Abstract
RAD51 (DNA repair gene) family genes play ubiquitous roles in immune response among species from plants to mammals. In this study, we cloned the ZmRAD51A gene (a member of RAD51) in maize and generated ZmRAD51A overexpression (ZmRAD51A-OE) in rice, tobacco, and Arabidopsis. The expression level of ZmRAD51A was remarkably induced by salicylic acid (SA) application in maize, and the transient overexpression of ZmRAD51A in tobacco induced a hypersensitive response. The disease resistance was significantly enhanced in ZmRAD51A- OE (overexpressing) plants, triggering an increased expression of defense-related genes. High-performance liquid chromatography (HPLC) analysis showed that, compared to control lines, ZmRAD51A-OE in rice plants resulted in higher SA levels, and conferred rice plants resistance to Magnaporthe oryzae. Moreover, the ZmRAD51A-OE Arabidopsis plants displayed increased resistance to Pseudomonas syringae pv. tomato DC3000 when compared to wild types. Together, our results provide the evidence that, for the first time, the maize DNA repair gene ZmRAD51A plays an important role in in disease resistance.
Keywords: disease resistance, ZmRAD51A, maize, rice, Arabidopsis
1. Introduction
In plant immune systems, PAMP (pathogen-associated molecular pattern)-triggered immunity (PTI) through pattern recognition is the first line of defense, which keeps most potential invaders in check [1]. Effector-triggered immunity (ETI) is a second line of defense by recognition of attacker-specific effector molecules [1]. The local activation of a PTI or ETI response triggers systemic acquired resistance (SAR) [2]. SAR is accompanied by the increased levels of SAR signal salicylic acid (SA), which then causes the accumulation, nuclear translocation, and turnover of the transcription cofactor NPR1 (non-expressor of PR1 genes), leading to the activation of pathogeneses-related (PR) genes [3,4,5]. SAR provides long-lasting, broad spectrum resistance to secondary infection [6]. Studies have shown that the DNA repaired protein RAD51D and SNI1 (suppressor of NPR1 inducible 1) coregulated NPR1-independent PR gene expression [7]. DNA damage repair proteins SSN2 (Suppressor of sni1 2) and RAD51D replace the transcription repressor SNI1 at PR gene promoters, and their coordinated action ensures plant immune gene expression during plant defense [8].
The RAD51 recombination protein was first discovered in Saccharomyces cerevisiae [9]. It is homologous to the Escherichia coli RecA protein, which plays a vital role in homologous recombination, a well-known repair process of DNA double strand breaks (DSBs) [9]. During the lifetime of plant, DNA damage always occurs, including DSBs, caused by environmental stresses or intercellular events [10]. DSBs influence genome stability and, if not repaired, they can substantially affect cell metabolism and viability [10]. RAD51 was found to express in both meiosis and mitosis and was involved in DNA repair and recombination [11]. Orthologues of RAD51 genes have been identified in several plant species, including Arabidopsis thaliana, Oryza sativa, Zea mays, Triticum aestivum, and Lycopersicon esculentum. In Arabidopsis, AtRAD51 is required during meiotic recombination [12] and its mRNA level increases after gamma irradiation [13]. In addition, AtRAD51B and AtRAD51C are reported to be involved in meiosis, mitosis and DNA repair process in somatic cells [14,15]. In rice, OsRAD51 (rice DNA repair gene) protein promoted homology dependent renaturation, as well as strand exchange reactions, in rice [16]. Another RAD51 gene of rice, OsRAD51D, is a negative factor for telomere lengthening and plays a critical role in reproductive growth in rice [17]. In maize, RAD51 shows higher levels of expression in mitotic and meiotic tissues [18], and recent studies have indicated that the RAD51 protein is involved in meiotic chromosome synapses and segregation [19,20]. In wheat, TaRAD51 expression is restricted to meiotic and highly increases during prophase I of meiosis [21]. A RAD-like gene of Gossypium barbadense GbRL1 is involved in cotton early ovule development and/or fiber initiation [22]. Except for the established roles in meiotic recombination, the presence of some RAD51 has also show the immune function in pathogen invasion; for instance, the rad51d mutant of Arabidopsis enhances disease susceptibility [7], abolishes PR genes transcriptional inducibility, and leads to disease susceptibility [23].
