Abstract
Background
Hepatic fibrosis is caused by chronic liver injury and may progress toward liver cirrhosis, and even hepatocellular carcinoma. However, current treatment is not satisfactory. Therefore, there is a mandate to find novel therapeutic targets to improve therapy, and biomarkers to monitor therapeutic response.
Methods
Liver fibrosis was induced by carbon tetrachloride (CCl4) or thioacetamide (TAA) in wild type (WT) or CTHRC1−/− mice, followed by immunofluorescence and immunohistochemical analyses. CTHRC1 monoclonal antibody (mAb) was used to abrogate the effect of CTHRC1 in vitro and in vivo.
Results
Here, we reported that collagen triple helix repeat containing 1 (CTHRC1), a secreted protein derived from hepatic stellate cells (HSCs), was significantly up-regulated in fibrotic liver tissues. CTHRC1 promoted HSCs transformation from a quiescent to an activated state, and enhanced migratory or contractile capacities of HSCs by activating TGF-β signaling. Meanwhile, CTHRC1 competitively bound to Wnt noncononical receptor and promoted the contractility but not activation of HSCs. CCl4 or TAA-induced liver fibrosis was attenuated in CTHRC−/− mice compared with littermate control, while a monoclonal antibody of CTHRC1 suppressed liver fibrosis in WT mice treated with CCl4 or TAA.
Interpretation
We demonstrated that CTHRC1 is a new regulator of liver fibrosis by modulating TGF-β signaling. Targeting CTHRC1 could be a promising therapeutic approach, which can suppress TGF-β signaling and avoid the side effects caused by directly targeting TGF-β. CTHRC1 could also be a potential biomarker for monitoring response to anti-fibrotic therapy.
Fund
This study was supported by the National Natural Science Foundation of China (ID 81672358, 81871923, 81872242, 81802890), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (ID 20181708), the Natural Science Foundation of Shanghai (ID 17ZR1428300, 18ZR1436900), and Shanghai Municipal Health Bureau (ID 2018BR32). The funders did not play a role in manuscript design, data collection, data analysis, interpretation nor writing of the manuscript.
Keywords: Liver fibrosis, CTHRC1, HSCs, TGF-β signaling
Research in context.
Evidence before this study
There is evidence to indicate that collagen triple helix repeat containing 1 (CTHRC1), a secreted protein, is involved in many physiological and pathological processes. In the progression of liver fibrosis, in which the activation of hepatic stellate cell (HSCs) is considered as the central event, the role of CTHRC1 remain unclear and need to be investigated.
Added value of this study
This study demonstrated that CTHRC1 was significantly up-regulated in activated HSCs and fibrotic liver tissues. CTHRC1 promoted the activation, contractility and migration of HSCs by activating TGF-β signaling. Moreover, CTHRC1 selectively activated non-canonical Wnt signaling and promoted the contractility and migration but not activation of HSCs. CCl4 or TAA-induced liver fibrosis was attenuated in CTHRC−/− mice, while a monoclonal antibody of CTHRC1 suppressed liver fibrosis in WT mice treated with CCl4 or TAA.
Implications of all the available evidence
Our research revealed the role of CTHRC1 in the progression of liver fibrosis and uncovered the underlying mechanisms. CTHRC1 might be used as a potential biomarker to monitor the therapeutic response in the treatment of liver fibrosis.
Alt-text: Unlabelled Box
1. Introduction
Liver fibrosis is a common disease worldwide mainly caused by various chronic liver injuries including viral, alcohol, drug-induced, cholestatic and metabolic diseases [1,2]. It is a serious and dangerous threaten to human health especially when liver fibrosis progress to cirrhosis or even hepatocellular carcinoma [[3], [4], [5], [6]]. Although some essential factors for resolution were identified in recent years, the clinical trials still lack the effective methods to treat the disease and reliable biomarkers to monitor its progression [[7], [8], [9], [10], [11]]. Therefore, it is urgent to discover more efficient therapeutic targets for improving therapy and potential biomarkers for measuring fibrosis progression. We searched for secreted proteins, which have been shown promise as therapeutic targets and biomarkers in other diseases [[12], [13], [14]].
Collagen triple helix repeat containing 1 (CTHRC1), a secreted protein, is highly conserved in chordates with no homologues found in lower species such as flies and worms. CTHRC1 was initially found in a screen for differentially expressed genes in balloon-injured versus normal rat arteries [15]. Subsequent studies found that CTHRC1 is involved in many physiological and pathological processes, including vascular remodeling, bone formation, developmental morphogenesis, inflammatory arthritis, and cancer progression [[16], [17], [18], [19], [20]].
In this study, we found CTHRC1 is significantly up-regulated in activated hepatic stellate cell (HSC) and cirrhotic liver tissue. In vitro and in vivo studies revealed that CTHRC1 is an important microenvironmental factor, which promotes HSC transformation from a quiescent to an activated state, and aggravates liver fibrosis. We further demonstrated that the promotive effect of CTHRC1 on HSC activation and liver fibrosis is mainly mediated through transforming growth factor-β (TGF-β) receptor and its downstream Smad2 and Smad3 signaling by using functional blocking antibodies and the specific antagonist. Together, these data suggest that CTHRC1 might serve as a promising biomarker and therapeutic target for liver fibrosis.
2. Methods
2.1. Clinical samples
Human normal liver and cirrhotic liver tissues were obtained from Department of Liver Surgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. The microarray containing forty fibrotic and thirty normal liver tissue samples was purchased from Alenabio (BC03117). All of the human materials were obtained with informed content, and protocols were approved by the ethical review committee of the World Health Organization Collaborating Center for Research in Human Production (authorized by the Shanghai Municipal Government).
2.2. Cell culture
Human LX-2 cell line was a gift from professor Friedman S.L.. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum (Gibco, 16000-044) and 1% antibiotics at 37 °C in a humidified incubator under 5% CO2 condition.
2.3. Animals
Male C57BL/6J mice (5 weeks old) and male Sprague Dawley rats (5 weeks old) were purchased from SLAC Laboratory Animal. Mice were housed and manipulated according to protocols approved by the East China Normal University Animal Care Commission. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
2.4. CTHRC1−/− mice
Male CTHRC1−/− mice (5 weeks old) were obtained from Riken BioResource Center (accession No: CDB0502K, link: http://www2.clst.riken.jp/arg/mutant_list_file/CDB0502K.html). CTHRC1 mutant mice (CDB0502K) were generated as following: a targeting vector was constructed by inserting a cassette consisting of LacZ-pA and PGK-neopA into the first exon of CTHRC1 to replace the coding sequence with LacZ. Primer sequences used for mouse CTHRC1 detection was shown in Supplementary Table 1.
2.5. CCl4 and TAA models
For Sprague Dawley rats, CCl4 in 50% olive oil at a dose of 0.2 ml/100 g body weight was injected twice weekly at equal intervals. For C57BL/6J or CTHRC1−/− mice, CCl4 in 25% olive oil at a dose of 0.5 μl/g body weight was injected twice weekly at equal intervals. For TAA model, TAA was injected into mice at a dose of 0.2 mg/g three times weekly. After 8 weeks, the livers of above rats or mice were collected.
2.6. Primary hepatic stellate cells isolation
Sprague Dawley rats or C57BL/6J mice were sacrificed, and their livers were perfused with a solution containing 0.1% collagenase, 0.25% pronase E and 0.01% DNase, followed by dissection. The liver was excised and incubated at 37 °C for 30 min in a solution as above. Then the suspension from rats was filtered through a 100 μm iron mesh and centrifuged on 18% Nycodenz (Axis-Shield, Qk1002424-1). And the suspension from mice was filtered through a 100 μm iron mesh and centrifuged on 8.2% Nycodenz. After isolation, cells were resuspended in DMEM with 20% fetal calf serum and 1% antibiotics and cultured at 37 °C. After 24 h, cell debris and non-adherent cells were removed and replaced with fresh complete medium.
2.7. Recombinant CTHRC1 protein expression, purification and verification
CTHRC1 ORF were cloned into the episomal expression vector with pCEP-Pu-Strep II-tag. CTHRC1 was recombinant expressed in 293 T cells after transfecting reconstructed plasmid by using X-tremeGENE 9 DNA Transfecting Reagent (Roche, 6365779001). After 48 h, the 293 T cells were screening with puromycin (Sigma, P7130) at a dose of 2 μg/ml in DMEM supplemented with 10% FBS for 7 days, then the culture media were collected and applied to the Strep Tactin sepharose column (IBA, 2-1202-101). The column was washed with binding buffer and eluted by elution buffer containing 2.5 mM desthiobiotin. The collected fractions were further quantified by Nanodrop 2000 spectrophotometer (Thermo) and identified by western blotting.
2.8. CTHRC1 monoclonal antibody generation
His-tag fusion proteins of CTHRC1 (encoding 33-168 amino acids of human CTHRC1) was constructed into PET28 vectors and expressed according to manufacturer's protocols. The purified His-tag fusion protein was used as the antigen to immunize mice for generation of anti-CTHRC1 monoclonal antibodies and for ELISA assay to screen monoclonal antibodies (Huabio). An monoclonal antibody was produced and labeled as D6-A6, the immunoglobulin subtype of which was IgG2c. The purity of this monoclonal antibody was 98.25%, and the concentration was 1.25 mg/ml.
