Abstract
During standard expansion culture (i.e., plasma osmolarity, 280 mOsm) human articular chondrocytes dedifferentiate, making them inappropriate for autologous chondrocyte implantation to treat cartilage defects. Increasing the osmolarity of culture media to physiological osmolarity levels of cartilage (i.e., 380 mOsm), increases collagen type II (COL2A1) expression of human articular chondrocytes in vitro, but the underlying molecular mechanism is not fully understood. We hypothesized that TGF-β superfamily signaling may drive expression of COL2A1 under physiological osmolarity culture conditions. Human articular chondrocytes were cultured in cytokine-free medium of 280 or 380 mOsm with or without siRNA mediated TGF-β2 knockdown (RNAi). Expression of TGF-β isoforms, and collagen type II was evaluated by RT-qPCR and immunoblotting. TGF-β2 protein secretion was evaluated using ELISA and TGF-β bioactivity was determined using an established reporter assay. Involvement of BMP signaling was investigated by culturing human articular chondrocytes in the presence or absence of BMP inhibitor dorsomorphin and BMP bioactivity was determined using an established reporter assay. Physiological cartilage osmolarity (i.e., physosmolarity) most prominently increased TGF-β2 mRNA expression and protein secretion as well as TGF-β bioactivity. Upon TGF-β2 isoform-specific knockdown, gene expression of chondrocyte marker COL2A1 was induced. TGF-β2 RNAi under physosmolarity enhanced TGF-β bioactivity. BMP bioactivity increased upon physosmotic treatment, but was not related to TGF-β2 RNAi. In contrast, dorsomorphin inhibited COL2A1 mRNA expression in human articular chondrocytes independent of the osmotic condition. Our data suggest a role for TGF-β superfamily member signaling in physosmolarity-induced mRNA expression of collagen type II. As physosmotic conditions favor the expression of COL2A1 independent of our manipulations, contribution of other metabolic, post-transcriptional or epigenetic factors cannot be excluded in the underlying complex and interdependent regulation of marker gene expression. Dissecting these molecular mechanisms holds potential to further improve future cell-based chondral repair strategies.
Keywords: chondrocyte, osmolarity, TGF-β superfamily, signalling, collagen type II, bone morphogenetic proteins
1. Introduction:
Articular cartilage defects do not heal spontaneously and recent data suggest that treatment of these cartilage defects by microfracture (MF) procedures are inferior to autologous chondrocyte implantation (ACI) [1,2] due to biomechanically inferior repair tissue [3]. Despite ACI being superior to microfracture, it is not always characterized by hyaline cartilage repair tissue [4]. Further improvements of currently available chondrocyte-based treatment approaches such as the ACI are thus needed.
Chondrocytes are the main cell type found in cartilage (i.e., about 1–5% v/v) and responsible for maintaining the cartilage extracellular matrix (ECM) [5]. ACI involves in vitro expansion of human articular chondrocytes (HACs) prior to re-implantation into the cartilage defect. During in vitro expansion chondrocytes inevitably lose their specific phenotype and de-differentiate with increasing passages making them gradually less suitable for autologous cell therapies [6]. Dedifferentiation of HACs is characterized by reduced expression of aggrecan (ACAN) and collagen type II (COL2A1) mRNA, probably being the most important cartilage-specific markers, and accompanied by an increased fibroblast-like phenotype [6].
Cartilage requires a high osmotic value of its interstitial fluid to maintain its hydrostatic pressure and viscoelastic properties, for which negatively charged glycosaminoglycan side chains of the proteoglycans (PGs) are crucial to attract mobile cations and water into the ECM environment. The intact collagen network, in contrast, restricts the inherent swelling force of the ECM and determines the relatively high osmolarity of this extracellular fluid [5]. The latter ranges between 380 and 450 mOsm in healthy cartilage (i.e., physiological osmolarity) [5], which is markedly higher than that of plasma levels or standard culture medium (around 280 mOsm) [7]. In analogy to using “physoxia” to describe physiological oxygenation levels in cartilage, avoiding the term hypoxia [8], we would like to introduce physiological osmolarity as “physosmotic”. We and others have already shown that cultured chondrocytes are osmo-responsive and improve ECM synthesis accordingly [5,9,10]. However, the underlying molecular mechanism is poorly understood.
Transforming growth factor (TGF-β) plays an indispensable role in cartilage repair and homeostasis [11,12]. This pleiotropic growth factor is the prototypic member of the TGF-β superfamily, which further includes, among others, the large subfamily of bone morphogenetic proteins (BMPs) [13]. Three mammalian isoforms of TGF-β have been described, with non-redundant functions in vivo [14,15]. In chondrocytes, TGF-β has been implicated in the transcription of collagen type II [16]. Canonically, TGF-β ligands bind to the TGF-β type II receptor (TGFBRII) which, in most cells, then recruits the type I receptor activin-like kinase 5 (ALK5) to phosphorylate intracellular effector molecules SMAD-2 and -3 to regulate transcriptional activity [17]. It was shown that in chondrocytes TGF-β can also signal through an alternative receptor, ALK1 [18] to phosphorylate SMAD1/5/8, rather than SMAD2/3. The ALK5/ALK1 ratio now appears to be responsible for the seemingly contradictory actions of TGF-β in chondrocytes [19]. Context-dependent ambivalent actions of TGF-β on articular chondrocytes have been reviewed [12], underscoring its key role in chondrocyte ECM synthesis and homeostasis.
Since TGF-β is known to induce COL2A1 expression in chondrocytes [18], it is tempting to speculate that increased COL2A1 expression in in vitro HAC cultures upon physosmotic treatment, may be caused by stimulated TGF-β signaling. Currently, however, little is known about how osmolarity may induce endogenous TGF-β signalling, which holds especially for chondrocytes. Dissecting molecular mechanisms underlying physosmotic induction of chondrocyte markers might aid in further improving cell-based chondral repair strategies, as well as improving HAC culture conditions for research purposes [20].
In the present study, we therefore aim to elucidate whether changes in TGF-β signalling underlie the cartilage physosmolar induction of chondrocyte marker gene expression in in vitro HAC cultures.
