Abstract
Background
Genetic overexpression or pharmacological activation of heme oxygenase (HO) are identified as potential therapeutic target for spinal cord injury (SCI); however, the role of carbon monoxide (CO), which is a major product of haem degenerated by HO, in SCI remains unknown. Applying hemin or chemicals which may regulate HO expression or activity to increase CO production are inadequate to elaborate the direct role of CO. Here, we assessed the effect of CO releasing molecule-3 (CORM-3), the classical donor of CO, in SCI and explained its possible protective mechanism.
Methods
Rat SCI model was performed with a vascular clip (30 g) compressing at T9 vertebral level for 1 min and CO was delivered immediately after SCI by CORM-3. The neurological deficits and neuron survival were assessed. Inflammasome and inositol-requiring enzyme 1 (IRE1) pathway were measured by western blot and immunofluorescence. For in vitro study, oxygen glucose deprivation (OGD) simulated the SCI-inflammasome change in cultured the primary neurons.
Findings
CORM-3 suppressed inflammasome signaling and pyroptosis occurrence, which consequently alleviated neuron death and improved motor functional recovery following SCI. As a pivotal sensor involving in endoplasmic reticulum stress-medicated inflammasome signaling, IRE1 and its downstream X-box binding protein 1 (XBP1) were activated in SCI tissues as well as in OGD neurons; while inhibition of IRE1 by STF-083010 in SCI rats or by si-RNA in OGD neurons suppressed inflammasome signaling and pyroptosis. Interestingly, the SCI/OGD-stimulated IRE1 activation was attenuated by CORM-3 treatment.
Interpretations
CO may alleviate neuron death and improve motor functional recovery in SCI through IRE1 regulation, and administration of CO could be a promising therapeutic strategy for SCI.
Keywords: Spinal cord injury, Neuron death, Inflammasome, Carbon monoxide
Research in context.
Evidence before this study
Genetic or pharmacological regulation of HO are identified as potential therapeutic target for SCI. As a heme degradation product, although CO has various bioactivity such as anti-inflammatory, anti-apoptotic and antioxidant in low concentration, the role of CO in SCI is still unknown. Applying hemin or chemicals which may regulate HO expression or activity to increase CO production are inadequate to elaborate the direct role of CO in SCI.
Added value of this study
In this study, we demonstrated CORM-3 improved functional recovery and diminished the neuron death in the rat following SCI. Moreover, the SCI-induced neuronal pyroptosis occurrence and inflammasome signaling was inhibited by CORM-3.
Implications of all the available evidence
Our data suggest CORM-3 offer therapeutic benefit for SCI patients.
Alt-text: Unlabelled Box
1. Introduction
Traumatic spinal cord injury (SCI) is a devastating disease, leading to sensory disorders and physical disabilities [1]. The primary mechanical damage and the secondary sequential damage are the crucial pathophysiological features of SCI. The latter is orchestrated by a series of detrimental events such as inflammatory response, endoplasmic reticulum (ER) stress, mitochondrial dysfunction and excitotoxicity [[2], [3], [4], [5]]. Neurons are of vital importance for central nervous system (CNS), however they could not regenerate when impaired [6,7]. Due to the irreversibility of mechanical injury, alleviating the secondary neuron death and ameliorating the surviving neuronal function are considered as the key strategy for SCI therapy.
Heme oxygenase (HO) is a highly conserved enzyme involved in the secondary injury process, it degrades heme into biliverdin, carbon monoxide, and free iron (Fe3+). Genetic or pharmacological upregulation of HO-1 activity preserves spinal cord function and restrains the neuron death after SCI [[8], [9], [10], [11], [12], [13]]. As a heme degradation product, carbon monoxide (CO) has been proved to have various bioactivity such as anti-inflammatory, anti-apoptotic and antioxidant in low concentration [[14], [15], [16]], which might explains the HO-1-induced protection following SCI. However, the direct role of CO in SCI is still unknown.
Neuroinflammation plays a key role in the secondary phase of SCI after initial cell death. Activation of cytoplasmic inflammasome complexes is regarded as the essential step of neuroinflammation and a key trigger for neuron death called pyroptosis [17]. Unlike traditional apoptosis, pyroptosis is defined as an exceptional category of inflammatory necrosis, characterized by the cell swell, rupture, the pore formation in cell membrane and the release of cytosolic contents [18]. And growing evidences proved that pyroptosis contributes to neuron death in acute CNS injury, such as ischemic stroke, subarachnoid hemorrhage and traumatic injury of brain and spinal cord [17,[19], [20], [21], [22], [23]]. During the CNS damage, inflammasomes are activated by the danger signals including high extracellular K+, ATP, β-amyloid and subsequently induced the pyroptosis occurrence [9,22]. The process of inflammasomes-medicated pyroptosis is a complex cascade and still remains many unanswered doubts [24,25]. In brief, during the inflammasome signaling is primed and activatied, the activated inflammasomes assembly binds and cleaves the pro-caspase1 to form active subunits, which further leads to inflammatory response and pyroptosis by activating the pro IL-1β, pro IL-18, and Gasdermin D (GSDMD, pore forming protein) [22]. The pharmacologically or genetically suppression of inflammasomes signaling or direct ablation of caspase1 have been demonstrated the protection of neuron in brain and spinal cord injury model [23,[26], [27], [28], [29]].
Several murine brain injuried models demonstrated that delivering CO by inhalation or the exogenous CO donor, CO-releasing molecule (CORM)-3, suppresses neuroinflammation, blood-brain barrier disruption and promotes neurogenesis [[30], [31], [32]]. Except for the anti-inflammatory, anti-apoptotic and regenerative effects, recent studies implied that CO possesses regulatory effects on inflammasomes signaling and pyroptosis occurrence [[33], [34], [35]]. Nevertheless, the relationship between CO and inflammasome-stimulated pyroptosis in neuron was unknown in SCI.
In this study, we firstly measured CO content variation within 7 days following SCI, revealing that the CO content variation is consistent with the expression of HO-1 after SCI. Next, we showed that exogenously increasing CO by CORM-3 improved functional recovery and diminished the neuron death in the rat model of SCI. Moreover, CORM-3 treatment attenuated pyroptosis occurrence and inflammasome priming in neuron in vivo and in vitro, the potential mechanism for CORM-3-regulated inflammasomes in SCI might be associated with abnormal activation of the kinase/endoribonuclease inositol-requiring enzyme (IRE1). This study demonstrates the direct role of CO in SCI, showing its potential as well as working mechanism for SCI therapy.
2. Materials and methods
2.1. Reagents and antibodies
Carbon monoxide releasing molecule 3 (CORM-3) and STF-083010 were purchased from Selleck (Houston, Texas, USA). Antibody against IL-1β was purchased from R&D system (Minneapolis, Minnesota, USA). Antibody against NLRP1 was purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against HO-1, caspase1, caspase11, IL-18, NLRP3, p-IRE1, IRE1, NeuN, GSDMD and GAPDH were the products of Abcam (Cambridge, MA, USA).
2.2. Animal and SCI model
Adult female Sprague–Dawley rats (220–250 g, 8-week old) were purchased from the Animal Center of the Chinese Academy of Sciences in Shanghai, China, housed in standard temperature conditions with a 12 h light/dark cycle and regularly fed with food and water. The protocol for animal care and use conformed to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and was approved by the Animal Care and Use Committee of Wenzhou Medical University.