Maize (Zea mays L.) is one of the most important crops in the world, but it is susceptible to many diseases that cause reduced crop yield. So far, a number of genes involved in maize disease resistance have been identified. For example, ZmRxo1 and ZmRp1-D, two genes containing nucleotide-binding site (NBS), are involved in resistance to diverse pathogen strains and confer resistance to rice bacterial streak disease [24] and rust [25]. ZmHtn1 encoding a putative wall-associated receptor-like kinase confers partial northern corn leaf blight resistance for maize by delaying the beginning of lesion formation [26]. RAD51 genes are another class of genes that function in plant disease response. In maize, there are two closely related RAD51 genes, ZmRAD51A and ZmRAD51B. Previous studies have indicated that maize ZmRAD51 can function in the homology search phase of chromosome pairing and meiotic recombination [18], as well as in the repair of MuDR (autonomous mutator transposon)-induced DSBs [27]. However, little is known about the roles of ZmRAD51 genes in maize immune response. In this study, we isolate ZmRAD51A and functionally characterize the role of ZmRAD51A in conferring disease resistance to rice and Arabidopsis.
2. Results
2.1. Cloning and Characterization of ZmRAD51A
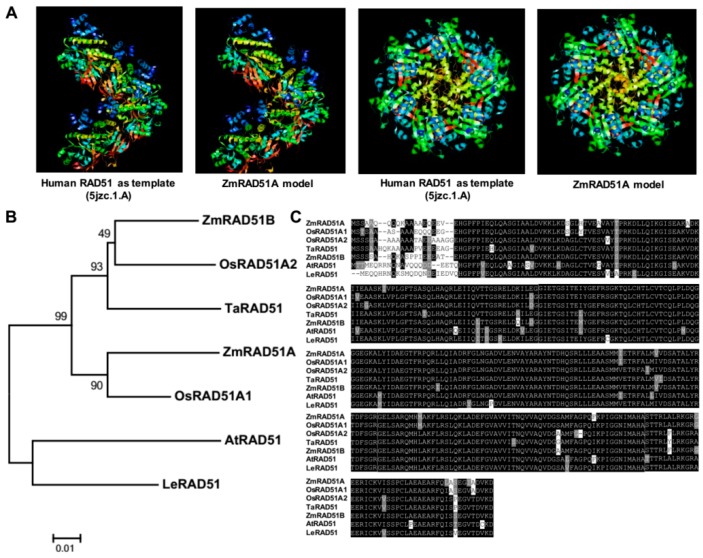
The full-length of ZmRAD51A (NP_001104918.2) coding sequence was obtained from the cDNA of maize B73 leaves by using PCR with specific primers. The ZmRAD51A transcript has 9 exons, encoding 340 amino acids (Figure S1). The predicted 3D model of ZmRAD51A showed similar structures with that of human RAD51 (Figure 1A). An unrooted phylogenetic tree generated by known RAD51 proteins exhibited a high association between ZmRAD51A and OsRAD51A1, and the two proteins share 92.46% sequence identity (Figure 1B). Amino acid sequence alignment of ZmRAD51A and other RAD51 proteins from Zea mays, Arabidopsis thaliana, Lycopersicon esculentum, Triticum aestivum and Oryza sativa Japonica suggested conservation of RAD51 across the species (Figure 1C).
Figure 1.
Characterization of ZmRAD51A. (A) The 3D structures of the ZmRAD51A model. (B) Phylogenetic analysis of ZmRAD51A and other RAD51 proteins from model species. Bootstrap values (1000 replicates) are shown as percentages at the branch nodes. Bar = 0.01. The GenBank accession numbers are: ZmRAD51B (NP_001104919.1) from Zea mays, AtRAD51 (OAO95923.1) from Arabidopsis thaliana, LeRAD51 (Q40134.1) from Lycopersicon esculentum, TaRAD51 (ACM47239.1) from Triticum aestivum, OsRAD51A1 (BAB85490.1) and OsRAD51A2 (ABI58231.1) from Oryza sativa Japonica. (C) Conserved domain comparisons between the amino acid sequence of ZmRAD51A and other RAD51 proteins. Black color represents identical amino acids sequences and gray color represents similar amino acid.
