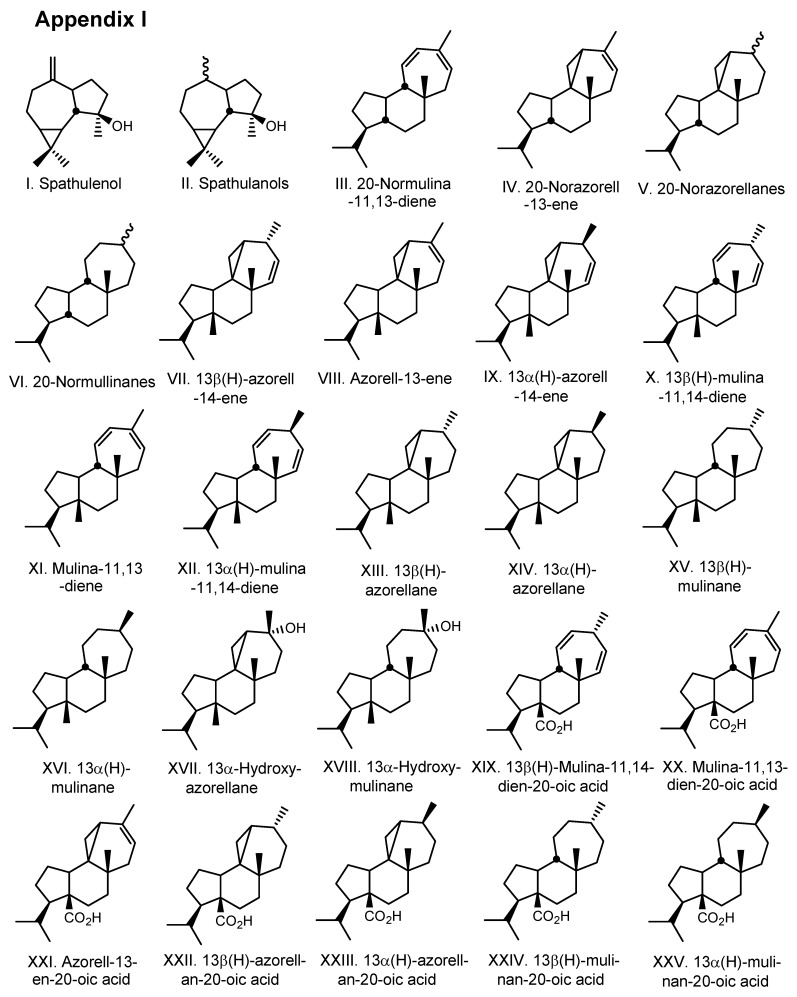
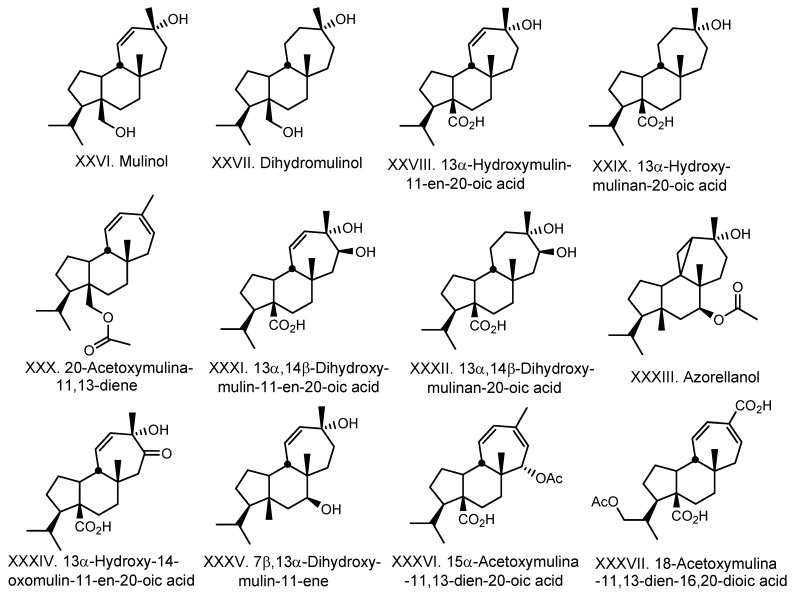
Abstract
Extracts of bled resin from Azorella compacta, of the Azorelloideae family from the Andes (>4000 m), were analyzed by gas chromatography-mass spectrometry. The mass spectra of the dominant compounds of the resin and its hydrogenation products were documented. The most abundant compounds were oxygenated diterpenoids, namely mulinadien-20-oic (Δ11,13 and Δ11,14) acids, azorell-13-en-20-oic acid, 13α,14β-dihydroxymulin-11-en-20-oic acid, and azorellanol, with a group of azorellenes and mulinadienes. The mass spectra of the novel diterpenoid hydrocarbons with the azorellane and mulinane skeletons were also presented. This study documents the molecular diversity of these diterpenoid classes, and could be of great utility for future organic geochemical, environmental, archeological, pharmaceutical, and forensic chemistry studies.
Keywords: Azorella compacta, diterpenoids, GC-MS, standards
1. Introduction
Natural products or their derivatives in the ambient environment or geological record are used by organic geochemists as tracers for sources, transport and alteration processes of organic matter, e.g., [1,2,3,4,5,6,7]. Remote locales, for example, the high Andes region in South America, are ideal sites as pristine references for background studies of such processes. An early report on the lipids and biomarkers in sediment from Lake Lejia in a caldera in Antofagasta, Chile, found traces of diterpenoids typical of resinous vegetation [8]. The region is barren of trees, especially gymnosperms that produce resin, and potential sources such as the Araucariaceae grow far to the south [9]. Subsequently, we reported on the lipid and resin composition of a dried sample of Laretia compacta (common name Llareta) from San Pedro de Atacama [10]. The dried material had a strong resinous odor and thus was implied as a possible source of diterpenoids in the Altiplano environment. The extract yield of the whole plant based on dry weight comprised 4.2 mg/g (0.2%) lipids and 2.5 mg/g (0.12%) terpenoids [10]. The terpenoid distribution consisted of mono-, sesqui- and diterpenoids, and the latter are of interest here because tri- and tetracyclic diterpenoids were the dominant compounds.
Laretia compacta is now called Azorella compacta of the Apiaceae:Azorelloideae family, also known as Umbelliferae [11]. These plants grow slowly at high altitudes (>4000 m) in the northern Andes of Chile and Peru as dense pillow-like structures close to the ground with subterranean stems and roots. The Azorelloideae family evolved from the first Umbelliferae during the Cretaceous, e.g., [12,13].