The rats were randomly divided into four groups. All rats were anesthetized by an intraperitoneal injection of sodium pentobarbital (65 mg/kg). Following shaved and sterilized in the back, the skin was incised along the midline of the dorsum to expose the vertebral column and a laminectomy was performed at the T9 level. The exposed spinal cord was subjected to crush injury by compression with a vascular clip (30 g force; Oscar, China) for 1 min. The same surgical procedure was performed in sham group rats, but there is no crush to the spinal cord is exposed for 1 min. Postoperative care involved manual urinary bladder emptying twice daily until the return of bladder function and the administration of cefazolin sodium (50 mg/kg, i.p.). And the rats experiencing the SCI were randomly divided into three groups, respectively, treatment with CORM-3 or iCORM-3 or saline. To confirm the CO effect, we prepared the inactive CORM-3 (iCORM-3), that was produced by leaving CORM-3 in saline (pH = 7.4) overnight at room temperature to allow all CO to be released from the molecule [34]. CORM-3 was diluted with normal saline and achieved a final CORM-3 concentration of 8 mg/ml. After surgery, the CORM-3 solution was immediately injected to tail veins with a dose of 8 mg/kg/day until the rats were sacrificed. Equivalent normal saline and iCORM-3 injections were administered for vehicle control. In addition, for IRE1 inhibitor experiment, the SCI rats were divided into two groups. The rats were immediately injected with STF-083010 (10 mg/kg/d) or DMSO after SCI, both given in 16% (vol/vol) Cremophor EL (Sigma-Aldrich) saline solution via i.p. injections. For pyroptosis inhibitor experiment, VX-765 (150 mg/kg/d; meilunbio, Da Lian, China) was dissolved in 20% cremophor and injected intraperitoneally in rats after SCI. All animals showed no significant side effects resulting from drug treatment such as mortality or signs of infectious disease during these experiments.
2.3. Locomotion recovery assessment
The Basso, Beattie, and Bresnahan (BBB) scores were assessed by three trained investigators who were blinded to experiment in an open-field scale at 1, 3, 7, 14, 21 and 28 days post-operation. Briefly, the BBB scores range from 0 points (complete paralysis) to 21 points (normal locomotion). The scale was developed using the natural progression of locomotion recovery in rats with thoracic SCI [36]. Moreover, the footprint analysis was performed by dipping the rat's hindpaws with blue dye at 28 days after SCI. Ten rats for each group were used to assess the motor function.
2.4. Hematoxylin-Eosin staining and Nissl staining
The rats of each group (n = 5) were euthanized with an overdose of sodium pentobarbital, followed by 4% paraformaldehyde in 0.01 M phosphate buffered saline (PBS, pH = 7.4) at 7 days after SCI. Tissue segments containing the lesion (1 cm on each side of the lesion) were paraffin embedded. Transverse paraffin sections (5 μm thick) were mounted on poly-l-lysine-coated slides for Hematoxylin-Eosin (HE) staining and Nissl staining and examined under a light microscope. The cellular stain HE was used to observe the cavity, at 5 mm from the lesion site. The measurements were reported as the percent of preserved area in relation to the total area of each section analyzed [37]. For Nissl staining, the number of ventral motor neuron (VMN) in sections were assessed as in previous report [38]. Transverse sections were collected at rostral, caudal 5 mm, and the lesion site and stained with cresyl violet acetate. After determination of the cells located in the lower ventral horn, cells larger than half of the sampling square (20 × 20 μm) were counted as a VMN. The cells above the line at 150 μm ventral from the central canal were excluded. The cells were manually counted from each field using Metamorph software.
2.5. Carbon monoxide content detection
The CO content in spinal cord was detected with endogenous carbon monoxide assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as previously described [39,40]. The specific steps were conducted according to the manufacturer's instructions. Briefly, the spinal cord tissue samples were removed at 1,3,5,7 days after SCI. The spinal cord segment (0.5 cm length) at the contusion epicenter was dissected and homogenized in PBS. Mix 1 mL of Hb solution (0.25 mL of fresh-packed erythrocytes in 50 mL of ammonia solution) with 0.5 mL of a sample or an equivalent amount of water, which is used as a blank to measure the endogenous CO that presented in the Hb solution, vortex-mix and let stand for 10 min. Read the absorbance at 541 nm and 555 nm against a reference curvette containing water for three times and get the average. Then, the ratio(R) of the 541 to 555 readings was record and further to calculate the CO content in sample. Eight rats for each group were used to detect the CO change after SCI.
2.6. Primary neuron culture and treatment
Primary neurons were cultured as previously described [41]. Briefly, primary neurons were obtained from cerebral cortices of 17-day-old SD rat embryos, and cultured in Neuronal Basal medium containing 2% B27 and 1% l-glutamine in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The medium was replaced every 2 days and cells were cultured for additional seven days before use. For OGD, the medium was replaced by glucose-free Dulbecco's Modified Eagle Medium, and placed in a hypoxic chamber containing 5% CO2, 0.02% O2 and 94.98% N2 at 37 °C for 6 h. The CORM-3 (100 μm) and STF-083010 (50 μm) was pretreated (3 h) before OGD.
2.7. Small interfering RNA transfection
siRNA for rat IRE1 gene was purchased from Santa Cruz Biotechnology (sc-270028; Dallas, TX, USA). Neurons were seeded in a six-well plate and cultured for 24 h to 60–70% confluency. The cells were transfected with 50 nM negative control or siRNA duplexes using Lipofectamine 2000 siRNA transfection reagent (Thermo Fisher, UT, USA). After the following further treatments, cells were harvested for western blot experiments.
2.8. LDH releasing assay
The supernatant from serum-free media was filtered using 0.2-μm syringe filters to use for LDH release detection. The detection was using a commercially available kit (Solarbio, Beijing). One supernatant was transferred to 96-well plates, and then the reaction mixture was added and incubated in the dark for 30 min at RT. LDH concentration was quantified by measuring the absorbance at 490 nm.
2.9. ELISA
Spinal cord samples (n = 5) were homogenized in phosphate-buffered saline (PBS), subsequently centrifuged at 5000 × g at 4 °C for 10 min. IL-1β and IL-18 concentrations in the supernatant were detected using enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher, UT, USA).
2.10. Real-time PCR
The total RNA was extracted from spinal cord by TRIzol reagent (Invitrogen, Grand Island, NY). One microgram of total RNA was used to synthesize cDNA (MBI Fermantas, Germany). For the quantitative realtime PCR (qPCR), a total 10 μl of reaction volume was used, including 5 μl of 2 × SYBR Master Mix, 0.25 μl of each primer and 4.5 μl of diluted cDNA. Parameters of RT-PCR were: 10 min 95 °C, followed by 40 cycles of 15 s 95 °C and 1 min 60 °C. The reaction was performed using CFX96Real-Time PCR System (BioRad Laboratories, California, USA). The cycle threshold (Ct) values were collected and normalized to the level of GAPDH. The level of relative mRNA of each target gene was calculated by using the 2-ΔΔCt method. The primer sequences were as follow: caspase1 (F) 5′-GACCGAGTGGTTCCCTCAAG-3′ and (R) 5′-GACGTGTACGAGTGGGTGTT-3′; IL-1β (F) 5′-TGTCTGACCCATGTGAGCTG-3′ and (R) 5′-GCCACAGGGATTTTGTCGTT-3′; IL-18 (F) 5′-ATATCGACCGAACAGCCAAC-3′ and (R) 5′-TTCCATCCTTCACAGATAGGG-3′; NLRP1 (F) 5′-GTGGCTGGACCTCTGTTTGA-3′ and (R) 5′-GGCGTTTCTAGGACCATCCC-3′; NLRP3 (F) 5′-CCAGAGCCTCACTGAACTGG-3′ and (R) 5′-AGCATTGATGGGTCAGTCCG-3′; GAPDH (F) 5′-ATGACATCAAGAAGGTGGTG-3′ and (R) 5′-CATACCAGGAAATGAGCTTG-3′.
2.11. Western blot analysis
RIPA lysis buffer (Beyotime, Shanghai, China) containing 1 mM PMSF was used to extract total protein followed by protein concentration measurement with an Enhanced BCA Protein Assay Kit (Beyotime, Shanghai, China) using a Microplate Reader (Molecular Devices Flexstation 3, USA). 40 ng of tissue protein was separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS PAGE) and transferred to a polyvinylidene difluoride membrane (Bio-Rad, California, USA). After blocking with 5% nonfat milk for 2 h, the membranes were incubated with the primary antibody against HO-1 (1:500), GSDMD (1:500), caspase1 (1:1000), caspase11 (1:500), IL-1β (1:500), IL-18 (1:200), NLRP1 (1:1000), NLRP3 (1:500), p-IRE1 (1:500), IRE1 (1:500), GAPDH (1:5000). Then, the membranes were washed with TBS for 5 min three times, and treated with horseradish peroxidase-conjugated secondary antibodies. After 3 times washing with TBST, the blots were visualized by electrochemiluminescence plus reagent (Invitrogen, Carlsbad, USA). Finally, the intensity of these blots were quantifed with Image Lab 3.0 software (Bio-Rad, California, USA).
