Abstract
Background
Dental rehabilitation surgery is associated with significant fear and anxiety with subsequent psychological disturbances. Midazolam has been used frequently as a premedication. However, it may be associated with side effects. Dexmedetomidine and ketamine combination has been suggested as an effective premedication in improving preoperative sedation and analgesia.
Objectives
This study compared the effects of combined intranasal dexmedetomidine and oral ketamine versus intranasal midazolam on anxiolysis and postoperative analgesia.
Methods
Seventy-six children (aged two to six years) undergoing dental rehabilitation under general anesthesia were assigned randomly to one of the two groups (n = 38 each) receiving either intranasal dexmedetomidine at 2 µg/kg and oral ketamine at 3 mg/kg (Group DK) or intranasal midazolam at 0.2 mg/kg (group M) 30 minutes prior to the anesthesia induction. The sedation levels and parental separation state were evaluated. Time to recovery, postoperative rescue analgesia, and postoperative adverse effects were assessed.
Results
Seventy-six children completed the study. Patients in group DK had significantly lower sedation scores than those in group M after 20 and 30 min (P < 0.05). The rate of satisfactory separation showed no statistically significant difference between the two groups 30 minutes after the administration of premedication (P = 0.926). A significantly higher number of patients in group M required rescue analgesic (42%) compared to those in group DK (16%) (P = 0.012).
Conclusions
Premedication with intranasal dexmedetomidine 2 µg/kg and oral ketamine 3 mg/kg is a rapid and effective alternative in children undergoing dental rehabilitation when compared to intranasal midazolam 0.2 mg/kg.
Keywords: Premedication, Dexmedetomidine, Ketamine, Midazolam.
1. Background
Children undergoing dental rehabilitation usually experience fear and anxiety from the anesthesia and surgery, particularly at the time of parental separation and during anesthesia induction (1). The anxiety leads to the stimulation of sympathetic and parasympathetic systems resulting in changes in HR and BP, as well as psychological disturbances and behavioral changes (2, 3).
Sedative premedication reduces anxiety and facilitates anesthesia induction (4). Although many studies examined the effects of different premedication drugs including midazolam, dexmedetomidine, clonidine, and ketamine, until now, there is no widely accepted drug of choice. The ideal premedication drug should have an easy and effective route of administration with no or little adverse reactions. Moreover, it should have a rapid onset of action with a little effect on cardiovascular stability (5).
Dexmedetomidine is a highly selective α2-adrenergic agonist. Its sedative effect is dose-dependent and characterized by being easily arousable (2). It also has an analgesic and anxiolytic effect without causing respiratory depression. The intranasal administration of dexmedetomidine is an effective and well-tolerated choice for children premedication (6).
Ketamine is an N-methyl-d-aspartate receptor antagonist exerting a desirable sedative and analgesic effect (7). When administered orally, as a sole premedication, it produces adverse effects such as salivation and anxiety (8). The combination of dexmedetomidine and ketamine results in an attenuation of the undesirable cardiovascular effects and reduces the incidence of the postoperative delirium produced by ketamine (9).
Midazolam is a γ-aminobutyric acid (GABA) receptor inhibitor. It is used frequently as premedication in pediatrics due to its sedative, anxiolytic, and amnesic effect. It is the most frequently used premedication in pediatrics (10-12). Undesirably, its side effects include paradoxical reactions, restlessness, and behavioral changes (13-15).
2. Objectives
The aim of the current study was to compare the effect of the administration of combined dexmedetomidine and ketamine versus midazolam as sedative premedication when administered 30 min before general anesthesia in children undergoing dental rehabilitation procedures.
3. Methods
A double-blind, prospective, randomized study, involving 76 children aged two to six years, was performed at Magrabi Center in Doha, Qatar. Written informed consent was obtained from the children’s parents after being informed about the objective and the procedure of our study. In addition, the study was approved by the Medical Ethics Committee. The patients were ASA I or II scheduled to undergo dental rehabilitation under general anesthesia. The exclusion criteria included a known allergy to dexmedetomidine, ketamine or midazolam, prematurity, delayed neurological development, and parental refusal.
