Abstract
Background
Locally advanced pancreatic cancer (LAPC) has a dismal prognosis with current treatment modalities and one-third of patients die from local progression of disease. Preclinical studies with orthotopic PC demonstrated dramatic synergy between radiotherapy (RT) and the poly(ADP-ribose) polymerase-1/2 inhibitor (PARPi), veliparib. We conducted a phase I trial of gemcitabine, radiotherapy and dose-escalated veliparib in LAPC.
Methods
This was a single institution investigator-initiated open-label, single-arm phase 1 clinical trial (NCT01908478). Weekly gemcitabine with daily IMRT and veliparib dose escalated using a Bayesian adaptive design were administered in treatment naïve LA or borderline resectable PC. The primary end point was identification of the MTD. Secondary endpoints included efficacy, characterization of PAR levels using ELISA, DDR alterations with targeted next generation sequencing and transcriptome analysis, tumor mutation burden (TMB) and microsatellite instability (MSI) status.
Findings
Thirty patients were enrolled. The MTD of veliparib was 40 mg BID with gemcitabine 400 mg/m2 and RT (36 Gy/15 fractions). Sixteen DLTs were identified in 12 patients. Grade ≥ 3 adverse events included lymphopenia (96%) and anemia (36%). Median OS for all patients was 15 months. Median OS for DDR pathway gene altered and intact cases was 19 months (95% CI: 6.2–27.2) and 14 months (95% CI: 10.0–21.8), respectively. There were no significant associations between levels of PAR, TMB, or MSI with outcomes. The DDR transcripts PARP3 and RBX1 significantly correlated with OS.
Interpretation
This is the first report of a PARPi-chemoradiotherapy combination in PC. The regimen was safe, tolerable at the RP2D, and clinically active as an upfront treatment strategy in patients biologically unselected by upfront chemotherapy. Expression of the DDR transcripts, PARP3 and RBX1, were associated with OS suggesting validation in a follow up phase 2 study.
Fund
Phase One Foundation; National Institutes of Health [1R01CA188480-01A1, P01 CA098912]. Veliparib was provided by Abbvie.
Keywords: Parp inhibitor, Radiation, Gemcitabine, Pancreas cancer, Veliparib
Research in context.
Evidence before this study
Pancreatic cancer (PC) has the lowest 5 year survival of any solid tumor. Randomized studies have shown modest clinical improvements with non-biomarker selected chemotherapies. Molecular subtypes with associated therapeutic targets are rare in PC, yet pathogenic mutations associated with DNA damage repair (DDR) have been identified providing rationale for investigating DNA damaging agents such as poly(ADP-ribose) polymerase-1 and 2 (PARP-1/2) inhibitors (PARPi) and radiotherapy (RT). Veliparib, an orally bioavailable PARPi, has efficacy in germline BRCA-1/2 mutated metastatic PC when used in combination with platinum chemotherapy. However, minimal clinical data exists with PARPi in patients with LAPC, either as single agent or in rational combinations, including potential synergy with RT. Preclinical studies with non metastatic orthotopic pancreatic tumors demonstrated dramatic synergy between RT and veliparib suggesting that the combination may be a promising therapeutic strategy.
Added value of this study
Here we report the first PARPi-chemoradiotherapy combination in LAPC. We demonstrate the combination of veliparib, gemcitabine and radiotherapy was well-tolerated, and demonstrated an acceptable safety profile at the recommended phase 2 dose. Pathogenic DDR gene mutations did not correlate with clinical response, yet alterations in expression of the DDR proteins PARP3 and RBX1 were associated with improved OS. Similar to other studies using PARPi, baseline and relative change of PAR levels during treatment, TMB and microsatellite instability were not associated with PFS or OS highlighting the need to identify better biomarkers of response to PARPi. The overall clinical outcomes for this trial were favorable compared to contemporary studies utilizing the chemotherapy-first approach suggesting validation in a larger follow up phase 2 study combining the two strategies may be warranted.
Implications of all the available evidence
Chemoradiation is used after standard multiagent chemotherapy in patients with LAPC. In spite of this, local progression rates are high (30–40%) and downstaging rates are low (10–15%). Radiation dose escalation to PC is limited by dose to adjacent normal tissues and rationale combination strategies are warranted to overcome these limitations. Additionally, no biomarker selected therapies exist in PC. This is the first study investigating safety and clinical efficacy of PARPi with CRT in LAPC with comprehensive interrogation of potential genomic, transcriptomic and protein based-biomarkers of response.
Alt-text: Unlabelled Box
1. Introduction
Despite currently available therapies locally advanced pancreatic cancer (LAPC) carries a poor prognosis with a 5-year overall survival of 3%, median progression free survival (mPFS) of 6 months and median overall survival (mOS) of 11 months [1]. Approximately 30% of patients die due to progression of the primary tumor. Radiotherapy (RT) is currently recommended after multiagent chemotherapy in patients without progression of disease, but rates of downstaging with cytotoxic therapies and RT regimens is only 4–15% highlighting the need for novel therapeutic approaches [1,2].
