Abstract
Vegetarian diets may lower symptomatic gallstone disease via cholesterol lowering. This study aimed to examine the risk of symptomatic gallstone disease (GSD) in Taiwanese vegetarians vs. nonvegetarians in a prospective cohort and to explore if this association is related to cholesterol concentration. We prospectively followed 4839 participants, and in the 29,295 person-years of follow-up, 104 new incident GSD cases were confirmed. Diet was assessed through a validated food frequency questionnaire. Symptomatic GSD was ascertained through linkage to the Taiwan National Health Insurance Research Database. Blood cholesterol profiles were measured at recruitment. Cox regression was applied to assess the effect of diet on symptomatic GSD, adjusting for age, education, smoking, alcohol, physical activities, diabetes, kidney diseases, body mass index, lipid-lowering medication, and hypercholesterolemia. Vegetarian diet was associated with a decreased risk of symptomatic GSD compared with nonvegetarian diet in women (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.28–0.96) but not in men. In women, nonvegetarians with hypercholesterolemia had 3.8 times the risk of GSD compared with vegetarians with normal cholesterol (HR, 3.81, 95% CI, 1.61–9.01). A vegetarian diet may therefore protect against GSD independent of baseline hypercholesterolemia. A nonvegetarian diet and hypercholesterolemia may have an additive effect in increasing GSD risk in women.
Keywords: vegetarian diet, gallstone disease, cholesterol, prospective cohort
1. Introduction
Gallstone disease (GSD) is a worldwide disease. Its prevalence appears to be higher in Western countries (>10%) [1,2] than in Asian countries (3%–10%) [1,3]. Female sex, older age, higher body mass index (BMI), hyperlipidemia, alcohol consumption, and diabetes mellitus have been reported as risk factors for GSD [1]. In Western countries, gallstones are composed mainly of cholesterol in 75%–80% of cases [4].
The associations between GSD and plant-based foods have been explored in previous literature; however, the results were controversial. Some studies have suggested that fruit and vegetable consumption decreases the risk of cholecystectomy in women [5,6,7,8], but the European EPIC-Oxford study showed that a vegetarian diet is a risk factor for symptomatic GSD [9]. Most of these studies are from the Western population, so the effect of vegetarian dietary patterns on the development of symptomatic GSD in the Asian population is unknown.
The pathologic factors for cholesterol gallstones include genetic background, hepatic hypersecretion of cholesterol, and supersaturated bile, which give life to precipitating cholesterol crystals that accumulate and grow in a sluggish gallbladder [4]. The relationship between blood cholesterol levels and gallstone formation is multifactorial and complex. The role of blood cholesterol levels in the development of symptomatic GSD is not yet well known.
Systemic review and meta-analysis studies have provided evidence that vegetarian diets effectively lower blood concentrations of total cholesterol (TCH), low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol [10,11,12]. Vegetarian dietary patterns have been associated with reductions in risk of several diseases, such as hypertension, metabolic syndrome, diabetes mellitus, ischemic heart disease, diverticular disease, and colorectal cancer [13,14,15,16,17]. A vegetarian diet might be a useful nonpharmaceutical means for managing dyslipidemia, especially hypercholesterolemia [10].
We hypothesized that a vegetarian diet lowers the incidence of symptomatic GSD due to lowering of blood cholesterol. The purpose of this study was to examine the effect of vegetarian vs. nonvegetarian diet on the incidence of symptomatic GSD using a prospective cohort study in Taiwan and to explore if this association varies by cholesterol concentration.
2. Materials and Methods
2.1. Study Design and Population
The Tzu Chi Health Study is a prospective cohort study that recruited 6002 participants (age 18 to 87) from 2007 to 2009 at the Buddhist Dalin Tzu Chi Hospital. About 77% of the study participants were Tzu Chi volunteers. Tzu Chi volunteers are devoted Buddhist who volunteer regularly for the Buddhist Tzu Chi Foundation for a variety of charity works and disaster relief. These volunteers are encouraged, though not required, to become vegetarians.
2.2. Assessment of Diet, Cholesterol, and Other Covariates
At the time of enrollment, all participants received a comprehensive health examination and were interviewed on a questionnaire for demographics (sex, education), medical history, lifestyle habits (smoking, alcohol, physical activities), and diet (a questionnaire on vegetarian dietary practice [type and duration] and a food frequency questionnaire [FFQ]).
Participants were interviewed on a 64-item quantitative FFQ that had been validated to show good reliability and validity in the present study population [18]. Vegetarian diet was defined as avoidance of meat and fish as assessed by both face-to-face confirmation and the FFQ by trained interviewers.
Blood was drawn for health examination after an overnight fast. Total cholesterol, LDL cholesterol, and HDL cholesterol were assessed using Integra 800 System (Roche Diagnostics, Indianapolis, IN). Participants were categorized into two cholesterol levels: hypercholesterolemia (≧200 mg/dL) or normal cholesterol (<200 mg/dL). Height and weight were measured using an electronic scale and were used to calculate the BMI. BMI levels were further subdivided to two categories by 24 kg/m2, according to Taiwan’s definition of overweight and obesity [19].
2.3. Cohort Follow-Up and Case Ascertainment
The baseline data of the participants were linked to the National Health Insurance Research Database (NHIRD) of Taiwan. Incident cases of gallstone disease were identified through the International Classification of Diseases, Ninth Revision (ICD-9) codes 574, 575.0, and 575.1 in the NHIRD. The NHIRD covers medical benefit claims for over 23 million people (approximately 99% of Taiwan’s population) [20]. Taiwan’s National Health Insurance (NHI) provides universal insurance coverage and is a single-payer system with the government as the sole insurer. The database was monitored for completeness and accuracy by Taiwan’s Department of Health.