Children were randomly allocated to one of the two groups (38 patients each) to receive either intranasal dexmedetomidine at 2 µg/kg and oral ketamine at 3 mg/kg (group DK) or intranasal midazolam at 0.2 mg/kg (group M). Medications in both groups were given 30 minutes before the anesthesia induction. Intranasal dexmedetomidine (Precedex, Hospira, IL, USA) was prepared from 100 µg/mL parenteral preparation in a 1-mL syringe that reached a final volume of 0.5 mL with adding 0.9% saline. Oral ketamine syrup was prepared by adding 5% glucose to racemic ketamine in a 1:2 ratio while intranasal midazolam (Dormicum, Roche Products Ltd, UK) was prepared from a 5 mg/mL parenteral preparation by adding 0.9% saline to reach a total volume of 0.5 mL in a 1-mL syringe. Children were in the recumbent position when we dripped the intranasal drug equally in both nostrils.
Heart rate (HR), mean arterial pressure (MAP), and pulse oxygen saturation (SpO2) were recorded before giving the premedication and every 15 min until patient transfer to the operating room. A five-point sedation scale (SS-5) was used to assess the sedation level 10 min, 20 min, and 30 min following premedication administration (Table 1). A four-point emotional state scale (ESS-4) (Table 1) was used to assess the parental separation state of children 30 min after premedication. A score of two or less was considered a satisfactory result. The onset time of sedation, which was the time between the premedication administration and achieving an SS-5 score of three, was also recorded.
Table 1. Sedation Score and Emotional State Score.
| Score | Detail |
|---|---|
| Sedation score | |
| 1 | Rarely awake, needs shaking or shouting to wake up |
| 2 | Asleep, eyes closed, wakes up when called softly or lightly touched |
| 3 | Sleepy but eyes open spontaneously |
| 4 | Awake |
| 5 | Agitated |
| Emotional state score | |
| 1 | Calm |
| 2 | Apprehensive, not smiling, tentative behavior, withdrawn |
| 3 | Crying |
| 4 | Thrashing, crying with the movement of arm and leg or resisting |
GA was induced in all children by inhalation of 8% sevoflurane in oxygen via a facemask. Endotracheal intubation was facilitated by giving IV fentanyl 2 µg/kg and IV cisatracurium 0.15 mg/kg via an inserted intravenous line after gas induction. IV ketorolac 0.5 mg/kg was given for intraoperative analgesia. Anesthesia was then maintained with 2% sevoflurane in a mixture of 50% N2O and 50% O2 to keep the end-tidal carbon dioxide pressure (PaCo2) between 30 and 35 mmHg. When the surgery was finished, sevoflurane discontinued, and residual neuromuscular blockade was antagonized with IV atropine 0.02 mg/kg and IV neostigmine 0.05 mg/kg. All patients were transferred to PACU after tracheal extubation. The total duration of anesthesia was recorded.
In the PACU, the postoperative adverse effects were recorded, which included postoperative nausea and vomiting (PONV), shivering, bradycardia, hypotension, and oxygen desaturation. Rescue analgesic in the form of IV paracetamol 15 mg/kg was given according to the facial expression and crying. The number of children required rescue analgesic was also recorded.
Patients were transferred from the PACU when the Aldrete score reached nine. The time needed to meet the eligibility criteria for discharge from the PACU was recorded.
3.1. Statistical Analysis
A total sample size of 78 patients (36 patients in each group) was needed to achieve a power of 80% to detect a significant difference (P < 0.05).
The statistical software used for data analysis was IBM SPSS Statistics version 25 for Windows. The numerical data were presented as mean and standard deviation (SD). The analysis was done using the student’s t-test. The Mann-Whitney U test was used to compare variables with non-normal distribution while the chi-square test was used for comparison of qualitative data. A statistical significance was considered when the P value was less than 0.05 in any test.
