Abstract
Raphanus sativus (Radish) belongs to the Brassicaceae family and is a widely consumed root vegetable all around the world. The nutritional and medicinal values of radishes have been proven by several researches. Extracts prepared from the aerial and underground parts of radishes have been used in the treatment of stomach disorders, urinary infections, hepatic inflammation, cardiac disorders and ulcers in folk medicine since the ancient times. The pharmaceutical potential of radishes is attributed to the presence of its beneficial secondary metabolites, such as glucosinolates, polyphenols and isothiocyanates. The present review has focused on the impact of radish extract administration under pathological complications, such as cancer, diabetes, hepatic inflammation and oxidative stress. In addition, a comprehensive view of molecular mechanism behind the regulation of molecular drug targets associated with different types of cancers and diabetes by the bioactive compounds present in the radish extracts have been discussed in detail.
Keywords: Anticancer, Anti-diabetics, Antioxidants, Glucosinolates, Hepatoprotection
1. Introduction
Diets enriched with plants have numerous health benefits to humans. It reduces the risk of various ailments, such as cancer, cardiovascular diseases, neurodegenerative disorders and aging related problems. Moreover, the plant-based diets supply plenty of antioxidants that are necessary for combating the harmful effects of free radicals, which are the inevitable byproducts of vital metabolisms. Plants consist of diverse pharmacologically important secondary metabolites. In contrast to primary metabolites, secondary metabolites occur in lower abundance and distribution and are deposited in specialized cells and organelles [1]. The plant based secondary metabolites can be classified into three major families, such as phenols, steroids and terpenes, and alkaloids [2]. Among the families, phenols, which are a wide range of compounds with one or more hydroxylated aromatic rings biosynthesized via shikimate pathway, are a major class of plant metabolites [3]. Generally, secondary metabolites aid the plant fitness by enhancing the plant–environment interaction. Consequently, the secondary metabolites in most cases acts as an antimicrobial and antioxidant in addition to being involved in plant defense against biotic and abiotic stresses.
Vegetables belonging to cruciferous plants have generated a wide range of dietary interest due to their higher nutritional and pharmaceutical potentials. Several reports illustrated that cruciferous vegetables consists of glucosinolates, phenolic compounds, tocopherols, carotenoids and ascorbic acid [4,5,6]. The principal antioxidative effects of the phytochemicals are manifested by the capability of the compound to scavenge the toxic free radicals or by hindering the oxidation of low-density lipoproteins [7,8,9]. Moreover, polyphenolic compounds have become the focus of present pharmaceutical industries, which is largely due to their health-promoting effects [5,6]. The radish (Raphanus sativus L., 2n = 18) is a well-known root vegetable crop belonging to the Brassicaceae family. The tap root of radishes has been consumed worldwide in the form of pickles, salads and curries due to its high nutritional values [6,7,8,10]. Apart from the roots, leaves and sprouts have also been reported to have nutritional and medicinal importance [9]. The extracts of radishes have been employed to treat stomach disorders, constipation, urinary infections, hepatic inflammation, cardiac disorders and ulcers in folk medicine since the ancient times [8]. In addition, various reports have recorded the antimicrobial [11,12], anticancer [13], antioxidant [14,15] and anxiety reducing properties [16] of radishes. The secondary metabolites with pharmaceutical benefits in radishes include glucosinolates, isothiocyanates and polyphenols [17,18,19]. Glucosinolates (GSL) are secondary metabolites that are exclusively found in cruciferous vegetables [4]. The chemical conformation of GSL possess ß-D-thioglucosides residue bonded to (Z)-N-hydroximinosulfate ester. GSLs are majorly classified into three types based on their precursor amino acids, such as aliphatic glucosinolates (AGSLs), aromatic glucosinolates (ArGSLs) and indolic glucosinolates (IGSLs) [20,21,22]. Recently, the GSLs have gained enormous interest in the pharmaceutical industry, especially in the designing of anticancer and antiinflammatory drugs. Hence, the present review will provide a comprehensive overview of the current research progress on the antioxidant, chemopreventive, hepatoprotective and antidiabetic properties of radishes.
2. Antioxidant Effects of Radishes
The roots and leaves of radishes consist of vital nutritional values and diverse secondary metabolites with antioxidant properties. When compared with roots, leaves possessed higher levels of proteins, calcium and ascorbic acid whereas the total phenol contents were two-fold higher in leaves than roots which corresponded with the free radical scavenging ability [8]. The study has reported different forms of polyphenol constituents in the tissues. For instance, the elevated ranges of pyrogallol (free form) and vannilic acid (bounded form) were identified in roots whereas leaves consisted of epicatechin (free form) and coumaric acid in bounded form [8]. Interestingly, the leaves encompassed levels of flavonoids that were four-fold higher than in roots. Flavonoids are the major members of polyphenols with multiple hydroxyl groups and high free radical scavenging potential [23]. Hence, the leafy part of radishes can be considered as an excellent source of bioactive compounds with antioxidant potentials. A series of in vitro assays conducted by Wang et al. [24] illustrated the antioxidant and prooxidant properties of red radishes. The red radish has higher levels of anthocyanin dominated by the acylated pelargonidin derivative. In detail, the acylated pelargonidin derivatives present in the radish extract scavenged the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS+) radicals and exerted free radical scavenging activity in a concentration-dependent manner. The ABTS+ assay is a predominant test to measure the antioxidant capacity of a compound using a spectrophotometer. Likewise, the acylated pelargonidin derivatives in radish had a higher reducing power potential. The ferric ion based reducing assay validated the free radical detoxification capacity of radishes. In general, the metal chelation hinders the formation of ROS and the compounds that possess the ability to chelate iron are considered to combat the ROS. Apart from the above assays, the prooxidant capacity of acylated pelargonidin derivatives in the radish extracts was determined using the plasmid DNA damage assay. The roles of radish extract as the antioxidant or prooxidant is determined by the concentration and the reaction condition. In addition, the prooxidant activity of radish extract is also based on the nature of the radicals and the prooxidant activity of radish varied between the cancer cells and plasmid DNA. However, deeper insights on the molecular mechanisms that trigger the prooxidant and antioxidant capacity need to be obtained by future researches. Anthocyanins are well-known antioxidants involved in the donation of hydrogen, metal chelation and protein binding [25,26,27]. In addition, anthocyanin also act as a chemoprotective agent by triggering the phase II antioxidant enzymes, preventing cell proliferation and enhancement of apoptosis [28,29,30,31,32]. Secondary metabolites with antioxidant properties identified in radishes have been listed in Table 1.
Table 1.
List of secondary metabolites with antioxidant properties identified in different parts of radish.