2.9. Immunohistochemical and sirius red staining
The formalin fixed and paraffin-embedded liver tissue slices (5 μm thickness) were deparaffinized and rehydrated for histopathological evaluation [21,22]. For sirius red staining, the sections were stained by hematoxylin and sirius red. For immunohistochemical staining, the sections were incubated with 0.3% hydrogen peroxide/phosphate-buffered saline for 30 min and blocked with 10% BSA (Sangon, AD0023-100). Slides were first incubated using the antibody for CTHRC1 (Huabio), α-SMA (Sigma, A5228) or desmin (Abcam, ab15200) at 4 °C overnight with optimal dilution, labeled by HRP second antibody of mouse (Cell Signaling, 5470S) or rabbit (Abcam, ab136817) at room temperature for 1 h. Then the sections were treated with DAB substrate liquid (Thermo, S21024-2) and counterstained by hematoxylin. All the sections were observed and photographed with a microscope (Carl Zeiss).
2.10. Immunofluorescence staining
For cell staining, LX-2 cells were seeded on slides in 24-well plates and incubated at 37 °C. For F-actin staining, cells were incubated with phalloidin-FITC (Sigma, P5282) for 75 min at room temperature. For CTHRC1 or α-SMA staining, cells were incubated with primary antibodies against CTHRC1 (Huabio) or α-SMA (Sigma, A5228) for 75 min, followed by an Alexa Fluor 594-conjugated secondary antibody. For coimmunostaining of liver tissues, samples were subjected to heat-mediated antigen retrieval in PH 6.0 citric acid, and blocked by 10% BSA. Slides were co-incubated with CTHRC1 and α-SMA antibodies and then labeled with Alexa Fluor 594-conjugated anti-rabbit antibody and Fluor 488 conjugated anti-mouse antibody. The nucleus was stained with DAPI (Sigma, D9542) and the immunofluorescence signals were captured using confocal-scopy (Carl Zeiss).
2.11. Lentivirus production and cell transduction
HA tagged Full-length cDNA encoding human CTHRC1 and extracellular domain of TGFBR1, TGFBR2, TGFBR3 and Endoglin were amplified by PCR and cloned into pEZ-lv105 vector (GeneCopoeia, Ex-T0451; Ex-Y4135; Ex-Z4152). Virus packaging was performed in 293 T cells after cotransfection of CTHRC1 or mock vector with Lipofectamine 2000 (Invitrogen, 11668-019). Viruses were harvested at 24 h, 48 h and 72 h after transfection, and virus titers were determined. 1 × 105 LX-2 cells were infected with 1 × 106 recombinant lentivirus-transducing units in the presence of 6 μg/ml polybrene (Sigma, H9268).
2.12. Western blotting
Cells were lysed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton-X 100, 1 mM each MgCl2, MnCl2 and CaCl2, 1 mM PMSF and 10 mM sodium fluoride) [23,24]. Tissues were homogenized in T-PER tissue protein extraction reagent (Thermo, 78510), in which protease inhibitor cocktail (Biotool, B14001) was added. Insoluble material was removed by centrifugation at 10,000 g for 10 min. Proteins were separated by SDS-PAGE under reducing condition, followed by blocking in phosphate-buffered saline/Tween-20 containing 1% BSA. The membrane was incubated with antibodies for CTHRC1 (Huabio), TGFBR1 (Cell Signaling, 3712S), TGFBR2 (Cell Signaling, 11888S), TGFBR3 (Cell Signaling, 2519S), Endoglin (Cell Signaling, 4335S), phospho-Smad2 (Cell Signaling, 3108P), Smad2 (Cell Signaling, 5339P), phospho-Smad3 (Cell Signaling, 9520P), Smad3 (Cell Signaling, 9523P), Smad4 (Cell Signaling, 9515P), phospho-TAK1 (Cell Signaling, 4508S), TAK1 (Cell Signaling, 5206S), phospho-p38 (Cell Signaling, 4511S), p38 (Cell Signaling, 8690S), phosphor-JNK (Cell Signaling, 4668S), JNK (Cell Signaling, 9252S), Wnt5a (Cell Signaling, 2392S), and GAPDH (Huabio, M1211-1), followed by incubation of species-specific secondary antibodies. Bound secondary antibodies (LI-COR, 926-32213; 926-68051) were revealed by Odyssey imaging system (LI-COR).
2.13. siRNA transfection
Small interfering RNAs duplexes for TGFBR2, Wnt3a, Wnt5a and TGF-β1 were produced by Genepharma. Transfection steps were following the manufacture's protocols.
2.14. Quantitative real-time PCR
Total RNA extracted using Trizol reagent (Takara, A7603-1), and reversely transcribed through Prime Script RT-PCR kit (Takara, PR036A-1) according to the protocol. Real-time PCR analyses were performed with Bestar™ qPCR Master Mix (DBI, DBI-2043) on a 7500 Real-time PCR system, Applied Biosystems at the recommended thermal cycling settings: one initial cycle at 95 °C for 30 s followed by 40 cycles of 5 s at 95 °C and 31 s at 60 °C. Primer sequences used for human or rat CTHRC1 detection, and for mouse Acta2, Col1a1, Timp1, Mmp9 detection were shown in Supplementary Table 1.
2.15. Collagen gel contraction assay
LX-2 at a density of 5 × 104 cells per ml were seeded onto 32 mm bacteriological plates (2 ml per dish) in DMEM supplemented with 10% fetal bovine serum, antibiotics and 0.3 mg/ml of acid-extracted collagen I from Sprague Dawley rat tail as previously described. Recombinant CTHRC1 and/or TGFBR2 neutralizing antibody (R&D, AF-241-NA), TGF-βR inhibitor, LY2109761 (SELLECK, S2704) were added in the medium simultaneously. The cells were cultured at 37 °C for 60 min to allow collagen polymerization. The gels were released from plates by tilting plates slightly and gel contraction was monitored by measuring the gel area at time points up to 6 h. Each data was performed in three independent experiments.
2.16. Migration assay
Cell migration assays were performed using transwell chambers, Millipore (PIEP12R48). 5 × 104 cells in 200 μl serum-free DMEM were seeded in the upper chamber and 500 μl medium supplemented with 10% fetal bovine serum was added to the lower chamber. Migrated cells were fixed and stained with 0.1% (w/v) crystal violet 24 h later. Three randomly selected fields were photographed and the numbers were counted.
2.17. Statistical analysis
Data were presented as the means ± standard error of the mean (SEM). Statistical analyses were done using GraphPad Prism 5 for windows. One-way ANOVA or two-tailed student's t-test was used for comparison between groups. Values of P < .05 were considered statistically significant.
3. Results
3.1. CTHRC1 is up-regulated in liver fibrosis and mainly derived from HSCs
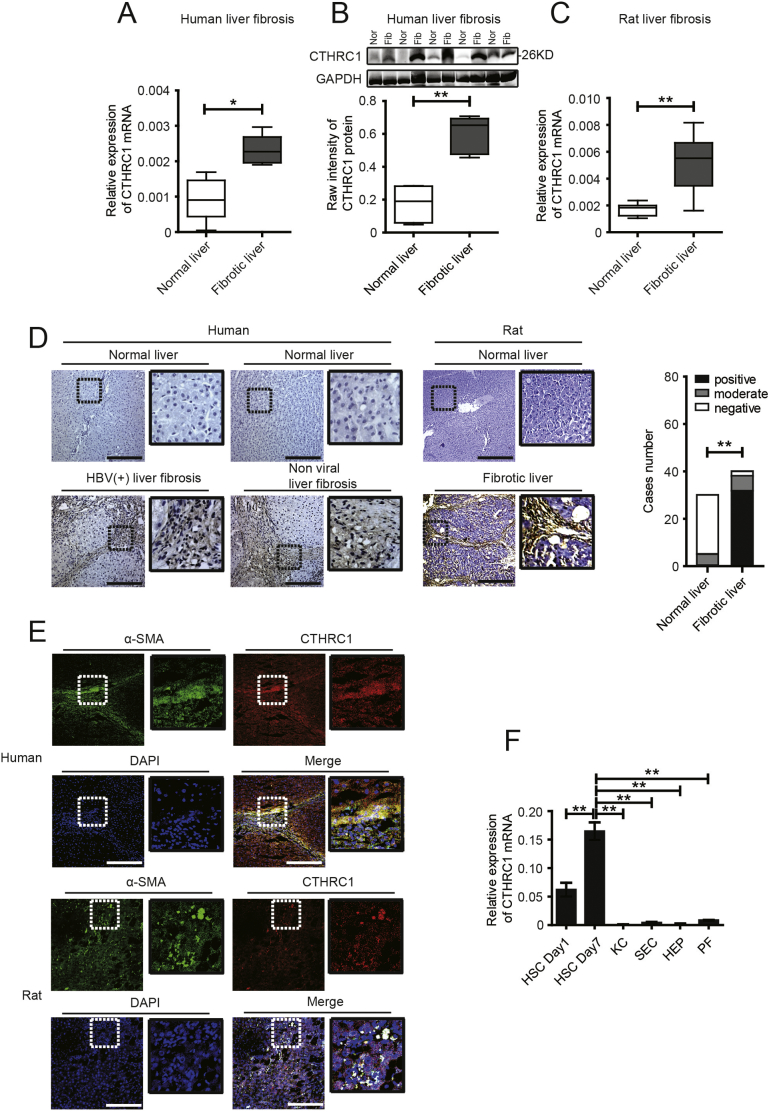
To discover potential biomarkers and therapeutic targets, we searched for secreted proteins by comparing differential gene expression at a genome-wide level in fibrotic liver tissues compared to normal liver tissues (carbon tetrachloride (CCl4)-induced mice liver tissues, GSE73985, and bile duct ligated-induced fibrotic rat liver tissues, GSE13747), and activated human and mouse HSCs compared to matched quiescent HSCs (GSE68001 and GSE34949). Three secreted proteins were significantly up-regulated in all of these datasets (log fold change (FC) > 3 and P < .05, Supplementary Fig. 1 in the Supplementary Material). Among them, CTHRC1 is of particular interest to us. We then conducted a preliminary validation with five pairs of human normal and fibrotic liver tissues by real-time quantitative polymerase chain reaction (qPCR) and western blotting, and the results confirmed that CTHRC1 was significantly up-regulated in fibrotic human liver tissues (Fig. 1A, B). Similar results were also obtained in CCl4-induced rat fibrotic liver tissues (Fig. 1C). We then performed an immunohistochemical analysis of a tissue microarray that contained forty fibrotic human liver tissue and thirty normal liver tissue samples. The results revealed that CTHRC1 expression level was significantly higher in fibrotic human liver than that in normal human liver tissues (Fig. 1D, left photos). Likewise, immunohistochemical staining of rat fibrotic liver tissues exhibited similar results (Fig. 1D, right photos). We further found that CTHRC1 was specifically expressed in the fibrous septa and mainly co-localized with α-SMA in fibrotic liver tissues (Fig. 1E), suggesting that CTHRC1 might be derived from myofibroblast-like cells in liver fibrosis. To identify the source of CTHRC1 in liver fibrosis, we isolated primary rat resident liver cells, including HSCs, Kupffer cells (KC), sinusoidal endothelial cells (SEC), hepatocytes (HEP), and portal fibroblasts (PF), and found that CTHRC1 was mainly derived from activated HSCs (Fig. 1F).