2.12. Immunofluorescence staining
Spinal cord tissue samples were obtained 3 days after injury. All spinal cords were postfixed in 4% PFA, washed, and embedded in paraffin. Transverse sections of 5-μm thickness were cut, deparaffinized in xylene, and rehydrated by ethanol washes. And the sections were incubated with 10% normal goat serum for 1 h at roomtemperature in PBS containing 0.1% Triton X-100. They were then incubated with the appropriate primary antibodies overnight at 4 °C in the same buffer. The following primary antibodies were used, based on differing targets: HO-1 (1:100), Caspase1 (1:100), NeuN (1:1000), p-IRE (1:100). After primary antibody incubation, sections were washed for 4 × 10 min and then incubated with Alexa Fluor 488/594 goat anti-rabbit/mouse secondary antibodies for 1 h at room temperature. Sections were rinsed three times with PBS and incubated with 4,6-diamidino-2-phenylindole (DAPI) for 10 min and finally washed in PBS and sealed with a coverslip. The images were captured with a fluorescence microscope (Olympus Inc., Tokyo, Japan), positive neurons in each section were counted by three observers who were blinded to the experimental groups. The rates of Corresponding-protein positive cells per section was calculated from values obtained by counting 30–40 random sections throughout the lesion site of each animal, with five animals examined per group.
2.13. Statistical analysis
The results were presented as mean ± S.D. Statistical analyses were performed using SPSS statistical software program 20.0 (IBM, Armonk, NY, USA). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's test for comparison between control and treatment groups. BBB score data were analyzed by Mann–Whitney test. P < .05 was considered statistically significant.
3. Results
3.1. CO content varies in spinal cord tissues after SCI
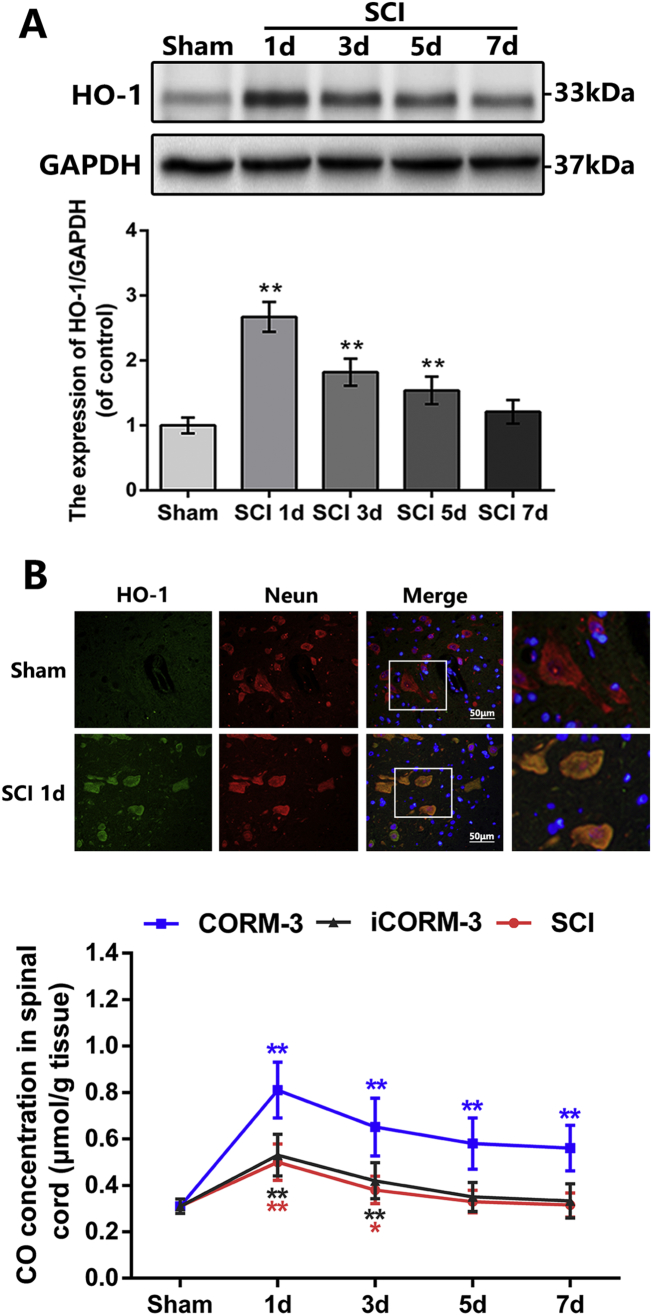
We detected the expression of HO-1, which is the most pivotal enzymes of autogenous CO generation [42], at 1, 3, 5 and 7 days after SCI. The western blotting results revealed the time course of HO-1 expression level increased at 1 day after SCI, declined at 3 days and then gradually tended to be stabilized at 5 and 7 days (Fig. 1A). By immunofluorescence double staining, we observed that the HO-1 mainly co-localized with neuron in injured spinal cord (Fig. 1B). To ascertain the change of CO level after SCI, we quantify the CO content in spinal cord at the same post-SCI time points; the results showed that the time course of CO concentration is basically consistent with the HO-1 expression after SCI (Fig. 1C). These results indicated that endogenous CO and HO-1 expression in spinal cord was increased after SCI.
Fig. 1.
Carbon monoxide content and HO-1 expression in the early stages after SCI.
(A) The protein expression of HO-1 in spinal cord at early time points after SCI (n = 5). (B) Double immunofluorescence of HO-1 and NeuN in sections from tissue at 1 day after SCI (bar: 50 μm) (n = 5). (C) Carbon monoxide concentration at early time points after SCI (n = 10). All data represent mean ± S.D. (n = 5). **P < .01.
3.2. CORM-3 contributes to increased CO content in spinal cord tissues
When SCI model was established in rats, the water-soluble exogenous CO donor, CORM-3, was continuously applied on SCI; meanwhile the inactive CORM-3 (iCORM-3), which does not release CO, was taken as the negative control. The results showed that CORM-3 administration efficiently multiplied CO content in the spinal cord tissues at 1 day post-SCI (Fig. 1C). Although the CO concentration in CORM-3 group appeared to decline slightly after 1 day, its total level was prominently higher than the iCORM-3 group (Fig. 1C). Meanwhile, there was no significant discrepancy in CO content among the SCI group and iCORM-3 group (Fig. 1C).
