Abstract
Necrotizing enterocolitis (NEC) is a devastating disease affecting premature infants with intestinal inflammation and necrosis. The neonatal intestinal inflammatory response is rich in macrophages, and blood monocyte count is low in human NEC. We previously found that NF-κB mediates the intestinal injury in experimental NEC. However, the role of NF-κB in myeloid cells during NEC remains unclear. Herein, inhibitor of kappaB kinase β (IKKβ), a critical kinase mediating NF-κB activation, was deleted in lysozyme M (Lysm)–expressing cells, which were found to be Cd11b+Ly6c+ monocytes but not Cd11b+Ly6c− macrophages in the dam-fed neonatal mouse intestine. NEC induced differentiation of monocytes into intestinal macrophages and up-regulation of monocyte recruitment genes (eg, L-selectin) in the macrophage compartment in wild-type mice, but not in pups with IKKβ deletion in Lysm+ cells. Thus, NF-κB is required for NEC-induced monocyte activation, recruitment, and differentiation in neonatal intestines. Furthermore, pups with Lysm-IKKβ deletion had improved survival and decreased incidence of severe NEC compared with littermate controls. Decreased NEC severity was not associated with an improved intestinal barrier. In contrast, NEC was unabated in mice with IKKβ deletion in intestinal epithelial cells. Together, these data suggest that recruitment of Ly6c+ monocytes into the intestine, NF-κB activation in these cells, and differentiation of Ly6c+ monocytes into macrophages are critical cellular and molecular events in NEC development to promote disease.
Necrotizing enterocolitis (NEC) is a devastating disease of premature infants characterized by intestinal inflammation and necrosis. Although studies suggest that NEC is mediated by an underdeveloped and compromised epithelial barrier allowing bacterial exposure to an immature immune system,1, 2 the mechanism remains unclear and no specific treatment is currently available. Several studies suggest that monocyte activation plays a role in NEC,3, 4, 5 and animal and human NEC tissues display a macrophage-rich infiltrate.3, 6 Infiltrating macrophages during inflammation typically result from the differentiation of monocytes that have left the bloodstream. However, not much is known about the early formative myeloid cell changes in the intestine during NEC, and whether monocyte activation and differentiation contribute to its pathogenesis remains unknown.
Many myeloid cell functions are mediated via the transcription factor NF-κB. On activation of receptors such as toll-like receptors, the catalytic subunit of the IKK complex, inhibitor of kappaB kinase β (IKKβ), becomes activated, which leads to NF-κB nuclear translocation and transcription of NF-κB–target genes, including cytokines/chemokines, adhesion molecules, and cell surface receptors.7 Our laboratory has previously shown that NF-κB is persistently activated in the intestine in a neonatal rat model of NEC8 and treatment with a specific NF-κB–inhibitory peptide decreased NEC-associated mortality and bowel injury.8 In a murine NEC model, NF-κB was found to be activated in both intestinal inflammatory cells and epithelial cells (IECs) before acute bowel injury.9 However, whether NF-κB activation in myeloid cells or epithelial cells plays a role in NEC-like injury remains unknown.
In the murine small intestine at birth, myeloid cells are mostly embryonic macrophages, which originate from the yolk sac or fetal liver.10 At 2 to 3 weeks of age, as the small intestine matures, macrophages derived from monocytes that originated in the bone marrow begin to replace most embryonic macrophages.10 Although both intestinal macrophages derived from monocytes and embryonic macrophages can be phenotypically identified by Cx3cr1 expression, these populations are functionally unique. Embryonic macrophages have proliferative potential and play a role in tissue debris removal11, 12, 13 and vascular development and remodeling.14, 15, 16, 17 In contrast, intestinal macrophages derived from monocytes are terminally differentiated, nonproliferative, and short lived, needing to be continuously replenished by recruitment and differentiation of blood monocytes. During their differentiation into monocyte-derived macrophages, monocytes up-regulate major histocompatibility complex (MHC)-II, which is sequentially followed by down-regulation of Ly6c and Ccr2 and up-regulation of Cx3cr1.18, 19, 20 Intestinal resident macrophages are anti-inflammatory and unresponsive to toll-like receptor stimulation.19 However, monocytes are toll-like receptor responsive and during inflammation can differentiate into inflammatory macrophages.19, 21 In the dam-fed (DF) neonatal intestine, the embryonic macrophage–rich macrophage compartment predominates.10 Herein, we hypothesize that during NEC, monocytes are recruited and differentiated into monocyte-derived macrophages in the neonatal intestine in an NF-κB–dependent manner and this process contributes to NEC pathogenesis.
To test this hypothesis, IKKβ, the upstream kinase of NF-κB activation in myeloid cells, was deleted using lysozyme M (Lysm)Cre/+–IKKβf/f mice, and it was determined whether NEC development was affected in a neonatal mouse model. Using this strategy, IKKβ was targetedly deleted in monocytes, followed by examination of whether monocyte recruitment and differentiation into monocyte-derived macrophages was increased during NEC and affected by deletion of IKKβ.
Materials and Methods
Animal Experiments
LysmCre/+ IKKβf/f, Villin Cre+/− IKKβf/f, and IKKβf/f were kindly provided by Drs. Michael Karin and Lars Eckmann (University of California, San Diego, San Diego, CA).
B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (mT/mG), Cx3cr1GFP/+, and wild-type C57BL/6J (B6) mice were purchased from Jackson Laboratory (Bar Harbor, ME). NF-κB luciferase reporter mice were a gift from Fiona Yull (Vanderbilt University, Nashville, TN). NEC was initiated in mice within 24 hours of birth as follows: Pups were separated from the dams and placed into a 37°C humidified incubator (Air-Shield Vickers Medical, Hatboro, PA). At entry into the 72-hour NEC protocol, the mice were given a dose of 108 colony-forming unit bacteria from our standardized murine adult commensal bacterial preparation22 and 5 mg/kg lipopolysaccharide by gavage. Pups were gavage fed with Esbilac formula (PetAg, Inc., Hampshire, IL) every 3 hours and exposed to brief episodes of asphyxia (60 seconds in 100% N2) followed by cold stress (4°C for 10 minutes) twice daily. Animals were observed for clinical signs of NEC, including severe abdominal distension, lethargy, and apnea. At signs of distress or at the end of the experimental period, animals were euthanized by decapitation. Tail biopsy was collected for genotyping. Survival time was recorded. Whole intestinal tissues were fixed in formalin, and tissue sections were stained with hematoxylin and eosin for evaluation by an investigator unaware of genotype results. Because the intestinal injury is typically patchy and scattered in this model, the entire intestine was fixed, sectioned, and examined. The injury was presented in two ways: both the grade and the extent of the injury. Because the prognosis of clinical NEC is determined by severe bowel injury, which leads to perforation and septic shock, the grade of histologic injury severity of the most affected area was first assigned after thorough examination of the bowel: grade I, injury limited to the tip of the villi; grade II, midvillous necrosis; grade III, complete villous necrosis; and grade IV, transmural necrosis. Severe NEC was defined as histologic grade ≥ II. The extent of the injury was then quantified. To calculate the injury score, the approximate percentage of each injured area was multiplied by its histologic injury index [1, tip of the villi, epithelial only; 2, villous injury with involvement of lamina propria (limited to mucosa); and 3, transmural necrosis]. The sum of the numbers obtained from each area was calculated for each pup. Pups that died before tissue collection were excluded from intestinal tissue assessment. Time points described refer to hours after NEC induction in experimental pups and their littermate DF controls. Specifically, 24-hour NEC and 24-hour DF controls are approximately 48 hours old and 48-hour NEC and 48-hour DF controls were approximately 72 hours old. For vascular perfusion studies, neonatal pups were anesthetized with 65 mg/kg of pentobarbital and injected by intracardiac infusion of 500 μL of 40 μg/mL Alexa Fluor–conjugated wheat germ agglutinin. Intestinal tissues were collected and fixed in formalin, paraffin embedded, and sectioned. Confocal vascular imaging was performed. The percentage of tissue section positive for wheat germ agglutinin was normalized to total tissue area using Fiji software version 1.51w (NIH, Bethesda, MD; https://fiji.sc), and results were expressed as fold-changes to controls. To block L-selectin, 20 μg of a neutralizing antibody (clone MEL-14) or IgG control antibody (Biolegend, San Diego, CA) was injected intraperitoneally into wild-type pups 2 hours before NEC induction. mRNA relative fold-increases were determined compared with DF IgG-injected littermates, and percentage changes between IgG-injected NEC or L-selectin antibody–injected NEC were reported. Mice were maintained in the Stanley Manne Research Institute barrier facility, and experiments were approved by the Institutional Animal Care and Use Committee.
Immunohistochemistry/Immunofluorescence
Formalin-fixed, paraffin-embedded sections were stained using standard immunohistochemical methods for Cd31 (Abcam, Cambridge, MA) or green fluorescent protein (GFP; Abcam) Vina Green. Briefly, slides were deparaffinized in a series of xylene and alcohol washes, followed by antigen retrieval with heated citrate buffer. Endogenous peroxidase was blocked, and slides were incubated with primary antibody overnight at 4°C. Slides were washed and incubated for 1 hour with horseradish peroxidase–conjugated anti-rabbit or anti-chicken secondary antibody (Dako, Carpinteria, CA) to detect anti-Cd31 and anti-GFP antibodies, respectively. After washing, slides were developed using chromogen detection (Dako). Immunofluorescence for Selectin-L (SELL), CD11b, CX3CR1, and GFP was performed after citrate buffer retrieval using antibodies from Santa Cruz Biotechnology (Dallas, TX), Novus (Centennial, CO), Biolegend, and Abcam, respectively. Negative controls omitting the primary antibody and single-stained controls were performed concomitantly. Immunofluorescence terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining was performed using the In Situ Cell Death Detection Kit following the manufacturer's instructions (Sigma, St. Louis, MO), followed by incubation with an antibody against GFP. Slides were mounted with antifade gold with DAPI (Life Technologies, Grand Island, NY), and images were acquired using an LSM510 Zeiss confocal microscope (Oberkochen, Germany) or Leica DMR-HC upright microscope (Wetzlar, Germany). CD68 immunohistochemical staining of human sections was performed on unstained paraffin-embedded sections (4 μm thick). Slides were deparaffinized with serial xylene treatments and subjected to heat-induced epitope retrieval with cell conditioning solution 2 (Ventana Medical Systems, Tucson, AZ). Immunolabelling was performed using a mouse anti-CD68 monoclonal antibody (KP-1, prediluted) on the automated Ventana Benchmark XT system using the biotin-free Ventana Optiview DAB IHC Detection Kit (Ventana Medical Systems).
