Abstract
The complex relationship between diet and metabolism is an important contributor to cellular metabolism and health. Over the past few decades, a central role for mammalian target of rapamycin (mTOR) in the regulation of multiple cellular processes, including the response to food intake, maintaining homeostasis, and the pathogenesis of disease, has been shown. Herein, we first review our current understanding of the biochemical functions of mTOR and its response to fluctuations in hormone levels, like insulin. Second, we highlight the role of mTOR in lipogenesis, adipogenesis, β-oxidation of lipids, and ketosis of carbohydrates, lipids, and proteins. Special attention is paid to recent advances in mTOR signaling in white versus brown adipose tissues. Finally, we review how mTOR regulates cardiovascular health and disease. Together, these insights define a clearer picture of the connection between mTOR signaling, metabolic health, and disease.
The increasing worldwide prevalence of metabolically linked diseases in humans, including obesity, cancer, and cardiovascular disease, has spurred efforts to define the underlying biological factors that cause these conditions. Metabolic state depends on various factors and processes, such as fluctuations in hormone and cytokine levels, oxidative stress and hypoxia, and metabolic by-products from the oxidation of carbohydrates, lipids, and proteins. In multicellular eukaryotes, these diverse signals are, in part, integrated through the phosphatidylinositol 3-kinase–related serine/threonine protein kinase mammalian target of rapamycin (mTOR).1 mTOR senses, integrates, and responds to various nutrient signals in a variety of tissues, like adipose and cardiac tissue. These signals are sensed by receptors that transduce the signal via cascades that control mTOR activity, which ultimately regulates several cellular pathways. These include metabolic pathways, cell growth, proliferation, and survival (Figure 12, 3). Depending on the outcome of this signaling cascade in a particular tissue, mTOR can promote metabolic health or disease.
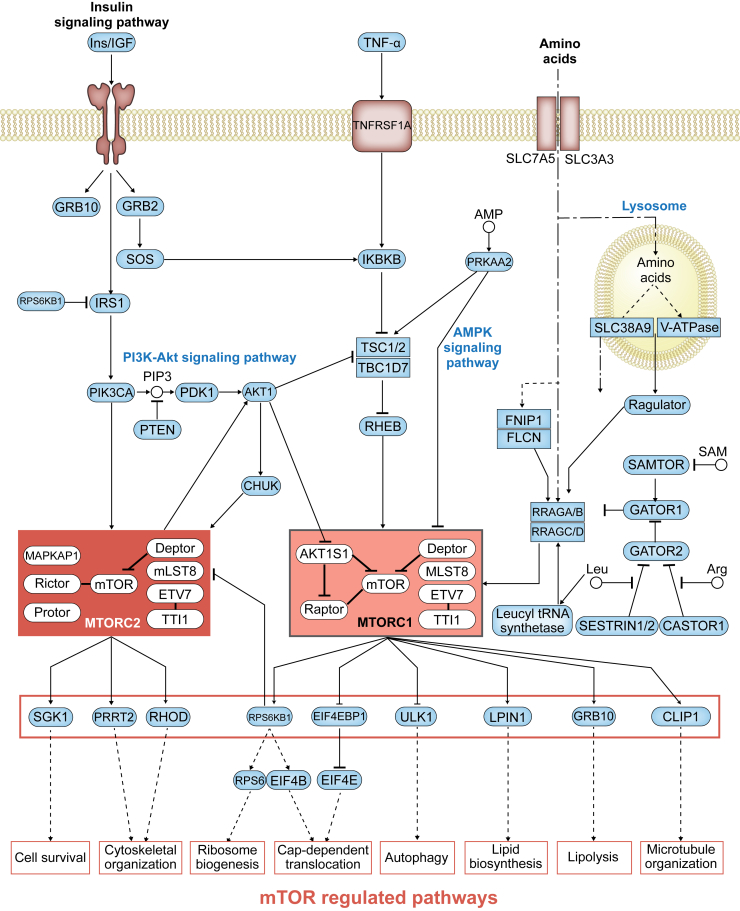
Figure 1.
The mammalian target of rapamycin (mTOR) regulatory network. The mTOR signaling pathway in Homo sapiens senses a variety of upstream signals, with distinctive downstream inputs. The insulin signaling pathway, cytokines such as tumor necrosis factor (TNF), and amino acids stimulate a variety of signaling molecules, such as phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), AKT1, and ras related GTP binding A (RRAGA), respectively, which in turn activate the mTOR complex 1 (MTORC1) and mTOR complex 2 (MTORC2). These complexes activate a variety of cell processes. Adapted from Kanehisa et al,3 with permission from Kyoto Encyclopedia of Genes and Genomes database. CHUK, conserved helix-loop-helix ubiquitous kinase; IRS1, insulin receptor–related substrate 1; PDK1, phosphoinositide-dependent kinase-1; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; SAM, S-adenosyl methionine; SGK, serum/glucocorticoid regulated kinase.
Several metabolically linked diseases, including heart disease,4 diabetes,5 and Alzheimer disease6 (all top 10 causes of death worldwide), have been linked, at least in part, to dysregulation of mTOR signaling. In fact, many of these diseases have been associated with dysregulation of mTOR signaling, importantly through imbalanced dietary intake.7, 8, 9 More evidence for the role of diet in regulating mTOR signaling comes from studies of calorie restriction, which have been shown to extend life span,10 in a diverse array of eukaryotes ranging from yeasts to humans.11, 12 Therefore, gaining a deeper understanding of mTOR's role and function in these poorly defined processes is critical to understanding disease pathogenesis in tissues where mTOR plays a central role.
Herein, we review the relationship between cellular metabolism, energy balance, and mTOR, including their influence on physiological and pathophysiological states. Specifically, we review how lipid metabolism is regulated by mTOR, from lipolysis and β-oxidation, to ketosis in adipose tissues. Finally, we describe known connections between mTOR function and cardiovascular disease, as well as how the mTOR signaling pathway may play a role in maintaining cardiovascular health.
Biochemistry of mTOR: Function and Signaling
The mTOR signaling pathway involves two distinct multiprotein complexes, and each has different upstream inputs and downstream functions: mTOR complex 1 (MTORC1) and mTOR complex 2 (MTORC2).
MTORC1 consists of mTOR, the regulatory protein Raptor, and mammalian lethal protein associated with SEC13 protein 8. Two additional proteins constitutively interact with MTORC1: ETS variant 7 (ETV7; alias Tel2) and TELO2 interacting protein 1 (TTI1)13 (Figure 1). Together, they form a nutrient-energy–oxidation-reduction sensor and they control protein synthesis, autophagy, microtubule organization, and lipid metabolism.