3.3. SCI-mediated neuron loss and neurological deficits are alleviated by CORM-3 treatment
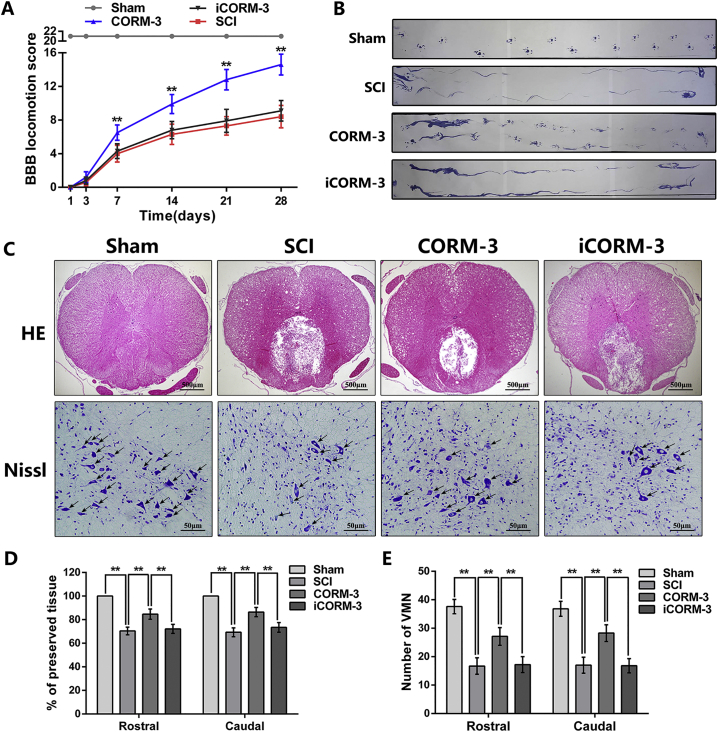
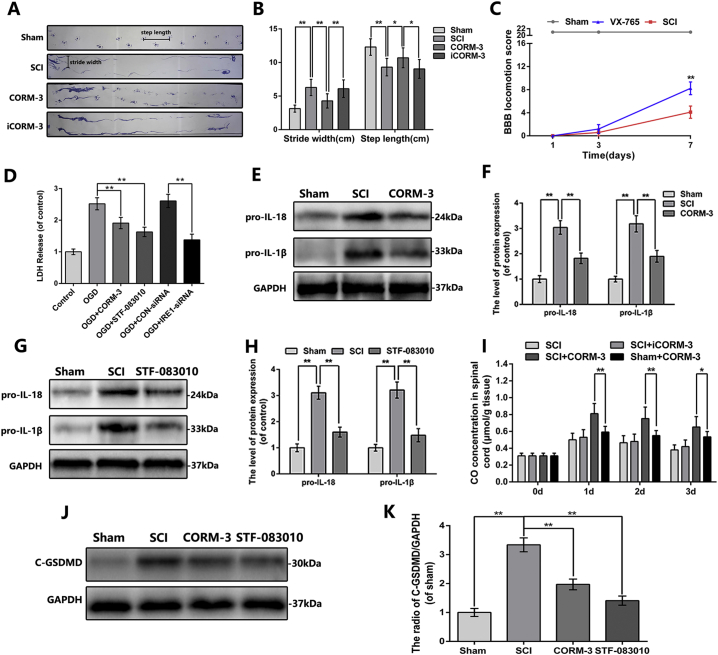
To investigate whether CO exerted a protective effect on the SCI, the BBB scores and footprint analysis were executed to assess the recovery of motor function in different groups. As shown in Fig. 2A, the BBB scores of rats in the Sham group maintained at the maximum score of 21, while the score of rats suffering from SCI dropped immediately to 0 points at 1 day after surgery. Compared to the SCI group and iCORM3 group, rats in CORM-3 treatment group exhibited significant promoted locomotor restoration, as indicated by increased BBB score at 7, 14, 21, 28 days after injury (Fig. 2A). Meanwhile, the footprint vividly showed the gait of the rat's hind limb, was performed at 28 days post SCI. The results appeared as a wavy line in SCI and iCORM-3 group, suggesting the hind limb in the rats of groups above lost its function of supporting body weight and coordinating forelimb movement (Fig. 2B). We also analyzed step length and stride width of footprint. As shown in Fig. S1A&B, rats with SCI exhibits shorter step length and longer stride width relative to the sham group, however CORM-3 treatment ameliorated this phenomenon. Nevertheless, we can found obvious paw prints along with harmonized alternate gait in rats of CORM-3 group (Fig. 2B).
Fig. 2.
CORM-3 attenuate neuron death and improve functional recovery after SCI.
(A) The Basso, Beattie and Bresnahan (BBB) scores of each group (n = 10). (B) The footprint result of each group (n = 10). (C) The HE staining at 7 days post SCI (bar: 500 μm) and Nissl staining at 3 days (bar: 50 μm). (D) The percent of preserved tissue in relation to the transverse area of the spinal cord by HE staining (n = 5). (E) Counting number of VMN by Nissl staining (n = 5). All data represent mean ± S.D. **P < .01.
Fig. S1.
CORM-3 effect in in vivo and in vitro experiments.
Histomorphological differences were accessed by HE staining. As shown in Fig. 2C & D, CORM-3 treatment decreased the size of the lesion cavity at 7 days post SCI. We also applied Nissl staining to detect neuronal survival at 3 days after SCI. The counting analysis of Nissl staining revealed that CORM-3 injection increased the number of ventral motor neuron (VMN) as compared to the SCI non-treatment and iCORM treatment (Fig. 2C, E). Interestingly, there is no significant difference in the results of the BBB scores, footprint, HE staining and Nissl staining between the SCI group and the iCORM-3 group, suggesting that the protective effects of CORM-3 on SCI was based on its CO donor capability.
3.4. CORM-3 inhibites pyroptosis and inflammasome expression following SCI
Unlike other types of cell death, pyroptosis is redefined as gasdermin-mediated programmed necrotic cell death which depends on caspase1 or caspase11/4/5 activation, leading to IL-1β and IL-18 stimulation, cytomembrane pore formation and subsequently the release of inflammatory mediators and cellular contents [18,[43], [44], [45]]. Rats treated with VX-765, the classical pyroptosis inhibitor, has a higher BBB scores at 7 days after SCI relative to the SCI group (Fig. S1C). Although it is controversial about which death form, namely apoptosis, pyroptosis and necrosis dominates the acute stage of central nervous system (CNS) injury, pharmacological or genetical suppression of pyroptotic death has been proved to be a potential therapeutic target for CNS injury [9,20,27]. Previous studies have reported the anti-apoptotic effect of CO in in vitro and in vivo models of traumatic brain injury (TBI) [31], however the effect of CO on pyroptosis in CNS is unknown.
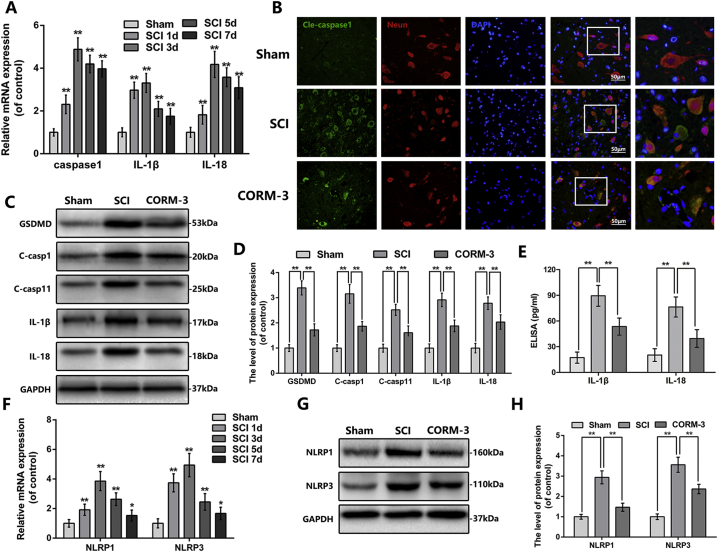
We firstly measured the mRNA level of pyroptosis-related key genes at different time points after SCI. The mRNA level of caspase1 elevated at 1 day after SCI, peaked at 3 days and persisted at the high level at 7 days; the expression of IL-1β and IL-18 showed the similar pattern (Fig. 3A). Next, we analyzed the pyroptosis level in spinal cord tissues by western blot and immunofluorescence. Immunofluorescence double staining of cleaved-caspase1 and NeuN showed that cleaved-caspase1 positive neuron in spinal cord anterior horn was lessen by CORM-3 administration following SCI (Fig. 3B). As shown in Fig. 3C & D, CORM-3 reversed the increased expression of GSDMD, cleaved-caspase1, cleaved-caspase11, IL-1β and IL-18 induced by SCI; the expression of IL-1β and IL-18 had further been confirmed by ELISA analysis (Fig. 3E).
Fig. 3.
CORM-3 inhibit pyroptosis and inflammasomes signaling at 3 days after SCI.
(A) The mRNA expression of caspase1, IL-1β and IL-18 of each group at early time points after SCI. (B) Double immunofluorescence of cleaved-caspase1 and NeuN in sections from tissue at 3 day after SCI (bar: 50 μm) (C) and (D) The protein expression of GSDMD, cleaved-caspase1, cleaved-caspase11, IL-1β and IL-18 in spinal cord at 3 days after SCI. (E) The ELISA of IL-1β and IL-18 at 3 days after SCI. (F) The mRNA expression of NLRP1 and NLRP3 of each group at early time points after SCI. (F) and (G) The protein expression of NLRP1 and NLRP3 in spinal cord at 3 days after SCI. All data represent mean ± S.D. (n = 5) **P < .01.