2. Results
2.1. Cartilage Physosmotic Culture Induces Specific TGF-β Isoform Expression in HACs
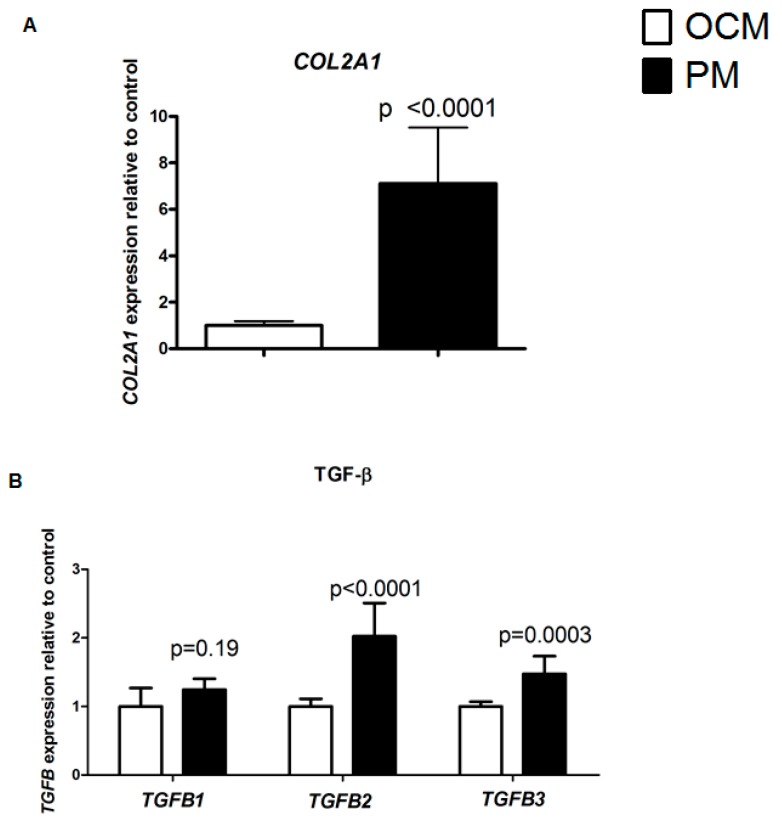
Confirming our earlier results [10], culturing HACs for seven days in physosmotic conditions (380 mOsm, physosmotic medium, PM) results in significantly (p ≤ 0.0001) elevated COL2A1 mRNA expression, as compared to 280 mOsm (i.e., osmotic control medium, OCM) (Figure 1A). To determine whether this increased COL2A1 expression upon physosmotic treatment may be caused by stimulated specific TGF-β isoform induced signalling, mRNA expression of the three human TGF-β isoforms (TGFB1-3) was measured. PM selectively induced expression of TGFB2 (two-fold, p < 0.0001)) and TGFB3 (1.5-fold, p < 0.0001), respectively. In contrast, expression of TGFB1 was not significantly altered by changes in medium osmolarity (Figure 1B).
Figure 1.
Physosmolarity-induced changes in gene expression of chondrocytes in vitro. Isolating and expanding HACs at PM for 7 days (black bars) significantly increased gene expression of COL2A1 (A), as well as TGFB2 and TGFB3 as compared to control (OCM; white bars) (B). Gene expression of TGFB1 was not significantly affected (B). mRNA levels were determined relatively to control OCM conditions by RT-qPCR (normalized for housekeeper expression) in HACs. Data are from six donors measured in duplicate and presented as the mean ± standard deviation.
2.2. Physosmolarity Increases Secretion of Bioactive TGF-β2
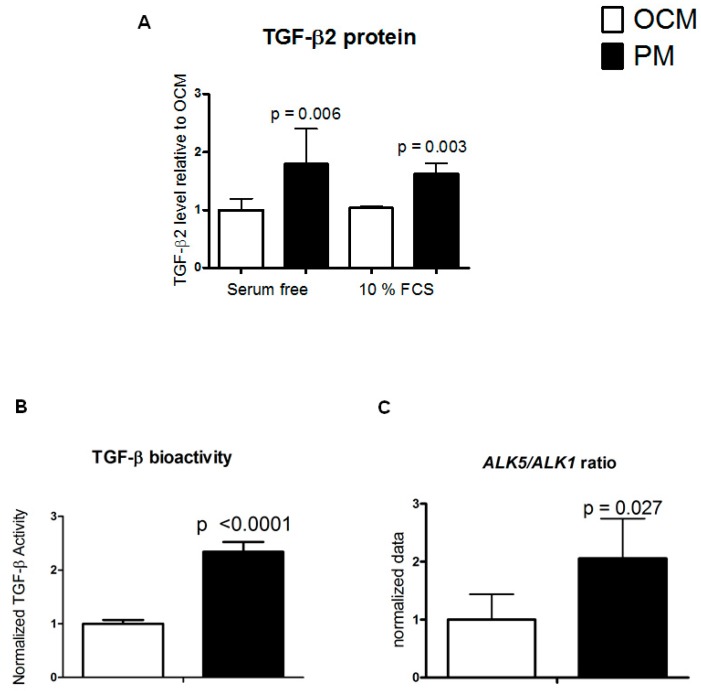
As PM most prominently increased TGFB2 mRNA abundance, we next aimed at confirming if this resulted in increased TGF-β2 protein level, using a TGF-β2 isoform-specific ELISA assay. HACs were cultured in either serum free (SF) medium or with 10% FCS and TGF-β2 secretion in culture supernatants was measured after seven days. TGF-β2 secretion was largely independent of the presence of serum in the culture medium and showed a 1.6-fold (10% FCS, p = 0.003) and two-fold (serum-free, p = 0.006) increase in PM as compared to OCM (Figure 2A). The osmolarity-dependent change in TGFB2 gene expression is thus predictive for TGF-β2 protein secretion in culture supernatant in response to PM. To determine whether the secreted TGF-β2 is also bioactive, we performed an established bioassay that reports a TGF-β specific activation of the TGF-β signaling pathway. Briefly, the TGF-β-responsive SMAD response element-mediated firefly luciferase signal is normalized to a constitutively expressed Renilla luciferase signal, to provide relative activity [21]. In agreement with increased TGF-β2 secretion in PM, this conditioned PM had a significantly (p < 0.0001) increased TGF-β bioactivity (2.3-fold; Figure 2B), as compared to conditioned OCM.
Figure 2.
Physosmotic culture medium (PM) increases TGF-β2 secretion and TGF-β bioactivity in HACs. In HACs cultured for seven days (serum-free cultures on the left and 10% FCS cultures on the right) TGF-β2 protein levels were determined by specific TGF-β2 ELISA in PM (black bars) or OCM (white bars) (A). TGF-β bioactivity was determined by TGF-β bioassay in 72 h serum starved conditioned medium from PM and OCM HAC cultures (B). Corresponding ALK5/ALK1 gene expression ratio was determined in samples from Figure 1 (C). Data are from 3 donors measured in duplicate and are presented as mean ± standard deviation relative to control OCM conditions.
On the receptor level ALK5, but not ALK1, appears to be essential for TGF-β induced phosphorylation of SMAD3 [18], and therefore we further investigated the expression ratio of ALK5/ALK1 in response to PM to get an indication if the increased TGF-β might be able to induce canonical signaling. In accordance with the TGF-β bioassay, the ALK5/ALK1 expression ratio was significantly shifted in favor of increased ALK5 expression in chondrocytes cultured in PM as compared to OCM (p = 0.027) (Figure 2C). These data indicate that physosmotic culture conditions improve activation of ALK5 driven TGF-β signaling.
