Abstract
Background: Antibiotic-resistant H. pylori was increasingly found in infected individuals, which resulted in treatment failure and required alternative therapeutic strategies. Daphnetin, a coumarin-derivative compound, has multiple pharmacological activities. Methods: The mechanism of daphnetin on H. pylori was investigated focusing on its effect on cell morphologies, transcription of genes related to virulence, adhesion, and cytotoxicity to human gastric epithelial (GES-1) cell line. Results: Daphnetin showed good activities against multidrug resistant (MDR) H. pylori clinical isolates, with minimal inhibitory concentration (MIC) values ranging from 25 to 100 μg/mL. In addition, daphnetin exposure resulted in H. pylori morphological changes. Moreover, daphnetin caused increased translocation of phosphatidylserine (PS), DNA damage, and recA expression, and RecA protein production vs. control group. Of great importance, daphnetin significantly decreased H. pylori adhesion to GES-1 cell line vs. control group, which may be related to the reduced expression of colonization related genes (e.g., babA and ureI). Conclusions: These results suggested that daphnetin has good activity against MDR H. pylori. The mechanism(s) of daphnetin against H. pylori were related to change of membrane structure, increase of DNA damage and PS translocation, and decrease of H. pylori attachment to GES-1 cells.
Keywords: Helicobacter pylori, daphnetin, mechanism of action, colonization
1. Introduction
Helicobacter pylori (H. pylori), a Gram-negative bacterium that specifically colonizes in the human stomach, has developed numerous strategies to survive in the high acidity environment in the stomach lumen [1]. It has been reported that this pathogen chronically infects over half of all humans [2]. Colonization of H. pylori can lead to gastritis and peptic ulcers, mucosa-associated lymphoid tissue lymphoma, and gastric cancer [3]. Therefore, H. pylori has been categorized as a Class I carcinogen by the World Health Organization (WHO) [4]. In addition, H. pylori infections are more common in developing countries and are mostly developed during childhood [5]. Of note, anti-H. pylori therapy has been used for decades, but the efficacy of the treatment has declined during the last decade because of increasing antibiotic resistance [6,7]. In 2017, WHO listed 12 bacteria that threaten human health the greatest, among which clarithromycin-resistant H. pylori was considered to be one of the high priorities [8]. A recent review demonstrated that H. pylori resistance rate to clarithromycin was 28.9%, whereas the primary antibiotic resistance of H. pylori was metronidazole in China (around 70%) [9]. Therefore, new antibacterial agents against H. pylori are needed to overcome this concern.
Daphnetin (7,8-dihydroxycoumarin)—a major bioactive component extracted from the genus Daphne as well as several other genera—is a coumarin-derivative compound of aromatic odor, with structure comprising o-hydroxy cinamic acid lactones (Figure 1) [10]. In China, daphnetin has been used clinically to treat Buerger’s disease for many years [11]. Its multiple pharmacological activities, including anti-inflammatory, -diarrheal, -parasitic, -hypoxia, etc., have been reported [12,13]. Daphnetin exhibited selective cytotoxicity to human renal cell carcinoma cells, relative to noncarcinoma proximal tubular cells [14]. So far, neither toxic effects [15] nor genetic toxicity [15,16] were found in daphnetin. Therefore, it has attracted extensive research interests to investigate the activity and mechanism(s) of daphnetin against MDR H. pylori.
Figure 1.
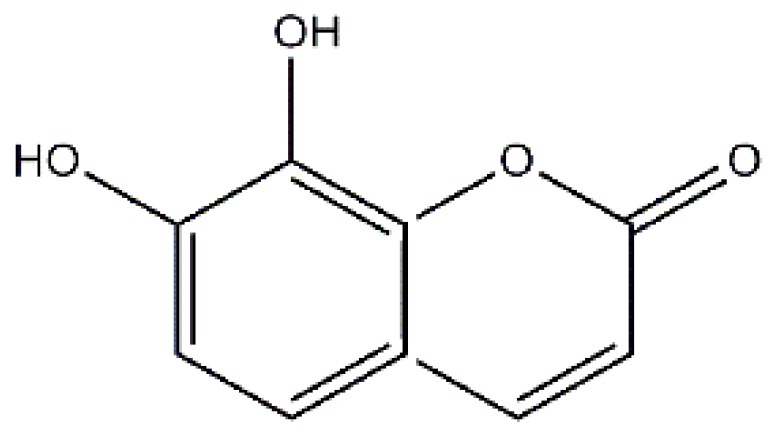
Structure of daphnetin (Molecular Weight 178.143 g/mol).
In the current studies, we examined the antibacterial activity of daphnetin against 20 H. pylori clinical isolates, including MDR strains, and investigated its anti-H. pylori mechanisms. Our findings suggest that daphnetin may offer a significant advantage in the prevention of H. pylori infections.
2. Results
2.1. Antibacterial Activity of Daphnetin Against H. pylori Strains
A total of 20 H. pylori strains isolated from human gastric antrum were used in this study (Table S1). Daphnetin inhibited all the tested H. pylori strains, regardless of their resistance profiles to other common used antibiotics, at concentrations ranging from 25 to 100 μg/mL (Table 1 and Table S1). The percentage of clarithromycin resistance in the studied H. pylori strains was 25%, with MICs ranging from 0.016 to 4 μg/mL (Table 1). Eighty-five percent of the clinical H. pylori strains were resistant to metronidazole with MICs ranging from 4 to 256 μg/mL, and daphnetin still had good activity against these highly metronidazole resistant H. pylori strains with MICs of 25 μg/mL (Table 1 and Table S1). H. pylori ATCC43504 strain was used as a quality control with expected MICs of clarithromycin and metronidazole (Table 1).
Table 1.
Minimum inhibitory concentrations (MICs) of daphnetin, metronidazole, and clarithromycin against H. pylori strains.
| Antibiotics | MICs (μg/mL) | Percent of Resistance (%) b | |
|---|---|---|---|
| 20 Clinical Isolates | ATCC43504 a | ||
| Daphnetin | 25–100 | 25 | NA c |
| Clarithromycin | 0.016–4 | 0.016 | 25% |
| Metronidazole | 4–256 | 128 | 85% |
aH. pylori ATCC43504 strain served as MIC quality control (metronidazole: 64–256 μg/mL; clarithromycin: 0.015–0.12 μg/mL). b Metronidazole: ≤ 8 μg/mL for susceptible and > 8 μg/mL for resistant; clarithromycin: ≤ 0.25 μg/mL for susceptible, 0.5 μg/mL for intermediate and > 0.5 μg/mL for resistant. c NA: not applicable.