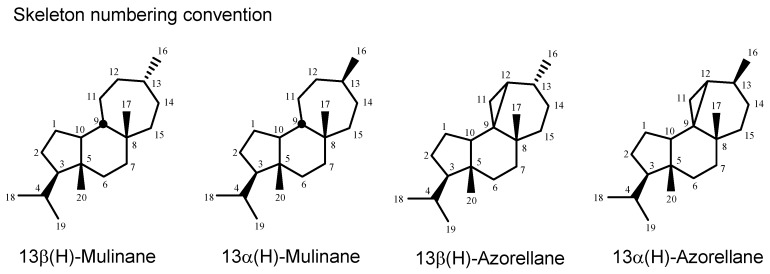
Azorella sp. have historically been used on the Altiplano as firewood and cooking fuel by local cultures, and later in early industrial and mining activities as fuel, which contributed to its scarcity and current protection. The species is now more common, and represents the dominant biomass in these Andean environments. The plants and resin exudates have been used by Andean cultures for medicinal purposes where, for example, hot water infusions serve as herbal remedies for various ailments [14,15]. Consequently, natural product characterization and pharmacological studies have been carried out on A. compacta and related species. Numerous investigations reported structure determinations and pharmacological potential studies of the resin compounds, that were isolated and purified from the major Andean Apiaceae species, namely Mulinum and Azorella [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. This has led to a description of mainly two novel diterpenoid skeletons among the natural products, specifically the mulinanes and azorellanes (Figure 1), with the assignment of their skeletal numbering conventions [16,21]. The biosynthetic pathway for the mulinane and azorellane diterpenoids has been proposed as starting from a phytatetraene [24]. Laboratory syntheses of polar mulinane diterpenoids have also recently been reported [36,37].
Figure 1.
Carbon numbering convention for the mulinane and azorellane skeletons.
Here, we characterize the molecular composition of Azorella compacta resin based on mass spectrometric interpretation, and correlation and comparison with known standards. We report the mass spectra and gas chromatography-mass spectrometry (GC-MS) characteristics of the dominant compounds of the resin and of the hydrogenated resin as the free and derivatized products. This information is unique, because prior reports in the natural product literature only provided mass spectrometric data on some compounds. Our data further documents the molecular diversity of this novel diterpenoid class (mulinane and azorellane), information which could be of great utility for future organic geochemical, environmental, archeological, pharmaceutical, and forensic chemistry studies.
2. Results and Discussion
The relative composition of the fresh resin was 1% sesquiterpenoid and 99% diterpenoids, excluding a low amount of bornyl acetate (<0.5%). No lipid components from plant wax were detected. It is of interest to note the breakdown of the diterpenoids into 19% hydrocarbons and 80% polar oxygenated compounds, and the same proportions were retained upon hydrogenation. This observation was unexpected because most bled conifer resins are characterized by higher oxygenated diterpenoid contents (Simoneit, unpublished data).
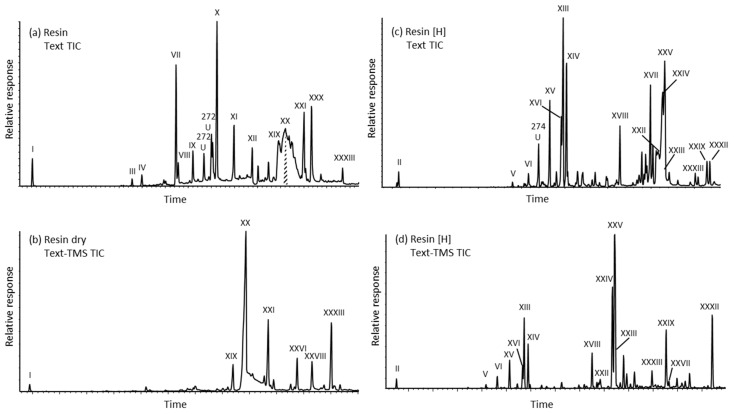
Typical examples of GC-MS total ion current traces for A. compacta resin and hydrogenated resin indicated the molecular complexity of the compound mixtures (Figure 2). The poor resolution of the underivatized resin acids (cf. Figure 2a) was due to their polarity. Sample components were classified into three groups of compounds, sesquiterpenoid, diterpenoid hydrocarbons and oxygenated diterpenoids, and are discussed as such. Only one sesquiterpenoid, namely spathulenol (I, all structures are shown in Appendix A), which hydrogenates to two isomers of spathulanol (II), was observed. The relative concentrations of all identified compounds are listed in Table 1.
Figure 2.
Examples of GC-MS total ion current traces for A. compacta resin and hydrogenated resin analyzed as: (a,c) total extracts, and (b,d) silylated extracts. Roman numerals refer to structures in Table 1 and Appendix A.
Table 1.
Relative concentrations of the major compounds identified in resin and hydrogenated resin of Azorella compacta.
