Abstract
In this study, for the environmental development, the antifungal, antibacterial, and antioxidant activities of a water extract of flowers from Acacia saligna (Labill.) H. L. Wendl. were evaluated. The extract concentrations were prepared by dissolving them in 10% DMSO. Wood samples of Melia azedarach were treated with water extract, and the antifungal activity was examined at concentrations of 0%, 1%, 2%, and 3% against three mold fungi; Fusarium culmorum MH352452, Rhizoctonia solani MH352450, and Penicillium chrysogenum MH352451 that cause root rot, cankers, and green fruit rot, respectively, isolated from infected Citrus sinensis L. Antibacterial evaluation of the extract was assayed against four phytopathogenic bacteria, including Agrobacterium tumefaciens, Enterobacter cloacae, Erwinia amylovora, and Pectobacterium carotovorum subsp. carotovorum, using the micro-dilution method to determine the minimum inhibitory concentrations (MICs). Further, the antioxidant capacity of the water extract was measured via 2,2′-diphenylpicrylhydrazyl (DPPH). Phenolic and flavonoid compounds in the water extract were analyzed using HPLC: benzoic acid, caffeine, and o-coumaric acid were the most abundant phenolic compounds; while the flavonoid compounds naringenin, quercetin, and kaempferol were identified compared with the standard flavonoid compounds. The antioxidant activity of the water extract in terms of IC50 was considered weak (463.71 μg/mL) compared to the standard used, butylated hydroxytoluene (BHT) (6.26 μg/mL). The MIC values were 200, 300, 300, and 100 µg/mL against the growth of A. tumefaciens, E. cloacae, E. amylovora, and P. carotovorum subsp. carotovorum, respectively, which were lower than the positive control used (Tobramycin 10 μg/disc). By increasing the extract concentration, the percentage inhibition of fungal mycelial was significantly increased compared to the control treatment, especially against P. chrysogenum, suggesting that the use of A. saligna flower extract as an environmentally friendly wood bio-preservative inhibited the growth of molds that cause discoloration of wood and wood products.
Keywords: acacia saligna, antibacterial activity, antifungal activity, antioxidant activity, flowers, wood-treated extract
1. Introduction
Wide global issue of foodborne diseases influenced significantly on environmental development and health. Consumers demand growing day by day for natural preservatives as alternatives to solve the bad reputation of toxic chemical compounds. The Plant extracts had antimicrobial compounds must be thoroughly characterized for their endo potential to serve as biocontrol or biopreservative agents. Comprehensive papers focused on plant extracts as antimicrobial agents for use in preservation and control foodborne pathogens in foods. Medicinal and aromatic plants rich in phytochemical compounds such as polyphenols, flavonoids, saponins, alkaloids, and others in their different parts (leaves, bark, flowers, seeds, wood, and branches) have broad applications as antioxidants and antimicrobials and are known for their pharmaceutical and biopesticide properties [1,2,3,4,5,6]. Several mold species, such as Fusarium, Paecilomyces, Rhizoctonia, Penicillium, Aspergillus, Alternaria, and Trichoderma, can colonize and cause pigmentation in, colored spores on, and the discoloration of different wood and wood-based products in humid conditions [7,8,9,10]. Molds produce hydrolyzing enzymes that hydrolyze cellulose into glucose [11], xylanase enzymes [12], and β-xylosidases that hydrolyze hemicelluloses [13]. In-service wood can be fortified against mold growth by using natural products as a surface experiment application [14,15,16].
Fusarium culmorum is a ubiquitous soil-borne fungus able to cause root rot on citrus specimens, particularly oranges and lemons [17]. However, the most frequently isolated fungi from the rotted roots of lemon transplants were F. oxysporum, F. solani, and R. solani, showing root rot and wilt disease complexes. The average percentage of root rot/wilt incidence in surveyed districts was 34.0% in lemon [18]. Meanwhile, the Penicillium spp., considered the most important postharvest fungal pathogen, reported on citrus and stone fruits were P. chrysogenum, P. crustosum, and P. expansum [19,20,21,22], and accounted for up to 90% of total losses [23,24]. Fusarium species and their fumonisin mycotoxins are toxic, causing maize ear rot disease and contaminating maize grains, leading to major problems in pre- and post-harvest losses [25,26]. Additionally, Panama wilt disease is caused by F. oxysporum in bananas (Musa paradisiaca) [27].
Phytopathogenic bacteria Agrobacterium tumefaciens, Bacillus pumilus, Dickeya solani, Enterobacter cloacae, Ralstonia solanacearum, and Pectobacterium carotovorum subsp. carotovorum are causal agents of different infectious plant symptoms, such as blackleg, brown or soft rot on potato tuber and stems, and tumors on olive and other ornamental plants [28,29,30,31]. Therefore, several studies have been carried out to study the effects of natural extracts on these bacteria and have shown a range of weak to strong activity [22,24,29,30,31].
A wide array of polymerase chain reaction (PCR) and real-time PCR tools, as well as complementary methods, have been developed for the detection and quantification of F. culmorum, R. solani, and P. chrysogenum in isolated pure cultures and in naturally infected plant tissue [32,33,34,35]. Presently, a great challenge in agriculture is the control of plant diseases caused by phytopathogenic bacteria and fungi. The emergence of antifungal/antibacterial-resistant strains is increasing, emphasizing the urgent need for the development of novel antifungal agents with properties and mechanisms of action different from the existing ones [32].
Currently, the fungicides/bactericides used are costly and environmentally toxic [36]. Also, phytopathogenic fungi and bacteria have developed resistance to most of the conventional pesticides and antibiotics [37,38]. Therefore, a search for new sources of biocides is needed from tropical and subtropical plants rich in phytochemicals that could be used as defense compounds for the protection of crop plants [39,40].
Acacia saligna (Labill.) H. L. Wendl. (Acacia cyanophylla Lindl.), native to Western Australia and belonging to the family Fabaceae, has been planted in Egypt and other Mediterranean countries in Africa, become an invasive species, and is considered a fast-growing tree [41,42]. Different Acacia plants produce allelopathic materials as bioactive compounds [43,44]; where phenolics, tannins, flavonoids, phenols, and proanthocyanidins are the most common compounds identified in various parts of the Acacia species [45,46,47,48,49]. The methanol extract of flowers and leaves was observed to have allelopathic effects greater than those of an aqueous extract on the germination percentage of Hordeum murinum [50], which was caused by a variety of active components [51]. The ethyl acetate extract of Acacia saligna leaves was more effective against Staphylococcus aureus, S. pyogenes, Bacillus cereus, B. subtilis, and Candida albicans than methanolic and water extracts [52].