2.2. Effect of Daphnetin on H. pylori Morphology
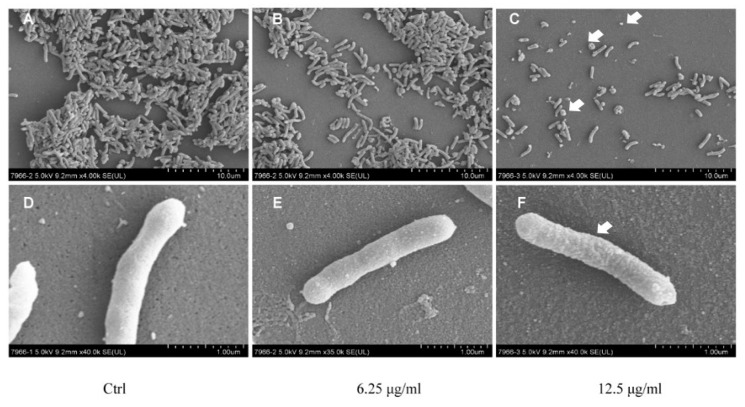
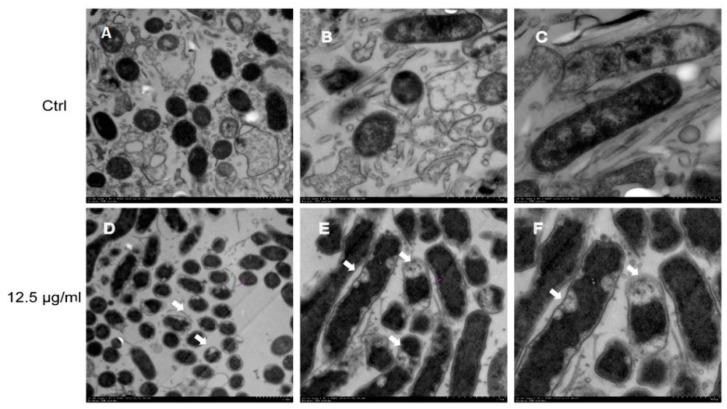
We first visualized the morphology of entire H. pylori cells by scanning electron microscopy (SEM). Control cells without daphnetin exposure demonstrated smooth, homogenous cell surfaces and rod-shaped morphotypes (Figure 2A,D). After exposure to daphnetin at sub-MIC concentrations (e.g., 6.25 or 12.5 μg/mL) for three days, some H. pylori cells showed extensive surface damage (e.g., budding structures), heterogeneous populations of cells, and increased coccid forms in a concentration-dependent manner (Figure 2B,E for daphnetin at 6.25 μg/mL exposure; 2C,F for daphnetin at 12.5 μg/mL exposure). Transmission electron microscopy (TEM) images displayed the organization of H. pylori with a clearly defined cytoplasm and cell membrane. The ultrastructural characteristics of H. pylori cells without daphnetin exposure showed homogeneous cytoplasm and intact cell membrane (Figure 3A–C). However, daphnetin-treated (at 12.5 μg/mL) H. pylori cells showed visual morphological changes, including reduced bacterial size (relative diameter: control:daphnetin exposure group = 1.00:0.77), rough outer membrane, granular-textured cytoplasm, peculiar detachments between membrane and cytoplasm, numerous vesicles, and/or typical “holes” attached to the inner membrane. In addition, vesicles emerged in ~40% H. pylori cells in the daphnetin exposure group, while no vesicle was found in control group (Figure 3D–F). Taken together, electron microscopy images showed that daphnetin exposed H. pylori had substantial visual morphological changes.
Figure 2.
The morphology of H. pylori cells with/without daphnetin exposure observed by SEM. Control (A,D); H. pylori were treated with 6.25 μg/mL (B,E) or 12.5 μg/mL of daphnetin (C,F). Magnification: A–C = 4000; D–F = 40000.
Figure 3.
The morphology of H. pylori cells with/without daphnetin exposure observed by TEM. Control (A–C); H. pylori treated with 12.5 μg/mL daphnetin (D–F). Magnification: A and D = 3000; B and E = 5000; and C and F = 8000.
2.3. Daphnetin-Induced Membrane Changes
The extent of phosphatidylserine (PS) exposure on the outer membrane was determined using annexin V. We observed that daphnetin at sub-MIC concentration (12.5 μg/mL) resulted in significant increased annexin V-mediated labeling of PS, from 5.93% to 56.99% (Table 2). These data indicated that daphnetin could change H. pylori’s outer membrane structure. We also employed the bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC) and propidium iodide (PI) dye to monitor membrane polarity and permeability. As shown in Table 2, no significant fluorescence changes were observed between control and daphnetin-treated H. pylori cells. Subsequently, we demonstrated that protein leakage was slightly increased in daphnetin treated group vs. control group (Table 2). However, these differences did not reach statistical significance. These results suggested that daphnetin treatment led to outer membrane structure change, while had no significant effect on membrane permeability and depolarization in H. pylori.
Table 2.
Membrane changes induced by daphnetin.
| Groups | Mean of the Positive Fluorescence ± SD (%) | Protein Leakage (μg/mL) | ||
|---|---|---|---|---|
| PS Translocation | Membrane Permeability | Membrane Depolarization | ||
| Control | 5.93 ± 1.25 | 7.78 ± 0.62 | 9.26 ± 1.34 | 0.56 ± 0.01 |
| Daphnetin (12.5 μg/mL) |
56.99 ± 5.78 * | 5.06 ± 3.40 | 8.87 ± 2.71 | 0.60 ± 0.03 |
* P < 0.001 vs. control.