Real-Time RT-PCR
RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer's instructions from tissues that were preserved in RNAlater (Qiagen) or from isolated cells. Reverse transcription was performed using the cDNA Archive kit (Life Technologies), and real-time PCR was performed using Power SYBR Green 2X master mix (Life Technologies) on an ABI 7500 Fast Real-Time PCR System (Thermo Fisher Scientifc, Waltham, MA). Primers spanned genomic DNA exon junctions to ensure specific amplification of mRNA. Sample values were normalized to glyceraldehyde-3-phosphate dehydrogenase, and fold-increases were calculated using the ΔΔCT method. Primer sequences were as follows: Ccl2, 5′-AAGCTGTAGTTTTTGTCACCAAGCT-3′ (forward) and 5′-TGGTTCCGATCCAGGTTTTTA-3′(reverse); IL-1β, 5′-TGACAGTGATGAGAATGACCTGTTC-3′ (forward) and 5′-GGACAGCCCAGGTCAAAGG-3′ (reverse); iNOS, 5′-CATCAGGTCGGCCATCACT-3′ (forward) and 5′-CGTACCGGATGAGCTGTGAA-3′ (reverse); TNF, 5′-GACCCTCACACTCAGATCATCTTCT-3′ (forward) and 5′-CCACTTGGTGGTTTGCTACGA-3′ (reverse); Il-6, 5′-TCGGAGGCTTAATTACACATGTTC-3′ (forward) and 5′-TGCCATTGCACAACTCTTTTCT-3′ (reverse); Mpo, 5′- CAGGACGTGAGGGTGACATG-3′ (forward) and 5′-GCTTCGTCTGTTGTTGCAGTGT-3′ (reverse); Mip2, 5′- TCAAGGGCGGTCAAAAAGTT-3′ (forward) and 5′-TTGCCTTTGTTCAGTATCTTTTGG-3′ (reverse); Mmp9, 5′-CCAGACGTGGGTCGATTCC-3′ (forward) and 5′-AGTAGTTTTGGATCCAGTATGTGATGTT-3′ (reverse); L-selectin, 5′-TGACGCCTGTCACAAACGA-3′ (forward) and 5′-GGCTGGCAAGAGGCTGTGT-3′ (reverse); and Itga4, 5′-CGACTTGAGAGGTGCTGTCTACATT-3′ (forward) and 5′-CAACGGCTACATCAACATATCCA-3′ (reverse).
LP Preparation and Flow Cytometry
To make lamina propria (LP) cell preparations, small intestines were cut longitudinally under a dissection microscope, carefully stripping away the mesenteric vasculature and pancreatic tissues. Tissue pieces were digested by shaking for 30 minutes at 37°C in phosphate-buffered saline supplemented with 5 mmol/L EDTA, 15 mmol/L HEPES, 1 mmol/L dithiothreitol, and 10% fetal bovine serum to release epithelial cells. Supernatants were discarded, and the tissues were washed in phosphate-buffered saline. The tissues were digested for 20 minutes in a Dulbecco's modified Eagle's medium buffer containing 1 mg/mL collagenase VIII (Sigma), 15 mmol/L HEPES, 0.1 mmol/L CaCl2, and 2% fetal bovine serum. Cells were filtered through a 40-μm strainer, centrifuged, washed, and enumerated. For flow cytometry, 1 × 106 cells were blocked in staining buffer (phosphate-buffered saline and 2% fetal bovine serum) with Fc block (Miltenyi Biotec, San Diego, CA) for 15 minutes on ice. Antibodies were then added for 20 minutes on ice. The antibody panel included Cd11b and MHC-II (BD Bioscience, Franklin Lakes, NJ); CD45 and F4/80 (ebioscience, San Diego, CA); and Ly6c, Ly6g, CD11c, and Cx3cr1 (Biolegend). Zombie live/dead discrimination (Biolegend) was used to gate out dead cells. When necessary for intracellular staining, cells were fixed and permeabilized (eBioscience), according to the manufacturer's instructions, and GFP was detected with anti-GFP antibody (Abcam). Data were collected on a BD LSR Fortessa and analyzed using FlowJo software version 10 (Tree Star, Ashland, OR). In the flow cytometry studies, Cd11b+ cells were defined as live, single, CD45+Cd11b+Ly6g−Side Scatterlo. For absolute cell counts CountBright absolute counting beads were added before flow cytometry acquisition, according to the manufacturer's instructions (Life Technologies).
Cell Isolation
Small intestinal LP cells were prepared, as described above, and incubated with biotin-labeled Cx3cr1 antibody (Biolegend), followed by anti-biotin microbeads, according to the manufacturer's instructions (Miltenyi Biotec). Cells were pelleted and lysed in RNA lysis buffer (Qiagen), and extraction was performed as described above.
Intestinal Permeability
Mouse litters were divided into two groups. One group was subjected to the NEC model for 24 hours, and the other group was left with the dam to be nursed (controls). At the end of the experiment, the pups in both groups were fasted for 2 hours, and then received 750 mg/kg of 10-kDa fluorescein isothiocyanate–dextran (FD-10S; Sigma) via gavage. Four hours later, blood samples were collected for fluorescence determination and the tails of the mice were biopsied for genotyping. Serum fluorescence was measured with a fluorescence spectrophotometer, and fluorescein isothiocyanate–dextran serum concentration was determined by comparison to a standard curve of known fluorescein isothiocyanate–dextran concentrations.
Luciferase Assay
Tails and small intestinal tissues were homogenized in 1 mL of lysis buffer. Freshly reconstituted luciferase assay buffer (100 μL) was added to 20 μL of tissue homogenate, and luciferase activity was detected with a luciferase assay system (Promega, Madison, WI). Results were expressed as relative light units normalized to intestinal protein concentration, as measured by the Bradford assay.
In Vivo Imaging
Pups were given luciferin (Gold Biotechnology, St. Louis, MO), 0.1 mg each, intraperitoneally, 10 minutes before each imaging. Pups were gently restrained with tape while bioluminescence images were taken using an intensified charge-coupled device camera (Xenogen IVIS imaging system; Xenogen Corp., Hopkinton, MA). Images were obtained at low binning for 5 seconds at the F8-sensitivity threshold, as these parameters were found to best detect intensity differences in neonatal pups while avoiding color saturation during image acquisition. A standard area over the abdomen was defined and used to analyze integrated photon intensity.
Statistical Analysis
Two-tailed t-test was used to evaluate differences between the two groups. When more than two groups were compared, analysis of variance analysis with a Tukey post test was performed. To evaluate differences in the incidence of severe NEC (grade ≥II), χ2 analysis was used. Animal survival data were analyzed by log-rank test. Differences were considered statistically significant when P ≤ 0.05. Error was calculated using SEM.
Results
NF-κB Activation Precedes Intestinal Injury as Early as 12 Hours, Followed by Expression of NF-κB–Target Genes in Intestinal Myeloid Cells during NEC Development
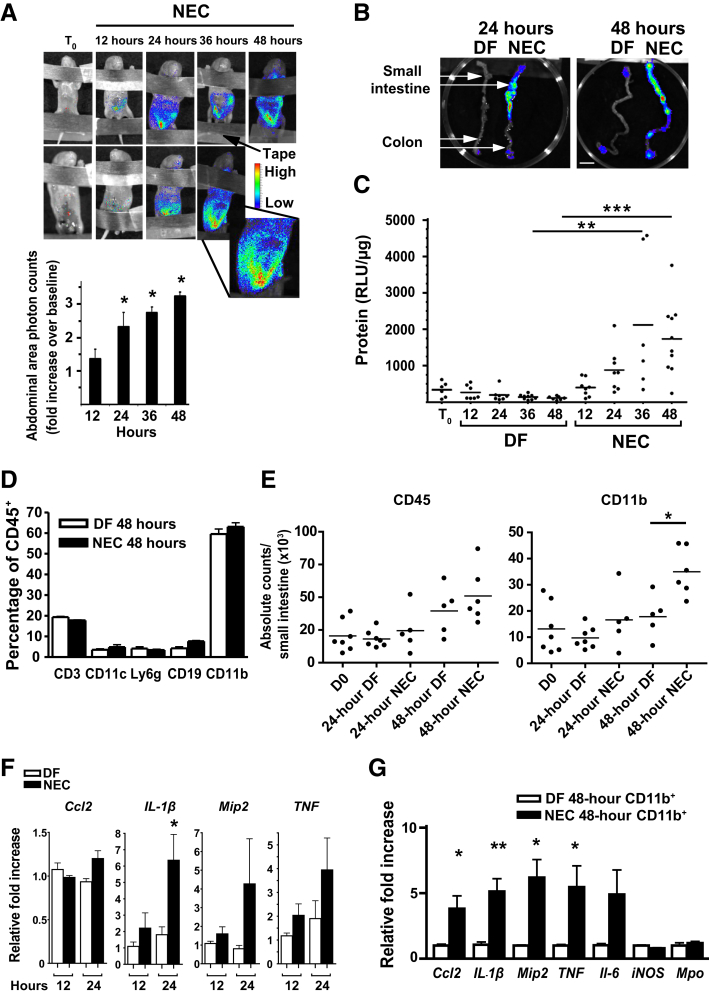
In an extensively characterized22 neonatal mouse NEC model, NEC typically develops between 48 and 72 hours, with intestinal injury only rarely detected before 36 hours.2 The studies presented herein examine pups between 12 and 48 hours because these early time points study the formative changes before disease onset. The term NEC will refer to pups that have been exposed to the NEC protocol for indicated times. Intestinal NF-κB–mediated transcription was directly investigated during the first 48 hours of NEC induction using mouse pups expressing NF-κB–driven luciferase. Luminescence induced by NF-κB–dependent luciferase was assessed by whole body imaging. A progressive increase was found in bioluminescence over the abdominal area of NEC pups (Figure 1A). This was detectable by 12 hours and strong at 24 and 36 hours compared with baseline (Figure 1A). To investigate whether NF-κB activation also occurred in other organs in NEC mice, intestines, lungs, hearts, livers, kidneys, and spleens were dissected from DF and NEC mice at 24 and 48 hours. When visualized with an IVIS camera, the NF-κB–dependent bioluminescence was predominantly observed in the small intestine, with lower detection in the colon (Figure 1B). However, no significant luminescence was detected in other organs (data not shown). Induction of luciferase during NEC was further examined on homogenized small intestinal tissues by luciferase assay. Although intestinal luciferase activity decreased over 48 hours in DF pups, intestines of NEC pups had a 2.5-fold increase in luciferase activity at 24 hours and a 3.125-fold increase at 48 hours compared with DF controls (Figure 1C).