One strategy to enhance the efficacy of DNA damaging agents including RT and gemcitabine is by targeting the cellular DNA repair machinery. Poly(ADP-ribose) polymerase-1 and 2 (PARP-1/2) are nuclear proteins involved in DNA damage repair (DDR). Following genotoxic stresses, PARP-1/2 are recruited to DNA strand breaks and lead to polymerization of poly(ADP-ribose) (PAR) and play a key role in the base excision repair pathway [3]. PARPi mechanistically act in two ways: first by interacting with the binding site of the PARP enzyme cofactor, β nicotinamide adenine dinucleotide (β-NAD+), in the catalytic domain of PARP1 and PARP2 preventing auto PARylation, and second by trapping PARP1 on DNA preventing PARP1/2 release from sites of DNA damage, although the latter depends on the affinity of the molecule for DNA. Veliparib (ABT-888; Abbvie, Chicago, Illinois) is an orally bioavailable inhibitor of PARylation with limited capacity to trap PARP1/2. Single-agent PARPi have shown efficacy in homologous recombination repair (HRR) deficient tumors such as ovarian cancers with germline BRCA-1/2 mutations, however minimal clinical data exists with PARPi in patients with pancreatic cancer, either as single agent or in rational combinations, including potential synergy with RT. [4] Preclinical studies with orthotopic pancreatic tumors demonstrated dramatic synergy between RT and veliparib suggesting that the combination may be a promising therapeutic strategy [5].
We undertook a phase I trial combining veliparib with gemcitabine-based chemoradiation (V + CRT) to explore synergy between PARPi and DNA damaging agents, such as RT, in LAPC. The primary objective was to determine the safety, toxicity profile and maximum tolerated dose (MTD) dose of veliparib. Secondary end points included efficacy and characterization of PAR, DDR and TMB alterations.
2. Methods
An institutional review board approved investigator-initiated, single-arm phase 1 study was opened to accrual July 2013 (NCT01908478). Treatment naïve patients age 18 years or older, ECOG 0–1, with LAPC or BRPC were eligible. Patients were treated with weekly gemcitabine (1000 mg/m2), daily intensity modulated RT (36 Gy/15 fractions) and a starting dose of veliparib 20 mg BID daily (dose level 1) for 3 weeks escalated using a Bayesian adaptive design [6]. The CRT regimen based on previous experience from the University of Michigan and the MD Anderson Cancer Center included full-dose gemcitabine and a hypofractionated regimen of RT to maximize synergy of both agents with veliparib allowing for systemic and local control [7]. Patients received 4DCT simulation to assess for respiration-induced tumor motion. Intravenous and oral contrast were administered to all patients unless medically contraindicated. The gross tumor volume included the primary tumor plus any involved (≥1.5 cm) regional lymph nodes identifiable on imaging. The clinical target volume margin was 0.5 cm, planning target volume was 0.5 cm and the internal target volume was calculated from 4DCT and applied. All patients were treated using intensity modulated RT or 3D-conformal RT if IMRT was not reimbursable. Image guidance was performed using daily cone-beam CT with alignment to intra-pancreatic fiducials and soft tissue. Additional details on treatment planning and constraints are included in protocol (Supplement).
Treatment on this protocol requires placement of 3–5 gold radio- opaque fiducials for targeting purposes.
Patients were evaluated for dose limiting toxicities (DLT) for 6 weeks and adverse events (AE) were monitored for the length of study participation using the Common Terminology Criteria for Adverse Events (CTCAE) version 4. DLTs included: G ≥ 4 thrombocytopenia/anemia, G3 thrombocytopenia with bleeding, G ≥ 3 febrile neutropenia, ANC < 100 for ≥3 days, ANC < 500 for ≥5 days, any non-hematologic grade ≥ 3 except grade 3 nausea, vomiting, and diarrhea that resolved within 5 days, or any other G4 toxicity. Patients with Grade 3–4 toxicity at least possibly attributable to veliparib except asymptomatic grade 3 lymphopenia stopped veliparib until toxicity resolved to ≤G1 or baseline. Standard of care chemotherapy was administered following DLT evaluation. Safety monitoring was conducted by an independent committee. Response assessment for LAPC and borderline resectable patients was conducted at baseline, 6–8 weeks post completion of study therapy, and every 2–3 months until resection or progression with computed tomography (CT) using RECIST 1.1 classification and assessment for resectability using NCCN guidelines.
Protocol mandatory treatment naïve core tumor biopsies were formalin-fixed paraffin-embedded and snap frozen for correlative analyses. Blood was collected pre-treatment, weekly for 6 weeks and then bimonthly. Targeted next generation sequencing (NGS), tumor mutational burden (TMB) and microsatellite (MSI) testing of treatment naïve pancreatic tumors was performed (Foundation Medicine, Cambridge, MA, USA). Genetic mutations (homozygous deletion or deleterious mutation) involved in DDR were identified. RNA sequencing was performed and the database for annotation, visualization, and integrated discovery (DAVID) v6.7 was used to interpret differential expression of genes. ELISA (Trevigen, Gaithersburg, MD) was used to quantitate PAR protein levels in peripheral blood mononuclear cells.