In fact, MTORC1 activity can be regulated by insulin,14, 15 growth factors,16 phosphatidic acid,17 certain amino acids,18, 19 mechanical stimuli,20 and oxidative stress.21, 22 On upstream insulin receptor stimulation, proline-rich AKT serine/threonine kinase 1 (AKT1 substrate 1) is activated and regulates MTORC1 activity. This occurs through a biphasic mechanism involving both AKT1 substrate 1 and TSC complex subunit 1/2 (TSC1/2),23 and TSC1/2 signaling can contribute to MTORC1 activation (Figure 1).
mTOR's role in these growth processes was established by the discovery of the MTORC1 inhibitor rapamycin (alias Sirolimus). Rapamycin is a macrocyclic lactone produced by Streptomyces hygroscopicus.24 Rapamycin forms an inhibitory complex with MTORC1 on binding to the protein FKBP prolyl isomerase 1A (FKBP1A), inhibiting downstream MTORC1 activation. Interestingly, prolonged treatment of cells with rapamycin also has effects on MTORC2 through a mechanism not fully understood.
The second mTOR complex, MTORC2, is a multifunctional complex composed of mTOR, the rapamycin-insensitive companion of mTOR, the mammalian stress-activated protein kinase interacting protein 1 (alias mSIN1), a proline-rich protein 5 (alias PROTOR), as well as three proteins that also make up MTORC1—mammalian lethal protein associated with SEC13 protein 8, ETV7, and TTI1 (Figure 1). As a component of MTORC2, mTOR responds to the activation of insulin receptors on cells through phosphorylation separately from MTORC1. In addition, MTORC2 regulates cytoskeletal organization through stimulation of F-actin stress fibers, ras homolog family member A (RHOA), Rac family small GTPase 1 (RAC1), cell division cycle 42 (CDC42), and protein kinase C. Finally, MTORC2 phosphorylates the serine/threonine protein kinase AKT1 on serine residue Ser473, thus promoting cell metabolism and cell survival.25, 26 Moreover, phosphorylation of AKT1-S473 results in AKT1 activation,27 which, as mentioned, regulates MTORC2 activity. In addition, AKT1 activation stimulates conserved helix-loop-helix ubiquitous kinase (CHUK; IKKα), which itself regulates MTORC2, thus generating a feedback loop that is stimulated by insulin signaling.28
Both MTORC1 and MTORC2 are inhibited by Deptor, whose dysregulation has been linked to a variety of disease states, such as multiple myeloma, in humans (Figure 1).29 Conversely, decreased Deptor activity increases S6 kinase β-1 (RPS6KB1 or S6K1) activity, and in turn, protein synthesis, as well as AKT1 and serum/glucocorticoid regulated kinase 1 (SGK1) activity via MTORC2, which ultimately promotes cell growth and survival. Besides DEPTOR, the activity of the mTOR complexes is also affected by the expression levels and activity of the accessory proteins that make up these complexes.
Current mTOR research is focused on how the mTOR-regulated signaling pathways function in specific tissues in the body, as well as how they are altered in disease states. Of particular interest is mTOR function in adipose tissue and in cardiovascular health. Hypertension, diabetes, obesity, and high total cholesterol comprise the major four risk factors for death in humans older than 50 years. Thus, a clearer understanding of the biological mechanisms underlying obesity and heart disease, as well as mTOR's potential involvement in these pathologic states, is merited.
The Role of mTOR in Mediating Nutrient Metabolism
Three dietary energy sources (namely, sugars, amino acids, and fatty acids) provide humans their essential nutrients, and mTOR plays an important role in processing/metabolizing all three.
mTOR and Glucose Metabolism
The breakdown of ingested sugars via glycolysis is a critical metabolic pathway in humans. Indeed, ingested glucose is used for ATP production, lipogenesis, and glycogenesis, among other pathways. The liver is the primary site of glucose uptake, a process which is regulated by the glucose transporter solute carrier family 2 member 1-4 (SLC2A1-4; GLUT1-4). Glucose transporter expression determines peripheral glucose levels, and their dysregulation is associated with diabetes and liver disease. mTOR controls glucose transporter expression in several tissues, including the liver.30 Two glucose transporters in particular mediate glucose homeostasis [ie, SLC2A1 (GLUT1) and SLC2A4 (GLUT4)] (Figure 2). SLC2A4 is a highly efficient glucose transporter expressed in the liver, adipose tissue, skeletal muscle, and cardiac muscle. Its expression is up-regulated via the insulin receptor signaling pathway and aids in the response to glucose levels in brown adipose tissue.30 SLC2A1, on the other hand, is ubiquitously expressed, but functions similarly to SLC2A4 in that it promotes glucose uptake into cells. SLC2A1 is directly up-regulated by MTORC2 through SGK1 signaling (Figure 2). Together, these glucose transporters determine the amount of glucose that enters a cell, and consequently glucose levels drive what metabolic processes a particular cell will engage. In the organism, after glucose is metabolized, the relative amount of energy storage versus expenditure is partially dependent on two main types of adipose tissue: brown adipose tissue (BAT) and white adipose tissue (WAT). There are major differences in how these tissues use glucose (ie, BAT converts glucose to heat, whereas WAT promotes lipogenesis).31 Diet can directly affect the size of WAT adipose tissue depots,32 and adipose tissue metabolism is controlled by hormonal stimuli.
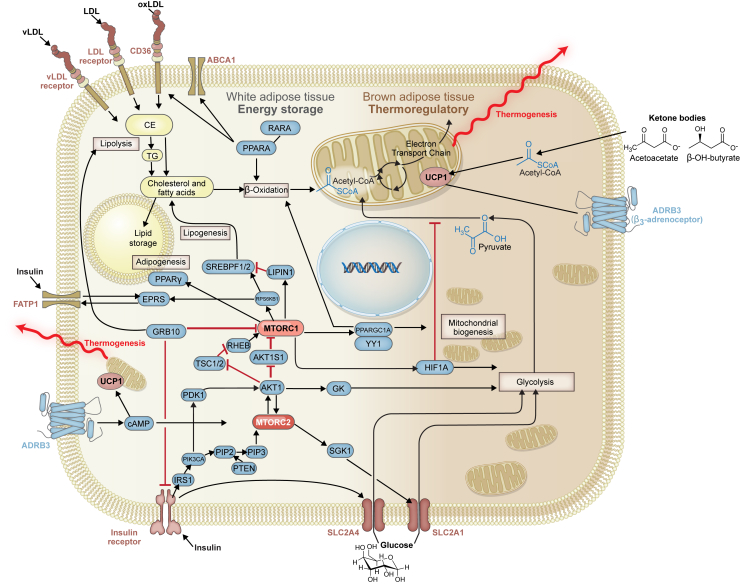
Figure 2.