| Structure Number a | Compound | Composition b | MW | ID c | Resin (n = 7) | Hydrogenated Resin (n = 4) |
|---|---|---|---|---|---|---|
| I | Spathulenol | C15H24O | 220 | S | 1.4 | |
| II | Spathulanol | C15H26O | 222 | S | 3 | |
| III | 20-Normulina-11,13-diene | C19H30 | 258 | T | 0.5 | |
| IV | 20-Norazorell-13-ene | C19H30 | 258 | T | 1 | |
| V | 20-Norazorellane, α + β | C19H32 | 260 | T | 1 | |
| VI | 20-Normulinane, α + β | C19H34 | 262 | T | 0.3 | |
| VII | 13β(H)-Azorell-14-ene | C20H32 | 272 | T | 9 | |
| VIII | Azorell-13-ene | C20H32 | 272 | I | 2 | |
| IX | 13α(H)-Azorell-14-ene | C20H32 | 272 | T | 2.5 | |
| X | 13β(H)-Mulina-11,14-diene | C20H32 | 272 | I | 16 | |
| XI | Mulina-11,13-diene | C20H32 | 272 | L | 7 | |
| XII | 13α(H)-Mulina-11,14-diene | C20H32 | 272 | I | 4 | |
| XIII | 13β(H)-Azorellane | C20H34 | 274 | I | 26 | |
| XIV | 13α(H)-Azorellane | C20H34 | 274 | I | 15 | |
| XV | 13β(H)-Mulinane | C20H36 | 276 | I | 11 | |
| XVI | 13α(H)-Mulinane | C20H36 | 276 | I | 8 | |
| XVII | 13-Hydroxyazorellanes | C20H34O | 290 | L | 14 | |
| XVIII | 13α-Hydroxymulinane | C20H36O | 292 | L | 22 | |
| XIX | 13β(H)-Mulina-11,14-dien-20-oic acid | C20H30O2 | 302 | S | 10 | |
| XX | Mulina-11,13-dien-20-oic acid | C20H30O2 | 302 | S | 100 | |
| XXI | Azorell-13-en-20-oic acid | C20H30O2 | 302 | S | 17 | |
| XXII | 13β(H)-Azorellan-20-oic acid | C20H32O2 | 304 | S | 5 | |
| XXIII | 13α(H)-Azorellan-20-oic acid | C20H32O2 | 304 | S | 30 | |
| XXIV | 13β(H)-Mulinan-20-oic acid | C20H34O2 | 306 | S | 44 | |
| XXV | 13α(H)-Mulinan-20-oic acid | C20H34O2 | 306 | S | 100 | |
| XXVI | Mulinol | C20H34O2 | 306 | L | 7 | |
| XXVII | Dihydromulinol | C20H36O2 | 308 | I | 6 | |
| XXVIII | 13α-Hydroxymulin-11-en-20-oic acid | C20H32O3 | 320 | S | 8 | |
| XXIX | 13α-Hydroxymulinan-20-oic acid | C20H34O3 | 322 | S | 25 | |
| XXX | 20-Acetoxymulina-11,13-diene | C22H34O2 | 330 | L | 15 | |
| XXXI | 13α,14β-Dihydroxymulin-11-en-20-oic acid | C20H30O4 | 336 | L | 11 | |
| XXXII | 13α,14β-Dihydroxymulinan-20-oic acid | C20H34O4 | 338 | I | 32 | |
| XXXIII | Azorellanol | C22H36O3 | 348 | L | 16 | 12 |
a Structures are shown in Appendix A. b Listed as the natural products, polar compounds analyzed as methyl esters and/or trimethylsilyl derivatives. c Identification: I = interpretation and correlation of MS fragmentation pattern and GC retention time as derivative from standard, T = suggested interpretation based on MS and GC retention time only, S = standard, L = literature citation, n = number of analyses.
2.1. Diterpenoid Hydrocarbons
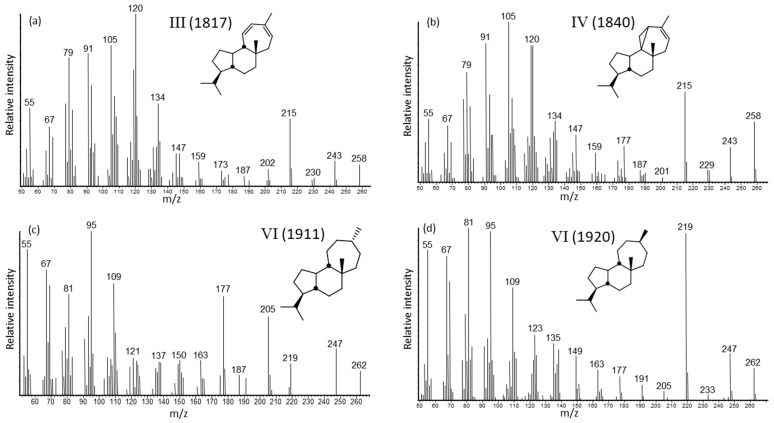
There were two norditerpenes, 20-normulina-11,13-diene (III) and 20-norazorell-13-ene (IV), which upon hydrogenation yielded four isomers, namely 20-normulinane (VI) and 20-norazorellane (V) both as the 13β(H) and 13α(H)-epimers (Figure 3a–f, also see Figure SM-2b). We inferred the absence of C-20 for these novel norditerpenes, rather than C-16 or C-17, based on the facile elimination of the functionalized C-20 group in the mass spectra of the acid standards discussed below. The configuration at C-5 is most likely 5β(H), but based on the preferential loss or absence of a C-20 functional group, could also be represented as 5α(H).
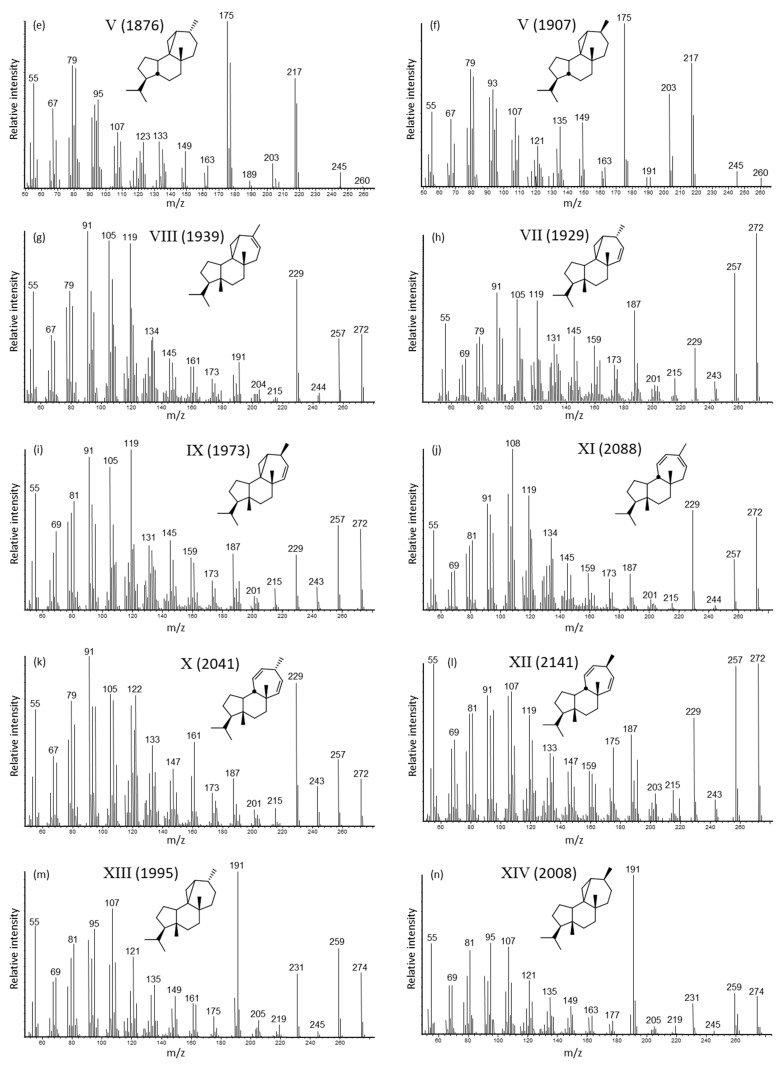
Figure 3.
