Abstract
Background/Objectives: The acute impact of Hibiscus sabdariffa calyces (HSC) extract on postprandial vascular function and other cardiometabolic risk factors have not been studied previously. This study investigated the acute impact of HSC extract consumption on blood pressure (BP), vascular function and other cardiometabolic risk markers. Subjects/Methods: Twenty-five men with 1% to 10% cardiovascular disease (CVD) risk (determined by QRISK®2) were randomised to consume either 250 mL of the aqueous extract of HSC or water with breakfast in a randomised, controlled, single-blinded, 2-meal cross-over study (ClinicalTrials.gov, NTC02165553) with a two weeks washout period between study days. BP was measured at baseline and hourly for 4 h. Flow mediated dilatation (FMD) of the branchial artery was measured at baseline, 2 and 4 h post intervention drink consumption. Results: Acute consumption of aqueous extract of HSC caused a significant increase in % FMD (p < 0.001), a non-significant decrease in systolic BP (SBP) and diastolic BP (DBP); non-significant increase in urinary and plasma nitric oxide (NOx) and reduced response of serum glucose, plasma insulin, serum triacylglycerol and C-reactive protein (CRP) levels; significant (p = 0.026) improvement in the area under systemic antioxidant response curve (0 to 2 h); no significant changes in arterial stiffness following the acute consumption of the extract of HSC. Gallic acid, 4-O-methylgallic acid, 3-O-methylgallic acid and hippuric acid reached a maximum plasma concentration at 1 to 2 h post consumption of the extract of HSC. Conclusion: The extract of HSC improved postprandial vascular function and may be a useful dietary strategy to reduce endothelial dysfunction and CVD risk, although this requires confirmation.
Keywords: Hibiscus sabdariffa calyces, postprandial blood pressure, vascular function
1. Introduction
Cardiovascular diseases (CVD) are the leading cause of death worldwide and have become a global public health concern. CVD are the main cause of death in developed [1,2] and developing countries [3]. According to the World Health Organisation (WHO), CVD cause 17.5 million deaths in the world each year [4,5]. It is projected that annual death due to CVD will increase from 17 million in 2008 to 25 million in 2030 [5]. Hypertension is the first and most important modifiable risk factors for CVD. In addition, markers of vascular endothelial function are regarded as novel or emerging CVD risk markers. Measurement of flow mediated dilatation (FMD) of the brachial artery is regarded as the gold standard measure of vascular endothelial function. Dietary modification is believed to be a major contributor to CVD risk reduction. Foods and beverages rich in polyphenols are thought to have a potential positive impact on CVD risk.
There are over 300 species of Malvaceae, and Hibiscus sabdariffa is the most commonly known. Infusions of Hibiscus sabdariffa calyces (HSC) are consumed in most parts of the world as a cold beverage or a hot drink and are believed to have antihypertensive and hypolipidaemic activities [6,7]. HSC are polyphenol-rich and are considered by some to have nutraceutical potential which is under exploited [8]. It has been postulated that the hypotensive effect of the extract of HSC could be associated with a number of potential mechanisms, including direct vasorelaxant effects [9,10]. The hypotensive effect of the extract of HSC was proposed to be related to its ability to induce endothelium-dependant effects related to the nitric oxide synthase (NOS) activation by active components within the extract. In addition, the extract of HSC has been found to act as an inhibitor of angiotensin converting enzyme (ACE) in vitro, [11,12]. ACE catalyses the conversion of angiotensin I to angiotensin II (vasoconstrictor) leading to an increase in blood pressure (BP). Angiotensin II promotes an increase in extracellular fluid volume through the increased sodium and water intake and retention coupled with a decrease in excretion of sodium and water, which results in a BP increase. ACE inhibitors are potent hypotensive agents. HSC anthocyanins (delphinidine-3-O-sambubioside and cyanidine-3-O-sambubioside) were reported to competitively inhibit ACE from rabbit lung [12]. This mechanism of action is supported in human studies which related hypotensive activity of the extract of HSC to inhibition of ACE and decrease serum sodium [13]. The polyphenols in the extract of HSC were thought to act through multi-faceted metabolic regulation [14].
Biochemical markers of dyslipidaemia, insulin resistance/sensitivity, inflammation and oxidative stress are among the modifiable cardiovascular risk markers shown to be responsive to dietary (fruits and vegetables) intervention, but evidence from human intervention trials appears to be limited as most studies called for further research [15,16,17,18,19,20,21,22,23,24,25,26].
The limited human studies on the impact of hibiscus drink consumption on cardiometabolic risk markers were of chronic design. The biological activities of phenolic compounds in the promotion of health and disease prevention depend on the intake, absorption, transport to target organs and metabolism [27]. Despite claims that the benefits of the consumption of the extract of HSC to cardiovascular health are related to its polyphenols content, only one study has investigated the pharmacokinetics of HSC anthocyanins in humans to date [28]. In addition, there have been no human studies that investigate the acute impact of the consumption of the extract of HSC on vascular function, arterial stiffness and nitric oxide.
Based on the limited data and suggestions for more clinical trials to confirm the benefits of the consumption of HSC extract, this study investigated the acute impact of the consumption of the aqueous extract of HSC on postprandial FMD, BP and other cardiometabolic risk markers in men.
2. Subjects and Methods
2.1. Ethical Approval
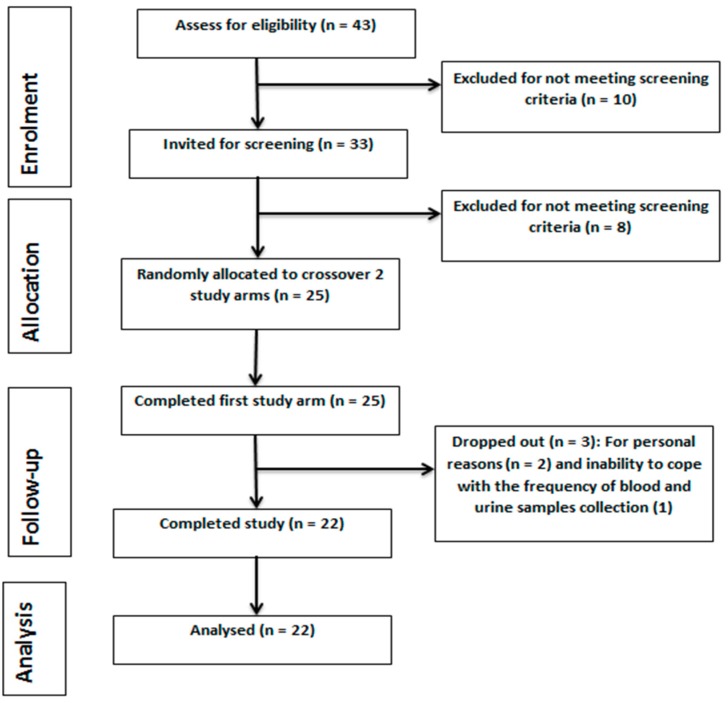
The study protocol was reviewed and given a favourable ethical opinion for conduct by the University of Reading Research Ethics Committee (UREC) (Project No. 13/40). The study protocol was registered at the ClinicalTrials.gov (NTC02165553). The study was conducted in accordance with the Declaration of Helsinki [29,30] and adhered to the Consolidated Standards of Reporting Trials (CONSORT) [31], as shown in Figure 1.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT)-guided study flow diagram.
2.2. Study Design
This study was a randomised, controlled, single-blinded, acute, cross-over trial conducted in the Hugh Sinclair Unit of Human Nutrition, University of Reading. The study consisted of two dietary intervention arms: polyphenol-rich hibiscus drink and control drink (water) and investigated the impact of their acute consumption on CVD risk markers in human volunteers with 1 to 10% CVD risk in ten years (measured by QRISK®2 see below). Volunteers were randomised using a computer-based randomisation software (Research Randomizer, Geoffrey C. Urbaniak and Scott Plous) and were assigned to consume a glass (250 mL) of hibiscus or water with a high fat meal, at time 0 min followed by a medium fat lunch at 120 min in a random order separated by a two-week washout period.
The study breakfast and lunch were prepared in the Hugh Sinclair Unit of Human Nutrition, University of Reading, UK. The breakfast consisted of 120 g buttered croissants, 30 g butter, a spoonful (15 g) of clear honey and 250 mL of hibiscus drink or low nitrate mineral water (Buxton, Nestle Waters UK Ltd, Gatwick, West Sussex, UK). The lunch consisted of 2 slices (89 g) of thick soft white bread, 33 g of soft cheese, 2 shortbread biscuit fingers (35 g), a bag (18 g) of ready salted crisps and Buxton mineral water. A summary of nutrients in the study meals is shown in Table 1.
Table 1.
Selected nutrient content of study breakfast and lunch.
| Breakfast | Lunch | |
|---|---|---|
| Energy (MJ) | 3.21 | 2.36 |
| Fat (Saturated) (g) | 50.1 (32.3) | 24.9 (11.4) |
| Carbohydrate (g) | 70.2 | 70.1 |
| Protein (g) | 8.6 | 12.8 |
| Sodium salt (g) | 1.7 | 1.5 |
2.3. Sample Size and Power Calculation
This study considered systolic BP (SBP) as the primary outcome measure. Previous chronic study findings [6] showed an average of 12.7 mmHg reduction of SBP with a standard deviation of 6.8 to 11.8 mmHg after consumption of Hibiscus drink. On this basis, and assuming two-sided test criteria with alpha of 0.05, mean SBP difference of 5 mmHg and standard deviation of 6 mmHg for an acute study, a sample size of 23 was required for a statistical power of 80%. The calculation was performed as previously described [32,33]. Twenty-five (25) participants were recruited to account for a 10% drop-out.