Figure 1.
NF-κB is activated in the intestine of mice within 12 hours of exposure to a necrotizing enterocolitis (NEC) protocol, and myeloid cells express NF-κB–target genes on NEC induction. A: Less than 12-hour–old heterozygous NF-κB–reporter mice were subjected to NEC protocol and imaged every 12 hours with an IVIS camera. Luciferin (0.1 mg, intraperitoneally) was administered 10 minutes before each image acquisition. Top panel: Representative bioluminescence images from two mice obtained at time 0 (T0) and then every 12 hours during exposure to the NEC protocol are shown. Bottom panel: Abdominal area bioluminescence images were quantified, and results are presented as count-fold increase over baseline. Three to five pups were used per time point. B: Bioluminescence of NEC or dam-fed (DF) control intestines obtained at 24 and 48 hours. DF and NEC pups were euthanized simultaneously, and intestines were immediately dissected by two different investigators and placed on a petri dish to be immediately imaged. White arrows indicate the small intestine and the colon. C: Heterozygous NF-κB–reporter pups were placed on NEC protocol or allowed to be DF for different time periods, and luciferase activity of homogenized small-intestinal tissues was measured by in vitro assay. Luciferase activity normalized to protein concentration [relative light units (RLU)/μg] is presented. D: Percentage of specific leukocyte subsets from intestinal lamina propria of 48-hour DF control (open bars) and NEC pups (closed bars) determined by flow cytometry. Cells were gated on live, single, CD45+ cells. Cd11c+ refers to Cd11c+MHC-II+. Cd11b+ refers to Cd11b+Ly6g−. E: Absolute counts determined by flow cytometry. Gates were made on live, single cells and evaluated for CD45 expression or further gated on CD45+Ly6g−SSClo Cd11b+ cells. F: Inflammatory gene expression of isolated Cd11b+ cells from 12- to 24-hour NEC (closed bars) or control (open bars) pups, as measured by real-time PCR. G: Inflammatory gene expression of isolated Cd11b+ cells from either 48-hour NEC (closed bars) or control (open bars) pups, as measured by real-time PCR. Data are expressed as means ± SEM. n = 3 per group (D); n = 6 per group (F); n = 5 per group (G). ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001. Scale bar = 1 cm (B). D0, day 0.
In rats, NF-κB is activated in the inflammatory cells of the LP during acute bowel injury.8 Therefore, we sought to define the different leukocyte populations in the neonatal murine intestine during NEC compared with baseline. Small intestinal LP cells of DF and NEC pups were isolated at 48 hours, and the different populations of CD45+ cells were quantified by flow cytometry. Cd11b+ myeloid cells were found to be the largest population of leukocytes in both DF and NEC mice at this age (Figure 1D). Cd3+ (T cells and natural killer T cells), Cd19+ (B cells), Cd11b+Ly6g+ (neutrophils), and Cd11c+MHC-II+ (dendritic cells) were substantially less (Figure 1D). Smaller lymphoid populations (ie, natural killer and innate lymphoid) were not evaluated. The impact of NEC was then determined on the absolute count of CD45+ and CD11b+ cells in the small intestine at day 0, 24 hours, and 48 hours. Although the absolute number of CD45+ cells increased over time, the Cd11b population was significantly increased during NEC at 48 hours but not at 24 hours (Figure 1E).
To investigate NF-κB activity specifically in myeloid cells, Cd11b+ cells were isolated from 12-, 24-, and 48-hour DF and NEC pups by magnetic bead enrichment, and the expression of inflammatory genes was analyzed by real-time RT-PCR. When myeloid cells from NEC pups were compared with their DF littermates, the expression of IL-1β was increased at 24 hours (Figure 1F), and the expression of Ccl2, Mip2, and TNF was trending up at 24 hours (Figure 1F), becoming significantly up-regulated at 48 hours (Figure 1G). Because Cd11b+ cells enriched by this method also include neutrophils, Mpo mRNA was additionally examined. Relatively low levels of Mpo transcripts were detected in both DF and NEC pups, which was consistent with the low percentage of neutrophils detected by flow cytometry (Figure 1G).
These data show that NF-κB activation occurs in the intestine before significant tissue injury in the murine NEC model and that, by 48 hours, Cd11b+ myeloid cells are robustly producing NF-κB–dependent inflammatory genes in the neonatal intestine in NEC pups.
Monocytes (Ly6c+), which Are Specifically Targeted by Lysm-Cre Deletion in the Intestine of DF Pups, Differentiate into Macrophages during NEC Development through an IKKβ-Dependent Mechanism
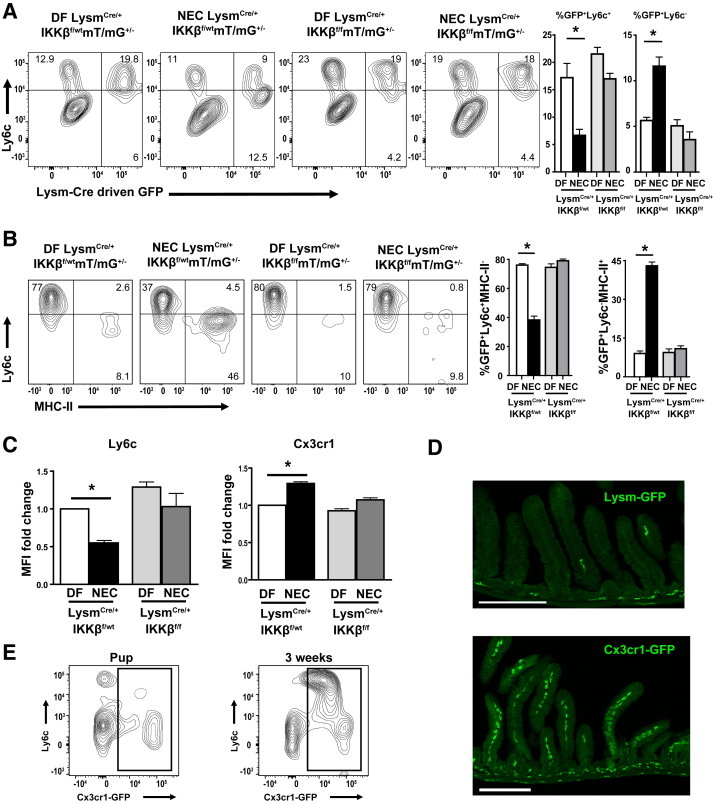
To interrogate the role of NF-κB in the myeloid cell compartment in this model, IKKβ was specifically deleted in myeloid cells using LysmCre/+-IKKβf/f mice. Although Lysm has been used as a global myeloid cell promoter to drive Cre expression in adult mice,23, 24 this strategy has never been tested to study neonatal intestinal myeloid cells. Thus, the populations of myeloid cells that express Lysm in the neonatal intestine and, therefore, would have IKKβ gene deletion in LysmCre/+-IKKβf/f mice, were first identified. LysmCre/Cre-IKKβf/wt mice were crossed with IKKβf/f mT/mG+/+ mice (mT/mG mice express GFP after Cre-dependent recombination), and litters containing approximately half LysmCre/+-IKKβf/f-mT/mG+/− (Lysm-IKKβ–deleted) and half LysmCre/+-IKKβf/wt-mT/mG+/− (heterozygous IKKβ–sufficient) littermate controls were generated. This strategy allowed the tracking of Lysm-Cre–expressing cells by GFP expression in both IKKβ-sufficient mice and in their Lysm-IKKβ–deficient littermates. When the myeloid cell population was examined by flow cytometry (gating strategy and F4/80 expression confirmation) (Supplemental Figure S1, A and B), Lysm-GFP+ cells were found to be mostly positive for Ly6c, an antigen expressed on blood monocytes but not on macrophages, in the LP of DF mice (Figure 2A). When IKKβ-sufficient mice were subjected to NEC for 24 hours and compared with their DF littermates, NEC induced a decrease in the percentage of Lysm+ cells expressing Ly6c+ and an increase in the percentage of Lysm+ cells that were Ly6c− (Figure 2A). However, in Lysm-IKKβ–deleted pups, NEC affected neither the percentage of Lysm+ cells expressing Ly6c nor the low baseline percentage of Lysm+ cells that were Ly6c− (Figure 2A).
Figure 2.
