Abstract
Bone metastasis is associated with significant morbidity for cancer patients and results in a reduced quality of life. The bone marrow is a fertile soil containing a complex composition of immune cells that may actually provide an immune-privileged niche for disseminated tumor cells to colonize and proliferate. In this unique immune milieu, multiple immune cells including T cells, natural killer cells, macrophages, dendritic cells, myeloid-derived suppressor cells, and neutrophils are involved in the process of bone metastasis. In this review, we will discuss the crosstalk between immune cells in bone microenvironment and their involvement with cancer cell metastasis to the bone. Furthermore, we will highlight the anti-tumoral and pro-tumoral function of each immune cell type that contributes to bone metastasis. We will end with a discussion of current therapeutic strategies aimed at sensitizing immune cells.
Keywords: bone metastasis, immune system, immunotherapy
1. Introduction
Accompanied by an increase in the incidence of cancer over the past several decades, bone metastasis has become an ongoing clinical problem which is a major cause of mortality for thousands of patients suffering from cancer. Over 80% of patients with advanced breast cancer or prostate cancer develop bone metastasis, followed by patients with thyroid cancer (60%), lung cancer (30–40%), and renal cancer (20–25%) [1]. Although there have been advances in the diagnosis and treatment of cancer, bone metastasis is still incurable.
In mineralized bone marrow, multiple cell types release signaling molecules that together make the bone microenvironment an attractive site for metastatic cancer cells to home. A “vicious cycle” develops that promotes metastasis to the bone. Osteoblasts and/or osteoclasts release various growth factors in the bone microenvironment, which further promote metastatic tumor growth and cause incurable osteoblastic and osteolytic lesions [2]. Early studies focused on the interactions between cancer cells and bone progenitor cells during bone metastasis. The significance of the contribution of the immune system in this process remains largely unexplored. Likewise, in vivo models that recapitulate the cancer cell-bone microenvironment interaction are lacking. It is most commonly accepted that the immune system functions as a major defense against cancer cells. However, increasing evidence suggests that metastasis may be dependent on the specific factors in the tumor microenvironment [3]. For example, an antitumoral or protumoral effect of the immune microenvironment may depend on the presence of accessory stromal cells, the local cytokine milieu, tumor-specific interactions and the specific types of immune cells present. As represented in Figure 1, for instance, cytotoxic T cells and natural killer cells indeed function as mediators of tumor clearance. Conversely, many other subtypes of immune cells including regulatory T cells (Tregs), CD4+ helper T cells, suppressive dendritic cells, and myeloid-derived suppressor cells (MDSCs) traffic to the bone-tumor microenvironment and are more prone to promote tumor progression and metastasis [4]. Likewise, as a response to the immune-suppressive cytokines secreted by tumor cells, the M1 macrophages and N1 neutrophils are subverted to tumor-associated M2 macrophages and N2 neutrophils which are characterized as having potent tumor-promoting activity [5]. In the current review, the detailed functions of different immune cells and their impact on cancer cell metastasis to the bone will be discussed. Additionally, the development of current therapeutic strategies for bone metastasis will be described.
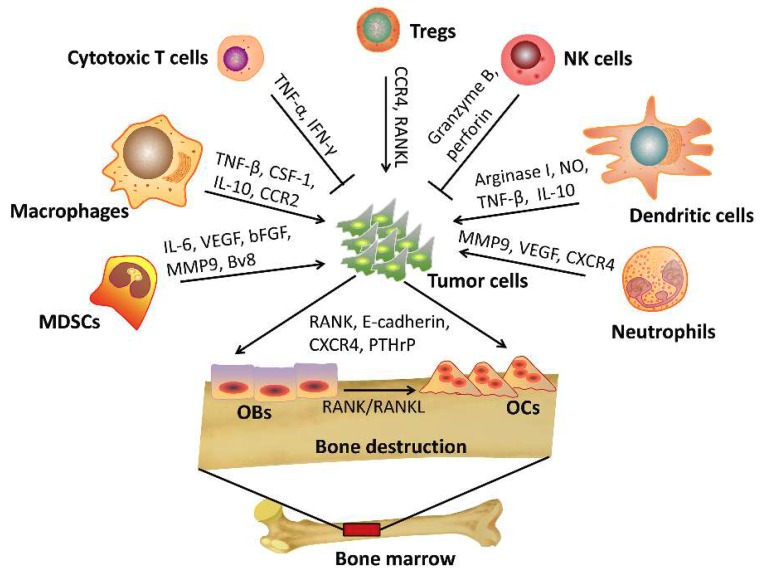
Figure 1.
The interaction of immune cells and cancer cells during bone metastasis. Cytotoxic CD8+ T cells release TNF-α and IFN-γ to eliminate tumor cells. Natural killer cells (NK cells) kill tumor cells through granzyme B- and perforin-mediated apoptosis. Regulatory T cells (Tregs) promote tumor cell to bone metastasis through CXCR4/CXCL12 signaling or RANK/RANKL axis. Tumor-associated macrophages (TAMs) promote tumor cell to bone metastasis through CCL2/CCR2 or CSF-1/ CSF-1R signaling. Meanwhile, TAMs secret high levels of IL-10 and TGF-β to decrease the activation of CD4+ and CD8+ T cells. Dendritic cells (DCs) suppress the cytotoxic capacity of CD8+ T cells via production of arginase I, nitric oxide (NO), TGF-β, interleukin-10 (IL-10) to promote tumor progression. Myeloid-derived suppressor cells (MDSCs) release chemokines including IL-6, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and matrix metalloproteinase (MMP)-9 to promote cancer progression and bone metastasis. Tumor-associated neutrophils (TANs) are able to release CXCR4, VEGF and MMP9 to promote tumor bone metastasis. Tumor cells also release factors such as RANK, E-cadherin, CXCR4, and parathyroid hormone-related protein (PTHrP) that promote osteolytic bone lesions.
2. Crosstalk among Cancer Cell, Immune Cells and the Bone Microenvironment
2.1. Bone Microenvironment
In multiple types of human cancer, the bone is the third most common site for metastasis [6]. The bone microenvironment plays a critical role in the development of metastases. In 1889, Stephen Paget proposed the “seed and soil” hypothesis: the dissemination of cancer cells (seed) from primary sites invades the metastatic sites (soil) to form metastatic lesions [7,8]. This hypothesis highlights that the bone microenvironment is fertile soil for metastasis, primarily due to: (1) high blood flow in the red marrow in bone; (2) tumor cell-stromal cell interactions; (3) multiple cells in the bone marrow that produce growth factors, angiogenic factors and bone-resorbing factors that stimulate tumor growth.
In order to understand the role of the bone microenvironment in tumor metastasis, the structure and function of bone and the cells that constitute the bone microenvironment are important to consider. As the connective tissue of the human body, bone provides structural support, protective function, and regulation of calcium levels [9]. Bones can be classified as long bones and flat bones, both of which contain 95% type-I collagen, 5% proteoglycans and other non-collagenous proteins [10]. Two major types of cells located within the bone microenvironment contribute to the metastatic bone niche: osteoblasts and osteoclasts. Osteoblasts develop from pluripotent mesenchymal stem cells in the bone marrow stroma that can transform to osteocytes which synthesize new bone matrix. Osteoblastic activity in bone metastases is primarily found in patients with prostate cancer [11]. In osteoblastic metastases, osteoblasts produce many factors including insulin-like growth factor 1 (IGF-1), insulin-like growth factor 2 (IGF-2), TGF-β, TNF-α and IL-1β that act as chemoattractants for cancer cells [12]. Meanwhile, osteoblasts interact with tumor cells to activate the Wnt signaling pathway and consequently increases bone formation which leads to osteoblastic lesions [13,14]. Osteoclasts are derived from monocytes and are responsible for bone resorption in the bone marrow [10]. Osteolytic lesions are more common than osteoblastic-type lesions in breast cancer, lung cancer, and especially renal cell cancer (RCC) [15]. The formation of osteolytic lesions is mediated by osteoclasts via the release of multiple osteoclastogenic factors from tumor cells (e.g., IL-1, IL-6, IL-11, PDGF, MIP1α, TNF, M-CFS, RANKL and PTHrP) [16,17]. These growth factors and cytokines are capable of stimulating metastatic growth in the bone, thereby establishing the vicious cycle of subsequent tumor adhesion and proliferation as well as further bone destruction [18]. In addition to immune cells, the bone microenvironment also contains stromal cells such as fibroblasts, adipocytes, vascular endothelial cells, chondrocytes, and osteoblasts, as well as transient cells such as erythrocytes, immune cells, and platelets that may play a role in metastasis. [10,19,20]. The non-cellular components of the bone, such as growth factors, cell adhesion molecules, cytokines, chemokines and calcium ions are released by multiple cell types including tumor cells in the bone marrow and also contribute to making the skeleton an attractive soil for metastatic cancer cells.
2.2. Cancer Cells Metastasize to Bone
For cancer cells to migrate from the primary tumor to the bone to initiate metastasis, disseminated tumor cells (DTCs) must escape from immune surveillance in order to survive in the circulation, and then extravasate into foreign tissues by attaching and adapting to the microenvironment at the metastatic site. For survival in the circulation, DTCs are covered by platelets in the bloodstream allowing them to evade the immune system and escape perforin/granzyme-mediated NK cell cytotoxicity and TNF-α-mediated cell death [21,22]. DTCs can acquire resistance to apoptosis by expressing prosurvival proteins such as BCL-2, MCL-1 and survivin-C that also protect DTCs against NK cell- or cytotoxic T cell-mediated killing [23]. Additionally, a common immune escape strategy includes the loss of MHC class-I molecules and the expression of programmed death ligand 1 (PD-L1) on tumor cells [24,25].