| Name | Class | Tissue | Reference |
|---|---|---|---|
| 1,2-dihydroxyferuloyl-gentiobiose | Phenolic acid | Leaves | [33] |
| 13Z-ß-Carotene | Carotenoids | Sprouts | [34] |
| 3-Butenyl isothiocyanate | Isothiocyanates | Pod & flower | [13] |
| 4-methoxyglucobrassicin | Glucosinolate | Sprouts | [35] |
| 4-OH-glucobrassicin | Glucosinolate | Sprouts | [35] |
| 6-Prenyl-naringenin | Flavonone | Root | [33] |
| 6,7,30,40-Tetrahydroxyisoflavone | Isoflavonoids | Leaves | [33] |
| 9Z-ß–carotene | Carotenoids | Sprouts | [34] |
| α-Carotene | Carotenoids | Sprouts | [34] |
| Antheraxanthin | Carotenoids | Sprouts | [34] |
| Anthocyanin-3-O-(cinnamoyl) sophoroside-5-O-glucoside derivatives | Anthocyanin | Sprouts | [36] |
| Anthocyanin 3-O-sophoroside-5-O-(malonyl) glucoside derivatives | Anthocyanin | Sprouts | [36] |
| Apigenin | Flavonoids | Sprouts & seeds | [37] |
| Apigenin-7-O-neohesperidoside | Flavone | Leaves | [33] |
| Apigenin-7-O-rutinoside | Flavone | Leaves | [33] |
| Caffeic acid | Phenolic acid | Sprouts & seeds | [37] |
| Caffeoylmalic acid | Polyphenols | Leaves | [38] |
| Chrysoeriol-7-O-apiosyl-glucoside | Flavone | Leaves | [33] |
| Cyanidin-3-O-caffeoyl-p-coumaroyl-sophoroside- 5-O-glucoside |
Anthocyanin | Root | [33] |
| Cyanidin-3-O-di-p-coumaroyl-sophoroside-5-Omalonylglucoside | Anthocyanin | Root | [33] |
| Cyanidin-3-O-glucoside | Anthocyanin | Leaves | [33] |
| Cyanidin-3-O-p-coumaroyl-feruloyl-sophoroside- 5-O-glucoside |
Anthocyanin | Root | [33] |
| Cyanidin-3-O-rhamnoside | Anthocyanin | Leaves | [33] |
| Cyanidin-3-O-sophoroside-5-O-glucoside | Anthocyanin | Leaves | [33] |
| Cyanidin-3-O-sophoroside-5-O-malonylglucoside | Anthocyanin | Leaves, root | [33] |
| Cyanidin-3-O-xylosyl-p-coumaroyl-glucosylgalactoside | Anthocyanin | Leaves | [33] |
| Delphinidin-3-O-rutinoside | Anthocyanin | Root | [33] |
| Dihydro-caffeoyl-3-O-glucuronide | Phenolic acid | Root | [33] |
| Dihydro-kaempherol-3-O-rutinoside | Dihydroflavonol | Leaves | [33] |
| E- ß –carotene | Carotenoids | Sprouts | [34] |
| Ferulic acid | Phenolic acid | Aerial parts | [39] |
| Ferulic acid | Phenolic acid | Sprouts & seeds | [37] |
| Feruloylmalic acid | Phenolic acid | Leaves | [38] |
| Gallic acid | Phenolic acid | Sprouts & seeds | [37] |
| Genistin | Isoflavonoids | Leaves | [33] |
| Glucobrassicin | Glucosinolate | Sprouts | [40] |
| Glucodehydroerucin | Glucosinolate | Sprouts | [40] |
| Glucoraphasatin | Glucosinolate | Whole plant, sprouts | [40,41] |
| Glucoraphenin | Glucosinolate | Sprouts | [42] |
| Indole-3-carbinol | Isothiocyanates | Sprouts | [15] |
| Isorhamnetin-3-O-p-coumaroyl-caffeoylsophorotrioside- 7-O-malonyl-glucoside |
Flavanol | Root | [33] |
| Isorhamnetin-3-O-p-coumaroyl-sophorotrioside- 7-O-glucoside |
Flavanol | Leaves | [33] |
| Kaemferol | Flavonoids | Sprouts & seeds | [37] |
| Kaempferitrin | Polyphenols | Leaves | [38] |
| Kaempferol-3-O-caffeoyl-sophoroside-7-Oglucoside | Flavanol | Root | [33] |
| Kaempferol-3-O-feruloyl-sophoroside-7-Oglucoside | Flavanol | Root | [33] |
| Kaempferol-3-O-glucoside | Flavanol | Leaves | [33] |
| Kaempferol-3-O-glucosyl-rhamnosyl-glucoside | Flavanol | Leaves | [33] |
| Kaempferol-3-O-p-coumaroyl-sinapoylsophorotrioside-7-O-malonyl-glucoside | Flavanol | Leaves, root | [33] |
| Kaempferol-3-O-p-coumaroyl-sophorotrioside- 7-O-glucoside | Flavanol | Leaves | [33] |
| Kaempferol-3-O-rhamnoside(I) | Flavanol | Leaves | [33] |
| Kaempferol-3-O-rutinoside | Flavanol | Leaves | [33] |
| Kaempferol-3-O-xylosyl-rutinoside | Flavanol | Leaves | [33] |
| Lutein | Carotenoids | Sprouts | [33] |
| Luteolin-7-O-glucoside | Flavone | Leaves | [33] |
| m-Coumaric acid | Phenolic acid | Leaves | [33] |
| Methylgalangin | Flavanol | Leaves | [33] |
| Naringenin-40-O-glucuronide | Flavonone | Leaves | [33] |
| Naringenin-7-O-glucuronide | Flavonone | Leaves | [33] |
| p-Coumaric acid | Phenolic acid | Sprouts & seeds | [37] |
| p-Coumarylmalic acid | Polyphenols | Leaves | [38] |
| Pelargonidin-3-O-caffeoyl-caffeoyl-diglucoside-5-O-malonyl-glucoside | Anthocyanin | Root | [33] |
| Pelargonidin-3-O-feruloyl-diglucoside-5-Oglucoside | Anthocyanin | Leaves | [33] |
| Pelargonidin-3-O-p-coumaroyl-diglucoside-5-Oglucoside | Anthocyanin | Leaves | [33] |
| Pelargonidin-3-O-sambubioside | Anthocyanin | Leaves | [33] |
| Protocatechuic acid | Phenolic acid | Sprouts & seeds | [37] |
| Quercetin | Flavonoids | Sprouts & seeds | [37] |
| Quercetin-3-O-p-coumaroyl-sophoroside-7-Oglucoside | Flavanol | Leaves | [33] |
| Quercetin-3-O-rhamnoside | Flavanol | Leaves | [33] |
| Quercetin-3-O-rhamnosyl-galactoside | Flavanol | Leaves | [33] |
| Sinapic acid | Phenolic acid | Sprouts & seeds | [37] |
| Spinacetin-3-O-(200-p-coumaroyl-glucosyl)(1-6)-(apiosyl(1_2))-glucoside | Flavanol | Root | [33] |
| ß-Cryptoxanthin | Carotenoids | Sprouts | [34] |
| Stigmasterol | Phytosterol | Aerial parts | [39] |
| Sulforaphane | Isothiocyanates | Pod & flower | [13] |
| Sulforaphene | Isothiocyanates | Pod & flower | [13] |
| Violaxanthin | Carotenoids | Sprouts | [34] |
| Zeaxanthin | Carotenoids | Sprouts | [34] |
| β-sitosterol | Phytosterol | Aerial parts | [39] |
| β-sitosterol-3-β-O-D-glucopyranoside | Phytosterol | Aerial parts | [39] |
3. Hepatoprotective Effects of Radishes
The hepatoprotective effects of the radish extract have been recorded by various researchers [36,42,43,44,45,46,47]. Bioactive compounds, such as indole-3-carbinol, 3-[ethoxy- (methylthio)methyl]-2-pyrrolidinethione and 3-(E)-(methylthio)-methylene-2-pyrrolidinethione, present in the radish root and sprouts decreased the severity of fatty liver disease in mouse models [48]. Moreover, the extracts of black radishes alleviated the negative effects of carbon tetrachloride (CCl4)-induced liver injury in rats [43]. The administration of a radish extract resulted in the inhibition of lipid accumulation caused by the oxidative stress. According to Ahn et al. [43], the radish extract upregulated the expression of cytochrome P 450 (CYP) CYP2E1, nuclear factor erythroid 2-related factor-2 (Nrf-2) and Heme oxygenase-1(HO-1) in a concentration-dependent manner. Moreover, the report suggested the possible mechanism behind the hepatoprotective effects rendered by radish could be due to the mediation of Nrf-2/HO-1 antioxidant pathway [43]. In general, Nrf-2 targets the HO-1 molecule, which plays a vital role in the amelioration of oxidative stress and helps in the regulation of genes associated with inflammation, cytoprotection and antioxidant activity [49]. Similarly, the ingestion of fresh radish juice reduced the hepatotoxicity induced by CCl4 in albino rats by the prevention of lipid peroxidation, which replenished the levels of non-protein sulfhydryl moiety (NP-SH) and enhanced the detoxification system of liver [50]. In addition, the phytochemical characterization of the fresh radish juice revealed the presence of hepatoprotective sulphurated compounds, phenols and terpenoids [50]. Similarly, Lee et al. [47] evidenced the hepatoprotective effects of radish enzyme extract in human liver derived cells (HepG2) and rats against tarcine and CCl4 induced hepatotoxicity. The histopathological investigations and biochemical analysis revealed that the radish enzyme extract efficiently provided protection against membrane fragility and reduced the leakage of glutamate oxaloacetate (GOT) and glutamate pyruvate transaminase (GPT) [47]. In liver, the disruption of cellular integrity by CCl4 increases the activities of GOT and GPT enzymes, which are considered to be biomarkers for the identification of liver damage [51,52]. Likewise, the triglycerides (TG) and total cholesterol (TC) in serum can indicate the status of the liver damage [53,54]. The supplementation of radish enzyme extract significantly reduced the levels if TC and TG in CCl4-treated rats [47]. Thus, the decrease in the activities of GOT and GPT and the levels of TC and TG by radish enzyme extract suggested the hepatoprotective potential of radishes.