2.4. Daphnetin Caused DNA Damage
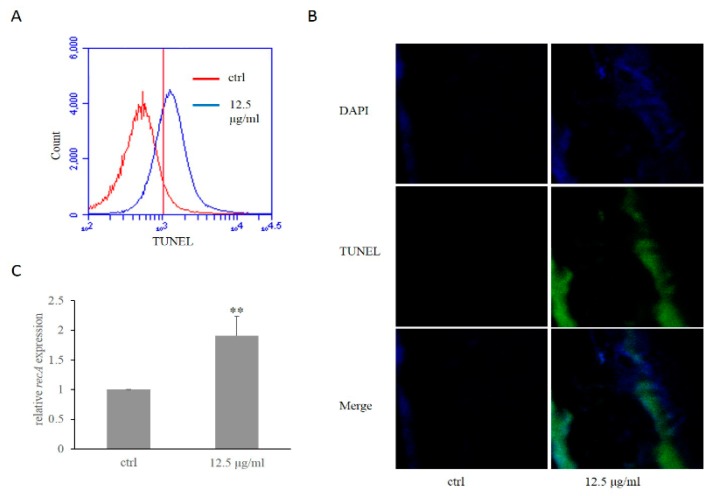
In order to determine whether daphnetin was able to cause DNA damage in H. pylori, flow cytometry and confocal were used to detect terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining cells. As shown in Figure 4A, significant higher fluorescence signal was observed in daphnetin-treated H. pylori cells vs. control (8.11% vs. 68.02% for control and daphnetin- treated group, respectively). These results were confirmed with confocal analyses (Figure 4B). It is well known that RecA is linked between DNA damage and membrane structure changes [17]. Thus, we tested the transcription level of recA and found that recA expression was significantly increased by daphnetin exposure as compared to untreated cells (Figure 4C). Corresponding to recA expression results, RecA protein production was also significantly increased in the daphnetin exposure group vs. control (66.9 ± 6.1 to 133.1 ± 6.1 for control and daphnetin-treated group, respectively; p < 0.001, Figure S1).
Figure 4.
Detection of DNA damage and recA expression in H. pylori. DNA damage detected using TUNEL by flow cytometry (A) and confocal (B). (C) The expression of recA in H. pylori with/without daphnetin exposure. (** p < 0.001 vs. control). The expression of the study genes without daphnetin exposure was normalized as 1.
2.5. Daphnetin Decreased H. pylori Adherence to Immortalized Human Gastric Epithelial Cell Line (GES-1) and Inhibited Colonization-Associated Gene Expression
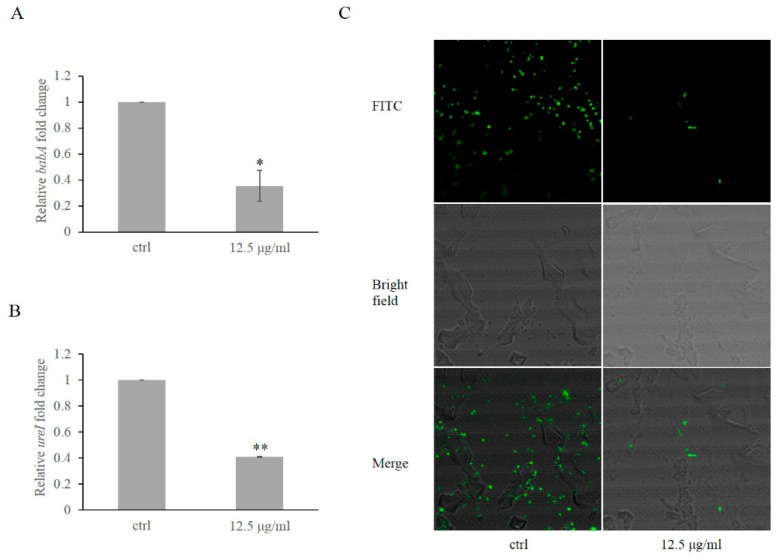
In order to investigate the effect of daphnetin on colonization, the expression of two key genes (babA and ureI) related to colonization were measured in H. pylori by qRT-PCR. The transcriptions of the two genes were significantly repressed by daphnetin exposure as compared to untreated cells (Figure 5A,B). In addition, we tested the production of BabA and UreI protein by LC–MS/MS, and found that consistent to the gene expression data, BabA protein level was also decreased in the daphnetin exposure group (from 113.9 ± 10.8 to 86.1 ± 10.8, p < 0.05; Figure S2), while the UreI level was below the limit of detection. Moreover, to test the adherence ability of H. pylori to the GES-1 cells, H. pylori cells with/without daphnetin exposure were labeled with fluorescein-isothiocyanate (FITC) and analyzed by confocal microscopy. We demonstrated that daphnetin exposure significantly decreased the adherence of H. pylori to the GES-1 cells vs. control group (Figure 5C). These results suggested that daphnetin may prevent H. pylori colonization in human stomach.
Figure 5.
The transcription of babA (A) and ureI (B) in H. pylori with/without daphnetin exposure. The expression of the study genes without daphnetin exposure was normalized as 1. Inhibitory effect of daphnetin on adhesion of H. pylori to GES-1 cells (C). The level of adherence of H. pylori was detected by confocal (magnification: 600). All the data were presented as mean and standard deviations (SD). * p < 0.05, ** p < 0.01 vs. control.
2.6. The Cytotoxic Effect of Daphnetin on GES-1
The cytotoxic effect of daphnetin to GES-1 cells in medium (with/without serum) was investigated by using cell counting kit-8 (CCK-8) assay. The results showed that sub-MIC of daphnetin was well tolerated by GES-1 cells and there was no significant cytotoxic difference under both conditions (Table 3).
Table 3.
The cytotoxic effects of daphnetin to GES-1 cells in medium (with/without serum).
| Groups | Viability (mean ± SD/%) a | |
|---|---|---|
| DMEM | DMEM + 10% FBS b | |
| Control | 100.00 ± 7.79 | 100.00 ± 2.30 |
| Daphnetin 12.5 μg/mL |
84.43 ± 5.95 | 81.14 ± 11.52 |
a: The viability of control group without daphnetin exposure was normalized as 100%. b: DMEM: Dulbecco’s modified Eagle’s medium; FBS: fetal bovine serum.
3. Discussion
The increasing prevalence of MDR H. pylori around the world has become one of the major causes of treatment failure in H. pylori infections [18,19]. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) resistance breakpoints of clarithromycin and metronidazole to H. pylori were >0.5 μg/mL f and >8 μg/mL, respectively [20]. The global H. pylori antibiotic resistance rates were 17.2% for clarithromycin and 26.7% for metronidazole. In general, the resistance rate in developing countries is higher than that in developed countries [7,19]. Of note, metronidazole resistance rate of H. pylori isolated in the southeast coastal region of China is close to 100% according to a recent report [21]. In our study, the resistance rates for clarithromycin and metronidazole were much higher that the global H. pylori antibiotic resistance rates. According to the study from De Francesco et al., the clarithromycin- and metronidazole-resistance levels can be further classified into low level resistance (MICs from > 0.5 to ≤ 8 μg/mL for clarithromycin and from > 8 to ≤ 32 μg/mL for metronidazole) and high level resistance (MICs from > 8 to 256 μg/mL for clarithromycin and from > 32 to 256 μg/mL for metronidazole) [22]. All the 20 clinical strains in our study were low level resistance to clarithromycin and 50% (10/20) of clinical strains were high level resistance to metronidazole (See Table S1). The similar result was reported by Bai et al., indicating that antibiotic resistance in Chinese patients (MIC50 = 0.38 μg/mL for clarithromycin, and MIC50 = 128 μg/mL for metronidazole) [23]. Our study demonstrated that daphnetin has anti-H. pylori activity, with MICs ranging from 25 to 100 μg/mL, regardless of their resistance patterns to other antibiotics. Daphnetin was also reported to have antibacterial activity against other bacterial species, including S. aureus, E. coli, P. aeruginosa, and R. solanacearum [24,25]. However, the mechanism of daphnetin against bacteria has not been well studied.