Fig. 1.
CTHRC1 is up-regulated in fibrotic liver tissues and mainly derived from activated hepatic stellate cells (HSCs).
A and B. Real-time quantitative PCR (A) and western blotting (B) analysis of CTHRC1 in human fibrotic (Fib) (n = 5) and normal liver tissues (Nor) (n = 5).
C. Real-time quantitative PCR analysis of CTHRC1 in CCl4-induced rat fibrotic (n = 7) and normal liver tissues (n = 7).
D. Immunohistochemical staining of CTHRC1 in a human tissue microarray that contained fibrotic (n = 40) and normal liver tissue (n = 30) samples (D, left photos), and CCl4-induced fibrotic and normal rat liver tissues (D, right photos). Scale bars, 100 μm. The percentage of tissue cores displaying negative, moderate or positive CTHRC1 staining. Statistical analysis of CTHRC1 expression in human fibrotic and normal liver tissue microarray was shown right.
E. Immunofluorescence images of α-SMA (green), CTHRC1 (red), DAPI (blue) and merge (yellow) in human and rat fibrotic liver tissues. Scale bars, 100 μm.
F. Relative CTHRC1 mRNA expression in primary HSCs, Kupffer cells (KC), sinusoidal endothelial cells (SEC), hepatocytes (HEP) and portal fibroblasts (PF), isolated from rat livers (n = 5). *P < .05, **P < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
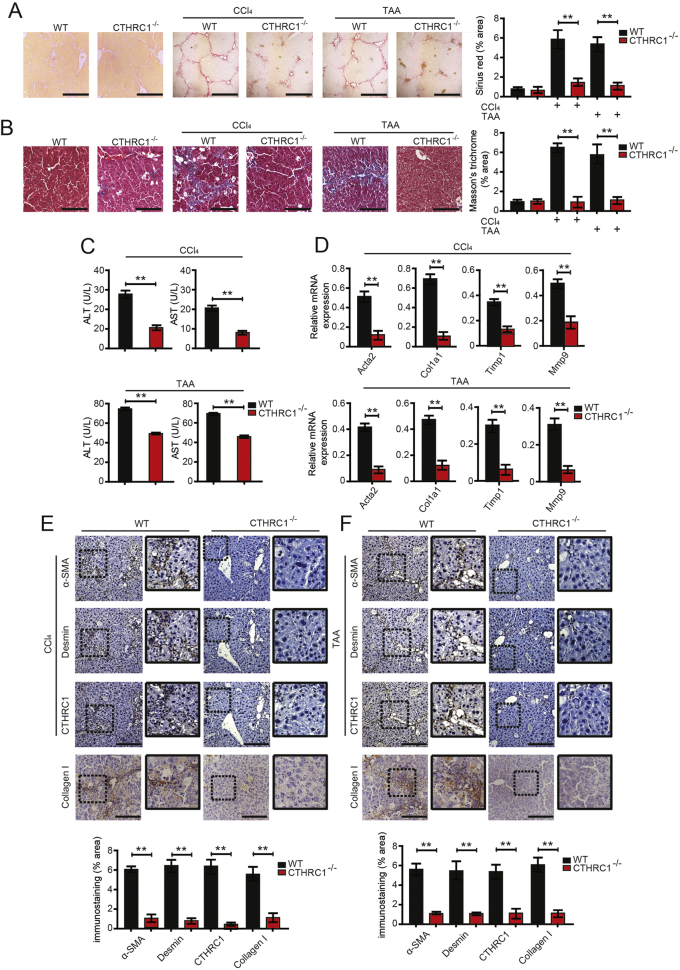
3.2. CTHRC1 supports liver fibrosis in vivo
To explore the role of CTHRC1 on hepatic fibrosis in vivo, CTHRC1−/− mice were used [[25], [26], [27]]. CTHRC1−/− mice had not any abnormalities in the livers. According to the schedule, CTHRC1−/− mice and wild-type littermates were subjected to CCl4-induced or thioacetamide (TAA)-induced fibrosis. The results showed that after consecutive CCl4 and TAA injections, collagen deposition in CTHRC1−/− mice was significantly reduced compared with wild-type (WT) mice (Fig. 2A, B). Liver injury was evaluated by measuring serum levels of ALT and AST. The results showed that the serum levels of ALT and AST in CTHRC1−/− mice were also significantly lower than those in WT mice (Fig. 2C). Further, qPCR analysis of the livers also showed that CTHRC1 knockdown reduced the CCl4-induced or TAA-induced up-regulation of the classical profibrogenic markers Acta2, Col1a1, Timp1, and Mmp9 (Fig. 2D). Meanwhile, the relative mRNA expression level of TGF-β1 was reduced in CTHRC1−/− mice (Supplementary Fig. 2A in the Supplementary Material). Hepatic α-SMA, desmin and collagen I expression, the markers of HSC activation, were also significantly reduced in CTHRC1−/− mice compared to WT mice (Fig. 2E, F). Moreover, it has been reported that CTHRC1 is associated with cell survival and proliferation [28]. We found that CTHRC1 could promote the proliferation of HSCs after 24 h, 48 h and 72 h (Supplementary Fig. 2B in the Supplementary Material).
Fig. 2.
CTHRC1 contributes to liver fibrosis in vivo.
A and B. Sirius red and Masson's trichrome staining in the livers of WT (n = 5), CTHRC1−/− (n = 5), WT (n = 10) and CTHRC1−/− mice (n = 10) consecutively injected intraperitoneally with CCl4 (A) or TAA (B) for 8 weeks. Scale bars, 100 μm. Quantification of sirius red and Masson's trichrome staining was shown right.
C. The serum levels of ALT and AST in WT (n = 10) and CTHRC1−/− mice (n = 10) consecutively injected intraperitoneally with CCl4 or TAA for 8 weeks.
D. The hepatic levels of Acta2, Col1a1, Timp1 and Mmp9 mRNA in WT (n = 10) and CTHRC1−/− mice (n = 10) were determined by qPCR.
E. WT and CTHRC1−/− mice were intraperitoneally injected with consecutive CCl4 for 8 weeks and their liver serial sections were subjected to immunostaining with α-SMA, desmin, collagen I and CTHRC1 antibodies. Scale bars, 100 μm. Quantification of immunostaining was shown below.
F. Mice were intraperitoneally injected with consecutive TAA and their liver serial sections were subjected to immunostaining with α-SMA, desmin, collagen I and CTHRC1 antibodies. Scale bars, 100 μm. Quantification of immunostaining was shown below. **P < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Taken together, CTHRC1−/− mice showed attenuated liver fibrosis and less hepatic injury upon CCl4 and TAA treatment compared to WT mice.