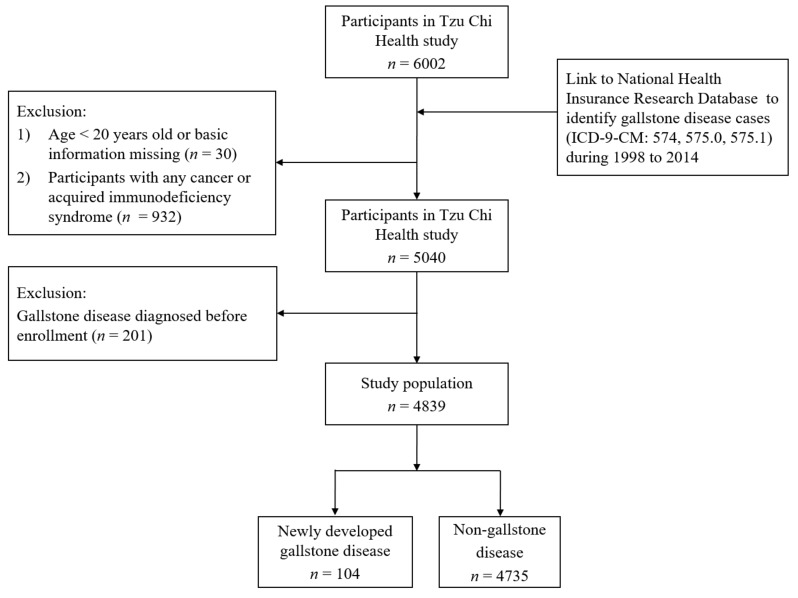
Participants with any cancer or acquired immunodeficiency syndrome (n = 932), those who did not have a complete dietary and lifestyle questionnaire or were aged below 20 at recruitment (n = 30), and those who already had prior gallstone disease as identified by ICD-9 code in NHIRD before recruitment (n = 201) were excluded (Figure 1).
Figure 1.
Flow chart for identifying the study cohort.
Participants were followed from recruitment until December 31, 2014. During this period, gallstone diseases that were coded at least three times in the outpatient records or at least one time for inpatient treatment were considered as incident cases; this was done because the first couple of diagnoses in outpatient clinics may be made to enable further examination for rule out purposes and therefore may not be the true diagnosis. Person-years were calculated from the date of recruitment until the date GSD was coded for the first time in NHIRD or until the end of follow-up (death or December 31, 2014).
We identified participants’ comorbidities through the NHIRD using ICD-9 codes: hypertension (ICD-9 codes 401, 402, 405), heart disease (ICD-9 codes 410-414), diabetes (ICD-9 code 250), renal diseases (ICD-9 codes 403, 404, 580-588), and liver disease (ICD-9 codes 571, 572). Prescription of lipid-lowering medications such as statins was also determined through NHIRD.
2.4. Ethics Statements
The study was approved by the Institutional Review Board for ethics in the Buddhist Dalin Tzu Chi Hospital. All participants gave written informed consents. The study has been registered at ClinicalTrials.gov (ID: NCT: 03204552).
2.5. Statistical Analysis
The SAS 9.4 (SAS Institute, Cary, NC, USA) was used for data analysis. A p-value of less than 0.05 was considered statistically significant. Because sex is an important predictor of GSD, we stratified all of our analyses by sex.
Baseline characteristics between vegetarians and nonvegetarians were compared using independent sample t-test (continuous variables) and chi-square test (categorical variables). Dietary intakes of vegetarians and nonvegetarians were compared using Wilcoxon sign rank test.
Cox proportional hazards regression was used to calculate the risk of GSD with adjustments for age, education, drinking, smoking, diabetes and kidney diseases, lipid-lowering medications, TCH and BMI. We also conducted a subgroup analysis by cholesterol concentrations.
A sensitivity test was conducted by eliminating the cases of symptomatic GSD diagnosed within one year of enrollment.
3. Results
3.1. Baseline Characteristics
A total of 4839 participants were included in the current study. Among them, 1403 were vegetarians, and 3436 were nonvegetarians. Their characteristics are summarized in Table 1. Vegetarians were slightly older, with a higher percentage of female, never-smokers, and habitual alcohol drinkers. Vegetarians had a lower prevalence of diabetes and kidney disease. Vegetarians also had lower cholesterol concentrations and BMI.
Table 1.
Baseline characteristics of vegetarians and nonvegetarians.
| All | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonvegetarians | Vegetarians | p-Value | Nonvegetarians | Vegetarians | p-Value | Nonvegetarians | Vegetarians | p-Value | |
| n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | n (%)/mean ± SD | ||||
| Age, mean ± SD years | 51.8 ± 10.5 | 54.0 ± 9.3 | <0.001 | 51.8 ± 10.9 | 54.2 ± 8.8 | <0.001 | 51.7 ± 10.1 | 54.0 ± 9.4 | <0.001 |
| Sex | <0.001 | ||||||||
| Male | 1625 (47.3%) | 345 (24.6%) | |||||||
| Female | 1811 (52.7%) | 1058 (75.4%) | |||||||
| Education degree | <0.001 | <0.001 | <0.001 | ||||||
| Elementary school or less | 733 (21.3%) | 397 (28.3%) | 232 (14.3%) | 60 (17.4%) | 501 (27.7%) | 337 (31.9%) | |||
| High school | 1654 (48.2%) | 719 (51.2%) | 765 (47.1%) | 178 (51.6%) | 889 (49.1%) | 541 (51.1%) | |||
| College or higher | 1049 (30.5%) | 287 (20.5%) | 628 (38.6%) | 107 (31.0%) | 421 (23.2%) | 180 (17.0%) | |||
| Smoking status | <0.001 | <0.001 | 0.014 | ||||||