DTCs are attracted to the “nutrients” from fertile bone environments that allow tumor cells to colonize. In bone marrow, DTCs bind with osteoblasts through CXCR4/CXCL12 and annexin II/annexin II receptor interactions [26,27,28]. Furthermore, DTCs expressing E-cadherin on their surface can form adherin junctions with N-cadherin expressed on the surface of osteoblasts [29,30]. In addition, DTCs expressing RANK can interact with RANKL which is secreted by osteoblasts and osteocytes in bone environments [31,32]. DTCs also interact with multiple factors, such as VCAM-1, ICAM-1, vitronectin, osteopontin (OPN) and bone sialoprotein (BSP) via integrin receptors [19,33,34,35]. Once DTCs establish residency in the bone marrow, they can proliferate and compete with hematopoietic stem cells (HSC) for the endosteal niche, or enter a state of dormancy [36]. It is possible that dormant DTCs may never develop bone metastasis. Alternatively, they may exit the state of dormancy and form bone metastasis many years after initial diagnosis and treatment [37]. Despite the clinical significance of dormancy on tumor metastasis, the mechanisms underlying it remain poorly understood. Some evidence suggests that factors such as CXCL12, E-selectin, thrombospondin-1 (TSP1), Notch-1, bone morphogenetic protein (BMP) and TGFβ2 are involved in the regulation and maintenance of dormancy [38,39,40,41]. Once cancer cells exit the latency period of dormancy, they start to re-proliferate and form macrometastases. Evidence suggests that the release of stem cell signals by osteoclasts may trigger the NF-κB pathway to cause cancer cell reactivation [42]. Moreover, the release of Ca2+ during normal bone remodeling binds with CaSR, a calcium-sensing receptor to stimulate parathyroid hormone-related protein (PTHrP), that leads to tumor cell reactivation [43]. TGF-β1 secreted from neovascular tips and periostin secreted from cancer stem cells (CSCs) have also been associated with tumor cell reactivation in the bone microenvironment [38]. Furthermore, the activation of TGF-β, BMP, IGFs and PDGF family members released from the bone matrix has also been shown to stimulate tumor cell proliferation [44,45]. Once macrometastases are established, multiple factors such as M-CSF, TNF-α, IL-8 and IL-11 released from tumor cells drive osteoclasts to induce osteolytic lesions via the stimulation of RANKL [6,46].
2.3. Interaction between Immune Cells and Bone Microenvironment
Immune cells in the bone marrow are in close proximity to and associate with osteoclasts and osteoblasts in the bone microenvironment. For instance, immune cells interact with osteoclasts mainly through osteoprotegerin (OPG) /RANKL/RANK. CD4+ T cells can release factors such as IL-6, IL-11, IL-15, and TNF-α to enhance osteoclastogenesis and the formation of osteolytic lesions [47,48]. Activated CD4+ T cells can enhance OPG-L-mediated osteoclast activity and bone destruction [49]. The knockdown of RANKL in tumor-specific T cells reduced bone destruction and metastasis. Conversely, there is also evidence suggesting that the production of an inflammatory factor, interferon-γ (IFN-γ), by activated CD4+ T cells can inhibit the activity of osteoclasts [50,51,52]. In addition to the activation of T-cells, T cells can also establish a feedback loop that is regulated by osteoclasts. For example, osteoclasts secrete chemokines that recruit CD8+ T cells [53]. Taken together, this suggests that the balance between pro-tumorigenic and anti-tumorigenic effects of the immune cells in the bone microenvironment will determine the likely hood of bone metastasis.
Unlike osteoclasts, osteoblasts play a role in the regulation and the differentiation of all stages of B cell development [54]. Both B cells and macrophages have been shown to interact with osteoblasts. Additionally, macrophages can regulate osteoblast differentiation and mineralization both in vitro and in vivo [55]. The results from a recent study showed that CD169+ macrophages within the bone-tumor microenvironment were essential for tumor-induced bone formation by osteoblasts [56]. Hence, the osteoblastic niches should be considered a therapeutic target to prevent bone metastasis in certain tumors. In general, the mutual interaction between immune cells, osteoclasts and osteoblasts in the bone marrow microenvironment complicate the study and identification of the mechanisms that drive bone metastasis.
3. The Role of Immune Cells in Bone Metastasis
3.1. T Cells
Bone marrow is vascularized and represents a major part of the lymphocyte recirculation network, with billions of lymphocytes recirculating through it per day. The bone marrow microenvironment can provide lymphocytes with the appropriate support to develop, even in the absence of the thymus [57]. Approximately 8%–20% of mononuclear cells in the bone marrow are T cells or B cells with a ratio of 5:1 [58,59]. Among T cells, there are about 1.5% CD4+ T cells and 2.0%–2.5% CD8+ T cells. About one-third of CD4+ T cells are CD4+CD25+ regulatory T (Treg) cells [60,61,62]. Among T cells, the CD8+ cytotoxic T cell is one of the most important immune-mediated cells for tumor destruction. In the human body, T cells cannot recognize host proteins due to the process of immune tolerance to self-antigens. However, they can recognize the tumor-antigen-MHC-I-complex in the presence of antigen presenting cells (APCs), and thereby destroy tumor cells through the perforin-granzyme B- and/or Fas-Fas ligand axis-mediated apoptosis [3]. Unfortunately, the T cell-mediated anti-tumor immune responses are inhibited by TGF-β released from osteoclasts [63]. Thus, inhibitors of TGF-β may be effective enhancers of antitumor immune responses and ultimately prevent bone metastasis.
Among CD4+ T cells, Tregs are known to be potent immune suppressors that play important roles in maintaining homeostasis in the immune system. An increased number of activated Tregs have been observed in nearly all cancer patients, and dampen the immune response against cancer cells [64]. More importantly, the presence of Tregs in cancer patients predicts poor prognosis [65,66]. Maj et al. found that bone marrow Treg cells are significantly increased in patients with prostate cancer that has metastasized to the bone [67]. Zou et al. demonstrated that CXCR4/CXCL12 signaling mediates Treg cell trafficking to the bone marrow [68]. The results from those studies suggested that bone marrow is a preferential site for migration, selective retainment and function of Treg cells [68,69,70]. In addition to the immunosuppressive functions, FOXP3+ Tregs have been demonstrated as a major source of RANKL [71], the critical cytokine required for osteoclasts differentiation as well as cancer cell mobility and bone metastasis [72], suggesting RANKL+ Tregs may promote DTC recruitment to the bone.
CD4+ helper T cells (Th17 cells) are another important subset of CD4+ T cells that might be important in enhancing osteoclastogenesis and bone metastasis through the RANKL pathway [73,74]. Evidence suggests that tumor-specific Th17 cells enhance the activation of osteoclasts and induce osteolytic bone disease by producing RANKL [75]. Moreover, RANKL+ Th17 cell adoptive transfer into mice, orthotopically injected with 4T1 breast cancer cells, increases tumor-cell to bone colonization [75]. Interestingly, Th17 cells can differentiate into Treg cells during an immune response and in the presence of TGF-β1, aryl hydrocarbon receptor (AhR) activation promotes this conversion [76]. These results highlight the critical role and promising therapeutic potential of targeting Tregs and Th17 to prevent bone metastasis.
3.2. NK Cells
Apart from the cytotoxic T cell, another important cell-type in immune-mediated tumor killing is natural killer (NK) cells which belong to the innate immune system. There are approximately 0.4%–4% NK cells in the bone marrow [77,78]. Generally, NK cells do not recognize tumor-specific antigens, whereas, they recognize cancer cells directly through antigen-specific receptors such as NKG2D, CD16, DNAM1 and NCRs, which recognize ligands expressed on the surface of cancer cells [3]. Another way for NK cells to recognize cancer cells is through the recognition by “missing-self”, which is caused by the down-regulation of MHC molecules on cancer cells causing them to evade T cell recognition. Once NK cells bind to cancer cells, apoptosis occurs through granule-mediated-exocytosis or Fas-Fas ligand interactions [79]. Depletion of NK cells causes uncontrolled tumor growth and metastasis [80,81]. The Core2 β-1,6-N-acetylglucosaminyltransferase (C2GnT) plays an important role in NK cell-mediated tumor immunity. In the bone microenvironment, cancer cells expressing C2GnT disrupts the ligand-receptor-mediated (NKR/NKR-L and TRAIL/DR4) immune response blocking the apoptosis of cancer cells [82]. In addition to C2GnT, the E3 ubiquitin ligase Casitas B cell lymphoma-b (Cbl-b) attenuates the anti-tumor activity of NK cells by enhancing the activity of TAM tyrosine kinase receptors (Tyro3, Axl or Mer) on NK cells [81]. More importantly, TAM receptor inhibitors largely reduced breast cancer metastasis in animal models [81]. Additionally, NK cell dysfunction had been reported in esophageal squamous cell cancer, gastric cancer as well as prostate cancer [83]. An imbalance in the activating and inhibitory cell surface receptors on NK cells such as NKG2D and CD161, low expression of the signal transducing ζ chain, and down-regulation of cytotoxic machinery caused by immunosuppressive cytokines such as IL-10 and TGF-β may explain the mechanisms behind NK cell dysfunction frequently observed in the tumor microenvironments [84]. Reactive oxygen species (ROS) produced by granulocytes, macrophages and tumor cells in tumor microenvironment also play a role in NK cell dysfunction [85]. Thus, the improvement of NK cell survival and activation will enhance tumor-specific targeting.