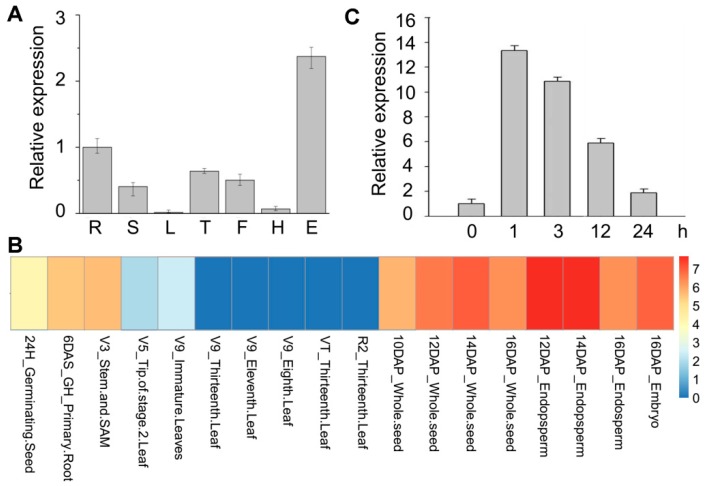
The spatial expression pattern analysis revealed that ZmRAD51A is highly expressed in maize ears, tassels, filaments, roots, and stems, but is relatively lower in leaves (Figure 2A). By employing previously reported transcriptome database, we further investigated the expression profiles of ZmRAD51A gene in tissues at different developmental stages. ZmRAD51A showed high expression levels in seeds, endosperm, embryos, SAM (shoot apical meristem), and roots (Figure 2B). ZmRAD51A was lower expressed in leaves, supported by the qRT-PCR (Quantitative real time polymerase chain reaction) result (Figure 2B). To test whether ZmRAD51A expression in maize leaves responds to biotic stress in SA signaling pathways, we applied SA to maize leaves at the three-leaf stage. The transcript level of ZmRAD51A increased from 1 to 24 h after SA treatment, where the highest transcript level (~13-fold up-regulation) was observed in the first hour after SA treatment (Figure 2C).
Figure 2.
Gene expression patterns of ZmRAD51A in maize. (A) Spatial expression pattern of ZmRAD51A in maize. Data represent means relative expression values + SD. R = root, S = stem, L = leaf, T = tassel, F = flower, H = husk, E = ear. (B) Heat map of ZmRAD51A gene expression in maize. The expression scale of high, medium, and low are represented as red, yellow, and blue colors at right, respectively. V = vegetative growth stage, R = reproductive growth stage, H = hours, DAS = days after sowing, and DAP = days after pollination, GH = greenhouse, V = vegetative, VT = vegetative tasseling, R = reproductive. (C) Gene expression levels of ZmRAD51A in leaf samples response to salicylic acid (SA) application. Three-leaf stage maize seedlings received SA and leaves were harvested at 0 h, 1 h, 3 h, 12 h and 24 h after SA treatment. ZmActin [28] was used for normalization. The expression levels of 0 h was used as the control and assigned value of 1. Data represent means SD. Three biological replicates were performed.
2.2. Transient Overexpression of ZmRAD51A in Nicotiana Benthamiana Leaves Induced a Hypersensitive Response
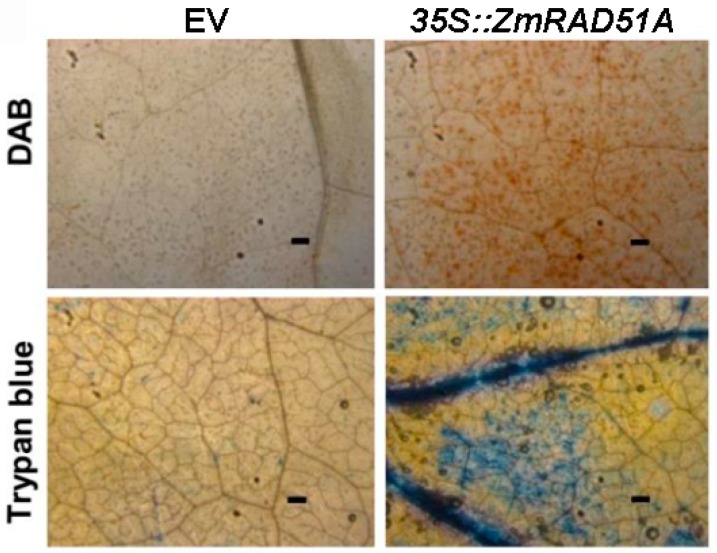
The 35S:ZmRAD51A construct, driven by CaMV35S promoter, was generated. The A. tumefaciens GV3101 containing 35S:ZmRAD51A vector and pCAMBIA1301 (control) infiltrated N. benthamiana leaves to verify hypersensitive response cell death. DAB (Diaminobenzidine) staining showed a large amount of H2O2 was accumulated in transformed N. benthamiana leaf overexpressing ZmRAD51A after 48 h (Figure 3). Trypan blue staining analysis correlated with the visual damage index [29]. ZmRAD51A transient overexpression leaf showed darker trypan blue staining than that of pCAMBIA1301 (Figure 3). These results suggest that transient overexpression of ZmRAD51A in tobacco leaves induced a hypersensitive response and H2O2 accumulation in response to stress.