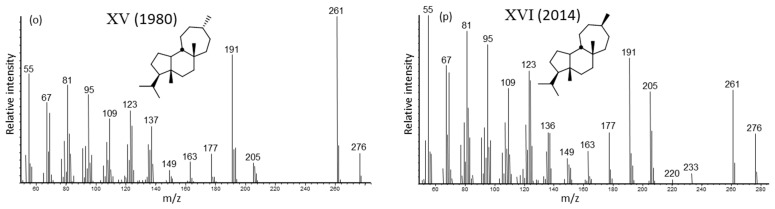
Mass spectra of the diterpenoid hydrocarbons in A. compacta resins. The KI values relative to n-alkanes on a DB-5 column are shown in parentheses on each mass spectrum: (a) 20-normulina-11,13-diene (III), (b) 20-norazorell-13-ene (IV), (c) 13β(H)-20-normulinane (VI), (d) 13α(H)-20-normulinane (VI), (e) 13β(H)-20-norazorellane (V), (f) 13α(H)-20-norazorellane (V), (g) azorell-13-ene (VIII), (h) 13β(H)-azorell-14-ene (VII), (i) 13α(H)-azorell-14-ene (IX), (j) mulina-11,13-diene (XI), (k) 13β(H)-mulina-11,14-diene (X), (l) 13α(H)-mulina-11,14-diene (XII), (m) 13β(H)-azorellane (XIII), (n) 13α(H)-azorellane (XIV), (o) 13β(H)-mulinane (XV), and (p) 13α(H)-mulinane (XVI).
There were six isomers of C20 diterpene hydrocarbons, which after hydrogenation yielded four isomers of the saturated diterpanes. The interpretations of the mass spectra as mulina-11,13-diene (XI), 13β(H)-mulina-11,14-diene (X) and 13α(H)-mulina-11,14-diene (XII) (Figure 3j–l) were inferred from the literature [32], although the match was poor. The identifications of azorell-13-ene (VIII), 13β(H)-azorell-14-ene (VII) and 13α(H)-azorell-14-ene (IX) were based on the GC elution order and interpretations of the mass spectrometric fragmentation patterns (Figure 3g–i; details are given in Figure SM-2b) when compared to the C-20 functionalized standards.
Upon hydrogenation to the mulinane and azorellane skeletons (Figure 1), we obtained two isomers, i.e., 13α(H) and 13β(H), for the position of the methyl group at C-13. We assigned the elution order as was observed for the α and β kaurane and phyllocladane standards [38,39], that is the 13β(H) isomer eluted prior to the 13α(H) epimer. The diterpanes, namely 13β(H)-azorellane (XIII), 13α(H)-azorellane (XIV), 13β(H)-mulinane (XV), and 13α(H)-mulinane (XVI), have similar mass spectra (Figure 3m–p), but different molecular weights. The key ion for all is m/z 191, with M-C3H7 (m/z 231) for the azorellanes and M-C5H11 (m/z 205) for the mulinanes (cf. Figure SM-2b). These four compounds may be potential biomarkers for the geologic record and of interest to organic geochemistry. The mass spectra of both C20 hydrocarbon groups are the first report of these novel parent diterpenoid skeletons, and they do not match with any mass spectra of other reported tri- and tetracyclic diterpanes (e.g., abietanes, atisanes, pimaranes, kauranes, phyllocladanes, etc.).