3.3. Macrophages
Once monocytes exit the bone marrow and peripheral blood, they can enter tissues and differentiate into macrophages. Macrophages are mononuclear myeloid lineage cells originally known for their protective role in eliminating undesired pathogens [86]. Under the influence of various cytokines, macrophages can polarize into two different types of populations: the proinflammatory M1 subtype and the anti-inflammatory M2 subtype macrophage. M1 macrophages are commonly regarded as tumor-suppressing immune cells that secrete proinflammatory cytokines such as IL-1, IL-6, IL-23, IFN-γ and IL-12 which activate cytotoxic T cells and NK cells to eliminate cancer cells [87,88,89]. Conversely, M2 macrophages are regarded as tumor-associated macrophages (TAMs). TAMs have been studied extensively in primary tumors, and have been considered as one of the most important regulators of tumor progression, angiogenesis, invasion, and metastasis [86,90,91]. Many clinical studies have demonstrated that high levels of TAMs correlate with poor prognosis in many cancer types [91,92,93]. Distinct from M1, TAMs secret high levels of cytokines including IL-10 and TGF-β which decreases the activation of CD4+ and CD8+ T cells [94]. Likewise, emerging evidence including clinical data and animal experiments demonstrate that TAMs potentiate tumor metastasis [95,96,97], particularly bone metastasis [98]. Increased numbers of CD206+ M2-like macrophages have been found in prostate cancer with bone metastatic lesions [98,99]. Depleting macrophages via gene targeted or pharmacologic approaches inhibits tumor growth in bone in animal models [99]. Moreover, CD68 (phagocytic capacity marker) positive macrophages are increased in metastatic breast and prostate cancers compared to matched primary tumors [100,101]. Research into the potential mechanism of the role of TAMs in promoting bone metastasis has uncovered that chemokine (C-C motif) ligand 2 (CCL2)-expressing breast tumor cells engage CCR2+ stromal cells of monocytic origin, including macrophages and preosteoclasts, to facilitate colonization in lung and bone [102]. Additionally, CSF-1, which is a potent chemokine for regulating proliferation and differentiation of osteoclasts, monocytes and macrophages, has also been implicated in the contribution of macrophage-driven bone metastasis [103]. However, therapeutic targeting of CSF-1R restricts the recruitment of TAMs to primary sites in the MMTV-PyMT breast cancer model [104] and reduces osteolytic bone lesions in nude mice injected intracardially with MDA-MB-231 breast cancer cells [105]. Given the important role of macrophages in supporting cancer cell metastasis to bone, targeting macrophages will be an effective therapeutic treatment for bone metastasis.
3.4. Dendritic Cells
Dendritic cells (DCs), also known as professional antigen-presenting cells (APC), play a key role in the regulation of cytotoxic T-cell immune response activation by virtue of their antigen-presenting capacities. Many studies have demonstrated that tumor antigen-pulsed DCs are capable of inducing activation and proliferation of both T-helper cells and cytotoxic T cells to mediate anti-tumor immune responses [106,107]. A number of studies have evaluated the therapeutic potential of DC-based cancer vaccines for some tumors such as breast, lung, colon and prostate cancers [108]. Compared with spleen, liver or lung tissues, circulating DCs are prone to migrate to the bone marrow where microvessels constitutively express VCAM-1 and endothelial selectins which helps to retain DCs in the bone marrow microenvironment [109]. Although DCs are known for their powerful role in anti-tumor immune response, cancer cells may still influence DCs to promote an immunosuppressive phenotype.
In 2012, Sawant et al. found increased amounts of plasmacytoid DCs (pDCs) within the bone marrow of mice inoculated with 4T1 breast cancer cells [47]. PDCA-1 mediated deficiency of pDCs significantly reduced lung and bone metastasis [47], suggesting the potential for therapeutically targeting pDCs for the treatment of bone metastasis. Evidence also demonstrated that purified DCs from patients with breast cancer showed a significantly decreased ability to stimulate allogeneic T cells [110]. It has also been found that tumor-infiltrating DCs could suppress the cytotoxic capacity of CD8+ T cells via production of TGF-β, nitric oxide (NO), IL-10, VEGF and arginase I [5]. Furthermore, the recruitment of other immunosuppressive immune cells including Tregs and myeloid-derived suppressor cells (MDSCs) by pDCs could promote, rather than protect from, tumor progression and metastasis [111].
3.5. Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells comprised of immature myeloid cells that are generated in the bone marrow. Under normal conditions, the immature myeloid cells (IMCs) can differentiate into mature myeloid cells such as macrophages, dendritic cells and granulocytes. However, under pathological conditions including cancer, IMC differentiation is inhibited resulting in the accumulation and activation of immunosuppressive MDSCs by the production of immune suppressive factors such as arginase I, inducible nitric oxidase synthase and TGF-β from the IMCs [112]. By suppressing both innate and adaptive immune response, MDSCs assist in cancer progression and metastasis. Studies in pre-clinical animal models and human patients have demonstrated that MDSCs accumulate in almost all cancers, both in primary and metastatic solid tumors [89]. In the tumor microenvironment, MDSCs suppress T cell function by suppressing proliferation and promoting apoptosis of T cells. In addition to suppressing effector T cell populations, MDSCs can promote the expansion and activation of Tregs and in turn regulate immunosuppression. Also, MDSCs can secrete factors to promote angiogenesis and lymphangiogenesis which favor tumor growth. Meanwhile, pro-angiogenic growth factors produced by MDSCs can directly incorporate into the tumor endothelium [112,113]. Thus, many different mechanisms used by MDSCs allow cancer cell proliferation and metastasis to distant organs including the bone [114]. Zhuang et al. found that MDSCs can differentiate into osteoclasts and contribute to bone destruction in myelomas [115]. Sawant et al. reported that increased numbers of MDSCs in breast cancer could also drive bone metastasis during breast cancer progression in animal models [116]. During cancer cell colonization of bone, dysregulation of this process leads to increased osteoclast activation and osteolysis [116]. It should be noted that only MDSCs isolated from a bone microenvironment with bone metastasis were able to differentiate into mature and functional osteoclasts; whereas, MDSCs isolated from a tumor-bearing mouse without bone metastasis did not differentiate into active osteoclasts. This suggests that cancer cells resident in the bone microenvironment contribute to an increased number of activated osteoclasts. Given the potent effects of MDSCs on suppressing host immunity and promoting bone damage, bone marrow MDSCs may serve as a potential therapeutic target for bone metastasis.
3.6. Neutrophils
Neutrophils are an important component of the innate immune system and play key roles in the initiation, modulation, and resolution of the host immune response [117]. In the normal adult human body, neutrophils are generated at a rate of 1 to 2 × 1011 cells per day under normal conditions [118]. The bone marrow is a large pool for mature neutrophils and plays an important role in neutrophil homeostasis. The CXCR4/CXCL12 signaling pathway is required to maintain neutrophils [119]. Early studies have reported that neutrophils attack tumor cells through antigen 1-dependent recognition [120]. However, increasing evidence has revealed that a group of tumor-associated neutrophils (TANs) have a pro-tumor effect rather than an anti-tumor effect [5]. More specifically, the function of neutrophils in cancer is dependent on their subtype: a tumor-inhibitory N1 phenotype or a tumor-promoting N2 phenotype. The N1 phenotype neutrophils have anti-tumor and anti-metastatic function; whereas N2 phenotype neutrophils promote tumor angiogenesis, tumor cell dissemination, and metastatic seeding in distant organs including the bone [121]. TGF-β plays an important role in determining the neutrophil phenotype, by shifting the balance from an antitumor (N1) phenotype toward a pro-tumor (N2) phenotype [122]. TANs are able to release CXCR4, VEGF and MMP9, all of which have been implicated in the metastatic process [123]. Liu et al. showed that host Toll-like receptor 3 (TLR3) promotes lung pre-metastatic niche formation via neutrophil recruitment which further predicted poor patient survival [124]. Additional studies demonstrated that the neutrophil-to-lymphocyte ratio in cancer patients could be considered a prognostic biomarker for predicting the overall survival rate of cancer patients [125,126], and may also be a useful predictor of bone metastasis [127].
4. Therapeutic Potential for Bone Metastasis by Modulating the Immune System
4.1. Targeting T Cells
The increased understanding of the role of the immune system in the bone–tumor microenvironment has been translated into the development of immune system-modulating therapies. For instance, to enhance the immune response against tumors, CD8+ T cells can be stimulated by vaccination or engineering the T cell receptor (TCR) or chimeric antigen receptor (CAR) [3]. The therapeutic application of regulatory T cells has been well demonstrated in the clinic. Evidence suggests that certain chemotherapy regimens such as cyclophosphamide, fludarabine and paclitaxel-based chemotherapy are able to reduce Tregs (CD4+CD25high regulatory T cells) through Fas-mediated cell death [128,129,130]. Studies also show that chemotherapeutic efficacy is improved with the addition of anti-CD25 treatments by mediating Treg regulation. Daclizumab and basiliximab are antihuman CD25 mAbs approved for use in cancer treatment as well as the treatment of other diseases. In metastatic breast cancer patients, treatment with daclizumab durably reduced circulating CD25highFOXP3+ Tregs favoring the population of tumor-specific cytotoxic CD8+T cells (CTLs) after vaccination with cancer antigen peptides (hTERT/survivin) [131]. The anti-CTLA-4 antagonist mAb such as ipilimumab and tremelimumab are used to block Foxp3+CD4+CD25high Treg suppressive function by binding to Tregs receptors. In addition to anti-CTLA-4 antagonists, Anti-PD-1 antibodies nivolumab have also been used to restrict the suppressive function of Tregs [132]. In addition, the use of sunitinib and sorafenib which targets vascular endothelial growth factor receptor 2 (VEGFR2) reduces the number of peripheral blood Tregs [133].