Apoptosis was always occurred when stimulated by appropriate trigger in both eukaryotic multicellular organisms and in prokaryotic cells [26,27]. The cell morphology of apoptosis includes morphological transition from spiral to coccoid, increased in electrondense bodies, appear vacuoles [26,27]. Our morphological data were consistent with results of Cellini et al., who demonstrated that H. pylori cells change from typical spiral morphology to coccoid form as a response to environmental stress [28,29]. In addition, these phenomena were also similar with results of Shu et al., who observed a reduction in size and empty bubble degeneration in the daphnetin treatment group in synovial cells [30].
A stereotypical set of biochemical hallmarks of apoptosis (e.g., PS translocation, membrane depolarization, and DNA damage) have been proved in both eukaryotic and prokaryotic organisms [17,27,31,32]. In our experiments, we observed PS translocation and DNA damage significantly increased after daphnetin exposure as compared to control group. It is well known that DNA damage and membrane structure changes are the specific characteristics of apoptosis [33]. These phenotypes were also confirmed by other research groups studying prokaryotic organisms during apoptosis [17,28]. Although we could not detect one of the phenotypes related to apoptosis (e.g., membrane depolarization) in the current study [17]. The outcome may be related to decreasing reactive oxygen species (ROS) formation by daphnetin exposure (daphnetin-treated vs. control: 73.8 ± 7.11% vs. 10.42 ± 2.42%), due to there is a positive interaction between ROS accumulation and depolarization [34]. In addition, RecA plays a central role in the exhibition of these phenotypes [17], and we actually observed its expression significantly increased after daphnetin exposure. Therefore, our observations suggested that daphnetin exposure could induce a non-typical apoptosis in H. pylori.
RecA not only mediates cell death, but also plays an important role during stomach colonization [35]. For instance, RecA negatively regulates colonization-related babA gene expression [35]. In our current studies, we observed recA gene expression and RecA protein production were significantly increased by daphnetin exposure as compared to control. BabA is the best-characterized adhesion protein in H. pylori, which contributes the bacterium to attach to the glycosylated gastric epithelial cells [36]. Inconsistent with RecA’s function on babA expression, we found that the transcription of babA and BabA protein production were significantly decreased with daphnetin treatment vs. controls. UreI is a proton-gated urea channel and plays an important role in H. pylori colonization on acidic stomach surface [37]. We found that urel gene was significantly decreased with daphnetin exposure vs. control group by qRT-PCR. However, we could not detect UreI protein expression in our study, which might be related to the limitation of our LC–MS/MS analyses, as well as the solubility of UreI protein [37]. As babA and urel play important roles in cell colonization, the decreased expression of these two genes may lead to decreased adherence of H. pylori to cells. Consistently, decreased adherence of H. pylori to GES-1 cells was observed with daphnetin exposure. In addition, we found no significant cytotoxicity of daphnetin to the GES-1 cell line, which is in agreement with published data [14,16]. These results suggested that daphnetin may have ability to prevent H. pylori colonization on the stomach.
4. Materials and Methods
4.1. Bacterial Strains and Materials
Twenty H. pylori strains from CAMS Collection Center of Pathogen Microorganisms (CCPM) were isolated from gastric antrum in Beijing, China (see Table S1). H. pylori ATCC43504 was a standard strain isolated from human gastric antrum in Australia. It is a metronidazole-resistant strain, while sensitive to other clinical antibiotics (e.g., clarithromycin). For the isolation of H. pylori strains, gastric mucosal specimens were collected, inoculated on agar plates containing 5% defibrinated sheep blood, and cultured at 37 °C under microaerobic conditions (10% CO2, 5% O2, 85% N2) for 3 days [38]. The isolated H. pylori strains were confirmed by standard biochemical tests (urease, catalase), 16S rRNA sequencing, and morphological analyses. The study H. pylori strains were frozen (BHI media with 30% glycerine) in cryobank tubes at −80 °C. Clarithromycin and metronidazole were purchased from National Institutes for Food and Drug Control, Beijing, China. Vancomycin was purchased from INALCO SPA in Milano, Lombardia, Italy. Trimethroprim, polymyxin B sulfate, amphotericin B, and cefsulodin sodium salt were purchased from Sangon Biotech Co., Ltd., Shanghai, China. Daphnetin was purchased from Meilun Biotech Co., Ltd., Dalian, China. β-cyclodextrin, fluorescein-isothiocyanate (FITC) and human serum albumin (HSA) were purchased from Sigma, St. Louis, MO, USA. DMEM and FBS were obtained from Gibco, Waltham, MA, USA.
4.2. Cell Cultures
Agar-based culture of H. pylori: Frozen H. pylori strains were revitalized and maintained on Columbia blood agar plates containing selective antibiotics (e.g., vancomycin, trimethroprim, polymyxin B sulfate, amphotericin B, and cefsulodin sodium salt), and cultured at 37 °C under microaerobic conditions (10% CO2, 5% O2, 85% N2) for 3 days [38].
Liquid broth-based culture of H. pylori: H. pylori cells were swap from agar plate, resuspended in Brucella broth containing 10% FBS and 1% vancomycin, and cultured at 37 °C under microaerobic conditions (10% CO2, 5% O2, 85% N2) for 3 days [38].
GES-1, an immortalized human gastric epithelial cell line was cultured in DMEM medium supplemented with 10% FBS in a humidified incubator [39].
4.3. MICs of Daphnetin, Metronidazole, and Clarithromycin on H. pylori Strains
The antibacterial activities of daphnetin, metronidazole and clarithromycin against H. pylori were examined by standard agar dilution test (CLSI [M45]). Briefly, a saline suspension equivalent to a 2.0 McFarland standard (about 108 CFU/mL) was prepared from a Mueller-Hinton agar plate plus selective antibiotics [38]. The inoculum is replicated directly onto the antimicrobial agent-containing agar dilution plates (daphnetin: 3.125–400 μg/mL; metronidazole: 1–512 μg/mL; clarithromycin: 0.015–8 μg/mL). The plates were incubated at 37 °C for 3 days. H. pylori ATCC43504 strain was used as a control. The MIC was determined as the lowest concentration of drug showing no growth, a haze, one discrete colony, or multiple tiny colonies [40].