4. Results
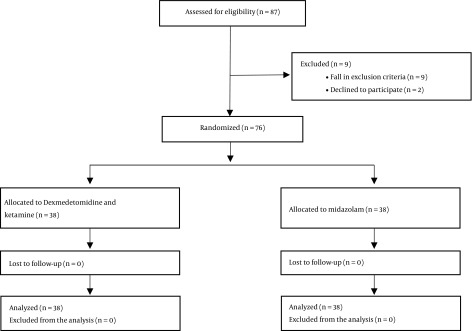
Of 87 patients enrolled in the study, nine patients were excluded as they fall in the exclusion criteria while two parents declined to participate in the study. The remaining 76 patients completed the study and they were divided into two equal groups (38 each) (Figure 1). The patients in the two groups were comparable concerning age, body weight, sex, ASA class, anesthesia duration, and surgery duration as the time passed between the start and end of the procedure (Table 2).
Figure 1. Flow chart of the study.
Table 2. Patients Characteristicsa.
| Group DK, n = 38 | Group M, n = 38 | P Value | |
|---|---|---|---|
| Age, y | 4.3 ± 1.1 | 3.9 ± 1.0 | 0.132 |
| Weight, kg | 17.7 ± 3.8 | 16.8 ± 4.1 | 0.309 |
| Sex (M/F) | 21/17 | 16/22 | 0.359 |
| ASA classification I/II | 33/5 | 30/8 | 0.544 |
| Anesthesia duration, min | 117.2 ± 19.0 | 122.4 ± 19.7 | 0.249 |
| Surgery duration, min | 100.1 ± 20.0 | 106.3 ± 17.9 | 0.139 |
aValues are expressed as mean ± SD or number of children.
Premedication with intranasal dexmedetomidine and oral ketamine significantly decreased the sedation score at 20 and 30 min compared to premedication with intranasal midazolam (P < 0.05). The median sedation scores after 20 min were 2 and 3 for groups DK and M, respectively. Moreover, the median sedation scores after 30 min of premedication were 1 and 2 for groups DK and M, respectively (P < 0.05) (Table 3). No statistically significant difference was observed in the rate of satisfactory separation between the groups 30 minutes after premedication administration (P > 0.05). The onset time of sedation showed a significant decrease in group DK compared to group M (P < 0.05) (Table 3).
Table 3. Onset Time of Sedation, Sedation Scores, and Emotional State Scorea.
| Group DK, n = 38 | Group M, n = 38 | P Value | |
|---|---|---|---|
| Onset time of sedation, min | 16.5 ± 3.8 | 19.6 ± 4.9b | 0.003 |
| Sedation score, min | |||
| 10 | 4 (1) | 4 (0.3) | 0.106 |
| 20 | 2 (0) | 3 (0.3)b | < 0.05 |
| 30 | 1 (0) | 2 (1)b | < 0.05 |
| Emotional state score 30 min | 1.5 (1) | 1 (1) | 0.926 |
aValues are expressed as mean ± SD or median (IQR).
bSignificant differences (P < 0.05).
Both groups displayed similar values in HR, MAP, and SpO2 at the baseline and every 10 min until the time they were transferred to the operating room (P > 0.05). No patient in both groups developed bradycardia, hypotension, or desaturation.
In the PACU, the time needed to reach an Aldrete score of nine showed no statistically significant difference between the two groups (P > 0.05) (Table 4).
Table 4. Recovery Time, Rescue Analgesic, and Adverse Reactionsa.
| Group DK, n = 38 | Group M, n = 38 | P Value | |
|---|---|---|---|
| Time to reach Aldrete score of 9, min | 24.2 ± 4.4 | 23.4 ± 4.7 | 0.498 |
| Rescue analgesic | 6 (15.8) | 16 (42.1)b | 0.012 |
| PONV | 6 (15.8) | 5 (13.2) | 0.290 |
| Shivering | 3 (7.9) | 10 (26.3)b | 0.034 |
aValues are expressed as mean ± SD or No. (%).
bSignificant differences (P < 0.05).
Six children (15.8%) who were premedicated with intranasal dexmedetomidine and ketamine required paracetamol as rescue analgesic compared to 16 children (42.1%) who received intranasal midazolam (P < 0.05) (Table 4).
Comparison of the incidence of shivering between the groups showed a significantly higher number of children in the midazolam group than in the dexmedetomidine and ketamine group during the postoperative period (P < 0.05). On the other hand, PONV showed no statistically significant difference between the groups (P > 0.05) (Table 4).