Figure 3.
Transient expression of ZmRAD51A in Nicotiana benthamiana affected immunity induction. DAB (Diaminobenzidine) staining and Trypan blue staining in N. benthamiana leaves 48 h after 35S:ZmRAD51A-Agrobacterium and pCAMBIA1301 (EV)-Agrobacterium infiltration. Bars = 0.5 mm.
2.3. Transgenic Overexpression of ZmRAD51A Increased SA Synthesis and Conferred Rice Resistance to M. oryzae
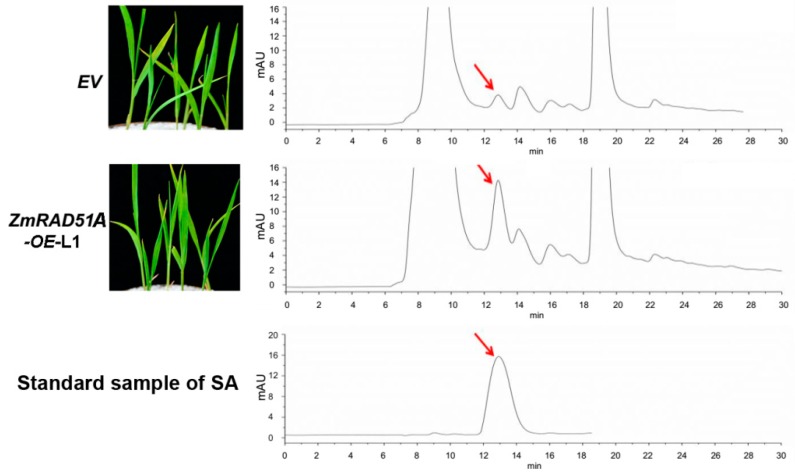
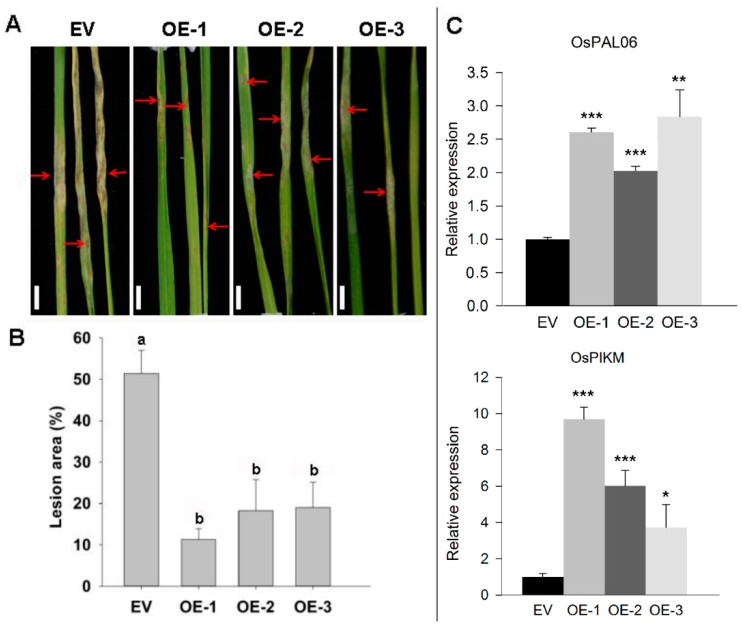
To test whether ZmRAD51A overexpression confers disease resistance in rice, 70 pieces of rice calli were induced and 19 transgenic lines expressing ZmRAD51A (ZmRAD51A-OE) were generated. Three transgenic lines renamed OE-1, 2, and 3, were randomly selected for further analysis. Transgenic expression analysis showed the three transgenic lines had different expression levels (Figure S2). The growth of ZmRAD51A-OE transgenic rice at the seedling stage was similar to the empty vector (EV) transgenic lines (Figure 4). Demonstrated by high-performance liquid chromatography (HPLC), we found that SA accumulation drastically increased in ZmRAD51A-OE plants (peak area 400.596 g–1) compared with control plants (peak area 74.749 g–1) without any pathogen inoculation (Figure 4). ZmRAD51A-OE transgenic plants at 4–5-leaf stage were challenged with M. oryzae, which can cause rice blast. Interestingly, overexpression of ZmRAD51A reduced macroscopic blast symptoms, with fewer and smaller blast lesions than the control at 7 dpi of M. oryzae (Figure 5A). ZmRAD51A-OE plants showed the lesion area ranging from 11.3 (OE-1) to 19.0% (OE-3) at 7 dpi, while that of the control plants was 51.4% (Figure 5B). In addition, the expression levels of rice blast resistant genes, OsPAL06, and OsPIKM [30], were respectively increased 2.3-fold and 5.1-fold in ZmRAD51A-OE transgenic lines at 7 dpi of M. oryzae (Figure 5C). These results together suggest that the overexpression of ZmRAD51A provides an enhanced resistance to rice blast in ZmRAD51A-OE lines.