| Ever | 718 (20.9%) | 116 (8.3%) | 676 (41.6%) | 105 (30.4%) | 42 (2.3%) | 11 (1.0%) | |||
| Never | 2718 (79.1%) | 1287 (91.7%) | 949 (58.4%) | 240 (69.6%) | 1769 (97.7%) | 1047 (99.0%) | |||
| Alcohol drinking status | <0.001 | 0.076 | <0.001 | ||||||
| Ever | 614 (17.9%) | 115 (8.2%) | 539 (33.2%) | 97 (28.1%) | 75 (4.1%) | 18 (1.7%) | |||
| Never | 2822 (82.1%) | 1288 (91.8%) | 1086 (66.8%) | 248 (71.9%) | 1736 (95.9%) | 1040 (98.3%) | |||
| Sport habit | 0.003 | 0.428 | 0.098 | ||||||
| Yes | 2365 (68.8%) | 904 (64.4%) | 1176 (72.4%) | 242 (70.1%) | 1189 (65.7%) | 662 (62.6%) | |||
| No | 1071 (31.2%) | 499 (35.6%) | 449 (27.6%) | 103 (29.9%) | 622 (34.3%) | 396 (37.4%) | |||
| Hypertension * | 0.564 | 0.2641 | 0.471 | ||||||
| Yes | 752 (21.9%) | 296 (21.1%) | 391 (24.1%) | 73 (21.2%) | 361 (19.9%) | 223 (21.1%) | |||
| No | 2684 (78.1%) | 1107 (78.9%) | 1234 (75.9%) | 272 (78.8%) | 1450 (80.1%) | 835 (78.9%) | |||
| Diabetes mellitus * | 0.005 | 0.423 | 0.021 | ||||||
| Yes | 318 (9.3%) | 95 (6.8%) | 161 (9.9%) | 29 (8.4%) | 157 (8.7%) | 66 (6.2%) | |||
| No | 3118 (90.7%) | 1308 (93.2%) | 1464 (90.1%) | 316 (91.6%) | 1654 (91.3%) | 992 (93.8%) | |||
| Heart disease * | 0.461 | 0.186 | 0.642 | ||||||
| Yes | 338 (9.8%) | 148 (10.5%) | 172 (10.6%) | 45 (13.0%) | 166 (9.2%) | 103 (9.7%) | |||
| No | 3098 (90.2%) | 1255 (89.5%) | 1453 (89.4%) | 300 (87.0%) | 1645 (90.8%) | 955 (90.3%) | |||
| Liver disease * | 0.403 | 0.677 | 0.409 | ||||||
| Yes | 689 (20.1%) | 266 (19.0%) | 391 (24.1%) | 79 (22.9%) | 298 (16.5%) | 187 (17.7%) | |||
| No | 2747 (79.9%) | 1137 (81.0%) | 1234 (75.9%) | 266 (77.1%) | 1513 (83.5%) | 871 (82.3%) | |||
| Kidney disease * | 0.040 | 0.241 | 0.275 | ||||||
| Yes | 170 (4.9%) | 50 (3.6%) | 93 (5.7%) | 14 (4.1%) | 77 (4.3%) | 36 (3.4%) | |||
| No | 3266 (95.1%) | 1353 (96.4%) | 1532 (94.3%) | 331 (95.9%) | 1734 (95.7%) | 1022 (96.6%) | |||
| Body mass index | <0.001 | <0.001 | <0.001 | ||||||
| ≧24 | 1611 (46.9%) | 471 (33.6%) | 914 (56.2%) | 135 (39.1%) | 697 (38.5%) | 336 (31.8%) | |||
| <24 | 1825 (53.1%) | 932 (66.4%) | 711 (43.8%) | 210 (60.9%) | 1114 (61.5%) | 722 (68.2%) | |||
| Total cholesterol | <0.001 | <0.001 | <0.001 | ||||||
| ≧200 | 1526 (44.4%) | 369 (26.3%) | 696 (42.8%) | 69 (20.0%) | 830 (45.8%) | 300 (28.4%) | |||
| <200 | 1910 (55.6%) | 1034 (73.7%) | 929(57.2%) | 276 (80.0%) | 981 (54.2%) | 758 (71.6%) | |||
| Lipid-lowering medications | <0.001 | <0.001 | <0.001 | ||||||
| Yes | 872 (25.4%) | 224 (16.0%) | 424 (26.1%) | 56 (16.2%) | 448 (24.7%) | 168 (15.9%) | |||
| No | 2564 (74.6%) | 1179 (84.0%) | 1201 (73.9%) | 289 (83.8%) | 1363 (75.3%) | 890 (84.1%) | |||
| Menopause | <0.001 | ||||||||
| Yes | 1032 (57.0%) | 694 (65.6%) | |||||||
| No | 779 (43.0%) | 364 (34.4%) | |||||||
| Total cholesterol, mean ± SD | 196.7 ± 36.7 | 180.7 ± 33.1 | <0.001 | 194.0 ± 36.6 | 175.0 ± 34.4 | <0.001 | 199.0 ± 36.6 | 183.0 ± 32.4 | <0.001 |
| HDL cholesterol, mean ± SD | 54.2 ± 14.7 | 52.4 ± 13.7 | <0.001 | 48.7 ± 12.4 | 44.9 ± 10.4 | <0.001 | 59.1 ± 14.9 | 54.9 ± 13.7 | <0.001 |
| LDL cholesterol, mean ± SD | 129.0 ± 33.4 | 116.0 ± 29.3 | <0.001 | 130.0 ± 33.3 | 115.0 ± 28.1 | <0.001 | 129.0 ± 33.5 | 117.0 ± 29.7 | <0.001 |
| Triglyceride, mean ± SD | 117.0 ± 85.4 | 114.0 ± 77.8 | 0.230 | 133.0 ± 104.0 | 129.0 ± 93.6 | 0.481 | 102.0 ± 61.4 | 109.0 ± 71.3 | 0.014 |
* Comorbidity was diagnosed through the NHIRD using ICD-9 codes. Abbreviations: SD, standard deviation; NHIRD, National Health Insurance Research Database; ICD-9, International Classification of Diseases, Ninth Revision; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
3.2. Dietary Intakes of Vegetarians and Nonvegetarians
Dietary intakes of vegetarians and nonvegetarians, as assessed by FFQ, are shown in Table 2. While completely avoiding meat and fish, vegetarians had higher intakes of soy and vegetables. Intakes of dairy (median = 0.2 serving/day) and eggs (median = 0.3 serving/day) were low for both nonvegetarians and vegetarians. Vegetarians consumed more carbohydrates and fiber but less fat and protein compared with nonvegetarians.
Table 2.