2.2. Oxygenated Diterpenoids
The oxygenated diterpenoids comprised mainly mulina-11,13-dien-20-oic acid (XX) with the isomers 13β(H)-mulina-11,14-dien-20-oic (XIX) and azorell-13-en-20-oic (XXI) acids. Their identification was based on comparison of the mass spectra of standard natural products as the free and derivatized compounds (Figure 4d–f,k–o and Figure SM-1o–p, respectively), and their respective GC retention indices. One characteristic of the mass spectra of all C-20 carboxylic acids and C-20 methyl or TMS carboxylates is the facile loss of that moiety from the respective molecular ion (cf. Figure SM-2a).
Figure 4.
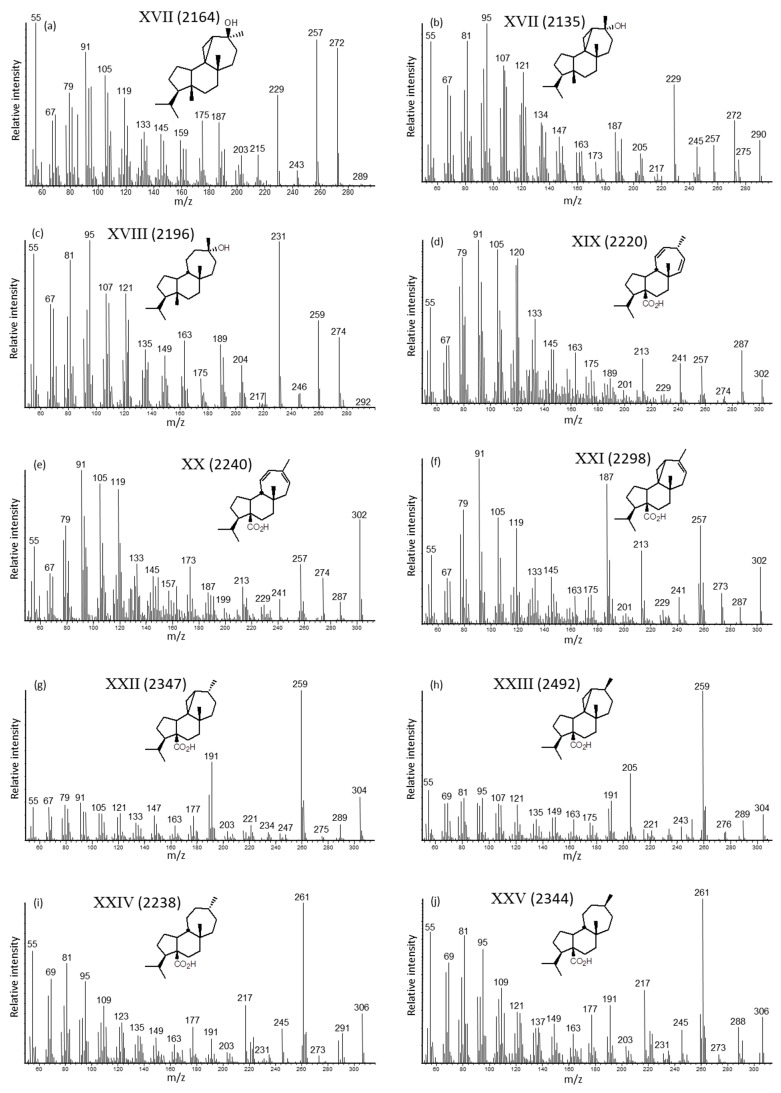
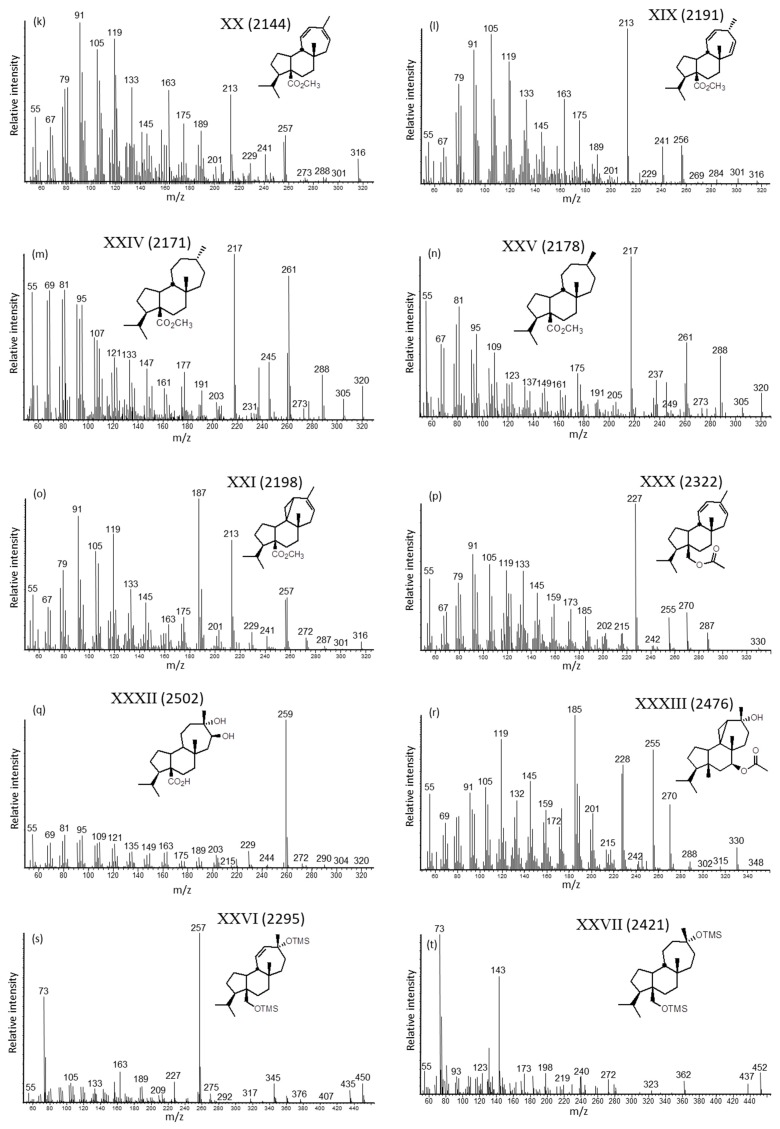
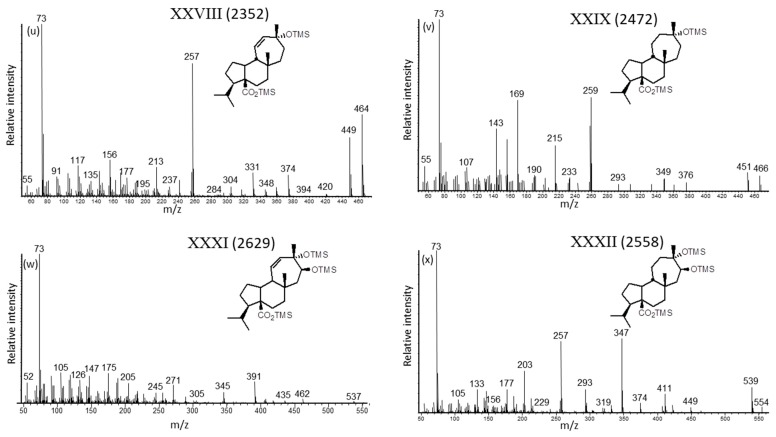
Mass spectra of the oxygenated diterpenoids in A. compacta resins, including KI values shown in parentheses: (a) 13β-hydroxyazorellane (XVII), (b) 13α-hydroxyazorellane (XVII), (c) 13α-hydroxymulinane (XVIII), (d) 13β(H)-mulina-11,14-dien-20-oic acid (XIX, standard), (e) mulina-11,13-dien-20-oic acid (XX, standard), (f) azorell-13-en-20-oic acid (XXI, standard), (g) 13β(H)-azorellan-20-oic acid (XXII, standard), (h) 13α(H)-azorellan-20-oic acid (XXIII, standard), (i) 13β(H)-mulinan-20-oic acid (XXIV, standard), (j) 13α(H)-mulinan-20-oic acid (XXV, standard), (k) methyl mulina-11,13-dien-20-oate (XX, standard), (l) methyl 13β(H)-mulina-11,14-dien-20-oate (XIX, standard), (m) methyl 13β(H)-mulinan-20-oate (XXIV, standard), (n) methyl 13α(H)-mulinan-20-oate (XXV, standard), (o) methyl azorell-13-en-20-oate (XXI, standard), (p) 20-acetoxymulina-11,13-diene (XXX), (q) 13α,14β-dihydroxymulinan-20-oic acid (XXXII), (r) azorellanol (XXXIII, standard), (s) mulinol-diTMS (XXVI), (t) dihydomulinol-diTMS (XXVII), (u) 13α-hydroxymulin-11-en-20-oic acid-diTMS (XXVIII, mulinolic acid-diTMS, standard), (v) 13α-hydroxymulinan-20-oic acid-diTMS (XXIX, standard), (w) 13α,14β-dihydroxymulin-11-en-20-oic acid-triTMS (XXXI), and (x) 13α,14β-dihydroxymulinan-20-oic acid-triTMS (XXXII).