The Toll-like receptors (TLR) are expressed on human Tregs. Studies showed that agonist TLR signaling (PAM2CSK4, PAM3CSK4, FSL-1) reduces Treg suppressive function via mechanisms involving downregulation of the Cdk-inhibitor p27Kip1 and restoration of the PI3K-Akt pathway [134]. Additionally, blocking Treg trafficking into the tumor site can be achieved by blocking the CCR4/CCL22 axis. Thus, Bayry et al. identified small-molecule chemokine receptor antagonists or mAb that block CCL22-mediated recruitment of human Tregs and Th2 cells in in vitro experiments [135].
4.2. Targeting NK Cells
NK cells have been recognized as promising agents for cell-based cancer therapies. Several clinical studies have been performed with adoptive autologous NK cells and allogeneic NK cell products in an attempt to target breast cancer, lung cancer, lymphoma, glioma, renal cell carcinoma, adenocarcinoma, leukemia, colorectal cancer, hepatocellular cancer, and melanoma [83]. There are other NK cell-based anti-cancer strategies such as genetic modification of NK cells. In this strategy, NK cells are modified to produce cytokines such as IL-2 and IL-15 which increase their survival capacity and proliferation and promote anti-tumor activity in vivo [136,137]. Similarly, to enhance their specificity for the target cells, NK cells can be modified to recognize antigens specifically expressed on the surface of cancer cells [83]. The combination of drug therapy with NK cell stimulating cytokines (IL-2, IL-12, IL-15, and IL-21) or immunomodulatory drugs (IMiDs) can enhance NK cell-mediated tumor killing [138]. Likewise, NK cell infusion may synergize with chemotherapy to enhance tumor targeting and elimination [139].
4.3. Targeting Macrophages
Many preclinical strategies targeting macrophages for suppression of the primary tumor as well as for metastasis are now being evaluated in the clinic, and provide proof of concept that targeting macrophages may enhance current anticancer therapies. Current strategies for targeting TAMs can be characterized into three main categories: depletion, reprogramming and molecular targeting. Given that bone marrow-derived macrophages (BDMs) are recruited to the tumor by chemokine (C–C motif) ligand 2/CCL receptor 2 (CCL2/CCLR2) or colony-stimulating factor-1 (CSF-1)/CSF-1R axis, inhibitors targeting these ligands and receptors have been developed [140]. Preclinical studies showed that blocking the CCL2/CCLR2 axis can suppress the accumulation of TAMs in tumors as well as reduce metastasis in animal models [141,142]. Discontinuation of CCL2 therapy accelerates breast cancer metastasis by promoting tumor angiogenesis [143]. Similarly, targeting CSF-1/CSF-1R signaling by small molecule pexidartinib (PLX3397) and mAb therapy (emactuzumab, cabiralizumab and PD-0360324) have been tested clinically and shown to reduce the number of TAMs in solid tumors as well as prevent metastasis [140,144,145,146]. Several compounds such as trabectedin, clodronate, and zoledronic acid have been demonstrated to deplete macrophages by inducing apoptosis [147,148]. To reprogram the suppressive effects of TAMs, sunitinib, sorafenib and fenretinide [4-hydroxy(phenyl) retinamide] have been used to inhibit STAT3 or STAT6 in macrophages, thereby inhibiting IL-10 secretion or skewing macrophage polarization [149,150]. Moreover, the strategies that exist for converting TAMs to antitumor macrophages including CSF-1R agonists, CD40 antagonists toll-like receptor (TLR) inhibitors, VEGF inhibitors, phosphatidylinositol-4,5-bisphosphate 3-kinase-g (PI3Kg) inhibition, and Class-IIa histone deacetylase (HDAC) inhibition, have been shown to result in macrophage-mediated reduction in primary tumor burden and distant metastasis [148].
4.4. Targeting Myeloid-Derived Suppressor Cells
MDSCs exhibit immunosuppressive activity by blocking the proliferation and activity of both T and NK cells [151]. Thus, MDSCs are a promising target in anticancer therapy. Currently, there are a variety of different therapeutic strategies that have been developed to target MDSCs including the following: (1) anti-Gr-1 antibodies and peptibodies that target membrane proteins on MDSCs eliminate MDSCs in various murine tumor models [152]; (2) chemotherapeutic agents (5FU, paclitaxel, gemcitabine, cisplatin, docetaxel and lurbinectedin), phosphodiesterase 5 (PDE-5) inhibitors (sildenafil, tadalafil and vardenafil), vemurafenib as well as zoledronic acid cause MDSC apoptosis thus reducing circulating MDSCs in patients [153,154]; (3) mTOR inhibitors (rapamycin) or STAT3 inhibitors (AG490, CPA7, S3I-201, and stattic) deactivate MDSCs [155,156]; (4) all-trans-retinoic acid (ATRA) or vitamin D promote the MDSC differentiation s into mature, non-suppressive cells such as macrophages and DCs [157]; (5) the COX2 inhibitor (celecoxib), PDE-5 inhibitors and nonsteroidal anti-inflammatory drugs NSAID (nitroaspirin) inhibit the suppressive function of MDSCs [158]; (6) tyrosine kinase inhibitors (sunitinib and sorafenib) inhibit both hematopoiesis and MDSCs production [154,159]; (7) antagonists for chemokine receptors (CCR2, CXCR2 and CXCR4) or chemokines (CCL2, CXCL5 and CXCL12) inhibitors prevent the recruitment of MDSCs from the bone marrow into tumor microenvironment [154,160,161].
4.5. Targeting Dendritic Cells and Tumor-Associated Neutrophils
Given that immature and/or dysfunctional DCs with immunosuppressive effects tend to accumulate in the tumor microenvironment, the ability to convert dysfunctional DCs into functional DCs could be a potential approach to enhance therapeutic efficacy. Studies have demonstrated that two families of microtubule destabilizing agents (dolastatin 10 and ansamitocin P3) can switch DCs from immunosuppressive to immune activating by provoking phenotypic and functional DC maturation [162]. Furthermore, vaccine immunotherapy with DCs is reported to prevent tumor metastasis [163]. Additionally, the therapeutic combination of mature DCs with 5-FU has been reported to suppress tumor metastasis [163].
Since TANs also act as a protumoral immune cells in many human cancers, targeting TANs may be a potential anticancer treatment strategy. Clinical studies found that CXCR2 or IL-17 inhibition could reduce neutrophil migration into the tumor [164,165]. In addition, the combination of immunotherapy with sunitinib could increase antitumor efficacy by interfering with immunosuppressive neutrophils in renal cell carcinoma patients [166]. Furthermore, since infiltrating neutrophils are driven by TGF-β to acquire a polarized N2 pro-tumor phenotype. Blockage of TGF-β leads to a shift from N2 to N1 phenotype of neutrophils with subsequent acquisition of antitumor activity [122].
5. Other Drugs Inhibiting Bone Metastasis
As described above, RANKL, which is secreted by bone marrow stromal cells, osteocytes, and tumor cells, is an essential mediator of osteoclast activity and osteolytic lesions, and is important for stimulating metastatic growth in the bone. Denosumab is a human monoclonal antibody that binds to and neutralizes human RANKL, inhibiting osteoclast activity and osteoclast-mediated bone destruction [167]. Many studies have been shown that denosumab reduce the frequency of skeletal-related events (SREs, defined as pathological fractures, surgery or radiation to bone, or spinal cord compression) in patients with advanced solid tumors, and increase their quality-adjusted life years [168,169,170]. Therefore, osteoclast inhibition with denosumab has provided an improvement for the management of patients with solid tumors and bone metastasis. Additionally, TGF-β, which secreted by osteoclasts and tumor cells, is also an important driver of tumor growth and immune suppression. Thus, inhibitors of TGF-β may be effective enhancers of antitumor immune responses and ultimately prevent bone metastasis. Galunisertib, a small molecule inhibitor of TGFβ receptor I (TGFβRI) [171], has been shown to inhibition of TGFβ-mediated immune-suppression decreasing tumor growth and bone metastasis [172,173,174,175].
6. Conclusions
Bone metastasis is a frequent occurrence in cancer patients, particularly patients with breast and prostate cancer. The abundant and specific cellular and molecular niches (such as hypoxia and tumor-derived factors) in the bone microenvironment, including high levels of multiple immune cell types, may impact tumor-to-bone metastasis, as well as contribute to bone pathologies in patients with bone metastasis [176,177]. Therefore, understanding the immune regulatory mechanisms in the bone marrow microenvironment is important for the development of cancer therapies. There are many promising therapeutic options based on reprogramming immune cells in bone marrow in current pre-clinical and clinical trials that give hope for improved treatments and outcomes in patients with metastatic bone cancer (Table 1). Furthermore, the combination of immunotherapy and conventional chemotherapy may synergistically result in reduced tumor progression and metastasis, as well as prolonged survival for cancer patients. Enhancing our understanding of this field will be required to develop effective therapeutic strategies that are urgently needed.
Table 1.