Dietary intakes of vegetarians and nonvegetarians as assessed by food frequency questionnaire.
| Food and Nutrients | All | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonvegetarians | Vegetarians | p-Value | Nonvegetarians | Vegetarians | p-Value | Nonvegetarians | Vegetarians | p-Value | |
| Median (P25, P75) | Median (P25, P75) | Median (P25, P75) | Median (P25, P75) | Median (P25, P75) | Median (P25, P75) | ||||
| Energy, kcal | 1739 (1312, 2290) | 1702 (1273, 2262) | 0.118 | 2066 (1616, 2669) | 2234 (1653, 2799) | 0.068 | 1486 (1119, 1931) | 1608 (1218, 2028) | <0.0001 |
| Meat, servings | 0.6 (0.2, 1.6) | - | - | 1.0 (0.3, 2.2) | - | - | 0.4 (0.1, 1.1) | - | - |
| Fish, servings | 0.5 (0.1, 1.1) | - | - | 0.6 (0.2, 1.3) | - | - | 0.3 (0.1, 0.9) | - | - |
| Soy, servings | 0.9 (0.5, 1.7) | 1.5 (0.8, 2.5) | <0.0001 | 1.0 (0.5, 1.7) | 1.6 (1.0, 2.9) | <0.0001 | 0.9 (0.5, 1.6) | 1.4 (0.8, 2.3) | <0.0001 |
| Dairy, servings | 0.2 (0, 0.7) | 0.2 (0, 0.6) | <0.0001 | 0.2 (0, 0.7) | 0.2 (0, 0.7) | 0.626 | 0.2 (0, 0.7) | 0.2 (0, 0.6) | <0.0001 |
| Eggs, servings | 0.3 (0.1, 0.6) | 0.3 (0.1, 0.4) | <0.0001 | 0.4 (0.2, 0.6) | 0.3 (0.1, 0.5) | <0.0001 | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.4) | <0.0001 |
| Vegetables, servings | 3.8 (2.3, 5.7) | 4.5 (2.9, 6.8) | <0.0001 | 3.5 (2.2, 5.5) | 4.9 (3.0, 7.3) | <0.0001 | 3.9 (2.4, 5.9) | 4.4 (2.9, 6.8) | <0.0001 |
| Fruits, servings | 1.0 (0.5, 2.0) | 1.0 (0.6, 2.0) | 0.020 | 1.0 (0.5, 2.0) | 1.0 (0.6, 2.0) | 0.112 | 1.0 (0.5, 2.0) | 1.0 (0.5, 2.0) | 0.226 |
| Vitamin C, mg | 164 (112, 233) | 172 (118, 249) | 0.001 | 165 (112, 226) | 184 (130, 262) | <0.0001 | 163 (112, 237) | 168 (115, 243) | 0.191 |
| Dietary fiber, g | 19 (14, 26) | 23 (16, 31) | <0.0001 | 19 (15, 27) | 25 (19, 35) | <0.0001 | 19 (14, 26) | 21 (16, 29) | <0.0001 |
| Protein (% energy) | 13 (12, 15) | 12 (11, 13) | <0.001 | 13 (11, 15) | 12 (10, 13) | <0.001 | 13 (12, 15) | 12 (11, 14) | <0.001 |
| Fat (% energy) | 27 (21, 33) | 25 (20, 30) | <0.001 | 26 (20, 32) | 22 (18, 29) | <0.001 | 28 (22, 34) | 25 (20, 30) | <0.001 |
| Carbohydrate (% energy) | 60 (54, 67) | 64 (59, 70) | <0.001 | 61 (54, 68) | 66 (59, 71) | <0.001 | 59 (53, 66) | 64 (58, 69) | <0.001 |
One serving of meat, fish, soy is defined as 7 g of protein, one serving of eggs is defined as 1 regular size egg, one serving of vegetables is defined as 100 g, one serving of fruit is defined as 15 g of carbohydrates. p-value is assessed using Wilcoxon sign rank test. Abbreviations: P25, lowest 25%; P75, highest 75%.
3.3. Vegetarian Diet and Gallstone Disease
In the 29,295 person-years (average 6.05 years) of follow-up, 104 individuals developed GSD. Among them, 22 were vegetarians, and 82 were nonvegetarians. Symptomatic GSD occurred at a higher incidence in nonvegetarians than in vegetarians (2.3% versus 1.5%).
Table 3 shows the association between a vegetarian diet and symptomatic GSD in Cox proportional hazards regression analysis. After adjustment for age, education, smoking, alcohol drinking, physical activities, diabetes, kidney diseases, BMI, hypercholesterolemia, lipid-lowering medication, and menopause (in women only), vegetarian diet was found to be associated with a nearly 50% decrease in symptomatic GSD risk compared with nonvegetarian diet (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.28–0.96) in women but not in men. In the whole population, age and hypercholesterolemia were two risk factors for symptomatic GSD (HR, 1.03; 95% CI, 1.01–1.06; and HR, 1.69; 95% CI, 1.12–2.55, respectively). In men, age (HR, 1.04; 95% CI, 1.01–1.08), hypercholesterolemia (HR, 1.89; 95% CI, 1.00–3.59), and diabetes (HR, 2.25; 95% CI, 1.01–5.03) were significantly associated with GSD.
Table 3.
Cox proportional hazards regression for risk of gallstone disease.
| Cases/Person-Years | All | Male | Female |
|---|---|---|---|
| 104/29295 | 4311964 | 61/17332 | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Crude model | 0.66 (0.41, 1.05) | 1.08 (0.50, 2.33) | 0.51 (0.28, 0.92) |
| Adjusted model | |||
| Vegetarians vs. nonvegetarians | 0.70 (0.43, 1.14) | 1.26 (0.57, 2.77) | 0.52 (0.28, 0.96) |
| Age | 1.03 (1.01, 1.06) | 1.04 (1.01, 1.08) | 1.06 (1.02, 1.10) |
| Sex (male vs. female) | 0.86 (0.53, 1.40) | - | - |
| High school vs. elementary | 1.11 (0.68, 1.82) | 1.58 (0.62, 3.99) | 0.83 (0.44, 1.56) |
| College or higher vs. elementary | 1.07 (0.59, 1.96) | 1.67 (0.62, 4.52) | 0.71 (0.30, 1.68) |
| Alcohol drinker | 1.37 (0.76, 2.49) | 1.68 (0.87, 3.23) | 0.39 (0.05, 2.92) |
| Smoking | 0.90 (0.48, 1.70) | 0.78 (0.4, 1.54) | 2.94 (0.68, 12.6) |
| Sport habit | 1.01 (0.66, 1.56) | 1.44 (0.66, 3.16) | 0.89 (0.52, 1.52) |
| Diabetes mellitus | 1.75 (0.98, 3.12) | 2.25 (1.01, 5.03) | 1.38 (0.59, 3.23) |
| Kidney disease | 1.27 (0.58, 2.77) | 0.66 (0.16, 2.76) | 1.99 (0.78, 5.09) |
| BMI ≧ 24 kg/m2 | 1.36 (0.92, 2.03) | 1.49 (0.79, 2.81) | 1.35 (0.80, 2.27) |
| Lipid-lowering medications | 0.82 (0.51, 1.32) | 0.90 (0.44, 1.85) | 0.72 (0.37, 1.39) |
| TCH ≧ 200 mg/dL | 1.69 (1.12, 2.55) | 1.89 (1.00, 3.59) | 1.74 (1.00, 3.03) |
| Menopause | - | - | 0.34 (0.16, 0.73) |
Abbreviations: BMI, body mass index; HR, hazard ratio; CI, confidence interval; TCH, total cholesterol.