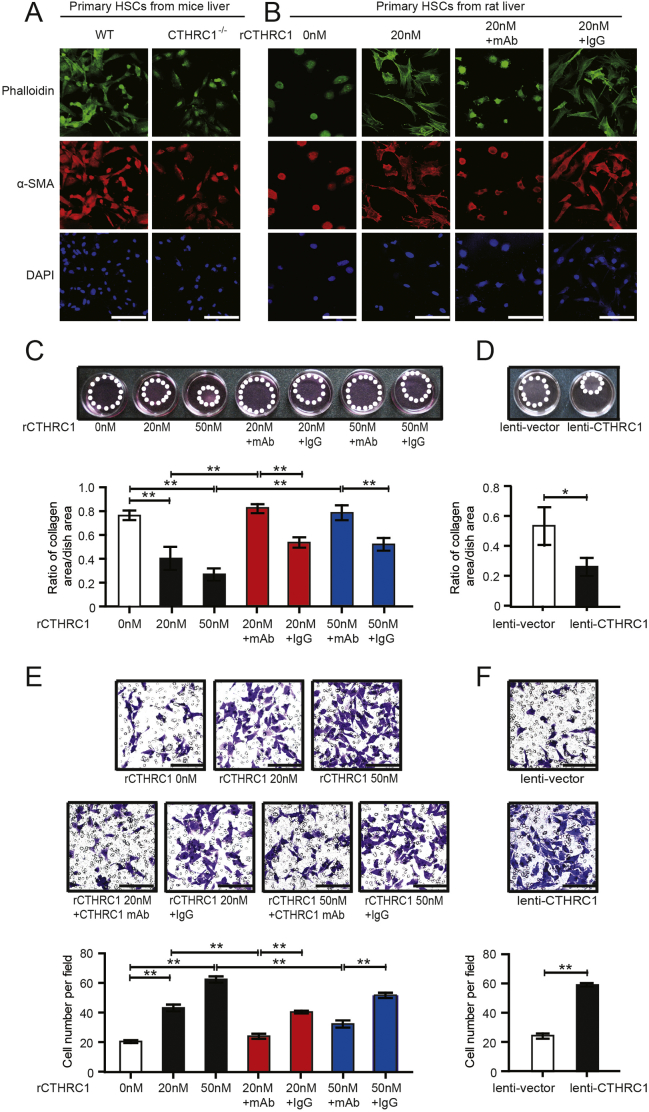
3.3. CTHRC1 promotes HSC activation, migratory and contractility in vitro
As HSCs play important roles in liver fibrosis, we further investigated the effects of CTHRC1 on HSCs. We first compared the activation of primary HSCs isolated from CTHRC1−/− mice and WT littermates. The results revealed that the activation of HSCs from CTHRC1−/− mice was significantly delayed compared with HSCs from WT mice in vitro (Fig. 3A). Then, we treated primary rat HSCs with recombinant CTHRC1 (rCTHRC1) protein. The activation of primary rat HSCs was significantly accelerated after treatment with rCTHRC1 protein, while the effect of rCTHRC1 protein on HSC activation was reversed by monoclonal antibody (mAb) of CTHRC1 (Fig. 3B). Acquiring migratory and contractile capacities are also very important properties of activated HSCs, so we investigated the effects of CTHRC1 on these properties. rCTHRC1 protein significantly enhanced the migratory and contractile capacities of LX2 cells, which were reversed by mAb of CTHRC1 (Fig. 3C, E). Pro-migratory and pro-contractile effects of CTHRC1 were also observed after lentiviral-vector-mediated overexpression of CTHRC1 in LX-2 cell (Fig. 3D, F).
Fig. 3.
CTHRC1 promotes HSC activation, migratory and contractile capacities in vitro.
A. Representative immunofluorescence images of phalloidin (green) and α-SMA (red) in primary HSCs after 7 days isolated from WT and CTHRC1−/− mice. Nuclei are stained with DAPI (blue). Scale bars, 50 μm.
B. Immunofluorescence images of phalloidin (green) and α-SMA (red) in primary rat HSCs after 4 days, which were treated with vehicle, 20 nM purified recombinant CTHRC1 (rCTHRC1) protein alone, and 20 nM rCTHRC1 protein plus CTHRC1 mAb or IgG. Nuclei are stained with DAPI (blue). Scale bars, 50 μm.
C. Collagen gel contraction assay of LX-2 treated with 0 nM, 20 nM or 50 nM rCTHRC1 protein alone, and 20 nM or 50 nM rCTHRC1 protein plus CTHRC1 mAb or IgG (n = 3 each group). Statistical analysis of collagen gel contractionis was shown below.
D. Collagen gel contraction assay of LX-2/lenti-vector and LX-2/lenti-CTHRC1 (n = 3 each group). Statistical analysis was shown below.
E. Representative images of LX-2 migration treated with 0 nM, 20 nM or 50 nM rCTHRC1 protein alone, and 20 nM or 50 nM rCTHRC1 protein plus CTHRC1 mAb or IgG, respectively (n = 3 each group). Scale bars, 100 μm. Statistical analysis of cell migration of LX-2 treated with 0 nM, 20 nM or 50 nM rCTHRC1 protein alone, and 20 nM or 50 nM rCTHRC1 protein plus CTHRC1 mAb or IgG was shown below.
F. Representative images of LX-2/lenti-vector and LX-2/lenti-CTHRC1 cell migration (n = 3 each group). Scale bars, 100 μm. Statistical analysis of cell migration of LX-2/lenti-vector and LX-2/lenti-CTHRC1 was shown below. *P < .05, **P < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
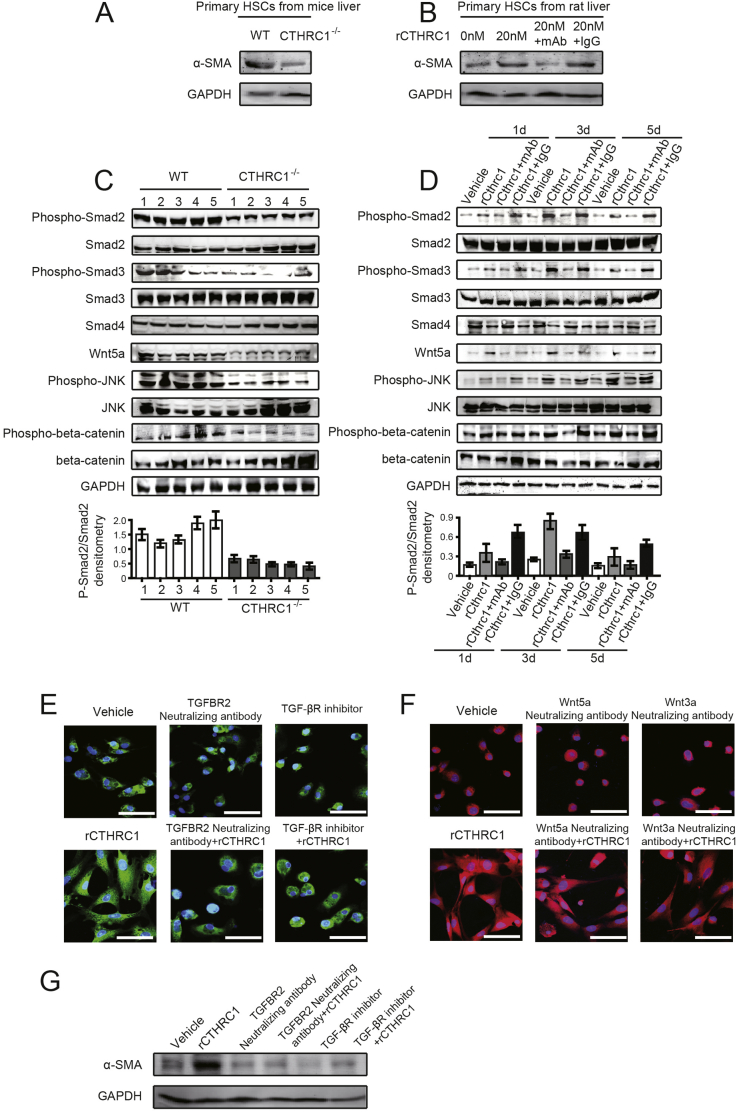
Furthermore, primary HSCs proliferation require self-activated in vitro culture [29]. So we detected the expression of α-SMA to confirm the delayed activation of HSCs in CTHRC1−/− mice and the effects of rCTHRC1 protein and CTHRC1 mAb on HSC activation by western blotting (Fig. 4A, B).
Fig. 4.
CTHRC1 activates both TGF-β and Wnt signaling, while the promotive effect of CTHRC1 on HSC activation is mainly dependent on TGF-β signaling.
A. The expression of α-SMA in primary HSCs after 7 days isolated from WT and CTHRC1−/− mice.
B. The expression of α-SMA in primary rat HSCs after 4 days, which were treated with vehicle, 20 nM rCTHRC1 protein alone, and 20 nM rCTHRC1 protein plus CTHRC1 mAb or IgG.
C. Western blotting analysis of phosphorylation of Smad2, Smad3, JNK, total Smad4 and expression of Wnt5a in five liver tissues of WT or CTHRC1−/− mice intraperitoneally injected with CCl4. GAPDH was the loading control. The densitometry of p-Smad2/Smad2 was shown below.
D. Phosphorylation of Smad2, Smad3, JNK, total Smad4 and expression of Wnt5a in primary rat HSCs, which were treated with vehicle, 20 nM rCTHRC1 protein, and 20 nM rCTHRC1 protein plus CTHRC1 mAb or IgG for 1, 3, 5 days, individually. GAPDH was the loading control. The densitometry of p-Smad2/Smad2 was shown below.
E and F. Representative immunofluorescence images of α-SMA (green in E, red in F) in primary rat HSCs after 4 days, which were treated with vehicle, 20 nM rCTHRC1 protein, and 20 nM rCTHRC1 protein plus neutralizing antibodies or inhibitor as follows: TGFBR2 neutralizing antibody or TGF-β receptor inhibitor (E), Wnt5a or Wnt3a neutralizing antibody (F). Nuclei are stained with DAPI (blue). Scale bars, 50 μm.