The role of immune cells in cancer progression and metastasis, and their therapeutic strategies.
| Immune Cells | Functions | Therapeutic Strategies | |
|---|---|---|---|
| Anti-Tumoral | Pro-Tumoral | ||
| CD8+ T cells | Recognize and eliminate cancer cells | — | Enhance CD8+ T cells immune response by vaccination or engineering the T cell receptor or chimeric antigen receptor |
| Tregs | — | Suppress immune response of cytotoxic T cells and NK cells that promote cancer cells survival and metastasis | Suppression of Tregs by chemotherapy with/or anti-CD25 treatment, anti-CTLA-4 antagonist mAb, anti-PD-1 antibodies, tyrosine kinase inhibitors, PI3K-Akt inhibitors, toll-like receptors inhibitors, CCR4/CCL22 antagonists |
| NK cells | Recognize and eliminate cancer cells | — | Stimulation of NK cells by cytokines such as IL-2, IL-15 and IL-21, or immunomodulatory drugs (IMiDs), or genetic modification of NK cells |
| Macrophages | Killing of cancer cells directly; Antigen-presenting function | Immunosuppression functions: TAMs promote survival and metastasis of cancer cells and angiogenesis | Suppress the accumulation of TAMs by CSF-1 inhibitors, CCL2 inhibitors; Depletion of TAMs by trabectedin, clodronate, and zoledronic acid; Reprogramming of TAMs by CD40 antagonism, toll-like receptor inhibitors, VEGF inhibitors, PI3Kg and HDAC inhibition, tyrosine kinase inhibitors, and fenretinide |
| Dendritic cells | Antigen presentation to T cells to stimulate immune response | Suppress the cytotoxic capacity of T cells and recruit immunosuppressive immune cells to promote tumor progression and metastasis | Targeting of DCs by microtubule destabilizing agents (dolastatin 10 and ansamitocin P3), chemotherapy, and vaccine immunotherapy |
| MDSCs | — | Suppress T cell functions; Regulate the immunosuppression; Stimulate angiogenesis and lymphangiogenesis | Targeting of MDSCs by chemotherapy, PDE-5 inhibitors, mTOR inhibitors, STAT3 inhibitors, COX2 inhibitors, tyrosine kinase inhibitors, chemokine receptors antagonists, peptibodies, vemurafenib, zoledronic acid, differentiation agents (ATRA), and vitamin D |
| Neutrophils | Attack cancer cells through antigen 1-dependent recognition | Release protumoral factors including CXCR4, VEGF and MMP9 to promote metastasis | Targeting of TANs by CXCR2 or IL-17 inhibition, or combination of immunotherapy with sunitinib |
Acknowledgments
The authors would like to acknowledge the researchers who contributed to the work cited in this review and to apologize for any work that was not cited due to space constraints. Work in the author’s laboratory is supported by U54-CA210173, R00-CA181352, V Scholar Foundation, Susan G. Komen Foundation (CCR17483484), The Jayne Koskinas Ted Giovanis Foundation for Health and Policy and the Breast Cancer Research Foundation, and The Emerson Collective.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Soeharno H., Povegliano L., Choong P.F. Multimodal Treatment of Bone Metastasis—A Surgical Perspective. Front. Endocrinol. 2018;9:578. doi: 10.3389/fendo.2018.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingsley L.A., Fournier P.G.J., Chirgwin J.M., Guise T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 3.Janssen L.M.E., Ramsay E.E., Logsdon C.D., Overwijk W.W. The immune system in cancer metastasis: Friend or foe? J. Immunother. Cancer. 2017;5:79. doi: 10.1186/s40425-017-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith H.A., Kang Y.B. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013;91:411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capietto A.H., Faccio R. Immune regulation of bone metastasis. Bonekey Rep. 2014;3:600. doi: 10.1038/bonekey.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundy G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 7.Sleeman J.P. The metastatic niche and stromal progression. Cancer Metastasis Rev. 2012;31:429–440. doi: 10.1007/s10555-012-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidler I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 9.Marks S.C., Jr., Popoff S.N. Bone cell biology: The regulation of development, structure, and function in the skeleton. Am. J. Anat. 1988;183:1–44. doi: 10.1002/aja.1001830102. [DOI] [PubMed] [Google Scholar]
- 10.Bussard K.M., Gay C.V., Mastro A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 11.Suva L.J., Washam C., Nicholas R.W., Griffin R.J. Bone metastasis: Mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchie C.K., Andrews L.R., Thomas K.G., Tindall D.J., Fitzpatrick L.A. The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis: Implications for the development of metastatic disease. Endocrinology. 1997;138:1145–1150. doi: 10.1210/endo.138.3.4974. [DOI] [PubMed] [Google Scholar]
- 13.Hall C.L., Bafico A., Dai J., Aaronson S.A., Keller E.T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 14.Sottnik J.L., Hall C.L., Zhang J., Keller E.T. Wnt and Wnt inhibitors in bone metastasis. BoneKEy Rep. 2012;1:101. doi: 10.1038/bonekey.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddington J.A., Mendez G.A., Ching A., Kubicky C.D., Klimo P., Jr., Ragel B.T. Imaging characteristic analysis of metastatic spine lesions from breast, prostate, lung, and renal cell carcinomas for surgical planning: Osteolytic versus osteoblastic. Surg. Neurol. Int. 2016;7(Suppl. 13):S361–S365. doi: 10.4103/2152-7806.182549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M.Y., Li C.J., Yiang G.T., Cheng Y.L., Tsai A.P., Hou Y.T., Ho Y.C., Hou M.F., Chu P.Y. Molecular Regulation of Bone Metastasis Pathogenesis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;46:1423–1438. doi: 10.1159/000489184. [DOI] [PubMed] [Google Scholar]
- 17.Lipton A. Future treatment of bone metastases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006;12:6305s–6308s. doi: 10.1158/1078-0432.CCR-06-1157. [DOI] [PubMed] [Google Scholar]
- 18.Loberg R.D., Logothetis C.J., Keller E.T., Pienta K.J. Pathogenesis and treatment of prostate cancer bone metastases: Targeting the lethal phenotype. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23:8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- 19.Michigami T., Shimizu N., Williams P.J., Niewolna M., Dallas S.L., Mundy G.R., Yoneda T. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–1960. [PubMed] [Google Scholar]
- 20.Chavez-Macgregor M., Aviles-Salas A., Green D., Fuentes-Alburo A., Gomez-Ruiz C., Aguayo A. Angiogenesis in the bone marrow of patients with breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005;11:5396–5400. doi: 10.1158/1078-0432.CCR-04-2420. [DOI] [PubMed] [Google Scholar]
- 21.Philippe C., Philippe B., Fouqueray B., Perez J., Lebret M., Baud L. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am. J. Pathol. 1993;143:1713–1723. [PMC free article] [PubMed] [Google Scholar]
- 22.Palumbo J.S., Talmage K.E., Massari J.V., La Jeunesse C.M., Flick M.J., Kombrinck K.W., Jirouskova M., Degen J.L. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 23.Akfirat C., Zhang X., Ventura A., Berel D., Colangelo M.E., Miranti C.K., Krajewska M., Reed J.C., Higano C.S., True L.D., et al. Tumour cell survival mechanisms in lethal metastatic prostate cancer differ between bone and soft tissue metastases. J. Pathol. 2013;230:291–297. doi: 10.1002/path.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubenik J. Tumour MHC class I downregulation and immunotherapy (Review) Oncol. Rep. 2003;10:2005–2008. doi: 10.3892/or.10.6.2005. [DOI] [PubMed] [Google Scholar]
- 25.Baschuk N., Rautela J., Parker B.S. Bone specific immunity and its impact on metastasis. BoneKEy Rep. 2015;4:665. doi: 10.1038/bonekey.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., McClanahan T., Murphy E., Yuan W., Wagner S.N., et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 27.Golan K., Kollet O., Lapidot T. Dynamic Cross Talk between S1P and CXCL12 Regulates Hematopoietic Stem Cells Migration, Development and Bone Remodeling. Pharmaceuticals. 2013;6:1145–1169. doi: 10.3390/ph6091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiozawa Y., Havens A.M., Jung Y., Ziegler A.M., Pedersen E.A., Wang J., Wang J., Lu G., Roodman G.D., Loberg R.D., et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J. Cell. Biochem. 2008;105:370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunasinghe N.P., Wells A., Thompson E.W., Hugo H.J. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012;31:469–478. doi: 10.1007/s10555-012-9377-5. [DOI] [PubMed] [Google Scholar]
- 30.Wang H., Yu C., Gao X., Welte T., Muscarella A.M., Tian L., Zhao H., Zhao Z., Du S., Tao J., et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27:193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones D.H., Nakashima T., Sanchez O.H., Kozieradzki I., Komarova S.V., Sarosi I., Morony S., Rubin E., Sarao R., Hojilla C.V., et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 32.Arrigoni C., De Luca P., Gilardi M., Previdi S., Broggini M., Moretti M. Direct but not indirect co-culture with osteogenically differentiated human bone marrow stromal cells increases RANKL/OPG ratio in human breast cancer cells generating bone metastases. Mol. Cancer. 2014;13:238. doi: 10.1186/1476-4598-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Bachelier R., Treilleux I., Pujuguet P., Peyruchaud O., Baron R., Clement-Lacroix P., Clezardin P. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 34.Clezardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr. Cancer Drug Targets. 2009;9:801–806. doi: 10.2174/156800909789760348. [DOI] [PubMed] [Google Scholar]
- 35.Dhawan A., Friedrichs J., Bonin M.V., Bejestani E.P., Werner C., Wobus M., Chavakis T., Bornhauser M. Breast cancer cells compete with hematopoietic stem and progenitor cells for intercellular adhesion molecule 1-mediated binding to the bone marrow microenvironment. Carcinogenesis. 2016;37:759–767. doi: 10.1093/carcin/bgw057. [DOI] [PubMed] [Google Scholar]