Figure 4.
Chromatogram of SA extracted from ZmRAD51A-OE and pCAMBIA1301 transgenic rice without pathogen inoculation. The SA chromatogram from top to bottom represents pCAMBIA1301 (EV), ZmRAD51A-OE, and standard SA. The arrow indicates the SA peak; mAU indicates peak height. EV represents pCAMBIA1301.
Figure 5.
ZmRAD51A-OE transgenic rice plants enhanced resistance to M. oryzae at seedling stages. (A) Phenotype of ZmRAD51A-OE and pCAMBIA1301 (EV) transgenic rice at 7 dpi (days post infection) of M. oryzae. OE-1, OE-2, OE-3 represent three transgenic lines of ZmRAD51A-OE transgenic rice plant. Red arrows represent blast lesions. Bars = 50 mm. (B) Statistical analysis of lesion area in M. oryzae infected leaves. Different letters above the columns indicate significant differences at p < 0.05 level among EV, OE-1, OE-2 and OE-3. (C) Gene expression patterns of defense-related genes in ZmRAD51A-OE and pCAMBIA1301 (EV) inoculated with M. oryzae. Student’s test was performed between pCAMBIA1301 and ZmRAD51A-OE transgenic lines (* p < 0.05, ** p < 0.01 and *** p < 0.001). OsActin [31] was used for normalization. The expression levels of inoculated EV was used as the control and assigned value of 1. Data represent means SD. Three biological replicates were performed.
To investigate whether ZmRAD51A gene affect rice yield, we measured rice yield-related traits, including the seed size and 1000-grain weight, among EV control and ZmRAD51A-OE lines. The seeds of ZmRAD51A-OE rice showed no significant size (in terms of length and width) and 1000-grain weight when compared to EV control rice (Figure S3).