Significant amounts of 20-acetoxymulina-11,13-diene (XXX), azorellanol (7β-acetoxy-13α-hydroxyazorellane, XXXIII), and 13α,14β-dihydroxymulin-11-en-20-oic acid (XXXI) were also identified. The major fragment ions of the mass spectrum of 20-acetoxymulina-11,13-diene (Figure 4p) matched with those listed in the literature for its direct insertion probe mass spectrum [26]. The mass spectrum of the azorellanol standard (Figure 4r and Figure SM-1v as the TMS derivative) matched with its literature listing [21]. There was also a trace of the tentatively identified 13-epi-azorellanol (Figure SM-1k), which eluted prior to azorellanol. The structure of 13α,14β-dihydroxymulin-11-en-20-oic acid (Figure 4w) was based on correlation with the standard 13α-hydroxy-14-oxomulin-11-en-20-oic acid (XXXIV, Figure SM-1j,w,z). Mulinol (13α,20-dihydroxymulin-11-ene, XXVI) and 13α-hydroxymulin-11-en-20-oic acid (mulinolic acid, XXVIII) were observed as minor components. The mass spectrum of mulinol (Figure 4s) was correlated with that of its hydrogenation product (Figure 4v) and the published MS listing [20]. The mass spectra of mulinolic acid matched with those of the standard (Figure 4u and Figure SM-1d,h).
The hydrogenated resin sample contained the corresponding saturated diterpenoids (Figure 2c,d). As discussed above for the hydrocarbons, the elution order assumed for the dominant 13β(H)- and 13α(H)-mulinan-20-oic acids (XXIV and XXV, respectively) was beta before alpha (Figure 4i,j,m,n and Figure SM-1r,s, respectively). The same was the case for the 13β(H)- and 13α(H)-azorellan-20-oic acids (XXII and XXIII, respectively) and their mass spectra are shown in Figure 4g,h and Figure SM-1r,s, respectively (refer to Figure SM-2a). These diterpanoid acids may be potential diagenetic products in the geologic record and thus are of interest to organic geochemistry. The hydrogenation products of mulinol and dihydroxymulin-11-en-20-oic acid were also detectable as dihydromulinol (XXVII, Figure 4t) and 13α,14β-dihydroxymulinan-20-oic acid (XXXII, Figure 4x).
3. Samples and Experimental Methods
A sample of A. compacta resin was obtained by cutting to bleed an Azorella compacta plant growing off the road B245 from El Tatio to San Pedro de Atacama in the Paso la Vizcacha (Chile, Region II, 22°22′34″S, 68°1′0″W, altitude 4556 m).
The fresh resin was split into two parts, one dissolved completely in dichloromethane/methanol (DCM/MeOH, 3:1 v/v), and the duplicate sample was dissolved in n-hexane. Aliquots (50 µL) of these total extracts were converted to trimethylsilyl derivatives by reaction with N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA, Sigma-Aldrich, St. Louis, MO, USA) and pyridine for 3 h at 70 °C, e.g., [40]. Prior to analysis, the excess silylating reagent was evaporated by nitrogen blow down and the sample mixture dissolved in an equivalent volume of n-hexane. Other aliquots of sample solutions were treated with trimethylsilyldiazomethane (2M in hexane, Sigma-Aldrich) at room temperature for 30 min to methylate carboxylic acids [41]. The excess reagent was reacted with concentrated acetic acid, the solution dried by nitrogen blow down, and the products dissolved in DCM/MeOH (3:1) for analysis.
The following reference standards were also derivatized and analyzed by these methods: spathulenol (I), mulina-11,13-dien-20-oic acid (XX), azorell-13-en-20-oic acid (XXI), 13α-hydroxymulin-11-en-20-oic acid (XXVIII), azorellanol (XXXIII), 13α-hydroxy-14-oxomulin-11-en-20-oic acid (XXXIV), 7β,13α-dihydroxymulin-11-ene (XXXV), 15α-acetoxymulina-11,13-dien-20-oic acid (XXXVI), and 18-acetoxymulina-11,13-diene-16,20-dioic acid (XXXVII).
Aliquots of the total extracts and mulina-11,13-dien-20-oic acid were hydrogenated. The samples were diluted with DCM/MeOH (3:1) in a round bottom flask (50 mL) with a magnetic stir bar and PtO2 (Arcos Organics, Fisher, Pittsburgh, PA, USA, 83% Pt) as catalyst. H2 at atmospheric pressure was installed with a latex balloon (500 mL) over the neck of the flask and hydrogenation allowed to proceed at room temperature for 8 h. The catalyst was filtered off and the sample concentrated by nitrogen blow down for analysis.
The gas chromatography-mass spectrometry (GC-MS) analyses of the total extracts and aliquots of the derivatized and hydrogenated total extracts (typical injection volume 1 µL) were performed on a Hewlett-Packard model 6890 GC coupled to a Hewlett-Packard model 5973 MSD (Palo Alto, CA, USA)). Separation of compounds was achieved on a fused silica capillary column coated with DB-5MS (Agilent, Santa Clara, CA, USA) 30 m × 0.25 mm i.d., 0.25 µm film thickness). The GC operating conditions were as follows: temperature held at 65 °C for 2 min, increased from 65 to 300 °C at a rate of 6 °C min−1, and final isothermal held at 300 °C for 20 min. Helium was used as carrier gas. The samples were injected splitless with the injector temperature at 280 °C. The mass spectrometer was operated in the electron impact mode at 70 eV and scanned from 50 to 650 da. Data were acquired and processed with the Chemstation software (Hewlett-Packard, Palo Alto, CA, USA). Compounds were identified by comparison of mass spectra and retention times with those of authentic standards, with literature data, and interpretation of mass spectrometric fragmentation patterns of unknowns. The Kovats [42] GC retention indices (KI) relative to n-alkanes are shown on each mass spectrum and are determined for elution on a DB-5 capillary column.
The extract of the dried plant (prior report by [10]) was reanalyzed. The dominant diterpenoids were the same as found in the new resin sample and are not discussed further.
4. Conclusions
In this study, we document the molecular characteristics and diversity of novel diterpenoid classes of natural products, namely mulinanes and azorellanes, from resin of the Andean Apiaceae plant species Azorella compacta. The dominant compounds were oxygenated diterpenoids, mainly mulinadien-20-oic (Δ11,13 and Δ11,14) acids, azorell-13-en-20-oic acid, 13α,14β-dihydroxymulin-11-en-20-oic acid, and azorellanol, as confirmed by standards. The diterpenoid hydrocarbons included mulinadienes and azorellenes, which are novel compounds of potential interest to organic geochemistry. The mulinane and azorellane diterpenoid hydrocarbons, resulting from the hydrogenation of the resin, may be potential biomarkers for the geologic record, while the corresponding oxygenated diterpenoids may be applied for environmental studies.
Acknowledgments
We thank Ljubomir Tomasevic for the bled resin sample, and Leszek Marynowski and S. Kurkiewicz for some GC-MS data. This is publication number 897 from the Southeast Environmental Research Center and Institute of Water and Environment at Florida International University.