The MTD was defined to be the dose level of veliparib that resulted in a probability equal to θ = 0.25 that a DLT would manifest within 6 weeks. The design is Bayesian adaptive and is an extension of escalation with overdose control (EWOC) algorithm [6]. Details of the statistical methodology and operating characteristics can be found in the clinical protocol. The dose for each subsequent patient after starting dose of 20 mg BID was determined so that the probability that it exceeds the MTD was equal to a pre- specified feasibility bound of α = 0.25 and increased α in small increments of 0.05 until α = 0.5.
PFS was defined as the time from diagnosis to date of disease progression or death from any cause, and OS defined as the time from diagnosis to the date of death. Median follow-up was calculated using the reverse Kaplan-Meier method [8]. Survival functions were estimated by the Kaplan-Meier method [9]. Cox proportional hazards model was employed to identify variables associated with OS or PFS [10]. The least absolute shrinkage and selection operator (LASSO) method for the Cox proportional hazards model was used to select candidate variables. Multivariable analysis was carried out with candidate variables using a backward variable selection procedure with an alpha level of 0.15 for a removal criterion. The proportional hazards assumption was assessed graphically and analytically with scaled Schoenfeld residuals [11]. Analyses were performed using SAS 9.4 and R package version 3.4.1 with two-sided tests and a significance level of 0.05. Response and progression were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria. Best percent change in target primary tumor diameter and change in tumor burden over time were calculated.
Role of the funding source: Abbvie provided the study drug, Veliparib. The Phase One Foundation, the National Institutes of Health, and the National Center for Research Resources [now the National Center for Advancing Translational Sciences] funded this work.
3. Results
3.1. Safety
From July 2013 to May 2016, 34 patients were enrolled. All patients received at least one dose of veliparib. Three patients were removed due to treatment non-compliance and one patient withdrew consent. Patient demographics and clinical characteristics are provided in Table 1. Four simultaneous DLTs were experienced in the first patient treated with veliparib dose level 1 (20 mg BID), gemcitabine (1000 mg/m2) and RT (36 Gy; Table 2). Subsequent dose reductions of gemcitabine to 750 mg/m2 and 500 mg/m2 resulted in DLTs in patients 2 and 3, respectively. No DLTs occurred in patients treated with veliparib (20 mg BID), gemcitabine (400 mg/m2) and RT (36 Gy) resulting in veliparib dose escalation. The MTD of veliparib was 40 mg BID in combination with gemcitabine 400 mg/m2 and RT (36 Gy). In all, 16 DLTs were identified in 12 of 30 treated patients (lymphopenia (n = 10), neutropenia (n = 1), febrile neutropenia (n = 1), abdominal infection (n = 1), abdominal pain (n = 1), hyponatremia (n = 1), and leukopenia (n = 1)). The most commonly occurring AEs (≥50% of patients) were gastrointestinal (GI), hematologic and fatigue consistent with the known veliparib toxicity profile (Table 3). No grade 5 toxicities were encountered.
Table 1.
Patient demographics and clinical characteristics.
| Characteristics | N = 30 |
|---|---|
| Age (Years) | 68 (60–77) |
| Male Gender | 12 (40) |
| Resectability | |
| Borderline | 4 (13) |
| Locally advanced | 26 (87) |
| Clinical stage T | |
| T3 | 5 (17) |
| T4 | 25 (83) |
| Clinical stage N | |
| N1 | 15 (50) |
| N0 | 15 (50) |
| Ecog | |
| 0 | 21 (70) |
| 1 | 9 (30) |
| Resected | 8 (27) |
| Borderline | 2 (7) |
| Locally advanced | 6 (20) |
| Pathologic stage T | |
| T1 | 1 (12.5) |
| T3 | 6 (75) |
| T4 | 1 (12.5) |
| Pathologic stage N | |
| N1 | 4 (50) |
| N0 | 4 (50) |
| Margin status | |
| R0 | 8 (100) |
| Adjuvant chemotherapy | |
| Gemcitabine | 8 (26.67) |
| Gem/Abraxane | 22 (73.33) |
| CA 19–9 | |
| </= 90 | 14 (46.67) |
| >90 | 16 (53.33) |
Note: Data are presented as the number of patients (%) or median (IQR, interquartile range).
Table 2.
Treatment related dose limiting toxicities.
|
Veliparib (mg) BID |
Dose level (N = 30) |
Radiotherapy (Gy) | Number of patients dosed | Number of patients with DLTs | Number of DLTs | Treatment days |
|---|---|---|---|---|---|---|
| Gemcitabine (mg/m2) | ||||||
| 20 | 1000 | 36 | 1 | 1 | 4 | 959 |
| 20 | 750 | 36 | 1 | 1 | 2 | 879 |
| 20 | 500 | 36 | 1 | 1 | 1 | 212 |
| 20 | 400 | 36 | 1 | 0 | 0 | 795 |
| 40 | 400 | 36 | 8 | 1 | 1 | 319.71 (±300.53)a |
| 60 | 400 | 36 | 18 | 7 | 7 | 403 (±200.82)a |
Mean (±standard deviation).