2.4. Subjects Enrolment
The study was advertised within and around the University of Reading, Reading, UK via posters, emails to volunteers on the Hugh Sinclair volunteer database (who consented to be contacted for potential participation on intervention trials), targeted social media and word of mouth. Volunteers who declared interest were sent a detailed study information sheet and were required to fill and return a health and lifestyle questionnaire to assess their eligibility for screening.
The inclusion criteria were that volunteers should be male, have 1 to 10% CVD (QRISK®2) risk in 10 years, not taking BP medication, not having kidney, liver or chronic diseases, smoking or not smoking and signed informed consent. Those who were eligible for screening were invited to the Hugh Sinclair Unit of Human Nutrition and requested to give informed written consent after any questions were answered. This was followed by BP and anthropometric measurement collection, and a screening fasted blood sample was collected and tested for glucose, lipids, liver and renal function markers and full blood count. Volunteers who were found eligible for the study were informed and with the aid of a computer-based randomisation software randomly assigned to an intervention order.
2.5. CVD Risk Calculation
An online version of QRISK®2 CVD risk calculator recommended by the National Institute for Health and Care Excellence (NICE) was used to estimate the CVD risk of volunteers [34].
2.6. Hibiscus Drink Preparation
Hibiscus drink was prepared in the Hugh Sinclair Unit of Human Nutrition kitchen in batches on a weekly basis. A batch was prepared in comparison with the usual method employed by habitual tea consumers. Briefly, a total of 30 g (average of 15 hibiscus tea bags) of H. sabdariffa calyces were steeped in 1 litre of low nitrate water (Buxton mineral water, nitrate content <0.1 mg/L) at 100 °C for 10 min. The tea bags were squeezed to ensure maximum extraction of the bioactive. The tea was allowed to cool at room temperature and a 250 mL aliquot was added to an opaque, food grade aluminium bottle, cap labelled and refrigerated at 4 °C until required for use. Each volunteer was served an equivalent extract of 7.5 g Hibiscus sabdariffa calyces in 250 mL Buxton water. Each serving contained a mean of 150 mg total anthocyanins and 311 mg gallic acid.
2.7. Blood and Urine Samples Collection and Processing
Volunteers arrived at the Hugh Sinclair Unit of Human Nutrition fasted having drunk only low-nitrate mineral water (Buxton mineral water) for the past 12 h and abstained from polyphenol-rich foods 24 h prior to the study visit. A flexible cannula was inserted into the antecubital vein of their left arm to enable regular blood sampling. Blood samples were collected into BD K3-EDTA, heparin and serum separation vacuette (Greiner Bio-One, Monroe, Louisiana, USA). EDTA and heparinised samples were immediately centrifuged at 4 °C and 3000 rpm for 10 min. Blood in serum separation tubes (SST) were allowed to stand at room temperature for 30 min before centrifugation. Plasma and serum samples were aliquoted and stored at −20 °C until future analysis.
Urine samples were collected into calibrated plastic containers and the volume measured at each point. The samples were mixed, aliquot into centrifuge tubes and centrifuge at 4 °C and 3000 rpm for 10 min; the supernatant was collected into labelled sample tubes and stored at −20 °C until required for analysis.
2.8. Measurement of FMD of the Brachial Artery
FMD of the brachial artery was measured at baseline (0), 2, and 4 h after consumption of the high fat breakfast with either hibiscus drink or water. FMD of the brachial artery was measured in accordance with the guidelines in the Report of the International Brachial Artery Reactivity Task Force [35] and previous descriptions [36,37,38,39,40]. FMD measurements were captured using HDI® 5000 ALT Ultrasound System (Philips Medical Systems, Nashville, Tennessee, USA) connected with a 5–10 MHz hockey-stick probe with a dimension of 3.5 × 1.0 cm. Brachial artery images captured by the ultrasound machine were analysed using brachial analyser software (Medical Applications, LLC, Coralville, Iowa, USA) on the computer connected to the ultrasound. The diameter of the branchial artery throughout the length of the lumen was captured and used for analysis.
Brachial artery FMD was calculated as the percentage change in artery diameter relative to baseline as in the following equation:
| % FMD = [PAD (mm) − BAD (mm)/BAD (mm)] × 100 |
where; PAD is Peak Artery Diameter = maximum artery diameter obtained at post-occlusion BAD is the Baseline Artery Diameter = average of baseline (pre-occlusion) artery diameters.
2.9. Assessment of Arterial Stiffness
The central BP and indices of arterial stiffness were measured using radial artery pulse wave analysis (SphygmoCor® software version 9.0, AtCor Medical Pty Ltd., Sydney, Australia) as described in the manufacturer’s manual and published previously [38,39] and following recommendations of the experts’ consensus on arterial stiffness measurement methods [41]
2.10. BP Measurement
On arrival at the Hugh Sinclair Unit of Human Nutrition volunteers were requested to relax in bed in an air-conditioned (23 °C) clinical room for 30 min before BP measurement. BP was measured using automatic BP monitor (OMRON Healthcare Co. Ltd., Kuoto, Japan) at baseline (0) and 1, 2, 3 and 4 h after consumption of a high fat breakfast with either hibiscus drink or water. The mean of three consecutive readings taken on the right arm within 10 min was taken at each measurement point. The heart rate was also determined. Pulse pressure (PP) was measured as the difference between SBP and DBP [42,43].
2.11. Solid Phase Extraction (SPE) and HPLC Analysis of Plasma Polyphenols
The preparation of plasma samples for analysis of anthocyanins, phenolic acids and their metabolites was performed as previously described [38,44,45] with modifications. In brief, plasma (500 µL) samples were diluted by addition of 1 mL of 0.5% glacial acetic acid. To this, 50 µL of 100 µM 3-(4-Hydroxy-3-methoxyphenylpropionic acid), 3hmPA, was added as a recovery standard. The mixture was vortexed and centrifuged at 17,000× g, 4 °C for 15 min. The supernatant was loaded onto an Oasis HLB 60 mg 3 cc cartridge (Waters Limited, Elstree, Hertfordshire, UK) earlier conditioned by sequential addition of 1 mL each of acidified methanol (0.1% Trifluoroacetic acid, TFA, pH 2, in methanol) and acidified water (0.5% glacial acetic acid in distilled water). The SPE cartridge was then washed by sequential addition of 3 mL acidified water (0.5% glacial acetic acid in distilled water) and 1 mL of 0.5% acidified water in 20% methanol (water:methanol:acetic acid, 79.5:20:0.5 respectively). The cartridge was then dried by applying a vacuum for 5 to 10 s. This was followed by elution of phenolics, into speedvac tubes containing 200 µL of acidified methanol (0.5% acetic acid in methanol), by adding 1 mL of acidified methanol (0.1% Trifluoroacetic acid, pH 2, in methanol) onto the solid phase extraction cartridge twice, and passing through the SPE cartridge gravitationally. Elusion was completed with the application of a vacuum for 5 to 10 s to maximise recovery.
The eluent was dried using a speedvac system (Thermo Fisher Scientific Inc. Basingstoke, Hampshire, UK) and redissolved with 200 µL of HPLC mobile phase A (0.1% formic acid in HPLC water). The redissolved eluent was then filtered through a 0.20 µm filter (Sartorius Stedim Biotech, Gottingen, Germany) into HPLC vial and ran on an Agilent 1100 series (Agilent Technologies Ltd. Cheadle, Manchester, UK). The HPLC system is equipped with a diode array detector and Nova-Pak C18 column (250 × 4.6 mm; 4 µm particle size; 30 °C; Waters Limited, Elstree, Hertfordshire, UK). The mobile phase A is 0.1 % formic acid in HPLC water while mobile phase B contained 0.1% HPLC water in acetonitrile and were pumped through the column at 0.3 mL per minute. Samples (50 µL) were injected and separated using the following gradient system (min/% mobile phase A/%mobile phase B): 0/95/5, 1/95/5, 20/70/30, 25/20/80, 30/20/80, 31/95/5 and 45/95/5. The eluent was monitored by photodiode array detection at 280 nm (phenolic acids and their metabolites) and 520 nm (anthocyanins and their metabolites) with spectra of products obtained between 220–600 nm. Retention time and peak spectra were used to identify phenolic acids, anthocyanins and their metabolites in the samples through comparison with that of authentic anthocyanidin, anthocyanin (Sigma Aldrich, Gillingham, Dorset, UK) and phenolic acid (Extrasynthese, Genay, France) standards. Standard curves (R2 ranges from 0.95 t0 0.99) prepared were used to quantify the identified anthocyanidins and phenolic acids in each sample. Loses due to extraction procedures were corrected with the recovery standard, 3hmPA.