4.4. H. pylori Morphology Analyses by SEM and TEM
The morphology of H. pylori with/without daphnetin exposure was performed by SEM and TEM as previously reported with some modifications [41,42]. For SEM, H. pylori strains were incubated with/without 6.25 or 12.5 μg/mL daphnetin for 3 days, then collected and fixed with 2.5% glutaraldehyde. Postfixing, the samples were centrifuged to remove glutaraldehyde and resuspended in phosphate buffer. The bacterial suspensions were spotted on a polished silicon wafer and allowed to dry overnight in a biosafety cabinet. The samples were then coated with chromium before SEM imaging. For TEM, H. pylori cells were exposed with/without 12.5 μg/mL of daphnetin for 3 days, then collected and fixed with 2.5% glutaraldehyde at least 2 h at 4 °C. The fixed organisms were washed and postfixed with 1% osmium tetroxide. Then the samples were washed, dehydrated in a graded series of ethanol and embedded in Epon Araldite. Ultrathin sections containing the cells were placed on copper grids, stained with uranyl acetate and lead citrate, observed, and photographed with a TEM microscope (Hitachi, Tokyo, Japan).
4.5. Detection of Membrane Changes
H. pylori cells were grown as described in the ‘Cell culture’ section above. Briefly, H. pylori cells were scraped from the Mueller-Hinton agar plates with or without daphnetin. To monitor the degree of cell membrane structural changes [17], a TransDetect Annexin V-FITC/PI Cell Apoptosis Detection Kit (Transgen Biotech, Beijing, China) was used. For membrane depolarization experiment [17], staining of cells were performed using DiBAC (Invitrogen, Waltham, MA, USA). To determine the integrity of cell membrane, a bicinchoninic acid (BCA) protein assay kit was used. Briefly, H. pylori cells (0.5 McFarland) were cultured with or without daphnetin in Brucella broth for 24 h. The samples were centrifuged at 4 °C, the supernatants were treated with BCA assay reagent, and OD at 595 nm was measured [43].
4.6. Detection of DNA Damage
To detect DNA damage in H. pylori [44], a TransDetect In Situ Fluorescein TUNEL Cell Apoptosis Detection Kit (Transgen Biotech, Beijing, China) was employed. Accuri C6 (BD, Franklin Lakes, Germany) flow cytometer and LSM510 confocal (Zeiss, Oberkochen, Germany) were used to detect the fluorescence signal changes. All flow cytometry data were collected using the Accuri C6 software. At least 10,000 cells were collected and analyzed for each sample. Flow data were processed and analyzed with CFlow Plus (BD, Franklin Lakes, Germany).
4.7. RNA Isolation and Quantitative Real-Time PCR
Briefly, H. pylori cells were incubated with/without 12.5 μg/mL daphnetin for 3 days, then collected. Total RNA was isolated using an RNAprep pure Cell/Bacteria Kit (TianGen Biotech, Beijing, China). Primers used in this study are listed in Table 4. Primer Premier 5 was used to design the primers, and nucleotide search was performed based on Helicobacter pylori strain 26695 chromosome locus (HP0071 for ureI; HP1243 for babA; and HP0153 for recA). A housekeeping gene 16S rRNA was used as control [45]. qRT-PCR was performed on the 7500 fast using an SYBR Green Supermix, Life Technologies (AB & Invitrogen, Waltham, MA, USA). All assays were carried out at least in three independent experiments in triplicates. Relative quantification was calculated by the ΔΔCt method.
Table 4.
The qRT-PCR primers used in this study.
| Primers | Sequence |
|---|---|
| ureI | Forward: CCCCTGTAGAAGGTGCTGAA Reverse: GCCGCATACAAGTAGGTGAAAC |
| babA | Forward: AAGCCTATCAAATCCTCCAAACG Reverse: TGGCGAGCAGTTATTATTCCCT |
| recA | Forward: CTAAGAGGTTGGGCGTGGA Reverse: CAATCCCTCCGCTTCTGGT |
| 16s rRNA | Forward: GTGCCAGCMGCCGCGGTAA Reverse: GACTACHVGGGTATCTAATCC |
4.8. Membrane Preparation and Proteomics by Liquid Chromatography–Mass Spectrometry/Mass Spectrometry Analyses
The membrane fraction of H. pylori was prepared as described previously with modifications [46,47]. In brief, H. pylori cells were harvested, washed in 20mM Tris-HCl (pH 7.5), and pelleted twice by centrifugation (4000× g for 5 min). Bacterial cells were suspended in 20 mM Tris-HCl (pH 7.5) and broken by repeated ultrasonication. The mixture was incubated at room temperature for 30 min. Cell debris were removed by centrifugation (9000× g for 20 min, 4 °C). Total membrane pellet was collected by centrifugation (50,000× g for 20 min, 4 °C), then resuspended in 20 mM Tris-HCl (pH 7.5) containing 2.0% (w/v) sodium lauryl sarcosine.
For shotgun proteomics [48], proteins were reduced by dithiothreitol at 95 °C for 5 min and alkylated with iodoacetamide in dark for 1 h. Proteins were digested by sequencing grade modified trypsin (1:50 w/w) overnight at 37 °C. Lastly, the sample was desalted by C18 reverse-phase ZipTip. Standard shotgun proteomics techniques [48,49] were used to analyze the samples on a Thermo Scientific Orbitrap Fusion Lumos equipped with a Thermo Scientific™ Nanospray Flex Ion Source and nano-LC 1200 (Thermo Fisher Scientific, Bremen, Germany). Briefly, protein digests were enriched on a trap column (Thermo Scientific™ Acclaim™ PepMap™ 100 C18 LC Column 164946 (75 μm × 20 mm)) and separated with another column (Thermo Scientific™ Acclaim™ PepMap™ 100 C18 LC Column 164943 (0.050 mm × 150 mm)). After sample loading, the gradient started from 2 to 8% of solvent buffer (acetonitrile with 0.1% formic acid) for 1 min and then from 8 to 30% of solvent buffer for 69 min. Then, the gradient quickly changed from 30 to 40% of solvent buffer for 14 min and from 40 to 100% of solvent buffer for 1 min. In the final stage, the mobile phase was kept at 100% of solvent buffer for 5 min. The eluted peptides were ionized online by electrospray ionization and transferred into an Orbitrap Fusion Lumos mass spectrometer which was operated in the positive mode to measure full scan Mass Spectrometry (MS) spectra (from m/z 350–1550 in the Orbitrap analyzer at resolution R = 120,000 (MS1) and 15,000 (MS2). Higher-energy C-trap dissociation collision Energy was 32%.