Table 3.
Treatment related adverse events.
| Adverse Events (Type) | All Grades (N = 30) | Grades 3–4 (N = 26) |
|---|---|---|
| Gastrointestinal | ||
| Nausea | 27 (90) | 3 (11.54) |
| Vomiting | 20 (66.67) | 2 (7.69) |
| Diarrhea | 14 (46.67) | 1 (3.85) |
| Abdominal Pain | 28 (93.33) | 3 (11.54) |
| Colitis | 2 (6.67) | 1 (3.85) |
| Gastritits | 15 (50) | 0 |
| Dyspepsia | 25 (83.33) | 0 |
| Hematologic | ||
| Neutropenia | 1 (3.33) | 1 (3.85) |
| Febrile neutropenia | 1 (3.33) | 1 (3.85) |
| Lymphopenia | 30 (100) | 25 (96.15) |
| Anemia | 30 (100) | 10 (38.46) |
| Thrombocytopenia | 28 (93.33) | 5 (19.23) |
| Anorexia | 24 (80) | 5 (19.23) |
| Fatigue | 28 (93.33) | 1 (3.85) |
Note: Data are presented as the number of patients (%).
3.2. Efficacy
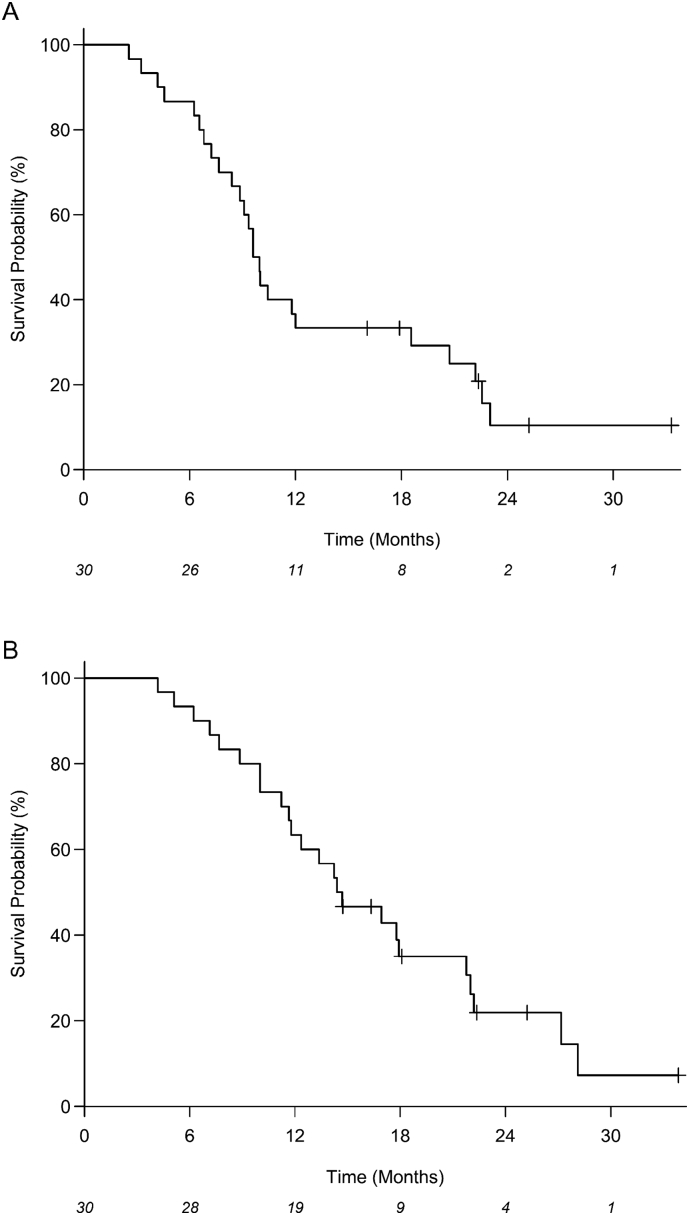
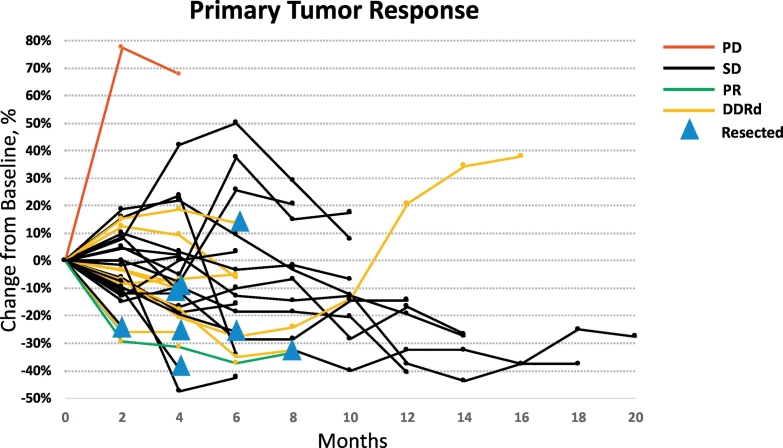
Median PFS (mPFS) and OS (mOS) for the 30 eligible patients were 9.8 months (95% CI: 8.4–18.6) and 14.6 months (95% CI: 11.6–21.8), respectively (Fig. 1A-B). Median OS for DDR pathway gene altered and DDR intact cases was 19 months (95% CI: 6.2–27.2) and 14 months (95% CI: 10.0–21.8), respectively. At the time of data cutoff, 24 of 30 patients had died (80%) and 25 patients had progressed (83%). On univariate analysis, CA 19–9 ≤ 90 (p = .048) and higher level of PARP3 expression (p = .024) identified through transcriptome analysis were associated with improved OS (Table 4). On multivariable analysis, higher levels of PARP3 (HR: 0.46; 95% CI: 0.21–0.99; p = .048) and lower levels of RBX1 (HR: 3.01; 95% CI: 1.12–8.59; p = .030) gene expression were associated with an improved OS. However, we stress the fact that this multivariable analysis is purely exploratory and these associations need to be validated with larger trials. Objective response rates (ORR) measured using RECIST 1.1 included one patient with partial response (PR; 3%) and another patient with progressive disease (3%; Fig. 2). The single patient with PR Given the lack of radiographic correlation seen in unresectable pancreatic cancers treated with radiotherapy, the majority of patients (28/30, 93%) experienced stable disease [12]. Definitive resection was performed in 8 of 30 patients (27%) and this did not correlate with ORR.
Fig. 1.
Kaplan-Meier estimates for (A) progression-free survival (PFS) and (B) overall survival (OS) for all patients. The number at risk for each time point is shown at the bottom of the plots.