Lysozyme M (Lysm) expression is predominantly limited to monocytes in dam-fed (DF) pups but is detected in both monocytes and newly differentiated macrophages during necrotizing enterocolitis (NEC) in an inhibitor of kappaB kinase β (IKKβ)–dependent manner. A: Lamina propria (LP) cells were gated on live, single, CD45+Cd11b+Ly6g− SSClo to select the monocyte/macrophage population (total Cd11b+ cells). To identify intestinal LP Cd11b+ cells undergoing Lysm-dependent Cre-recombination, LP cells from NEC (24 hours) or DF control IKKβ sufficient (LysmCre/+-IKKβf/wt-mT/mG+/−) and IKKβ deleted (LysmCre/+-IKKβf/f-mT/mG+/−) mice were analyzed for Ly6c and for Lysm-Cre–dependent green fluorescent protein (GFP) expression on total Cd11b+Ly6g− cells by flow cytometry. Bar graphs show the percentage of Cd11b+Ly6g− cells that were GFP+Ly6c+ (left panel) and those that were GFP+Ly6c− (right panel). B: Cells were further gated on the Lysm-GFP+ population and analyzed for Ly6c and major histocompatibility complex (MHC)-II. Bar graphs show the percentage of GFP+ cells that were Ly6c+MHC-II− (left panel) and those that were Ly6c−MHC-II+ (right panel). C: The mean fluorescence intensity (MFI) of Ly6c and Cx3cr1 in Lysm-GFP+ cells was assessed. Bar graph represents the fold-increase in Ly6c or Cx3cr1 MFI over DF LysmCre/+-IKKβf/wt pup value. All experiments were repeated at least twice, and cells were fixed before data acquisition. D: Representative images of immunofluorescence staining for Lysm-GFP (top panel) from a DF 24-hour LysmCre/+IKKβf/wtmT/mG+/− (IKKβ-sufficient) pup or Cx3cr1-GFP (bottom panel) from a DF 24-hour Cx3cr1GFP/+ pup on formalin-fixed, paraffin-embedded tissue sections (16 μm thick). Z-stack confocal images are displayed as maximum projection. E: Representative flow cytometry plots from 24-hour (left panel) or 3-week–old (right panel) Cx3cr1GFP/+ mice showing lack of Cx3cr1 expression on Ly6c+ cells. Data are expressed as means ± SEM. n = 3 per group (C). ∗P ≤ 0.05. Scale bar = 200 μm (D). Original magnification, ×10 (D).
It was then determined whether the NEC-induced decrease in intestinal monocytes in IKKβ-sufficient pups was due to differentiation of Lysm+ monocytes into macrophages. Lysm-GFP+ cells were gated, and the expression of Ly6c and MHC-II was evaluated, which are down-regulated and up-regulated, respectively, during differentiation into monocyte-derived macrophages. In DF IKKβ-sufficient pups, most Lysm+ cells were monocytes (Ly6c+MHC-II−), with a small percentage of macrophages (Ly6c−MHC-II+) (Figure 2B). In contrast, during NEC, the largest portion of Lysm+ cells were macrophages (Ly6c−MHC-II+) with a much smaller population of monocytes (Ly6c+MHC-II−) (Figure 2B). A large portion of these macrophages (Ly6c−) expressed both Cx3cr1 and MHC-II (Supplemental Figure S1D). However, in DF and NEC Lysm-IKKβ–deleted pups, Lysm+ cells were mainly detected in the monocyte (Ly6c+MHC-II−) compartment but not the macrophage (Ly6c−MHC-II+) compartment (Figure 2B). In addition, in the Lysm-GFP+ population of IKKβ-sufficient pups subjected to NEC for 24 hours, mean fluorescence intensity (MFI) of Ly6c was found to be decreased (Figure 2C), MFI of Cx3cr1 was found to be increased (Figure 2C), and the CD64 (macrophage marker)/CD115 (monocyte marker) ratio was found to be increased (Supplemental Figure S1C). These findings are all consistent with increased monocyte differentiation in IKKβ-sufficient mice subjected to NEC for 24 hours but not in Lysm-IKKβ–deficient mice.
In the neonatal intestine, Lysm-GFP was only expressed in a low number of intestinal LP leukocytes when examined by immunofluorescence (Figure 2D). These data are consistent with the flow cytometry studies in which Lysm-GFP expression was limited to a small percentage of the total Cd11b population (Figure 2A). This is in contrast to abundant Cx3cr1 expression at this age (Figure 2D and Supplemental Figure S2). Notably, unlike mature Ly6c+ monocytes, neonatal Ly6c+ monocytes lack Cx3cr1 expression (Figure 2E).
Together, these results demonstrate that, in the neonatal intestine, Lysm is expressed predominantly in monocytes (Ly6c+) in DF pups. However, during NEC, Lysm is not only detected in Ly6c+ cells but also in Ly6c− cells in an IKKβ-dependent manner and suggests that intestinal Ly6c+ monocyte differentiation into macrophages is dependent on NF-κB signaling.
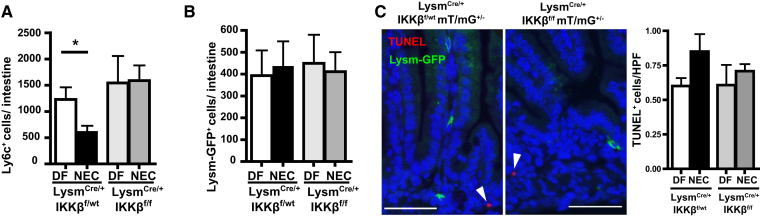
To determine whether the differentiation process led to a decrease in the absolute number of monocytes in intestinal tissues, the absolute cell counts were determined by flow cytometry. In IKKβ-sufficient pups, significantly fewer Ly6c+ cells were found in the small intestine of NEC compared with DF littermates. However, in Lysm-IKKβ–deleted pups, the numbers of Ly6c+ cells in NEC and DF were not significantly different (Figure 3A). The decrease in Ly6c+ cells during NEC in IKKβ-sufficient pups was not consequent to a decrease in Lysm+ cell number, which remained unchanged in all four groups (Figure 3B). Because only rare apoptotic cells were observed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining in the LP at 24 hours, the decrease in Ly6c+ cells is unlikely to be consequent to their apoptosis (Figure 3C). These rare apoptotic cells were not Lysm-GFP+ in either genotype. Moreover, there was no significant difference in terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling–positive cells at this early time point (Figure 3C). These data show that the decrease in intestinal Ly6c+ cells in NEC is not due to their apoptosis, and further confirm that decreased Ly6c expression is likely indicative of differentiation into intestinal monocyte-derived macrophages, which is abrogated when IKKβ is deleted in Lysm+ cells.
Figure 3.
Necrotizing enterocolitis (NEC) induces a decrease in intestinal Ly6c+ cells in inhibitor of kappaB kinase β (IKKβ)–sufficient mice, but not in lysozyme M (Lysm)–IKKβ–deleted pups. A and B: Absolute counts of Ly6c+ (A) and Lysm–green fluorescent protein–positive (GFP+) (B) expressing cells per small intestine were determined from at least three mice per group. C: Representative LysmCre/+IKKβf/wtmT/mG+/− and LysmCre/+IKKβf/fmT/mG+/− formalin-fixed, paraffin-embedded tissue sections from NEC pups stained for GFP (green), terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; red), and DAPI (blue); white arrowheads indicate TUNEL+ cells. Bar graph indicates TUNEL+ cells/high-power field (HPF) on four mice per group and four HPFs per slide. Data are expressed as means ± SEM. ∗P ≤ 0.05. Scale bar = 50 μm (C). DF, dam fed.
In NEC Development, the Intestinal Macrophage Compartment Is Enriched in Cells Expressing Genes Involved in Monocyte Recruitment and Infiltration in an IKKβ-Dependent Manner
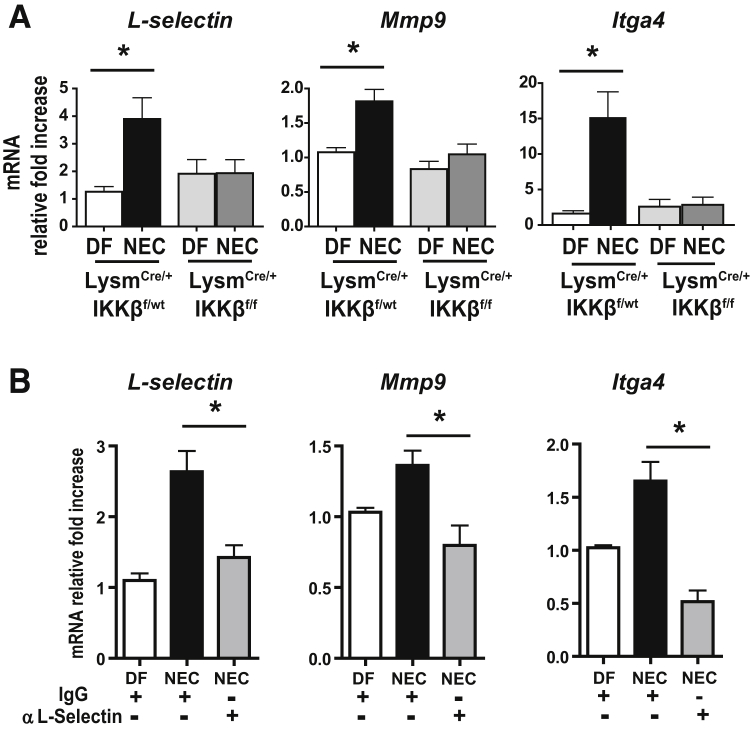
To determine whether NF-κB signaling may be involved in monocyte recruitment to intestinal tissues during NEC, the intestinal macrophage compartment was examined for its expression of genes associated with leukocyte recruitment [eg, L-selectin (Sell) and Mmp9], which are typically not expressed by embryonic macrophages.25, 26 These genes are also expressed in other leukocytes (L-selectin, eg, is present on neutrophils and T cells). However, because only isolated macrophages were studied in this experiment, these genes, described as monocyte-associated genes, were used to distinguish macrophages derived from monocytes from embryonic macrophages. Both L-selectin and Mmp9 were significantly increased during NEC in the Cx3cr1+ fraction of IKKβ-sufficient pups but not in Lysm-IKKβ–deleted pups (Figure 4A). Because both of these genes are NF-κB target genes, the NF-κB–independent leukocyte recruitment gene integrin α4 (Itga4) was also examined. Interestingly, this gene was also significantly increased in NEC in the Cx3cr1+ fraction of IKKβ-sufficient pups but not in Lysm-IKKβ–deleted pups. These results demonstrate that, during NEC, the Cx3cr1 fraction becomes enriched in monocyte-derived cells in IKKβ-sufficient pups but not Lysm-IKKβ–deleted pups. To determine whether blocking monocyte recruitment with antibodies against L-selectin would prevent monocyte enrichment of the Cx3cr1 fraction in NEC, wild-type pups were injected with a neutralizing antibody against L-selectin or IgG control 2 hours before NEC induction and LP Cx3cr1+ cells were isolated 24 hours after NEC induction. When L-selectin was blocked, decreased mRNA transcripts of L-selectin (45.91% ± 12.9%; P ≤ 0.01), Mmp9 (41.51% ± 12.51%; P ≤ 0.01), and Itga4 (59.76% ± 15.26%; P ≤ 0.05) were found in the Cx3cr1+ population compared with those treated with IgG control antibodies (Figure 4B). These results establish that the increase in monocyte-associated genes in the Cx3cr1 population during NEC occurs because of NF-κB–mediated monocyte recruitment.