In our subgroup analysis by cholesterol concentration (Table 4), we found vegetarian women had a 66% decrease in symptomatic GSD risk compared with nonvegetarian women (HR, 0.34; 95% CI, 0.14–0.82) among those with normal TCH but only a nonsignificant 23% (HR, 0.77; 95% CI, 0.33–1.79) among those with hypercholesterolemia, though the interaction of cholesterol concentration and vegetarian diet was not statistically significant (p-interaction = 0.365).
Table 4.
Subgroup analysis for risk of gallstone disease by total cholesterol concentration.
| All | Male | Female | ||
|---|---|---|---|---|
| Total cholesterol ≧ 200 mg/dL | Cases/person-years | 56/11,421 | 23/4638 | 33/6783 |
| HR (95% CI) | 0.89 (0.44, 1.81) | 1.39 (0.41, 4.76) | 0.77 (0.33, 1.79) | |
| Total cholesterol < 200 mg/dL | Cases/person-years | 48/17,874 | 20/7326 | 28/10,549 |
| HR (95% CI) | 0.56 (0.28, 1.11) | 1.31 (0.47, 3.70) | 0.34 (0.14, 0.82) |
Model adjusted for age, sex, education level, smoking status, drinking status, sport, diabetes, chronic kidney disease, BMI, lipid-lowering medications, menopause (for female). Abbreviations: HR, hazard ratio; CI, confidence interval.
The combined effect of a nonvegetarian diet and hypercholesterolemia is shown in Table 5. After adjusting for age, education, smoking, alcohol, physical activities, diabetes, kidney diseases, BMI, and prescription of lipid-lowering medications, we found that nonvegetarian women with TCH ≧ 200 mg/dL had 3.8 times the risk of developing symptomatic GSD compared with vegetarian women with a normal TCH (HR, 3.81; 95% CI, 1.61–9.01).
Table 5.
Hazard ratio of diet with normal and high total cholesterol concentration for gallstone disease.
| All | Male | Female | |
|---|---|---|---|
| Vegetarian with TCH < 200 mg/dL | 1 | 1 | 1 |
| Nonvegetarian with TCH < 200 mg/dL | 1.72 (0.88, 3.33) | 0.89 (0.32, 2.46) | 2.48 (1.05, 5.88) |
| Vegetarian with TCH ≧ 200 mg/dL | 2.20 (0.94, 5.14) | 2.12 (0.50, 8.99) | 2.64 (0.91, 7.65) |
| Nonvegetarian with TCH ≧ 200 mg/dL | 2.71 (1.40, 5.21) | 1.65 (0.60, 4.50) | 3.81 (1.61, 9.01) |
Adjusted for age, sex, education, smoking status, drinking status, sport, diabetes, chronic kidney disease, BMI, lipid-lowering medications, menopause (for female). Abbreviation: TCH, total cholesterol.
Sensitivity analysis of eliminating GSD diagnosed within one year of recruitment showed a similar protective association between vegetarian diet and GSD (HR, 0.42; 95% CI, 0.14–0.90) (Supplement Materials, Table S1).
4. Discussion
In this prospective cohort study, we found that a vegetarian diet has an inverse association with symptomatic GSD in women but not in men. This protective association in women persists after adjustment for overweight/obesity and hypercholesterolemia. The potential protective effect of a vegetarian diet is most significant among those with normal cholesterol concentration. Nonvegetarian diet and hypercholesterolemia may have an additive effect in promoting GSD development.
4.1. Vegetarian Diet and Gallstone Disease as Related to Cholesterol and Metabolic Risk Factors
One potential reason that a vegetarian diet may contribute to a lower GSD risk may be through lowering of cholesterol. Both vegetarian diets and plant protein have been shown to reduce cholesterol levels in meta-analyses [10,12,21]. In our study, we noticed that high total cholesterol concentration (TCH > 200 mg/dL) is a risk factor for symptomatic gallstone disease. Cholesterol, lecithin, and bile acid are the major components in bile [22]. Cholesterol supersaturation of the gallbladder bile is a necessary cause for gallstone formation [23]. Unphysiological supersaturation, generally from hypersecretion of cholesterol, is essential for the formation of cholesterol gallstones [24]. Previous studies have found a positive correlation between blood TCH, bile cholesterol [25], and cholesterol stone formation [26]. Although some studies have shown that abnormal serum lipid profile does not seem to be an essential feature for cholesterol GSD, patients with cholesterol GSD are more likely to have serum lipid parameters toward the undesirable cutoff levels of their respective normal ranges [27,28]. Our study found that vegetarian female with TCH < 200 mg/dL was a strong protective factor for symptomatic GSD. This suggests that even among those with normal TCH concentrations, adopting a healthy vegetarian diet could exert additional protection toward GSD. On the other hand, high cholesterol may attenuate the health benefit typically expected of a vegetarian diet.