2.12. Plasma and Urine Nitric Oxide Determination
Nitric oxide was measured as nitrite and nitrate using an HPLC-based ENO-30 NOx Analyser (Eicom Corporation, Kuoto, Japan) as described in the ENO-30 NOx analyser manual and previous studies [46,47]. ENO-30 NOx analyser is designed to perform a high sensitivity analysis of nitrite and nitrate in biological samples by combining colorimetric diazo coupling (Griess reaction) method with a reverse phase high performance liquid chromatography (HPLC). Nitrite and nitrate peaks were detected by the detector with the nitrite detection limit of 0.1 pmol at 540 nm.
The method is based on the separation of nitrate and nitrite in the sample by reverse phase HPLC followed by reduction of nitrate (NO3) to nitrite (NO2) as a result of reaction with the cadmium and reduced copper in the reduction column. The concentration of nitrite and nitrate in the sample are obtained with the aid of nitrite and nitrate standard calibration curves.
2.13. Biochemical Analyses
Serum glucose, triacylglycerol (TAG), non-esterified fatty acids (NEFA), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and C-reactive protein ultra sensitive (CRP-US) were analysed using clinical chemistry automated analyser (Instrumentation Laboratory (UK) Limited, Warrington, UK) as previously described [48]. Serum LDL-C was calculated using Friedewald equation [49]. Enzyme-based IL Test TM kits (Instrumentation Laboratory (UK) Limited, Warrington, UK) were used for the analysis of glucose, TAG, TC and HDL-C. NEFA was measured in serum using NEFA-HR (2) enzymatic colorimetric assay kit (Wako Chemicals GmbH, Nuess, Germany) as described previously [50]. CRP-US was assayed using quantex ultrasensitive kit (Biokit, Barcelona, Spain). Total antioxidant capacity (TAC) was determined using Trolox equivalent antioxidant capacity (TEAC) assay kit (Medicon, Gerakas, Greece). Plasma insulin was analysed using an enzyme-linked immunosorbent assay (ELISA)-based assay kit (Agilent Technologies Company, Dako, Denmark). Insulin sensitivity/resistance and β-cell function were estimated with the aid of a computer-based Homeostasis Model Assessment 2 (HOMA2) [51,52].
2.14. Statistical Analysis
All data were analysed using IBM Statistical Package for Social Sciences (SPSS) software version 21 (International Business Machines Corporation, New York, NY, USA). Data were presented as mean ± standard error in both tables and charts. Normal distribution of all datasets was checked using the Kolmogorov-Smirnov and Shapiro-Wilk, Q-Q plots and histograms. Where necessary, data were normalised, and normality confirmed using z-score calculated by dividing either skewness or kurtosis values by the standard error. A full factorial two-way repeated measure ANOVA was performed with treatment and time as factors. Statistical comparison of main effect (treatment) was further confirmed by Bonferroni posthoc test. Any difference that resulted in a p-value of less than 0.05 (95% confidence interval) was considered statistically significant.
3. Results
3.1. Baseline Characteristics of Study Volunteers
The baseline characteristics of volunteers are summarised in Table 2. There were no significant changes in the baseline parameters between hibiscus drink and water consumption study visits. Responses of volunteers to questionnaire suggested no adverse reaction as a result of hibiscus drink consumption.
Table 2.
Baseline characteristics of study volunteers at water and hibiscus drink study visits.
| Characteristics | Water Consumption Visit | Hibiscus Consumption Visit | p-Values for Water vs. Hibiscus |
|---|---|---|---|
| CVD risk, age and anthropometric measures | |||
| Age (Years) | 49 ± 2.0 | 49 ± 2.0 | 1 |
| BMI (kg/m2) | 26.9 ± 0.7 | 26.7 ± 0.8 | 0.227 |
| Blood pressure and vascular function measures | |||
| SBP (mmHg) | 126± 3 | 129 ± 3 | 0.063 |
| DBP (mmHg) | 74 ± 2 | 75 ± 2 | 0.225 |
| Pulse Pressure (mmHg) | 52 ± 2 | 54 ± 2 | 0.381 |
| Heart Rate (Pulse/minute) | 63 ± 3 | 65 ± 3 | 0.788 |
| FMD (%) | 3.67 ± 0.3 | 3.36 ± 0.2 | 0.838 |
| Augmentation Index | 3.45 ± 1.00 | 3.51 ± 0.9 | 0.371 |
| Biochemical data | |||
| TC (mmol/L) | 5.35 ± 0.2 | 5.37 ± 0.2 | 0.679 |
| HDL-C (mmol/L) | 1.29 ± 0.1 | 1.32 ± 0.1 | 0.645 |
| TC:HDL-C | 4.30 ± 0.2 | 4.23 ± 0.2 | 0.881 |
| LDL-C (mmol/L) | 3.28 ± 0.2 | 3.32 ± 0.2 | 0.67 |
| TAG (mmol/L) | 1.71 ± 0.3 | 1.62 ± 0.2 | 0.901 |
| NEFA (mmol/L) | 454.9 ± 36.4 | 454.9 ± 38.7 | 0.819 |
| Glucose (mmol/L) | 5.51 ± 0.1 | 5.44 ± 0.1 | 0.843 |
| Insulin (pmol/L) | 51.90 ± 7.9 | 55.34 ± 9.8 | 0.449 |
| HOMA2 IR | 0.99 ± 0.2 | 1.05 ± 0.2 | 0.465 |
All values are mean ± SEM. CVD, cardiovascular disease, BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FMD, flow mediated dilatation; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TAG, triacylglycerol; NEFA, non-esterified fatty acid; HOMA, Homeostasis Model Assessment; IR, insulin resistance.
3.2. Bioavailability of Hippuric Acid, Gallic Acid and Its Metabolites
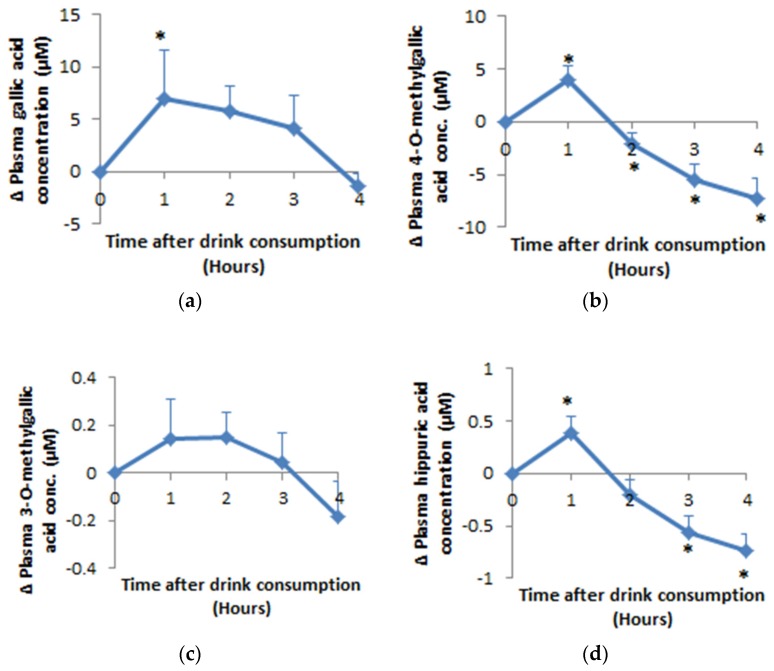
Gallic acid, 3-O-methyl gallic acid (3OMGA), 4-O-methyl gallic acid (4OMGA) and hippuric acid were the main phenolic metabolites detected (Figure 2). Apart from 3OMGA, plasma concentrations for all the phenolic acids reached maximum concentration within 90 min following acute consumption of the extract of HSC. Relative to baseline, mean plasma gallic acid concentration increased by 6.94 µM (p = 0.017), 4OMGA increased by 3.94 µM (p = 0.006), 3OMGA increased by 0.15 µM (p = 0.09) and hippuric acid increased by 0.39 µM (p = 0.01). Intact anthocyanins were not detected in the plasma but their metabolite: hippuric acid reached maximum concentration at 55 ± 5 (mean ± SEM) minutes. A total of 0.6% of the ingested gallic acid was detected in plasma as 0.38% gallic acid, 0.22 4OMGA and 0.01% 3OMGA. The plasma hippuric acid detected represents 0.09% of the ingested anthocyanin.
Figure 2.
Mean (±SEM) plasma difference relative to baseline of (a) gallic aid, (b) 4-O-Methylgallic acid, (c) 3-O-Methylgallic acid and (d) hippuric acid after hibiscus drink consumption with a high fat meal followed by a medium fat meal at 2 h post drink consumption. An asterisk (*) means significant difference relative to baseline; Δ means change in.