The aim of this study was to evaluate the bioactivity of a water extract from flowers of Acacia saligna in terms of the fungal resistance of wood treated with the water extract, antibacterial activity against some phytopathogenic bacteria, and antioxidation properties. Further, the main phenolic and flavonoid compounds in the water extract were analyzed using an HPLC method.
2. Results
2.1. Isolated Fungi
The isolation trails and the ITS sequences revealed that the three fungal isolates were Fusarium culmorum, Rhizoctonia solani, and Penicillium chrysogenum. GenBank accession numbers of the isolated fungi are listed in Table 1.
Table 1.
Accession numbers of fungal isolates used for antifungal activity evaluation.
2.2. Antifungal Activity of Wood Treated with Water Extract
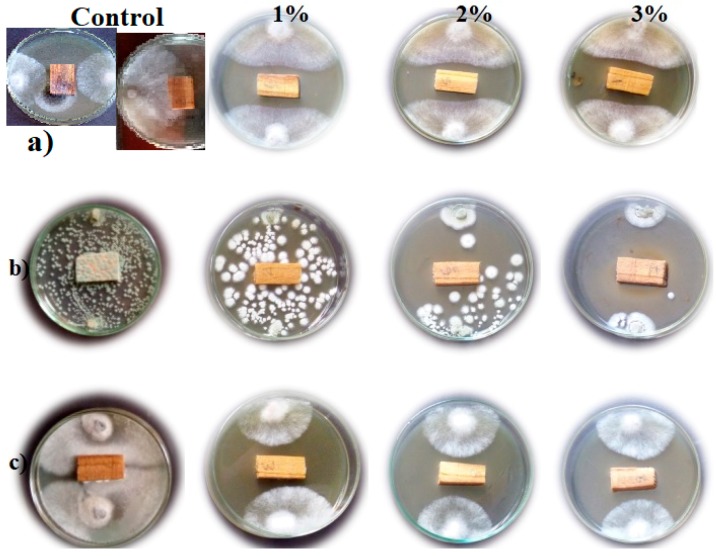
Figure 1 shows that wood samples treated with water extracts of different concentrations (1, 2, and 3%) of A. saligna flower presented different degrees of inhibitions to fungal growth compared to the control treatment (wood samples treated with 10% DMSO). The water extract of flowers at 3% exhibited the highest inhibition percentage of mycelial growth of F. culmorum, P. chrysogenum, and R. solani, with values of 38.51%, 65.92%, and 41.48%, respectively, compared to the control. It can be seen from Table 2 that there is no significant difference among the three concentrations (1, 2, and 3%) of water extracts for the inhibition percentage of R. solani. However, with an increasing concentration, the percentage inhibition of fungal mycelia of P. chrysogenum was significantly increased. Furthermore, there is no significant difference between the concentrations 1% and 2% for the inhibition percentage of F. culmorum, but the concentration 3% showed the highest inhibition percentage with a significant value against the same fungus.
Figure 1.
Wood treated with water extracts of A. saligna flowers and exposed to the growth of three fungi: (a) Rhizoctonia solani; (b) Penicillium chrysogenum; and (c) Fusarium culmorum.
Table 2.
Mycelia percentage inhibited of F. culmorum, P. chrysogenum, and R. solani by wood treated with A. saligna flower water extracts at different concentrations.
| Conc. (%) | Inhibition Percentage of Mycelial Growth (%) | ||
|---|---|---|---|
| F. culmorum | P. chrysogenum | R. solani | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 0 | 0.00 c | 0.00 c | 0.00 b |
| 1 | 31.11 b ± 2.22 | 14.07 c ± 7.14 | 40.74 a ± 1.28 |
| 2 | 31.11 b ± 2.22 | 36.29 b ± 1.28 | 41.48 a ± 1.28 |
| 3 | 38.51 a ± 1.28 | 65.92 a ± 1.28 | 41.48 a ± 1.28 |
| p-value | <0.0001 | <0.0001 | <0.0001 |
| LSD0.05 | 3.195 | 6.938 | 2.092 |
Conc. = Concentration. Means with the same superscript letters within the same column are not significantly different according to LSD0.05.
2.3. Antibacterial Activity
Table 3 presents the antibacterial activity of the water extracts, where the MIC values in µg/mL of 200 (A. tumefaciens), 300 (E. cloacae), 300 (E. amylovora), and 100 (P. carotovorum subsp. carotovorum) were observed and all the values were lower than those reported from the positive control used (Tobramycin 10 μg/disc).
Table 3.
The MIC (µg/mL) values against the growth of four phytopathogenic bacteria.
| Tested Material | MIC (µg/mL) | |||
|---|---|---|---|---|
| A. tumefaciens | E. cloacae | E. amylovora | P. carotovorum subsp. carotovorum | |
| Extract | 200 | 300 | 300 | 100 |
| Tobramycin (10 μg/disc) | 32 | 35 | 35 | 16 |
2.4. Phytochemical Constituents and DPPH Activity of Extract
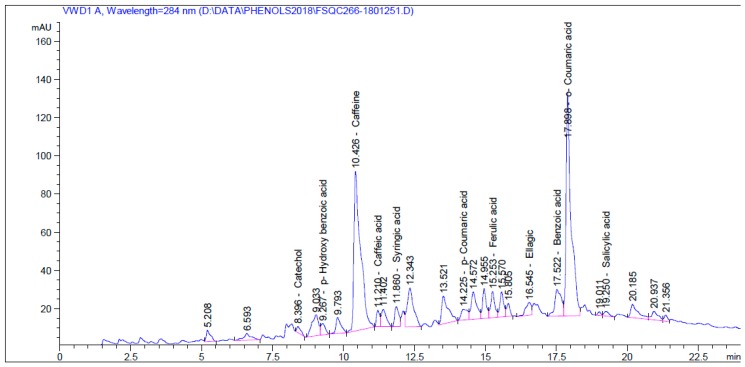
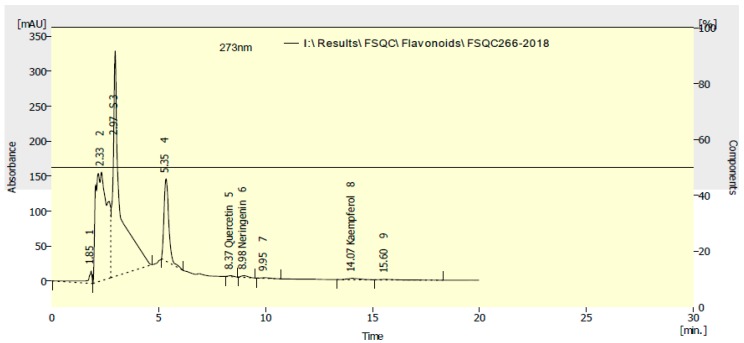
The HPLC chromatograms for the identified phenolic and flavonoid compounds are shown in Figure 2 and Figure 3, respectively. Table 4 presents the phenolic and flavonoid compounds identified in the water extract of A. saligna flowers. The most abundant phenolic compounds were benzoic acid (161.68 mg/100 g), caffeine (100.11 mg/100 g), o-coumaric acid (42.09 mg/100 g), p-hydroxy benzoic acid (14.13 mg/100 g), and ellagic acid (12.17 mg/100 g); while the identified flavonoid compounds were naringenin (145.03 mg/100 g), quercetin (111.96 mg/100 g), and kaempferol (44.49 mg/100 g).