2.3. TGF-β2 Isoform-Specific Knockdown in HAC Cultures In Vitro
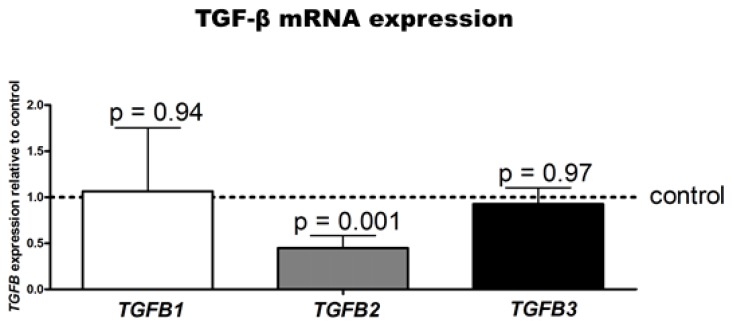
To investigate whether a causal relation exists between the increased COL2A1 expression and TGF-β2 secretion after treatment with PM, we performed TGF-β2 knockdown experiments using RNAi (siRNA) in our HAC cultures. First, we evaluated the isoform-specificity of RNAi before treatment with PM at t = 0: TGF-β2 gene expression was specifically and significantly down-regulated upon RNAi when compared to control and other TGF-β isoforms (Figure 3) (p = 0.001).
Figure 3.
TGF-β2 RNAi efficacy and specificity in HACs. TGFB1, TGFB2, and TGFB3 gene expression was determined by RT-qPCR upon TGF-β2-specific knockdown using transfected siRNAs (in OCM conditions). Data are the means ± standard deviation of duplicate measurements from three donors relative to control conditions (scrambled siRNA) and were normalized to housekeeper expression. Control condition is indicated by the dotted line (set to 1).
2.4. TGF-β2 RNAi Combined with Physosmotic Treatment Increases COL2A1 Gene Expression in HACs
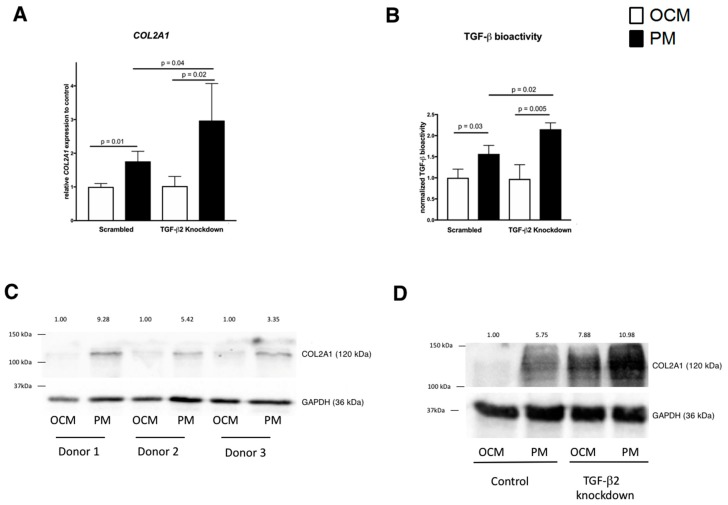
We subsequently determined the consequence of TGF-β2 RNAi on COL2A1 gene expression 48 h upon stimulation with PM as compared to OCM. PM significantly increased COL2A1 after 48 h (i.e., 1.8-fold, p = 0.01) (Figure 4A).
Figure 4.
TGF-β2 RNAi combined with physosmotic treatment increases COL2A1 gene expression and TGF-β bioactivity in HACs. Expression of COL2A1 was measured by RT-qPCR replicates (n = 3) in control conditions (left) and after TGF-β2 RNAi (right) in OCM (white bars) or PM (black bar) conditions 48 h after physosmotic stimulation (A). TGF-β bioactivity in media from SW1353 cultures with or without TGF-β knockdown from PM and OCM cultures. Data are normalized means ± standard deviation relative to control OCM condition (B). Collagen type II (COL2A1) protein expression was confirmed by immunoblotting in HACs from three donors. Molecular weight markers in kDa are shown on the left. The numbers across the top depict the relative quantity (C,D).
TGF-β2 knockdown, did not alter COL2A1 expression in OCM (Figure 4A). However, TGF-β2 RNAi with PM stimulation after 48 h resulted in higher COL2A1 expression compared to OA HACs cultured at PM without TGF-β2 RNAi (p = 0.04) (Figure 4A).
To determine if the increased COL2A1 expression upon TGF-β2 knockdown under physosmotic conditions (i.e., in PM) was accompanied by an increased reporter assay activity, we repeated the experiment in the established chondrosarcoma cell line SW1353 [22,23]. We measured CAGA-12 luciferase activity 48 h after stimulation with PM with TGF-β2 RNAi. Bioactivity of TGF-β displayed a similar pattern as COL2A1 expression: stimulation with PM increased CAGA-12 luciferase activity (p = 0.03) as compared to the control condition (i.e., OCM, Figure 4B). While TGF-β2 knockdown at OCM did not alter CAGA-12 luciferase activity, stimulation with PM in combination with TGF-β2 knockdown significantly increased the activity of this TGF-β signaling-specific reporter as compared to the same osmotic stimulation without TGF-β2 knockdown (p = 0.02).
COL2A1 gene expression data were confirmed on protein level. As shown in Figure 4C, PM increased COL2A1 protein levels. In addition, COL2A1 protein levels were higher at PM combined with TGF-β2 knockdown than in HACs cultured at PM alone (Figure 4D).
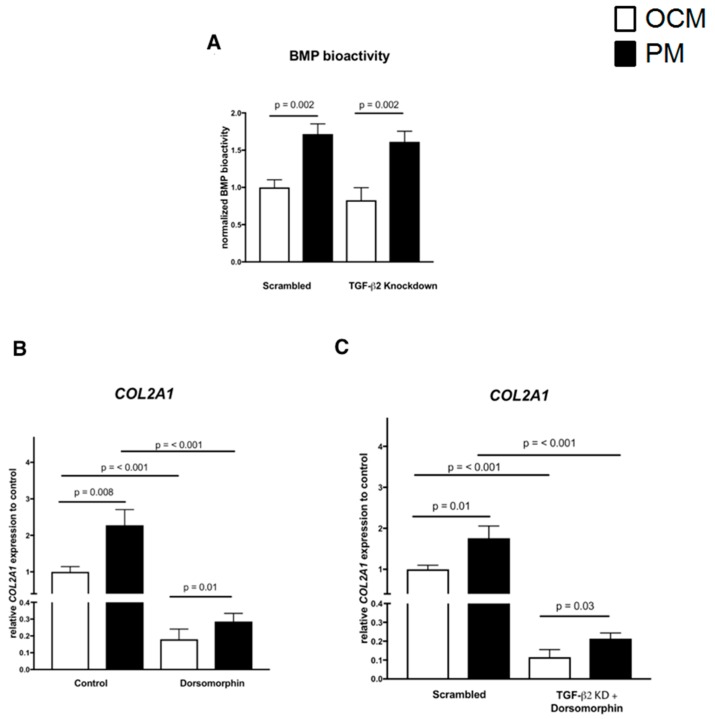
Since BMP signaling has been shown to be also involved in COL2A1 expression, we next evaluated BMP-specific BRE reporter activity under the same in vitro conditions. As shown in Figure 5A, PM significantly increased BRE activity compared to the OCM control condition (p = 0.002). In contrast, TGF-β2 knockdown did not alter BRE activity at both PM and OCM compared to conditions without TGF-β2 knockdown. Under TGF-β2 knockdown, PM again increased BMP reporter activity as compared to OCM (p = 0.002, Figure 5A).