3.3. Impact of Hibiscus Drink Consumption on Systolic (SBP), Diastolic (DBP) and Pulse Pressure (PP) and Heart Rate
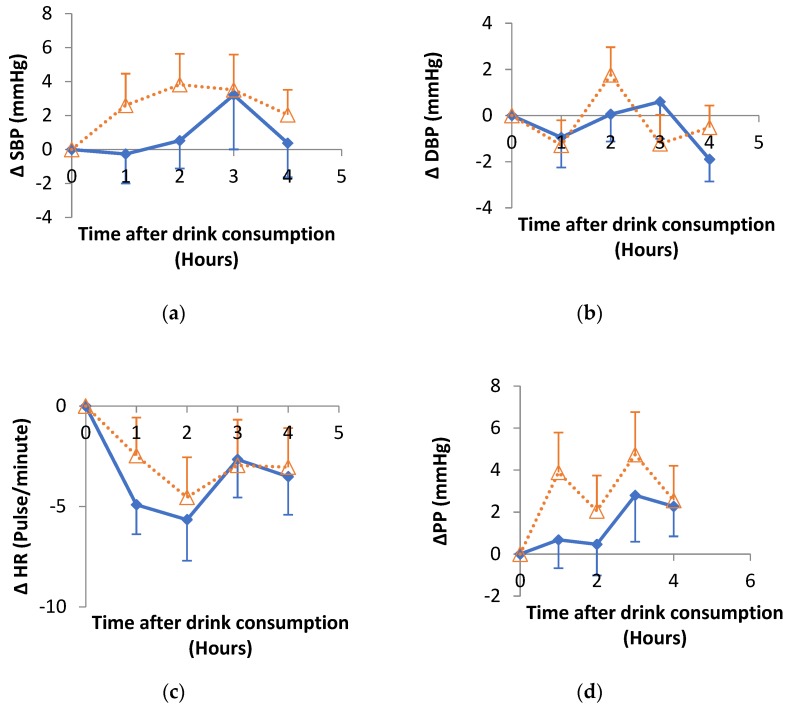
The change in SBP, DBP, PP and heart rate up to 4 h after hibiscus drink or water consumption relative to baseline were summarised in Figure 3. There was no significant effect of treatment, time or treatment-time interaction for SBP or DBP up to 4 h post hibiscus drink consumption. Significant (p = 0.003) time effect was observed for the heart rate and PP (p = 0.011) while no significant effect of treatment or treatment-time interaction was observed. No significant difference was observed in any of the summary response measures (Table 3) when hibiscus drink was compared with water.
Figure 3.
Changes in (a) systolic blood pressure (SBP), (b) diastolic blood pressure (DBP), (c) heart rate (HR), (d) pulse pressure (PP), after hibiscus drink (solid lines) or water consumption (dotted lines) relative to baseline. There were no significant differences between hibiscus and water groups in each case. Δ means change in.
Table 3.
Summary response measures for BP, heart rate and pulse pressure.
| SBP | DBP | Heart Rate | Pulse Pressure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | |
| AUC0–240min | 31,134 ± 496 | 30,992 ± 571 | 0.426 | 17,924 ± 325 | 17,692 ± 338 | 0.312 | 14,580 ± 341 | 14,347 ± 374 | 0.324 | 13,210 ± 383 | 13,300 ± 413 | 0.437 |
| IAUC0–240min | 219 ± 361 | 658 ± 346 | 0.193 | −86 ± 217 | −61 ± 183 | 0.466 | −343 ± 632 | −690 ± 416 | 0.345 | 305 ± 283 | 719 ± 351 | 0.182 |
| Tmax0–240min | 142 ± 16 | 108 ± 18 | 0.083 | 101 ± 18 | 119 ± 17 | 0.237 | 95 ± 20 | 65 ± 17 | 0.133 | 138 ± 18 | 109 ± 18 | 0.130 |
| Pmax0–240min | 138 ± 3 | 135 ± 3 | 0.191 | 79 ± 1 | 78 ± 2 | 0.325 | 68 ± 2 | 66 ± 2 | 0.246 | 62 ± 2 | 61 ± 2 | 0.343 |
| Tmin0–240min | 95 ± 18 | 90 ± 19 | 0.428 | 130 ± 16 | 119 ± 18 | 0.328 | 91 ± 14 | 112 ± 17 | 0.179 | 92 ± 17 | 82 ± 20 | 0.344 |
| Pmin0–240min | 123 ± 2 | 122 ± 2 | 0.425 | 69 ± 1 | 69 ± 1 | 0.387 | 57 ± 1 | 56 ± 2 | 0.300 | 50 ± 1 | 49 ± 2 | 0.424 |
Values are mean ± SEM of 22 volunteers. Area under curve (AUC), incremental area under curve (IAUC), time to reach maximum pressure (Tmax), maximum pressure (Pmax), time to reach minimum pressure (Tmin) and minimum pressure (Pmin). AUC and IAUC are expressed as mmHg.minutes; Tmax and Tmin are in minutes while Pmin and Pmax are in mmHg.
3.4. Impact of Hibiscus Drink Consumption on FMD of the Branchial Artery, Augmentation Index, Plasma and Urine Nitrate and Nitrite
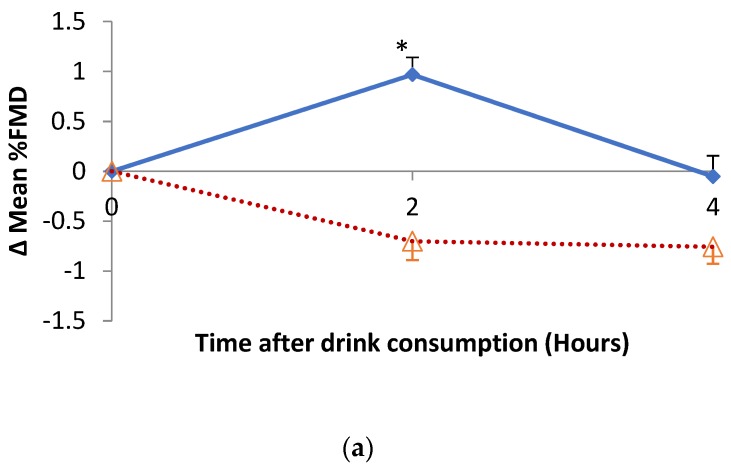
The changes in percentage FMD of the brachial artery are shown in Figure 4a. There was a significant treatment (p = 0.017), time (p = 0.006) and treatment-time interaction (p < 0.001) for the mean FMD of the brachial artery after hibiscus compared to water consumption. Changes in the FMD of the brachial artery relative to baseline were similar with treatment (p < 0.001), time (p = 0.006) and treatment-time interaction (p < 0.001).
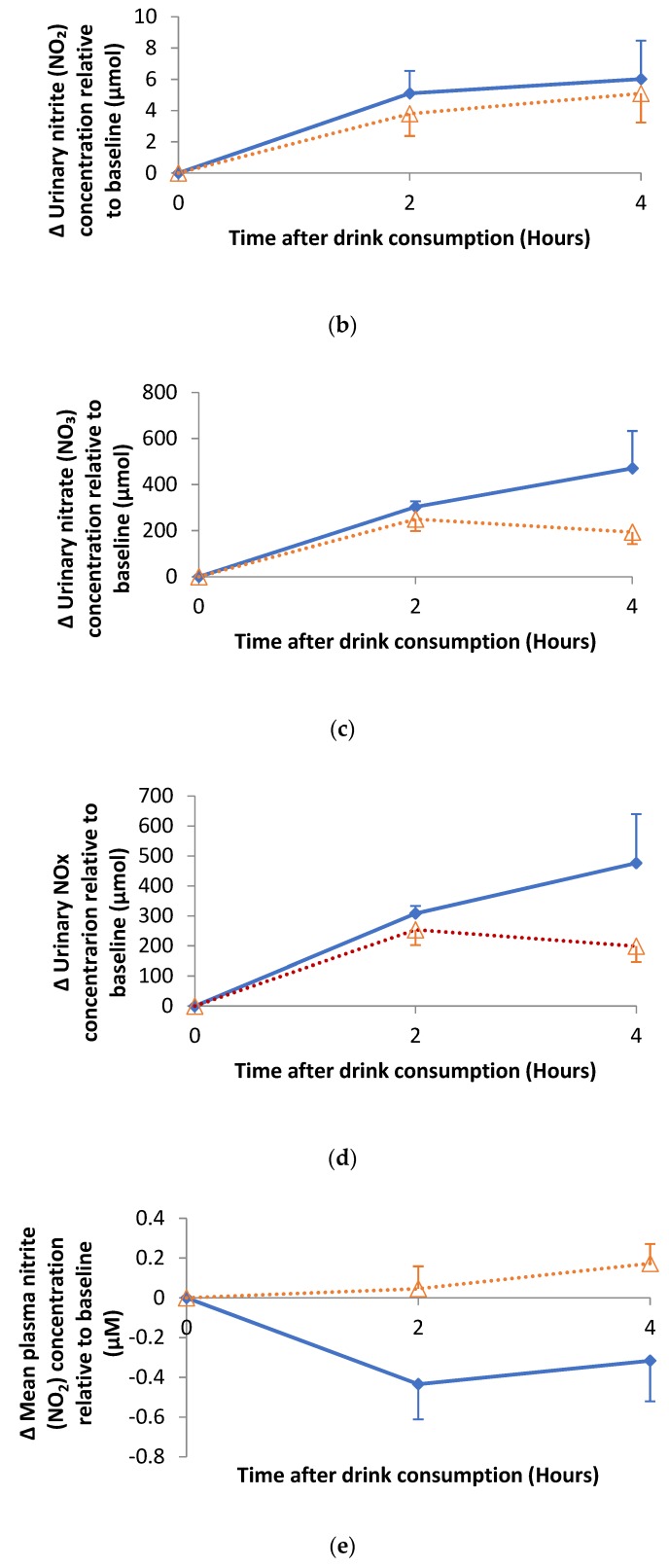
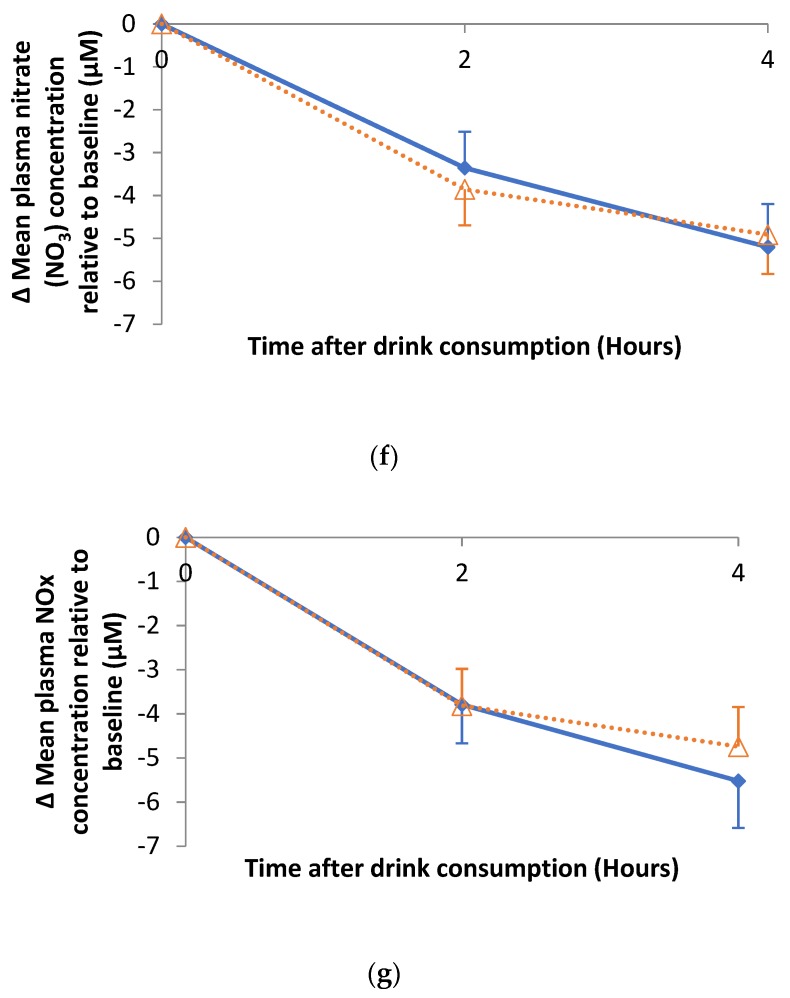
Figure 4.
Changes in (a) % FMD, (b) urinary nitrite (NO2), (c) urinary nitrate (NO3), (d) urinary NOx, (e) plasma nitrite (NO2), (f) plasma nitrate (NO3) and (g) plasma NOx relative to baseline after hibiscus drink (solid lines) or water (dotted lines) consumption. An asterisk (*) means p < 0.001. Δ means change in.
There was no significant effects observed for the changes in Augmentation Index (AIx) for the postprandial response of AIx relative to baseline. There was an overall significant (p < 0.001) time effect for both mean AIx and changes in mean AIx relative to baseline after the consumption of a glass of hibiscus compared to water.
Figure 4b–d respectively summarise the changes in urinary nitrite (NO2), nitrate (NO3) and NOx concentrations following hibiscus drink or water consumption relative to baseline. There was no statistical difference in the urinary NO2 and NO3 at 2 h post-consumption of hibiscus drink compared with water, yet a borderline statistical increase (p = 0.048) in urinary NO3 at 4 h post-consumption of hibiscus drink compared with water was observed. No significant effect of postprandial urinary NO2 response was observed. However, there was a significant (p < 0.001) time effect of the urinary NO2. Although urinary NOx increased from baseline to 4 h post hibiscus drink consumption, it decreased post water consumption, yet there was no statistical treatment (p = 0.05) or treatment-time interaction. There was a significant time effect (p < 0.001) for the urinary NOx concentration when hibiscus drink was compared with water consumption.