- 36.Ghajar C.M. Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer. 2015;15:238–247. doi: 10.1038/nrc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croucher P.I., McDonald M.M., Martin T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer. 2016;16:373–386. doi: 10.1038/nrc.2016.44. [DOI] [PubMed] [Google Scholar]
- 38.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H., Almeida D., Koller A., Hajjar K.A., Stainier D.Y., et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragado P., Estrada Y., Parikh F., Krause S., Capobianco C., Farina H.G., Schewe D.M., Aguirre-Ghiso J.A. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat. Cell Biol. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S., Xing F., Liu Y., Wu K., Said N., Pochampally R., Shiozawa Y., Lin H.K., Balaji K.C., Watabe K. Secreted Protein Acidic and Rich in Cysteine (SPARC) Mediates Metastatic Dormancy of Prostate Cancer in Bone. J. Biol. Chem. 2016;291:19351–19363. doi: 10.1074/jbc.M116.737379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler I.G., Barbier V., Nowlan B., Jacobsen R.N., Forristal C.E., Patton J.T., Magnani J.L., Levesque J.P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 42.Lu X., Mu E., Wei Y., Riethdorf S., Yang Q., Yuan M., Yan J., Hua Y., Tiede B.J., Lu X., et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams G.B., Chabner K.T., Alley I.R., Olson D.P., Szczepiorkowski Z.M., Poznansky M.C., Kos C.H., Pollak M.R., Brown E.M., Scadden D.T. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 44.Buijs J.T., Stayrook K.R., Guise T.A. The role of TGF-beta in bone metastasis: Novel therapeutic perspectives. BoneKEy Rep. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Pape F., Vargas G., Clezardin P. The role of osteoclasts in breast cancer bone metastasis. J. Bone Oncol. 2016;5:93–95. doi: 10.1016/j.jbo.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawant A., Hensel J.A., Chanda D., Harris B.A., Siegal G.P., Maheshwari A., Ponnazhagan S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 2012;189:4258–4265. doi: 10.4049/jimmunol.1101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roato I., Grano M., Brunetti G., Colucci S., Mussa A., Bertetto O., Ferracini R. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005;19:228–230. doi: 10.1096/fj.04-1823fje. [DOI] [PubMed] [Google Scholar]
- 49.Teng Y.T., Nguyen H., Gao X., Kong Y.Y., Gorczynski R.M., Singh B., Ellen R.P., Penninger J.M. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 2000;106:R59–R67. doi: 10.1172/JCI10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., Takaoka A., Yokochi T., Oda H., Tanaka K., et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y., Grassi F., Ryan M.R., Terauchi M., Page K., Yang X., Weitzmann M.N., Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Investig. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Z., Hurchla M.A., Deng H., Uluckan O., Bu F., Berdy A., Eagleton M.C., Heller E.A., Floyd D.H., Dirksen W.P., et al. Interferon-gamma targets cancer cells and osteoclasts to prevent tumor-associated bone loss and bone metastases. J. Biol. Chem. 2009;284:4658–4666. doi: 10.1074/jbc.M804812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiesel J.R., Buchwald Z.S., Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J. Immunol. 2009;182:5477–5487. doi: 10.4049/jimmunol.0803897. [DOI] [PubMed] [Google Scholar]
- 54.D’Amico L., Roato I. The Impact of Immune System in Regulating Bone Metastasis Formation by Osteotropic Tumors. J. Immunol. Res. 2015;2015:143526. doi: 10.1155/2015/143526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang M.K., Raggatt L.J., Alexander K.A., Kuliwaba J.S., Fazzalari N.L., Schroder K., Maylin E.R., Ripoll V.M., Hume D.A., Pettit A.R. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 56.Wu A.C., He Y., Broomfield A., Paatan N.J., Harrington B.S., Tseng H.W., Beaven E.A., Kiernan D.M., Swindle P., Clubb A.B., et al. CD169(+) macrophages mediate pathological formation of woven bone in skeletal lesions of prostate cancer. J. Pathol. 2016;239:218–230. doi: 10.1002/path.4718. [DOI] [PubMed] [Google Scholar]
- 57.Dejbakhsh-Jones S., Jerabek L., Weissman I.L., Strober S. Extrathymic maturation of alpha beta T cells from hemopoietic stem cells. J. Immunol. 1995;155:3338–3344. [PubMed] [Google Scholar]
- 58.Schirrmacher V., Feuerer M., Fournier P., Ahlert T., Umansky V., Beckhove P. T-cell priming in bone marrow: The potential for long-lasting protective anti-tumor immunity. Trends Mol. Med. 2003;9:526–534. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Feuerer M., Beckhove P., Mahnke Y., Hommel M., Kyewski B., Hamann A., Umansky V., Schirrmacher V. Bone marrow microenvironment facilitating dendritic cell: CD4 T cell interactions and maintenance of CD4 memory. Int. J. Oncol. 2004;25:867–876. [PubMed] [Google Scholar]
- 60.Mazo I.B., Honczarenko M., Leung H., Cavanagh L.L., Bonasio R., Weninger W., Engelke K., Xia L., McEver R.P., Koni P.A., et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Zeng D., Hoffmann P., Lan F., Huie P., Higgins J., Strober S. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: Impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.V99.4.1449. [DOI] [PubMed] [Google Scholar]
- 62.Price P.W., Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCR Vbeta repertoire. Eur. J. Immunol. 1999;29:1051–1056. doi: 10.1002/(SICI)1521-4141(199903)29:03<1051::AID-IMMU1051>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 63.Fournier P.G., Chirgwin J.M., Guise T.A. New insights into the role of T cells in the vicious cycle of bone metastases. Curr. Opin. Rheumatol. 2006;18:396–404. doi: 10.1097/01.bor.0000231909.35043.da. [DOI] [PubMed] [Google Scholar]
- 64.Najafi M., Farhood B., Mortezaee K. Contribution of regulatory T cells to cancer: A review. J. Cell. Physiol. 2018 doi: 10.1002/jcp.27553. [DOI] [PubMed] [Google Scholar]
- 65.Bates G.J., Fox S.B., Han C., Leek R.D., Garcia J.F., Harris A.L., Banham A.H. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 66.Wolf A.M., Wolf D., Steurer M., Gastl G., Gunsilius E., Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 67.Zhao E., Wang L., Dai J., Kryczek I., Wei S., Vatan L., Altuwaijri S., Sparwasser T., Wang G., Keller E.T., et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–161. doi: 10.4161/onci.1.2.18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou L., Barnett B., Safah H., Larussa V.F., Evdemon-Hogan M., Mottram P., Wei S., David O., Curiel T.J., Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 69.Wei S., Kryczek I., Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei S., Kryczek I., Edwards R.P., Zou L., Szeliga W., Banerjee M., Cost M., Cheng P., Chang A., Redman B., et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 71.Tan W., Zhang W., Strasner A., Grivennikov S., Cheng J.Q., Hoffman R.M., Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakashima T. Bone metastasis and RANKL. Clin. Calcium. 2014;24:1201–1208. [PubMed] [Google Scholar]
- 73.Roy L.D., Ghosh S., Pathangey L.B., Tinder T.L., Gruber H.E., Mukherjee P. Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer. 2011;11:365. doi: 10.1186/1471-2407-11-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okamoto K., Takayanagi H. Regulation of bone by the adaptive immune system in arthritis. Arthritis Res. Ther. 2011;13:219. doi: 10.1186/ar3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monteiro A.C., Leal A.C., Goncalves-Silva T., Mercadante A.C., Kestelman F., Chaves S.B., Azevedo R.B., Monteiro J.P., Bonomo A. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE. 2013;8:e68171. doi: 10.1371/journal.pone.0068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W., de Zoete M.R., Licona-Limon P., Paiva R.S., Ching T., et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng D., Gazit G., Dejbakhsh-Jones S., Balk S.P., Snapper S., Taniguchi M., Strober S. Heterogeneity of NK1.1+ T cells in the bone marrow: Divergence from the thymus. J. Immunol. 1999;163:5338–5345. [PubMed] [Google Scholar]
- 78.Higuchi M., Zeng D., Shizuru J., Gworek J., Dejbakhsh-Jones S., Taniguchi M., Strober S. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J. Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 79.Wu J., Lanier L.L. Natural killer cells and cancer. Adv. Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 80.Bidwell B.N., Slaney C.Y., Withana N.P., Forster S., Cao Y., Loi S., Andrews D., Mikeska T., Mangan N.E., Samarajiwa S.A., et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 2012;18:1224–1231. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- 81.Paolino M., Choidas A., Wallner S., Pranjic B., Uribesalgo I., Loeser S., Jamieson A.M., Langdon W.Y., Ikeda F., Fededa J.P., et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okamoto T., Yoneyama M.S., Hatakeyama S., Mori K., Yamamoto H., Koie T., Saitoh H., Yamaya K., Funyu T., Fukuda M., et al. Core2 O-glycan-expressing prostate cancer cells are resistant to NK cell immunity. Mol. Med. Rep. 2013;7:359–364. doi: 10.3892/mmr.2012.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dahlberg C.I., Sarhan D., Chrobok M., Duru A.D., Alici E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front. Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe M., Kono K., Kawaguchi Y., Mizukami Y., Mimura K., Maruyama T., Izawa S., Fujii H. NK cell dysfunction with down-regulated CD16 and up-regulated CD56 molecules in patients with esophageal squamous cell carcinoma. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus. 2010;23:675–681. doi: 10.1111/j.1442-2050.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 85.Izawa S., Kono K., Mimura K., Kawaguchi Y., Watanabe M., Maruyama T., Fujii H. H(2)O(2) production within tumor microenvironment inversely correlated with infiltration of CD56(dim) NK cells in gastric and esophageal cancer: Possible mechanisms of NK cell dysfunction. Cancer Immunol. Immunother. CII. 2011;60:1801–1810. doi: 10.1007/s00262-011-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinder B.P., Pettit A.R., McCauley L.K. Macrophages: Their Emerging Roles in Bone. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015;30:2140–2149. doi: 10.1002/jbmr.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mantovani A., Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Reviews. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karnevi E., Andersson R., Rosendahl A.H. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol. Cell Biol. 2014;92:543–552. doi: 10.1038/icb.2014.22. [DOI] [PubMed] [Google Scholar]