Mammalian target of rapamycin (mTOR)–regulated pathways in white adipose tissue and brown adipose tissue. mTOR-related signaling regulates the metabolic capacity of adipose tissue, as well as its lipid storage and thermogenic potential. For example, a variety of modified lipoproteins, once bound to their receptors, can activate free and esterified cholesterol pathways affecting fatty acid oxidation, sterol storage, lipogenesis and lipolysis, and adipogenesis. Transcription factors, such as the peroxisome proliferator-activated receptors (PPARs) and sterol regulatory element-binding protein 1/2 (SREBP1/2), can be activated by the mTOR complexes once they are stimulated by effector molecules, such as phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase (PTEN), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), and phosphatidylinositol (4,5)-bisphosphate (PIP2). PPAR and SREBP are important in the regulation of cholesterol metabolism and its storage in the cell. EPRS, glutamyl-prolyl tRNA synthetase; LDL, low-density lipoprotein; MTORC1, mTOR complex 1; MTORC2, mTOR complex 2; PDK1, phosphoinositide-dependent kinase-1; PIP3, phosphatidylinositol (3,4,5)-trisphosphate.
In response to low glucose levels, gluconeogenesis and ketone body formation are up-regulated by MTORC1 in a peroxisome proliferator-activated receptor A (PPARA)–dependent mechanism. Mutagenesis experiments on molecules of the MTORC1 pathway have demonstrated PPARA's critical role in maintaining homeostasis during bouts of low glucose intake: ras-related GTP-binding protein A (RRAGA),33 Sestrin,34 and pancreatic β cell−specific TSC135 mutations inhibit the ability to respond to low blood glucose. Moreover, fasting-induced inhibition of MTORC1 and increases in free fatty acids work synergistically to activate PPARA, whose downstream effects on metabolism are multifold. PPARA activates the retinoic acid receptor through the formation of a PPARA−retinoic acid receptor heterodimeric complex, and this interaction is required for the activation of genes implicated in fatty acid oxidation (Figure 2).36 In addition, the interaction of MTORC1 with PPAR coactivator activates fibroblast growth factor 21, which is involved in maintaining and increasing thermogenesis.37 Thus, MTORC1 is critical in modulating glucose levels and its trafficking into cells.
Amino Acid Metabolism
Another critical metabolic pathway is the metabolism of proteins into amino acids. Provided by both the diet and de novo biosynthesis, amino acids are the building blocks of proteins, and are additionally used for a variety of signaling pathways. Amino acids are transported into cells by several amino acid exchanger proteins, including SLC7A5 and SLC3A3.38 These transporter proteins are abundantly expressed on a variety of cells, including hepatocytes. Depriving cells of amino acids leads to suppression of MTORC1 activity, which in turn increases autophagy,39 a form of intracellular amino acid recycling.40 Globally, several experiments demonstrate that amino acid removal or depletion mimics MTORC1 inhibition through rapamycin treatment.41, 42 Recently, it was discovered that the RNA-binding protein nuclear FMR1 interacting protein 1 (NUFIP1) is a novel regulator of autophagy, which is required for MTORC1 reactivation after prolonged arginine starvation and ribosome autophagy.43 Given the importance of amino acid metabolism, this finding may have broad implications for how cells handle nutrient stress, and directly implicates mTOR in amino acid metabolism.
MTORC1 regulation by amino acids involves the lysosomal-bound Ras homolog, mTORC1 binding (RHEB) and the heterodimeric RRAGA/B-RRAGC/D proteins.18 Guanine-nucleotide exchange factor (alias GEF) activity regulates the function of the lysosomal-bound Ragulator complex, which senses intralysosomal amino acid concentrations. MTORC1 comprises LAMTOR3 (late endosomal/lysosomal adaptor, MAPK and MTOR activator 3; alias MP1), p14, p18, HBXIP, and C7ORF59.19 When RRAG is GTP bound, it interacts with MTORC1 and recruits it to the lysosomal surface. On the lysosomal surface, MTORC1 interacts with RHEB and becomes activated, thus acting as a positive regulator of cellular growth.44 RHEB is independently regulated by TSC1/2, as well as a variety of growth factor signals and oxygen. It also appears that MTORC1 activity in response to amino acid concentrations is also regulated via other protein-protein signaling interactions. For example, increased lysosomal arginine concentration disrupts CASTOR1-GATOR2 binding, leading to MTORC1 activation (Figure 1).45 Similarly, in the absence of leucine, cytosolic sestrin2 interacts with GATOR2 to inhibit MTORC1 signaling (Figure 1).46 Thus, cells contain sensors that modulate MTORC1 activity when arginine or leucine concentrations change. Given mTOR's role as a nutrient sensor, these recent findings highlight the need for further research into how amino acids collaborate with cellular metabolism.
Additional evidence that mTOR is important for leucine metabolism comes from recent work showing that leucyl-tRNA synthetase binds RRAGD, functions as a GTPase-activating protein for RRAGD, and is dependent on leucine concentration (Figure 1).47 Mutations in the tumor suppressor gene folliculin (FLCN), a gene required for proper amino acid sensing, have been associated with increased numbers of mitochondria in adipocytes.48 FLCN is believed to interact with mTOR via a unique arm of the mTOR pathway involving transcription factor E3, and in the absence of FLCN, browning of white fat can occur.48 This has been found to have relevance for obesity and diabetes, as BAT has been found to be protective against both.
Recent discoveries regarding the mechanism of mTOR's sensing of amino acids have revealed the complexities of this process. For instance, the structure of GATOR1 via cryo–electron microscopy revealed new binding modes necessary for function.49 Furthermore, it was also recently discovered that MTORC1 is inhibited by S-adenosyl methionine through an additional regulatory protein SAMTOR, when bound to GATOR1 (Figure 1).50 This signaling has implications in the regulation of food intake and levels of cellular metabolites.50 Despite this, it is not known how S-adenosyl methionine limitation in the context of the MTORC1 regulatory pathway can affect health or disease. Collectively, these findings highlight a unique role for mTOR in amino acid metabolism, and further details of the contribution of mTOR to this cellular process remain to be fully elucidated.