Table 4.
Univariate and multivariable analyses for overall survival.
| Variable | N | Univariate |
Multivariable |
||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | ||
| GM-CSF (Human): difference in measurement from baseline to 21 days | 26 | 2.49 (0.90–6.86) | 0.078 | 3.14 (0.90–11.03) | 0.074 |
| PARP2 | 29 | 0.91 (0.56–1.47) | 0.692 | 1.71 (0.88–3.31) | 0.113 |
| PARP3 | 29 | 0.48 (0.25–0.91) | 0.024 | 0.46 (0.21–0.99) | 0.048 |
| RBX1 | 29 | 1.26 (0.67–2.36) | 0.475 | 3.10 (1.12–8.59) | 0.030 |
| CA 19–9 | |||||
| >90 | 16 | 2.37 (1.01–5.60) | 0.048 | 3.39 (0.96–12.00) | 0.059 |
| ≤90 | 14 | 1 (Reference) | 1 (Reference) | ||
Note: Out of 30 patients, 25 observations with complete data were used in the multivariable Cox proportional hazards model. Out of 25 patients, there were 20 deaths. A backward selection procedure was applied to 8 candidate markers (GM-CSF difference from baseline to 21 days, CETN2, NEIL3, PARP2, PARP3, RBX1, and CA 19–9) after an initial lasso regression analysis.
Fig. 2.
Spider plot depicting primary pancreatic tumor burden over time. PD – progressive disease; SD – stable disease; PR – partial response; DDRd – DNA damage repair deficient tumors.
3.3. Next generation sequencing
NGS was available for 26 of 30 patients (87%; Table 5) [13]. Four samples were inadequate for testing. Known or predicted deleterious mutations in assessed DDR genes included ARID1A [4], ATM [1], CHEK2-D293fs*1 [2], PALB2-V836I [1], PTEN [1] and MLH1-loss [1]. No BRCA1/2 mutations were identified. Of these 10 alterations, 1 patient (11%) harbored alterations in more than one gene. There was no statistically significant improvement in mPFS or mOS for patients with DDR pathway mutations compared to patients without pathogenic DDR mutations (p = .38, p = .9), respectively. Patient 8 with both a CHEK2 and MLH1 mutation was sufficiently downstaged during therapy and subsequently underwent a margin negative resection for initially unresectable LAPC. This patient had a PFS and OS of 20 months and 24 months, respectively. Additionally, the single LAPC patient with a deleterious PALB2 mutation was downstaged, underwent a margin negative resection, and remains without evidence of disease at 23 months as of the data cutoff and submission.
Table 5.