Figure 5.
Blocking of BMP signalling decreases COL2A1 expression of chondrocytes irrespective of osmolarity. Bioassays in SW1353 cells show a largely TGF-β2 knockdown-independent, but osmolarity-dependent induction of BMP-responsiveness. Data are the normalized means ± standard deviation (n = 3) relative to control OCM condition (A). Physosmolar conditions further significantly elevated COL2A1 expression as compared to vehicle controls at OCM (B). Dorsomorphin (10 μM)-mediated inhibition of BMP signaling significantly suppressed COL2A1 expression in both osmolarities (B), but COL2A1 expression remained relatively higher in PM as compared to OCM irrespective of BMP signaling. Combining TGF-β2 knockdown and dorsomorphin showed the same pattern as dorsomorphin alone (C). Data from five donors were measured in duplicates and are presented as normalized means ± standard deviation relative to control OCM condition. Data is normalized means ± standard relative to control OCM condition (replicate measurements, three donors).
We further evaluated the effect of an established BMP signaling inhibitor, Dorsomorphin, on COL2A1 expression levels when TGF-β2 knockdown was combined with physosmotic stimulation.
First, we confirmed the attenuating effect of inhibiting BMP signaling under culture conditions without TGF-β2 knockdown. BMP signaling inhibition significantly decreased COL2A1 expression levels at both PM and OCM compared to vehicle controls, while COL2A1 expression levels at PM were still significantly elevated compared to OCM (Figure 5B). Under culture conditions with TGF-β2 knockdown, dorsomorphin again decreased COL2A1 expression, while COL2A1 expression was again significantly elevated at PM as compared to OCM (Figure 5B). COL2A1 expression levels in conditions with TGF-β2-specific knockdown in combination with blocked BMP signaling were suppressed in both OCM and PM, while COL2A1 expression remained relatively always higher in PM as compared to OCM irrespective of the TGF-β2 knockdown and blocked BMP signalling (Figure 5C).
3. Discussion
In the present study, we investigated whether physiological osmolarity (PM) is able to improve expression of key cartilage ECM marker COL2A1 in chondrocytes (HACs), through facilitating TGF-β superfamily signalling.
We reported earlier that expansion of HACs in PM (i.e., 380 mOsm) improved COL2A1 marker gene and protein expression [10] and now reveal a correlation between improved expression of key chondrocyte marker COL2A1 and upregulated TGF-β family member ligands (e.g., TGF-β2, -β3) in PM. Interestingly, of all three prototypic isoforms of this family, only TGF-β2 was prominently osmo-dependently regulated in our study. Of all three human isoforms, several reports support a relatively more potent role of TGF-β2 in stimulating ECM synthesis: first, expression of TGF-β2, but not TGF-β1, is reduced in cartilage of old compared to young mice, suggesting a possible chondroprotective role [24]. Secondly, only TGF-β2 is characterized by unique upstream response elements in its P1 promoter [25], suggesting its isoform specific regulation. TGF-β2 can further suppress collagenase mediated proteolytic degradation of collagen type II in human cartilage [26] and preserved the chondrocyte phenotype in physoxic high density in vitro cultures [2]. TGF-β2 also more potently induces SMAD2 phosphorylation at 10 and 250 pM as compared to TGF-β1 and TGF-β3 [16]. Furthermore, TGF-β1 and TGF-β3, but not TGF- β2, bind to endoglin [27], which is known to inhibit SMAD3 driven ECM production in chondrocytes [28]. Collectively, this prompted us to postulate that TGF-β2 signalling might trigger the increased collagen type II expression.
More than twice as much TGF-β2 protein was measured in conditioned PM compared to medium of plasma osmolarity (OCM). As TGF-β can be secreted noncovalently attached to its prodomain, requiring additional processing to be activated from latency [16], we subsequently used a functional bio-assay to investigate TGF-β activity. A twofold increase in TGF-β activity in conditioned PM was observed, confirming our gene- and protein-expression data. We next used a functional TGF-β bioassay with a SMAD3 responsive element as TGF-β2 induced SMAD3-driven transcriptional activity may cause subsequent expression of COL2A1 in chondrocytes [18]. The endogenous TGF-β activity measured in the conditioned PM reached 50% of the activity of the exogenously added positive TGF-β control (10 ng/mL) used in the bioassay. This translates to a concentration of about 5 ng/mL active TGF-β in conditioned PM, a concentration which is sufficient for SMAD3 phosphorylation [29]. As the TGF-β bioassay is not isoform specific, we are unfortunately unable to exclude a potential contribution of other TGF-β isoforms to the observed effects.
Thus, a limitation of our study is that we cannot rule out a contribution of antagonistic TGFBRI-like pseudo-receptors, MAPKs, like p38 MAPK or ERK1/2, in our experiments [30]. These have been shown to contribute to effects of hyperosmotic stimulation in chondrocytes [30], at least at a high osmotic value (380 vs. 550 mOsm). Tew et al. further underscored the importance of post-transcriptional regulation in response to osmotic changes and, interestingly, while downstream transcriptional regulators of TGF-β signaling were induced, expression of TGF-β receptors (i.e., TGFBR2) and certain SMADs were downregulated [30].
Of note, our physosmotic treatment also shifted receptor expression from TGF-β type I receptor ALK1 towards relatively more canonical TGF-β type I receptor ALK5 abundance. This is in agreement with our TGF-β bioassay, since it is well established that ALK5 potentiates TGF-β-induced SMAD3-driven transcriptional activity of the COL2A1 promotor [18]. Cartilage-specific deletion of ALK5 induces an osteoarthritis-like phenotype in mice, underscoring its chondroprotective function in cartilage [31]. A limitation of the present study is that we could not study receptor expression on protein level to answer this, due to their low expression levels [16].
A bioinformatics-driven approach suggested an involvement of TGF-β3 at high end osmotic values [30], which we cannot confirm nor rule out as we focused on TGF-β2. The latter appeared to be the most prominently regulated isoform under physiological conditions. High osmolarity is able to down-regulate not only TGFRI mRNA expression, but also TAB1, SMAD3 and BMP signaling inhibitor Smad7 [30]. Altogether, the response to extracellular osmotic values appears complex and seems to depend not only on the species, but also on cell type and its pathological state. Even the exact zonal location the chondrocytes are derived from may also influence the extent of the response to osmotic stimulation [32].