- 91.Lee H.W., Choi H.J., Ha S.J., Lee K.T., Kwon Y.G. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. Biophys. Acta. 2013;1835:170–179. doi: 10.1016/j.bbcan.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 92.Zabuawala T., Taffany D.A., Sharma S.M., Merchant A., Adair B., Srinivasan R., Rosol T.J., Fernandez S., Huang K., Leone G., et al. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–1333. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawa-Wejksza K., Kandefer-Szerszen M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. 2018;66:97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 95.Grossman J.G., Nywening T.M., Belt B.A., Panni R.Z., Krasnick B.A., DeNardo D.G., Hawkins W.G., Goedegebuure S.P., Linehan D.C., Fields R.C. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology. 2018;7:e1470729. doi: 10.1080/2162402X.2018.1470729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu F., Cui W.Q., Wei Y., Cui J., Qiu J., Hu L.L., Gong W.Y., Dong J.C., Liu B.J. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018;37:207. doi: 10.1186/s13046-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao H., Li L., Zhang J., Zheng C., Ding K., Xiao H., Wang L., Zhang Z. C-C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles to Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl. Mater. Interfaces. 2018;10:31124–31135. doi: 10.1021/acsami.8b11645. [DOI] [PubMed] [Google Scholar]
- 98.Lo C.H., Lynch C.C. Multifaceted Roles for Macrophages in Prostate Cancer Skeletal Metastasis. Front. Endocrinol. 2018;9:247. doi: 10.3389/fendo.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soki F.N., Cho S.W., Kim Y.W., Jones J.D., Park S.I., Koh A.J., Entezami P., Daignault-Newton S., Pienta K.J., Roca H., et al. Bone marrow macrophages support prostate cancer growth in bone. Oncotarget. 2015;6:35782–35796. doi: 10.18632/oncotarget.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindholm P.F., Lu Y., Adley B.P., Vladislav T., Jovanovic B., Sivapurapu N., Yang X.J., Kajdacsy-Balla A. Role of monocyte-lineage cells in prostate cancer cell invasion and tissue factor expression. Prostate. 2010;70:1672–1682. doi: 10.1002/pros.21202. [DOI] [PubMed] [Google Scholar]
- 101.Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332:3–10. doi: 10.1016/j.canlet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 102.Lu X., Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem. 2009;284:29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sullivan A.R., Pixley F.J. CSF-1R signaling in health and disease: A focus on the mammary gland. J. Mammary Gland Biol. Neoplasia. 2014;19:149–159. doi: 10.1007/s10911-014-9320-1. [DOI] [PubMed] [Google Scholar]
- 104.DeNardo D.G., Brennan D.J., Rexhepaj E., Ruffell B., Shiao S.L., Madden S.F., Gallagher W.M., Wadhwani N., Keil S.D., Junaid S.A., et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fend L., Accart N., Kintz J., Cochin S., Reymann C., Le Pogam F., Marchand J.B., Menguy T., Slos P., Rooke R., et al. Therapeutic effects of anti-CD115 monoclonal antibody in mouse cancer models through dual inhibition of tumor-associated macrophages and osteoclasts. PLoS ONE. 2013;8:e73310. doi: 10.1371/journal.pone.0073310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schuler G., Steinman R.M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porgador A., Snyder D., Gilboa E. Induction of antitumor immunity using bone marrow-generated dendritic cells. J. Immunol. 1996;156:2918–2926. [PubMed] [Google Scholar]
- 108.Abdul Hafid S.R., Chakravarthi S., Nesaretnam K., Radhakrishnan A.K. Tocotrienol-adjuvanted dendritic cells inhibit tumor growth and metastasis: A murine model of breast cancer. PLoS ONE. 2013;8:e74753. doi: 10.1371/journal.pone.0074753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cavanagh L.L., Bonasio R., Mazo I.B., Halin C., Cheng G., van der Velden A.W., Cariappa A., Chase C., Russell P., Starnbach M.N., et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gabrilovich D.I., Corak J., Ciernik I.F., Kavanaugh D., Carbone D.P. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 111.Shurin M.R., Naiditch H., Zhong H., Shurin G.V. Regulatory dendritic cells: New targets for cancer immunotherapy. Cancer Biol. Ther. 2011;11:988–992. doi: 10.4161/cbt.11.11.15543. [DOI] [PubMed] [Google Scholar]
- 112.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Reviews. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sawant A., Ponnazhagan S. Myeloid-derived suppressor cells as osteoclast progenitors: A novel target for controlling osteolytic bone metastasis. Cancer Res. 2013;73:4606–4610. doi: 10.1158/0008-5472.CAN-13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Danilin S., Merkel A.R., Johnson J.R., Johnson R.W., Edwards J.R., Sterling J.A. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology. 2012;1:1484–1494. doi: 10.4161/onci.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhuang J., Zhang J., Lwin S.T., Edwards J.R., Edwards C.M., Mundy G.R., Yang X. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PLoS ONE. 2012;7:e48871. doi: 10.1371/journal.pone.0048871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sawant A., Deshane J., Jules J., Lee C.M., Harris B.A., Feng X., Ponnazhagan S. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013;73:672–682. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jenne C.N., Liao S., Singh B. Neutrophils: Multitasking first responders of immunity and tissue homeostasis. Cell Tissue Res. 2018;371:395–397. doi: 10.1007/s00441-018-2802-5. [DOI] [PubMed] [Google Scholar]
- 118.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 119.Martin C., Burdon P.C., Bridger G., Gutierrez-Ramos J.C., Williams T.J., Rankin S.M. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/S1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 120.Di Carlo E., Forni G., Lollini P., Colombo M.P., Modesti A., Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.V97.2.339. [DOI] [PubMed] [Google Scholar]
- 121.Shaul M.E., Levy L., Sun J., Mishalian I., Singhal S., Kapoor V., Horng W., Fridlender G., Albelda S.M., Fridlender Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFbeta modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology. 2016;5:e1232221. doi: 10.1080/2162402X.2016.1232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schernberg A., Blanchard P., Chargari C., Deutsch E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol. 2017;56:1522–1530. doi: 10.1080/0284186X.2017.1348623. [DOI] [PubMed] [Google Scholar]
- 124.Liu Y., Gu Y., Han Y., Zhang Q., Jiang Z., Zhang X., Huang B., Xu X., Zheng J., Cao X. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 125.Chua W., Charles K.A., Baracos V.E., Clarke S.J. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br. J. Cancer. 2011;104:1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee S., Oh S.Y., Kim S.H., Lee J.H., Kim M.C., Kim K.H., Kim H.J. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang S., Zhang Z., Fang F., Gao X., Sun W., Liu H. The neutrophil/lymphocyte ratio is an independent prognostic indicator in patients with bone metastasis. Oncol. Lett. 2011;2:735–740. doi: 10.3892/ol.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. CII. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shaffer J., Villard J., Means T.K., Alexander S., Dombkowski D., Dey B.R., McAfee S., Ballen K.K., Saidman S., Preffer F.I., et al. Regulatory T-cell recovery in recipients of haploidentical nonmyeloablative hematopoietic cell transplantation with a humanized anti-CD2 mAb, MEDI-507, with or without fludarabine. Exp. Hematol. 2007;35:1140–1152. doi: 10.1016/j.exphem.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L., Dermawan K., Jin M., Liu R., Zheng H., Xu L., Zhang Y., Cai Y., Chu Y., Xiong S. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin. Immunol. 2008;129:219–229. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 131.Rech A.J., Vonderheide R.H. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. N. Y. Acad. Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 132.Wang S.D., Li H.Y., Li B.H., Xie T., Zhu T., Sun L.L., Ren H.Y., Ye Z.M. The role of CTLA-4 and PD-1 in anti-tumor immune response and their potential efficacy against osteosarcoma. Int. Immunopharmacol. 2016;38:81–89. doi: 10.1016/j.intimp.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 133.Busse A., Asemissen A.M., Nonnenmacher A., Braun F., Ochsenreither S., Stather D., Fusi A., Schmittel A., Miller K., Thiel E., et al. Immunomodulatory effects of sorafenib on peripheral immune effector cells in metastatic renal cell carcinoma. Eur. J. Cancer. 2011;47:690–696. doi: 10.1016/j.ejca.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 134.Oberg H.H., Juricke M., Kabelitz D., Wesch D. Regulation of T cell activation by TLR ligands. Eur. J. Cell Biol. 2011;90:582–592. doi: 10.1016/j.ejcb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 135.Bayry J., Tchilian E.Z., Davies M.N., Forbes E.K., Draper S.J., Kaveri S.V., Hill A.V., Kazatchkine M.D., Beverley P.C., Flower D.R., et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc. Natl. Acad. Sci. USA. 2008;105:10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klingemann H. Improving natural killer cells. Cytotherapy. 2008;10:225–226. doi: 10.1080/14653240802028376. [DOI] [PubMed] [Google Scholar]