Figure 2.
HPLC chromatogram of phenolic compounds identified in water extract of A. saligna flowers.
Figure 3.
HPLC chromatogram of flavonoid compounds identified in water extract of A. saligna flowers.
Table 4.
Chemical composition analysis of phenolic and flavonoid compounds of water extract from A. saligna flowers by HPLC.
| Compound | Conc. (mg/100 g) |
|---|---|
| Phenolic compounds | |
| Gallic acid | ND * |
| Catechol | 6.54 |
| p-Hydroxy benzoic acid | 14.13 |
| Caffeine | 100.11 |
| Vanillic acid | ND |
| Caffeic acid | 2.50 |
| Syringic acid | 5.83 |
| Vanillin | ND |
| p-Coumaric acid | 2.45 |
| Ferulic acid | 6.65 |
| Ellagic acid | 12.17 |
| Benzoic acid | 161.68 |
| o-Coumaric acid | 42.09 |
| Salicylic acid | 4.43 |
| Cinnamic acid | ND |
| Flavonoid compounds | |
| Rutin | ND |
| Myricetin | ND |
| Quercetin | 111.96 |
| Naringenin | 145.03 |
| Kaempferol | 44.49 |
| Apigenin | ND |
* ND: not detected.
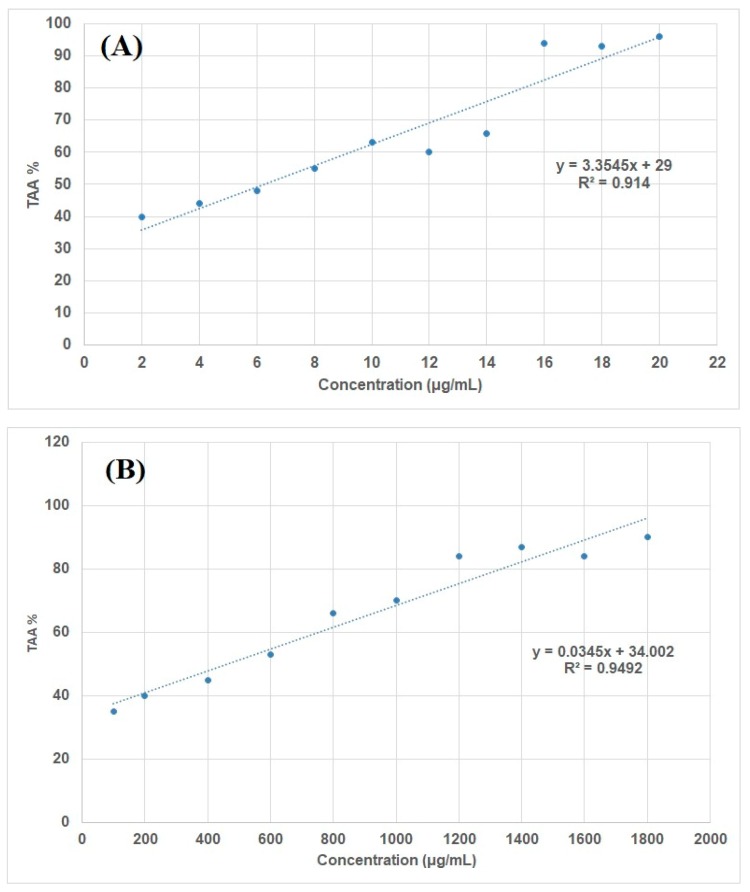
A spectrophotometric DPPH assay was used to evaluate the antioxidant properties of the flower water extract. Results of the antioxidant activity of the water extract from A. saligna flowers showed that with increasing concentration, the total antioxidant activity (TAA%) increased (Figure 4). The results obtained show that the total flower extract presents a weak scavenging capacity. The measured antioxidant activity in terms of the IC50 value (the concentration of the extract able to scavenge 50% of the DPPH free radical) was 463.71 μg/mL, which was lower than the value reported with BHT (6.26 μg/mL).
Figure 4.
TAA % curve of BHT (A) and water extract from A. saligna flowers (B).
3. Discussion
Bioactive compounds extracted from plant materials are recognized to be the first step for the utilization of phytochemicals in dietary supplement preparation, as well as of food ingredients, pharmaceutical products, and antimicrobial agents [2]. Several studies have examined the antimicrobial activity of water extracts from Acacia species. The aqueous extract of A. cyanophylla leaves showed different levels of activity against the growth of Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Xanthomonas, Escherichia coli, and Citrobacter [53]. The extract obtained with A. saligna leaves was effective against the tested bacteria B. subtilis, E. coli, Klebsiella pneumoniae, P. aeruginosa, S. aureus, and Micrococcus luteus (Gumgumjee and Hajar, 2015). The ethanol extract of A. saligna showed good activity against A. niger, A. fumigatus, A. flavus, and C. albicans at a concentration of 200 mg/mL [54]. To the best our knowledge, no studies have been carried out to study the effects of flower extracts against the phytopathogenic bacteria. Other studies showed that alkaloidal extracts from Conocarpus lancifolius leaves had MICs value of 20–50 μg/mL and >200 μg/mL against the growth of A. tumefaciens and E. amylovora, respectively [55]; while it was 125, 250, 125, and 16 μg/mL against P. carotovorum subsp. carotovorum when treated with acetone extract of Callistemon viminalis flower, n-butanol extract of C. viminalis flower, essential oil from the aerial parts of Conyza dioscoridis, and n-butanol extract from the bark of Eucalyptus camaldulensis, respectively; and against A. tumefaciens with values of 16, 250, >4000, and 250 μg/mL with the same extracts [3]. The leaf ethanol extract of A. cyanophylla showed activity against Aspergillus niger, A. fumigatus, A. flavus, and Candida albicans, where A. fumigatus was the most susceptible fungi and A. niger the most resistant [56].