4. Anticancer Effects of R. sativus
In recent days, the interest in diets enriched with potential bioactive natural compounds with anticancer properties is increasing. Ingestion of cruciferous vegetables has significant benefits of chemoprevention due to the presence of secondary metabolites, such as glucosinolates, which are highly noted for their anticancer properties. Several studies have recorded the antiproliferative effects of isothiocyanates, the hydrolyzed forms of glucosinolates in different forms of cancers [12,13,41,55]. According to Rampal et al. [56], the isothiocyanates exhibit multiple anticancer mechanisms with pharmaceutical interest, such as regulation of detoxification enzymes, activation of apoptosis and prevention of cell cycle progression. In this section, a comprehensive overview on the effects of radish extracts on various cancers was provided.
4.1. Liver Cancer
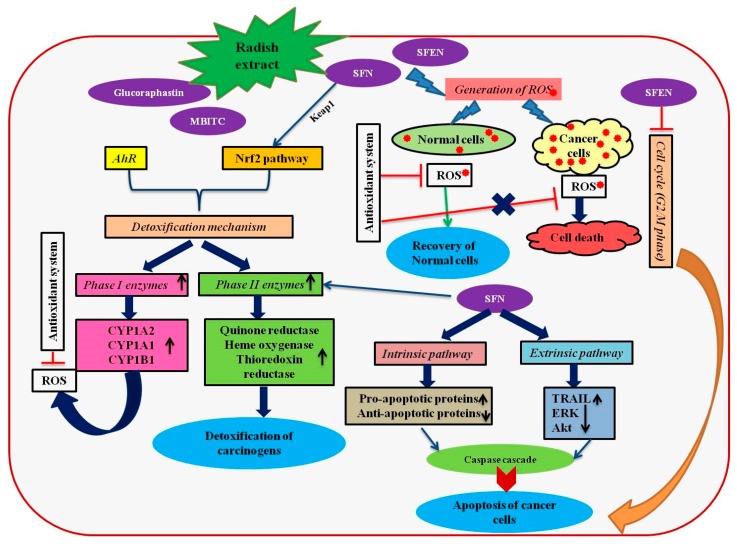
The extract of Spanish black radishes significantly inhibited the proliferation of HepG2 cells by the regulation of phase I and phase II detoxification system [41]. The anticancer property of the extract has been attributed to the glucosinolate compounds, which are namely glucoraphasatin and 4-methylthio-3-butenyl isothiocyanate. According to the report, the crude extract improved the activity of phase II detoxification enzymes, such as quinone reductase, heme oxygenase 1 and thioredoxin reductase 1. In addition, the mRNA levels of phase I detoxification enzymes, such as cytochrome P450 (CYP) 1A1, CYP1A2 and CYP1B1, were also increased upon the addition of radish extracts. The results suggested that the radish extract activated the detoxifying enzymes by activating the aryl hydrocarbon receptor (AhR) and NF-E2-related factor 2 (Nrf2) pathways [57,58,59,60,61,62]. However, the activation of phase I detoxification appeared to be of concern because of the reactive intermediates formation, which increases the toxicity, but the synergistic induction of phase II detoxification system is necessary for the elimination of toxic compounds [41].
4.2. Colon Cancer
The extracts of Thai rat tailed radishes displayed effective cytotoxicity against colon cancer cell line (HCT116) [13]. The occurrences of bioactive phytochemicals, such as sulforaphane and sulforaphane, have been identified in the extract using gas chromatography–mass spectrometry (GC–MS). The radish extract induced apoptosis in the colon cancer cell line. Sulforaphane induced free radicals in cancer cells and was involved in the disruption of microtubule polymerization [63,64,65]. In general, the cancer cells has higher levels of basal ROS. Thus, tailoring the ROS to target the cancer cells could be a potential strategy for designing anticancer drugs with higher selectivity. The administration of sulforaphane and sulforaphene resulted in the death of cancerous cells whereas the normal cells were unaffected. The presence of an active antioxidant mechanism in normal cells could have prevented the death of normal cells. In addition, the sulforaphane triggered the intrinsic and extrinsic apoptosis pathway in cancer cells. For instance, in the intrinsic pathway, sulforaphane induced the regulation of mitochondrial membrane proteins and enhanced the proapoptotic protein expression whereas it decreased the levels of antiapoptic proteins, resulting in the activation of caspase cascade [64]. Similarly, the administration of sulforaphane improved the apoptosis by the induction of TNF-related apoptosis inducing ligand (TRAIL) and alleviation of extracellular signal-regulated kinase (ERK) and Akt in the extrinsic pathway [63,66,67]. On the other hand, the antimutagenic effect of sulforaphene is robust compared to sulforaphane [68]. According to Papi et al. [69], the sulforaphene elicited the intrinsic apoptosis pathway in human colon cancer cell lines although the exact molecular rationale studies behind the anticancer activity of sulforaphene is still in rudimentary stages. Both sulforaphane and sulforaphene contains highly electrophilic central atoms, which interact with nucleophilic cellular targets, such as glutathione synthase hydrogenase (GSH) and cysteine amino acid in Keap1 protein involved in the stabilization of Nrf2 [69]. Apart from the induction of cytotoxicity and apoptosis, the bioactive compounds stimulated the phase II enzymes, which aids in the detoxification of carcinogens.
4.3. Breast Cancer
The aerial extract of radishes actively induced cytotoxicity in the breast cancer cell line (MDA-MB-231) via the ErbB-Akt pathway [70]. The epidermal growth factor receptor (EGFR) is a potential oncogene in breast cancer, with the repression of EGFR amplification reported to have antiproliferative benefits in breast cancer [71]. EGFR is composed of ErbB1, ErbB2, ErbB3 and ErbB4 proteins, an increase in the overexpression of ErbB proteins has been linked to the development of breast cancer [72]. In general, the docking of EGFR ligands to the EGF receptors initiates the formation of heterodimers, which triggers the autophosphorylation of tyrosine kinase. The active phosphorylated EGFRs serves as the binding sites for the proteins involved in signaling cascades for cellular proliferation and survival [73,74]. The application of radish aerial extracts downregulated the mRNA and protein expression levels of ErbB2 and ErbB3 in MDA-MB-231 cell lines [70]. Moreover, the EGF receptor-ligand interaction activates the PI3K/Akt pathway, which plays an important role in tumorigenesis [75,76,77]. Akt is involved in the enhancement of cell survival and suppression of cell death [78]. In addition, Akt is also involved in the phosphorylation of caspase 9, Bad and proapoptotic transcription factors, which prevents the antiapoptotic property [79,80,81]. The administration of a radish extract also decreased the expression of Akt in a dose-dependent manner, thus increasing the antitumor activity [70]. Hence, the aerial extract of radishes was proved to be a valuable source for antitumor drug discovery. Likewise, the active isothiocyante component sulforaphene in radish significantly reduced the viability of SKBR-3 breast cancer cell line with less toxicity towards normal cells [82]. According to Pawlik et al. [82], sulforaphene arrested the cell cycle in G2/M phase, disrupted the organization of cytoskeleton, decreased the colonization of cancer cells and induced apoptosis. The cells cultured with sulforaphene displayed increased Bax protein (proapoptotic protein) whereas the Bcl-2 (antiapoptotic) levels were decreased. Similarly, the level of ADRP proteins, which is involved in the lipid coating during cellular stress due to mitochondrial dysfunction, increased upon sulforaphene treatment [55,83]. Previous reports suggested that the sulforaphene treatment results in the disintegration of mitochondrial potential and leads to the inhibition of mitochondrial respiratory chain complexes I and III [84,85]. In addition, sulforaphene also reduced the caspase-dependent degradation of PARP protein, which can be related to the existence of other suicidal proteases [86,87]. Overall, the sulforaphene present in the radish extract can be a potential anticancer agent with higher efficacy to cancer cells and has comparatively less toxicity to normal cells.