Supplementary Materials
Additional mass spectra of related and derivatized natural products are collected in the Supplemental Materials section available with this article.
Appendix A
Figure A1.
Chemical structures cited and listed in Table 1.
Author Contributions
B.R.T.S. and B.M.D. conceived and designed the work; B.R.T.S., R.J., C.A. and B.S. performed the experiments; B.R.T.S. and D.R.O. analyzed and interpreted the data; B.R.T.S. wrote the paper, and all coauthors reviewed and edited the paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Johns R.B. Biological Markers in the Sedimentary Record. Elsevier; Amsterdam, The Netherlands: 1986. p. 364. [Google Scholar]
- 2.Simoneit B.R.T. The organic chemistry of marine sediments. In: Riley J.P., Chester R., editors. Chemical Oceanography. 2nd ed. Volume 7. Academic Press; New York, NY, USA: 1978. pp. 233–311. [Google Scholar]
- 3.Simoneit B.R.T. Organic matter of the troposphere-V: Application of molecular marker analysis to biogenic emissions into the troposphere for source reconciliations. J. Atmos. Chem. 1989;8:251–275. doi: 10.1007/BF00051497. [DOI] [Google Scholar]
- 4.Simoneit B.R.T. A review of biomarker compounds as source indicators and tracers for air pollution. Environ. Sci. Pollut. Res. 1999;6:153–163. doi: 10.1007/BF02987621. [DOI] [PubMed] [Google Scholar]
- 5.Simoneit B.R.T., Mazurek M.A. Organic matter of the troposphere-II: Natural background of biogenic lipid matter in aerosols over the rural western United States. Atmos. Environ. 1982;16:2139–2159. doi: 10.1016/0004-6981(82)90284-0. [DOI] [Google Scholar]
- 6.Otto A., White J.D., Simoneit B.R.T. Natural product terpenoids in Eocene and Miocene conifer fossils. Science. 2002;297:1543–1545. doi: 10.1126/science.1074225. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros P.M., Simoneit B.R.T. Multi-biomarker characterization of sedimentary organic carbon in small rivers draining the northwestern United States. Org. Geochem. 2008;39:52–74. doi: 10.1016/j.orggeochem.2007.10.001. [DOI] [Google Scholar]
- 8.Simoneit B.R.T., Halpern H.I., Didyk B.M. Lipid productivity of a high Andean lake. In: Trudinger P.A., Walter M.R., Ralph B.J., editors. Biogeochemistry of Ancient and Modern Environments. Australian Academy of Sciences; Canberra, Australia: 1980. pp. 201–210. [Google Scholar]
- 9.Muñoz C. Sinopsis de la Flora Chilena. 2nd ed. Edition de la Universidad de Chile; Santiago, Chile: 1966. p. 500. [Google Scholar]
- 10.Simoneit B.R.T., Didyk B.M. The lipid and resin composition of Laretia compacta Phil. from the Andes of Chile. Z. Naturforsch. C. 1999;54:309–313. doi: 10.1515/znc-1999-5-603. [DOI] [Google Scholar]
- 11.Nicolas A.N., Plunkett G.M. Untangling generic limits in Azorella, Laretia, and Mulinum (Apiaceae: Azorelloideae): Insights from phylogenetics and biogeography. Taxon. 2012;61:826–840. doi: 10.1002/tax.614008. [DOI] [Google Scholar]
- 12.Creceau-Larrival M.-T. Morphologie pollinique et corrélation phytogénétique chez les Ombelifères. In: Heywood V.H., editor. The Biology and Chemistry of the Umbelliferae. Academic Press; London, UK: 1971. pp. 109–135. [Google Scholar]
- 13.Saldivia P., Faundez L., Urbina-Casanova R., Scherson R.A. Demeykoa andina (Apiaceae; Azorelloideae), a new species from northern Chile. Syst. Bot. 2016;41:457–463. doi: 10.1600/036364416X691821. [DOI] [Google Scholar]
- 14.Suárez Contreras I. Contribución al estudio Botánico y Químico de le Laretia compacta Phil. (Llareta) Facultad de Biología y Ciencias Médicas, Universidad de Chile; Santiago, Chile: 1935. [Google Scholar]
- 15.Wickens G.E. Llareta (Azorella compacta, Umbelliferae): A review. Econ. Bot. 1995;49:207–212. doi: 10.1007/BF02862926. [DOI] [Google Scholar]
- 16.Loyola L.A., Morales G., Rodríguez B., Jiménez-Barbero J., de la Torre M.C., Perales A., Torres M.R. Mulinic and isomulinic acids, rearranged diterpenes with a new carbon skeleton from Mulinum crassifolium. Tetrahedron. 1990;46:5413–5420. doi: 10.1016/S0040-4020(01)87848-0. [DOI] [Google Scholar]
- 17.Loyola L.A., Morales G., de la Torre M.C., Pedreros S., Rodríguez B. 17-Acetoxymulinic acid, a rearranged diterpenoid from Mulinum crassifolium. Phytochemistry. 1990;29:3950–3951. doi: 10.1016/0031-9422(90)85372-M. [DOI] [Google Scholar]
- 18.Loyola L.A., Morales G., Rodríguez B., Jiménez-Barbero J., Pedreros S., de la Torre M.C., Perales A. Mulinenic acid a rearranged diterpenoid from Mulinum crassifolium. J. Nat. Prod. 1991;54:1404–1408. doi: 10.1021/np50077a028. [DOI] [Google Scholar]
- 19.Loyola L.A., Bórquez J., Morales G., San-Martín A. Diterpenoids from Azorella compacta. Phytochemistry. 1997;44:649–651. doi: 10.1016/S0031-9422(96)00603-6. [DOI] [PubMed] [Google Scholar]
- 20.Loyola L.A., Bórquez J., Morales G., San-Martín A. Mulinol, a diterpenoid from Azorella compacta. Phytochemistry. 1997;45:1465–1467. doi: 10.1016/S0031-9422(97)00178-7. [DOI] [Google Scholar]
- 21.Loyola L.A., Bórquez J., Morales G., San-Martín A., Manríquez V., Wittke O. Azorellanol: A diterpenoid with a new carbon skeleton from Azorella compacta. Tetrahedron. 1998;54:15533–15540. doi: 10.1016/S0040-4020(98)01016-3. [DOI] [Google Scholar]
- 22.Loyola L.A., Bórquez J., Morales G., San-Martín A. 11,12-Epoxy-mulin-13-en-20-oic acid, a diterpenoid from Azorella compacta. Phytochemistry. 1998;49:1091–1093. doi: 10.1016/S0031-9422(97)01062-5. [DOI] [Google Scholar]