For database analyses, unbiased data-dependent MS/MS acquisition was employed in peptide/protein identification. These initial data-dependent runs were searched against H. pylori ATCC 43504 and ATCC 26695 databases. Thermo Scientific™ Proteome Discoverer™ version 2.2 was used to analyze the quantitative data. The search parameters were set to MS accuracy 10 ppm, MS/MS accuracy 0.02 Da, dynamic modification (protein terminus) for acetyl, dynamic modification for oxidation, and static modification for carbamidomethyl.
4.9. H. pylori Adhesion Assays
To test the effect of daphnetin on H. pylori adherence to GES-1 cells, an adhesion assay was performed as described previously with minor modifications [50]. Briefly, GES-1 cells were seeded on cover glass bottom dishes and cultured at 37 °C with 5% CO2, until appropriate confluence (80–90%) was reached. Samples were then infected with FITC-labeled H. pylori (with or without daphnetin exposure). For H. pylori FITC-labeling, a previously described method [51] with minor modifications was used. Briefly, after 3 days incubation, H. pylori cells were harvested from agar plates with/without daphnetin exposure, and resuspended in 1.0 mL of 0.15 M NaCl and 0.1 M Na2CO3, pH 9.0 in double-distilled water by gentle pipetting. H. pylori cells were adjusted to 1.0 McFarland. Ten microliters of freshly prepared 1% FITC in dimethyl sulfoxide (DMSO) were added to the suspension, then incubated for 1 h at room temperature in the dark. Bacteria were recovered by centrifugation at 3000× g for 5 min, resuspended by gentle pipetting in 1.0 mL PBS supplemented with 5% inactivated fetal bovine serum, 0.2% BSA and 0.05% Tween 20. Add the FITC-labeled H. pylori cells into the dishes and incubated 4 h at 37 °C. After incubation, three washes were performed with PBS to remove nonadherent bacteria. LSM710 confocal was used to observe H. pylori adherence to GES-1 cells (Zeiss, Germany).
4.10. Cell Cytotoxicity Assays
Cell cytotoxicity was tested by the CCK-8 assay [52]. Briefly, GES-1 cells were plated in a 96-well plate. After overnight incubation, the medium were replaced by with/without serum medium, and then different concentrations of daphnetin were added. After 24 h of incubation, the cells were treated with CCK-8 assay reagent, and OD at 450 nm was measured.
4.11. Statistical Analyses
Descriptive statistics of samples in the detection of the cell-related changes were presented as means and SD from at least two independent experiments. Comparisons between control and daphnetin-treated groups were performed via unpaired 2-tailed Student’s t-test. p < 0.05 was considered statistically significant.
5. Conclusions
In conclusion, the anti-H. pylori activity of daphnetin and relevant mechanisms of its action were reported in the current study. Daphnetin exhibited anti-MDR H. pylori activities. The mechanisms of its action attributed to induce membrane structure changes, DNA damage, and increase RecA expression. In addition, daphnetin exposure resulted with decreased colonization related gene expression (e.g., babA and urel) and adherence to GES-1 cells with no significant cytotoxicity to the cell line (Figure 6). Taken together, these results suggested that daphnetin has a potential to be an effective anti-H. pylori agent. Future studies, including in vivo anti-H. pylori activity evaluation and synthesis of daphnetin-derivatives with better biological activity, are expected.
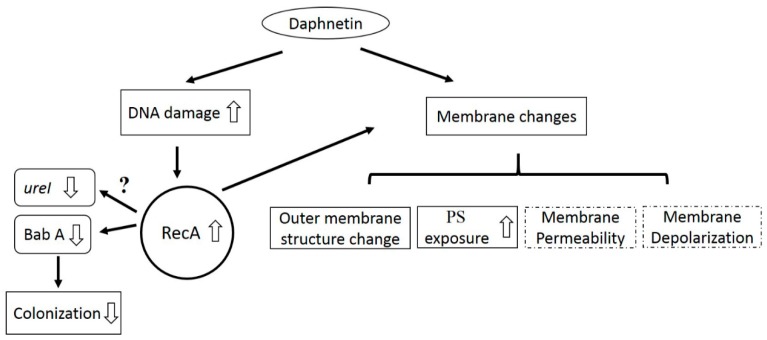
Figure 6.
Hypothesized model of the mechanism(s) of daphnetin against H. pylori. Daphnetin exposure caused DNA damage and subsequently induced recA expression. In addition, recA negatively regulated babA transcription. To our best knowledge, no study indicated a direct interaction between recA and urel. Lower babA and urel transcription, and their respective protein production could reduce H. pylori adherence to GES-1 cells. Moreover, daphnetin exhibited effect on membrane changes (e.g., outer membrane structural change and increased PS exposure). In the current study, no significant impact of daphnetin on membrane permeability and depolarization was observed (dotted line indicates no statistical significance between control and daphnetin exposure groups).
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/4/850/s1. Table S1. MICs of metronidazole, clarithromycin and daphnetin against H. pylori strains. Figure S1. The expression of RecA in H. pylori with/without daphnetin exposure. Figure S2. The expression of BabA in H. pylori with/without daphnetin exposure.