In addition, the radish extract prevented the deleterious effects of zearalenone (zen), a mycotoxin synthesized by Fusarium spp [88]. The zen toxicity has been widely associated with the liver and breast cancer. The administration of radish extract significantly improved the immune cells, such as lymphocytes, immunoglobulins, T-cells, B-cells and interleukins, which were able to act against zen toxicity. The oral ingestion of radish extracts enhanced the release of tumor necrosis factor (TNF-α), which is a vital antitumor drug candidate under study. Overall, the intake of radish extract detoxified the zen toxicity by the improvement of immune cells, inhibition of lipid peroxidation and elicitation of TNF-α.
4.4. Cervical, Lung and Prostate Cancer
The chemopreventive effects of different parts of radishes have been evaluated in cervical (HeLa), lung (A549) and prostate (PC-3) and breast (MCF-7) cancer cell lines by Beevi et al. [89]. The report illustrated the molecular rationale behind the radish extract mediated apoptosis in cancer cell lines. In detail, the hexane extract obtained from the roots of radishes was comprised of isothiocyanates (ITCs), such as 4-(methylthio)-3- butenyl isothiocyanate (MTBITC), 4- methylthio)-3-butyl isothiocyanate (erucin), 4-methylpentyl isothiocyanate, 4-pentenyl isothiocyanate and sulforaphene. Radish extracts caused apoptosis in both p53 deficient and proficient cell lines, which denoted the effect of extract induced apoptosis signaling irrespective of the status of p53 in the cells. Additionally, the apoptosis process involved the interaction of Bcl2family genes and caspase-3 activation [89]. Interestingly, the radish extract selectively targeted the cancer cells without affecting the normal cells, which is a prerequisite for the potential anticancer drug. The radish extract treatment resulted in the detachment of cancer cells, inhibition of cell elongation, induction of cell shrinkage and DNA fragmentation [89]. Variation in the expression levels of genes involved in apoptosis have been determined in cancer cell lines with different tissue lineages treated with radish extract [55,89]. Similarly, the in vivo administration of sulphoraphene actively inhibited tumor growth in mice [90]. The ingestion of sulphoraphene in Balb/C mice with lung cancer prevented the tumor growth by the inhibition of P13K-AKT signaling, reducing the expression of PTEN and ceasing the phosphorylation of AKT in mice. Thus, the study evidenced the anticancer effects of a major isothiocyanate present in radishes in the animal model. Different mechanisms exhibited by radish extracts to prevent cancer cell proliferation have been illustrated in Figure 1.
Figure 1.
A schematic representation of anticancer mechanisms induced by the bioactive compounds present in radish extracts. The figure has been conceived based on the interpretation of the literatures cited in Section 4. AhR, aryl hydrocarbon receptor; Akt, alpha serine-threonine protein kinase; CYP, cytochrome P450; ERK, extracellular signal-regulated kinase; MTBITC, 4-(methylthio)-3-butenyl isothiocyanate; Nrf2, NF-E2-related factor 2; ROS, reactive oxygen species SFN, sulforaphane; SFEN, sulforaphene; TRAIL, TNF-related apoptosis inducing ligand.
5. Antidiabetic Effects of Radish
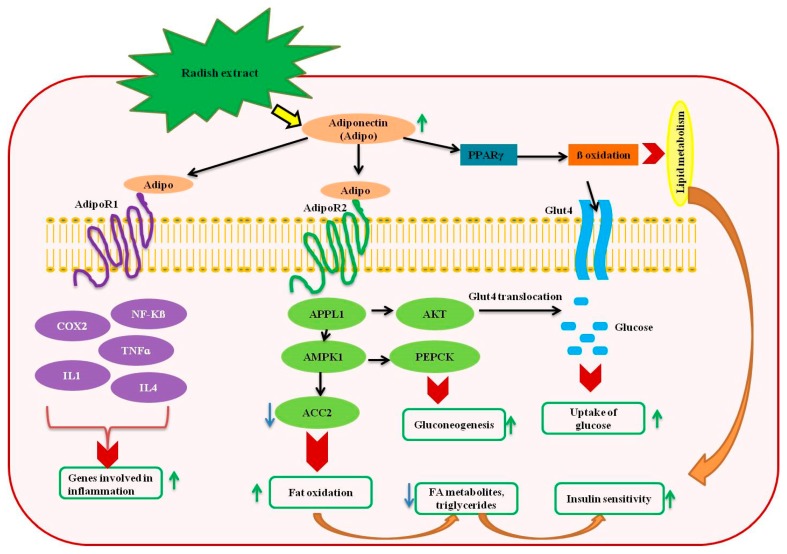
Recently, the incidence of diabetes has become a major health threat worldwide. Diabetes is one of the leading causes of mortality in humans [91]. In order to prevent the disease, several countries have adapted various therapeutic approaches and scientists are continuously striving to discover potential antidiabetic agents [92]. However, the need to circumvent the uncontrolled homeostasis of glucose metabolism has led to the search of plant-based antidiabetic compounds [92,93,94]. The usage of radish extracts in the treatments of digestive or stomach-related ailments since the ancient times has given a clue for the occurrence of phytochemicals with antidiabetic properties in radishes. The water soluble extract of radish displayed hypoglycemic properties due to the presence of insulin-like polyphenols or glucose-inhibiting compounds [95,96]. Likewise, several studies have recorded the antidiabetic effects of radish in the in vitro and in vivo environment [15,97,98]. The antidiabetic nature of radish extracts can be due to the following mechanisms: (a) regulation of glucose related hormones, (b) prevention of diabetes-induce oxidative stress and (c) balancing the glucose uptake and absorption [92]. The radish extracts enhanced the synthesis of adiponectin, a central regulatory protein involved in the regulation of lipid and glucose metabolism secreted by adipose tissue [99,100]. Adiponectin increases insulin sensitivity and enhances the bodyweight reduction [101]. It synchronizes various metabolic processes and aids in the maintenance of glucose uptake and lipid oxidation processes [101,102]. Moreover, adiponectin regulates several genes involved in inflammation, cellular proliferation, cell death, endosomal trafficking and chromatin remodeling [103]. An increase in the production of adiponectin triggers the adiponectin receptors (ADIPOR1 and 2) and peroxisome proliferator-activated receptor gamma (PPARγ) [101]. The ADIPOR1 stimulates the genes involved in inflammation and regulation of oxidative stress whereas ADIPOR2 activates adaptor protein, phosphotyrosine interaction, pH domain and leucine zipper containing 1 (APPL1), which in turn increases the expression of genes that are vital for gluconeogenesis and glucose uptake [104,105]. On the other hand, PPARγ maintains the beta oxidation in lipid metabolism. The adiponectin interaction with its receptors results in the phosphorylation of acetyl-CoA carboxylase 2 (ACC2), which increases the oxidation of fatty acids and enhances the insulin sensitivity [101,106,107]. In addition, the increase in the ROS levels have been alleviated by adiponectin mediated regulation of transcription of genes involved in antioxidant machinery, such as superoxide dismutase (SOD) [108]. Overall, the stimulatory effects of radish extract on adiponectin could be an important tactic to combat the diabetes. A detailed illustration of the possible mechanisms involved in the adiponectin mediated prevention of diabetes by radish extract has been shown in Figure 2.
Figure 2.