Besides cholesterol, GSD is also associated with other metabolic risk factors, including physical inactivity, insulin resistance, diabetes mellitus, and obesity [4]. Vegetarian diets have been reported to reduce insulin resistance [29] and body weight [30] and lower the risk of diabetes [16,31]. Nevertheless, we found that even after adjustment for cholesterol, BMI, diabetes, and physical activities, vegetarian diet was still associated with a lower risk of GSD in women. This suggests that a vegetarian diet may exert protection beyond cholesterol and these metabolic risk factors among women.
4.2. Vegetarian Diet and Gallstone Disease as Related to Female Sex
Epidemiological and clinical studies have found that GSD is more common in women than men [2,32]. Studies have found that higher estrogen level increases the risk of GSD [4,32,33,34,35]. It has been proposed that estrogen increases the risk of developing cholesterol gallstones by increasing the hepatic secretion of biliary cholesterol, which leads to an increase in cholesterol saturation of bile [32]. Previous studies have suggested that in premenopausal women, low-fat diets [36,37,38] and low meat diets [39,40] may reduce serum estrogens levels. In postmenopausal women, vegetarians have been shown to have higher concentrations of sex hormone-binding globulin (SHBG, which inhibits sex hormones and reduces their bioavailability) and a lower level of free estradiol than omnivores [41,42]. This may explain, in part, the lower risk of developing symptomatic GSD in vegetarian females in our study. Our study also demonstrated that menopause was a protective factor for GSD, and this finding is consistent with the understanding that estrogen is a risk factor for GSD.
In contrast, the EPIC-Oxford study found a positive association between a vegetarian diet and GSD after BMI adjustment [9]. In their study, the vegetarian population was much younger than the nonvegetarian group: 42 vs. 50 years of age in men and 38 vs. 46 years of age in women. The significant age gap may have potentially induced bias through the difference in estrogen levels in females as menopausal status was not adjusted.
It has been reported that GSD patients have delayed intestine transit time and higher small intestine bacterial overgrowth [43]. Studies have shown that women’s intestinal transits are slower than men’s [44,45]. A slow colonic transit may, in turn, increase the risk of GSD by increasing the intestinal absorption of cholesterol [46]. Vegetarian diets are rich in dietary fiber, which may shorten the intestinal transit time [47] and may partly contribute to a lower risk of GSD.
4.3. Strength and Limitation
The strength of this study is the availability of participants’ biomarkers at recruitment. This enabled us to examine the associations with potential mediators, such as blood cholesterol concentration. Moreover, the diagnosis of GSD in this cohort study was based on NHIRD records, which is based on physician’s clinical diagnosis rather than self-reported medical history or sonography result. Sonography reports may indicate the existence of gallstone or sludge but are less clinically significant; it does not refer to symptomatic GSD as up to 80% of gallstone cases may remain asymptomatic [48,49,50].
There are some limitations to this study. First, the diagnosis of patients’ symptomatic GSD and identification of comorbidities were completely dependent on ICD codes, although the NHI Bureau of Taiwan randomly reviews the charts and interviews patients in order to verify diagnostic accuracy. In addition, the use of three outpatient diagnosis records for case ascertainment (stringent criteria) to avoid uncertainty and misdiagnosis such as biliary colic or acute gastritis in order to decrease false positive rate may have led to an under-identification of cases. Second, the follow-up period was only from five to seven years for the participants; nevertheless, the significant association suggests adequate power. Third, change in diet over time is possible, but only baseline diet pattern was available in the current study. However, any misclassification due to change in diet over time would tend to attenuate the results toward the null hypothesis. Fourth, the length of time as a vegetarian before recruitment was not certain. Therefore, we did the sensitivity test by eliminating GSD diagnosed within one year of recruitment, and the result was robust. Finally, the study was conducted in Taiwanese Buddhists who generally consume a healthy diet (adequate amount of fiber and vegetables), with little to moderate amount of animal products compared with Western populations, so the generalizability to other population requires additional studies. However, the small amount of meat consumption in our nonvegetarians would have narrowed the dietary range, making it more difficult to detect any potential diet–disease association. Therefore, the actual effect of a vegetarian diet may be even stronger if compared with high meat diets typical of Western populations.
5. Conclusions
This prospective cohort study shows that a vegetarian diet is associated with a lower incidence of symptomatic GSD in Taiwanese women but not in men. A vegetarian diet may strongly protect against GSD in women with normal cholesterol concentration.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/11/2/335/s1. Table S1: Sensitivity test by eliminating symptomatic GSD cases diagnosed within one year of enrollment.
Author Contributions
Conceptualization, C.-M.C., T.H.T.C. and C.-L.L.; methodology, C.-M.C., T.H.T.C., and C.-C.C.; software, T.H.T.C. and C.-C.C.; validation, C.-M.C., T.H.T.C., M.-N.L., and C.-L.L.; formal analysis, C.-C.C., T.H.T.C.; investigation, C.-L.L., M.-N.L., and T.H.T.C.; resources, C.-L.L., M.-N.L., and T.H.T.C.; data curation, C.-L.L., M.-N.L., and T.H.T.C.; writing—original draft preparation, C.-M.C.; writing—review and editing, T.H.T.C. and C.-L.L.; visualization, C.-M.C. and T.H.T.C.; supervision, C.-L.L.; project administration, T.H.T.C.; funding acquisition, C.-L.L., C.-M.C., and T.H.T.C.