Lipid Metabolism
Lipid metabolism is vastly important for the proper function of tissues, and the metabolic pathways that regulate this process are thus important for health. MTORC1 and MTORC2 play a critical role in lipid metabolism.51, 52 Lipid catabolism involves CD36, which scavenges lipoproteins and oxidized lipids. CD36 is regulated by PPARA, which itself is directly regulated by MTORC1. Moreover, carnitine acyl transferase, which facilitates free fatty acid transfer to the inner mitochondrial membrane, is also regulated by MTORC1. Evidence for this is provided by the fact that rapamycin-mediated inhibition of MTORC1 increases the expression of carnitine acyl transferase and, subsequently, β-oxidation of lipids.53, 54, 55 Finally, a recent discovery involving the fatty acid transporter SLC27A1 (alias FATP1) demonstrated that glutamyl-prolyl tRNA synthetase is phosphorylated and regulated by the MTORC1-linked protein RPS6KB1 (Figure 2).56 Thus, the mTOR pathway regulates various signaling molecules involved in the regulation of fat metabolism.
MTORC1 is also involved in the oxidation of fatty acids, both in terms of β-oxidation and ketosis. For example, deletion of mTOR specifically in cardiac tissues depletes enzymes that mediate fatty acid β-oxidation.57 Moreover, MTORC1 activates PPAR coactivator via a multistep signaling cascade. This results in an increased mitochondrial metabolic rate through β-oxidation. MTORC1 activity also regulates several acyl-CoA dehydrogenase enzymes, which are involved in the first of four steps of β-oxidation.58
Lipid catabolism also involves ketogenesis, a set of chemical reactions that generate acetyl-CoA and drive ATP synthesis. The anabolic portion of ketogenesis occurs in the mitochondria of hepatocytes, where enzymes responsible for acetyl-CoA synthesis are up-regulated in response to low insulin. During ketosis, biosynthesis of glycogen, proteins, and lipids decreases, whereas ketone body synthesis increases (acetoacetate and β-hydroxybutyrate). Ketone bodies then circulate throughout the organism to allow continued ATP synthesis even in glucose-deprived conditions and prevent protein degradation and muscle tissue breakdown. Inhibition of mTOR by rapamycin and two different states of ketosis (caloric restriction and low carbohydrate diets) elicits similar beneficial effects, suggesting potential overlap between ketogenic regulatory mechanisms and mTOR signaling.59
Finally, ketone bodies have diverse effects during different ketogenic states. When ketone body concentration is low in mice, AKT1 signaling is universally decreased. This yields decreased mTOR kinase activity in the brain and liver and increased AMP kinase signaling in the liver.60 Recently, it was shown that hepatic IQ motif containing GTPase activating protein 1 (IQGAP1) expression, which is known to regulate serum glucose levels, and even bind directly to mTOR,61, 62 also increases β-oxidation and ketogenesis in a PPARα- and mTOR-related manner.63 In the brain, ketogenic metabolism regulated via MTORC1 reduces levels of phosphorylated S6, which, in turn, inhibits protein synthesis and cell growth.64 Moreover, the thermogenic potential of BAT is directly stimulated by ketone bodies and mitochondrial size (Figure 2).32
To date, evidence-based medicine lacks a true coherent understanding of the benefits and risks of low carbohydrate diets, especially given the proclivity to conflate health benefits with weight loss benefits.65 In the future, systematic analyses of human patients in ketosis may reveal more useful, interpretable end points that will separate misleading health claims from actual clinical outcomes. Undoubtedly, mTOR biology will continue to play a central role in understanding how these diets contribute to overall health.
mTOR and Adipose Tissue Regulation
mTOR plays a central role in adipose tissue development and metabolic activity. Energy homeostasis, including adipogenesis, lipid metabolism, and thermogenesis, is linked to mTOR signaling.66 The relative proportions of WAT and BAT partially determine an individual's resting metabolic rate.67 Collectively, these observations have implications for the pathogenesis of both diabetes and heart disease.
Insulin and mTOR
One of the most important signaling molecules is insulin. The insulin receptor is differentially expressed and exerts different downstream signaling effects across tissue types, collectively contributing to an individual's stores of WAT and BAT (Figure 2).68 Interestingly, MTORC1 can be refractory to stimulation by insulin, particularly when there are low levels of amino acids present in the cell. Hence, mTOR may be a nutrient sensor under this scenario and regulate storage of fat.
In addition to regulating lipogenesis and adipogenesis, the insulin receptor also plays important roles in regulating mTOR signaling.69 In its bound (and active) conformation, the insulin receptor dimerizes and autophosphorylates itself, which in turn activates insulin receptor–related substrate 1. Phosphoinositide 3-kinase is subsequently recruited and activated, and converts phosphatidylinositol (4,5)-bisphosphate into phosphatidylinositol (3,4,5)-trisphosphate.70 Finally, phosphatidylinositol (3,4,5)-trisphosphate activates the phosphoinositide 3-kinase–AKT1 signaling pathway, which directly activates MTORC2. AKT1 can also be activated by phosphoinositide-dependent kinase-1, which is an additional activation target of phosphoinositide 3-kinase. Thus, mTOR is pivotal in the control of cell proliferation and survival, given insulin's important role in maintaining nutritional homeostasis.
Activation of MTORC1 by the insulin receptor via AKT1 eventually leads to increased expression of the sterol regulatory element-binding proteins, which are the master regulatory proteins that control lipogenic and steroidogenic genes. MTORC1 does this via two mechanisms: phosphorylation of the ribosomal protein RPS6KB171 and phosphorylation and inhibition of nucleus translocation of lipin 1 (LPIN1), a protein that inhibits sterol regulatory element-binding protein binding to and regulation of lipogenic genes (Figure 2).72 Thus, there is a dual MTORC1-mediated control system over sterol regulatory element-binding protein–regulated lipogenesis and steroidogenesis in adipose tissue.
Finally, insulin receptor signaling activates the mitogen-activated protein kinase pathway via growth factor receptor–bound protein 2, a promotor of MTORC1 activity. MTORC1 activation can also induce lipolysis via a different adaptor protein, growth factor receptor–bound protein 10, which is a negative regulator of MTORC1 and insulin signaling.73 Growth factor receptor–bound protein 10, which is overexpressed in BAT similar to uncoupling protein 1 (UCP1), and is induced in WAT through exposure to cold, causes WAT to become increasingly thermogenic (browning).74 Thus, the inhibition of MTORC1 in WAT via any pathway regulated by insulin has the effect of partially phenocopying BAT.
In summary, in response to feeding and subsequent insulin release, mTOR can promote increased lipogenesis through a complex and multifaceted set of tightly controlled and highly interwoven metabolic regulatory pathways. In adipocytes in particular, MTORC2 is especially important in regulating lipogenesis because it regulates insulin sensitivity via phosphorylation of AKT1. Thus, mTOR is a universal rheostat able to dictate how cells metabolize, store, and use ingested nutrients. This is driven by both the types of food eaten (eg, carbohydrates) as well as by the hormonal milieu (eg, insulin).