- 137.Konstantinidis K.V., Alici E., Aints A., Christensson B., Ljunggren H.G., Dilber M.S. Targeting IL-2 to the endoplasmic reticulum confines autocrine growth stimulation to NK-92 cells. Exp. Hematol. 2005;33:159–164. doi: 10.1016/j.exphem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Romee R., Leong J.W., Fehniger T.A. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014;2014:205796. doi: 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miller J.S., Soignier Y., Panoskaltsis-Mortari A., McNearney S.A., Yun G.H., Fautsch S.K., McKenna D., Le C., Defor T.E., Burns L.J., et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 140.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I., et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 141.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kitamura T., Qian B.Z., Soong D., Cassetta L., Noy R., Sugano G., Kato Y., Li J., Pollard J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bonapace L., Coissieux M.M., Wyckoff J., Mertz K.D., Varga Z., Junt T., Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 144.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Linde N., Casanova-Acebes M., Sosa M.S., Mortha A., Rahman A., Farias E., Harper K., Tardio E., Reyes Torres I., Jones J., et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 2018;9:21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cannarile M.A., Weisser M., Jacob W., Jegg A.M., Ries C.H., Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.D’Incalci M., Badri N., Galmarini C.M., Allavena P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br. J. Cancer. 2014;111:646–650. doi: 10.1038/bjc.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guerriero J.L. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol. Med. 2018;24:472–489. doi: 10.1016/j.molmed.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dong R., Gong Y., Meng W., Yuan M., Zhu H., Ying M., He Q., Cao J., Yang B. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer. Cancer Lett. 2017;388:43–53. doi: 10.1016/j.canlet.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 150.Edwards J.P., Emens L.A. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE(2) in murine macrophages. Int. Immunopharmacol. 2010;10:1220–1228. doi: 10.1016/j.intimp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bronte V., Brandau S., Chen S.H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S., et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qin H., Lerman B., Sakamaki I., Wei G., Cha S.C., Rao S.S., Qian J., Hailemichael Y., Nurieva R., Dwyer K.C., et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat. Med. 2014;20:676–681. doi: 10.1038/nm.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kuroda H., Mabuchi S., Kozasa K., Yokoi E., Matsumoto Y., Komura N., Kawano M., Hashimoto K., Sawada K., Kimura T. PM01183 inhibits myeloid-derived suppressor cells in vitro and in vivo. Immunotherapy. 2017;9:805–817. doi: 10.2217/imt-2017-0046. [DOI] [PubMed] [Google Scholar]
- 154.Mabuchi S., Yokoi E., Komura N., Kimura T. Myeloid-derived suppressor cells and their role in gynecological malignancies. Tumour Biol. 2018;40 doi: 10.1177/1010428318776485. [DOI] [PubMed] [Google Scholar]
- 155.Bu L.L., Li Y.C., Yu G.T., Liu J.F., Deng W.W., Zhang W.F., Zhang L., Sun Z.J. Targeting phosphorylation of STAT3 delays tumor growth in HPV-negative anal squamous cell carcinoma mouse model. Sci. Rep. 2017;7:6629. doi: 10.1038/s41598-017-06643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu T., Zhao Y., Wang H., Li Y., Shao L., Wang R., Lu J., Yang Z., Wang J., Zhao Y. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci. Rep. 2016;6:20250. doi: 10.1038/srep20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chesney J.A., Mitchell R.A., Yaddanapudi K. Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy. J. Leukoc. Biol. 2017;102:727–740. doi: 10.1189/jlb.5VMR1116-458RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wesolowski R., Markowitz J., Carson W.E., 3rd Myeloid derived suppressor cells—A new therapeutic target in the treatment of cancer. J. Immunother. Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heine A., Schilling J., Grunwald B., Kruger A., Gevensleben H., Held S.A., Garbi N., Kurts C., Brossart P., Knolle P., et al. The induction of human myeloid derived suppressor cells through hepatic stellate cells is dose-dependently inhibited by the tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but not sunitinib. Cancer Immunol. Immunother. CII. 2016;65:273–282. doi: 10.1007/s00262-015-1790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang L., Huang J., Ren X., Gorska A.E., Chytil A., Aakre M., Carbone D.P., Matrisian L.M., Richmond A., Lin P.C., et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zollo M., Di Dato V., Spano D., De Martino D., Liguori L., Marino N., Vastolo V., Navas L., Garrone B., Mangano G., et al. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin. Exp. Metastasis. 2012;29:585–601. doi: 10.1007/s10585-012-9473-5. [DOI] [PubMed] [Google Scholar]
- 162.Muller P., Martin K., Theurich S., von Bergwelt-Baildon M., Zippelius A. Cancer chemotherapy agents target intratumoral dendritic cells to potentiate antitumor immunity. Oncoimmunology. 2014;3:e954460. doi: 10.4161/21624011.2014.954460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Luo G., He Y., Zhao Q., Yu X. Immune Cells Act as Promising Targets for the Treatment of Bone Metastasis. Recent Pat. Anti-Cancer Drug Discov. 2017;12:221–233. doi: 10.2174/1574892812666170606123113. [DOI] [PubMed] [Google Scholar]
- 164.Rennard S.I., Dale D.C., Donohue J.F., Kanniess F., Magnussen H., Sutherland E.R., Watz H., Lu S., Stryszak P., Rosenberg E., et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015;191:1001–1011. doi: 10.1164/rccm.201405-0992OC. [DOI] [PubMed] [Google Scholar]
- 165.Benevides L., da Fonseca D.M., Donate P.B., Tiezzi D.G., De Carvalho D.D., de Andrade J.M., Martins G.A., Silva J.S. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer Res. 2015;75:3788–3799. doi: 10.1158/0008-5472.CAN-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guislain A., Gadiot J., Kaiser A., Jordanova E.S., Broeks A., Sanders J., van Boven H., de Gruijl T.D., Haanen J.B., Bex A., et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol. Immunother. CII. 2015;64:1241–1250. doi: 10.1007/s00262-015-1735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Raje N., Terpos E., Willenbacher W., Shimizu K., Garcia-Sanz R., Durie B., Legiec W., Krejci M., Laribi K., Zhu L., et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 168.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Brown J.E., Yardley D.A., Richardson G.E., Siena S., Maroto P., Clemens M., et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 169.Yee A.J., Raje N.S. Denosumab for the treatment of bone disease in solid tumors and multiple myeloma. Future Oncol. 2018;14:195–203. doi: 10.2217/fon-2017-0403. [DOI] [PubMed] [Google Scholar]
- 170.Gul G., Sendur M.A., Aksoy S., Sever A.R., Altundag K. A comprehensive review of denosumab for bone metastasis in patients with solid tumors. Curr. Med Res. Opin. 2016;32:133–145. doi: 10.1185/03007995.2015.1105795. [DOI] [PubMed] [Google Scholar]
- 171.Herbertz S., Sawyer J.S., Stauber A.J., Gueorguieva I., Driscoll K.E., Estrem S.T., Cleverly A.L., Desaiah D., Guba S.C., Benhadji K.A., et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Dev. Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Song B., Park S.H., Zhao J.C., Fong K.W., Li S., Lee Y., Yang Y.A., Sridhar S., Lu X., Abdulkadir S.A., et al. Targeting FOXA1-mediated repression of TGF-beta signaling suppresses castration-resistant prostate cancer progression. J. Clin. Investig. 2019;129:569–582. doi: 10.1172/JCI122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fields S.Z., Parshad S., Anne M., Raftopoulos H., Alexander M.J., Sherman M.L., Laadem A., Sung V., Terpos E. Activin receptor antagonists for cancer-related anemia and bone disease. Expert Opin. Investig. Drugs. 2013;22:87–101. doi: 10.1517/13543784.2013.738666. [DOI] [PubMed] [Google Scholar]
- 174.Gore J., Imasuen-Williams I.E., Conteh A.M., Craven K.E., Cheng M., Korc M. Combined targeting of TGF-beta, EGFR and HER2 suppresses lymphangiogenesis and metastasis in a pancreatic cancer model. Cancer Lett. 2016;379:143–153. doi: 10.1016/j.canlet.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spender L.C., Ferguson G.J., Hughes G.D., Davies B.R., Goldberg F.W., Herrera B., Taylor R.G., Strathearn L.S., Sansom O.J., Barry S.T., et al. Preclinical Evaluation of AZ12601011 and AZ12799734, Inhibitors of Transforming Growth Factor beta Superfamily Type 1 Receptors. Mol. Pharmacol. 2019;95:222–234. doi: 10.1124/mol.118.112946. [DOI] [PubMed] [Google Scholar]
- 176.Gilkes D.M. Implications of Hypoxia in Breast Cancer Metastasis to Bone. Int. J. Mol. Sci. 2016;17:1669. doi: 10.3390/ijms17101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Futakuchi M., Fukamachi K., Suzui M. Heterogeneity of tumor cells in the bone microenvironment: Mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Pt BAdv. Drug Deliv. Rev. 2016;99:206–211. doi: 10.1016/j.addr.2015.11.017. [DOI] [PubMed] [Google Scholar]