A schematic representation of anti-diabetic mechanisms induced by the radish extracts. The figure has been conceived based on the interpretation of the literatures cited in Section 5. ACC2, acetyl-CoA carboxylase 2; APPL1: adaptor protein, phosphotyrosine interaction, pH domain and leucine zipper containing 1; ADIPOR, adiponectin receptors; Akt, alpha serine-threonine protein kinase; COX2, cyclooxygenase 2; AMPK, adenosine monophosphate-activated protein kinase; NF-kβ, nuclear factor kappa-light-chain-enhancer of activated B cells; TNFα, tumor necrosis factor alpha; IL, interleukin; PPARγ, peroxisome proliferator-activated receptor gamma; PEPCK, phosphoenolpyruvate carboxykinase.
Similarly, the extracts of Japanese radish sprouts displayed antidiabetic activity in streptozotocin-induced diabetic rats [97]. In addition, the administration of radishes decreased the starch-stimulated glycemic load, which provided evidence for the prevention of diabetes [109]. Moreover, the occurrence of polyphenols, such as catechin in radishes, improved the insulin secretion [110]. Apart from the regulation of glucose metabolism, the antioxidant activity of radish prevented the oxidative stress induced by diabetes. For instance, radishes enhanced the synthesis of superoxide dismutase (SOD) like proteins and endogenous glutathione and catalase enzymes to scavenge the free radicals and prevented the peroxidation of lipids under diabetic conditions [111,112]. Similarly, radish extracts with pelargonidin (anthocyanin derivative) aid in the antioxidant activity by decreasing the generation of free radicals and formation of a thiobarbituric acid reactive moiety [113]. Another important antioxidant compound, sulforaphane (isothiocyanate), induces the phase II antioxidant enzymes and maintains the redox balance upon oxidative stress [114]. In addition, the nutritional and secondary metabolites content of radishes can be varied by using a different method of processing for consumption [115]. Overall, the radish extracts consist of high antidiabetic values although the exact mechanism associated with antidiabetic properties has to be determined in the future, which can be utilized in the antidiabetic drug designing pipelines.
6. Conclusions
Radishes is of great pharmaceutical importance, most of which has been attributed to its antioxidant property. The administration of radish extracts under numerous pathological conditions aids in the recovery of diseases and in the prevention of harmful ailments because of their attributed bioactivities. Bioactive compounds present in different parts of radishes, such as leaves, sprouts, stem and roots, act on a variety of potential drug targets associated with ailments, such as cancer, inflammation, liver injury and diabetes. However, the in-depth molecular mechanistic studies are required to address the regulatory roles of bioactive compounds in radish extracts. In future, researches focusing on the determination and pharmacokinetic elucidation of the bioactive compounds in radishes could facilitate the designing of plant based drugs for life threatening disorders, such as cancer and diabetes. Overall, the knowledge gained from the present researches in radish should be utilized in the discovery of novel drug molecules with higher efficacy towards drug targeting with less side effects.
Acknowledgments
This study was supported by the National Institute of Horticultural & Herbal Science, Rural Development Administration, Republic of Korea (Project No. PJ01185402). Abinaya Manivannan was supported by the RDA Research Associate Fellowship Program of National Institute of Horticultural and Herbal Science, Rural Development Administration, Republic of Korea. The authors thank Ms. Sena Choi, Researcher, Vegetable research division, National Institute of Horticulture and Herbal Science, Rural Development Administration, Wan-ju, for the extensive review of the references section.
Author Contributions
A.M. designed the manuscript outline, collected literature and wrote the manuscript. J.-H.K., H.-E.L. and D.-S.K. reviewed and edited the manuscript. E.-S.L. and H.-E.L. participated in literature collection and figure designing.
Conflicts of Interest
These authors have declared no conflict of interest.
References
- 1.Rhodes M.J.C. Physiological roles for secondary metabolites in plants: Some progress, many outstanding problems. Plant Mol. Biol. 1994;24:1–20. doi: 10.1007/BF00040570. [DOI] [PubMed] [Google Scholar]
- 2.Luckner M. Secondary Metabolism in Microorganisms, Plants and Animals. Springer Science & Business Media; Berlin, Germany: 2013. [Google Scholar]
- 3.Seca A., Pinto D. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018;19:263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soundararajan P., Kim J. Anticarcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancer. Molecules. 2018;23:2983. doi: 10.3390/molecules23112983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beevi S.S., Mangamoori L.N., Gowda B.B. Polyphenolics profile and antioxidant properties of Raphanus sativus L. Nat. Prod. Res. 2012;26:557–563. doi: 10.1080/14786419.2010.521884. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Torres I.G., De la O-Arciniega M., Gallegos-Estudillo J., Naranjo-Rodríguez E.B., Domínguez-Ortíz M.Á. Raphanus sativus L. var. niger as a source of phytochemicals for the prevention of cholesterol gallstones. Phytother. Res. 2014;28:167–171. doi: 10.1002/ptr.4964. [DOI] [PubMed] [Google Scholar]
- 7.Curtis I.S. The noble radish: Past, present and future. Trends Plant Sci. 2003;8:305–307. doi: 10.1016/S1360-1385(03)00127-4. [DOI] [PubMed] [Google Scholar]
- 8.Goyeneche R., Roura S., Ponce A., Vega-Galvez A., Quispe-Fuentes I., Uribe E., Di Scala K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods. 2015;16:256–264. doi: 10.1016/j.jff.2015.04.049. [DOI] [Google Scholar]
- 9.Takaya Y., Kondo Y., Furukawa T., Niwa M. Antioxidant constituents of radish sprout (Kaiware-daikon), Raphanus sativus L. J. Agric. Food Chem. 2003;51:8061–8066. doi: 10.1021/jf0346206. [DOI] [PubMed] [Google Scholar]
- 10.Tsouvaltzis P., Brecht J.K. Changes in Quality and Antioxidant Enzyme Activities of Bunched and Topped Radish (Raphanus sativus L.) Plants during Storage at 5 or 10C. J. Food Quality. 2014;37:157–167. doi: 10.1111/jfq.12082. [DOI] [Google Scholar]
- 11.Beevi S.S., Mangamoori L.N., Dhand V., Ramakrishna D.S. Isothiocyanate profile and selective antibacterial activity of root, stem and leaf extracts derived from Raphanus sativus L. Foodborne Pathog. Dis. 2009;6:129–136. doi: 10.1089/fpd.2008.0166. [DOI] [PubMed] [Google Scholar]
- 12.Rakhmawati R., Anggarwulan E., Retnaningtyas E. Potency of Lobak leaves (Raphanus sativus L. var. Hortensis Back) as anticancer and antimicrobial candidates. Biodivers. J. Biol. Divers. 2009;10 doi: 10.13057/biodiv/d100310. [DOI] [Google Scholar]
- 13.Pocasap P., Weerapreeyakul N., Barusrux S. Cancer preventive effect of Thai rat-tailed radish (Raphanus sativus L. var. caudatus Alef) J. Funct. Foods. 2013;5:1372–1381. doi: 10.1016/j.jff.2013.05.005. [DOI] [Google Scholar]