G. The expression of α-SMA in primary rat HSCs after 4 days, which were treated with vehicle, 20 nM rCTHRC1 protein, and 20 nM rCTHRC1 protein plus TGFBR2 neutralizing antibody or TGF-β receptor inhibitor. GAPDH was the loading control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. CTHRC1 activates TGF-β and Wnt signaling, while HSC activation is mainly dependent on TGF-β signaling
Previous studies have demonstrated that CTHRC1 is related to TGF-β and non-canonical Wnt signaling [[25], [26], [27]], so we further examined the signaling molecules involved in the TGF-β and the non-canonical Wnt pathway in liver tissues from CTHRC1−/− or wild type mice induced by CCl4, and in primary rat HSCs treated with rCTHRC1 protein. Phosphorylation of Smad2 and Smad3, which are regarded as the main downstream signaling molecules of TGF-β signaling [[30], [31], [32], [33], [34], [35]], and phosphorylation of JNK and expression of Wnt5A, which are involved in the non-canonical Wnt pathway [[36], [37], [38], [39]], were all significantly reduced in CTHRC1−/− mice liver tissues compared to wild type mice treated with CCl4 (Fig. 4C). The phosphorylation of Smad2, Smad3, JNK and the expression of Wnt5A were also significantly elevated in primary rat HSCs by treatment with rCTHRC1 protein during the transition process from quiescent to activated state (Fig. 4D, Supplementary Fig. 3A in the Supplementary Material), and these effects were reversed by mAb of CTHRC1 (Fig. 4D). Thus, CTHRC1 significantly activated both TGF-β and non-canonical Wnt signaling in liver fibrosis and HSC activation. We further investigated whether the effect of CTHRC1 on HSC activation is dependent on TGF-β or non-canonical Wnt signaling. To block TGF-β signaling, the primary rat HSCs were treated with TGFBR2 neutralizing antibody or TGF-β receptor inhibitor LY2109761 before rCTHRC1 protein was added. The results showed the promotive effect of rCTHRC1 protein on HSC activation was dramatically decreased (Fig. 4E). To block Wnt signaling, the primary rat HSCs were treated with neutralizing antibody for Wnt5a, the ligand for the non-canonical Wnt pathway, or neutralizing antibody for Wnt3a, the ligand in the canonical Wnt pathway. The stimulatory effect of rCTHRC1 on primary HSC activation was affected by neither Wnt5A nor Wnt3A neutralizing antibody (Fig. 4F). Together, CTHRC1 can activate both TGF-β and non-canonical Wnt signaling, while the stimulatory effect of CTHRC1 on HSC activation was mainly dependent on TGF-β signaling. Moreover, we detected α-SMA expression to confirm the inhibitory effects of TGFBR2 neutralizing antibody and TGF-β receptor inhibitor on CTHRC1 induced HSC activation by western blotting (Fig. 4G).
After activation, HSC acquired migratory and contractile capacities. We further investigated whether the pro-migratory and pro-contractile effects of CTHRC1 on HSC are dependent on TGF-β or non-canonical Wnt signaling. The stimulatory effects of rCTHRC1 protein on HSC motility and contractile capacity were significantly inhibited by treatment with TGFBR2 neutralizing antibody, TGF-β receptor inhibitor, knockdown of TGFBR2 or Wnt5a, suggesting that these effects might be dependent on both TGF-β and non-canonical Wnt signaling (Supplementary Fig. 4, Supplementary Fig. 5, Supplementary Fig. 6, Supplementary Fig. 7 in the Supplementary Material). Additionally, CTHRC1 was reported to regulate YAP/TAZ, a key molecule of HSC activation [40,41]. We performed the immunostaining of YAP and found that YAP expression was increased and transferred to nucleus in the livers of CTHRC1−/− compared with WT mice (Supplementary Fig. 3B in the Supplementary Material).
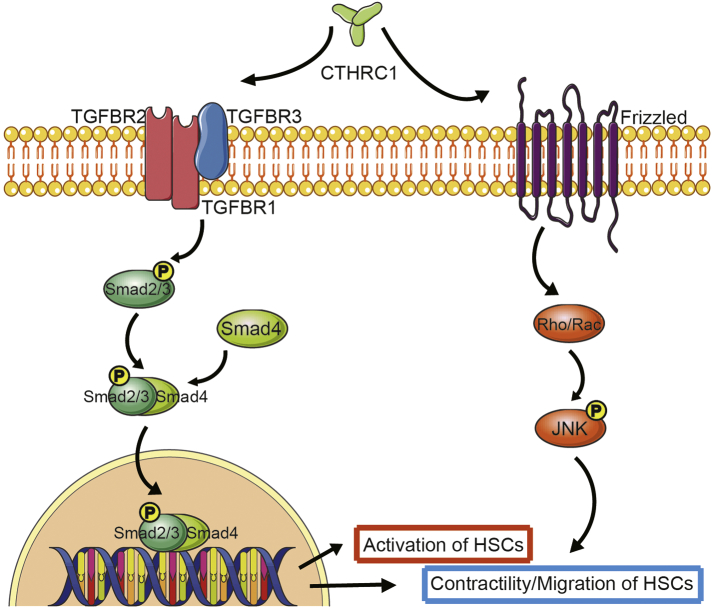
Taken together, these data indicated that rCTHRC1 protein accelerated primary HSC activation mainly through TGF-β signaling, while it enhanced motility and contractile capacity of activated HSC by activating both TGF-β and non-canonical Wnt signaling (Fig. 6).
Fig. 6.
A model for CTHRC1 modulating HSC behaviors through TGF-β and Wnt signaling pathways.
CTHRC1 induces activation of TGF-β downstream signaling molecules: Smad2, Smad3 and Smad4, which mediates the activation, migration and contractility of HSCs. In addition, CTHRC1 competitively binds to Wnt noncononical receptor and induces the phosphorylation of JNK, which mediates the contractility but not activation of HSCs.
3.5. CTHRC1 monoclonal antibody attenuates liver fibrosis and TGF-β signaling
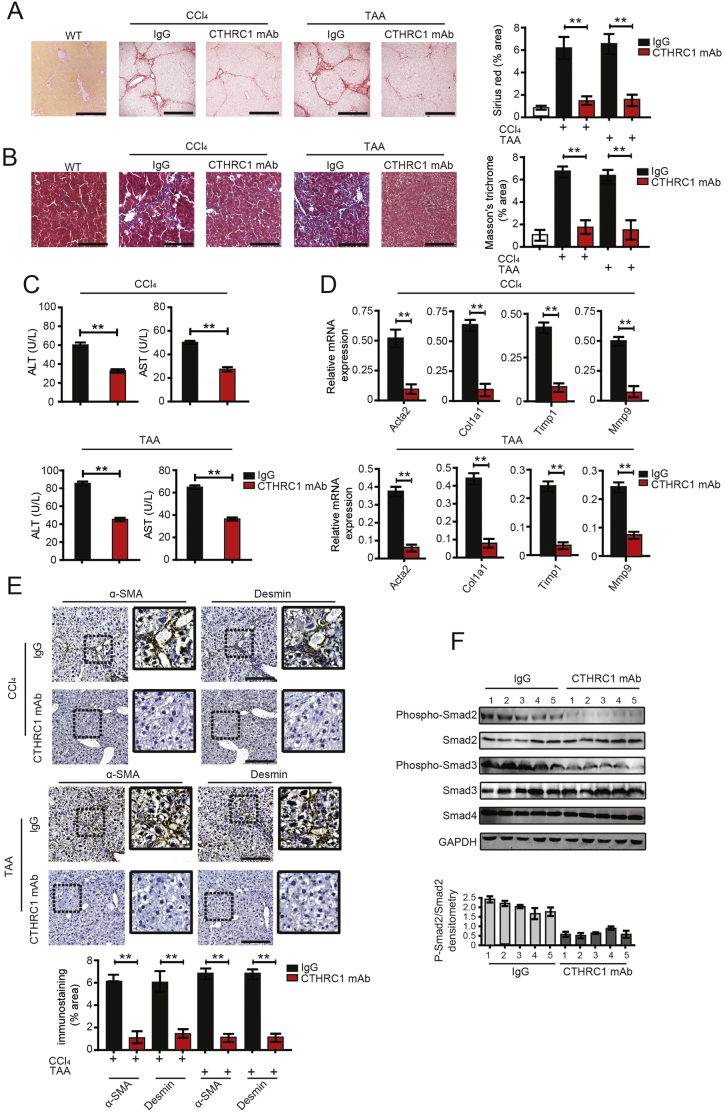
Following on from the in vitro experimental results, we subsequently investigated the anti-fibrotic effect of CTHRC1 mAb in a CCl4- or TAA-induced liver fibrosis mouse model. C57 WT mice treated with CTHRC1 mAb showed much less hepatic collagen deposition compared with IgG control (Fig. 5A, B). ALT and AST activity were significantly reduced in the mice treated with CTHRC1 mAb compared to IgG control (Fig. 5C). Further, qPCR analysis of the livers showed that CTHRC1 mAb administration reduced the CCl4-induced or TAA-induced up-regulation of the classical profibrogenic markers Acta2, Col1a1, Timp1, and Mmp9 (Fig. 5D). Meanwhile, the relative mRNA expression level of TGF-β1 was reduced in CTHRC1 mAb injection group (Supplementary Fig. 2C in the Supplementary Material). Hepatic α-SMA and desmin expression were also significantly reduced in the CTHRC1 mAb injection group compared to the IgG injection group (Fig. 5E).
Fig. 5.
CTHRC1 monoclonal antibody attenuates promotive effects of CTHRC1 on liver fibrosis.