Various Acacia species have condensed and hydrolysable tannins, as well as flavonoids [57,58]; which exhibit various bioactivities, such as antioxidant, anti-inflammatory, and antimicrobial properties [59]. A. saligna extracts showed steroidal saponins and biflavonoid glycosides [60], with high antimicrobial activity. Catechin, 7-O-galloylcatechin, myricetin-3-O-α-l-arabinopyranoside, quercetin-3-O-β-d-glucopyranoside, quercetin-3-O-α-l-arabinopyranoside, apigenin-7-O-β-d-glucopyranoside, and luteolin-7-O-β-d-glucopyranoside were isolated from A. saligna leaves [52]. Several flavonoid compounds (astragalin, myricitrin, and quercitrin) have been isolated from the leaves of A. saligna [60]. Myricetin 3-O-glucoside has also been found in A. saligna [61].
Phenolic and flavonoid compounds extracted from different plant materials have shown potential antioxidant properties, i.e., flavonoids from Larix decidua [62] and Abies spectabilis [63] bark extracts. The catechol group presented in the quercetin derivative is known to strongly increase activity in the DPPH assay [64]. On the other hand, moderate and lower antioxidant activity than standard antioxidants (butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and ascorbic acid), was found with ethanolic extracts of Salvia microstegia, S. brachyantha, and S aethiopis, while the main phenolic and flavonoid compounds were kaempferol, rosmarinic acid, apigenin, luteolin, p-coumaric acid, and chlorogenic acid [65]. In the present study, we found weak antioxidant activity, which could be the result of the poor solubility of polyphenols in the water extract [66]. For example, the S. aethiopis water extract and S. microstegia ethanol extract presented an activity similar to ascorbic acid, but the lowest activity was observed in the S. microstegia water extract [65].
Quercetin-7-O-diglucoside isolated from stem bark and wood extracts of Terminalia brownii possessed significant antifungal activity against Aspergillus and Fusarium strains [67]. In addition, quercetin and its derivatives have been reported to have good antifungal activities with low MIC values [68,69,70,71]. The growth of Fusarium spp. has been suppressed by dihydroquercetin isolated from barley [72]. Naringenin and its derivatives demonstrated some antifungal and antibacterial activity [73].
It should be mentioned that, while the flavonoid compounds relies on the comparison of their retention times with standard compounds, there still the biggest peaks in the chromatograms at 2.97 and 5.35 min which could be contributed properly the assessed biological activities, especially the identified flavonoids seem to be minor components in the water extract of A. saligna. These results supported the limitations of the HPLC analysis when using the available standard compounds.
Overall, it can be concluded from the current study that the water extract from flowers, containing various phenolic compounds, can be used as a bio-fungicide against certain wood-staining molds.
4. Materials and Methods
4.1. Plant Material and Preparation of the Extract
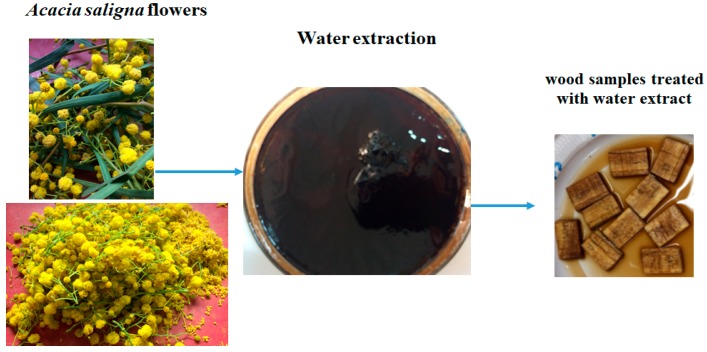
Flowers of Acacia saligna were collected from Alexandria, Egypt. The extraction procedure was carried out according to Salem et al. [74], with some modification, where approximately 25 g of the flowers were extracted with distilled water (200 mL) for 3 h under heat using a water bath at 50 °C. The extract was filtered with cotton plugs and then with filter paper (Whatman No. 1) (Mumbai, India) and concentrated to a small volume using a rotary evaporator (Rotavapor RII, Cole-Parmer GmbH, BucHI, Essen Germany). The water extract (7.45% w/w of fresh weight) was stored in a brown vial prior to chemical and bioactivity analyses.
4.2. Fungal Isolation, DNA Extraction, PCR, and Sequencing
During 2017, the tissues of infected Citrus sinensis L. trees showing root rots, cankers, and green fruit rot symptoms (Beheira, Egypt) were collected from Beheira governorate in Egypt and sent to the Plant Pathology laboratory of the Agricultural Botany Department, Alexandria University, Alexandria, Egypt, for isolation of the causal agents. The fungal isolates were isolated, kept on potato dextrose agar (PDA) plates, and incubated for seven days. The cultural and morphological characteristics of the isolated fungi were used for identification to the genus level, and for further molecular characterization, the DNA extraction was carried out on freshly growing mycelium using the GenElute™ Plant Genomic DNA Miniprep Kit (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. The PCR was carried out using primer pairs (ITS1/ITS4) to amplify the internal transcribed spacer (ITS) region of the rDNA [75,76].
The PCR reaction mixture consisted of a total volume of 25 µL; made up of 1 µL target DNA, 2.5 µL of 10 × PCR buffer (Sigma-Aldrich, St. Louis, MO, USA), and 1.25 µL deoxynucleotide triphosphates (dNTPs) mix (2.5 mM each); 1 µL each of the sense and antisense primer; 1 µL MgCl2 (50 mM); 0.1 µL Taq polymerase (Sigma-Aldrich, St. Louis, MO, USA); and RNAse free water, which was used to reach a total of 25 µL.