In physiological conditions, caspase1 presents as inactive precursor in the cytoplasm; however in pathological conditions, it could be cleaved by inflammasome to produce a tetramer consisting of active subunits [17]. Our PCR results showed that the time course of the NLRP1 and NLRP3 mRNA expression was broadly consistent with caspase1 after SCI (Fig. 3F), suggesting pyroptosis was correlated with inflammasome signaling in SCI. The western blot analysis also showed that the expression of NLRP1 and NLRP3 in spinal cord lysate was ascended following SCI, whereas CORM-3 reversed these trends, suggesting the priming step of inflammasome signaling was inhibited by CORM-3 treatment (Fig. 3G, H). The priming of inflammasome signaling is accompanied by the synthesis of pro-IL-1β and pro-IL-18. As shown in Fig. S1E&F and S1J&K, CORM-3 treatment down-regulated the SCI-upregulated expression of pro-IL-1β, pro-IL-18 and cleaved GSDMD.
These results suggest that CORM-3 inhibit pyroptosis and inflammasome signaling priming following SCI in rats.
3.5. CORM-3 suppresses SCI-induced IRE1 phosphorylation in neuron in vivo
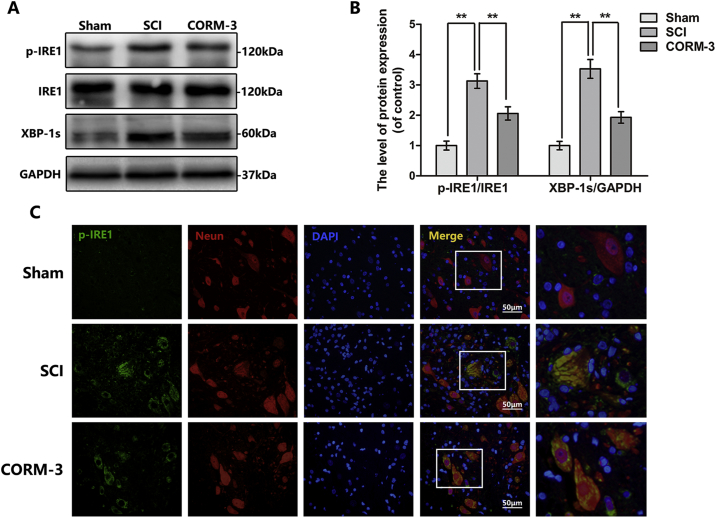
CO has emerged various bioactivities in multiple disease models [14,15]; however, the specific molecular target for CO is still not clear. Early researches mainly focus on the regulation of ion channels [[46], [47], [48]]; recently, researchers found that thapsigargin or tunicamycin (the classical agonist of ER stress) induced IRE1 phosphorylation was blocked by CORMs pretreatment [14,49,50], indicating CORM-3 may regulate ER stress. In our study, we examined the IRE1 phosphorylation level and its downstream protein XBP1s expression by western blot (Fig. 4A, B). And we demonstrated that CORM-3 treatment inhibited the SCI-mediated IRE1-XBP1 pathway stimulation. Moreover, we showed that p-IRE1α was mainly localized in neurons as indicated by the immunofluorescence analysis in spinal cord tissues (Fig. 4C). The green fluorescence intensity in neuron was increased in SCI group relative to the sham group, CORM-3 alleviated this phenomenon. These results suggest that CORM-3 suppress SCI-induced IRE1 phosphorylation in neuron.
Fig. 4.
CORM-3 restrain IRE1/XBP-1 s pathway at 3 days after SCI.
(A) and (B) The protein expression of p-IRE1, IRE1 and XBP-1 s in spinal cord at 3 days after SCI. (C) Double immunofluorescence of p-IRE1 and NeuN in sections from tissue at 3 days after SCI (bar: 50 μm). All data represent mean ± S.D. (n = 5). **P < .01.
3.6. CORM-3 restrains the IRE1 phosphorylation and inflammasome expression in OGD neuron in vitro
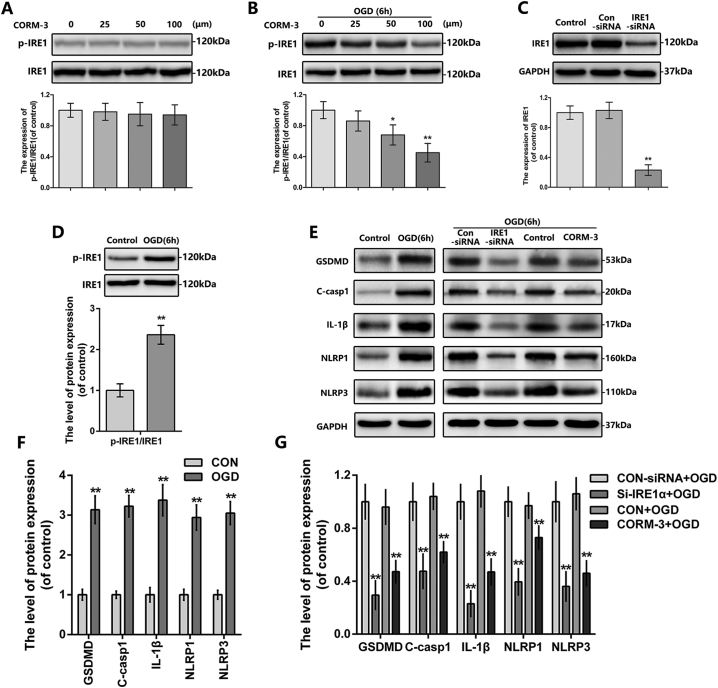
To further explain the working mechanism of CO-dependent protective effects on SCI, we exposed the primary neurons at OGD condition to mimic SCI model in vitro [31,51]. Interestingly, the suppression of IRE1 phosphorylation by CORM-3 was only observed at the neuron under OGD condition, and treated of neuron with CORM-3 alone had no significant effect on p-IRE1 and IRE1 expression (Fig. 5A, B). Consistent with our in vivo experiments results, CORM-3 treatment down-regulated the OGD-induced GSDMD, cleaved-caspase1, IL-1β, NLRP1 and NLRP3 expression in neuron (Fig. 5E-G). These results suggest that CORM-3 restrain the IRE1 phosphorylation and inflammasome expression in neuron after OGD.
Fig. 5.
The effect of CORM-3 and IRE1 in neuron under OGD condition.
(A) and (B) The protein expression of p-IRE1 and IRE1 in neuron treated as above. (C) The protein expression of IRE1 in neuron treated as above. (D-G) The protein expression of p-IRE1, IRE1, GSDMD, cleaved-caspase1, IL-1β, NLRP1 and NLRP3 in neuron treated as above. All data represent mean ± S.D. (n = 5). **P < .01.
3.7. Inhibition of IRE1 blocks inflammasome priming in OGD neuron in vitro
Based on the phenomena above, we questioned if OGD-induced IRE1 activation involves in inflammasome signaling and pyroptosis occurrence. Therefore, we transfected neuron with IRE1-siRNA, the knock-down efficiency was confirmed by western blot (Fig. 5C). As shown in Fig. 5E-G, IRE1 inhibition by siRNA interdicted GSDMD, cleaved-caspase1, IL-1β, NLRP1 and NLRP3 expression in OGD-exposed neuron. Furthermore, administering STF-083010, the specific antagonist of IRE1, to OGD-exposed neuron showed the similar results to IRE1-siRNA knock down (Fig. 6G, H). These results showed that inhibition of IRE1, either genetically by siRNA or pharmacologically by STF-083010, blocks regulatory effects of CORM-3 on inflammasome in OGD neuron in vitro, indicating the regulatory effects of CORM-3 on inflammasome is through IRE1.