5. Discussion
The results of our randomized, double-blind study revealed that premedication using a combination of intranasal dexmedetomidine and oral ketamine results in faster satisfactory preoperative sedation and a more potent immediate postoperative analgesia when compared to intranasal midazolam. Moreover, the former combination results in less postoperative shivering.
Various drugs and routes of administration have been tried with the aim of finding an ideal premedication drug in children. Selecting the appropriate premedication depends on its safety, rapid onset, effectiveness in reducing anxiety, and facilitating a smoother induction of anesthesia (16). Comparison of oral and nasal routes of administration of premedication in previous studies showed that the intranasal route was relatively accepted by children and was associated with a higher bioavailability compared to the oral route of administration (17). The most common premedication drugs used currently are dexmedetomidine, ketamine, and midazolam.
Midazolam has been used for a long time as a premedication in pediatrics via many routes of administration. While the intranasal route is considered a rapid noninvasive method and provides favorable pharmacokinetics, the main cons are causing nasal irritation during administration and producing negative behavioral changes in the postoperative period (18, 19).
Dexmedetomidine is a selective α2 adrenergic agonist that produces sedation, analgesia, and anxiolysis without causing respiratory depression. Its main action on the locus coeruleus in the central nervous system is to induce EEG activity like that seen during natural sleep (20).
Ketamine is commonly used as oral premedication in pediatrics owing to its sedative and analgesic properties (16). Nevertheless, its undesirable postoperative side effects, like salivation, nausea, vomiting, and psychiatric complications, have restricted its use as a single premedication choice.
When used together, dexmedetomidine decreases the side effects of ketamine such as cardiovascular changes and psychiatric disturbances (21). Jia et al. combined intranasal dexmedetomidine with oral ketamine as premedication in children. They concluded that combining intranasal dexmedetomidine 2 µg/kg and oral ketamine 3 mg/kg results in an easier separation process with less adverse reactions or postoperative complications (21). Furthermore, it was previously reported that combining nebulized ketamine and dexmedetomidine could result in better sedation, rapid recovery, and fewer adverse reactions when compared with nebulized ketamine or dexmedetomidine alone (22).
On the other hand, Behrle et al. (23) noted that the use of intranasal dexmedetomidine for pediatrics undergoing non-invasive procedures was associated with longer recovery time when compared with non-dexmedetomidine sedation.
Our study showed that dexmedetomidine plus ketamine was associated with a more rapid onset (16.5 minutes) of sedation compared with midazolam (19.6 minutes). Other studies have shown that adding oral ketamine to intranasal dexmedetomidine will shorten the time needed to achieve desirable sedation when compared with intranasal dexmedetomidine alone from 30 - 45 min to 25 - 30 min (21).
In our study, children who received a combination of ketamine and dexmedetomidine were significantly more sedated after 20 and 30 minutes of drug administration compared to those who received intranasal midazolam, while the emotional state scale showed no significant difference upon separation from parents.
Faritus et al. (24) studied the effect of oral midazolam in comparison with dexmedetomidine as premedication in pediatric patients undergoing congenital heart surgery. They reported that dexmedetomidine has better mask acceptance than midazolam while both drugs have similar effects on sedation scores. Another study by Sheta et al. (25) compared the effect of intranasally administered dexmedetomidine 1 µg/kg versus midazolam 0.2 mg/kg as premedication before dental rehabilitation and concluded that dexmedetomidine has better sedation compared to midazolam when the children were separated from their parents and during anesthesia induction.
In this study, we showed that the percentage of children who required rescue analgesia was significantly different between the DK group and the M group. Only 15.8% of the DK group needed rescue analgesia in the postoperative period in comparison with 42.1% in the M group. This immediate postoperative analgesic effect is produced by the complementary pharmacological characteristics that result from combining ketamine with dexmedetomidine.
In Akin et al. (26) study, patients who were premedicated with a similar dose of intranasal midazolam as in our study (0.2 mg/kg) showed weak evidence to require more analgesia postoperatively compared to those who received intranasal dexmedetomidine 1 µg/kg.