Author Contributions
X.Y. conceived and designed the experiments; G.W., J.P., X.H., T.N. X.L., X.W. and Y.L. performed the experiments and analyzed the data; C.L., Y.Q.X., X.Y., J.J., and Y.X. edited and modified the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 81621064, 81361138020), CAMS Initiative for Innovative Medicine (grant number 2016-I2M-3-014, 2017-I2M-1-012) and the National Mega-project for Innovative Drugs (grant number 2018ZX09721001).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Warren J.R., Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;321:1273–1275. [PubMed] [Google Scholar]
- 2.Zali M.R. Facing resistance of H. pylori infection. Gastroenterol. Hepatol. Bed Bench. 2011;4:3–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Walker M.M., Crabtree J.E. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann. N. Y. Acad. Sci. 1998;859:96–111. doi: 10.1111/j.1749-6632.1998.tb11114.x. [DOI] [PubMed] [Google Scholar]
- 4.McNulty C., Owen R., Tompkins D., Hawtin P., McColl K., Price A., Smith G., Teare L. Helicobacter pylori susceptibility testing by disc diffusion. J. Antimicrob. Chemother. 2002;49:601–609. doi: 10.1093/jac/49.4.601. [DOI] [PubMed] [Google Scholar]
- 5.Chey W.D., I Leontiadis G., Howden C.W., Moss S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y., Zhang Z., Hong J., Liu W., Lu H., Du Y., Wang W., Xu J., Wang X., Huo L., et al. Furazolidone-containing triple and quadruple eradication therapy for initial treatment for Helicobacter pylori infection: A multicenter randomized controlled trial in China. Helicobacter. 2018;23:e12496. doi: 10.1111/hel.12496. [DOI] [PubMed] [Google Scholar]
- 7.Thung I., Aramin H., Vavinskaya V., Gupta S., Park J.Y., Crowe S.E., Valasek M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2015;43:514–533. doi: 10.1111/apt.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. [(accessed on 14 February 2019)];2017 Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/
- 9.Hu Y., Zhu Y., Lu N.-H. Primary Antibiotic Resistance of Helicobacter pylori in China. Dig. Dis. Sci. 2017;51:70–1154. doi: 10.1007/s10620-017-4536-8. [DOI] [PubMed] [Google Scholar]
- 10.Zobel A.M., Brown S.A. Localization of daphnetin and umbelliferone in different tissues of Daphne mezereum shoots. Can. J. Bot. 1989;67:1456–1459. doi: 10.1139/b89-194. [DOI] [Google Scholar]
- 11.Yang Y.-Z., Ranz A., Pan H.-Z., Zhang Z.-N., Lin X.-B., Meshnick S.R. Daphnetin: A novel antimalarial agent with in vitro and in vivo activity. Am. J. Trop. Med. Hyg. 1992;46:15–20. doi: 10.4269/ajtmh.1992.46.15. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda H., Nakamura S., Chisaki Y., Takada T., Toda Y., Murata H., Itoh K., Yano Y., Takata K., Ashihara E. Daphnetin inhibits invasion and migration of LM8 murine osteosarcoma cells by decreasing RhoA and Cdc42 expression. Biochem. Biophys. Res. Commun. 2016;471:63–67. doi: 10.1016/j.bbrc.2016.01.179. [DOI] [PubMed] [Google Scholar]
- 13.Shen L., Zhou T., Wang J., Sang X., Lan L., Luo L., Yin Z. Daphnetin reduces endotoxin lethality in mice and decreases LPS-induced inflammation in Raw264.7 cells via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 2017;66:579–589. doi: 10.1007/s00011-017-1039-1. [DOI] [PubMed] [Google Scholar]
- 14.Finn G.J., Creaven B.S., Egan D.A. Daphnetin induced differentiation of human renal carcinoma cells and its mediation by p38 mitogen-activated protein kinase. Biochem. Pharmacol. 2004;67:1779–1788. doi: 10.1016/j.bcp.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Nanzhen K., Jieying W., Wenwei Z., Xiaoping Z., Yingyuan F. Toxicological studies of daphnetin. Pharmacogn. Mag. 2018;58:561–566. [Google Scholar]
- 16.Finn G.J., Kenealy E., Creaven B.S., Egan D.A. In vitro cytotoxic potential and mechanism of action of selected coumarins, using human renal cell lines. Cancer Lett. 2002;183:61–68. doi: 10.1016/S0304-3835(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer D.J., Camacho D.M., Kohanski M.A., Callura J.M., Collins J.J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol. Cell. 2012;46:561–572. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duck W.M., Sobel J., Pruckler J.M., Song Q., Swerdlow D., Friedman C., Sulka A., Swaminathan B., Taylor T., Hoekstra M., et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori–infected persons, United States. Emerg. Infect. Dis. 2004;10:1088–1094. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Francesco V., Giorgio F., Hassan C., Manes G., Vannella L., Panella C., Ierardi E., Zullo A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointestin. Liver Dis. 2010;19:409–414. [PubMed] [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 5.0, 2015. [(accessed on 14 February 2019)];2015 Available online: http://www.eucast.org.
- 21.Su P., Li Y., Li H., Zhang J., Lin L., Wang Q., Guo F., Ji Z., Mao J., Tang W., et al. Antibiotic resistance of Helicobacter Pylori isolated in the southeast coastal region of China. Helicobacter. 2013;18:274–279. doi: 10.1111/hel.12046. [DOI] [PubMed] [Google Scholar]
- 22.De Francesco V., Zullo A., Fiorini G., Saracino I.M., Pavoni M., Vaira D. Role of MIC levels of resistance to clarithromycin and metronidazole in Helicobacter pylori eradication. [(accessed on 26 November 2018)];J. Antimicrob. Chemother. 2018 doi: 10.1093/jac/dky469. Available online: https://academic.oup.com/jac/advance-article-abstract/doi/10.1093/jac/dky469/5210026. [DOI] [PubMed] [Google Scholar]
- 23.Bai P., Zhou L.Y., Xiao X.M., Luo Y., Ding Y. Susceptibility of Helicobacter pylorito antibiotics in Chinese patients. J. Dig. Dis. 2015;16:464–470. doi: 10.1111/1751-2980.12271. [DOI] [PubMed] [Google Scholar]
- 24.Yang L., Ding W., Xu Y., Wu D., Li S., Chen J., Guo B. New insights into the antibacterial activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules. 2016;21:468. doi: 10.3390/molecules21040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehman S.-U., Khan R., Bhat K.A., Raja A.F., Shawl A.S., Alam M.S. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall. ex G. Don, Ericaceae. Rev. Bras. Farm. 2010;20:886–890. doi: 10.1590/S0102-695X2010005000037. [DOI] [Google Scholar]
- 26.Häcker G. The morphology of apoptosis. Cell Tissue Res. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 27.Allocati N., Masulli M., Di Ilio C., De Laurenzi V. Die for the community: An overview of programmed cell death in bacteria. Cell Death Dis. 2015;6:e1609. doi: 10.1038/cddis.2014.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maraldi N.M., Cellini L., Robuffo I., Donelli G. Searching the point of no return in Helicobacter pylori life: Necrosis and/or programmed death? J. Appl. Microbiol. 2001;90:727–732. doi: 10.1046/j.1365-2672.2001.01300.x. [DOI] [PubMed] [Google Scholar]