We further aimed to elucidate the role of physosmotic-driven TGF-β2 signaling on collagen expression by a ligand-specific knockdown approach. Unexpectedly, anti-TGF-β2 RNAi increased collagen type II expression upon TGF-β2 specific knockdown. We, therefore, next investigated if a counter-regulatory increase in TGF-β bioactivity might have caused the increased COL2A1 expression and evaluated SMAD3 driven transcriptional activity. We did this in SW1353 chondrosarcoma cells as a model for human chondrocytes [23], which resulted in a similarly increased TGF-β bioactivity. Interestingly, however, the bioactivity increased even further when physosmotic treatment was combined with TGF-β2 knockdown as compared to physosmotic treatment alone. Given that SMAD3 can transcriptionally induce COL2A1 expression [18], this may indicate a compensatory increase in TGF-β bioactivity upon TGF-β2 knockdown under physosmotic conditions. Post-transcriptional processes may also interfere with collagen expression under these conditions and may explain the increase in type II collagen protein. Earlier, SOX9 mRNA decay was shown to be influenced by high osmolarity. This led to a p38 MAPK-dependent increase in SOX9 protein and consequently enhanced COL2A1 transcription. Of note, high end osmolarity destabilized COL2A1 mRNA and reduced its overall expression [30,33]. In freshly isolated chondrocytes, osmotic stimulation thus increased SOX9, but decreased COL2A1, mRNA levels post-transcriptionally [33]. In our study, for some experiments we used SW1353 and not freshly isolated chondrocytes. Furthermore, we shifted the osmotic environment from plasma level to a (moderate) cartilage-specific physosmotic level and not from 380mOsm to high end osmolarity (>500 mOsm) as in the latter study. Together, differences in the osmotic baseline, the quality of the osmotic shift, and a different timing may at least partially explain contradictions between reported results.
Since BMP-signalling also regulates COL2A1 expression in chondrocytes, we additionally evaluated BMP bioactivity under physosmotic conditions in combination with TGF-β2 knockdown. Cross-talk between different TGF-β superfamily members can have synergistic or antagonistic effects [34]. Physosmotic treatment significantly increased BMP responsive element (BRE) activity under both in vitro culture conditions, with or without knockdown. There was no TGF-β2 knockdown specific effect on BRE signaling, while BMP-signaling inhibitor dorsomorphin was able to attenuate COL2A1 expression in both conditions. When combining TGF-β2 RNAi and pharmacological inhibition (i.e., dorsomorphin), COL2A1 expression appeared to be largely BMP-dependent (Figure 5) as BMP signaling inhibition reduced COL2A1 expression to 20% of the baseline level and 10% of the TGF-β2 knockdown control. Thus, while TGF-β superfamily members interdependently influence expression of this chondrocyte marker, physomotic conditions apparently always favor its expression independent of our means of TGF-β superfamily signalling manipulation.
The quantity of the osmotic pressure appears to be as important as its quality. To this end, recent studies confirm that using physiological salt solutions may be more appropriate than using sorbitol. Hyperosmolar potassium treatment attenuate protein production of catabolic and inflammatory OA markers in a 3D in vitro model, at values of ~490 mOsm [35]. A study of experimentally injured rat and bovine cartilage reported chondroprotection by raising osmolarity of irrigation solutions [36]. However, also here normal saline (300 mOsm) was compared to sucrose-mediated hyperosmolar saline solution (600 mOsm) [36]. We reported earlier that sodium is specifically enriched in the ECM of cartilage [10,37,38] and using NaCl to alter osmotic values appears to be more physiological than using e.g., sorbitol or sucrose solutions. Although chondrocytes in vitro do not tolerate hyperosmolar stresses above about 380 mOsm for too long [10], in vivo the situation may be different. All experiments of the present study were performed in 2D, while results might differ in 3D cultures [32], which is a limitation.
We showed that expression of several TGF-β superfamily member ligands, their receptors and target genes is altered in cultured human chondrocytes in physosmolar in vitro culture. However, the cellular responses to extracellular osmotic changes are complex [39] and both, macromolecular crowding and high ionic strength, may trigger a plethora of cell signaling cascades. High intracellular ionic strength enhances NFAT5 activity in chondrocytic cells [10,40] and more universally in mammalian cells [39]. Basically, elevating the extracellular osmolarity causes, at least temporarily, a state of molecular crowding from the presence of a total high weight per volume concentration of functionally unrelated soluble macromolecules [41]. This may indirectly affect interactions of soluble macromolecules with membranes, other structural elements or molecular chaperones.
In summary, physosmotic conditions preserve the phenotype of articular chondrocytes in vitro, a process to which TGF-β superfamily members seem to contribute. Although we could not proof a link between increased activity of this superfamily and increased COL2A1 expression in physosmotic cultures, the osmotic component appears prevailing. The TGF-β superfamily [42] consists of more than 35 members of which we only analyzed prototypic members (i.e., TGF-βs and BMPs) of each major branch [43] in more detail. Within this setting, our rational approach further cannot exclude TGF-β mediated responses that are independent of Smad proteins [44]. Molecular crowding might affect protein structure, folding, shape, conformational stability, binding of small molecules, enzymatic activity, protein-protein interactions, protein-nucleic acid interactions, and pathological aggregation [45] and should be addressed in future studies.
4. Materials and Methods
4.1. Cartilage and Chondrocyte Isolation
Human articular cartilage was explanted from macroscopically normal areas of the femoral condyles and tibial plateau of nine patients undergoing total knee replacement surgery for osteoarthritis (OA). The Erasmus MC and the Maastricht University Medical Centre institutional policy on the use of residual human surgical material specifically states that no informed consent is needed in the case of residual surgical material. However, an approval from the institutional Medical Ethical Committee (MEC) for the use of this material is required. The MEC approved this study and assigned approval ID: MEC2004-322 and MEC08-4-028 (23 June 2008).
Cartilage was separated from the subchondral bone and cut into small pieces using a sterile surgical blade. Cartilage pieces were digested overnight at 37 °C in collagenase type II solution (300 U/mL in HEPES buffered DMEM/F12 supplemented with antibiotics (Invitrogen, Carlsbad, CA, USA), 280 mOsm) under continuous agitation [10,46,47,48]. Medium osmolarity was experimentally adjusted to 380 mOsm (physosmotic medium i.e., PM) by adding sterile NaCl as reported earlier [10]. The preparation was rinsed with 0.9% NaCl over a 70 µm cell strainer and plated in culture flasks.
4.2. Chondrocyte Expansion and Culturing
Briefly, primary HACs were cultured (humidified atmosphere at 37 °C, 5% CO2) for expansion in monolayers at a seeding density of 7500 cells/cm2 in culture medium consisting of DMEM/F12 (Invitrogen) with 10% fetal calf serum (FCS) (Sigma-Aldrich, St. Louis, MO, USA) and 1% antibiotic/antimycotic (Invitrogen) corresponding to their isolation osmolarity; 280 mOsm (osmotic control medium, i.e., OCM) or 380 mOsm (physosmotic medium i.e., PM). Once cells reached confluency, they were trypsinized, resuspended, and replated into 175 cm2 flasks. Within four weeks after harvest early passage HACs (P1, P2) were seeded in high-density monolayers (20,000 cells/cm2) and were cultured in OCM or PM, respectively, for an additional 7 days before analysis. Moreover, HACs were cultured for 7 days, including a 72 h serum free period in order to collect conditioned medium for TGF-β protein analyses (TGF-β2 isoform-specific ELISA and TGF-β bioassay). Experiments were performed in technical replicates from six donors.