Figure 4.
Monocyte-specific genes involved in recruitment and tissue infiltration are up-regulated in Cx3cr1+ lamina propria (LP) macrophages during necrotizing enterocolitis (NEC) in an inhibitor of kappaB kinase β (IKKβ)–dependent manner. A:L-selectin, Mmp9, and Itga4 mRNA expression from Cx3cr1+ cells isolated from LP cells of 24-hour dam-fed (DF) or NEC littermates with or without lysozyme M (Lysm)–dependent IKKβ deletion. B: Wild-type mice were injected with a neutralizing antibody against L-selectin 2 hours before NEC induction, and L-selectin, Mmp9, and Itga4 mRNA expression levels from LP Cx3cr1+ cells were analyzed. Bar graphs representing mRNA relative fold-increases compared with DF are presented. Data are expressed as means ± SEM. n ≥ 7 per group (A); n = 5 per group (B). ∗P ≤ 0.05.
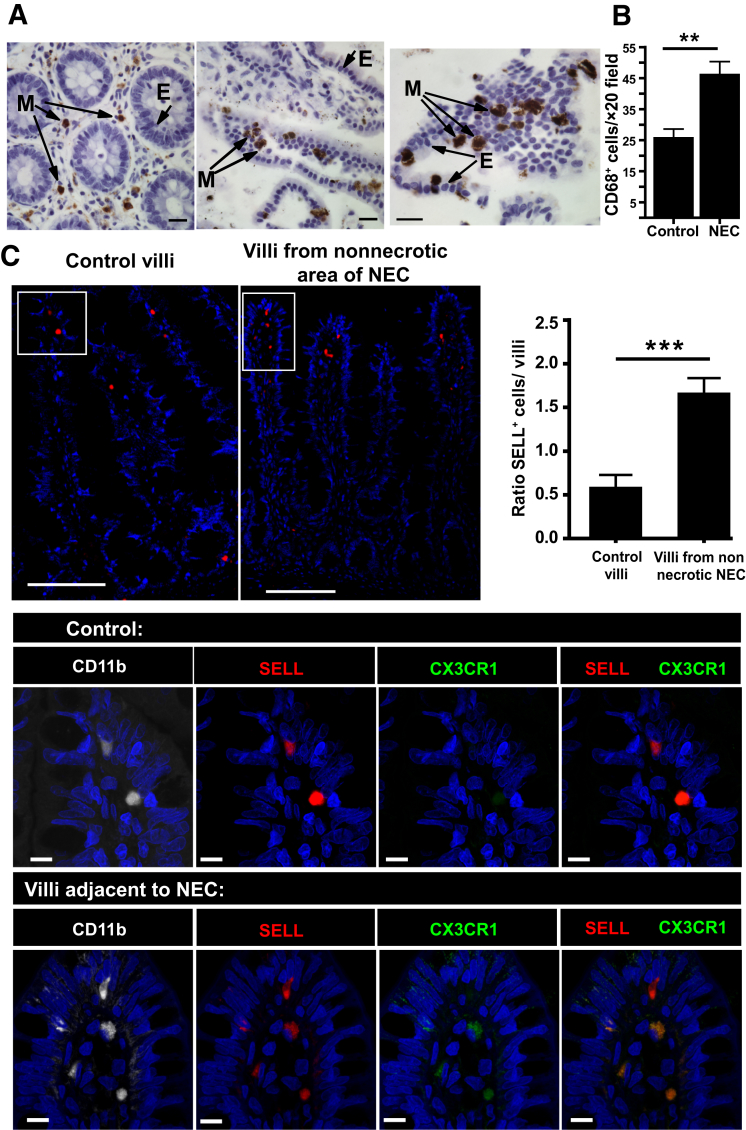
The Number of Myeloid Cells Expressing CD68 and SELL Is Increased in the Villi of Human NEC Tissues
When human tissues were examined by immunohistochemistry for CD68, it was found that myeloid cells were abundant in human control specimens but significantly increased in NEC tissues (Figure 5, A and B). To investigate whether SELL myeloid cells were also increased in human NEC, tissue sections from infants with or without NEC were stained for SELL, CD11b, and CX3CR1 (Figure 5C), and the numbers of SELL+ cells present in the LP of the villi in nonnecrotic areas of NEC and in control tissues were compared (Figure 5C). Control tissues were selected from small bowel without significant histopathological changes, such as from patients with Hirschsprung disease or ileal atresia. Although scarce in the neonatal intestine, the villous LP of NEC patients contained significantly more CD11b+ SELL+ cells compared with controls (Figure 5C). Furthermore, a slight change was found in the morphology of these cells from round to more irregular and these cells also had increased CX3CR1 staining (Figure 5C). Representative hematoxylin and eosin images of intestinal tissues are shown in the Supplemental Figure S3.
Figure 5.
Selectin-L (SELL)+ cell numbers are increased in the villous lamina propria (LP) of human necrotizing enterocolitis (NEC). A: Tissue specimens collected from the small intestine of patients undergoing resection for ileal atresia (left panel) or NEC (middle and right panels) were fixed in formalin and stained for the monocyte/macrophage marker CD68 by immunohistochemistry [myeloid cell (M) is brown; epithelial cell (E)]. B: Bar graph showing CD68+ cells/×20 field of control slides or from patients with NEC. C: Representative immunofluorescence images of intestinal tissues stained for SELL (top left panel) and SELL, CD11b, and CX3CR1 [bottom panels (magnified areas from the white boxed areas of the top panels)]. Top right panel: Bar graph shows the ratio of SELL cells per LP villi in NEC patients in areas adjacent to necrotic areas versus villi of non-NEC patient controls. Data are expressed as means ± SEM. n = 4 (B, patients per group with at least five ×20 fields per slide, and C, SELL+ cells per LP villi of non-NEC patient controls); n = 7 (C, SELL+ cells per LP villi in NEC patients in areas adjacent to necrotic areas). ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001. Scale bars: 20 μm (A); 100 μm (C, top panels); 10 μm (C, bottom panels). Original magnifications: ×10 (C, top panels); ×100 (C, bottom panels).
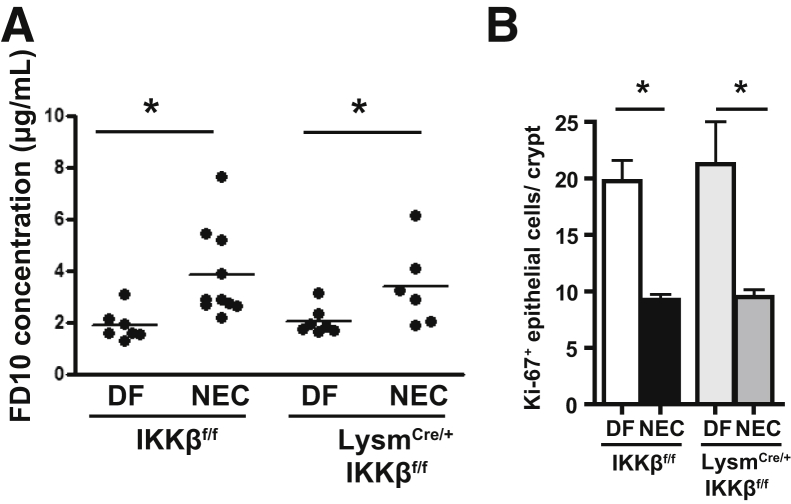
Deletion of IKKβ in Lysm+ Monocytes Does Not Ameliorate the NEC-Associated Increase in Gut Permeability
To determine whether decreased monocyte recruitment and differentiation in Lysm-IKKβ–deleted pups was indirectly due to an improved intestinal barrier, intestinal permeability was measured after oral gavage of fluorescein isothiocyanate–dextran (10 kDa). In these experiments, LysmCre/+-IKKβf/f or their Lysm+/+-IKKβf/f littermate controls (IKKβf/f; non-cre controls) were used because GFP reporter activity was unnecessary. It was first demonstrated that intestinal permeability is increased in IKKβ-sufficient pups after 24 hours of NEC compared with DF littermates (which is consistent with previous data from our laboratory2). The deletion of IKKβ in Lysm+ cells did not prevent NEC-induced increase in 24-hour intestinal permeability (Figure 6A). Next, epithelial cell proliferation was evaluated by Ki-67 staining at 24 hours. This parameter was used as an indicator of healthy epithelial cells, which should display robust proliferation. In NEC, both the IKKβ-sufficient and the Lysm-IKKβ–deleted pups displayed a similar decrease in epithelial cell proliferation (Figure 6B). These results indicate that the decreased monocyte recruitment and differentiation found in pups with Lysm-IKKβ deletion was not caused by an improved intestinal epithelial barrier.
Figure 6.