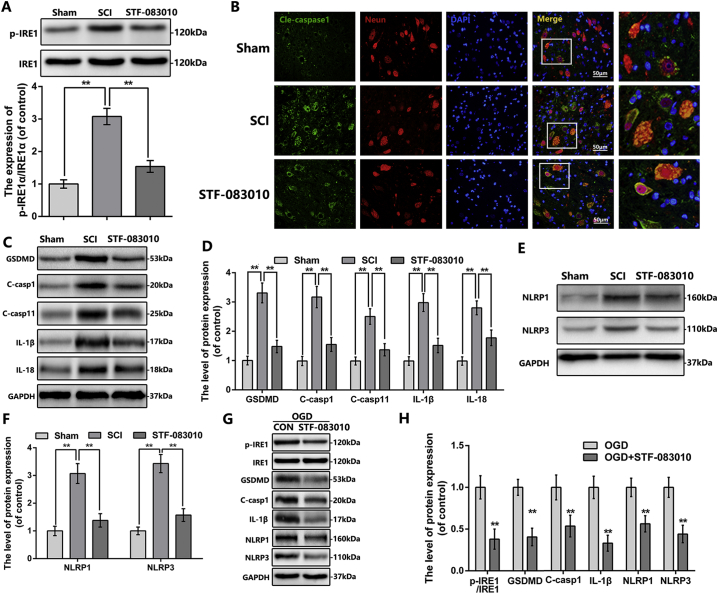
Fig. 6.
STF-083010 suppress the inflammasome signaling and pyroptosis after SCI and OGD-induced neuron.
(A) The protein expression of p-IRE1 in spinal cord at 3 days after SCI. (B) Double immunofluorescence of cleaved-caspase1 and NeuN in sections from tissue at 3 days after SCI (bar: 50 μm). (C—F) The protein expression of GSDMD, cleaved-caspase1, cleaved-caspase11, IL-1β, IL-18, NLRP1 and NLRP3 in spinal cord at 3 days after SCI. (G) and (H) The protein expression of p-IRE1, IRE1, cleaved-caspase1, IL-1β, NLRP1 and NLRP3 in neuron treated as above. All data represent mean ± S.D. (n = 5). **P < .01.
3.8. Pharmacological inhibition of IRE1 relieves inflammasome priming, activation and pyroptosis following SCI in vivo
To further confirm the effect of IRE1 signaling in SCI, we applied STF-083010 in rat SCI model. The western blot demonstrated that STF-083010 markedly inhibited the IRE1 phosphorylation following SCI (Fig. 6A). As shown in Fig. 6B, the fluorescence intensity of cleaved-caspase1 in neuron was reduced by STF-083010 administration post-SCI. By western bot results, the expression of GSDMD, cleaved GSDMD, cleaved-caspase1, cleaved-caspase11, IL-1β, IL-18, NLRP1, NLRP3, pro-IL-1β and pro-IL-18 were observed downregulated in STF-083010 treatment group (Fig. 6C-F and Fig. S1G-K). Together, these results suggest that pharmacological inhibition of IRE1 by STF-083010 relieves inflammasome priming and pyroptosis following SCI in vivo.
4. Discussion
As a product of haem degradation, CO has been demonstrated to have various biological functions such as anti-inflammatory, anti-apoptotic and anti-oxidative effects at low concentration [15,46]. Recently, it was reported that CO could prevent pericyte apoptosis following TBI [31]. We are the first to report that the dynamic change of CO content in spinal cord at the early stage of SCI and the role of CORM-3 in neuron death and functional recovery following SCI. Moreover, we further demonstrated the protective effects of CORM-3 might be through the regulation of IRE1-inflammasome pathway.
General dogma showed CO is poisonous, because of its high affinity with Hb. Therefore, we administered CORM-3 via tail vein thereby avoiding traditional pulmonary delivery systems, which greatly reduce the toxicity of CO. Prabhu et al. administered CORM-3 (40 mg/kg/d) to mice via intraperitoneal injections and found that a fairly constant level of carboxyhemoglobin (COHb; 6%) was maintained during the 24 days medication period, which is a safe concentration [16].
During the SCI process, the mechanical impact on spinal cord causes vascular rupture and tissue destruction which may subsequently enhance haem production (deriving from the dying cells or hemoglobin) [52,53]. Meanwhile, the expression and activity of HO-1 were up-regulated relative to the uninjured spinal cord [46]. These phenomena may explain SCI-induced CO content change in spinal cord.
The intervention of secondary injury-induced neuron death has been widely studied and identified as a potential therapeutic target for SCI. Besides apoptosis and necrosis (the traditional cell death categories), recent findings showed that a special death mechanism (pyroptosis) involves in the SCI pathology, which has been redefined as gasdermin-mediated programmed necrotic cell death. It is characterized by the following: [1] formation of discrete 1 to 2 nm pores in the plasma membrane with the cytolysis; [2] maturation and release of inflammatory cytokines such as IL-1β and IL-18; [3] without mitochondrial ultrastructure damage; [4] a caspase3/6/7 independent cell death [22,23,25,45]. We found cleaved-caspase 1, cleaved-caspase 11, IL-1β and IL-18 expression in spinal cord are increased after injury, suggesting pyroptosis is induced in SCI; while CORM-3 treatment could suppress it.
As a multiprotein complex initiating caspase 1 activation, inflammasome mainly consists of sensor Nod-like receptors (NLRs), adaptor protein apoptosis-associated speck-like protein containing a caspase-activating recruitment domain protein (ASC), and pro-caspase 1 [21]. According to the NLR protein distinction, inflammasomes can be divided into different types, such as NLRP1, NLRP2, NLRP3, NLRP6, NLRP7 et.al, neuron in the spinal cord mainly express NLRP1 and NLRP3 [22]. Interestingly, NLRP1 inflammasome in neurons differs from that in macrophages in that it also contains caspase 11 and the inhibitor of apoptosis proteinX-linked inhibitor of apoptosis protein (XIAP) [22,23,54]. The mechanism of caspase 11 activation in the CNS injury is still unknown. Whether NLRP1 inflammasome activation induced the cleavage of caspase 11 or whether caspase 11 forms its own inflammasome (independent of caspase 1) remains to be investigated [22]. Besides the canonical inflammasome pathways, caspase 11 could also be activated by other noncanonical inflammasome pathways [42]. For instance, during the bacterial infection, LPS in cytoplasm could cleavage caspase 11 [55,56].
The regulation of inflammasome is a multifactor involved process. Our study confirmed that the expression of NLRP1, NLRP3, pro-IL-1β and pro-IL-18 are elevated in spinal cord after SCI, suggesting NLRP1 and NLRP3 inflammasome signaling are priming in SCI. As an acute sterile injury, inflammasome priming in SCI is triggered by the interactions between the danger/damage-associated molecular patterns (DAMPs) and pattern recognition receptors (PRRs) [[57], [58], [59]]. DAMPs in CNS refers to those endogenous ligands such as high plasma glucose, β-amyloid, uric acid, K+ and ATP that released by dying or dysfunctional cells, these ligands may be discerned by PRRs e.g. toll-like receptors, C-type lectin receptors, rig-like receptors, and NLRs [22,60].
The signaling pathways involving in injury stimulated inflammasome signaling in the CNS have not been well demonstrated yet, only the mechanism of K+ efflux and ATP releasing has been described. After injury, the released ATP may activate P2X4 and P2X7 purinergic receptors, which may further facilitate K+ efflux into extracellular space [22,25]. Meanwhile, high concentration of extracellular K+ opens the pannexin (PANX1) channels which may result in ATP release and further stimulates P2X7 receptors and forms the interaction with inflammasomes [24,29]. This positive feedback involving P2X7 receptors and PANX1 channels may decrease intracellular K+ concentration and triggers NLRP1 and NLRP3 inflammasome activation [21,22,25,54]. Until recently, P2X receptors are identified as the only ligand-gated ion channels regulated by CO [47,48]. However, there is no in vivo or in vitro evidence supporting that CO could inhibit P2X4 and P2X7 receptors in the presence of ATP. Wilkinson et al. demonstrated that CORM-2, another CO donor, is an efficient inhibitor of P2X4 receptor [48,61]; therefore, we speculate that CORM-3 might exert the same effect on P2X4 receptors, which of cause needs further verification.