4.3. RNA Expression Analysis
According to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [49], we subsequently aim at reporting suggested essential information: (i) the experimental design is outlined elsewhere in this section, with the numbers within each group reported as “n” per analyses, (ii) the sample dissection, processing and storage has been reported earlier by us [10], (iii) total RNA extraction, purification, and quantification thereof are also reported in much detail [10], as was (iv) the RT reaction, both using commercially available Eurogentec kits. Other essential details on the reaction conditions of the cDNA synthesis has also been previously reported [10]. With respect to (v) qPCR target information, we aimed at using the latest Entrez Gene Symbols, designed our assays using the National Center for Biotechnology Information (NCBI)’s database RefSeq sequences [50], performed NCBI’s Primer-Blast to verify specificity in silico and report NCBI Gene IDs. Real-time quantitative PCR (RT-qPCR) was performed using MesagreenTM qPCR mastermix plus for SYBR Green (Eurogentec, Maastricht, The Netherlands) and a CFX96 Real-Time PCR Detection system (Biorad, Hercules, CA, USA for amplification with the following profile: initial denaturation 10 min at 95 °C, followed by 50 cycles of amplification (15 s at 95 °C and 1 min at 60 °C), and followed by a dissociation curve. Specificity of the Primer DesignerTM (ThermoFisher Scientific, Waltham, MA, USA) designed and mfold-checked amplicons (> 70 < 250 bp; default secondary structure exclusion settings) were checked by agarose gel electrophoresis to exclude primer dimers and post run melting curve evaluation and assays were selected to have PCR efficiencies of >0.95. Data analysis: data were normalized to an index of stably-expressed reference genes that were pre-evaluated to be stably expressed across samples by BestKeeper software (Microsoft, Redmond, WA, USA) as was reported elsewhere by our group [2,10]. All NTCs had Cqs ≥ 39 and GOIs with Cqs ≥ 35 were excluded from analyses. Relative expression was calculated according to the delta-delta CT method. Not earlier reported oligonucleotide sequences are listed in Table 1 and the statistical methods are reported elsewhere in this section.
Table 1.
Oligonucleotide sequences.
| Gene Amplicon |
ID Tm |
Forward/Sense (5′–3′) nt Positions |
Reverse/Antisense (5′–3′) nt Positions |
|---|---|---|---|
|
COL2A1 44 bp |
1280 62.0/62.4 |
TGGACGATCAGGCGAAACC 3570–3588 |
GCTGCGGATGCTCTCAATCT 3813–3794 |
|
TGFB1 209 bp |
7040 61.4/60.7 |
CTAATGGTGGAAACCCACAACG 334–355 |
TATCGCCAGGAATTGTTGCTG 542–522 |
|
TGFB2 154 bp |
7042 62.3/62.9 |
CCATCCCGCCCACTTTCTAC 434–453 |
AGCTCAATCCGTTGTTCAGGC 587–567 |
|
TGFB3 121 bp |
7043 60.9/60.3 |
GGAAAACACCGAGTCGGAATAC 279–300 |
GCGGAAAACCTTGGAGGTAAT 399–379 |
|
ALK1 194 bp |
94 62.3/62.3 |
CATCGCCTCAGACATGACCTC 777–797 |
GTTTGCCCTGTGTACCGAAGA 970–950 |
|
ALK5 167 bp |
7046 60.6/61.1 |
ACGGCGTTACAGTGTTTCTG 94–113 |
GCACATACAAACGGCCTATCTC 260–239 |
|
GAPDH
101 bp |
2597 62.0/62.9 |
CTGGGCTACACTGAGCACC 694–712 |
AAGTGGTCGTTGAGGGCAATG 794–774 |
|
TGF-β2
siRNA |
− | CTAATGGTGGAAACCCACAACG | TATCGCCAGGAATTGTTGCTG |
Primer sequences for RT-qPCR and oligonucleotide sequences for RNAi are listed in the 5′–3′ direction. Gene, NCBI Gene Symbol; ID, NCBI Gene ID; bp, base pairs; nt positions, location of primers. Note the following newer aliases for above-listed gene symbols: ALK1 (ACVRL1), ALK5 (TGFBR1).
4.4. Immunoblotting
HACs were washed with 0.9% NaCl and lysed in RIPA buffer. Extracts were sonicated on ice using the Soniprep 150 (MSE, London, UK). Insoluble material was removed by centrifugation (13,000× g, 4 °C). The BCA protein assay (Sigma-Aldrich) was used to determine protein concentration. Polypeptides were separated by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred to nitrocellulose membranes by electroblotting. Primary antibodies (all 1:100 dilution) for immunodetection were polyclonal goat anti-COL2A1 1320–01, Southern Biotech, Birmingham, AL, USA), and mouse monoclonal anti-GAPDH (10R-1261096, Fitzgerald, MA, USA). Bound primary antibodies were detected with secondary immunoglobulins conjugated with horseradish peroxidase (Dako) and visualized by enhanced chemiluminescence (ECL). ECL signals were quantified using ImageJ 1.46f software (National Institutes of Health, Bethesda, MA, USA), and relative differences, corrected for background and housekeeper, were determined as compared to control conditions.
4.5. TGF-β2 ELISA
To determine TGF-β2 in the secretome, medium was collected and centrifuged at 1200 rpm for 8 min to remove cell debris. The cleared supernatant was collected and stored at –80 °C until further use. TGF-β2 concentration was quantified spectrophotometrically using an enzyme-linked immunosorbent assay (ELISA; Demeditec Diagnostics GmbH, Kiel, Germany). TGF-β2 concentrations in the samples were calculated from a standard curve according to manufacturer’s instructions.
4.6. TGF-β Bioassay
To verify that the secreted TGF-β as measured by ELISA is biologically active, we next employed an established luciferase assay, using the CAGA-luc Smad2/3 reporter.
Human embryonic kidney cells were seeded at 3 × 104/well in 24-well plates and transiently transfected with 150 ng of the reporter construct and 75 ng of pRL-TK vector (Promega, Madison, WI, USA), an internal control for transfection efficiency, using FuGENE 6 transfection reagent (Roche Diagnostics, Basel, Switzerland). Twenty-four hours after transfection, cells were incubated for 2 h in medium containing 0.2% FCS, followed by 16 h incubation with 10 ng/mL TGF-β2 as a positive control, DMEM/F12 as a negative control or 300 μL conditioned medium (see above, ELISA). Twenty-four hours after stimulation, the firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
4.7. RNAi Experiments
Primary HACs from three OA patients were cultured at OCM for two weeks and then seeded in high-density monolayers (20,000 cells/cm2). A TGF-β2-specific siRNA duplex (Table 1) and a scrambled siRNA control duplex were used (Eurogentec, Seraing, Belgium) [47]. HACs were transfected with siRNAs (100 nM) at OCM using HiPerFect (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. One day after transfection, HACs were refreshed with either OCM or PM in the presence or absence of dorsomorphin (10 µM, Santa Cruz Biotechnologies, Santa Cruz, CA, USA). Forty-eight hours after stimulation cells were harvested for mRNA expression or protein analyses. HACs were also seeded and cultured with either DMSO (1:1000) or dorsomorphin (10 µM). One day after seeding, cells were either refreshed with OCM or PM, and 48 h later harvested for gene expression analyses. In another experiment, SW1353 cells were seeded in high-density monolayers (30,000 cells/cm2. Cells were transfected with the previously described CAGA-luc Smad2/3 reporter or the BRE-luc reporter using FuGene transfection reagent (Promega, Madison, WI, USA) at OCM [51]. The gaussia luciferase reporter construct was used as an internal control for transfection efficiency. One day after transfection of the reporters, the previously described TGF-β2-specific siRNA duplex and a scrambled siRNA control duplex were transfected (100 nM) at OCM using HiPerFect (Qiagen, Hilder, Germany). Five hours after siRNA transfection medium was refreshed with either OCM or PM. Forty-eight hours later, cells were processed and their luciferase activity was measured by a luciferase reporter assay system (Promega, Madison, WI, USA).