Inhibitor of kappaB kinase β (IKKβ) deletion in lysozyme M–positive (Lysm+) monocytes does not abrogate the increase in intestinal permeability associated with necrotizing enterocolitis (NEC). A: Intestinal permeability expressed as fluorescein isothiocyanate (FITC)–dextran plasma concentration 4 hours after administration of FITC-dextran by gavage in LysmCre/+-IKKβf/f and IKKβf/f mice exposed to the NEC protocol for 24 hours and dam-fed (DF) controls. B: Quantification of epithelial cell proliferation on formalin-fixed, paraffin-emebedded tissue sections stained for Ki-67 from 24-hour DF or NEC pups with or without Lysm-Cre expression. Counts represent the number of Ki-67+ epithelial cells per crypt in ≥20 well-oriented crypts from at least three mice per group. Data are expressed as means ± SEM. ∗P ≤ 0.05.
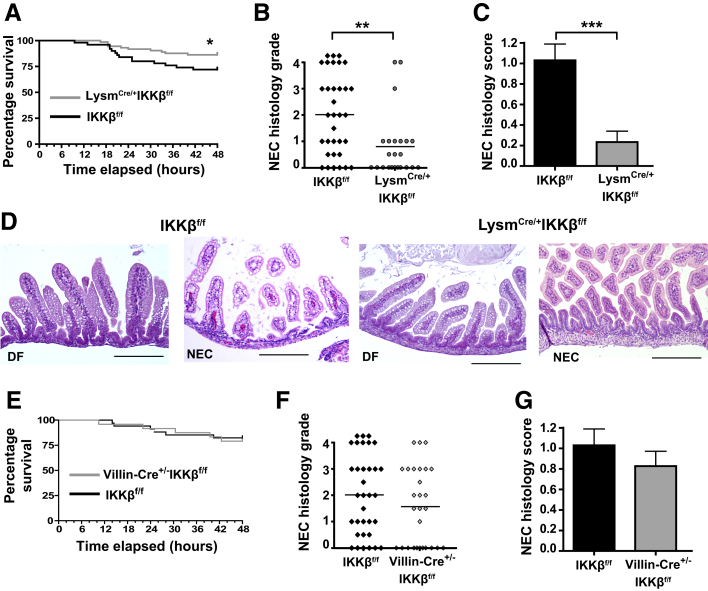
IKKβ in Lysm+ Cells Mediates Intestinal Tissue Injury in Experimental NEC
It was next determined if NF-κB activation in monocytes played a role in NEC pathogenesis. In Lysm-IKKβ–deleted mice, mortality was significantly decreased at 48 hours compared with their IKKβ-sufficient littermate controls (Figure 7A). Furthermore, the incidence of severe NEC was significantly reduced in the Lysm-IKKβ–deleted mice compared with the IKKβ-sufficient controls (Figure 7, B and D), as was the NEC histology score (Figure 7, C and D). Also, NEC-induced reduction in villous microvascular area was attenuated in Lysm-IKKβ–deleted mice compared with IKKβ-sufficient littermate controls (Supplemental Figure S4, D and E).
Figure 7.
Mice with inhibitor of kappaB kinase β (IKKβ)–deleted monocytes have increased survival and decreased intestinal disease severity when exposed to a necrotizing enterocolitis (NEC) protocol. A: Survival curve demonstrating decreased mortality in lysozyme M (Lysm)Cre/+-IKKβf/f mice when exposed to the NEC model. B and C: NEC histologic grades (χ2 = 8.89; B) and NEC histologic scores (C) of LysmCre/+-IKKf/f mice and controls (IKKβf/f). D: Representative hematoxylin and eosin staining of dam-fed (DF) and NEC LysmCre/+-IKKf/f mice and littermate controls (IKKβf/f) 72 hours into NEC induction. E: Survival curves demonstrating no change in mortality in Villin-Cre+/−-IKKβf/f mice when exposed to the NEC model. F and G: NEC histologic grades (F) and NEC histologic scores (G) of Villin-Cre+/−-IKKf/f and controls. Data are expressed as means ± SEM. n = 73 (A, LysmCre/+-IKKβf/f); n = 50 (A, IKKβf/f); n = 24 (E, Villin-Cre+/−-IKKβf/f); n = 34 (E, IKKβf/f). ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗P ≤ 0.001. Scale bar = 200 μm (D).
Given that our laboratory previously found NF-κB to be also activated in IECs during acute intestinal injury,9 IKKβ-deleted IECs (Villin-Cre+/−-IKKβf/f) were also examined in the NEC model. No difference was observed in NEC mortality between Villin-Cre+/−-IKKβf/f mice and their littermate controls (IKKβf/f) (Figure 7E). Furthermore, Villin-Cre+/−-IKKβf/f pups had a similar incidence of severe injury (NEC histology grade ≥II) (Figure 7F) and similar NEC histology scores (Figure 7G). Epithelial-specific Cre recombination in the neonatal period was confirmed by β-galactosidase staining of Villin-Cre+/−-ROSAlacZ mouse intestinal tissue (Supplemental Figure S4, A and B).
These data show that although IKKβ signaling in monocytes drives NEC-associated injury, no role was observed for IKKβ in IECs in this NEC model. Moreover, in Lysm-IKKβ–deleted mice, despite IKKβ deletion being limited to a relatively small population of monocyte-derived cells, these cells were found to play a role in NEC-associated histologic lesions and mortality. More important, these processes occur downstream of the compromised epithelial barrier.
Discussion
Herein, it was demonstrated that in the murine intestine during NEC, monocytes undergo differentiation into macrophages via an IKKβ-dependent mechanism. This differentiation process is associated with up-regulation of monocyte-associated gene expression in the intestinal macrophage compartment. It was prevented when monocyte adhesion to endothelial cells was inhibited by L-selectin antibody. Pups with Lysm-monocyte IKKβ deletion had improved survival and decreased incidence of severe NEC in comparison to their littermate controls. In human NEC specimens, a higher number of myeloid cells and SELL+ cells were found compared with control intestinal tissues. Together, these findings support a role for recruitment of Ly6c+ monocytes to the intestine and Ly6c+ monocyte differentiation into macrophages in NEC development, in a process mediated by NF-κB signaling.
This study, along with several previous studies, has observed that the cellular inflammatory response resulted in macrophage-rich infiltrates of intestinal tissues in human NEC27, 28, 29 and also in neonatal mouse NEC models. Most of this previous research dealt with late stages of disease when intestinal injury was already present.3, 30 Herein, the initial 24 hours during NEC development were studied to identify the formative changes in the myeloid compartment before disease. This early time point not only helps to discriminate the recently arrived monocytes but also allows the interrogation of NF-κB signaling events that may occur before robust up-regulation of inflammatory genes at 48 hours when myeloid cells are increased. NF-κB–mediated recruitment and differentiation of monocytes occurred within 24 hours into the NEC model. This process was mediated via L-selectin, suggesting that monocyte adhesion was one of the pathways involved. The significance of these data is strengthened by the recent study that identified nongranulocyte adhesion and diapedesis as top canonical pathways involved in human NEC.31
Several studies have demonstrated a specific role for monocytes in promoting NEC. Mechanisms reported include the promotion of type 17 helper T-cell differentiation that leads to an imbalance between type 17 helper T cells and regulatory T cells5 and increased M1 macrophage polarization that leads to an increase in IEC apoptosis.6 Myeloid cells may also be protective in NEC. A recent study showed that immunosuppressive myeloid-derived suppressor cells are found in the spleen and bone marrow of neonatal mice and humans, which potently inhibit proliferation of CD4+ and CD8+ T cells. Myeloid-derived suppressor cells were identified at days 4 through 14 of life and play a protective role in controlling experimental NEC in 4-day–old mice.4 Intriguingly, these cells were not present in the first 3 days, which the authors suggest is a period of increased NEC susceptibility.4 Whether macrophages derived from monocytes, which were found to be recruited during NEC in an IKKβ-dependent manner, impact the T-cell compartment remains unknown.
Our laboratory has reported that an increase in epithelial barrier permeability precedes NEC in this mouse model.2 Increased intestinal permeability has been associated with other NEC models32, 33 and with human NEC.34 Herein, it was shown that increased intestinal permeability and decreased epithelial proliferation in NEC were present independently of IKKβ status in Lysm monocytes. Because intestinal injury was markedly attenuated in Lysm-IKKβ–deleted NEC mice, increased intestinal permeability may be necessary but not sufficient to induce intestinal injury in this NEC model. In addition, these findings suggest that therapies targeting monocyte NF-κB signaling independently of epithelial effects may be promising in NEC.
Recent evidence challenges the current paradigm involving the adult small intestinal macrophage pool. Data suggest that this compartment is composed of three distinct subsets of equal proportions.35 Two of these populations are derived from blood monocytes but have different replenishment rates and are phenotypically described as Tim4−CD4+ (slow rate) and Tim4−CD4− (high rate). These populations both transition from Ly6c+ monocytes to Ly6c− macrophages through differentiation mechanisms described as the monocyte waterfall,19, 36, 37 which is named for the appearance of the flow cytometry plot as cells lose Ly6c and gain MHC-II or Cx3cr1. This waterfall is typically absent in the neonatal intestine, but it is apparent in the adult, where the continuous replacement of most intestinal macrophages by bone marrow–derived monocytes helps achieve the precise balance between tolerance and immune activation. The third population, previously thought to be replaced before reaching adulthood,10 is the embryonically derived Tim-4+CD4+ population, and it is locally maintained independently of monocytes (embryonic macrophages).35 In the intestine during the neonatal period, the Tim-4+CD4+ (embryonic macrophage) predominates35 and Ly6c+ monocytes are rare.10 These data concur with our findings describing a low number of Ly6c+ cells/Lysm+ cells in the neonatal intestine.
The gut embryonic macrophage population maintains the integrity of enteric neurons as well as submucosal and mucosal microvasculature in adults.38 Depletion of the embryonic macrophage population via diphtheria toxin in adult transgenic mice resulted in their replacement by blood monocytes that differentiated into gut Cx3cr1+ monocyte-derived macrophages38 and led to reduced abundance of the intestinal microvasculature and decreased enteric neuron density.38 During development, embryonic macrophages play a role in vascular maturation and remodeling14, 15, 16, 17 and in the removal of debris (such as dying epithelial cells).11, 12, 13 The intestinal microvasculature develops tremendously during the neonatal period,39 and NEC stresses decrease intestinal microvasculature density.40 A premature influx of monocyte-derived macrophages in the neonatal intestine induced by NEC stresses may disturb the embryonic macrophage compartment at a time of intense development of the intestinal microvasculature and likely impact the environment necessary to orchestrate its maturation at this stage. In support of this idea, it was demonstrated that the intestinal microvascular density was preserved in Lysm-IKKβ–deficient mice during NEC versus their IKKβ-sufficient littermates (Supplemental Figure S4, E and F). Further studies are needed to determine whether the early influx of monocyte-derived macrophages into the intestine impacts the self-maintaining gut macrophage compartment, thus affecting microvasculature development.