Figure 4e–g respectively summarise the changes in plasma nitrite (NO2), nitrate (NO3) and NOx concentration following hibiscus drink or water consumption relative to baseline. A significant treatment (p = 0.016) effect was observed for plasma NO2 up to 4 h post-consumption of hibiscus drink when compared with water, but no time (p = 0.190) or treatment-time interaction (p = 0.058) effects were observed. A significant (p < 0.001) time effect was observed for plasma NO3 and NOx up to 4 h post-consumption of hibiscus drink compared with water, but no significant treatment or treatment-time effect was observed.
3.5. Impact of Hibiscus Drink Consumption on Postprandial Lipids, Biomarkers of Insulin Resistance and Inflammation
A significant time effect (p < 0.001) was observed with a similar postprandial pattern of response for serum TAG from 0 to 4 h post hibiscus drink or water consumption with peak concentrations (approximately 1.6 and 1.9 mmol/L increase relative to baseline after hibiscus drink or water consumption respectively) at 210 and 90 min after breakfast/baseline and lunch respectively which then subsequently declined. There was no time by treatment interaction and although lower mean postprandial TAG concentrations were observed following hibiscus drink consumption compared with water control, this did not reach statistical significance. There was no significant difference in the AUC, IAUC, Cmax and Tmax of mean TAG response when hibiscus drink consumption was compared with water (Table 4).
Table 4.
Serum glucose, plasma insulin, serum TAG and plasma TAC postprandial response measures.
| Glucose | Insulin | TAG | TAC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | Hibiscus | Water | p Value | |
| AUC 0–240 min | 1384 ± 47 | 1455 ± 56 | 0.058 | 49,242 ± 8666 | 50,017 ± 7661 | 0.424 | 547± 55 | 605 ± 71 | 0.188 | 343 ± 50 | 318 ± 40 | 0.105 |
| IAUC 0–240 min | 80 ± 40 | 134 ± 51 | 0.090 | 35,962 ± 6588 | 37,561 ± 6554 | 0.465 | 157 ± 28 | 196 ± 26 | 0.128 | 79.0 ± 38 | 75.8 ± 25 | 0.478 |
| Tmax 0–240 min | 139 ± 14.5 | 135 ± 15 | 0.408 | 126 ± 13 | 123 ± 14 | 0.500 | 196 ± 6 | 196 ± 6 | 0.380 | 153 ± 14 | 175 ± 10 | 0.100 |
| Cmax 0–240 min | 7.5 ± 0.3 | 7.6 ± 0.3 | 0.052 | 387 ± 61 | 403 ± 49 | 0.482 | 3.3 ± 0.3 | 3.6 ± 0.4 | 0.246 | 2.2 ± 0.3 | 2.3 ± 0.4 | 0.460 |
| AUC 0–120 min | 706 ± 23 | 722 ± 30 | 0.143 | 23,629 ± 3932 | 23,388 ± 3551 | 0.303 | 222 ± 23 | 324 ± 34 | 0.143 | 150 ± 17 * | 130 ± 11 | 0.026 |
| IAUC 0–120 min | 53.8 ± 19.3 | 61.8 ± 24.6 | 0.209 | 16,988 ± 2935 | 17,160 ± 3100 | 0.383 | 27.5 ± 6.8 | 46.1 ± 19.5 | 0.209 | 18.4 ± 10.9 | 9.4 ± 5.3 | 0.209 |
| Tmax0–120 min | 68.4 ± 5.3 | 53.2 ± 5.2 | 0.059 | 64.4 ± 4.3 | 65.5 ± 5.1 | 0.191 | 110 ± 5 | 196 ± 6 | 0.060 | 96.3 ± 7.4 * | 75.0 ± 8.1 | 0.007 |
| Cmax0–120 min | 6.9 ± 0.3 | 7.1 ± 0.3 | 0.201 | 339 ± 60 | 338 ± 49 | 0.344 | 2.4 ± 0.2 | 3.3 ± 0.3 | 0.201 | 1.7 ± 0.3 | 1.4 ± 0.1 | 0.056 |
| AUC 121–240 min | 678 ± 37 | 733± 31 | 0.065 | 25,614 ± 4838 | 26,629 ± 4288 | 0.472 | 239 ± 29 | 365 ± 43 | 0.065 | 192 ± 33 | 187 ± 30 | 0.340 |
| IAUC 121–240 min | 95 ± 36 | 109 ± 33 | 0.272 | 10,457 ± 2477 | 10,782 ± 2141 | 0.449 | 34.7 ± 7.9 | 76.3 ± 16.2 | 0.272 | 21.5 ± 16.7 | 50.9 ± 15.6 | 0.067 |
| Tmax121–240 min | 189 ± 7 | 188± 6 | 0.398 | 185 ± 5 | 177 ± 4 | 0.072 | 108 ± 6 | 196 ± 6 | 0.398 | 176 ± 8 | 187 ± 8 | 0.140 |
| Cmax121–240 min | 7.2 ± 0.3 * | 7.5 ± 0.3 | 0.040 | 365 ± 61 | 347 ± 46 | 0.218 | 2.5 ± 0.3 | 3.6 ± 0.4 | 0.040 | 2.1 ± 0.4 | 2.2 ± 0.4 | 0.440 |
Values are mean ± SEM of 22 volunteers. Superscript * means significant difference when compared to water. Area under curve (AUC); incremental area under curve (IAUC); time to reach total maximum concentration (Tmax); maximum concentration (Cmax); triacylglycerol (TAG); total antioxidant capacity (TAC). AUC, IAUC, AUC and IAUC for glucose, insulin, TAG and TAC are expressed as mmol/L·minutes, pmol/L·minutes, mmol/L·minutes and mmol/L. Trolox Eq·minutes respectively. Tmax is in minutes, Cmax is in mmol/L for glucose and TAG; mmol/L Trolox Eq for TAC and as pmol/L for insulin.