A and B. Sirius red and Masson's trichrome staining in the livers of WT, WT C57 mice treated with CCl4 (A) or TAA (B) and intraperitoneally injected with monoclonal antibody (mAb) of CTHRC1 (n = 10) or IgG (n = 10). Each mouse was intraperitoneally injected with CTHRC1 mAb at a dose of 5 μg/g body weight. Scale bars, 100 μm. Quantification of sirius red and Masson's trichrome staining was shown right.
C. The serum levels of ALT and AST in WT C57 mice treated with CCl4 or TAA and intraperitoneally injected with mAb of CTHRC1 (n = 10) or IgG (n = 10).
D. The hepatic levels of Acta2, Col1a1, Timp1 and Mmp9 mRNA in WT C57 mice treated with CCl4 or TAA and intraperitoneally injected with CTHRC1 mAb (n = 10) or IgG (n = 10) were determined by qPCR.
E. Immunohistochemical staining of α-SMA and desmin in CCl4 (E, upper photos) and TAA (E, lower photos) induced liver fibrosis tissues of WT mice treated with CTHRC1 blocking antibody or IgG control, using serial sections. Scale bars, 100 μm. Quantification of immunostaining was shown below.
F. Western blotting analysis of phosphorylation of Smad2, Smad3 and total Smad4 in five liver tissues of WT mice treated with CTHRC1 mAb or IgG. GAPDH was detected as the loading control. The densitometry of p-Smad2/Smad2 was shown below. **P < .01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We further investigated whether TGF-β/Smad signaling was inhibited by CTHRC1 mAb. Phosphorylation of Smad2 and Smad3 were significantly suppressed in liver tissues of CTHRC1 mAb treated mice compared to those of IgG treated control mice (Fig. 5F).
CTHRC1 mAb significantly reduced liver injury through inhibition of TGF-β/Smad signaling in a CCl4- or TAA-induced liver fibrosis model, suggesting that blocking CTHRC1 might be a promising anti-fibrotic strategy.
4. Discussion
Liver fibrosis is marked by abnormal collagen accumulation and activation of HSCs. The activation of HSCs is always considered as the central event during the progression of liver fibrosis [1,3]. HSCs exist in Disse's space in normal liver, and activate into myofibroblasts when liver come in response to chronic liver injury. Upon activation, various cytokines and extracellular matrix components like collagens are produced by HSCs to further promote fibrogenesis. So in recent years HSCs are commonly regarded as the targets for anti-fibrotic therapies [2]. It is well known that TGF-β, a potent pro-fibrogenic cytokine, plays a key role in HSC activation. However, anti-fibrotic strategies to directly block TGF-β carry high risk of unwanted side effects, due to this cytokine playing central roles in cell proliferation, recognition, differentiation and apoptosis [33].
Our research revealed that CTHRC1 promoted activation, contractility and migration of HSCs through TGF-β signaling. Thus, targeting CTHRC1 provides an alternative approach to suppress TGF-β signaling and inhibit HSC activation, whilst avoiding the side effects caused by directly blocking TGF-β. Moreover, during the activation of HSCs, Kupffer cells were also activated and participated in the immune response. Meanwhile, lots of hepatocytes were deteriorated and produced some inflammatory cytokines [3], which further contributed to the activation of HSCs and induction of CTHRC1.
Previous study showed that CTHRC1 expression was highly correlated with HCC progression in HBV-infected patients, and HBV stimulated CTHRC1 expression by activating NF-κB and CREB, through ERK/c-JNK pathway [42]. Moreover, ROS could modulate many signaling, such as: Wnt/beta-catenin and TGF-β signaling [[43], [44], [45]]. It has been reported that Wnt signaling could regulate the expression of CTHRC1 [46]. So ROS might contribute to the production of CTHRC1 through Wnt signaling.
It has been reported that CTHRC1 inhibited TGF-β signaling in smooth muscle cells but not endothelial cells [25]. The authors concluded that the effect of CTHRC1 on TGF-β signaling is cell-type specific. Recently, it has been reported that CTHRC1 expression was increased in murine cholestatic liver fibrosis and administration of exogenous CTHRC1 with injection of adenovirus vector containing the CTHRC1 gene prevented cholestatic liver fibrosis [47]. CTHRC1 did not affected the induction of phospho-Smad2 and phospho-Smad3, but promoted the degradation of phospho-Smad3 [47]. CTHRC1 was also indicated to reduce fibrotic tissue formation in bleomycin-induced lung fibrosis [48]. While CTHRC1 may have other effects in liver fibrosis which is different from lung fibrosis or cholestatic fibrosis. In our study, we found that HSC-derived CTHRC1 was a novel pro-fibrotic secreted factor that acted by directly activating TGF-β signaling.
CTHRC1 selectively activates non-canonical Wnt pathway by stabilizing the Wnt-receptor complex [26]. A recent study revealed that non-canonical Wnt predominates in activated HSCs, influencing HSC survival and paracrine stimulation of Kupffer cells [49,50]. In this study, we found that CTHRC1 significantly activated non-canonical Wnt signaling in liver fibrosis and HSC activation. Non-canonical Wnt signaling is important for CTHRC1 induced migratory and contractile capacities of HSC. Therefore, targeting CTHRC1 provides a novel strategy to simultaneously suppress these two signaling, TGF-β and Wnt pathway, which are crucial for liver fibrosis and HSC activation.
Taken together both the clinical and experimental data, CTHRC1 is a promising therapeutic target for liver fibrosis. Furthermore, due to the fact that it is mainly derived from activated HSCs and significantly overexpressed in liver fibrosis, CTHRC1 might be also used as a biomarker to monitor the therapeutic response in any potential treatment of liver fibrosis.
Funding sources
This study was supported by the National Natural Science Foundation of China (ID 81672358, 81871923, 81872242, 81802890), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (ID 20181708), the Natural Science Foundation of Shanghai (ID 17ZR1428300, 18ZR1436900), and Shanghai Municipal Health Bureau (ID 2018BR32).
Declarations of interests
The authors have declared that no conflict of interest exists.
Author contributions
ZGZ and JL designed and supervised the overall study, analyzed data, and drafted the manuscript; JL and MZM performed immunohistochemical staining of human cirrhotic liver tissues, quantitative real-time PCR and Western blotting; JL, YHW and MZM designed CCl4 and TAA models, performed rat primary hepatic stallate cells isolation; MZM, YHW and XMY designed and performed in vitro collagen gel contraction assays and migration assays; SHJ and GAT assisted with the building of CCl4 and TAA models; XLZ, YLZ, YW, QL, LZ and HZN technically assisted with experiments and analyzed data; MXF and CJX assisted with analysis of immunohistochemical staining; QX provided human normal liver and cirrhotic liver tissues; QX, JRG supervised this study and edited the manuscript.
The following are the supplementary data related to this article.
Analysis of extracellular protein expression in four independent gene expression omnibus (GEO) databases. CTHRC1 expression was significantly upregulated in all these databases, Log (Fold change) > 3, P < .05. Agilent mouse LncRNA microarray data are available for download from the GEO under accession number GSE73985.
The relative mRNA expression level of TGF-β1 in the livers of WT or CTHRC1−/− mice, and the cell viability of HSCs treated with rCTHRC1 protein. A. The relative mRNA expression level of TGF-β1 in the livers of WT, CTHRC1−/−, WT and CTHRC1−/− mice consecutively injected intraperitoneally with CCl4 or TAA for 8 weeks. B. The cell viability of HSCs treated with 0 nM, 20 nM or 50 nM rCTHRC1 protein after 24 h, 48 h and 72 h. C. The relative mRNA expression level of TGF-β1 in the livers of WT, WT C57 mice treated with CCl4 or TAA and intraperitoneally injected with mAb of CTHRC1 or IgG. **P < .01.
Phosphorylation of Smad2, Smad3, TAK1 and total Smad4 in primary rat HSCs treated with rCTHRC1 protein. A. Western blotting analysis of phosphorylation of Smad2, Smad3, TAK1 and total Smad4 in primary rat HSCs treated with rCTHRC1 protein from the 1st to 7th day (from quiescent to activated states). GAPDH was the loading control. The densitometry of p-Smad2/Smad2 is shown below. B. The immunostaining of YAP in the livers of WT and CTHRC1−/− mice consecutively injected intraperitoneally with CCl4 or TAA for 8 weeks.
The pro-contractile effect of CTHRC1 on LX-2 cells is abrogated by neutralizing antibody, inhibitor, or siRNA of TGFBR2. A. Collagen gel contraction analysis of LX-2 cells treated with 20 nM rCTHRC1 protein alone, rCTHRC1 protein plus TGFBR2 neutralizing antibody, TGF-β receptor inhibitor or si-TGFBR2 (n = 3 each group), respectively. B. Statistical analysis of collagen area/dish area ratio is shown below. **P < .01.
The pro-contractile effect of CTHRC1 on LX-2 cells is abrogated by knockdown of Wnt5a, but not Wnt3a. A. Collagen gel contraction analysis of control, si-Wnt5a, or si-Wnt3a of LX-2 cells stimulated with 20 nM CTHRC1 (n = 3 each group). B. Statistical analysis of collagen area/dish area ratio is shown below. **P < .01.