A recent study by Imani et al. (27) used dexmedetomidine in addition to non-opioid analgesics for the control of post-cesarean pain. They found that the addition of dexmedetomidine to lower doses of paracetamol and ketorolac resulted in adequate analgesia.
We found that the incidence of postoperative shivering was higher in the midazolam group (26%) than in the dexmedetomidine and ketamine group (8%). Our findings are very similar to the findings of Sheta et al. (25) who reported that intranasal dexmedetomidine as premedication resulted in less postoperative shivering when compared with intranasal midazolam. Blaine Easley et al. (28) also found that using dexmedetomidine as a premedication was effective in the prevention of postoperative shivering.
The first limitation of this study is the lack of a control group. Second, we did not evaluate the nasal irritation produced by intranasal midazolam and the undesirable side effects resulting from oral ketamine, like increased airway secretions and psychiatric complications. Third, rescue analgesic was given according to crying and facial expression but we did not measure any pain score.
In conclusion, the combination of 2 µg/kg intranasal dexmedetomidine and 3 mg/kg oral ketamine produces a more satisfactory and rapid onset of sedation. This combination potentiates the postoperative analgesia and produces less postoperative shivering in comparison with 0.2 mg/kg intranasal midazolam when used as premedication in children undergoing dental rehabilitation.
Footnotes
Conflict of Interests: The author declares that there is no conflict of interests.
Ethical Considerations: This study was approved by the Medical Ethics Committee.
Funding/Support: This study was carried out without external funding.
Patient Consent: Written informed consent was obtained from the children’s parents after being informed about the objective and the procedure of our study.
References
- 1.Creedon LR, Dock M. Pharmacological management of patient behavior. In: McDonald RE, Avery DR, editors. Dentistry for the children and adolescent. 8th ed. China: Louis: Mosby CV; 2004. pp. 285–311. [Google Scholar]
- 2.Davidson A, McKenzie I. Distress at induction: Prevention and consequences. Curr Opin Anaesthesiol. 2011;24(3):301–6. doi: 10.1097/ACO.0b013e3283466b27. [DOI] [PubMed] [Google Scholar]
- 3.Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99(6):1648–54. doi: 10.1213/01.ANE.0000136471.36680.97. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Calipel S, Lucas-Polomeni MM, Wodey E, Ecoffey C. Premedication in children: Hypnosis versus midazolam. Paediatr Anaesth. 2005;15(4):275–81. doi: 10.1111/j.1460-9592.2004.01514.x. [DOI] [PubMed] [Google Scholar]
- 5.Kain ZN, Hofstadter MB, Mayes LC, Krivutza DM, Alexander G, Wang SM, et al. Midazolam: Effects on amnesia and anxiety in children. Anesthesiology. 2000;93(3):676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LH. A double-blind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg. 2007;105(2):374–80. doi: 10.1213/01.ane.0000269488.06546.7c. [DOI] [PubMed] [Google Scholar]
- 7.Cortinas M, Oya B, Caparros P, Cano G, Ibarra M, Martinez L. [Oral ketamine-midazolam premedication of uncooperative patients in major outpatient surgery.]. Rev Esp Anestesiol Reanim. 2010;57(8):479–85. doi: 10.1016/S0034-9356(10)70708-8. [DOI] [PubMed] [Google Scholar]
- 8.Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari TI. Ketamine: A review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. 2016;55(9):1059–77. doi: 10.1007/s40262-016-0383-6. [DOI] [PubMed] [Google Scholar]
- 9.Levanen J, Makela ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82(5):1117–25. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Premedication in children: A comparison of oral midazolam and oral clonidine. Paediatr Anaesth. 2007;17(12):1143–9. doi: 10.1111/j.1460-9592.2007.02332.x. [DOI] [PubMed] [Google Scholar]