The reaction was carried out in a Techne Prime thermal cycler (Techne, Cambridge, United Kingdom). The reaction cycle consisted of denaturation for 4 min at 95 °C; followed by 40 cycles of 1 min at 94 °C, 1 min at 50 °C, and 1 min at 72 °C; and a final extension of 8 min at 72 °C. Amplification products were separated in 1.2% (w/v) agarose gel (iNtRON Biotechnology, Inc., Seongnam, South Korea), and pre-stained with red safe (iNtRON Biotechnology, Inc., Seongnam, South Korea) along with a 100 bp plus ladder at 70 V. The DNA bands were observed under a UV transilluminator. Purified fragments of ITS were sequenced by Macrogen, Inc., Seoul, Korea. The generated sequences were deposited in GenBank under the accession numbers presented in Table 1.
4.3. Antifungal Activity of Wood Treated with Water Extract
The water extract from A. saligna flowers was prepared at concentrations of 0%, 1%, 2%, and 3% by dissolving the extract in 10% dimethyl sulfoxide (DMSO). A total of 36 wood samples of Melia azedarach with dimensions of 0.5 cm × 1 cm × 2 cm, air-dried, and autoclaved at 121 °C for 20 min, were used for the antifungal activity test (Figure 5). Three wood samples were used for each concentration. The antifungal activity was evaluated against the linear growths of Fusarium culmorum, Rhizoctonia solani, and Penicillium chrysogenum. The inhibition percentage of mycelial growth was calculated using the following equation: Mycelial growth inhibition (%) = [(AC − AT)/AC] × 100 [77], where AC and AT are average diameters of the fungal colony of the control and treatment, respectively. Wood samples soaked only with 10% DMSO were used as the control.
Figure 5.
Wood samples treated with water extract of A. saligna flowers
4.4. Antibacterial Activity
The minimum inhibitory concentrations (MICs) of water extracts from A. saligna flowers were evaluated against the four phytopathogenic bacteria Agrobacterium tumefaciens, Enterobacter cloacae, Erwinia amylovora, and Pectobacterium carotovorum subsp. carotovorum using the micro-dilution method with serial concentrations of 4–350 µg/mL [78], and compared with the positive control (Tobramycin 10 μg/disc).
4.5. Determination of Antioxidant Activity
The antioxidant capacity was assessed by the 2,2′-diphenylpicrylhydrazyl (DPPH) assay [79] in terms of IC50 (the concentration that caused a 50% inhibition of growth compared with control) using the calibration curve compared with butylated hydroxytoluene (BHT).
4.6. HPLC condition for Phenolic Compounds
An Agilent 1260 Infinity HPLC Series (Agilent, Santa Clara, CA, USA), equipped with a Quaternary pump and a Zorbax Eclipse plus C18 column (100 mm × 4.6 mm i.d.) (Agilent Technologies, Santa Clara, CA, USA), was operated at 30 °C. Separation was achieved using a ternary linear elution gradient with (A) HPLC grade water 0.2% H3PO4 (v/v), (B) methanol, and (C) acetonitrile. The injected volume was 20 μL. A VWD detector was set at 284 nm. The standard phenolic compounds used were gallic acid, catechol, p-hydroxy benzoic acid, caffeine, vanillic acid, caffeic acid, syringic acid, vanillin, p-coumaric acid, ferulic acid, ellagic acid, benzoic acid, o-coumaric acid, salicylic acid, and cinnamic acid.
4.7. HPLC Condition for Flavonoids
HPLC, Smart line, Knauer, Germany, equipped with a binary pump, a Zorbax Eclipse plusC18 (column 150 mm × 4.6 mm i.d.) (Agilent technologies, USA), operated at 35 °C, was used. The conditions used were: Eluent as methanol: H2O with 0.5% H3PO4, 50:50; a flow rate of 0.7 mL/min; and injected volume of 20 μL. The UV detector was set at 273 nm and data integration was done using ClarityChrom@ Version 7.2.0, Chromatography Software (Knauer Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin, Germany). The standard flavonoid compounds were rutin, myricetin, quercetin, naringenin, kaempferol, and apigenin.
4.8. Statistical Analysis
The results of the inhibition percentage of mycelial growth of the three fungi as affected by the four concentrations (0, 1, 2, and 3%) of the A. saligna flower water extract were statistically analyzed using one-way analysis of variance (ANOVA) SAS software (SAS Institute, Release 8.02, Cary, North Carolina State University, Raleigh, NC, USA), and the means were compared against the control treatment.
5. Conclusions
In the present study, the water extract of A. saligna flowers at 3% shows moderate antifungal properties against the three mold species tested (F. culmorum, R. solani, and P. chrysogenum). Therefore, it is possible to assert that there are some potential applications of this extract for wood protection. The water extract showed lower antibacterial and antioxidant activities than the standards used, Tobramycin and butylated hydroxytoluene (BHT), respectively. Overall, the presence of the activity of the water extract could be related to the presence of some phenolic and flavonoid compounds.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs. The authors also thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Author Contributions
S.I.B. and M.Z.M.S. designed the experiments, wrote parts of the manuscript, conducted laboratory analyses, and interpreted the results; A.A.A.-H., H.M.A., and M.H.S. contributed reagents/materials/analytical tools and wrote part of the manuscript; and A.Z.M.S. wrote part of the manuscript, and revised and amended the article for technical merits.