The pro-migratory effect of CTHRC1 on LX-2 cell is abrogated by neutralizing antibody, inhibitor or siRNA of TGFBR2. A. Cell migration analysis of LX-2 cells treated with 20 nM rCTHRC1 protein alone, rCTHRC1protein plus TGFBR2 neutralizing antibody, TGF-β receptor inhibitor, or si-TGFBR2 (n = 3 each group), respectively. B. Statistical analysis of cell number per field is shown below. Scale bars, 100 μm. **P < .01.
The pro-migratory effect of CTHRC1 on LX-2 cell is abrogated by knockdown of Wnt5a, but not Wnt3a. A. Cell migration analysis of control, si-Wnt5a, or si-Wnt3a of LX-2 cells stimulated with 20 nM CTHRC1 (n = 3 each group). B. Statistical analysis of cell number per field is shown below. Scale bars, 100 μm. **P < .01.
Primer sequences used for CTHRC1, Acta2, Col1α1, Timp1, Mmp9 and TGF-β1 detection.
Acknowledgments
We thank Dr. Qing Yang, Dr. Lipeng Hu, Dr. Xiaoxin Zhang, Dr. Lili Zhu, and Ms. Xiaoyan Cao for assistance with our experiments.
Contributor Information
Qing Xu, Email: renjixuqing@163.com.
Zhigang Zhang, Email: zzhang@shsci.org.
References
- 1.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005 Feb;115(2):209–218. doi: 10.1172/JCI24282. PubMed PMID: 15690074. Pubmed Central PMCID: 546435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuppan D., Afdhal N.H. Liver cirrhosis. Lancet. 2008 Mar 8;371(9615):838–851. doi: 10.1016/S0140-6736(08)60383-9. PubMed PMID: 18328931. Pubmed Central PMCID: 2271178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008 May;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. PubMed PMID: 18471545. Pubmed Central PMCID: 2888539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y.A., Wallace M.C., Friedman S.L. Pathobiology of liver fibrosis: a translational success story. Gut. 2015 May;64(5):830–841. doi: 10.1136/gutjnl-2014-306842. PubMed PMID: 25681399. Pubmed Central PMCID: 4477794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popov Y., Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009 Oct;50(4):1294–1306. doi: 10.1002/hep.23123. PubMed PMID: 19711424. [DOI] [PubMed] [Google Scholar]
- 6.Trautwein C., Friedman S.L., Schuppan D., Pinzani M. Hepatic fibrosis: concept to treatment. J Hepatol. 2015 Apr;62(1 Suppl):S15–S24. doi: 10.1016/j.jhep.2015.02.039. PubMed PMID: 25920084. [DOI] [PubMed] [Google Scholar]
- 7.Friedman S.L. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl. 1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. PubMed PMID: 12591185. [DOI] [PubMed] [Google Scholar]
- 8.Friedman S.L. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010 Aug;7(8):425–436. doi: 10.1038/nrgastro.2010.97. PubMed PMID: 20585339. [DOI] [PubMed] [Google Scholar]
- 9.Poelstra K. Liver fibrosis in 2015: crucial steps towards an effective treatment. Nat Rev Gastroenterol Hepatol. 2016 Feb;13(2):67–68. doi: 10.1038/nrgastro.2015.224. PubMed PMID: 26758787. [DOI] [PubMed] [Google Scholar]
- 10.Nanthakumar C.B., Hatley R.J., Lemma S., Gauldie J., Marshall R.P., Macdonald S.J. Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat Rev Drug Discov. 2015 Oct;14(10):693–720. doi: 10.1038/nrd4592. PubMed PMID: 26338155. [DOI] [PubMed] [Google Scholar]
- 11.Schuppan D., Kim Y.O. Evolving therapies for liver fibrosis. J Clin Invest. 2013 May;123(5):1887–1901. doi: 10.1172/JCI66028. PubMed PMID: 23635787. Pubmed Central PMCID: 3635731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S., Takahashi H., Lin W.W., Descargues P., Grivennikov S., Kim Y. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009 Jan 1;457(7225):102–106. doi: 10.1038/nature07623. PubMed PMID: 19122641. Pubmed Central PMCID: 2746432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015 Jul 9;523(7559):177–182. doi: 10.1038/nature14581. PubMed PMID: 26106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q., Fan J., Yang X.R., Tan Y., Zhao W., Xu Y. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012 Aug;13(8):817–826. doi: 10.1016/S1470-2045(12)70233-4. PubMed PMID: 22738799. [DOI] [PubMed] [Google Scholar]
- 15.Pyagay P., Heroult M., Wang Q., Lehnert W., Belden J., Liaw L. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005 Feb 4;96(2):261–268. doi: 10.1161/01.RES.0000154262.07264.12. PubMed PMID: 15618538. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.L., Wang T.H., Hsu H.C., Yuan R.H., Jeng Y.M. Overexpression of CTHRC1 in hepatocellular carcinoma promotes tumor invasion and predicts poor prognosis. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0070324. PubMed PMID: 23922981. Pubmed Central PMCID: 3726622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura H., Kwan K.M., Zhang Z., Deng J.M., Darnay B.G., Behringer R.R. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS One. 2008;3(9) doi: 10.1371/journal.pone.0003174. PubMed PMID: 18779865. Pubmed Central PMCID: 2527134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park E.H., Kim S., Jo J.Y., Kim S.J., Hwang Y., Kim J.M. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis. 2013 Mar;34(3):694–702. doi: 10.1093/carcin/bgs378. PubMed PMID: 23222813. [DOI] [PubMed] [Google Scholar]
- 19.Takeshita S., Fumoto T., Matsuoka K., Park K.A., Aburatani H., Kato S. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest. 2013 Sep;123(9):3914–3924. doi: 10.1172/JCI69493. PubMed PMID: 23908115. Pubmed Central PMCID: 3754269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P., Wang Y.C., Chen X.Y., Shen Z.Y., Cao H., Zhang Y.J. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-beta1 and may be associated with metastasis in human gastric cancer. Cancer Sci. 2012 Jul;103(7):1327–1333. doi: 10.1111/j.1349-7006.2012.02292.x. PubMed PMID: 22590977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Yang X.M., Wang Y.H., Feng M.X., Liu X.J., Zhang Y.L. Monoamine oxidase a suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J Hepatol. 2014 Jun;60(6):1225–1234. doi: 10.1016/j.jhep.2014.02.025. PubMed PMID: 24607627. [DOI] [PubMed] [Google Scholar]
- 22.Yi S.Q., Li J., Yamaguchi T., Hori K., Hayashi K., Itoh M. Immunolocalization of the PP family and its receptors in the gastrointestinal tract of house musk shrew, Suncus murinus. Neuro Endocrinol Lett. 2011;32(2):212–219. PubMed PMID: 21552195. [PubMed] [Google Scholar]
- 23.Liu X.J., Xu M.J., Fan S.T., Wu Z., Li J., Yang X.M. Xiamenmycin attenuates hypertrophic scars by suppressing local inflammation and the effects of mechanical stress. J Invest Dermatol. 2013 May;133(5):1351–1360. doi: 10.1038/jid.2012.486. PubMed PMID: 23303451. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z., Chometon G., Wen T., Qu H., Mauch C., Krieg T. Migration of epithelial cells on laminins: RhoA antagonizes directionally persistent migration. Eur J Cell Biol. 2011 Jan;90(1):1–12. doi: 10.1016/j.ejcb.2010.09.005. PubMed PMID: 20971525. [DOI] [PubMed] [Google Scholar]
- 25.LeClair R.J., Durmus T., Wang Q., Pyagay P., Terzic A., Lindner V. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circ Res. 2007 Mar 30;100(6):826–833. doi: 10.1161/01.RES.0000260806.99307.72. PubMed PMID: 17322174. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S., Nishimura O., Misaki K., Nishita M., Minami Y., Yonemura S. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008 Jul;15(1):23–36. doi: 10.1016/j.devcel.2008.05.007. PubMed PMID: 18606138. [DOI] [PubMed] [Google Scholar]
- 27.Kelley M.W. Leading Wnt down a PCP path: Cthrc1 acts as a coreceptor in the Wnt-PCP pathway. Dev Cell. 2008 Jul;15(1):7–8. doi: 10.1016/j.devcel.2008.06.008. PubMed PMID: 18606135. [DOI] [PubMed] [Google Scholar]
- 28.Wang C., Li Z., Shao F., Yang X., Feng X., Shi S. High expression of collagen triple helix repeat containing 1 (CTHRC1) facilitates progression of oesophageal squamous cell carcinoma through MAPK/MEK/ERK/FRA-1 activation. J Exp Clin Cancer Res. 2017 Jun 23;36(1):84. doi: 10.1186/s13046-017-0555-8. PubMed PMID: 28645305. Pubmed Central PMCID: 5481965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birukawa N.K., Murase K., Sato Y., Kosaka A., Yoneda A., Nishita H. Activated hepatic stellate cells are dependent on self-collagen, cleaved by membrane type 1 matrix metalloproteinase for their growth. J Biol Chem. 2014 Jul 18;289(29):20209–20221. doi: 10.1074/jbc.M113.544494. PubMed PMID: 24867951. Pubmed Central PMCID: 4106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X., Wang H., Liao H.J., Hu W., Gewin L., Mernaugh G. Integrin-mediated type II TGF-beta receptor tyrosine dephosphorylation controls SMAD-dependent profibrotic signaling. J Clin Invest. 2014 Aug;124(8):3295–3310. doi: 10.1172/JCI71668. PubMed PMID: 24983314. Pubmed Central PMCID: 4109532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inokuchi S., Aoyama T., Miura K., Osterreicher C.H., Kodama Y., Miyai K. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):844–849. doi: 10.1073/pnas.0909781107. PubMed PMID: 20080763. Pubmed Central PMCID: 2818947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Wang Z., Kwong S.Q., Lui E.L., Friedman S.L., Li F.R. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011 Sep;55(3):612–625. doi: 10.1016/j.jhep.2010.11.035. PubMed PMID: 21251937. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003 Jun 13;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. PubMed PMID: 12809600. [DOI] [PubMed] [Google Scholar]