Energy Storage and Use in WAT and BAT
In response to food intake, energy can be stored or used. Energy is stored in the form of triglycerides and cholesterol esters. When lipolysis is activated in adipose tissue, fatty acids are cleaved, then oxidized to form ATP. When energy is used, this use can be productive (ie, work necessary for the organism to survive) or unproductive (ie, accumulating chemiosmotic potential energy to use in the form of heat above and beyond the baseline need). Different adipose tissues perform these tasks, and mTOR is a key protein modulating this metabolic process.
In WAT, insulin-sensitive MTORC1 inhibits the activation of PPARA, and therefore downstream β-oxidation, leading to increased lipid storage,75 and potential changes in an individual's ratios of WAT/BAT also occur. For example, β-aminoisobutyric acid, a metabolite of the amino acid valine, which is produced during exercise and excreted into the periphery in low μmol/L quantities,76 binds PPARA receptors and, through activation of PPAR coactivator, can control the browning of WAT. This pathway overlaps with the insulin receptor signaling arm of the mTOR regulatory pathway. Recently, it was shown that protein kinase A activation through MTORC1 is also critical for the browning process of WAT.77 Thus, we are still defining how WAT responds to hormonal signals that also engage the mTOR pathway. Given that WAT and BAT perform such biologically divergent functions (storage versus use), further studies will be necessary to understand the metabolic and health implications of WAT metabolism.
BAT expresses UCP1, a protein responsible for decoupling the chemiosmotic gradient formed by the electron transport chain. This process occurs at a significantly higher rate in BAT than WAT and is thermogenic, meaning it releases heat instead of generating ATP. In an mTOR-regulated process, BAT uses glucose from the periphery for heat generation, which can influence overall blood glucose stores.30 Indeed, the expression of SLC2A4 on BAT directly correlates with thermogenesis. This process is directly opposed by insulin, specifically in BAT and some types of WAT.78 It has also been shown that β3-adrenoceptor–stimulated uptake of glucose in brown adipocytes occurs in a two-step process involving mTOR: first, cAMP drives the rapid expression of SLC2A1, and second, SLC2A1 is transported to the plasma membrane in an MTORC2-dependent manner.79 In effect, glucose uptake in BAT is directly regulated by mTOR, and drives thermogenesis in BAT.79 The connection between adipose tissue and overall metabolic activity is mTOR regulated, and the pharmacomodulatory potential of this metabolic activity can provide potential methods to target this pathway.
mTOR also regulates adipose tissue by modulating the differentiation of mesenchymal stem cells into mature brown adipocytes.80 Indeed, the RPS6KB1 protein, which is activated by MTORC1, is important for initial adipocyte differentiation. It has been shown that RPS6KB11−/− mice have reduced body fat mass and adipocyte size as well as an elevated rate of lipolysis.81, 82 Conversely, knockout of the eukaryotic translation initiation factor 4E binding protein 1 (EIF4EBP1) protein, which is inhibited by MTORC1 (Figure 1), results in obesity and increased resistance to insulin.83
Finally, mTOR has been shown to directly regulate the electron transport chain. Specifically, PPAR coactivator controls mitochondrial oxidative phosphorylation in response to food intake and subsequent changes in hormonal status84, 85 by interacting with the transcription factor yin-yang 1 in an MTORC1-dependent manner. This mTOR-regulated signaling ultimately influences WAT/BAT tissue distribution within an organism, as well as the browning of WAT, which may have important therapeutic implications for the treatment of metabolic diseases.86
The Role of mTOR in Cardiovascular Disease
There are specific examples where alterations of adipose tissue metabolism can impact cardiac health, particularly when there is an accumulation of triglycerides in adipocytes. This can lead to stress on the heart. As studies of mTOR advance, new insights into mTOR's therapeutic potential are becoming apparent because mTOR regulates cardiac health in several ways, including via its actions on adipose tissue. For instance, cardiac tissues secrete atrial natriuretic peptide and B-type natriuretic peptide when under stress. Although it was known that the β-adrenergic–cAMP–protein kinase A pathway increases browning of adipose tissue via mTOR signaling (Figure 2), it was recently shown that browning also occurs in response to cGMP via guanylyl cyclase and natriuretic peptide receptor A signaling as well.87
There are other examples of the role of mTOR in cardiac regulation. mTOR has been shown to be necessary for endothelial progenitor cell development, which has implications in angiogenesis, endothelial survival, and cardiomyocyte protection. In addition, the observation that inhibition of the mTOR pathways with rapamycin promotes endothelial progenitor cell death has been used clinically during heart transplant to reduce acute graft rejection.88 This fasting metabolic program is beneficial for tissue homeostasis and regeneration (including cardiac function). A novel therapeutic implication is that activation of this MTORC1 pathway through treatment with small molecules can mimic its beneficial effects, including the breakdown of lipids.89
In addition to cellular regeneration, the mTOR pathway plays an important role in the control of inflammation, a common feature of atherogenesis. Oxidative stress contributes to the onset of cardiovascular injury and affects metabolic and cardiopulmonary function. The release of reactive oxygen species leads to oxidative stress and promotes cell injury and aging. Both apoptosis and autophagy may result in cardiac injury through oxidative stress, which can eventually lead to cardiac failure. Moreover, autophagy can lead to the generation of oxygen and nitrogen free radicals, which can contribute to myocardial infarction. It was recently demonstrated that rapamycin treatment alleviates myocardial ischemia and reperfusion injury in an mTOR-dependent pathway.90
mTOR also senses the metabolic status of cells during angiogenesis in cardiac tissue. Angiogenesis is the process of new capillary formation, which is important for cardiac tissue protection and regeneration. Inhibition of mTOR signaling can lead to tissue remodeling through an increased matrix metalloproteinase-1 activity and, subsequently, impaired angiogenesis.
Furthermore, activation of mTOR can be protective against oxidant ischemic/reperfusion injury. Recent evidence from studies of Huntington disease patients highlights the connection between mTOR and cardiovascular function. In these patients, the mutant Huntingtin protein is expressed in heart tissue and causes cardiac malfunction, supporting epidemiologic studies, which reported higher rates of heart disease in this population.91 Two mouse models of Huntington disease have shown that inactivation of the MTORC1-RHEB complex and the resulting loss of MTORC1 function in cardiac tissue can cause cardiac malfunction in this disease.91
Finally, other studies have shown that cardiac function is tightly regulated by mTOR and requires glycogen synthase kinase 3 alpha (GSK3A)/B signaling. In GKS3A knockout mice, mTOR is activated and mice have age-related cardiac abnormalities.92 In addition, GKS3B inhibition exacerbates ischemic injury.93 Moreover, proteins that protect the heart against stress, like thioredoxin-1, are regulated by mTOR.94 Although it has been shown that mTOR is activated during myocardial tissue injury, it was not known until recently that mTOR directly binds to a thiol-disulfide oxidoreductase responsible for maintenance of cardiomyocytes during oxidative stress. These findings suggest a mechanism whereby mTOR can influence many of the intracellular events associated with atherogenesis.