4.8. Statistical Analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were tested for normality using Kolmogorov-Smirnov test and normality plots were visually assessed for skewness. All samples for gene expression analysis and TGF-β2 measurements in medium were processed and analyzed individually with replicate measurements per donor. Gene expression levels, TGF-β2 protein expression and luciferase activity between HACs cultured in PM or OCM were compared by an unpaired t-test. Data obtained from RNAi experiments were analyzed by a mixed linear model test. In the model, conditions with or without TGF-β2 knockdown and with or without physosmotic treatment were considered as fixed factors, while the cartilage donor was considered as a random factor. Adjustment for multiple comparisons was made by a Bonferonni’s post hoc comparison test. p < 0.05 was considered to indicate levels of statistically significant difference.
Author Contributions
U.T.T., M.C., G.v.d.A., T.W., and H.J. designed the study. U.T.T. and M.C. performed the experiments. U.T.T. wrote the manuscript, and analyzed and interpreted the data. M.C., A.v.d.W., T.W., H.J., P.E., L.v.R., H.W., and J.V. analyzed and interpreted the data, and critically revised the manuscript. All authors approved the final version of the manuscript.
Funding
H.J.: H.W. and U.T.T. are members of the D-BOARD Consortium; This project has received funding from the European Union’s Seventh Framework Programme for Research, Technological Development, and Demonstration under Grant Agreement no. 305815 (HEALTH.2012.2.4.5-2). We are further indebted to START of the Faculty of Medicine of the RWTH Aachen and the Aachen-Jülich-Haifa Umbrella Cooperation (acronym SmartCart). This project was also financially supported by the Dutch Arthritis Foundation (grant LLP14).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Brittberg M. Autologous chondrocyte transplantation. Clin. Orthop. Relat. Res. 1999:147–155. doi: 10.1097/00003086-199910001-00016. [DOI] [PubMed] [Google Scholar]
- 2.Das R., Timur U.T., Edip S., Haak E., Wruck C., Weinans H., Jahr H. Tgf-beta2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann. Anat. 2015;198:1–10. doi: 10.1016/j.aanat.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Chawla A., Twycross-Lewis R., Maffulli N. Microfracture produces inferior outcomes to other cartilage repair techniques in chondral injuries in the paediatric knee. Br. Med. Bull. 2015;116:93–103. doi: 10.1093/bmb/ldv040. [DOI] [PubMed] [Google Scholar]
- 4.Falah M., Nierenberg G., Soudry M., Hayden M., Volpin G. Treatment of articular cartilage lesions of the knee. Int. Orthop. 2010;34:621–630. doi: 10.1007/s00264-010-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban J.P., Hall A.C., Gehl K.A. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J. Cell. Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 6.Benya P.D., Padilla S.R., Nimni M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla A., Sankaralingam S., Dundas R., Swaminathan R., Wolfe C.D., Rudd A.G. Influence of raised plasma osmolality on clinical outcome after acute stroke. Stroke. 2000;31:2043–2048. doi: 10.1161/01.STR.31.9.2043. [DOI] [PubMed] [Google Scholar]
- 8.Anderson D.E., Markway B.D., Weekes K.J., McCarthy H.E., Johnstone B. Physioxia promotes the articular chondrocyte-like phenotype in human chondroprogenitor-derived self-organized tissue. Tissue Eng. Part A. 2017 doi: 10.1089/ten.tea.2016.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson Z.I., Shapiro I.M., Risbud M.V. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of tonebp. Matrix Biol. 2014;40:10–16. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Windt A.E., Haak E., Das R.H., Kops N., Welting T.J., Caron M.M., van Til N.P., Verhaar J.A., Weinans H., Jahr H. Physiological tonicity improves human chondrogenic marker expression through nuclear factor of activated t-cells 5 in vitro. Arthritis. Res. Ther. 2010;12:R100. doi: 10.1186/ar3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Kraan P.M., Buma P., van Kuppevelt T., van den Berg W.B. Interaction of chondrocytes, extracellular matrix and growth factors: Relevance for articular cartilage tissue engineering. Osteoarthr. Cartil. 2002;10:631–637. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 12.Van der Kraan P.M., Blaney Davidson E.N., Blom A., van den Berg W.B. Tgf-beta signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-smads. Osteoarthr. Cartil. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa K., Shinozaki M., Hara T., Furuya T., Miyazono K. Two major smad pathways in tgf-beta superfamily signalling. Genes Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 14.Roberts A.B., Sporn M.B., Assoian R.K., Smith J.M., Roche N.S., Wakefield L.M., Heine U.I., Liotta L.A., Falanga V., Kehrl J.H., et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanford L.P., Ormsby I., Gittenberger-de Groot A.C., Sariola H., Friedman R., Boivin G.P., Cardell E.L., Doetschman T. Tgfbeta2 knockout mice have multiple developmental defects that are non-overlapping with other tgfbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker W.L. Tgf-β Receptors on Human Chondrocytes: Hetero-Oligomerization and Function. McGill University; Montreal, QC, Canada: 2003. [Google Scholar]
- 17.Heldin C.H., Miyazono K., ten Dijke P. Tgf-beta signalling from cell membrane to nucleus through smad proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 18.Finnson K.W., Parker W.L., ten Dijke P., Thorikay M., Philip A. Alk1 opposes alk5/smad3 signaling and expression of extracellular matrix components in human chondrocytes. J. Bone Miner. Res. 2008;23:896–906. doi: 10.1359/jbmr.080209. [DOI] [PubMed] [Google Scholar]
- 19.Blaney Davidson E.N., Remst D.F., Vitters E.L., van Beuningen H.M., Blom A.B., Goumans M.J., van den Berg W.B., van der Kraan P.M. Increase in alk1/alk5 ratio as a cause for elevated mmp-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 20.Harrison P.E., Ashton I.K., Johnson W.E., Turner S.L., Richardson J.B., Ashton B.A. The in vitro growth of human chondrocytes. Cell Tissue Bank. 2000;1:255–260. doi: 10.1023/A:1010131729208. [DOI] [PubMed] [Google Scholar]