Funding
The establishment of the cohort was funded by Buddhist Tzu Chi General Hospital, grant number TCRD-I9605-02. The follow-up was funded by the Tzu Chi Medical Foundation, grant numbers: TCMMP104-08-02, TCMMP105-13-05, and TCMMP106-04-01.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen C.H., Huang M.H., Yang J.C., Nien C.K., Etheredge G.D., Yang C.C., Yeh Y.H., Wu H.S., Chou D.A., Yueh S.K. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: An epidemiological survey. J. Gastroenterol. Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 2.Portincasa P., Moschetta A., Palasciano G. Cholesterol gallstone disease. Lancet (Lond. Engl.) 2006;368:230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y.C., Chiou C., Lin M.N., Lin C.L. The prevalence and risk factors for gallstone disease in Taiwanese vegetarians. PLoS ONE. 2014;9:e115145. doi: 10.1371/journal.pone.0115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Ciaula A., Wang D.Q., Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr. Opin. Gastroenterol. 2018;34:71–80. doi: 10.1097/MOG.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai C.J., Leitzmann M.F., Willett W.C., Giovannucci E.L. Long-term intake of dietary fiber and decreased risk of cholecystectomy in women. Am. J. Gastroenterol. 2004;99:1364–1370. doi: 10.1111/j.1572-0241.2004.30153.x. [DOI] [PubMed] [Google Scholar]
- 6.Barre A., Gusto G., Cadeau C., Carbonnel F., Boutron-Ruault M.C. Diet and Risk of Cholecystectomy: A Prospective Study Based on the French E3N Cohort. Am. J. Gastroenterol. 2017;112:1448–1456. doi: 10.1038/ajg.2017.216. [DOI] [PubMed] [Google Scholar]
- 7.Nordenvall C., Oskarsson V., Wolk A. Fruit and vegetable consumption and risk of cholecystectomy: A prospective cohort study of women and men. Eur. J. Nutr. 2018;57:75–81. doi: 10.1007/s00394-016-1298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lander E.M., Wertheim B.C., Koch S.M., Chen Z., Hsu C.H., Thomson C.A. Vegetable protein intake is associated with lower gallbladder disease risk: Findings from the Women’s Health Initiative prospective cohort. Prev. Med. 2016;88:20–26. doi: 10.1016/j.ypmed.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McConnell T.J., Appleby P.N., Key T.J. Vegetarian diet as a risk factor for symptomatic gallstone disease. Eur. J. Clin. Nutr. 2017;71:731–735. doi: 10.1038/ejcn.2016.252. [DOI] [PubMed] [Google Scholar]
- 10.Wang F., Zheng J., Yang B., Jiang J., Fu Y., Li D. Effects of Vegetarian Diets on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015;4:e002408. doi: 10.1161/JAHA.115.002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu Y.F., Hsu C.C., Chiu T.H., Lee C.Y., Liu T.T., Tsao C.K., Chuang S.C., Hsiung C.A. Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: A matched cohort study. Br. J. Nutr. 2015;114:1313–1320. doi: 10.1017/S0007114515002937. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama Y., Levin S.M., Barnard N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017;75:683–698. doi: 10.1093/nutrit/nux030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlich M.J., Singh P.N., Sabate J., Jaceldo-Siegl K., Fan J., Knutsen S., Beeson W.L., Fraser G.E. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern. Med. 2013;173:1230–1238. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe F.L., Appleby P.N., Allen N.E., Key T.J. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): Prospective study of British vegetarians and non-vegetarians. BMJ (Clin. Res. Ed.) 2011;(343):d4131. doi: 10.1136/bmj.d4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orlich M.J., Singh P.N., Sabate J., Fan J., Sveen L., Bennett H., Knutsen S.F., Beeson W.L., Jaceldo-Siegl K., Butler T.L., et al. Vegetarian dietary patterns and the risk of colorectal cancers. JAMA Intern. Med. 2015;175:767–776. doi: 10.1001/jamainternmed.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu T.H.T., Pan W.H., Lin M.N., Lin C.L. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr. Diabetes. 2018;8:12. doi: 10.1038/s41387-018-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama Y., Nishimura K., Barnard N.D., Takegami M., Watanabe M., Sekikawa A., Okamura T., Miyamoto Y. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern. Med. 2014;174:577–587. doi: 10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 18.Chiu T.H., Huang H.Y., Chen K.J., Wu Y.R., Chiu J.P., Li Y.H., Chiu B.C., Lin C.L., Lin M.N. Relative validity and reproducibility of a quantitative FFQ for assessing nutrient intakes of vegetarians in Taiwan. Public Health Nutr. 2014;17:1459–1466. doi: 10.1017/S1368980013001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu N.F. Prevalence of obesity in Taiwan. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2005;6:271–274. doi: 10.1111/j.1467-789X.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 20.National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. [(accessed on 20 January 2015)]; Available online: http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=1023&WD_ID=1023&webdata_id=815.
- 21.Li S.S., Blanco Mejia S., Lytvyn L., Stewart S.E., Viguiliouk E., Ha V., de Souza R.J., Leiter L.A., Kendall C.W.C., Jenkins D.J.A., et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2017:6. doi: 10.1161/JAHA.117.006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zak A., Zeman M., Hrubant K., Vecka M., Tvrzicka E. Effect of hypolipidemic treatment on the composition of bile and the risk or cholesterol gallstone disease. Cas. Lek. Ceskych. 2007;146:24–34. [PubMed] [Google Scholar]
- 23.Holzbach R.T., Marsh M., Olszewski M., Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J. Clin. Investig. 1973;52:1467–1479. doi: 10.1172/JCI107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey M.C. Pathogenesis of gallstones. Am. J. Surg. 1993;165:410–419. doi: 10.1016/S0002-9610(05)80932-8. [DOI] [PubMed] [Google Scholar]
- 25.Fu X., Gong K., Shao X. The relationship between serum lipids, apolipoproteins level and bile lipids level, chemical type of stone. Zhonghua Yi Xue Za Zhi. 1995;75:656–659. [PubMed] [Google Scholar]