MSI, TMB and DDR.
| Patient # | MSI Status | TMB (mutations/Mb) | DDR Mutations | OS (months) |
|---|---|---|---|---|
| 1 | MSS | 1.8 | 21.8 | |
| 2 | NA | 1.3 | 13.4 | |
| 3 | NA | NA | 22.2 | |
| 5 | MSS | 0.9 | 11.2 | |
| 6 | NA | 0.9 | 28.1 | |
| 7 | NA | 1.8 | ARID1A | 14.4 |
| 8 | MSI-H | 23.4 | CHEK2, MLH1 | 22.0 |
| 9 | NA | 5.4 | CHEK2 | 14.7 |
| 10 | NA | NA | 5.1 | |
| 11 | NA | 2.7 | ARID1A | 16.9 |
| 12 | MSS | 2.7 | 4.2 | |
| 13 | MSS | 2.7 | PTEN | 10.0 |
| 14 | NA | NA | 8.9 | |
| 15 | NA | NA | 10.0 | |
| 16 | MSS | 0.9 | 33.9 | |
| 17 | MSS | 1.8 | 17.9 | |
| 18 | MSS | 0.9 | 17.8 | |
| 19 | MSS | 5.4 | ARID1A | 27.2 |
| 20 | MSS | 1.8 | 12.4 | |
| 21 | MSS | 5.4 | ATM | 6.2 |
| 24 | MSS | 8.1 | 22.4 | |
| 25 | MSS | 0 | 25.2 | |
| 26 | MSS | 1.8 | 7.1 | |
| 28 | MSS | 5.4 | 14.2 | |
| 29 | MSS | 1.8 | PALB2 | 18.1 |
| 30 | NA | NA | 11.6 | |
| 31 | NA | 3.6 | 16.4 | |
| 32 | MSS | 0.9 | 11.8 | |
| 33 | MSS | 1.8 | ARID1A | 14.7 |
| 34 | NA | 2.7 | 7.7 |
3.4. Tumor mutational burden (TMB) and microsatellite instability
TMB was available for 25 of 30 patients (Table 5). Median TMB was 1.8 mutations/Mb with a range of 0–23.4 mutations/Mb consistent with reported pancreatic series. Of 25 TMB-evaluable patients, only patient 8 with an MSI-high tumor and a CHEK2 and MLH1 mutation had a high TMB (mutations/Mb >20). DDR altered and wild-type cases had a mean TMB of 5.6 and 2.2, respectively (p = .08). Median OS for DDR altered and intact cases was 19 months (95% CI: 6.2–27.2) and 14 months (95% CI: 10.0–21.8), respectively. All patients identified as DDR wild type by NGS were TMB-low. Microsatellite status was available for 18 of 30 patients, and all MSS patients were evaluable to TMB. All MSS (17/18) patients were TMB-low.
3.5. PAR expression
Serial plasma PAR protein levels were assessed as a surrogate for PARPi and correlated with outcomes. All 30 patients had pretreatment and weekly assessable PAR levels. Whereas PAR levels (normalized to pretreatment baseline) decreased for the majority of patients during treatment, there were no statistically significant associations between PAR levels and PFS or OS.
4. Discussion
Here we present a rationally designed first in human trial of V + CRT and confirm veliparib to be safe and well-tolerated when combined with gemcitabine and RT in patients with LAPC. The MTD and recommended phase 2 dose for veliparib was 40 mg twice daily with CRT. Early efficacy signals are seen with a median OS of 15 months, and correlative readouts suggest improved outcomes in DDR-altered patients. The most significant toxicities of V + CRT were cytopenias which resolved with medical management.
A recent phase 1 dose-escalation study by Stoller et al. identified a similar MTD of 20 mg of veliparib twice daily with single agent weekly gemcitabine (750 mg/m2/21-day cycle) in patients with solid tumors [14]. Additionally, O'Reilly et al. identified the MTD of veliparib of 80 mg BID with gemcitabine and cisplatin in advanced PC patients. Notably, objective responses were identified in 78% of BRCA-mutated patients with a median OS of 23 months and grade 4 hematologic toxicities in 40% of patients at the MTD [15]. Historically, gemcitabine and RT in pancreatic cancer or advanced solid tumors has led to acute grade 4–5 toxicities in >40% of patients with the majority being hematologic and GI [1]. Whereas the majority of toxicities identified were hematologic, surprisingly few serious GI toxicities occurred in our patient cohort. The reduced rates of GI toxicities may be a result of using IMRT and motion management strategies where appropriate to minimize expansion margins and volume of normal incidental tissue irradiated [16]. Another explanation may be the reduced, albeit hypofractionated, dose of radiation we used though this does represent a biologically equivalent dose of 45 Gy using an alpha/beta ratio of 10.