A significant time effect (p < 0.001) was observed for the serum NEFA response relative to baseline with a similar postprandial response which sharply declined reaching approximately 214 µmol/L less than the baseline at 90 min post hibiscus drink or water consumption. No treatment or treatment by time interactions were observed. Consumption of hibiscus drink did not cause significant difference in the postprandial summary measures of NEFA response when compared with water (Table 5).
Table 5.
Non-esterified fatty acids (NEFA) postprandial response measures.
| Hibiscus | Water | p Value | |
|---|---|---|---|
| Tmin0–90 min | 76.8 ± 4.3 | 76.4 ± 4.7 | 0.413 |
| Cmin0–90 min | 230 ± 16 | 221 ± 14 | 0.367 |
| AUC 91–240 min | 46,593 ± 3313 | 46,523 ± 3284 | 0.462 |
| IAUC 91–240 min | 9665 ± 2711 | 10,364 ± 2507 | 0.388 |
| Tmax91–240 min | 186 ± 9 | 195 ± 9 | 0.216 |
| Cmax91–240 min | 423 ± 26 | 410 ± 25 | 0.317 |
| Tmin91–240 min | 127 ± 10 | 130 ± 12 | 0.198 |
| Cmin91–240 min | 216 ± 15 | 215 ± 14 | 0.459 |
Values are mean ± SEM of 22 volunteers. Area under curve (AUC); incremental area under curve (IAUC); time to reach total maximum concentration (Tmax); time to reach total minimum concentration (Tmin); maximum concentration (Cmax) and minimum concentration (Cmin) AUC and IAUC are expressed as µM.minutes. Tmax and Tmin in minutes, Cmax and Cmin are in µmol/L.
A significant (p < 0.001) time effect was observed with a biphasic serum glucose response relative to baseline for both hibiscus drink and water consumption. The first phase peaked at 60 min post breakfast and the second phase peaked at 60 min post lunch (equivalent to 180 min breakfast). No significant treatment or treatment-time interaction was observed, however there was a tendency for a lower postprandial glucose response after hibiscus consumption at 30 and 210 (p < 0.05) minutes post consumption, but this did not reach statistical significance after Bonferroni correction (p < 0.006). There were no significant differences in any summary measures of postprandial glucose (Table 4).
There was a significant time effect for the postprandial insulin response which showed a biphasic response similar to the glucose response, although there was no significant treatment or time-treatment interaction. However, there was a tendency for a lower postprandial insulin response after hibiscus consumption at 30, 150 and 210 min post consumption yet this did not reach statistical significance after Bonferroni correction (p < 0.006). There were no significant differences in any summary measures of the postprandial insulin response (Table 4).
No significant differences were observed for any surrogate fasting measure of insulin sensitivity (HOMA2 IR, %B and %S). Assessment of glucose: Insulin ratio (GIR) showed no significant (p > 0.05) treatment or time-treatment interaction effect when hibiscus drink and water consumption study arms were compared. However, there was a significant (p < 0.001) time effect.
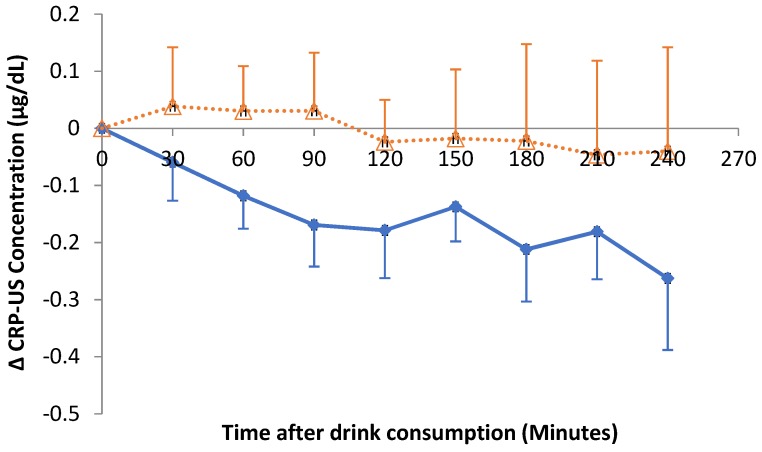
Acute consumption of hibiscus drink did not impact on postprandial CRP-US responses. Even though not statistically significant, the decreasing trend after HSC consumption was observed (Figure 5).
Figure 5.
Changes in postprandial CRP-US response relative to baseline after hibiscus drink (solid line) or water (dotted line) consumption. Δ means change in.
Changes in plasma total antioxidant capacity of volunteers were not significantly (p > 0.05) affected by treatment, time or time-treatment interaction. However, a significant increase (p = 0.026) in the area under the 0 to 2 h curve for the TAC response (Table 4) post hibiscus drink consumption compared to water was observed and a significantly later time to reach peak concentration (p = 0.007).
4. Discussion
In this novel human study, we were the first to determine the acute impact of HSC extract (containing 150 mg total anthocyanins and 311 mg gallic acid) consumption on BP, vascular function and other biochemical markers of CVD. As a result of acute consumption of HSC extract the postprandial SBP (primary outcome) was lower than after the water control, although this did not reach statistical significance. The use of digital automatic BP monitor and relatively small sample size could be potential limitations of our study and it is recommended that future research should consider larger sample size and the use of other acute BP measurement methods such as photoplethysmomanometry in order to record beat-to-beat BP data and short-term variability.
However, acute HSC consumption resulted in improvements in vascular function, measured by FMD of the brachial artery, and recognised as an important CVD risk marker. Our results are difficult to compare directly with other HSC extract studies as ours was the first acute postprandial study of HSC extract. However, acute consumption of anthocyanins-rich blueberries showed similar effects [38]. In a randomized, double-blind, placebo-controlled chronic study a decrease in SBP and DBP of 7.2 and 3.1 mmHg respectively resulted from consumption of HSC extract (as brewed hibiscus tea) at a daily dose of 3.75 g HSC/720 mL (containing 21.12 mg anthocyanin) for 6 weeks by pre- and mildly hypertensive adults not taking BP-lowering medication [53]. In a related study [54], SBP and DBP were decreased by 15.4 and 4.3 mmHg respectively following daily consumption of 4 g HSC/ 480 mL for 30 days by type II diabetic patients with mild hypertension. Reduction in SBP and DBP by 14.2 and 11.2 mmHg respectively was reported as a result of daily consumption of 10 g HSC/500 mL (containing 9.6 mg anthocyanin) for 4 weeks by patients with diagnosed hypertension and without antihypertensive treatment for at least 1 month [55]. Furthermore, patients with moderate essential hypertension had SBP and DBP decreases of 17.6 and 10.9 mmHg respectively following daily consumption of two spoonfuls of HSC (as sour tea)/glass of water for four weeks [56]. Therefore, it may suggest that habitual intake of hibiscus drink has a potential to reduce BP and CVD risk. If translated to a population level, the reduction in BP observed as a result of hibiscus drink consumption could give rise to reduction of CVD by up to 10% based on the assertion by Prospective Studies Collaboration [57] that suggested that a 2 mmHg usual reduction in SBP could give rise to a 10% decrease in mortality, due to stroke and 7% decrease in mortality from ischemic heart disease (IHD) and other vascular causes in middle age population.
The benefit of the consumption of the extract of HSC on BP could be because of the synergy of many nutrients. In this study we have investigated the bioavailability of anthocyanins and phenolic acids in the HSC extract following acute consumption with mixed meals in men with l-10% CVD risk. Gallic acid, 4-O-methylgallic acid, 3-O-methylgallic acid and hippuric acid were detected in the plasma following the acute intake of HSC extract. Contrary to a previous study [44] on the bioavailability of cranberry juice anthocyanins, we have not detected intact anthocyanins in the plasma, however the plasma hippuric acid detected could be a metabolite of anthocyanins contained in HSC. Our findings suggest that gallic acid and its metabolites were the most bioavailable phenolics of the HSC extract. The decline in the concentrations of 4-O-methylgallic acid and hippuric acid observed in this study after reaching peak plasma concentrations at 1hr relative to baseline, could be due to the metabolism, cellular uptake and excretion of the available gallic acid and anthocyanins ingested from the HSC extract.