During inflammation, recruited monocytes adhere to activated endothelial cells. This interaction induces MHC-II expression, leading to monocyte differentiation and subsequent tissue infiltration.18 This process sequentially involves down-regulation of Ly6c and Ccr2 and up-regulation of Cx3cr1.20 Herein, it was shown that genes preferentially associated with monocytes but not embryonic macrophages were up-regulated during NEC in the Cx3cr1+ population in IKKβ-sufficient but not in Lysm-IKKβ–deleted pups. When monocyte adhesion was blocked by L-selectin antibody, the Cx3cr1 population had decreased expression of these genes. Because Cx3cr1 is expressed at high level in yolk sac macrophages41 but not in fetal liver monocytes before tissue infiltration,25 our findings demonstrate that the intestinal macrophage compartment was enriched in monocyte-derived macrophages during NEC in IKKβ-sufficient pups but not in Lysm-IKKβ–deleted pups and that, in the intestine, NF-κB activation up-regulated the target genes L-selectin and Mmp9 in monocytes. More important, the number of SELL+ cells was also increased in the villi of human NEC tissue specimens compared with controls, which further supports monocyte recruitment in NEC pathogenesis.
Several studies have investigated the gene signatures of both macrophages derived from monocytes and embryonic macrophages14, 25, 26 and found Ly6c, Ccl2, Lysm, Mmp9, and L-selectin to be strongly associated with monocyte-derived cells, whereas Cx3cr1 was associated with an embryonic signature. This aligns with the finding that Lysm was expressed in Ly6c+ cells. However, it was surprising to find a lack of Lysm expression in most neonatal intestinal myeloid cells, especially given that Lysm-expressing cells are abundantly present in both their liver and spleen (Supplemental Figure S4D) as well as in the adult intestine (Supplemental Figure S4C). These data demonstrate that in the neonatal period, unlike the adult,23, 24 Lysm recombination should not been used to target the global intestinal myeloid population because its expression is mainly in monocyte-derived cells. Furthermore, it shows that in steady state, these Lysm+ cells are normally excluded from the intestine.
Together, our findings support a role for monocyte adhesion and recruitment to intestinal tissue during NEC in a process mediated by NF-κB signaling at a time when typically the intestinal tissue myeloid compartment is mostly constituted by embryonic macrophages. Preventing early monocyte recruitment to the neonatal intestine may be a novel and an effective strategy to prevent intestinal injury in NEC.
Acknowledgments
We thank Runlan Tian and Josalyn Mangrum (Lurie Children's Hospital of Chicago, Chicago, IL) for technical help; Dr. Harris Perlman (Northwestern University, Chicago, IL) for helpful insights; Dr. Lauren Pachman (Lurie Children's Hospital of Chicago, Chicago, IL) for carefully reviewing the manuscript draft; Drs. Michael Karin and Lars Eckmann (University of California, San Diego, San Diego, CA) for providing LysmCre/+ IKKβf/f, Villin Cre+/− IKKβf/f, and IKKβf/f; and Fiona Yull (Vanderbilt University, Nashville, TN) for providing NF-κB luciferase reporter mice.
Footnotes
Supported by NIH grants R01GM122406 and R01GM117628 (X.-D.T.), 2RO1 DK095662 (T.A.B.), and R01HD060876 (I.G.D.P.); Veterans Affairs Merit grant 1I01CX001353-01 (T.A.B.); Northwestern University Flow Cytometry Facility and National Cancer Institute grant CA060553; and the Stanley Manne Children's Research Institute of the Ann & Robert H. Lurie Children's Hospital of Chicago (I.G.D.P.).
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.11.015.
Supplemental Data
A: Lamina propria cells were gated on live, single, CD45+CD11b+Ly6g− SSClo. This gating strategy focuses on the monocyte/macrophage population and is referred to as total CD11b+ cells. For some experiments, green fluorescent protein–positive (GFP+) cells were gated. B: F4/80 expression confirms that the total CD11b+ population represents monocytes/macrophages. The histogram overlay shows F4/80 expression in dam-fed (DF) and necrotizing enterocolitis (NEC) pups compared with a negative control. C: To assess monocyte differentiation, GFP+ cells were analyzed for CD115 (increased on monocytes) and CD64 (increased on macrophages). Because these markers are expressed on both monocytes and embryonic macrophages, the degree of monocyte differentiation was quantified using the CD64/CD115 mean fluorescent intensity (MFI) ratio. D: GFP+ cells were then gated alternatively on either Ly6c+ (top panels) or Ly6c− (bottom panels) cells, and Cx3cr1 (antibody) versus major histocompatibility complex (MHC)-II was examined. ∗∗P ≤ 0.01. FSC, forward scatter; IKKβ, inhibitor of kappa-B kinase β; Lysm, lysozyme M; SSC, side scatter.
Contrasting with abundant Cx3cr1+ cells, there is only a low number of lysozyme M–positive (Lysm+) cells in the neonatal intestine, although with irregular distribution throughout the whole intestine. Representative images of double immunohistochemical staining for Cd31 (brown) and green fluorescent protein (GFP; Vina Green) on formalin-fixed tissue sections from 24-hour necrotizing enterocolitis (NEC) and dam-fed (DF) LysmCre/+- inhibitor of kappa-B kinase β (IKKβ)f/wt-mT/mG+/− (IKKβ-sufficient), LysmCre/+−IKKβf/f-mT/mG+/− (IKKβ-deficient), and Cx3cr1GFP/+ pups. Scale bar = 200 μm. Original magnification, ×20.
A: Because L-selectin is present on other leukocytes besides monocytes, flow cytometry was used to evaluate the effect of blocking L-selectin on individual populations. Lysozyme M (Lysm)Cre/+inhibitor of kappa-B kinase β (IKKβ)f/wtmT/mG+/− × 24-hour–old pups were injected with a neutralizing antibody against L-selectin before induction of necrotizing enterocolitis (NEC). Pups were sacrificed 24 hours later, and lamina propria cells were isolated and stained for flow cytometry analysis. A significant decrease in the percentage of Cd11b+Lysm–green fluorescent protein–positive (GFP+) cells is observed. Although not significant, a trend toward decreased CD3+ cells is also determined, but CD19 and Ly6g do not decrease. Two independent experiments were performed. B: Representative hematoxylin and eosin images from formalin-fixed, paraffin-embedded intestinal tissues from human control and NEC patients. ∗P ≤ 0.05. Scale bar = 200 μm (B).
A and B: X-Gal staining to detect β-galactosidase activity in the neonatal intestine of Villin Cre+/−-Rosa26 mice showing specific Cre-dependent recombination in the intestine (A) and intestinal epithelial cells (B). C: Representative staining of lysozyme M (Lysm)–Cre–green fluorescent protein (GFP) expression in LysmCre/+-inhibitor of kappa-B kinase β (IKKβ)f/wt -mT/mG+/− adult murine intestine. D: Representative staining of GFP expression in the liver and the spleen of LysmCre/+-IKKβf/wt -mT/mG+/− and LysmCre/+-IKKβf/f -mT/mG+/− 48-hour dam-fed pups. E and F: LysmCre/+-IKKβf/f and IKKβf/f littermate control mice were exposed to necrotizing enterocolitis (NEC) protocol for 48 hours and then injected with fluorescently labeled wheat germ agglutinin (WGA). E: Typical images of intact areas of formalin-fixed, paraffin-embedded small intestinal tissue sections immunostained for the endothelial cell marker Cd31 are shown herein. F: Bar graph depicting preserved villous microvascular area in IKKβ-deficient mice compared with IKKβ-sufficient controls in NEC, as determined by percentage WGA positivity of total intestinal tissue area. Sections from six NEC mice per group from three independent experiments were evaluated. ∗P ≤ 0.05. Scale bars: 100 μm (A, D, and E); 50 μm (C).