Funding
This research was funded by the Deanship of Scientific Research, King Saud University through the Vice Deanship of Scientific Research Chairs.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Wu C.Y., Chen R., Wang X.S., Shen B., Yue W., Wu Q. Antioxidant and Anti-Fatigue Activities of Phenolic Extract from the Seed Coat of Euryale ferox Salisb. and Identification of Three Phenolic Compounds by LC-ESI-MS/MS. Molecules. 2013;18:11003–11021. doi: 10.3390/molecules180911003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EL-Hefny M., Ashmawy N.A., Salem M.Z.M., Salem A.Z.M. Antibacterial activity of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathogen. 2017;113:348–356. doi: 10.1016/j.micpath.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Ashmawy N.A., Salem M.Z.M., EL-Hefny M., Abd El-Kareem M.S.M., El-Shanhorey N.A., Mohamed A.A., Salem A.Z.M. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb. Pathogen. 2018;121:331–340. doi: 10.1016/j.micpath.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 5.Salem M.Z.M., El-Hefny M., Ali H.M., Elansary H.O., Nasser R.A., El-Settawy A.A.A., El Shanhorey N., Ashmawy N.A., Salem A.Z.M. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb. Pathogen. 2018;120:119–127. doi: 10.1016/j.micpath.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 6.Salem M.Z.M., Elansary H.O., Ali H.M., El-Settawy A.A., Elshikh M.S., Abdel-Salam E.M., Skalicka-Woźniak K. Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and Corymbia citriodora leaves grown in Egypt. BMC Complem. Altern. Med. 2018;18:23–29. doi: 10.1186/s12906-018-2085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen B., Frisvad J.C., Søndergaard I., Rasmussen I.S., Larsen L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microb. 2011;77:4180–4188. doi: 10.1128/AEM.02513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X., Lee S., Wu Y., Wu Q. Borate-treated strand board from southern wood species: Resistance against decay and mold fungi. BioResources. 2013;8:104–114. doi: 10.15376/biores.8.1.104-114. [DOI] [Google Scholar]
- 9.Lee Y.M., Lee H., Jang Y., Cho Y., Kim G.-H., Kim J.-J. Phylogenetic analysis of major molds inhabiting woods. Part 4. Genus Alternaria. Holzforschung. 2014;68:247–251. doi: 10.1515/hf-2013-0089. [DOI] [Google Scholar]
- 10.Salem M.Z.M. EDX measurements and SEM examination of surface of some imported woods inoculated by three mold fungi. Measurement. 2016;86:301–309. doi: 10.1016/j.measurement.2016.03.008. [DOI] [Google Scholar]
- 11.Sohail M., Ahmad A., Khan S.A. Production of cellulases from Alternaria sp. MS28 and their partial characterization. Pak. J. Bot. 2011;43:3001–3006. [Google Scholar]
- 12.De Vries R.P., Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. R. 2001;65:497–522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubicek C.P. Fungi and Lignocellulosic Biomass. John Wiley & Sons, Inc.; Ames, IA, USA: 2012. Enzymology of hemicellulose degradation; pp. 69–97. [Google Scholar]
- 14.Mansour M.M.A., Salem M.Z.M. Evaluation of wood treated with some natural extracts and Paraloid B-72 against the fungus Trichoderma harzianum: Wood elemental composition, in-vitro and application evidence. Int. Biodeter. Biodegr. 2015;100:62–69. doi: 10.1016/j.ibiod.2015.02.009. [DOI] [Google Scholar]
- 15.Mansour M.M.A., Abdel-Megeed A., Nasser R.A., Salem M.Z.M. Comparative evaluation of some woody tree methanolic extracts and Paraloid B-72 against phytopathogenic mold fungi Alternaria tenuissima and Fusarium culmorum. BioResources. 2015;10:2570–2584. doi: 10.15376/biores.10.2.2570-2584. [DOI] [Google Scholar]
- 16.Mansour M.M.A., Salem M.Z.M., Khamis M.H., Ali H.M. Natural durability of Citharexylum spinosum and Morus alba woods against three mold fungi. BioResources. 2015;10:5330–5344. doi: 10.15376/biores.10.3.5330-5344. [DOI] [Google Scholar]
- 17.Ochoa J.L., Hernández-Montiel L.G., Latisnere-Barragán H., León de La Luz J.L., Larralde-Corona C.P. Isolation and identification of pathogenic fungi from orange Citrus sinensis L. Osbeck cultured in Baja California Sur, Mexico. Cienc. Tecnol. Aliment. 2007;5:352–359. doi: 10.1080/11358120709487712. [DOI] [Google Scholar]
- 18.Abdel-Monaim M.F., EL-Morsi M.E.A., Hassan M.A.E. Control of root rot and wilt disease complex of some evergreen fruit transplants by using plant growth promoting rhizobacteria in the New Valley Governorate, Egypt. J. Phytopathol. Pest Manag. 2014;1:23–33. [Google Scholar]
- 19.Barkai-Golan R. Chemical control. In: Barkai-Golan R., editor. Postharvest Diseases of Fruits and Vegetables: Development and Control. Elsevier Science; Oxford, UK: 2001. pp. 147–188. [Google Scholar]
- 20.Restuccia C., Giusino F., Licciardello F., Randazzo C., Caggia C., Muratore G. Biological control of peach fungal pathogens by commercial products and indigenous yeasts. J. Food Protect. 2006;69:2465–2470. doi: 10.4315/0362-028X-69.10.2465. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-Montiel L.G., Ochoa J.L., Troyo-Diéguez E., Larralde-Corona C.P. Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol. Technol. 2010;56:181–187. doi: 10.1016/j.postharvbio.2009.12.010. [DOI] [Google Scholar]
- 22.Molinu M.G., Pani G., Venditti T., Dore A., Ladu G., D’Hallewin G. Alternative methods to control postharvest decay caused by Penicillium expansum in plums (Prunus domestica L.) Commun. Agric. Appl. Biol. Sci. 2012;77:509–514. [PubMed] [Google Scholar]
- 23.Eckert J.W., Eaks I.L. Postharvest disorders and diseases of citrus fruits. In: Reuther W., Calavan E.C., Carman G.E., editors. The Citrus Industry. Volume 5. University of California Press; Berkeley, CA, USA: 1989. pp. 179–260. [Google Scholar]
- 24.Marcet-Houben M., Ballester A.-R., De la Fuente B., Harries E., Marcos J.F., González-Candelas L., Gabaldón T. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genom. 2012;13:646. doi: 10.1186/1471-2164-13-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atanasova-Penichon V., Bernillon S., Marchegay G., Lornac A., Pinson-Gadais L., Ponts N., Zehraoui E., Barreau C., Richard-Forget F. Bioguided isolation, characterization, and biotransformation by Fusarium verticillioides of Maize Kernel compounds that inhibit Fumonisin production. Mol. Plant Microbe Interact. 2014;27:1148–1158. doi: 10.1094/MPMI-04-14-0100-R. [DOI] [PubMed] [Google Scholar]