- 14.Kim S., Woo M., Kim M., Noh J.S., Song Y.O. Hot water extracts of pressure-roasted dried radish attenuates hepatic oxidative stress via Nrf2 upregulation in mice fed high-fat diet. Food Sci. Biotechnol. 2017;26:1063–1069. doi: 10.1007/s10068-017-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baenas N., Piegholdt S., Schloesser A., Moreno D.A., Garcia-Viguera C., Rimbach G., Wagner A.E. Metabolic activity of radish sprouts derived isothiocyanates in drosophila melanogaster. Int. J. Mol. Sci. 2016;17:251. doi: 10.3390/ijms17020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiq A., Younus I. The Radish, Raphanus sativus L. Var. caudatus reduces anxiety-like behavior in mice. Metab. Brain Dis. 2018 doi: 10.1007/s11011-018-0240-4. [DOI] [PubMed] [Google Scholar]
- 17.Yuan G., Wang X., Guo R., Wang Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010;121:1014–1019. doi: 10.1016/j.foodchem.2010.01.040. [DOI] [Google Scholar]
- 18.Salah-Abbès J.B., Abbès S., Zohra H., Oueslati R. Tunisian radish (Raphanus sativus) extract prevents cadmium-induced immunotoxic and biochemical alterations in rats. J. Immunotoxicol. 2015;12:40–47. doi: 10.3109/1547691X.2014.880534. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y., Nakamura K., Asai Y., Wada T., Tanaka K., Matsuo T., Okamoto S., Meijer J., Kitamura Y., Nishikawa A., et al. Comparison of the glucosinolate? myrosinase systems among daikon (Raphanus sativus, Japanese white radish) varieties. J. Agric. Food Chem. 2008;56:2702–2707. doi: 10.1021/jf7035774. [DOI] [PubMed] [Google Scholar]
- 20.Kliebenstein D.J., Kroymann J., Brown P., Figuth A., Pedersen D., Gershenzon J., Mitchell-Olds T. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 2001;126:811–825. doi: 10.1104/pp.126.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field B., Cardon G., Traka M., Botterman J., Vancanneyt G., Mithen R. Glucosinolate and amino acid biosynthesis in Arabidopsis. Plant Physiol. 2004;135:828–839. doi: 10.1104/pp.104.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson G.W., Hick A.J., Bennett R.N., Donald A., Pickett J.A., Wallsgrove R.M. Synthesis of glucosinolate precursors and investigations into the biosynthesis of phenylalkyl-and methylthioalkylglucosinolates. J. Biol. Chem. 1993;268:27154–27159. [PubMed] [Google Scholar]
- 23.Grassi D., Desideri G., Ferri C. Flavonoids: Antioxidants against atherosclerosis. Nutrients. 2010;2:889–902. doi: 10.3390/nu2080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.S., Sun X.D., Cao Y., Wang L., Li F.J., Wang Y.F. Antioxidant and prooxidant properties of acylated pelargonidin derivatives extracted from red radish (Raphanus sativus var. niger, Brassicaceae) Food Chem. Toxicol. 2010;48:2712–2718. doi: 10.1016/j.fct.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 25.Castaneda-Ovando A., de Lourdes Pacheco-Hernández M., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- 26.Kong J.M., Chia L.S., Goh N.K., Chia T.F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 27.Mazza G., Miniati E. Anthocyanins in Fruits, Vegetables and Grains. CRC Press; Boca Raton, FL, USA: 2018. [Google Scholar]
- 28.Bagchi D., Sen C.K., Bagchi M., Atalay M. Antiangiogenic, antioxidant and anticarcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry. 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- 29.Renis M., Calandra L., Scifo C., Tomasello B., Cardile V., Vanella L., Bei R., Fauci L.L., Galvano F. Response of cell cycle/stress-related protein expression and DNA damage upon treatment of CaCO2 cells with anthocyanins. Br. J. Nutr. 2008;100:27–35. doi: 10.1017/S0007114507876239. [DOI] [PubMed] [Google Scholar]
- 30.Shih P.H., Yeh C.T., Yen G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007;55:9427–9435. doi: 10.1021/jf071933i. [DOI] [PubMed] [Google Scholar]
- 31.Wang L.S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng R., Ni H.M., Wang S.Y., Tourkova I.L., Shurin M.R., Harada H., Yin X.M. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J. Biol. Chem. 2007;282:13468–13476. doi: 10.1074/jbc.M610616200. [DOI] [PubMed] [Google Scholar]
- 33.Koley T.K., Khan Z., Oulkar D., Singh B.K., Maurya A., Singh B., Banerjee K. High resolution LC-MS characterization of phenolic compounds and the evaluation of antioxidant properties of a tropical purple radish genotype. Arab. J. Chem. 2017 doi: 10.1016/j.arabjc.2017.11.007. [DOI] [Google Scholar]
- 34.Lee K.B., Kim Y.J., Kim H.J., Choi J., Kim J.K. Phytochemical profiles of Brassicaceae vegetables and their multivariate characterization using chemometrics. Appl. Biol. Chem. 2018;61:131–144. doi: 10.1007/s13765-017-0340-6. [DOI] [Google Scholar]
- 35.Wei J., Miao H., Wang Q. Effect of glucose on glucosinolates, antioxidants and metabolic enzymes in Brassica sprouts. Sci. Hortic. 2011;129:535–540. doi: 10.1016/j.scienta.2011.04.026. [DOI] [Google Scholar]
- 36.Baenas N., Ferreres F., Garcia-Viguera C., Moreno D.A. Radish sprouts? Characterization and elicitation of novel varieties rich in anthocyanins. Food Res. Int. 2015;69:305–312. doi: 10.1016/j.foodres.2015.01.009. [DOI] [Google Scholar]
- 37.Pajak P., Socha R., Gałkowska D., Roznowski J., Fortuna T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014;143:300–306. doi: 10.1016/j.foodchem.2013.07.064. [DOI] [PubMed] [Google Scholar]
- 38.Luo X., Zhang H., Duan Y., Chen G. Protective effects of radish (Raphanus sativus L.) leaves extract against hydrogen peroxide-induced oxidative damage in human fetal lung fibroblast (MRC-5) cells. Biomed. Pharmacother. 2018;103:406–414. doi: 10.1016/j.biopha.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 39.Ricardo L.L., Bernardi D.I., Mantovanelli G.C., Moreno B.P., Mito M.S., Silva A.A., de Oliveira R.S., Jr., Ishii-Iwamoto E.L., Sarragiotto M.H., Baldoqui D.C. Phytochemical investigation and phytotoxic activity of aerial parts of oilseed radish (Raphanus sativus var. oleifer Stokes) Biochem. Syst. Ecol. 2018;78:52–58. doi: 10.1016/j.bse.2018.03.009. [DOI] [Google Scholar]
- 40.Force L.E., O’Hare T.J., Wong L.S., Irving D.E. Impact of cold storage on glucosinolate levels in seed-sprouts of broccoli, rocket, white radish and kohl-rabi. Postharvest Biol. Technol. 2007;44:175–178. doi: 10.1016/j.postharvbio.2006.11.014. [DOI] [Google Scholar]
- 41.Hanlon P.R., Webber D.M., Barnes D.M. Aqueous extract from Spanish black radish (Raphanus sativus L. Var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. J. Agric. Food Chem. 2007;55:6439–6446. doi: 10.1021/jf070530f. [DOI] [PubMed] [Google Scholar]
- 42.Li R., Song D., Vriesekoop F., Cheng L., Yuan Q., Liang H. Glucoraphenin, sulforaphene and antiproliferative capacity of radish sprouts in germinating and thermal processes. Eur. Food Res. Technol. 2017;243:547–554. doi: 10.1007/s00217-016-2764-3. [DOI] [Google Scholar]
- 43.Ahn M., Kim J., Hong S., Kim J., Ko H., Lee N.H., Kim G.O., Shin T. Black Radish (Raphanus sativus L. var. niger) Extract mediates its hepatoprotective effect on carbon tetrachloride-induced hepatic injury by attenuating oxidative stress. J. Med. Food. 2018;21:866–875. doi: 10.1089/jmf.2017.4102. [DOI] [PubMed] [Google Scholar]
- 44.Syed S.N., Rizvi W., Kumar A., Khan A.A., Moin S., Ahsan A. In Vitro Antioxidant and In Vivo Hepatoprotective Activity of Leave Extract of Raphanus sativus in Rats Using CCL 4 Model. Afr. J. Tradit. Complement. Altern. Med. 2014;11:102–106. doi: 10.4314/ajtcam.v11i3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dash R.N., Habibuddin M., Baruah D.B. Anthocyanins fraction of red radish (Raphanus sativus L.) protects hepatic damage induced by carbon tetrachloride in albino rats. J. Exp. Integr. Med. 2013;3:43–50. doi: 10.5455/jeim.220812.or.046. [DOI] [Google Scholar]
- 46.Kim J., Ahn M., Kim S.E., Lee H.S., Kim H.K., Kim G.O., Shin T. Hepatoprotective effect of fermented black radish (Raphanus sativus L. var. niger) in CCl4 induced liver injury in rats. J. Prev. Vet. Med. 2017;41:143–149. doi: 10.13041/jpvm.2017.41.4.143. [DOI] [Google Scholar]