Conclusions
Although strong connections between mTOR signaling and metabolic diseases have been made, questions regarding these pathways remain. For instance, it remains to be seen whether the mTOR pathway can be manipulated to shift the balance between WAT and BAT to combat metabolic diseases. Second, future studies should assess whether pharmacologic modification of mTOR signaling can be used to treat cardiac disease. These insights can be used to develop therapeutics that target mTOR. In fact, the role of mTOR as a rheostat to control the metabolic profile of cells during metabolic homeostasis and disease could be applied to current and future clinical interventions.
Footnotes
Supported by the National Center for Advancing Translational Sciences of the NIH award TL1 TR002386 (M.F.W.) and the Rosenfeld Heart Foundation (D.P.H.).
Disclosures: A.M.G. and D.P.H. consult for KOWA Pharmaceutical Corp. A.M.G. is a member of the Data Safety Monitoring Board for Ionis Pharmaceuticals and a Board Member of Esperion.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.11.013.
Supplemental Data
References
- 1.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sciarretta S., Forte M., Frati G., Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardestani A., Lupse B., Kido Y., Leibowitz G., Maedler K. mTORC1 signaling: a double-edged sword in diabetic beta cells. Cell Metab. 2018;27:314–331. doi: 10.1016/j.cmet.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Yu J.T., Miao D., Wu Z.C., Tan M.S., Tan L. Targeting the mTOR signaling network for Alzheimer's disease therapy. Mol Neurobiol. 2014;49:120–135. doi: 10.1007/s12035-013-8505-8. [DOI] [PubMed] [Google Scholar]
- 7.Te Morenga L., Mallard S., Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 8.Orozco L.J., Buchleitner A.M., Gimenez-Perez G., Roque I.F.M., Richter B., Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;3:CD003054. doi: 10.1002/14651858.CD003054.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Estruch R., Ros E., Martinez-Gonzalez M.A. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med. 2013;369:676–677. doi: 10.1056/NEJMc1306659. [DOI] [PubMed] [Google Scholar]
- 10.Fang Y., Hill C.M., Darcy J., Reyes-Ordonez A., Arauz E., McFadden S., Zhang C., Osland J., Gao J., Zhang T., Frank S.J., Javors M.A., Yuan R., Kopchick J.J., Sun L.Y., Chen J., Bartke A. Effects of rapamycin on growth hormone receptor knockout mice. Proc Natl Acad Sci U S A. 2018;115:E1495–E1503. doi: 10.1073/pnas.1717065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaeberlein M., Powers R.W., 3rd, Steffen K.K., Westman E.A., Hu D., Dang N., Kerr E.O., Kirkland K.T., Fields S., Kennedy B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 12.Fontana L., Partridge L., Longo V.D. Extending healthy life span: from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaizuka T., Hara T., Oshiro N., Kikkawa U., Yonezawa K., Takehana K., Iemura S., Natsume T., Mizushima N. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285:20109–20116. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saucedo L.J., Gao X., Chiarelli D.A., Li L., Pan D., Edgar B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 15.Sancak Y., Thoreen C.C., Peterson T.R., Lindquist R.A., Kang S.A., Spooner E., Carr S.A., Sabatini D.M. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Edinger A.L., Thompson C.B. An activated mTOR mutant supports growth factor-independent, nutrient-dependent cell survival. Oncogene. 2004;23:5654–5663. doi: 10.1038/sj.onc.1207738. [DOI] [PubMed] [Google Scholar]
- 17.Joy J.M., Gundermann D.M., Lowery R.P., Jager R., McCleary S.A., Purpura M., Roberts M.D., Wilson S.M., Hornberger T.A., Wilson J.M. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab (Lond) 2014;11:29. doi: 10.1186/1743-7075-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sancak Y., Peterson T.R., Shaul Y.D., Lindquist R.A., Thoreen C.C., Bar-Peled L., Sabatini D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim E., Goraksha-Hicks P., Li L., Neufeld T.P., Guan K.L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanchi N.E., Lancha A.H., Jr. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol. 2008;102:253–263. doi: 10.1007/s00421-007-0588-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D., Yang J., Yang L. Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev. 2017;2017:6437467. doi: 10.1155/2017/6437467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heberle A.M., Prentzell M.T., van Eunen K., Bakker B.M., Grellscheid S.N., Thedieck K. Molecular mechanisms of mTOR regulation by stress. Mol Cell Oncol. 2015;2:e970489. doi: 10.4161/23723548.2014.970489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vander Haar E., Lee S.I., Bandhakavi S., Griffin T.J., Kim D.H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin D., Colombi M., Moroni C., Hall M.N. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 25.Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R., Reese C.B., Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 26.Vanhaesebroeck B., Alessi D.R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346 Pt 3:561–576. [PMC free article] [PubMed] [Google Scholar]
- 27.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y., Lai E., Liu J., Lin J., Yang C., Jia C., Li Y., Bai X., Li M. IKK interacts with rictor and regulates mTORC2. Cell Signal. 2013;25:2239–2245. doi: 10.1016/j.cellsig.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Peterson T.R., Laplante M., Thoreen C.C., Sancak Y., Kang S.A., Kuehl W.M., Gray N.S., Sabatini D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Wei C., Xi J., Tang Z., Liang C. Glucose transporter 3 performs a critical role in mTOR-mediated oncogenic glycolysis and tumorigenesis. Oncol Lett. 2015;9:2809–2814. doi: 10.3892/ol.2015.3075. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Lee P., Bova R., Schofield L., Bryant W., Dieckmann W., Slattery A., Govendir M.A., Emmett L., Greenfield J.R. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 2016;23:602–609. doi: 10.1016/j.cmet.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava S., Baxa U., Niu G., Chen X., Veech R.L. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life. 2013;65:58–66. doi: 10.1002/iub.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori H., Inoki K., Opland D., Munzberg H., Villanueva E.C., Faouzi M., Ikenoue T., Kwiatkowski D.J., Macdougald O.A., Myers M.G., Jr., Guan K.L. Critical roles for the TSC-mTOR pathway in beta-cell function. Am J Physiol Endocrinol Metab. 2009;297:E1013–E1022. doi: 10.1152/ajpendo.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng M., Yin N., Li M.O. Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell. 2014;159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Efeyan A., Zoncu R., Chang S., Gumper I., Snitkin H., Wolfson R.L., Kirak O., Sabatini D.D., Sabatini D.M. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorla-Bajszczak A., Juge-Aubry C., Pernin A., Burger A.G., Meier C.A. Conserved amino acids in the ligand-binding and tau(i) domains of the peroxisome proliferator-activated receptor alpha are necessary for heterodimerization with RXR. Mol Cell Endocrinol. 1999;147:37–47. doi: 10.1016/s0303-7207(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 37.Cornu M., Oppliger W., Albert V., Robitaille A.M., Trapani F., Quagliata L., Fuhrer T., Sauer U., Terracciano L., Hall M.N. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc Natl Acad Sci U S A. 2014;111:11592–11599. doi: 10.1073/pnas.1412047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen R., Zou Y., Mao D., Sun D., Gao G., Shi J., Liu X., Zhu C., Yang M., Ye W., Hao Q., Li R., Yu L. The general amino acid control pathway regulates mTOR and autophagy during serum/glutamine starvation. J Cell Biol. 2014;206:173–182. doi: 10.1083/jcb.201403009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blommaart E.F., Luiken J.J., Blommaart P.J., van Woerkom G.M., Meijer A.J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–2326. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 40.Crespo J.L., Hall M.N. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2002;66:579–591. doi: 10.1128/MMBR.66.4.579-591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calder P.C., Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17:227–241. doi: 10.1007/BF01366922. [DOI] [PubMed] [Google Scholar]
- 42.Kafkewitz D., Bendich A. Enzyme-induced asparagine and glutamine depletion and immune system function. Am J Clin Nutr. 1983;37:1025–1030. doi: 10.1093/ajcn/37.6.1025. [DOI] [PubMed] [Google Scholar]
- 43.Wyant G.A., Abu-Remaileh M., Frenkel E.M., Laqtom N.N., Dharamdasani V., Lewis C.A., Chan S.H., Heinze I., Ori A., Sabatini D.M. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science. 2018;360:751–758. doi: 10.1126/science.aar2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar-Peled L., Schweitzer L.D., Zoncu R., Sabatini D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chantranupong L., Scaria S.M., Saxton R.A., Gygi M.P., Shen K., Wyant G.A., Wang T., Harper J.W., Gygi S.P., Sabatini D.M. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell. 2016;165:153–164. doi: 10.1016/j.cell.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfson R.L., Chantranupong L., Saxton R.A., Shen K., Scaria S.M., Cantor J.R., Sabatini D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han J.M., Jeong S.J., Park M.C., Kim G., Kwon N.H., Kim H.K., Ha S.H., Ryu S.H., Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 48.Wada S., Neinast M., Jang C., Ibrahim Y.H., Lee G., Babu A., Li J., Hoshino A., Rowe G.C., Rhee J., Martina J.A., Puertollano R., Blenis J., Morley M., Baur J.A., Seale P., Arany Z. The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 2016;30:2551–2564. doi: 10.1101/gad.287953.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen K., Huang R.K., Brignole E.J., Condon K.J., Valenstein M.L., Chantranupong L., Bomaliyamu A., Choe A., Hong C., Yu Z., Sabatini D.M. Architecture of the human GATOR1 and GATOR1-Rag GTPases complexes. Nature. 2018;556:64–69. doi: 10.1038/nature26158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu X., Orozco J.M., Saxton R.A., Condon K.J., Liu G.Y., Krawczyk P.A., Scaria S.M., Harper J.W., Gygi S.P., Sabatini D.M. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science. 2017;358:813–818. doi: 10.1126/science.aao3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soliman G.A. The integral role of mTOR in lipid metabolism. Cell Cycle. 2011;10:861–862. doi: 10.4161/cc.10.6.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamming D.W., Sabatini D.M. A central role for mTOR in lipid homeostasis. Cell Metab. 2013;18:465–469. doi: 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng T., Golub T.R., Sabatini D.M. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y., Kwok-Shing Ng P., Kucherlapati M., Chen F., Liu Y., Tsang Y.H. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell. 2017;31:820–832.e3. doi: 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morita M., Prudent J., Basu K., Goyon V., Katsumura S., Hulea L., Pearl D., Siddiqui N., Strack S., McGuirk S., St-Pierre J., Larsson O., Topisirovic I., Vali H., McBride H.M., Bergeron J.J., Sonenberg N. mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol Cell. 2017;67:922–935.e5. doi: 10.1016/j.molcel.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Arif A., Terenzi F., Potdar A.A., Jia J., Sacks J., China A., Halawani D., Vasu K., Li X., Brown J.M., Chen J., Kozma S.C., Thomas G., Fox P.L. EPRS is a critical mTORC1-S6K1 effector that influences adiposity in mice. Nature. 2017;542:357–361. doi: 10.1038/nature21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Y., Soto J., Anderson B., Riehle C., Zhang Y.C., Wende A.R., Jones D., McClain D.A., Abel E.D. Regulation of fatty acid metabolism by mTOR in adult murine hearts occurs independently of changes in PGC-1alpha. Am J Physiol Heart Circ Physiol. 2013;305:H41–H51. doi: 10.1152/ajpheart.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laplante M., Sabatini D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts M.N., Wallace M.A., Tomilov A.A., Zhou Z., Marcotte G.R., Tran D., Perez G., Gutierrez-Casado E., Koike S., Knotts T.A., Imai D.M., Griffey S.M., Kim K., Hagopian K., McMackin M.Z., Haj F.G., Baar K., Cortopassi G.A., Ramsey J.J., Lopez-Dominguez J.A. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2018;27:1156. doi: 10.1016/j.cmet.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDaniel S.S., Rensing N.R., Thio L.L., Yamada K.A., Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–e11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tekletsadik Y.K., Sonn R., Osman M.A. A conserved role of IQGAP1 in regulating TOR complex 1. J Cell Sci. 2012;125:2041–2052. doi: 10.1242/jcs.098947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen F., Zhu H.H., Zhou L.F., Wu S.S., Wang J., Chen Z. IQGAP1 is overexpressed in hepatocellular carcinoma and promotes cell proliferation by Akt activation. Exp Mol Med. 2010;42:477–483. doi: 10.3858/emm.2010.42.7.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erickson H.L., Sayeepriyadarshini A. Identification of IQ motif–containing GTPase-activating protein 1 as a regulator of long-term ketosis. JCI Insight. 