Misawa et al. reported that spatial arrangement of mitochondria may promote activation of the NLRP3 inflammasome [62]. The NLRP3 inflammasome may also respond to mitochondrial stress such as reactive oxygen species (ROS) and oxidized mitochondrial DNA [63]. While ROS overproduction, which is mainly derived from dysfunctional mitochondira, may result in dissociated thioredoxin-interacting protein (TXNIP) from thioredoxin, binding to NLRP3 so as to form active inflammasome complex [63,64]. These studies suggest that inflammasome activation is also closely related to mitochondria function. CO has been reported to control mitochondrial quality via regulating mitochondrial biogenesis and mitophagy [33]. Therefore, the protective effect of CO against mitochondrial stress might provide a potential interpretation of inflammasome signaling by CORM-3.
The kinase/endoribonuclease inositol-requiring enzyme 1 (IRE1) is a pivotal endoplasmic reticulum (ER)-resident protein folding sensor, it is well known for its RNase activity that may degenerate ER-bound mRNA through activating X-box binding protein-1 (XBP1). And, it was also found to participate in NLRP1 and NLRP3 inflammasomes signaling [65,66]. Our results show that IRE1 phosphorylation level is elevated in rat SCI and neuron OGD model, accompanied by increased NLRP1/3 inflammasomes production and pyroptosis activity, whereas CORM-3 intervention reversed these changes. These results suggest that IRE1 may be involved in the regulation of CORM-3 on inflammasomes and pyroptosis. In an effort to explicate the role of IRE1 in SCI, we applied STF-083010, an IRE1 inhibitor, in SCI rats. Our results showed that SCI-induced GSDMD expression, caspase 1/11 cleavage, IL-1β and IL-18 production and NLRP1/3 inflammasomes priming was alleviated after STF-083010 treament, suggesting that CORM-3 may regulate inflammasomes signaling and pyroptosis through IRE1. However, how CORM-3 regulates IRE1 is still unknown. Chung et al. reported that CO may reverse TG (classical ER stress stimulator)-induced IRE1 phosphorylation via upregulating PERK phosphorylation [49,67,68]. Also, they found that CO-medicated PERK phosphorylation is ROS dependent and is abolished by ROS scavenger N-acetyl-L-cysteine (NAC) [68]. Whether CORM-3 regulates IRE1 through the same mechanism in SCI needs further verification.
In conclusion, the present study reports that exogenous administration of CORM-3 increase the concentration of CO in spinal cord tissues, and CORM-3 treatment could alleviate neuron pyroptosis after spinal cord injury, the mechanism may related to IRE1 mediated inflammasome signaling regulation. Our study suggests that CO may be beneficial for SCI recovery and CORM-3 could be a potential agent for SCI therapy.
The following are the supplementary data related to this article.
(A) Representative footprint picture of each group (n = 10). (B) The footprint analysis of each group (n = 10). (C) The Basso, Beattie and Bresnahan (BBB) scores of each group (n = 8). We applied the VX-765 to rats following SCI to assess the functional role of pyroptosis in our SCI model. (D) The LDH release assays in the cell culture supernatant and the cells were treated as above (n = 5). (E-H) The protein expression of pro-IL-1β and pro-IL-18 in spinal cord at 3 days after SCI (n = 5). (I) Carbon monoxide concentration at 1,2,3 days after SCI and rats in each groups were treated as above (n = 5). (J) and (K) The protein expression of cleaved GSDMD in spinal cord at 3 days after SCI (n = 5). All data represent mean ± S.D. (n = 5). *P < .05, **P < .01.
Acknowledgments
Acknowledgement
This study is supported by National Natural Science Foundation of China (81601963, 81873992, 81572227); Wenzhou Science and Technology Bureau Foundation (Y20170083, Y20170092).
Author contributions
GZ wrote the paper, performed the experiments and generated data; GZ, WHZ and YZ performed the experiments and generated data; ZCL, FHZ and YFZ analyzed data; YSW, YLZ and SW contributed reagents and materials tools; YW conceived and designed the experiments; CX and GHX performed the supplemental experiments and generate data; HZX, NFT and XLZ designed the experiments and helped write the manuscript.
Author disclosure statement
The authors declare no competing financial interest.
Contributor Information
Huazi Xu, Email: spinexu@163.com.
Naifeng Tian, Email: fengnq@163.com.
Xiaolei Zhang, Email: zhangxiaolei@wmu.edu.cn.
References
- 1.Agostinello J., Battistuzzo C.R., Skeers P., Bernard S., Batchelor P.E. Early spinal surgery following thoracolumbar spinal cord injury: process of care from trauma to theatre. Spine. 2016;42(10) doi: 10.1097/BRS.0000000000001903. [DOI] [PubMed] [Google Scholar]
- 2.Liu S., Sarkar C., Dinizo M. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6(1):e1582. doi: 10.1038/cddis.2014.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie M.S., Hermann G.E., Rogers R.C., Bresnahan J.C. Cell death in models of spinal cord injury. Prog. Brain Res. 2002;137:37. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 4.Di G.S., Knoblach S.M., Brandoli C., Aden S.A., Hoffman E.P., Faden A.I. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann. Neurol. 2003;53(4):454. doi: 10.1002/ana.10472. [DOI] [PubMed] [Google Scholar]
- 5.Park E., Velumian A.A., Fehlings M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J. Neurotrauma. 2004;21(6):754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 6.O'Shea T.M., Burda J.E., Sofroniew M.V. Cell biology of spinal cord injury and repair. J. Clin. Invest. 2017;127(9):3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X.Z., Xu X.M., Hu R. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997;17(14):5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin W., Wang S., Yang Z. Heme oxygenase-1 inhibits neuronal apoptosis in spinal cord injury through down-regulation of Cdc42-MLK3-MKK7-JNK3 axis. J. Neurotrauma. 2017;34(3) doi: 10.1089/neu.2016.4608. [DOI] [PubMed] [Google Scholar]
- 9.Lin W.P., Xiong G.P., Lin Q. Heme oxygenase-1 promotes neuron survival through down-regulation of neuronal NLRP1 expression after spinal cord injury. J. Neuroinflammation. 2016;13(1):52. doi: 10.1186/s12974-016-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei W., Shurui C., Zipeng Z. Aspirin suppresses neuronal apoptosis, reduces tissue inflammation, and restrains astrocyte activation by activating the Nrf2/HO-1 signaling pathway. NeuroReport. 2018;1 doi: 10.1097/WNR.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 11.Lu T., Wu X., Wei N. Lipoxin A4 protects against spinal cord injury via regulating Akt/nuclear factor (erythroid-derived 2)-like 2/heme oxygenase-1 signaling. Retour Au Numéro. 2018;97:905. doi: 10.1016/j.biopha.2017.10.092. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Ruiz A., Maldonado P.D., Mendez-Armenta M. Activation of heme oxygenase recovers motor function after spinal cord injury in rats. Neurosci. Lett. 2013;556(1):26–31. doi: 10.1016/j.neulet.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi T., Lin Y., Sharp F.R., Noble-Haeusslein L.J. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J. Neurotrauma. 2004;21(8):1017–1030. doi: 10.1089/0897715041651042. [DOI] [PubMed] [Google Scholar]
- 14.Kim K.M., Pae H.O., Zheng M., Park R., Kim Y.M., Chung H.T. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ. Res. 2007;101(9):919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- 15.Motterlini R., Otterbein L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010;9(9):728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 16.Wang G., Hamid T., Keith R.J. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121(17):1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei L., Chen Y., Jiao M. Ablation of caspase-1 protects against TBI-induced pyroptosis in vitro and in vivo. J. Neuroinflammation. 2018;15(1):48. doi: 10.1186/s12974-018-1083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Gao W., Shi X. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 19.Adamczak S.E., Vaccari J.P.D.R., Dale G. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 2014;34(4):621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fann Y.W., Lee S.Y., Manzanero S. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4(9) doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trendelenburg G. Molecular regulation of cell fate in cerebral ischemia: role of the inflammasome and connected pathways. J. Cereb. Blood Flow Metab. 2014;34(12):1857–1867. doi: 10.1038/jcbfm.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccari J.P.D.R., Dietrich W.D., Keane R.W. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 2014;34(3):369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaccari J.P.D.R., Lotocki G., Marcillo A.E., Dietrich W.D., Keane R.W. A molecular platform in neurons regulates inflammation after spinal cord injury. J. Neurosci. 2008;28(13):3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou K., Shi L., Wang Y., Chen S., Zhang J. Recent advances of the NLRP3 inflammasome in central nervous system disorders. J. Immunol. Res. 2016;2016(2):1–9. doi: 10.1155/2016/9238290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh J.G., Muruve D.A., Power C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014;15(2):84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 26.Zendedel A., Johann S., Mehrabi S. Activation and regulation of NLRP3 inflammasome by intrathecal application of SDF-1a in a spinal cord injury model. Mol. Neurobiol. 2016;53(5):3063–3075. doi: 10.1007/s12035-015-9203-5. [DOI] [PubMed] [Google Scholar]