- 29.Kusters J.G., Gerrits M.M., A Van Strijp J., Vandenbroucke-Grauls C.M. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect. Immun. 1997;65:3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu K., Kuang N., Zhang Z., Hu Z., Zhang Y., Fu Y., Min W. Therapeutic effect of daphnetin on the autoimmune arthritis through demethylation of proapoptotic genes in synovial cells. J. Transl. Med. 2014;12:287. doi: 10.1186/s12967-014-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice K.C., Bayles K.W. Death’s toolbox: Examining the molecular components of bacterial programmed cell death. Mol. Microbiol. 2003;50:729–738. doi: 10.1046/j.1365-2958.2003.t01-1-03720.x. [DOI] [PubMed] [Google Scholar]
- 32.Kohanski M.A., Dwyer D.J., Wierzbowski J., Cottarel G., Collins J.J. Mistranslation of Membrane Proteins and Two-Component System Activation Trigger Antibiotic-Mediated Cell Death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erental A., Kalderon Z., Saada A., Smith Y., Engelberg-Kulka H. Apoptosis-like death, an extreme SOS response in Escherichia coli. MBio. 2014;5:e01426-14. doi: 10.1128/mBio.01426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki-Karasaki M., Ochiai T., Suzuki-Karasaki Y. Crosstalk between mitochondrial ROS and depolarization in the potentiation of TRAIL-induced apoptosis in human tumor cells. Int. J. Oncol. 2013;44:616–628. doi: 10.3892/ijo.2013.2215. [DOI] [PubMed] [Google Scholar]
- 35.Amundsen S.K., Fero J., Hansen L.M., Cromie G.A., Solnick J.V., Smith G.R., Salama N.R. Helicobacter pyloriAddAB helicase-nuclease and RecA promote recombination-related DNA repair and survival during stomach colonization. Mol. Microbiol. 2008;69:994–1007. doi: 10.1111/j.1365-2958.2008.06336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hage N., Howard T. Structural basis of Lewis(b) antigen binding by the Helicobacter pylori adhesin BabA. Sci Adv. 2015;1:e1500315. doi: 10.1126/sciadv.1500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strugatsky D., McNulty R., Munson K., Chen C.-K., Soltis S.M., Sachs G., Luecke H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2012;493:255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitmire J.M., Merrell D.S. Helicobacter Species. Humana Press; Totowa, NJ, USA: 2012. Successful Culture Techniques for Helicobacter Species: General Culture Techniques for Helicobacter pylori; pp. 37–40. [DOI] [PubMed] [Google Scholar]
- 39.Cui J., Xing L., Li Z., Wu S., Wang J., Liu J., Wang J., Yan X., Zhang X. Ochratoxin A induces G2 phase arrest in human gastric epithelium GES-1 cells in vitro. Toxicol. Lett. 2010;193:152–158. doi: 10.1016/j.toxlet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Best L.M., Haldane D.J.M., Keelan M., Taylor D.E., Thomson A.B.R., Loo V., Fallone C.A., Lyn P., Smaill F.M., Hunt R., et al. Multilaboratory Comparison of Proficiencies in Susceptibility Testing of Helicobacter pylori and correlation between agar dilution and E test methods. Antimicrob. Agents Chemother. 2003;47:3138–3144. doi: 10.1128/AAC.47.10.3138-3144.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obonyo M., Zhang L., Thamphiwatana S., Pornpattananangkul D., Fu V., Zhang L. Antibacterial Activities of Liposomal Linolenic Acids against Antibiotic-Resistant Helicobacter pylori. Mol. Pharm. 2012;9:2677–2685. doi: 10.1021/mp300243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figura N., Marcolongo R., Cavallo G., Santucci A., Collodel G., Spreafico A., Moretti E. Polysorbate 80 and Helicobacter pylori: A microbiological and ultrastructural study. BMC Microbiol. 2012;12:217. doi: 10.1186/1471-2180-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soo-Hwan K., Lee H.S. Antibacterial Activity of Silver-nanoparticles Against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011;39:77–85. [Google Scholar]
- 44.Dwyer D.J., A Kohanski M., Hayete B., Collins J.J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan M., Wan C., Xie Q., Huang R., Tao X., Shah N.P., Wei H. Changes in gastric microbiota induced by Helicobacter pylori infection and preventive effects of Lactobacillus plantarum ZDY 2013 against such infection. J. Dairy Sci. 2016;99:970–981. doi: 10.3168/jds.2015-10510. [DOI] [PubMed] [Google Scholar]
- 46.Carlsohn E., Nyström J., Karlsson H., Svennerholm A.-M., Nilsson C.L. Characterization of the outer membrane protein profile from disease-related Helicobacter pylori isolates by subcellular fractionation and nano-LC FT-ICR MS analysis. J. Proteome Res. 2006;5:3197–3204. doi: 10.1021/pr060181p. [DOI] [PubMed] [Google Scholar]
- 47.Baik S.-C., Kim K.-M., Song S.-M., Kim D.-S., Jun J.-S., Lee S.-G., Song J.-Y., Park J.-U., Kang H.-L., Lee W.-K., et al. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J. Bacteriol. 2004;186:949–955. doi: 10.1128/JB.186.4.949-955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Hu Y., Pai P.-J., Chen D., Lam H. Label-free quantitative proteomics analysis of antibiotic response in staphylococcus aureus to oxacillin. J. Proteome Res. 2014;13:1223–1233. doi: 10.1021/pr400669d. [DOI] [PubMed] [Google Scholar]
- 49.Domon B., Aebersold R. Mass Spectrometry and Protein Analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 50.Salas-Jara M.J., Sanhueza E.A., Retamal-Díaz A., González C., Urrutia H., García A. Probiotic Lactobacillus fermentum UCO-979C biofilm formation on AGS and Caco-2 cells and Helicobacter pylori inhibition. Biofouling. 2016;32:1245–1257. doi: 10.1080/08927014.2016.1249367. [DOI] [PubMed] [Google Scholar]
- 51.Niehues M., Euler M., Georgi G., Mank M., Stahl B., Hensel A. Peptides from Pisum sativum L. enzymatic protein digest with anti-adhesive activity against Helicobacter pylori: Structure-activity and inhibitory activity against BabA, SabA, HpaA and a fibronectin-binding adhesin. Mol. Nutr. Food Res. 2010;54:1851–1861. doi: 10.1002/mnfr.201000021. [DOI] [PubMed] [Google Scholar]
- 52.Li N., Han L., Chen J., Lin X., Chen H., She F. Proliferative and apoptotic effects of gastric epithelial cells induced by coccoid Helicobacter pylori. J. Basic Microbiol. 2012;53:147–155. doi: 10.1002/jobm.201100370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.