- 47.Lee S.W., Yang K.M., Kim J.K., Nam B.H., Lee C.M., Jeong M.H., Seo S.Y., Kim G.Y., Jo W.S. Effects of white radish (Raphanus sativus) enzyme extract on hepatotoxicity. Toxicol. Res. 2012;28:165. doi: 10.5487/TR.2012.28.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You H., Hao R., Li R., Zhang L., Zhu Y., Luo Y. The effect of radish sourced 4-(Methylthio)-3-butenyl isothiocyanate on ameliorating the severity of high fat diet inducted nonalcoholic fatty liver disease in rats. Int. J. Clin. Exp. Med. 2015;8:15910. [PMC free article] [PubMed] [Google Scholar]
- 49.Niture S.K., Khatri R., Jaiswal A.K. Regulation of Nrf2? an update. Free Radic. Biol. Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafatullah S., Al-Sheikh A., Alqasoumi S., Al-Yahya M., ElTahir K., Galal A. Protective effect of fresh radish juice (Raphanus sativus L.) against carbon tetrachloride-induced hepatotoxicity. Int. J. Pharmacol. 2008;4:130–134. doi: 10.3923/ijp.2008.130.134. [DOI] [Google Scholar]
- 51.Gutiérrez R.M., Solís R.V. Hepatoprotective and inhibition of oxidative stress in liver of Prostechea michuacana. Rec. Nat. Prod. 2009;3:46. [Google Scholar]
- 52.Wolf P.L. Biochemical diagnosis of liver disease. Indian J. Clin. Biochem. 1999;14:59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seakins A., Robinson D.S. The effect of the administration of carbon tetrachloride on the formation of plasma lipoproteins in the rat. Biochem. J. 1963;86:401. doi: 10.1042/bj0860401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres-Durán P.V., Miranda-Zamora R., Paredes-Carbajal M.C., Mascher D., Díaz-Zagoya J.C., Juárez-Oropeza M.A. Spirulina maxima prevents induction of fatty liver by carbon tetrachloride in the rat. IUBMB Life. 1998;44:787–793. doi: 10.1080/15216549800201832. [DOI] [PubMed] [Google Scholar]
- 55.Gupta P., Kim B., Kim S.H., Srivastava S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014;58:1685–1707. doi: 10.1002/mnfr.201300684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rampal G., Khanna N., Thind T.S., Arora S., Vig A.P. Role of isothiocyanates as anticancer agents and their contributing molecular and cellular mechanisms. Med. Chem. Drug Discov. 2012;3:79–93. [Google Scholar]
- 57.Schmidt J.V., Bradfield C.A. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 58.Lee J.M., Johnson J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. BMB Rep. 2004;37:139–143. doi: 10.5483/BMBRep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Savas Ü., Alexander D.L., Jefcoate C.R. Characterization of the mouse CYP1B1 gene identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. J. Biol. Chem. 1998;273:5174–5183. doi: 10.1074/jbc.273.9.5174. [DOI] [PubMed] [Google Scholar]
- 60.Dong L., Ma Q., Whitlock J.P. DNA Binding by the Heterodimeric Ah Receptor Relationship to dioxin-induced CYP1A1 transcription in vivo. J. Biol. Chem. 1996;271:7942–7948. doi: 10.1074/jbc.271.14.7942. [DOI] [PubMed] [Google Scholar]
- 61.Li W., Harper P.A., Tang B.K., Okey A.B. Regulation of cytochrome P450 enzymes by aryl hydrocarbon receptor in human cells: CYP1A2 expression in the LS180 colon carcinoma cell line after treatment with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin or 3-methylcholanthrene. Biochem. Pharmacol. 1998;56:599–612. doi: 10.1016/S0006-2952(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 62.Favreau L.V., Pickett C.B. Transcriptional regulation of the rat NAD (P) H: Quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 63.Clarke J.D., Dashwood R.H., Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juge N., Mithen R.F., Traka M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007;64:1105. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh C.T., Yen G.C. Chemopreventive functions of sulforaphane: A potent inducer of antioxidant enzymes and apoptosis. J. Funct. Foods. 2009;1:23–32. doi: 10.1016/j.jff.2008.09.002. [DOI] [Google Scholar]
- 66.Ahn Y.H., Hwang Y., Liu H., Wang X.J., Zhang Y., Stephenson K.K., Boronina T.N., Cole R.N., Dinkova-Kostova A.T., Talalay P., et al. Electrophilic tuning of the chemoprotective natural product sulforaphane. Proc. Natl. Acad. Sci. USA. 2010;2010 doi: 10.1073/pnas.1004104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin C.Y., Moon D.O., Lee J.D., Heo M.S., Choi Y.H., Lee C.M., Park Y.M., Kim G.Y. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis through downregulation of ERK and Akt in lung adenocarcinoma A549 cells. Carcinogenesis. 2006;28:1058–1066. doi: 10.1093/carcin/bgl251. [DOI] [PubMed] [Google Scholar]
- 68.Kaur I.P. Inhibition of cooked food-induced mutagenesis by dietary constituents: Comparison of two natural isothiocyanates. Food Chem. 2009;112:977–981. [Google Scholar]
- 69.Papi A., Orlandi M., Bartolini G., Barillari J., Iori R., Paolini M., Ferroni F., Fumo M.G., Pedulli G.F., Valgimigli L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J. Agric. Food Chem. 2008;56:875–883. doi: 10.1021/jf073123c. [DOI] [PubMed] [Google Scholar]
- 70.Kim W.K., Kim J.H., Jeong D.H., Chun Y.H., Kim S.H., Cho K.J., Chang M.J. Radish (Raphanus sativus L. leaf) ethanol extract inhibits protein and mRNA expression of ErbB2 and ErbB3 in MDA-MB-231 human breast cancer cells. Nutr. Res. Pract. 2011;5:288–293. doi: 10.4162/nrp.2011.5.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slamon D.J., Clark G.M. Amplification of c-erbB-2 and Aggressive Human Breast Tumors?: Response. Science. 1988;240:1796–1798. doi: 10.1126/science.240.4860.1796. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi K., Ito F. EGF receptor in relation to tumor development: Molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010;277:316–326. doi: 10.1111/j.1742-4658.2009.07450.x. [DOI] [PubMed] [Google Scholar]
- 73.Burgess A.W., Cho H.S., Eigenbrot C., Ferguson K.M., Garrett T.P., Leahy D.J., Lemmon M.A., Sliwkowski M.X., Ward C.W., Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell. 2003;12:541–552. doi: 10.1016/S1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 74.Citri A., Yarden Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7:505. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 75.Janmaat M.L., Kruyt F.A., Rodriguez J.A., Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: Limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin. Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- 76.Yuan Z.Q., Sun M., Feldman R.I., Wang G., Ma X.L., Jiang C., Coppola D., Nicosia S.V., Cheng J.Q. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 77.Bellacosa A., De Feo D., Godwin A.K., Bell D.W., Cheng J.Q., Altomare D.A., Wan M., Dubeau L., Scambia G., Masciullo V., et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 78.Sen P., Mukherjee S., Ray D., Raha S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol. Cell. Biochem. 2003;253:241–246. doi: 10.1023/A:1026020101379. [DOI] [PubMed] [Google Scholar]
- 79.Cardone M.H., Roy N., Stennicke H.R., Salvesen G.S., Franke T.F., Stanbridge E., Frisch S., Reed J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 80.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 81.Tang E.D., Nuñez G., Barr F.G., Guan K.L. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 82.Pawlik A., Wała M., Hać A., Felczykowska A., Herman-Antosiewicz A. Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine. 2017;29:1–10. doi: 10.1016/j.phymed.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Mondal A., Biswas R., Rhee Y.H., Kim J., Ahn J.C. Sulforaphene promotes Bax/Bcl2, MAPK-dependent human gastric cancer AGS cells apoptosis and inhibits migration via EGFR, p-ERK1/2 downregulation. Gen. Physiol. Biophys. 2016;35:25–34. doi: 10.4149/gpb_2015033. [DOI] [PubMed] [Google Scholar]