- 34.Ueberham E., Low R., Ueberham U., Schonig K., Bujard H., Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology. 2003 May;37(5):1067–1078. doi: 10.1053/jhep.2003.50196. PubMed PMID: 12717387. [DOI] [PubMed] [Google Scholar]
- 35.Yang L., Inokuchi S., Roh Y.S., Song J., Loomba R., Park E.J. Transforming growth factor-beta signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte-specific deletion of TAK1. Gastroenterology. 2013 May;144(5) doi: 10.1053/j.gastro.2013.01.056. 1042-54 e4. PubMed PMID: 23391818. Pubmed Central PMCID: 3752402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang F., Parsons C.J., Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006 Sep;45(3):401–409. doi: 10.1016/j.jhep.2006.03.016. PubMed PMID: 16780995. [DOI] [PubMed] [Google Scholar]
- 37.Kordes C., Sawitza I., Haussinger D. Canonical Wnt signaling maintains the quiescent stage of hepatic stellate cells. Biochem Biophys Res Commun. 2008 Feb 29;367(1):116–123. doi: 10.1016/j.bbrc.2007.12.085. PubMed PMID: 18158920. [DOI] [PubMed] [Google Scholar]
- 38.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012 Dec;13(12):767–779. doi: 10.1038/nrm3470. PubMed PMID: 23151663. [DOI] [PubMed] [Google Scholar]
- 39.Xiong W.J., Hu L.J., Jian Y.C., Wang L.J., Jiang M., Li W. Wnt5a participates in hepatic stellate cell activation observed by gene expression profile and functional assays. World J Gastroenterol. 2012 Apr 21;18(15):1745–1752. doi: 10.3748/wjg.v18.i15.1745. PubMed PMID: 22553398. Pubmed Central PMCID: 3332287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C., Gu W., Sun B., Zhang Y., Ji Y., Xu X. CTHRC1 promotes osteogenic differentiation of periodontal ligament stem cells by regulating TAZ. J Mol Histol. 2017 Aug;48(4):311–319. doi: 10.1007/s10735-017-9729-0. PubMed PMID: 28647773. [DOI] [PubMed] [Google Scholar]
- 41.Zhao M.J., Chen S.Y., Qu X.Y., Abdul-Fattah B., Lai T., Xie M. Increased cthrc1 activates normal fibroblasts and suppresses keloid fibroblasts by inhibiting TGF-beta/Smad signal pathway and modulating YAP subcellular location. Curr Med Sci. 2018 Oct;38(5):894–902. doi: 10.1007/s11596-018-1959-1. PubMed PMID: 30341526. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R., Cao Y., Bai L., Zhu C., Li R., He H. The collagen triple helix repeat containing 1 facilitates hepatitis B virus-associated hepatocellular carcinoma progression by regulating multiple cellular factors and signal cascades. Mol Carcinog. 2015 Dec;54(12):1554–1566. doi: 10.1002/mc.22229. PubMed PMID: 25263696. [DOI] [PubMed] [Google Scholar]
- 43.Sandieson L., Hwang J.T., Kelly G.M. Redox regulation of canonical Wnt signaling affects extraembryonic endoderm formation. Stem Cells Dev. 2014 May 15;23(10):1037–1049. doi: 10.1089/scd.2014.0010. PubMed PMID: 24471440. [DOI] [PubMed] [Google Scholar]
- 44.Korswagen H.C. Regulation of the Wnt/beta-catenin pathway by redox signaling. Dev Cell. 2006 Jun;10(6):687–688. doi: 10.1016/j.devcel.2006.05.007. PubMed PMID: 16740470. [DOI] [PubMed] [Google Scholar]
- 45.Caliceti C., Nigro P., Rizzo P., Ferrari R. ROS, Notch, and Wnt signaling pathways: crosstalk between three major regulators of cardiovascular biology. Biomed Res Int. 2014;2014:318714. doi: 10.1155/2014/318714. PubMed PMID: 24689035. Pubmed Central PMCID: 3932294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu G., Sengupta P.K., Jamal B., Yang H.Y., Bouchie M.P., Lindner V. N-glycosylation induces the CTHRC1 protein and drives oral cancer cell migration. J Biol Chem. 2013 Jul 12;288(28):20217–20227. doi: 10.1074/jbc.M113.473785. PubMed PMID: 23703614. Pubmed Central PMCID: 3711289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bian Z., Miao Q., Zhong W., Zhang H., Wang Q., Peng Y. Treatment of cholestatic fibrosis by altering gene expression of Cthrc1: Implications for autoimmune and non-autoimmune liver disease. J Autoimmun. 2015 Sep;63:76–87. doi: 10.1016/j.jaut.2015.07.010. PubMed PMID: 26238209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binks A.P., Beyer M., Miller R., LeClair R.J. Cthrc1 lowers pulmonary collagen associated with bleomycin-induced fibrosis and protects lung function. Physiol Rep. 2017 Mar;5(5) doi: 10.14814/phy2.13115. PubMed PMID: 28292882. Pubmed Central PMCID: 5350163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corbett L., Mann J., Mann D.A. Non-canonical Wnt predominates in activated rat hepatic stellate cells, influencing HSC survival and paracrine stimulation of Kupffer cells. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142794. PubMed PMID: 26566235. Pubmed Central PMCID: 4643911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miao C.G., Yang Y.Y., He X., Huang C., Huang Y., Zhang L. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013 Dec;95(12):2326–2335. doi: 10.1016/j.biochi.2013.09.003. PubMed PMID: 24036368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of extracellular protein expression in four independent gene expression omnibus (GEO) databases. CTHRC1 expression was significantly upregulated in all these databases, Log (Fold change) > 3, P < .05. Agilent mouse LncRNA microarray data are available for download from the GEO under accession number GSE73985.
The relative mRNA expression level of TGF-β1 in the livers of WT or CTHRC1−/− mice, and the cell viability of HSCs treated with rCTHRC1 protein. A. The relative mRNA expression level of TGF-β1 in the livers of WT, CTHRC1−/−, WT and CTHRC1−/− mice consecutively injected intraperitoneally with CCl4 or TAA for 8 weeks. B. The cell viability of HSCs treated with 0 nM, 20 nM or 50 nM rCTHRC1 protein after 24 h, 48 h and 72 h. C. The relative mRNA expression level of TGF-β1 in the livers of WT, WT C57 mice treated with CCl4 or TAA and intraperitoneally injected with mAb of CTHRC1 or IgG. **P < .01.
Phosphorylation of Smad2, Smad3, TAK1 and total Smad4 in primary rat HSCs treated with rCTHRC1 protein. A. Western blotting analysis of phosphorylation of Smad2, Smad3, TAK1 and total Smad4 in primary rat HSCs treated with rCTHRC1 protein from the 1st to 7th day (from quiescent to activated states). GAPDH was the loading control. The densitometry of p-Smad2/Smad2 is shown below. B. The immunostaining of YAP in the livers of WT and CTHRC1−/− mice consecutively injected intraperitoneally with CCl4 or TAA for 8 weeks.
The pro-contractile effect of CTHRC1 on LX-2 cells is abrogated by neutralizing antibody, inhibitor, or siRNA of TGFBR2. A. Collagen gel contraction analysis of LX-2 cells treated with 20 nM rCTHRC1 protein alone, rCTHRC1 protein plus TGFBR2 neutralizing antibody, TGF-β receptor inhibitor or si-TGFBR2 (n = 3 each group), respectively. B. Statistical analysis of collagen area/dish area ratio is shown below. **P < .01.
The pro-contractile effect of CTHRC1 on LX-2 cells is abrogated by knockdown of Wnt5a, but not Wnt3a. A. Collagen gel contraction analysis of control, si-Wnt5a, or si-Wnt3a of LX-2 cells stimulated with 20 nM CTHRC1 (n = 3 each group). B. Statistical analysis of collagen area/dish area ratio is shown below. **P < .01.
The pro-migratory effect of CTHRC1 on LX-2 cell is abrogated by neutralizing antibody, inhibitor or siRNA of TGFBR2. A. Cell migration analysis of LX-2 cells treated with 20 nM rCTHRC1 protein alone, rCTHRC1protein plus TGFBR2 neutralizing antibody, TGF-β receptor inhibitor, or si-TGFBR2 (n = 3 each group), respectively. B. Statistical analysis of cell number per field is shown below. Scale bars, 100 μm. **P < .01.
The pro-migratory effect of CTHRC1 on LX-2 cell is abrogated by knockdown of Wnt5a, but not Wnt3a. A. Cell migration analysis of control, si-Wnt5a, or si-Wnt3a of LX-2 cells stimulated with 20 nM CTHRC1 (n = 3 each group). B. Statistical analysis of cell number per field is shown below. Scale bars, 100 μm. **P < .01.
Primer sequences used for CTHRC1, Acta2, Col1α1, Timp1, Mmp9 and TGF-β1 detection.