Induction chemotherapy has evolved to become first line therapy for all pancreatic patients, as approximately 25% will go on to develop local progression and/or metastases after chemotherapy [1]. The present study was designed before this paradigm shift. However, the mPFS and mOS for LAP-07 which utilized induction chemotherapy prior to randomization to chemoradiotherapy was only 9.9 and 13 months, respectively [2]. In LAP-07, 39% of patients did not reach the second randomization of chemoradiation mostly due to disease progression after chemotherapy and mOS of this group was 7.7 months. Although limited by size, our truly upfront V + CRT trial compares favorably with the chemotherapy followed by chemoradiotherapy LAP-07 approach, and suggests that biologic selection with upfront chemotherapy may offer opportunities for further optimization [1,2]. The MDACC group has reported extensively on the use of lower dose gemcitabine (300–500 mg/m2) concurrent with RT and identified PR in 24% of LAPC and successful pancreaticoduodenectomy in 74% of resectable PC treated with treated with upfront gemcitabine and RT. [17,18] Notably, there is no single acceptable chemotherapy regimen recommended by the NCCN for locally advanced pancreatic cancer (v2.2018), and we allowed investigator choice chemotherapy following completion of the DLT period, and chemotherapy type did not influence outcomes. Similar to published data, ORRs in this study were low [1,2,7]. Radiation dose intensification strategies have improved local control rates yet have resulted in disappointing rates of tumor downstaging largely due to inability to accurately assess the post-RT fibrotic tumor-vessel interaction using the triple-phase CT scan. RECIST also inadequately characterize response of borderline resectable pancreatic tumors to radiotherapy, as exemplified by high rates of post- radiotherapy margin negative resections in spite of minimal radiographic changes identified on CT [12].
Previous studies linking PARP inhibitors to DDR suggest that defects involving HRR may be the most important. However, biomarkers of PARP-i response or those that predict HRR deficiency remain elusive. To date, targeted multiplex sequencing allowing identification of germline and somatic mutations in genes involved in HRR has been used to identify tumors with sensitivity to PARP-inhibition with mixed results [19]. Statistical association of transcriptomic DDR biomarkers was challenging given the number of variables relative to the number of patients treated, yet expression levels of the DDR transcripts PARP3 was associated with OS. PARP3 shares structural and functional characteristics similar to PARP1 and PARP2, and is also inhibited by veliparib [20]. Recent evidence suggests that PARP3 is stimulated by DNA DSBs in vitro and accelerates chromosomal DSB repair through NHEJ [21]. RBX1, encoding the ring-box protein 1, is one of the first nucleotide excision repair factors recruited to sites of DNA damage as part of the Cullin-RING ubiquitin ligase (CRL4) complex (CUL4A–RBX1) [22]. Based on the findings from our study, we hypothesize that patients with increased PARP3 and decreased RBX1 expression are particularly sensitive to PARP inhibition which underlies the improved clinical outcomes we preliminarily observed. However, given the single arm nature of this study, synergy between PARP inhibition, RT and/or gemcitabine may play a significant role and could not be controlled for due to study design [23].
Prior genome-wide expression profiling studies have identified a HRR deficient gene expression signature associated with response to olaparib, rucaparib, and DNA damaging chemotherapies [24]. Prior sensitivity to platinum chemotherapy may also be a clinical independent predictor of response to niraparib in the absence of germline BRCA mutation in ovarian cancer patients [23]. However, a small prospective single arm phase 2 study of heavily pre-treated BRCA1/2 mutated pancreatic cancer patients treated with single agent veliparib showed no responses [25]. These data suggest that expression profiles identifying operational HR deficiency without specific genetic or epigenetic aberrations such as BRCA mutation may have more utility as indicators of potential response to PARP inhibition [23,24]. As PARP3 and RBX1 participate in NHEJ and NER respectively, we hypothesize that PARPi may function best when multiple pathways regulating DNA integrity are compromised, an observation seen in other studies. Fleury et al. also found that alterations of genes in the NER and MMR pathways in high grade serous ovarian cancer cell lines increased sensitivity to PARP inhibitors with the greatest response identified when more than one pathway was concomitantly down regulated supporting the notion that functional deficiency of DDR genes may predict for response to PARP inhibitors [26].
Patients with DDR mutations identified by NGS did not have improved clinical outcomes compared to patients without such alterations in spite of the relatively high frequency of such mutations (34%) compared to prior germline and/or somatic series (4–10%) [27]. Deleterious mutations in DDR genes such as BRCA1/2, p53 and ATM among others have been known to result in a higher frequency of deleterious changes within the genomic structure and genomic instability as a consequence of impaired DDR leading to increased TMB [28]. Only a single MSI-H patient was identified who also harbored a CHEK2 and MLH1 gene mutation, though the germline status is unknown. This was also the only patient with high TMB highlighting the association of DDR repair with genomic instability in pancreatic cancer [29]. The low observed TMB suggests the mutations observed in DDR pathways may not cause sufficient genomic instability thereby limiting the impact of these mutations on clinical outcomes.
Inhibition of PARP is expected to lead to decreased levels of systemic PAR. Patients in this study showed low baseline PAR levels. Further, although a trend towards decreasing PAR levels was seen during veliparib administration, there were no associations with clinical outcomes. Despite the rationale, measurement of PAR protein levels has not been validated as a potential biomarker of response to PARPi and our results are highly preliminary. The lack of association is likely because PAR levels measured in PBMCs are low at baseline and have high temporal variability especially when quantitated using ELISA [30]. PAR levels may only be predictive when higher baseline levels exist as in a recent trial of veliparib and carboplatin for germline BRCA1/2 mutated breast cancer in which higher baseline PAR levels were associated with clinical benefit [30].