- 27.Wu J., Li M., Fan H., Zhou S., Zhu L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J. Neuroinflammation. 2017;14(1):207. doi: 10.1186/s12974-017-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaccari J.P.D.R., Lotocki G., Alonso O.F., Bramlett H.M., Dietrich W.D., Keane R.W. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29(7):1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zendedel A., Mönnink F., Hassanzadeh G. Estrogen attenuates local inflammasome expression and activation after spinal cord injury. Mol. Neurobiol. 2017;55(2):1–12. doi: 10.1007/s12035-017-0400-2. [DOI] [PubMed] [Google Scholar]
- 30.Zeynalov E., Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox. Res. 2009;15(2):133. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi Y.K., Maki T., Mandeville E.T. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat. Med. 2016;22(11):1335–1341. doi: 10.1038/nm.4188. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Di Z., Fu X. Carbon monoxide-releasing molecule-3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood-brain barrier disruption. J. Neuroinflammation. 2018;15(1):188. doi: 10.1186/s12974-018-1226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suliman H.B., Piantadosi C.A. Mitochondrial quality control as a therapeutic target. Pharmacol. Rev. 2016;68(1):20–48. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W., Tao A., Lan T. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res. Cardiol. 2017;112(2):16. doi: 10.1007/s00395-017-0603-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang P., Huang J., Li Y. Exogenous carbon monoxide decreases sepsis-induced acute kidney injury and inhibits NLRP3 inflammasome activation in rats. Int. J. Mol. Sci. 2015;16(9):20595–20608. doi: 10.3390/ijms160920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H.-Y., Wang Z.-G., Wu F.-Z. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol. Neurobiol. 2013;48(3):452–464. doi: 10.1007/s12035-013-8432-8. [DOI] [PubMed] [Google Scholar]
- 37.Sugawara T., Lewén A., Gasche Y., Yu F., Chan P.H. Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J. 2002;16(14):1997–1999. doi: 10.1096/fj.02-0251fje. [DOI] [PubMed] [Google Scholar]
- 38.Takafumi M., Masaaki T., Satoshi Y. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res Ther. 2013;4(2):35. doi: 10.1186/scrt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piantadosi C.A. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 2008;45(5):562–569. doi: 10.1016/j.freeradbiomed.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geralds M., Hendrikj V., Jamesf M.L.B., Kanji N. Measurement of endogenous carbon monoxide formation in biological systems. Antioxid. Redox Signal. 2002;4(2):271–277. doi: 10.1089/152308602753666325. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Wang Q., Wang H. Lentivirus mediating FGF13 enhances axon regeneration after spinal cord injury by stablilizing microtubule and improving mitochondrial function. J. Neurotrauma. Feb 2018;1;35(3):548–559. doi: 10.1089/neu.2017.5205. [DOI] [PubMed] [Google Scholar]
- 42.Deng J., Lei C., Chen Y. Neuroprotective gases – fantasy or reality for clinical use? Prog. Neurobiol. 2014;115(2):210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Wu X., Zhang H., Qi W. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9(2):171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Liu X., Bai X. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J. Pineal Res. 2018;64(2) doi: 10.1111/jpi.12449. [DOI] [PubMed] [Google Scholar]
- 45.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Queiroga C.S., Vercelli A., Vieira H.L. Carbon monoxide and the CNS: challenges and achievements. Br. J. Pharmacol. 2015;172(6):1533. doi: 10.1111/bph.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peers C., Boyle J.P., Scragg J.L. Diverse mechanisms underlying the regulation of ion channels by carbon monoxide. Br. J. Pharmacol. 2015;172(6):1546–1556. doi: 10.1111/bph.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson W.J., Kemp P.J. Carbon monoxide: an emerging regulator of ion channels. J. Physiol. 2011;589(13):3055–3062. doi: 10.1113/jphysiol.2011.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng M., Zhang Q., Joe Y. Carbon monoxide-releasing molecules reverse leptin resistance induced by endoplasmic reticulum stress. Am. J. Physiol. Endocrinol. Metab. 2013;304(7) doi: 10.1152/ajpendo.00466.2012. (E780-E8) [DOI] [PubMed] [Google Scholar]
- 50.Chung J., Shin D.Y., Zheng M. Carbon monoxide, a reaction product of heme oxygenase-1, suppresses the expression of C-reactive protein by endoplasmic reticulum stress through modulation of the unfolded protein response. Mol. Immunol. 2011;48(15–16):1793–1799. doi: 10.1016/j.molimm.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Ye L.B., Yu X.C., Xia Q.H. Regulation of caveolin-1 and junction proteins by bFGF contributes to the integrity of blood–spinal cord barrier and functional recovery. Neurotherapeutics. 2016;13(4):844–858. doi: 10.1007/s13311-016-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gozzelino R., Jeney V., Soares M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010;50(1):323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 53.Grochot-Przeczek A., Dulak J., Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin. Sci. 2012;122(3):93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 54.Mortezaee K., Khanlarkhani N., Beyer C., Zendedel A. Inflammasome: its role in traumatic brain and spinal cord injury. J. Cell. Physiol. 2018;233(7) doi: 10.1002/jcp.26287. [DOI] [PubMed] [Google Scholar]
- 55.Shi J., Zhao Y., Wang Y. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 56.Kayagaki N., Stowe I.B., Lee B.L. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 57.Hanamsagar R., Hanke M.L., Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33(7):333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedra J.H.F., Cassel S.L., Sutterwala F.S. Sensing pathogens and danger signals by the inflammasome. Curr. Opin. Immunol. 2009;21(1):10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alnemri E.S. Sensing cytoplasmic danger signals by the inflammasome. J. Clin. Immunol. 2010;30(4):512–519. doi: 10.1007/s10875-010-9419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaccari J.P.D.R., Dietrich W.D., Keane R.W. Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 2016;167(1):35–45. doi: 10.1016/j.trsl.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkinson W.J., Hanne G., Harrison A.W.J., Allen N.D., Riccardi D., Kemp P.J. Carbon monoxide is a rapid modulator of recombinant and native P2X2 ligand-gated ion channels [Abstract] Purinergic Signal. Oct 2009;158(3):862–871. doi: 10.1111/j.1476-5381.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Misawa T., Takahama M., Kozaki T. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14(5):454. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 63.Han Y., Xu X., Tang C. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ROS-TXNIP-NLRP3 biological axis. Redox Biol. 2018;16:32. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 65.Tufanli O., Telkoparan Akillilar P., Acosta-Alvear D. Targeting IRE1 with small molecules counteracts progression of atherosclerosis. Proc. Natl. Acad. Sci. U. S. A. 2017;114(8) doi: 10.1073/pnas.1621188114. (E1395-E404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen D., Dixon B.J., Doycheva D.M. IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic–ischemic brain injury in rats. J. Neuroinflammation. 2018;15(1):32. doi: 10.1186/s12974-018-1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joe Y., Kim S., Kim H.J. FGF21 induced by carbon monoxide mediates metabolic homeostasisviathe PERK/ATF4 pathway. FASEB J. 2018;32(5):2630–2643. doi: 10.1096/fj.201700709RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeong K.H., Joe Y., Kim S.K. Carbon monoxide protects against hepatic steatosis in mice by inducing Sestrin-2 via the PERK-eIF2α-ATF4 pathway. Free Radic. Biol. Med. 2017;110:81–91. doi: 10.1016/j.freeradbiomed.2017.05.026. [DOI] [PubMed] [Google Scholar]