2018;3:e99866. doi: 10.1172/jci.insight.99866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cota D., Proulx K., Smith K.A., Kozma S.C., Thomas G., Woods S.C., Seeley R.J. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 65.Gardner C.D., Trepanowski J.F., Del Gobbo L.C., Hauser M.E., Rigdon J., Ioannidis J.P.A., Desai M., King A.C. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319:667–679. doi: 10.1001/jama.2018.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai H., Dong L.Q., Liu F. Recent advances in adipose mTOR signaling and function: therapeutic prospects. Trends Pharmacol Sci. 2016;37:303–317. doi: 10.1016/j.tips.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 68.Boucher J., Softic S., El Ouaamari A., Krumpoch M.T., Kleinridders A., Kulkarni R.N., O'Neill B.T., Kahn C.R. Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes. 2016;65:2201–2213. doi: 10.2337/db16-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H.H., Huang J., Duvel K., Boback B., Wu S., Squillace R.M., Wu C.L., Manning B.D. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Czech M.P. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 71.Duvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M.G., MacKeigan J.P., Finan P.M., Clish C.B., Murphy L.O., Manning B.D. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A., Madden K.L., Carpenter A.E., Finck B.N., Sabatini D.M. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He W., Rose D.W., Olefsky J.M., Gustafson T.A. Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor, and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain and a second novel domain located between the pleckstrin homology and SH2 domains. J Biol Chem. 1998;273:6860–6867. doi: 10.1074/jbc.273.12.6860. [DOI] [PubMed] [Google Scholar]
- 74.Liu M., Bai J., He S., Villarreal R., Hu D., Zhang C., Yang X., Liang H., Slaga T.J., Yu Y., Zhou Z., Blenis J., Scherer P.E., Dong L.Q., Liu F. Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab. 2014;19:967–980. doi: 10.1016/j.cmet.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goto T., Lee J.Y., Teraminami A., Kim Y.I., Hirai S., Uemura T., Inoue H., Takahashi N., Kawada T. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res. 2011;52:873–884. doi: 10.1194/jlr.M011320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts L.D., Bostrom P., O'Sullivan J.F., Schinzel R.T., Lewis G.D., Dejam A., Lee Y.K., Palma M.J., Calhoun S., Georgiadi A., Chen M.H., Ramachandran V.S., Larson M.G., Bouchard C., Rankinen T., Souza A.L., Clish C.B., Wang T.J., Estall J.L., Soukas A.A., Cowan C.A., Spiegelman B.M., Gerszten R.E. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu D., Bordicchia M., Zhang C., Fang H., Wei W., Li J.L., Guilherme A., Guntur K., Czech M.P., Collins S. Activation of mTORC1 is essential for beta-adrenergic stimulation of adipose browning. J Clin Invest. 2016;126:1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dallon B.W., Parker B.A., Hodson A.E., Tippetts T.S., Harrison M.E., Appiah M.M.A., Witt J.E., Gibbs J.L., Gray H.M., Sant T.M., Bikman B.T. Insulin selectively reduces mitochondrial uncoupling in brown adipose tissue in mice. Biochem J. 2018;475:561–569. doi: 10.1042/BCJ20170736. [DOI] [PubMed] [Google Scholar]
- 79.Olsen J.M., Sato M., Dallner O.S., Sandstrom A.L., Pisani D.F., Chambard J.C., Amri E.Z., Hutchinson D.S., Bengtsson T. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J Cell Biol. 2014;207:365–374. doi: 10.1083/jcb.201403080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vila-Bedmar R., Lorenzo M., Fernandez-Veledo S. Adenosine 5'-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology. 2010;151:980–992. doi: 10.1210/en.2009-0810. [DOI] [PubMed] [Google Scholar]
- 81.Um S.H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M., Fumagalli S., Allegrini P.R., Kozma S.C., Auwerx J., Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 82.Carnevalli L.S., Masuda K., Frigerio F., Le Bacquer O., Um S.H., Gandin V., Topisirovic I., Sonenberg N., Thomas G., Kozma S.C. S6K1 plays a critical role in early adipocyte differentiation. Dev Cell. 2010;18:763–774. doi: 10.1016/j.devcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Bacquer O., Petroulakis E., Paglialunga S., Poulin F., Richard D., Cianflone K., Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117:387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin J., Handschin C., Spiegelman B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Finck B.N., Kelly D.P. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 87.Liu D., Ceddia R.P., Collins S. Cardiac natriuretic peptides promote adipose “browning” through mTOR complex-1. Mol Metab. 2018;9:192–198. doi: 10.1016/j.molmet.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miriuka S.G., Rao V., Peterson M., Tumiati L., Delgado D.H., Mohan R., Ramzy D., Stewart D., Ross H.J., Waddell T.K. mTOR inhibition induces endothelial progenitor cell death. Am J Transplant. 2006;6:2069–2079. doi: 10.1111/j.1600-6143.2006.01433.x. [DOI] [PubMed] [Google Scholar]
- 89.Mihaylova M.M., Cheng C.W., Cao A.Q., Tripathi S., Mana M.D., Bauer-Rowe K.E., Abu-Remaileh M., Clavain L., Erdemir A., Lewis C.A., Freinkman E., Dickey A.S., La Spada A.R., Huang Y., Bell G.W., Deshpande V., Carmeliet P., Katajisto P., Sabatini D.M., Yilmaz O.H. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. 2018;22:769–778.e4. doi: 10.1016/j.stem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma L.L., Ma X., Kong F.J., Guo J.J., Shi H.T., Zhu J.B., Zou Y.Z., Ge J.B. Mammalian target of rapamycin inhibition attenuates myocardial ischaemia-reperfusion injury in hypertrophic heart. J Cell Mol Med. 2018;22:1708–1719. doi: 10.1111/jcmm.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Child D.D., Lee J.H., Pascua C.J., Chen Y.H., Mas Monteys A., Davidson B.L. Cardiac mTORC1 dysregulation impacts stress adaptation and survival in huntington's disease. Cell Rep. 2018;23:1020–1033. doi: 10.1016/j.celrep.2018.03.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou J., Freeman T.A., Ahmad F., Shang X., Mangano E., Gao E., Farber J., Wang Y., Ma X.L., Woodgett J., Vagnozzi R.J., Lal H., Force T. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhai P., Sciarretta S., Galeotti J., Volpe M., Sadoshima J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oka S.I., Hirata T., Suzuki W., Naito D., Chen Y., Chin A., Yaginuma H., Saito T., Nagarajan N., Zhai P., Bhat S., Schesing K., Shao D., Hirabayashi Y., Yodoi J., Sciarretta S., Sadoshima J. Thioredoxin-1 maintains mechanistic target of rapamycin (mTOR) function during oxidative stress in cardiomyocytes. J Biol Chem. 2017;292:18988–19000. doi: 10.1074/jbc.M117.807735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.