- 26.Xing F., Hua H., Selvaraj J.N., Yuan Y., Zhao Y., Zhou L., Liu Y. Degradation of fumonisin B1 by cinnamon essential oil. Food Control. 2014;38:37–40. doi: 10.1016/j.foodcont.2013.09.045. [DOI] [Google Scholar]
- 27.Ploetz R.C. Fusarium Wilt of Banana. Phytopathology. 2015;105:1512–1521. doi: 10.1094/PHYTO-04-15-0101-RVW. [DOI] [PubMed] [Google Scholar]
- 28.Pérombelon M.C.M. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathol. 2002;51:1–12. doi: 10.1046/j.0032-0862.2001.Shorttitle.doc.x. [DOI] [Google Scholar]
- 29.EL-Hefny M., Mohamed A.A., Salem M.Z.M., Abd El-Kareem M.S.M., Ali H.M. Chemical composition, antioxidant capacity and antibacterial activity against some potato bacterial pathogens of fruit extracts from Phytolacca dioica and Ziziphus spina-christi grown in Egypt. Sci. Hortic. 2018;233:225–232. doi: 10.1016/j.scienta.2018.01.046. [DOI] [Google Scholar]
- 30.Salem M.Z.M., Elansary H.O., Elkelish A.A., Zeidler A., Ali H.M., Hefny M.E.L., Yessoufou K. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. BioResources. 2016;11:9421–9437. doi: 10.15376/biores.11.4.9421-9437. [DOI] [Google Scholar]
- 31.Salem M.Z.M., Behiry S.I., Salem A.Z.M. Effectiveness of root-bark extract from Salvadora persica against the growth of certain molecularly identified pathogenic bacteria. Microb. Pathogen. 2018;117:320–326. doi: 10.1016/j.micpath.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 32.Meyer V. A small protein that fights fungi: AFP as a new promising antifungal agent of biotechnological value. Appl. Microbiol. Biot. 2008;78:17–28. doi: 10.1007/s00253-007-1291-3. [DOI] [PubMed] [Google Scholar]
- 33.Scherm B., Balmas V., Spanu F., Pani G., Delogu G., Pasquali M., Migheli Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013;14:323–341. doi: 10.1111/mpp.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ajayi-Oyetunde O.O., Bradley C.A. Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 2017;67:3–17. doi: 10.1111/ppa.12733. [DOI] [Google Scholar]
- 35.Castiblanco V., Castillo H.E., Miedaner T. Candidate genes for aggressiveness in a natural Fusarium culmorum population greatly differ between wheat and rye head blight. J. Fungi. 2018;4:14. doi: 10.3390/jof4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dias M.C. Phytotoxicity: An overview of the physiological responses of plants exposed to fungicides. Jpn. J. Bot. 2012;2012:135479. doi: 10.1155/2012/135479. [DOI] [Google Scholar]
- 37.Mazu T.K., Bricker B.A., Flores-Rozas H., Ablordeppey S.Y. The mechanistic targets of antifungal agents: An overview. Mini Rev. Med. Chem. 2016;16:555–578. doi: 10.2174/1389557516666160118112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villa F., Cappitelli F., Cortesi P., Kunova A. Fungal biofilms: Targets for the development of novel strategies in plant disease management. Front. Microbiol. 2017;8:654. doi: 10.3389/fmicb.2017.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh A.K., Kumar P., Nidhi R., Gade R.M. Allelopathy—A Sustainable Alternative and Eco-Friendly Tool for Plant Disease Management. Plant Dis. Sci. 2012;7:127–134. [Google Scholar]
- 40.Rodrigues A.M., Theodoro P.N., Eparvier V., Basset C., Silva M.R., Beauchêne J., Espíndola L.S., Stien D. Search for Antifungal Compounds from the Wood of Durable Tropical Trees. J. Nat. Prod. 2010;73:1706–1707. doi: 10.1021/np1001412. [DOI] [PubMed] [Google Scholar]
- 41.Midgely S.J., Turnbull J.W. Domestication and use of Australian acacias: Case studies of five important species. Aust. Syst. Bot. 2003;16:89–102. doi: 10.1071/SB01038. [DOI] [Google Scholar]
- 42.Shinwari Z.K., Gilani S.A., Khan A.L. Biodiversity loss, emerging infectious diseases and impact on human and crops. Pak. J. Bot. 2012;44:137–142. [Google Scholar]
- 43.Rafiqul Hoque A.T.M., Ahmed R., Uddin M.B., Hossain M.K. Allelopathic effect of different concentration of water extracts of Acacia auriculiformis leaf on some initial growth parameters of five common agricultural crops. J. Agron. 2003;2:92–100. [Google Scholar]
- 44.Alhammadi A.S.A. Allelopathic effect of Tagetes minuta L. water extracts on seeds germination and seedling root growth of Acacia asak. Assiut Univ. Bull. Environ. Res. 2008;11:17–24. [Google Scholar]
- 45.Bitende S.N., Ledin I. Effect of doubling the amount of low quality grass hay offered and supplementation with Acacia tortilis fruits or Sesbania sesban leaves, on intake and digestibility by sheep in Tanzania. Livest. Prod. Sci. 1996;45:39–48. doi: 10.1016/0301-6226(95)00085-2. [DOI] [Google Scholar]
- 46.Shayo C.M. Udén, PNutritional uniformity on neutral detergent solubles in some tropical browse leaf and pod diets. Anim. Feed Sci. Technol. 1999;82:63–73. doi: 10.1016/S0377-8401(99)00098-X. [DOI] [Google Scholar]
- 47.Dube J.S., Reed J.D., Ndlovu L.R. Proanthocyanidins and other phenolics in Acacia leaves of Southern Africa. Anim. Feed Sci. Technol. 2001;91:59–67. doi: 10.1016/S0377-8401(01)00229-2. [DOI] [Google Scholar]
- 48.Rubanza C.D.K., Shem M.N., Otsyina R., Bakengesa S.S., Ichinohe T., Fujihara T. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Technol. 2005;119:129–142. doi: 10.1016/j.anifeedsci.2004.12.004. [DOI] [Google Scholar]
- 49.Nakafeero A.L., Reed M.S., Moleele N.M. Allelopathic potential of five agroforestry trees, Botswana. Afr. J. Ecol. 2007;45:590–593. doi: 10.1111/j.1365-2028.2007.00776.x. [DOI] [Google Scholar]
- 50.Abd El-Gawad A.M., El-Amier Y.A. Allelopathy and potential impact of invasive Acacia saligna (Labill.) Wendl. on plant diversity in the Nile Delta coast of Egypt. Int. J. Environ. Res. 2015;9:923–932. [Google Scholar]