- 84.Lee S.J., Zhang J., Choi A.M., Kim H.P. Mitochondrial dysfunction induces formation of lipid droplets as a generalized response to stress. Oxid. Med. Cell. Longev. 2013;2013:327167. doi: 10.1155/2013/327167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao D., Powolny A.A., Antosiewicz J., Hahm E.R., Bommareddy A., Zeng Y., Desai D., Amin S., Herman-Antosiewicz A., Singh S.V. Cellular responses to cancer chemopreventive agent D, L-sulforaphane in human prostate cancer cells are initiated by mitochondrial reactive oxygen species. Pharm. Res. 2009;26:1729–1738. doi: 10.1007/s11095-009-9883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gobeil S., Boucher C.C., Nadeau D., Poirier G.G. Characterization of the necrotic cleavage of poly (ADP-ribose) polymerase (PARP-1): Implication of lysosomal proteases. Cell Death Differ. 2001;8:588. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- 87.Chaitanya G.V., Alexander J.S., Babu P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salah-Abbes J.B., Abbes S., Houas Z., Abdel-Wahhab M.A., Oueslati R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus) J. Pharm. Pharmacol. 2008;60:761–770. doi: 10.1211/jpp.60.6.0012. [DOI] [PubMed] [Google Scholar]
- 89.Beevi S.S., Mangamoori L.N., Subathra M., Edula J.R. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Food Hum. Nutr. 2010;65:200–209. doi: 10.1007/s11130-010-0178-0. [DOI] [PubMed] [Google Scholar]
- 90.Yang M., Wang H., Zhou M., Liu W., Kuang P., Liang H., Yuan Q. The natural compound sulforaphene, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget. 2016;7:76656. doi: 10.18632/oncotarget.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dal-Ré R. Worldwide clinical interventional studies on leading causes of death: A descriptive analysis. Ann. Epidemiol. 2011;21:727–731. doi: 10.1016/j.annepidem.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 92.Banihani S. Radish (Raphanus sativus) and diabetes. Nutrients. 2017;9:1014. doi: 10.3390/nu9091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vigersky R.A., Filmore-Nassar A., Glass A.R. Thyrotropin suppression by metformin. J. Clin. Endocrinol. Metab. 2006;91:225–227. doi: 10.1210/jc.2005-1210. [DOI] [PubMed] [Google Scholar]
- 94.Vidon N., Chaussade S., Noel M., Franchisseur C., Huchet B., Bernier J.J. Metformin in the digestive tract. Diabetes Res. Clin. Pract. 1988;4:223–229. doi: 10.1016/S0168-8227(88)80022-6. [DOI] [PubMed] [Google Scholar]
- 95.Taniguchi H., Muroi R., Kobayashi-Hattori K., Uda Y., Oishi Y., Takita T. Differing effects of water-soluble and fat-soluble extracts from Japanese radish (Raphanus sativus) sprouts on carbohydrate and lipid metabolism in normal and streptozotocin-induced diabetic rats. J. Nutr. Sci. Vitaminol. 2007;53:261–266. doi: 10.3177/jnsv.53.261. [DOI] [PubMed] [Google Scholar]
- 96.Broadhurst C.L., Polansky M.M., Anderson R.A. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J. Agric. Food Chem. 2000;48:849–852. doi: 10.1021/jf9904517. [DOI] [PubMed] [Google Scholar]
- 97.Taniguchi H., Kobayashi-Hattori K., Tenmyo C., Kamei T., Uda Y., Sugita-Konishi Y., Oishi Y., Takita T. Effect of Japanese radish (Raphanus sativus) sprout (Kaiware-daikon) on carbohydrate and lipid metabolisms in normal and streptozotocin-induced diabetic rats. Phytother. Res. 2006;20:274–278. doi: 10.1002/ptr.1851. [DOI] [PubMed] [Google Scholar]
- 98.Aly T.A., Fayed S.A., Ahmed A.M., Rahim E.A.E. Effect of Egyptian radish and clover sprouts on blood sugar and lipid metabolisms in diabetic rats. Glob. J. Biotechnol. Biochem. 2015;10:16–21. [Google Scholar]
- 99.Antonopoulos A.S., Margaritis M., Coutinho P., Shirodaria C., Psarros C., Herdman L., Krasopoulos G. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: The regulatory role of perivascular adipose tissue. Diabetes. 2015;64:2207–2219. doi: 10.2337/db14-1011. [DOI] [PubMed] [Google Scholar]
- 100.Okada Y., Okada M., Sagesaka Y. Screening of dried plant seed extracts for adiponectin production activity and tumor necrosis factor-alpha inhibitory activity on 3T3-L1 adipocytes. Plant Food. Hum. Nutr. 2010;65:225–232. doi: 10.1007/s11130-010-0184-2. [DOI] [PubMed] [Google Scholar]
- 101.Ghoshal K., Bhattacharyya M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J. Diabetes. 2015;6:151. doi: 10.4239/wjd.v6.i1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fruebis J., Tsao T.S., Javorschi S., Ebbets-Reed D., Erickson M.R.S., Yen F.T., Bihain B.E., Lodish H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA. 2001;98:2005–2010. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deepa S.S., Dong L.Q. APPL1: Role in adiponectin signaling and beyond. Am. J. Physiol.-Endoc. Metab. 2009;296:E22–E36. doi: 10.1152/ajpendo.90731.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lihn A.S., Pedersen S.B., Richelsen B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhu W., Cheng K.K., Vanhoutte P.M., Lam K.S., Xu A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008;114:361–374. doi: 10.1042/CS20070347. [DOI] [PubMed] [Google Scholar]
- 106.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 107.Yoon M.J., Lee G.Y., Chung J.J., Ahn Y.H., Hong S.H., Kim J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase and peroxisome proliferator–activated receptor α. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 108.Maassen J.A., Janssen G.M.C., Lemkes H.H.J.P. Mitochondrial diabetes mellitus. J. Endocrinol. Investig. 2002;25:477–484. doi: 10.1007/BF03344042. [DOI] [PubMed] [Google Scholar]
- 109.Tiwari A.K. Revisiting “Vegetables” to combat modern epidemic of imbalanced glucose homeostasis. Pharmacog. Mag. 2014;10:S207. doi: 10.4103/0973-1296.133211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang C.F., Chen Y.W., Yang C.Y., Lin H.Y., Way T.D., Chiang W., Liu S.H. Extract of lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J. Agric. Food Chem. 2011;59:1087–1094. doi: 10.1021/jf103382h. [DOI] [PubMed] [Google Scholar]
- 111.Chaturvedi P. Inhibitory response of Raphanus sativus on lipid peroxidation in albino rats. Evid.-Based Complment. Altern. Med. 2008;5:55–59. doi: 10.1093/ecam/nel077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lugasi A., Dworschák E., Blázovics A., Kery A. Antioxidant and free radical scavenging properties of squeezed juice from black radish (Raphanus sativus L. var niger) root. Phytother. Res. 1998;12:502–506. doi: 10.1002/(SICI)1099-1573(199811)12:7<502::AID-PTR336>3.0.CO;2-I. [DOI] [Google Scholar]
- 113.Forbes-Hernández T.Y., Gasparrini M., Afrin S., Cianciosi D., González-Paramás A.M., Santos-Buelga C., Bompadre S. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017;18:1149. doi: 10.3390/ijms18061149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fahey J.W., Talalay P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999;37:973–979. doi: 10.1016/S0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 115.Kumakura K., Kato R., Kobayashi T., Sekiguchi A., Kimura N., Takahashi H., Takahashi A., Matsuoka H. Nutritional content and health benefits of sun-dried and salt-aged radish (takuan-zuke) Food Chem. 2017;231:33–41. doi: 10.1016/j.foodchem.2017.03.096. [DOI] [PubMed] [Google Scholar]