In a related study [58], plasma hippuric acid increased by about 500% in 300 min relative to baseline after acute consumption of 400 mL of fruit and vegetable puree-based drink (FVPD) by apparently healthy volunteers. This suggested that phenolics within the fruits and vegetables were absorbed and metabolised within 3 h of consumption and were able to exert biological effects acutely. In addition, the plasma antioxidant potential, determined by the relative increase in ferric-reducing antioxidant potential (FRAP), was reported [59] to increase by 8% of baseline as a result of the acute consumption of FVPD. This supports the findings in our study, which showed a significant increase in the area under 0–2 h plasma antioxidant response curve, suggesting the potential of polyphenol-rich drinks to interact with oxidative stress signalling pathways, which could have a beneficial impact on vascular function.
A previous animal study [60] detected 17 polyphenols and metabolites including hibiscus acid, quercetin and kaempferol derivatives in the plasma of rats following acute oral ingestion (via gastric gavage) of 1200 mg/kg of HSC extract. They [60] found a plasma maximum concentration of 112.5, 6.07, 12.59, 3.77, 1.57, 2.96 and 0.81 µM of hibiscus acid, hibiscus acid hydroxyethyl ester, hydroxycitric acid, chlorogenic acid, quercetin, quercetin glucuronide and kaempferol respectively among others at ≥2 h post hibiscus extract consumption. Frank et al. [28] reported the presence of intact cyanidin and delphinidin-3-sambubiosides which reached a maximum plasma concentration of 2.23 and 1.26 ng/mL at 90 min following a single oral dose (150 mL) of the extract of HSC containing 147 mg of total anthocyanins. Variations in the plasma phenolic compounds reported could be due to differences in the calyces, extraction methods and doses. Marked inter-individual differences in plasma anthocyanins pharmacokinetics [44], the impact of the co-consumed foods or nutrients and colonic microflora [27,61] could be other reasons for the variation in bioavailability of dietary phenolic compounds.
The non-significant decrease in SBP observed in our study was consistent with the significant improvement in vascular reactivity measured by FMD and increases in the urinary excretion of nitrite/nitrate. A related study [59] has reported a 25% increase in plasma nitrite and nitrate coupled with improvement in vascular function as a result of acute consumption of fruit and vegetable puree-based drink (FVPD) by apparently healthy volunteers. However, HSC extract did not significantly affect plasma nitrite/nitrate or arterial stiffness measured by pulse wave velocity (PWA). In a previous in vitro study [62], endothelial cells pre-treated with 20 to 40 µg/mL of the extract of HSC significantly improved endothelial nitric oxide synthase (eNOS) expression and NO production. The involvement of NO and eNOS in the modulation of vascular tone and function has been well documented with NO recognised as a key vasodilator [63]. Therefore potential mechanisms for the hypotensive action of HSC drink could be multifaceted and possibly synergistic and may act through improved endothelium-dependent response [62]. Inhibition of ACE has also been suggested as a possible mechanism of the hypotensive activity of HSC drink [11,12].
In consideration of the possible link between BP and circulating lipids, we investigated the impact of the acute consumption of HSC extract on lipid profiles. We spend the majority of our day in a postprandial state and postprandial TAG is now recognised as an independent risk factor for CVD and therefore important to determine. However, we found no significant impact of either intervention on lipid responses. In addition, we did not observe statistically significant changes in the postprandial serum glucose, CRP, insulin and systemic antioxidant potential.
5. Conclusions
This study is the first to provide evidence that acute consumption of HSC extract is beneficial to vascular function through its ability to improve postprandial FMD of the brachial artery. Overall, HSC extract consumption may be a useful dietary strategy for improving postprandial vascular function and CVD risk reduction, although this requires confirmation in further studies.
Author Contributions
S.M.A., J.P.E.S., J.A.L. designed the study. S.M.A and M.T.U performed the study and sample analyses. S.M.A performed the statistical analyses and drafted the paper, which was reviewed by all authors.
Funding
S.M.A received PhD funding from the Tertiary Education Trust Fund (TETFund), Nigeria.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Vontilainen S., Nurmi T., Mursu J., Rissanen T.H. Carotenoids and Cardiovascular Disease. Am. J. Clin. Nutr. 2006;83:1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- 2.Ford E.S., Ajani U.A., Croft J.B. Explaining the Decrease in U.S. Death from Coronary Disease 1980–2000. N. Engl. J. Med. 2007;365:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 3.Gaziano T.A. The Growing Burden of Cardiovascular Disease in the Developing World. Health Aff. 2007;26:13–24. doi: 10.1377/hlthaff.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.British Heart Foundation BHF Statistics Factsheet. [(accessed on 20 January 2019)]; Available online: https://www.bhf.org.uk/
- 5.WHO . World Health Statistics 2012. World Health Organisation; Geneva, Switzerland: 2012. WHO Reports. [Google Scholar]
- 6.Hopkins A.L., Lamm M.G., Funk J.L., Ritenbaugh C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: A comprehensive review of animal and human studies. Fitoterapia. 2013;85:84–94. doi: 10.1016/j.fitote.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz Z., Wong S.Y., Chong N.J. Effects of Hibiscus sabdariffa L. on serum lipids: A systematic review and meta-analysis. J. Ethnopharmacol. 2013;150:442–450. doi: 10.1016/j.jep.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Patel S. Hibiscus sabdariffa: An ideal yet under-exploited candidate for nutraceutical applications. Biomed. Prev. Nutr. 2014;4:23–27. doi: 10.1016/j.bionut.2013.10.004. [DOI] [Google Scholar]
- 9.Adegunloye B.J., Omoniyi J.O., Owolabi O.A., Ajagbonna O.P., Sofola O.A., Coker H.A. Mechanisms of the blood pressure lowering effect of the calyx extract of Hibiscus sabdariffa in rats. Afr. J. Med. Med. Sci. 1996;25:235–238. [PubMed] [Google Scholar]
- 10.Ajay M., Chai H.J., Mustafa A.M., Gilani A.H., Mustafa M.R. Mechanisms of the anti-hypertensive effect of Hibiscus sadariffa L. calyces. J. Ethnopharmacol. 2007;10:388–393. doi: 10.1016/j.jep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Jonadet M., Bastide J., Bastide P., Boyer B., Carnat A.P., Lamaison J.L. Enzyme-inhibiting activities invitro and angioprotective activity invivo of roselle (Hibiscus-sabdariffa L.) extracts. J. Pharm. Belg. 1990;45:120–124. [PubMed] [Google Scholar]
- 12.Ojeda D., Jimenez-Ferrer E., Zamilpa A., Herrera-Arellano A., Tortoriello J., Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 13.Herrera-Arellano A., Miranda-Sanchez J., Avila-Castro P., Herrera-Alvarez S., Jimenez-Ferrer J.E., Zamilpa A., Roman-Ramos R., Ponce-Monter H., Tortoriello J. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Medica. 2007;73:6–12. doi: 10.1055/s-2006-957065. [DOI] [PubMed] [Google Scholar]
- 14.Beltrán-Debón R., Rodríguez-Gallego E., Fernández-Arroyo S., Senan-Campos O., Massucci F.A., Hernández-Aguilera A., Sales-Pardo M., Guimerà R., Camps J., Menendez J.A., et al. The acute impact of polyphenols from Hibiscus sabdariffa in metabolic homeostasis: An approach combining metabolomics and gene-expression analyses. Food Funct. 2015;6:2957–2966. doi: 10.1039/C5FO00696A. [DOI] [PubMed] [Google Scholar]
- 15.Chong M.F.F., Macdonald R., Lovegrove J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010;104:S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- 16.Kuntz S., Kunz C., Herrmann J., Borsch C.H., Abel G., Fröhling B., Dietrich H., Rudloff S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014;112:925–936. doi: 10.1017/S0007114514001482. [DOI] [PubMed] [Google Scholar]
- 17.Woodside J.V., Young I.S., McKinley M.C. Fruit and vegetable intake and risk of cardiovascular disease. Proc. Nutr. Soc. 2013;72:399–406. doi: 10.1017/S0029665113003029. [DOI] [PubMed] [Google Scholar]
- 18.Okuda N., Miura K., Okayama A., Okamura T., Abbott R.D., Nishi N., Fujiyoshi A., Kita Y., Nakamura Y., Miyagawa N., et al. Fruit and vegetable intake and mortality from cardiovascular disease in Japan: A 24-year follow-up of the NIPPON DATA80 Study. Eur. J. Clin. Nutr. 2015;69:482–488. doi: 10.1038/ejcn.2014.276. [DOI] [PubMed] [Google Scholar]
- 19.Vlachojannis C., Erne P., Schoenenberger A.W., Chrubasik-Hausmann S. A Critical Evaluation of the Clinical Evidence for Pomegranate Preparations in the Prevention and Treatment of Cardiovascular Diseases. Phytother. Res. 2015;29:501–508. doi: 10.1002/ptr.5280. [DOI] [PubMed] [Google Scholar]
- 20.Del Bo C., Martini D., Porrini M., Klimis-Zacas D., Riso P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015;6:2890–2917. doi: 10.1039/C5FO00657K. [DOI] [PubMed] [Google Scholar]