- 23.Loyola L.A., Bórquez J., Morales G., Araya J., González J., Neira I., Sagua H., San-Martín A. Diterpenoids from Azorella yareta and their trichomonicidal activities. Phytochemistry. 2001;56:177–180. doi: 10.1016/S0031-9422(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 24.Loyola L.A., Bórquez J., Morales G., San-Martín A., Darias J. Madreporanone, a unique diterpene with a novel skeleton from Azorella madreporica. Tetrahedr. Lett. 2002;43:6359–6362. doi: 10.1016/S0040-4039(02)01387-4. [DOI] [Google Scholar]
- 25.Loyola L.A., Bórquez J., Morales G., San-Martín A., Manríquez V., Boys D., Darias J. Yaretol, a norditerpenoid from Azorella madreporica. J. Nat. Prod. 2002;65:1678–1680. doi: 10.1021/np010529p. [DOI] [PubMed] [Google Scholar]
- 26.Loyola L.A., Bórquez J., Morales G., San-Martín A., Darias J., Flores N., Giménez A. Mulinane-type diterpenoids from Azorella compacta display antiplasmodial activity. Phytochemistry. 2004;65:1931–1935. doi: 10.1016/j.phytochem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Nicoletti M., di Fabio A., d’Andrea A., Salvatore G., van Baren C., Coussio J.D. Diterpenoid acids from Mulinum spinosum. Phytochemistry. 1996;43:1065–1067. doi: 10.1016/S0031-9422(96)00403-7. [DOI] [Google Scholar]
- 28.Wächter G.A., Matooq G., Hoffmann J.J., Maiese W.M., Singh M.P., Montenegro G., Timmermann B.N. Antibacterial diterpenoid acids from Azorella compacta. J. Nat. Prod. 1999;62:1319–1321. doi: 10.1021/np990134u. [DOI] [PubMed] [Google Scholar]
- 29.Areche C., Rojas-Alvarez F., Campos-Briones C., Lima C., Pérez E.G., Sepúlveda B. Further mulinane diterpenoids from Azorella compacta. J. Pharm. Pharmacol. 2013;65:1231–1238. doi: 10.1111/jphp.12083. [DOI] [PubMed] [Google Scholar]
- 30.Areche C., Sepúlveda B., San-Martín A., García-Beltrán O., Simirgiotis M., Cañete A. An unusual mulinane diterpenoid from the Chilean plant Azorella compacta (Gaertn) Pers. Org. Biomol. Chem. 2014;12:6406–6413. doi: 10.1039/C4OB00966E. [DOI] [PubMed] [Google Scholar]
- 31.Bórquez J., Ardiles A., Loyola L.A., Peña-Rodriguez L.M., Molina-Salinas G.M., Vallejos J., Collado I.G., Simirgiotis M.J. Further mulinane and azorellane diterpenoids isolated from Mulinum crassifolium and Azorella compacta. Molecules. 2014;19:3898–3908. doi: 10.3390/molecules19043898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bórquez J., Bartolucci N.L., Echiburú-Chau C., Winterhalter P., Vallejos J., Jerz G., Simirgiotis M.J. Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama Desert by high-speed counter-current chromatography. J. Sci. Food Agric. 2015;96:2832–2838. doi: 10.1002/jsfa.7451. [DOI] [PubMed] [Google Scholar]
- 33.Salgado F., Areche C., Sepúlveda B., Simirgiotis M.J., Caceres F., Quispe C., Quispe L., Cano T. A new mulinane diterpenoid from the cushion shrub Azorella compacta growing in Peru. Pharmacog. Mag. 2014;10:S542–S548. doi: 10.4103/0973-1296.139807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepúlveda B., Quispe C., Simirgiotis M., García-Beltrán O., Areche C. Gastroprotective effects of new diterpenoid derivatives from Azorella cuatrecasasii Mathias & Constance obtained using a β-cyclodextrin complex with microbial and chemical transformations. Bioorg. Med. Chem. Lett. 2016;26:3220–3222. doi: 10.1016/j.bmcl.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 35.San-Martín A., Bacho M., Núñez S., Rovirosa J., Soler A., Blanc V., León R., Olea A.F. A novel normulinane isolated from Azorella compacta and assessment of its antibacterial activity. J. Chil. Chem. Soc. 2018;63:4082–4085. doi: 10.4067/s0717-97072018000304082. [DOI] [Google Scholar]
- 36.Liu Y.-T., Li L.-P., Xie J.-H., Zhou Q.-L. Divergent asymmetric total synthesis of mulinane diterpenoids. Angew. Chem. Int. Edit. 2017;56:12708–12711. doi: 10.1002/anie.201706994. [DOI] [PubMed] [Google Scholar]
- 37.Reber K.P., Xu J., Guerrero C.A. Synthesis of mulinane diterpenoids. J. Org. Chem. 2015;80:2397–2406. doi: 10.1021/jo502602u. [DOI] [PubMed] [Google Scholar]
- 38.Noble R.A., Alexander R., Kagi R.I., Knox J. Tetracyclic diterpenoid hydrocarbons in some Australian coals, sediments and crude oils. Geochim. Cosmochim. Acta. 1985;49:2141–2147. doi: 10.1016/0016-7037(85)90072-9. [DOI] [Google Scholar]
- 39.Noble R.A., Alexander R., Kagi R.I., Knox J. Identification of some diterpenoid hydrocarbons in petroleum. Org. Geochem. 1986;10:825–829. doi: 10.1016/S0146-6380(86)80019-5. [DOI] [Google Scholar]
- 40.Shareef A., Angrove M.J., Wells J.D. Optimization of silylation using N-methyl-N-(trimethylsilyl)-trifluoroacetamide, N,O-bis-(trimethylsilyl)-trifluoroacetamide and N-(tert-butyldimethylsilyl)-N-methyltrifluoroactamide for the determination of the estrogens estrone and 17α-ethinylestradiol by gas chromatography-mass spectrometry. J. Chromatogr. A. 2006;1108:121–128. doi: 10.1016/j.chroma.2005.12.098. [DOI] [PubMed] [Google Scholar]
- 41.Kühnel E., Laffan D.D.P., Lloyd-Jones G.C., Martínez del Campo T., Shepperson I.R., Slaughter J.L. Mechanism of methyl esterification of carboxylic acids by trimethylsilyldiazomethane. Angew. Chem. Int. Ed. 2007;46:7075–7078. doi: 10.1002/anie.200702131. [DOI] [PubMed] [Google Scholar]
- 42.Kovats E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta. 1958;41:1915–1932. doi: 10.1002/hlca.19580410703. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.