- 21.Dennler S., Itoh S., Vivien D., ten Dijke P., Huet S., Gauthier J.M. Direct binding of smad3 and smad4 to critical tgf beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z., Le H., Chen W., Shen W., Ouyang H. Progress on treatment of tendinopathy with platelet-enriched plasma. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016;45:179–186. doi: 10.3785/j.issn.1008-9292.2016.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebauer M., Saas J., Sohler F., Haag J., Söder S., Pieper M., Bartnik E., Beninga J., Zimmer R., Aigner T. Comparison of the chondrosarcoma cell line sw1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to il-1beta. Osteoarthr. Cartil. 2005;13:697–708. doi: 10.1016/j.joca.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Blaney Davidson E.N., Scharstuhl A., Vitters E.L., van der Kraan P.M., van den Berg W.B. Reduced transforming growth factor-beta signaling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. Ther. 2005;7:R1338–R1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts A.B., Sporn M.B. Differential expression of the tgf-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol. Reprod. Dev. 1992;32:91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 26.Tchetina E.V., Antoniou J., Tanzer M., Zukor D.J., Poole A.R. Transforming growth factor-beta2 suppresses collagen cleavage in cultured human osteoarthritic cartilage, reduces expression of genes associated with chondrocyte hypertrophy and degradation, and increases prostaglandin e(2) production. Am. J. Pathol. 2006;168:131–140. doi: 10.2353/ajpath.2006.050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheifetz S., Bellon T., Cales C., Vera S., Bernabeu C., Massague J., Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 28.Finnson K.W., Parker W.L., Chi Y., Hoemann C.D., Goldring M.B., Antoniou J., Philip A. Endoglin differentially regulates tgf-beta-induced smad2/3 and smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthr. Cartil. 2010;18:1518–1527. doi: 10.1016/j.joca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Remst D.F., Blaney Davidson E.N., Vitters E.L., Bank R.A., van den Berg W.B., van der Kraan P.M. Tgf-ss induces lysyl hydroxylase 2b in human synovial osteoarthritic fibroblasts through alk5 signaling. Cell Tissue Res. 2014;355:163–171. doi: 10.1007/s00441-013-1740-5. [DOI] [PubMed] [Google Scholar]
- 30.Tew S.R., Vasieva O., Peffers M.J., Clegg P.D. Post-transcriptional gene regulation following exposure of osteoarthritic human articular chondrocytes to hyperosmotic conditions. Osteoarthr. Cartil. 2011;19:1036–1046. doi: 10.1016/j.joca.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., Tan Q., Xu W., Qi H., Chen D., Zhou S., Ni Z., Kuang L., Guo J., Huang J. Cartilage-specific deletion of alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthr. Cartil. 2017;25:1868–1879. doi: 10.1016/j.joca.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada E., Mizuno S. Reproduction of characteristics of extracellular matrices in specific longitudinal depth zone cartilage within spherical organoids in response to changes in osmotic pressure. Int. J. Mol. Sci. 2018;19:1507. doi: 10.3390/ijms19051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tew S.R., Peffers M.J., McKay T.R., Lowe E.T., Khan W.S., Hardingham T.E., Clegg P.D. Hyperosmolarity regulates sox9 mrna posttranscriptionally in human articular chondrocytes. Am. J. Physiol. Cell Physiol. 2009;297:C898–C906. doi: 10.1152/ajpcell.00571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo X., Wang X.F. Signaling cross-talk between tgf-beta/bmp and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erndt-Marino J., Trinkle E., Hahn M.S. Hyperosmolar potassium (K+) Treatment Suppresses Osteoarthritic Chondrocyte Catabolic and Inflammatory Protein Production in a 3-Dimensional In Vitro Model. Cartilage. 2017 doi: 10.1177/1947603517734028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltawil N.M., Ahmed S., Chan L.H., Simpson A.H.R.W., Hall A.C. Chondroprotection in models of cartilage injury by raising the temperature and osmolarity of irrigation solutions. Cartilage. 2018;9:313–320. doi: 10.1177/1947603516688511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Windt A.E., Jahr H., Farrell E., Verhaar J.A., Weinans H., van Osch G.J. Calcineurin inhibitors promote chondrogenic marker expression of dedifferentiated human adult chondrocytes via stimulation of endogenous tgfbeta1 production. Tissue Eng. Part A. 2010;16:1–10. doi: 10.1089/ten.tea.2009.0082. [DOI] [PubMed] [Google Scholar]
- 38.Van der Windt A.E., Haak E., Kops N., Verhaar J.A., Weinans H., Jahr H. Inhibiting calcineurin activity under physiologic tonicity elevates anabolic but suppresses catabolic chondrocyte markers. Arthritis Rheum. 2012;64:1929–1939. doi: 10.1002/art.34369. [DOI] [PubMed] [Google Scholar]
- 39.Bounedjah O., Hamon L., Savarin P., Desforges B., Curmi P.A., Pastré D. Macromolecular crowding regulates assembly of mrna stress granules after osmotic stress: New role for compatible osmolytes. J. Biol. Chem. 2012;287:2446–2458. doi: 10.1074/jbc.M111.292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caron M.M., van der Windt A.E., Emans P.J., van Rhijn L.W., Jahr H., Welting T.J. Osmolarity determines the in vitro chondrogenic differentiation capacity of progenitor cells via nuclear factor of activated t-cells 5. Bone. 2013;53:94–102. doi: 10.1016/j.bone.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 41.Rivas G., Minton A.P. Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem. Sci. 2016;41:970–981. doi: 10.1016/j.tibs.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massagué J. How cells read tgf-beta signals. Nat. Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 43.Van der Kraan P.M. The changing role of tgfβ in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 2017;13:155–163. doi: 10.1038/nrrheum.2016.219. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y., Massagué J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 45.Kuznetsova I.M., Turoverov K.K., Uversky V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014;15:23090–23140. doi: 10.3390/ijms151223090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caron M.M., Emans P.J., Coolsen M.M., Voss L., Surtel D.A., Cremers A., van Rhijn L.W., Welting T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2d and 3d cultures. Osteoarthr. Cartil. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Caron M.M., Emans P.J., Surtel D.A., van der Kraan P.M., van Rhijn L.W., Welting T.J. Bapx-1/nkx-3.2 acts as a chondrocyte hypertrophy molecular switch in osteoarthritis. Arthritis Rheumatol. 2015;67:2944–2956. doi: 10.1002/art.39293. [DOI] [PubMed] [Google Scholar]
- 48.Das R.H., van Osch G.J., Kreukniet M., Oostra J., Weinans H., Jahr H. Effects of individual control of ph and hypoxia in chondrocyte culture. J. Orthop. Res. 2009 doi: 10.1002/jor.20994. [DOI] [PubMed] [Google Scholar]
- 49.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The miqe guidelines: Minimum information for publication of quantitative real-time pcr experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 50.Maglott D., Ostell J., Pruitt K.D., Tatusova T. Entrez gene: Gene-centered information at ncbi. Nucleic Acids Res. 2011;39:D52–D57. doi: 10.1093/nar/gkq1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korchynskyi O., ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the id1 promoter. J. Biol. Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]