- 21.McKay D.L., Chen C.Y.O., Zampariello C.A., Blumberg J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015;168:233–240. doi: 10.1016/j.foodchem.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 22.Wightman J.D., Heuberger R.A. Effect of grape and other berries on cardiovascular health. J. Sci. Food Agric. 2015;95:1584–1597. doi: 10.1002/jsfa.6890. [DOI] [PubMed] [Google Scholar]
- 23.Bozzetto L., Annuzzi G., Pacini G., Costabile G., Vetrani C., Vitale M., Griffo E., Giacco A., De Natale C., Cocozza S., et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: A controlled randomised intervention trial. Diabetologia. 2015;58:1551–1560. doi: 10.1007/s00125-015-3592-x. [DOI] [PubMed] [Google Scholar]
- 24.Bondia-Pons I., Pöhö P., Bozzetto L., Vetrani C., Patti L., Aura A.M., Annuzzi G., Hyötyläinen T., Rivellese A.A., Orešič M. Isoenergetic diets differing in their n-3 fatty acid and polyphenol content reflect different plasma and HDL-fraction lipidomic profiles in subjects at high cardiovascular risk. Mol. Nutr. Food Res. 2014;58:1873–1882. doi: 10.1002/mnfr.201400155. [DOI] [PubMed] [Google Scholar]
- 25.Tresserra-Rimbau A., Rimm E., Medina-Remón A., Martínez-González M., de la Torre R., Corella D., Salas-Salvadó J., Gómez-Gracia E., Lapetra J., Arós F., et al. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2014;24:639–647. doi: 10.1016/j.numecd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 27.Velderrain-Rodríguez G., Palafox-Carlos H., Wall-Medrano A., Ayala-Zavala J., Chen C.O., Robles-Sánchez M., Astiazaran-García H., Alvarez-Parrilla E., González-Aguilar G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014;5:189–197. doi: 10.1039/C3FO60361J. [DOI] [PubMed] [Google Scholar]
- 28.Frank T., Janßen M., Netzel M., Straß G., Kler A., Kriesl E., Bitsch I. Pharmacokinetics of Anthocyanidin-3-Glycosides Following Consumption of Hibiscus sabdariffa L. Extract. J. Clin. Pharmacol. 2005;45:203–210. doi: 10.1177/0091270004270561. [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 30.Millum J., Wendler D., Emanuel E.J. The 50th anniversary of the Declaration of Helsinki: Progress but many remaining challenges. JAMA. 2013;310:2143–2144. doi: 10.1001/jama.2013.281632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noordzij M., Tripepi G., Dekker F.W., Zoccali C., Tanck M.W., Jager K.J. Sample size calculations: Basic principles and common pitfalls. Nephrol. Dial. Transplant. 2010;25:1388–1393. doi: 10.1093/ndt/gfp732. [DOI] [PubMed] [Google Scholar]
- 33.Florey C. Sample size for beginners. BMJ. 1993;306:1181. doi: 10.1136/bmj.306.6886.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hippisley-Cox J., Coupland C., Vinogradova Y., Robson J., Minhas R., Sheikh A., Brindle P. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475–1482. doi: 10.1136/bmj.39609.449676.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corretti M.C., Anderson T.J., Benjamin E.J., Celermajer D., Charbonneau F., Creager M.A., Deanfield J., Drexler H., Gerhard-Herman M., Herrington D., et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002;39:257–265. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 36.Barton M., Turner A.T., Newens K.J., Williams C.M., Thompson A.K. Minimum recovery time between reactive hyperemia stimulus in the repeated measurement of brachial flow-mediated dilatation. Ultrasound Med. Biol. 2011;37:879–883. doi: 10.1016/j.ultrasmedbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Kizhakekuttu T.J., Gutterman D.D., Phillips S.A., Jurva J.W., Arthur E.I., Das E., Widlansky M.E. Measuring FMD in the brachial artery: How important is QRS gating? J. Appl. Physiol. 2010;109:959–965. doi: 10.1152/japplphysiol.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Mateos A., Rendeiro C., Bergillos-Meca T., Tabatabaee S., George T.W., Heiss C., Spencer J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013;98:1179–1191. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 39.Vafeiadou K., Weech M., Altowaijri H., Todd S., Yaqoob P., Jackson K.G., Lovegrove J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015;102:40–48. doi: 10.3945/ajcn.114.097089. [DOI] [PubMed] [Google Scholar]
- 40.Newens K., Thompson A., Jackson K., Williams C. Endothelial function and insulin sensitivity during acute non-esterified fatty acid elevation: Effects of fat composition and gender. Nutr. Metab. Cardiovasc. Dis. 2015;25:575–581. doi: 10.1016/j.numecd.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen T., Pedersen E., Munk K., Hjortdal V., Emmertsen K., Andersen N.H. High pulse pressure is not associated with abnormal activation of the renin–angiotensin–aldosterone system in repaired aortic coarctation. J. Hum. Hypertens. 2015;29:268–273. doi: 10.1038/jhh.2014.75. [DOI] [PubMed] [Google Scholar]
- 43.de Simone G., Roman M.J., Koren M.J., Mensah G.A., Ganau A., Devereux R.B. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33:800–805. doi: 10.1161/01.HYP.33.3.800. [DOI] [PubMed] [Google Scholar]
- 44.Milbury P.E., Vita J.A., Blumberg J.B. Anthocyanins are Bioavailable in Humans following an Acute Dose of Cranberry Juice. J. Nutr. 2010;140:1099–1104. doi: 10.3945/jn.109.117168. [DOI] [PubMed] [Google Scholar]
- 45.Ottaviani J.I., Momma T.Y., Kuhnle G.K., Keen C.L., Schroeter H. Structurally related (−)-epicatechin metabolites in humans: Assessment using de novo chemically synthesized authentic standards. Free Radic. Biol. Med. 2012;52:1403–1412. doi: 10.1016/j.freeradbiomed.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polhemus D.J., Li Z., Pattillo C.B., Goodchild T.T., Gojon G., Giordano T., Krum H. A Novel Hydrogen Sulfide Prodrug, SG1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015;33:216–226. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson K.G., Walden C.M., Murray P., Smith A.M., Lovegrove J.A., Minihane A.M., Williams C.M. A sequential two meal challenge reveals abnormalities in postprandial TAG but not glucose in men with increasing numbers of metabolic syndrome components. Atherosclerosis. 2012;220:237–243. doi: 10.1016/j.atherosclerosis.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 49.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 50.Okabe H., Uji Y., Nagashima K., Noma A. Enzymic determination of free fatty acids in serum. Clin. Chem. 1980;26:1540–1543. [PubMed] [Google Scholar]
- 51.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 52.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 53.McKay D.L., Chen C.-Y.O., Saltzman E., Blumberg J.B. Hibiscus sabdariffa, L. Tea (Tisane) Lowers Blood Pressure in Prehypertensive and Mildly Hypertensive Adults. J. Nutr. 2010;140:298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 54.Mozaffari-Khosravi H., Jalali-Khanabadi B.-A., Afkhami-Ardekani M., Fatehi F. Effects of sour tea (Hibiscus sabdariffa) on lipid profile and lipoproteins in patients with type II diabetes. J. Altern. Complement. Med. 2009;15:899–903. doi: 10.1089/acm.2008.0540. [DOI] [PubMed] [Google Scholar]
- 55.Herrera-Arellano A., Flores-Romero S., Chávez-Soto M.A., Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine. 2004;11:375–382. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Haji Faraji M., Haji Tarkhani A.H. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. J. Ethnopharmacol. 1999;65:231–236. doi: 10.1016/S0378-8741(98)00157-3. [DOI] [PubMed] [Google Scholar]
- 57.Collaboration P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 58.George T.W., Waroonphan S., Niwat C., Gordon M.H., Lovegrove J.A. The Glu298Asp single nucleotide polymorphism in the endothelial nitric oxide synthase gene differentially affects the vascular response to acute consumption of fruit and vegetable puree based drinks. Mol. Nutr. Food Res. 2012;56:1014–1024. doi: 10.1002/mnfr.201100689. [DOI] [PubMed] [Google Scholar]
- 59.George T.W., Waroonphan S., Niwat C., Gordon M.H., Lovegrove J.A. Effects of acute consumption of a fruit and vegetable puree-based drink on vasodilation and oxidative status. Br. J. Nutr. 2013;109:1442–1452. doi: 10.1017/S0007114512003315. [DOI] [PubMed] [Google Scholar]
- 60.Fernández-Arroyo S., Herranz-López M., Beltrán-Debón R., Borrás-Linares I., Barrajón-Catalán E., Joven J., Fernández-Gutiérrez A., Segura-Carretero A., Micol V. Bioavailability study of a polyphenol-enriched extract from Hibiscus sabdariffa in rats and associated antioxidant status. Mol. Nutr. Food Res. 2012;56:1590–1595. doi: 10.1002/mnfr.201200091. [DOI] [PubMed] [Google Scholar]
- 61.Hollman P.C. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004;42:74–83. doi: 10.3109/13880200490893492. [DOI] [Google Scholar]
- 62.Joven J., March I., Espinel E., Fernandez-Arroyo S., Rodriguez-Gallego E., Aragones G., Beltrán-Debón R., Alonso-Villaverde C., Rios L., Martin-Paredero V., et al. Hibiscus sabdariffa extract lowers blood pressure and improves endothelial function. Mol. Nutr. Food Res. 2014;58:1374–1378. doi: 10.1002/mnfr.201300774. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y., Vanhoutte P.M., Leung S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]