, Minuto F, et al. Is there a real diagnostic impact of elastosonography and contrast-enhanced ultrasonography in the management of thyroid nodules? J Zhejiang Univ Sci B. (2013) 14:195–206. 10.1631/jzus.B1200106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang J, Shang X, Wang H, Xu Y-B, Gao Y, Zhou Q. Diagnostic value of contrast-enhanced ultrasound in thyroid nodules with calcification. Kaohsiung J Med Sci. (2015) 31:138–44. 10.1016/j.kjms.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou X, Zhou P, Hu Z, Tian SM, Zhao Y, Liu W, et al. Diagnostic efficiency of quantitative contrast-enhanced ultrasound indicators for discriminating benign from malignant solid thyroid nodules. J Ultras Med. (2018) 37:425–37. 10.1002/jum.14347 [DOI] [PubMed] [Google Scholar]
- 116.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. (2003) 56:1129–35. 10.1016/S0895-4356(03)00177-X [DOI] [PubMed] [Google Scholar]
- 117.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver-update 2012. Ultraschall Med. (2013) 34:11–29. 10.1016/j.ultrasmedbio.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 118.Lee T, Mendhiratta N, Sperling D, Lepor H. Focal laser ablation for localized prostate cancer: principles, clinical trials, and our initial experience. Rev Urol. (2014) 16:55. [PMC free article] [PubMed] [Google Scholar]
- 119.Friedrich-Rust M, Buggisch P, De Knegt R, Dries V, Shi Y, Matschenz K, et al. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepatitis. (2013) 20:240–7. 10.1111/j.1365-2893.2012.01646.x [DOI] [PubMed] [Google Scholar]
- 120.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. (2002) 59:913–8. 10.1016/S0090-4295(02)01509-1 [DOI] [PubMed] [Google Scholar]
- 121.Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. (2018) 31:241–74. 10.1016/j.echo.2017.11.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist for systematic reviews/meta-analysis.
Bibliographic Information of Included Studies.
Additional Pairwise Meta-analysis Figures.