- 26.Atamanalp S.S., Keles M.S., Atamanalp R.S., Acemoglu H., Laloglu E. The effects of serum cholesterol, LDL, and HDL levels on gallstone cholesterol concentration. Pak. J. Med Sci. 2013;29:187–190. doi: 10.12669/pjms.291.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weerakoon H.T., Ranasinghe S., Navaratne A., Sivakanesan R., Galketiya K.B., Rosairo S. Serum lipid concentrations in patients with cholesterol and pigment gallstones. BMC Res. Notes. 2014;7:548. doi: 10.1186/1756-0500-7-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aulakh R., Mohan H., Attri A.K., Kaur J., Punia R.P. A comparative study of serum lipid profile and gallstone disease. Indian J. Pathol. Microbiol. 2007;50:308–312. [PubMed] [Google Scholar]
- 29.Kahleova H., Matoulek M., Malinska H., Oliyarnik O., Kazdova L., Neskudla T., Skoch A., Hajek M., Hill M., Kahle M., et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2011;28:549–559. doi: 10.1111/j.1464-5491.2010.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang R.Y., Huang C.C., Hu F.B., Chavarro J.E. Vegetarian Diets and Weight Reduction: A Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2016;31:109–116. doi: 10.1007/s11606-015-3390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonstad S., Stewart K., Oda K., Batech M., Herring R.P., Fraser G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. NMCD. 2013;23:292–299. doi: 10.1016/j.numecd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H.H., Liu M., Clegg D.J., Portincasa P., Wang D.Q. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim. Biophys. Acta. 2009;1791:1037–1047. doi: 10.1016/j.bbalip.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bari O., Wang T.Y., Liu M., Paik C.N., Portincasa P., Wang D.Q. Cholesterol cholelithiasis in pregnant women: Pathogenesis, prevention and treatment. Ann. Hepatol. 2014;13:728–745. [PubMed] [Google Scholar]
- 34.Nordenvall C., Oskarsson V., Sadr-Azodi O., Orsini N., Wolk A. Postmenopausal hormone replacement therapy and risk of cholecystectomy: A prospective cohort study. Scand. J. Gastroenterol. 2014;49:109–113. doi: 10.3109/00365521.2013.858180. [DOI] [PubMed] [Google Scholar]
- 35.Racine A., Bijon A., Fournier A., Mesrine S., Clavel-Chapelon F., Carbonnel F., Boutron-Ruault M.C. Menopausal hormone therapy and risk of cholecystectomy: A prospective study based on the French E3N cohort. CMAJ. 2013;185:555–561. doi: 10.1503/cmaj.121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gann P.H., Chatterton R.T., Gapstur S.M., Liu K., Garside D., Giovanazzi S., Thedford K., Van Horn L. The effects of a low-fat/high-fiber diet on sex hormone levels and menstrual cycling in premenopausal women: A 12-month randomized trial (the diet and hormone study) Cancer. 2003;98:1870–1879. doi: 10.1002/cncr.11735. [DOI] [PubMed] [Google Scholar]
- 37.Boyd N.F., Lockwood G.A., Greenberg C.V., Martin L.J., Tritchler D.L. Effects of a low-fat high-carbohydrate diet on plasma sex hormones in premenopausal women: Results from a randomized controlled trial. Canadian Diet and Breast Cancer Prevention Study Group. Br. J. Cancer. 1997;76:127–135. doi: 10.1038/bjc.1997.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagga D., Ashley J.M., Geffrey S.P., Wang H.J., Barnard R.J., Korenman S., Heber D. Effects of a very low fat, high fiber diet on serum hormones and menstrual function. Implications for breast cancer prevention. Cancer. 1995;76:2491–2496. doi: 10.1002/1097-0142(19951215)76:12<2491::AID-CNCR2820761213>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Harmon B.E., Morimoto Y., Beckford F., Franke A.A., Stanczyk F.Z., Maskarinec G. Oestrogen levels in serum and urine of premenopausal women eating low and high amounts of meat. Public Health Nutr. 2014;17:2087–2093. doi: 10.1017/S1368980013002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fung T.T., Hu F.B., Barbieri R.L., Willett W.C., Hankinson S.E. Dietary patterns, the Alternate Healthy Eating Index and plasma sex hormone concentrations in postmenopausal women. Int. J. Cancer. 2007;121:803–809. doi: 10.1002/ijc.22728. [DOI] [PubMed] [Google Scholar]
- 41.Karelis A.D., Fex A., Filion M.E., Adlercreutz H., Aubertin-Leheudre M. Comparison of sex hormonal and metabolic profiles between omnivores and vegetarians in pre- and post-menopausal women. Br. J. Nutr. 2010;104:222–226. doi: 10.1017/S0007114510000619. [DOI] [PubMed] [Google Scholar]
- 42.Thomas H.V., Davey G.K., Key T.J. Oestradiol and sex hormone-binding globulin in premenopausal and post-menopausal meat-eaters, vegetarians and vegans. Br. J. Cancer. 1999;80:1470–1475. doi: 10.1038/sj.bjc.6690546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur J., Rana S.V., Gupta R., Gupta V., Sharma S.K., Dhawan D.K. Prolonged orocecal transit time enhances serum bile acids through bacterial overgrowth, contributing factor to gallstone disease. J. Clin. Gastroenterol. 2014;48:365–369. doi: 10.1097/MCG.0b013e3182a14fba. [DOI] [PubMed] [Google Scholar]
- 44.Heaton K.W., Emmett P.M., Symes C.L., Braddon F.E. An explanation for gallstones in normal-weight women: Slow intestinal transit. Lancet (Lond. Engl.) 1993;341:8–10. doi: 10.1016/0140-6736(93)92479-D. [DOI] [PubMed] [Google Scholar]
- 45.Meier R., Beglinger C., Dederding J.P., Meyer-Wyss B., Fumagalli M., Rowedder A., Turberg Y., Brignoli R. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol. Motil. 1995;7:235–238. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 46.Xie M., Kotecha V.R., Andrade J.D., Fox J.G., Carey M.C. Augmented cholesterol absorption and sarcolemmal sterol enrichment slow small intestinal transit in mice, contributing to cholesterol cholelithogenesis. J. Physiol. 2012;590:1811–1824. doi: 10.1113/jphysiol.2011.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillemeier C. An overview of the effects of dietary fiber on gastrointestinal transit. Pediatrics. 1995;96:997–999. [PubMed] [Google Scholar]
- 48.Shabanzadeh D.M., Sorensen L.T., Jorgensen T. A Prediction Rule for Risk Stratification of Incidentally Discovered Gallstones: Results From a Large Cohort Study. Gastroenterology. 2016;150:156–167.e1. doi: 10.1053/j.gastro.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Halldestam I., Enell E.L., Kullman E., Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br. J. Surg. 2004;91:734–738. doi: 10.1002/bjs.4547. [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim M., Sarvepalli S., Morris-Stiff G., Rizk M., Bhatt A., Walsh R.M., Hayat U., Garber A., Vargo J., Burke C.A. Gallstones: Watch and wait, or intervene? Clevel. Clin. J. Med. 2018;85:323–331. doi: 10.3949/ccjm.85a.17035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.