- 11.Feng JF, Wang XX, Lu YY, Pang DG, Peng W, Mo JL. Effects of dexmedetomidine versus midazolam for premedication in paediatric anaesthesia with sevoflurane: A meta-analysis. J Int Med Res. 2017;45(3):912–23. doi: 10.1177/0300060517704595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jannu V, Mane RS, Dhorigol MG, Sanikop CS. A comparison of oral midazolam and oral dexmedetomidine as premedication in pediatric anesthesia. Saudi J Anaesth. 2016;10(4):390–4. doi: 10.4103/1658-354X.177333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talon MD, Woodson LC, Sherwood ER, Aarsland A, McRae L, Benham T. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res. 2009;30(4):599–605. doi: 10.1097/BCR.0b013e3181abff90. [DOI] [PubMed] [Google Scholar]
- 14.Lonnqvist PA, Habre W. Midazolam as premedication: Is the emperor naked or just half-dressed? Paediatr Anaesth. 2005;15(4):263–5. doi: 10.1111/j.1460-9592.2005.01600.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanegaye JT, Favela JL, Acosta M, Bank DE. High-dose rectal midazolam for pediatric procedures: A randomized trial of sedative efficacy and agitation. Pediatr Emerg Care. 2003;19(5):329–36. doi: 10.1097/01.pec.0000092578.40174.85. [DOI] [PubMed] [Google Scholar]
- 16.Qiao H, Xie Z, Jia J. Pediatric premedication: A double-blind randomized trial of dexmedetomidine or ketamine alone versus a combination of dexmedetomidine and ketamine. BMC Anesthesiol. 2017;17(1):158. doi: 10.1186/s12871-017-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: A double-blinded randomized controlled trial. Anesth Analg. 2008;106(6):1715–21. doi: 10.1213/ane.0b013e31816c8929. [DOI] [PubMed] [Google Scholar]
- 18.Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine: An alternative to benzodiazepines for premedication in children. Curr Opin Anaesthesiol. 2005;18(6):608–13. doi: 10.1097/01.aco.0000191891.44314.36. [DOI] [PubMed] [Google Scholar]
- 19.Bergendahl H, Lonnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: Review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50(2):135–43. doi: 10.1111/j.1399-6576.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 20.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98(2):428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Jia JE, Chen JY, Hu X, Li WX. A randomised study of intranasal dexmedetomidine and oral ketamine for premedication in children. Anaesthesia. 2013;68(9):944–9. doi: 10.1111/anae.12312. [DOI] [PubMed] [Google Scholar]
- 22.Zanaty OM, El Metainy SA. A comparative evaluation of nebulized dexmedetomidine, nebulized ketamine, and their combination as premedication for outpatient pediatric dental surgery. Anesth Analg. 2015;121(1):167–71. doi: 10.1213/ANE.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 23.Behrle N, Birisci E, Anderson J, Schroeder S, Dalabih A. Intranasal dexmedetomidine as a sedative for pediatric procedural sedation. J Pediatr Pharmacol Ther. 2017;22(1):4–8. doi: 10.5863/1551-6776-22.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faritus SZ, Khazaee-Koohpar M, Ziyaeifard M, Mehrabanian MJ. Oral dexmedetomidine versus midazolam as anesthetic premedication in children undergoing congenital heart surgery. Anesth Pain Med. 2015;5(3):e25032. doi: 10.5812/aapm.5(3)2015.25032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: A double-blinded randomized controlled trial. Paediatr Anaesth. 2014;24(2):181–9. doi: 10.1111/pan.12287. [DOI] [PubMed] [Google Scholar]
- 26.Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth. 2012;22(9):871–6. doi: 10.1111/j.1460-9592.2012.03802.x. [DOI] [PubMed] [Google Scholar]
- 27.Imani F, Rahimzadeh P, Faiz HR, Nowruzina S, Shakeri A, Ghahremani M. Comparison of the post-caesarean analgesic effect of adding dexmedetomidine to paracetamol and ketorolac: A randomized clinical trial. Anesth Pain Med. 2018;8(5):e85311. doi: 10.5812/aapm.85311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaine Easley R, Brady KM, Tobias JD. Dexmedetomidine for the treatment of postanesthesia shivering in children. Paediatr Anaesth. 2007;17(4):341–6. doi: 10.1111/j.1460-9592.2006.02100.x. [DOI] [PubMed] [Google Scholar]