References
- 1.Sharma R., Tepas J.J., 3rd, Hudak M.L., Mollitt D.L., Wludyka P.S., Teng R.J., Premachandra B.R. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann K.R., Liu S.X., Tian R., Kushnir A., Turner J.R., Li H.L., Chou P.M., Weber C.R., De Plaen I.G. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182:1595–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MohanKumar K., Kaza N., Jagadeeswaran R., Garzon S.A., Bansal A., Kurundkar A.R., Namachivayam K., Remon J.I., Bandepalli C.R., Feng X., Weitkamp J.H., Maheshwari A. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol. 2012;303:G93–G102. doi: 10.1152/ajpgi.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y.M., Li X., Perego M., Nefedova Y., Kossenkov A.V., Jensen E.A., Kagan V., Liu Y.F., Fu S.Y., Ye Q.J., Zhou Y.H., Wei L., Gabrilovich D.I., Zhou J. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat Med. 2018;24:224–231. doi: 10.1038/nm.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang Y., Du X., Xu X., Wang M., Li Z. Monocyte activation and inflammation can exacerbate Treg/Th17 imbalance in infants with neonatal necrotizing enterocolitis. Int Immunopharmacol. 2018;59:354–360. doi: 10.1016/j.intimp.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Wei J., Besner G.E. M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J Surg Res. 2015;197:126–138. doi: 10.1016/j.jss.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiDonato J.A., Hayakawa M., Rothwarf D.M., Zandi E., Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 8.De Plaen I.G., Liu S.X., Tian R., Neequaye I., May M.J., Han X.B., Hsueh W., Jilling T., Lu J., Caplan M.S. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 2007;61:716–721. doi: 10.1203/pdr.0b013e3180534219. [DOI] [PubMed] [Google Scholar]
- 9.De Plaen I.G., Tan X.D., Chang H., Qu X.W., Liu Q.P., Hsueh W. Intestinal NF-kappaB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim Biophys Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 10.Bain C.C., Bravo-Blas A., Scott C.L., Gomez Perdiguero E., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., Mowat A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., Sher A., Litke A.M., Lambris J.D., Smith S.J., John S.W., Barres B.A. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Lang R.A., Bishop J.M. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 13.Varol C., Mildner A., Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 14.Leid J., Carrelha J., Boukarabila H., Epelman S., Jacobsen S.E., Lavine K.J. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res. 2016;118:1498–1511. doi: 10.1161/CIRCRESAHA.115.308270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Kleer I., Willems F., Lambrecht B., Goriely S. Ontogeny of myeloid cells. Front Immunol. 2014;5:423. doi: 10.3389/fimmu.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFalco T., Bhattacharya I., Williams A.V., Sams D.M., Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111:E2384–E2393. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantin A., Vieira J.M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S.W., Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakubzick C., Gautier E.L., Gibbings S.L., Sojka D.K., Schlitzer A., Johnson T.E., Ivanov S., Duan Q., Bala S., Condon T., van Rooijen N., Grainger J.R., Belkaid Y., Ma'ayan A., Riches D.W., Yokoyama W.M., Ginhoux F., Henson P.M., Randolph G.J. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bain C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., Mowat A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grainger J.R., Wohlfert E.A., Fuss I.J., Bouladoux N., Askenase M.H., Legrand F., Koo L.Y., Brenchley J.M., Fraser I.D., Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian R., Liu S.X., Williams C., Soltau T.D., Dimmitt R., Zheng X., De Plaen I.G. Characterization of a necrotizing enterocolitis model in newborn mice. Int J Clin Exp Med. 2010;3:293–302. [PMC free article] [PubMed] [Google Scholar]
- 23.Clausen B.E., Burkhardt C., Reith W., Renkawitz R., Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 24.Cross M., Mangelsdorf I., Wedel A., Renkawitz R. Mouse lysozyme M gene: isolation, characterization, and expression studies. Proc Natl Acad Sci U S A. 1988;85:6232–6236. doi: 10.1073/pnas.85.17.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P., Beaudin A.E., Lum J., Low I., Forsberg E.C., Poidinger M., Zolezzi F., Larbi A., Ng L.G., Chan J.K., Greter M., Becher B., Samokhvalov I.M., Merad M., Ginhoux F. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pucci F., Venneri M.A., Biziato D., Nonis A., Moi D., Sica A., Di Serio C., Naldini L., De Palma M. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 27.Remon J.I., Amin S.C., Mehendale S.R., Rao R., Luciano A.A., Garzon S.A., Maheshwari A. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J Perinatol. 2015;35:755–762. doi: 10.1038/jp.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pender S.L., Braegger C., Gunther U., Monteleone G., Meuli M., Schuppan D., Macdonald T.T. Matrix metalloproteinases in necrotising enterocolitis. Pediatr Res. 2003;54:160–164. doi: 10.1203/01.PDR.0000072326.23442.C3. [DOI] [PubMed] [Google Scholar]
- 29.Ballance W.A., Dahms B.B., Shenker N., Kliegman R.M. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6–S13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 30.Ginzel M., Feng X., Kuebler J.F., Klemann C., Yu Y., von Wasielewski R., Park J.K., Hornef M.W., Vieten G., Ure B.M., Kaussen T., Gosemann J.H., Mayer S., Suttkus A., Lacher M. Dextran sodium sulfate (DSS) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS One. 2017;12:e0182732. doi: 10.1371/journal.pone.0182732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MohanKumar K., Namachivayam K., Cheng F., Jiang R.H., Flores-Torres J., Torres B.A., Maheshwari A. Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr Res. 2017;81:99–112. doi: 10.1038/pr.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackam D.J., Upperman J.S., Grishin A., Ford H.R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 33.White J.R., Gong H., Pope B., Schlievert P., McElroy S.J. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Dis Model Mech. 2017;10:727–736. doi: 10.1242/dmm.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore S.A., Nighot P., Reyes C., Rawat M., McKee J., Lemon D., Hanson J., Ma T.Y. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg. 2016;51:1907–1913. doi: 10.1016/j.jpedsurg.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw T.N., Houston S.A., Wemyss K., Bridgeman H.M., Barbera T.A., Zangerle-Murray T., Strangward P., Ridley A.J.L., Wang P., Tamoutounour S., Allen J.E., Konkel J.E., Grainger J.R. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med. 2018;215:1507–1518. doi: 10.1084/jem.20180019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamoutounour S., Henri S., Lelouard H., de Bovis B., de Haar C., van der Woude C.J., Woltman A.M., Reyal Y., Bonnet D., Sichien D., Bain C.C., Mowat A.M., Reis e Sousa C., Poulin L.F., Malissen B., Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A.T., Friedlander G., Mack M., Shpigel N., Boneca I.G., Murphy K.M., Shakhar G., Halpern Z., Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 38.De Schepper S., Verheijden S., Aguilera-Lizarraga J., Viola M.F., Boesmans W., Stakenborg N., Voytyuk I., Smidt I., Boeckx B., Dierckx de Casterle I., Baekelandt V., Gonzalez Dominguez E., Mack M., Depoortere I., De Strooper B., Sprangers B., Himmelreich U., Soenen S., Guilliams M., Vanden Berghe P., Jones E., Lambrechts D., Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175:400–415.e13. doi: 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 39.Stappenbeck T.S., Hooper L.V., Gordon J.I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan X., Managlia E., Liu S.X., Tan X.D., Wang X., Marek C., De Plaen I.G. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G716–G725. doi: 10.1152/ajpgi.00273.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D.A., Perlman H., Malissen B., Zelzer E., Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Lamina propria cells were gated on live, single, CD45+CD11b+Ly6g− SSClo. This gating strategy focuses on the monocyte/macrophage population and is referred to as total CD11b+ cells. For some experiments, green fluorescent protein–positive (GFP+) cells were gated. B: F4/80 expression confirms that the total CD11b+ population represents monocytes/macrophages. The histogram overlay shows F4/80 expression in dam-fed (DF) and necrotizing enterocolitis (NEC) pups compared with a negative control. C: To assess monocyte differentiation, GFP+ cells were analyzed for CD115 (increased on monocytes) and CD64 (increased on macrophages). Because these markers are expressed on both monocytes and embryonic macrophages, the degree of monocyte differentiation was quantified using the CD64/CD115 mean fluorescent intensity (MFI) ratio. D: GFP+ cells were then gated alternatively on either Ly6c+ (top panels) or Ly6c− (bottom panels) cells, and Cx3cr1 (antibody) versus major histocompatibility complex (MHC)-II was examined. ∗∗P ≤ 0.01. FSC, forward scatter; IKKβ, inhibitor of kappa-B kinase β; Lysm, lysozyme M; SSC, side scatter.
Contrasting with abundant Cx3cr1+ cells, there is only a low number of lysozyme M–positive (Lysm+) cells in the neonatal intestine, although with irregular distribution throughout the whole intestine. Representative images of double immunohistochemical staining for Cd31 (brown) and green fluorescent protein (GFP; Vina Green) on formalin-fixed tissue sections from 24-hour necrotizing enterocolitis (NEC) and dam-fed (DF) LysmCre/+- inhibitor of kappa-B kinase β (IKKβ)f/wt-mT/mG+/− (IKKβ-sufficient), LysmCre/+−IKKβf/f-mT/mG+/− (IKKβ-deficient), and Cx3cr1GFP/+ pups. Scale bar = 200 μm. Original magnification, ×20.
A: Because L-selectin is present on other leukocytes besides monocytes, flow cytometry was used to evaluate the effect of blocking L-selectin on individual populations. Lysozyme M (Lysm)Cre/+inhibitor of kappa-B kinase β (IKKβ)f/wtmT/mG+/− × 24-hour–old pups were injected with a neutralizing antibody against L-selectin before induction of necrotizing enterocolitis (NEC). Pups were sacrificed 24 hours later, and lamina propria cells were isolated and stained for flow cytometry analysis. A significant decrease in the percentage of Cd11b+Lysm–green fluorescent protein–positive (GFP+) cells is observed. Although not significant, a trend toward decreased CD3+ cells is also determined, but CD19 and Ly6g do not decrease. Two independent experiments were performed. B: Representative hematoxylin and eosin images from formalin-fixed, paraffin-embedded intestinal tissues from human control and NEC patients. ∗P ≤ 0.05. Scale bar = 200 μm (B).
A and B: X-Gal staining to detect β-galactosidase activity in the neonatal intestine of Villin Cre+/−-Rosa26 mice showing specific Cre-dependent recombination in the intestine (A) and intestinal epithelial cells (B). C: Representative staining of lysozyme M (Lysm)–Cre–green fluorescent protein (GFP) expression in LysmCre/+-inhibitor of kappa-B kinase β (IKKβ)f/wt -mT/mG+/− adult murine intestine. D: Representative staining of GFP expression in the liver and the spleen of LysmCre/+-IKKβf/wt -mT/mG+/− and LysmCre/+-IKKβf/f -mT/mG+/− 48-hour dam-fed pups. E and F: LysmCre/+-IKKβf/f and IKKβf/f littermate control mice were exposed to necrotizing enterocolitis (NEC) protocol for 48 hours and then injected with fluorescently labeled wheat germ agglutinin (WGA). E: Typical images of intact areas of formalin-fixed, paraffin-embedded small intestinal tissue sections immunostained for the endothelial cell marker Cd31 are shown herein. F: Bar graph depicting preserved villous microvascular area in IKKβ-deficient mice compared with IKKβ-sufficient controls in NEC, as determined by percentage WGA positivity of total intestinal tissue area. Sections from six NEC mice per group from three independent experiments were evaluated. ∗P ≤ 0.05. Scale bars: 100 μm (A, D, and E); 50 μm (C).