5. Conclusion
The combination of V and CRTwas well tolerated in patients with LAPC. Alterations in expression of the DDR proteins PARP3 was associated with improved OS, however mutations in the DDR pathways, though prevalent, did not correlate with OS. The overall clinical outcomes for this trial were favorable compared to contemporary studies utilizing the chemotherapy-first approach suggesting validation in a larger follow up phase 2 study combining the two strategies may be warranted. We note that the inference regarding the association of PARP3 with OS is limited due to the small sample size and lack of adjustments of important baseline risk factors such as CA-19-9, tumor size, and dose of Veliparib.
Disclosure
RT has served as an advisor for Astra Zeneca. SJK has served as a consultant/advisor for Lilly Oncology, Astellas, and Boston Biomedical, and is on the speaker bureau for Foundation Medicine, Inc. All remaining authors have declared no conflicts of interest.
Acknowledgements of research support
Veliparib was provided by Abbvie. This work was supported by the Phase One Foundation; the National Institutes of Health [1R01CA188480-01A1 to M.T. and S.K., and P01 CA098912 to M.T.]; the National Center for Research Resources [UL1RR033176 and is now at the National Center for Advancing Translational Sciences UL1TR000124 to M.T. and A.R.].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.060.
Appendix A. Supplementary data
Supplementary material
References
- 1.Loehrer P.J., Sr., Feng Y., Cardenes H. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammel P., Huguet F., van Laethem J.L. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 3.de Murcia J.M., Niedergang C., Trucco C. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A. 1997;94(14):7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman B., Shapira-Frommer R., Schmutzler R.K. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuli R., Surmak A.J., Reyes J. Radiosensitization of pancreatic cancer cells in vitro and in vivo through poly (ADP-ribose) polymerase inhibition with ABT-888. Transl Oncol. 2014;7(3):439–445. doi: 10.1016/j.tranon.2014.04.003. PMID: 24836647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGinn C.J., Zalupski M.M., Shureiqi I. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19(22):4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 7.Tighiouart M., Liu Y., Rogatko A. Escalation with overdose control using time to toxicity for cancer phase I clinical trials. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0093070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 9.KJaP R.L. John Wiley & Sons, Inc; New York: 1980. The statistical analysis of failure time data. Wiley series in probability and mathematical statistics. [Google Scholar]
- 10.DR C. Regression models and life-tables. J R Stat Soc. 1972;34(2):187–220. [Google Scholar]
- 11.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 12.Katz M.H., Fleming J.B., Bhosale P. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118(23):5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 13.Frampton G.M., Fichtenholtz A., Otto G.A. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoller R., Schmitz J.C., Ding F. Phase I study of veliparib in combination with gemcitabine. Cancer Chemother Pharmacol. 2017;80(3):631–643. doi: 10.1007/s00280-017-3409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly E.M., Lee J.W., Lowery M.A. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer. 2018;124(7):1374–1382. doi: 10.1002/cncr.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy J.D., Adusumilli S., Griffith K.A. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 17.Evans D.B., Varadhachary G.R., Crane C.H. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 18.Wolff R.A., Evans D.B., Gravel D.M. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res. 2001;7(8):2246–2253. [PubMed] [Google Scholar]
- 19.Gelmon K.A., Tischkowitz M., Mackay H. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 20.Rouleau M., Patel A., Hendzel M.J., Kaufmann S.H., Poirier G.G. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rulten S.L., Fisher A.E., Robert I. PARP-3 and APLF function together to accelerate nonhomologous end-joining. Mol Cell. 2011;41(1):33–45. doi: 10.1016/j.molcel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Pines A., Vrouwe M.G., Marteijn J.A. PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J Cell Biol. 2012;199(2):235–249. doi: 10.1083/jcb.201112132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza M.R., Monk B.J., Herrstedt J. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 24.Peng G., Chun-Jen Lin C., Mo W. Genome-wide transcriptome profiling of homologous recombination DNA repair. Nat Commun. 2014;5:3361. doi: 10.1038/ncomms4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowery M.A., Kelsen D.P., Capanu M. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleury H., Carmona E., Morin V.G. Cumulative defects in DNA repair pathways drive the PARP inhibitor response in high-grade serous epithelial ovarian cancer cell lines. Oncotarget. 2017;8(25):40152–40168. doi: 10.18632/oncotarget.10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant R.C., Selander I., Connor A.A. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148(3):556–564. doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 29.Gudmundsdottir K., Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25(43):5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- 30.de Haan R., Pluim D., van Triest B. Improved pharmacodynamic (PD) assessment of low dose PARP inhibitor PD activity for radiotherapy and chemotherapy combination trials. Radiother Oncol. 2018;126(3):443–449. doi: 10.1016/j.radonc.2017.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material