- 51.Oskoueian E., Abdullah N., Ahmad S., Saad W.Z., Omar A.R., Ho Y.W. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int. J. Mol. Sci. 2011;12:5955–5970. doi: 10.3390/ijms12095955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Toumy S.A., Salib J.Y., Mohamed W.M., Morsy F.A. Phytochemical and antimicrobial studies on Acacia saligna leaves. Egypt. J. Chem. 2010;53:705–717. [Google Scholar]
- 53.Noreen I., Iqbal A., Fazl-e-Rabbi, Muhammad A., Shah Z., Ur Rahman Z. Antimicrobial activity of different solvents extracts of Acacia cyanophylla. Pak. J. Weed Sci. Res. 2017;23:79–90. [Google Scholar]
- 54.Gumgumjee N.M., Hajar A.S. Antimicrobial efficacy of Acacia saligna (Labill.) H.L. Wendl. and Cordia sinensis Lam. leaves extracts against some pathogenic microorganisms. Int. J. Microbiol. Immunol. Res. 2015;3:51–57. [Google Scholar]
- 55.Ali H.M., Salem M.Z.M., Abdel-Megeed A. In-vitro antibacterial activities of alkaloids extract from leaves of Conocarpus lancifolius Engl. J. Pure Appl. Microbiol. 2013;7:1903–1907. [Google Scholar]
- 56.Saleem A., Ahotupa M., Pihlaja K. Total phenolics concentration and antioxidant potential of extracts of medicinal plants of Pakistan. Z. Naturforsch. C. 2001;56:973–978. doi: 10.1515/znc-2001-11-1211. [DOI] [PubMed] [Google Scholar]
- 57.Seigler D.S. Phytochemistry of Acacia—Sensu lato. Biochem. Syst. Ecol. 2003;31:845–873. doi: 10.1016/S0305-1978(03)00082-6. [DOI] [Google Scholar]
- 58.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Photochemistry. 2000;55:481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 59.Gedara S.R., Galala A.A. New cytotoxic spirostane saponin and biflavonoid glycoside from the leaves of Acacia saligna (Labill.) H.L. Wendl. Nat. Prod. Res. 2014;28:324–329. doi: 10.1080/14786419.2013.863200. [DOI] [PubMed] [Google Scholar]
- 60.El Sissi H.I., El Sherbeiny A.E.A. The flavonoid components of the leaves of Acacia saligna. Qual. Plant Mater. Veg. 1967;14:257–266. doi: 10.1007/BF02419928. [DOI] [Google Scholar]
- 61.Thieme H., Khogali A. The occurrence of flavonoids and tannins in the leaves of some African acacia species. Pharmazie. 1975;30:736–743. [PubMed] [Google Scholar]
- 62.Baldan V., Sut S., Faggian M., Gassa E.D., Ferrari S., De Nadai G., Francescato S., Baratto G., Dall’Acqua S. Larix decidua bark as a source of phytoconstituents: An LC-MS study. Molecules. 2017;22:1974. doi: 10.3390/molecules22111974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dall’Acqua S., Minesso P., Shresta B.B., Comai S., Jha P.K., Gewali M.B., Greco E., Cervellati R., Innocenti G. Phytochemical and antioxidant-related investigations on bark of Abies spectabilis (D. don) spach. from Nepal. Molecules. 2012;17:1686–1697. doi: 10.3390/molecules17021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dall’Acqua S., Cervellati R., Loi M.C., Innocenti G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008;106:745–749. doi: 10.1016/j.foodchem.2007.06.055. [DOI] [Google Scholar]
- 65.Tohma H., Köksal E., Kılıç Ö., Alan Y., Yılmaz M.A., Gülçin İ., Bursal E., Alwasel S.H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. Species. Antioxidants. 2016;5:38. doi: 10.3390/antiox5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bravo L., Goya L., Lecumberri E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Res. Int. 2007;40:393–405. doi: 10.1016/j.foodres.2006.10.016. [DOI] [Google Scholar]
- 67.Salih E.Y.A., Fyhrquist P., Abdalla A.M.A., Abdelgadir A.Y., Kanninen M., Sipi M., Luukkanen O., Fahmi M.K.M., Elamin M.H., Ali H.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen) Antibiotics. 2017;6:37. doi: 10.3390/antibiotics6040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alves C.T., Ferreira I.C., Barros L., Silva S., Azeredo J., Henriques M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014;9:139–146. doi: 10.2217/fmb.13.147. [DOI] [PubMed] [Google Scholar]
- 69.Tempesti T.C., Alvarez M.G., de Arau’jo M.F., Ju’nior F.E., de Carvalho M.G., Durantini E.N. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res. 2012;21:2217–2222. doi: 10.1007/s00044-011-9750-x. [DOI] [Google Scholar]
- 70.Weidenbörner M., Hindorf H., Jha H.C., Tsotsonos P. Antifungal activity of flavonoids against storage fungi of the genus Aspergillus. Phytochemistry. 1990;29:1103–1105. doi: 10.1016/0031-9422(90)85412-9. [DOI] [Google Scholar]
- 71.Céspedes C.L., Salazar J.R., Ariza-Castolo A., Yamaguchi L., Avila J.G., Aqueveque P., Kubo I., Alarcón J. Biopesticides from plants: Calceolaria integrifolia sl. Environ. Res. 2014;132:391–406. doi: 10.1016/j.envres.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Mierziak J., Kostyn K., Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19:16240–16265. doi: 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orhan D.D., Özçelik B., Özgen S., Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010;165:496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 74.Salem M.Z.M., Zidan Y.E., El Hadidi N.M.N., Mansour M.M.A., Abo Elgat W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeter. Biodegr. 2016;110:206–226. doi: 10.1016/j.ibiod.2016.03.028. [DOI] [Google Scholar]
- 75.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press, Inc.; New York, NY, USA: 1990. [Google Scholar]
- 76.Geiser D.M., Jiménez-Gasco M.D.M., Kang S., Makalowska I., Veeraraghavan N., Ward T.J., Zhang N., Kuldau G.A., O’donnell K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- 77.Salem M.Z.M., Mansour M.M.A., Elansary H.O. Evaluation of the effect of inner and outer bark extracts of Sugar Maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 2019 doi: 10.1080/02773813.2018.1547763. [DOI] [Google Scholar]
- 78.Mohareb A.S.O., Kherallah I.E.A., Badawy M.E.I., Salem M.Z.M., Faraj H.A.Y. Chemical composition and antibacterial activity of essential oils isolated from leaves of different woody trees grown in Al-Jabel al-Akhdar region, Libya. Alex. Sci. Exchang. J. 2016;37:358–371. [Google Scholar]
- 79.Tepe B., Daferera D., Sokmen A., Sokmen M., Polissiou A. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem. 2005;90:333–340. doi: 10.1016/j.foodchem.2003.09.013. [DOI] [Google Scholar]