2.4. ZmRAD51A Overexpression in Arabidopsis Enhanced Resistance to Pst DC3000 Triggering by Increased SA-Related Genes Expression
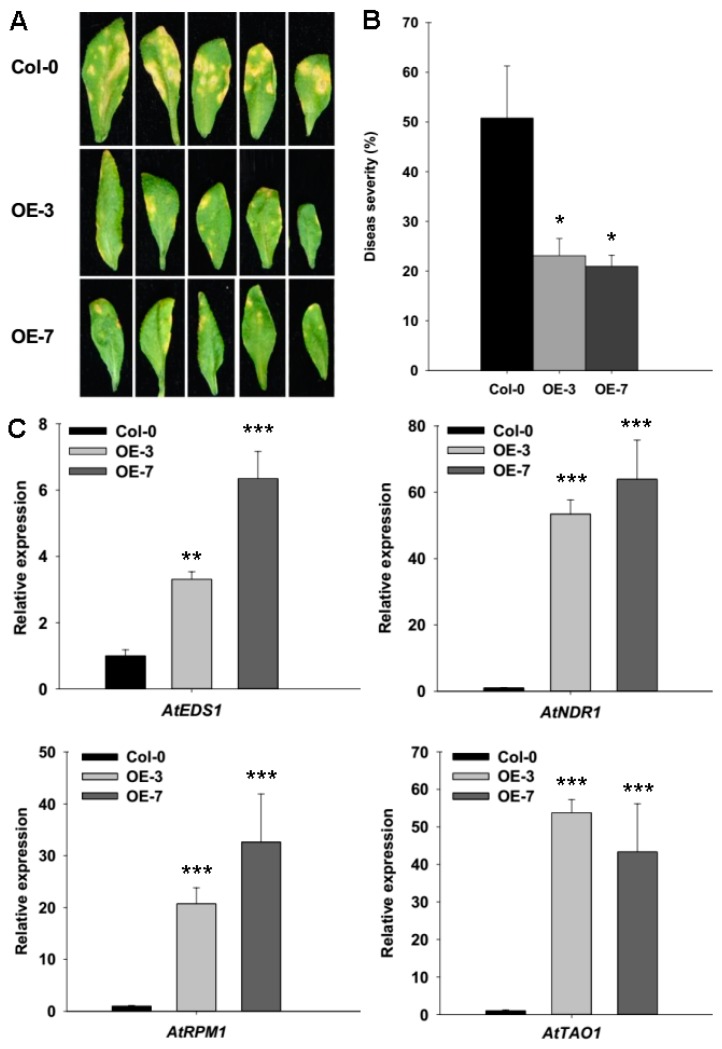
To investigate whether ZmRAD51A confers disease resistance to other species, we generated ZmRAD51A overexpression Arabidopsis plants. We firstly examined the impact of ZmRAD51A overexpression in Arabidopsis upon the infection of Pst DC3000 (Pseudomonas syringae pathovar tomato DC3000). The ZmRAD51A-OE lines showed significant resistance to Pst DC3000 at 7 dpi (Figure 6A,B). Col-0 plants had more lesions than ZmRAD51A-OE plants after Pst DC3000 infection (Figure 6A). The disease severity (DI, disease index) of ZmRAD51A-OE lines was 23.11% (OE-3) and 20.97% (OE-7) on average, but the Col-0 presented a DI of 50.78% at 7 dpi (Figure 6B). We further examined the expression levels of the genes that were involved in SA-dependent defense signaling pathway and pathogen resistance at 7 dpi, including AtEDS1 (Enhanced Disease Susceptibility 1) [32], AtNDR1 (Nonrace Specific Disease Resistance 1) [33], AtRPM1 (Resistance to P. syringae 1) [34], and AtTAO1 (Target of AvrB Operation) [35]. All the four genes that participated either in the SA signal pathway or in the resistance to Pst DC3000 pathogens were expressed significantly higher in the ZmRAD51A-OE plants than in the Col-0 plants (Figure 6C).
Figure 6.
Enhanced resistance of ZmRAD51A-OE transgenic Arabidopsis plants to Pst DC3000. (A) Phenotypes of ZmRAD51A-OE and Col-0 leaves after Pst DC3000 infected for 7 days. (B) Disease index of ZmRAD51A-OE and Col-0 plants infected by Pst DC3000. (C) Expression levels of defense-related genes in ZmRAD51A-OE and Col-0 plants infected by Pst DC3000. Data were normalized using the transcript level of AtUbiquitin [36]. The expression levels of genes in Col-0 plants infected by Pst DC3000 were used as the control and assigned value of 1. OE-3 and OE-7 represents two different transgenic lines of ZmRAD51A-OE Arabidopsis plants. Student’s test was performed between Col-0 plants and ZmRAD51A-OE transgenic lines (* p < 0.05, ** p < 0.01 and *** p < 0.001). Data represent means SD. Three biological replicates were performed.
3. Discussion
3.1. ZmRAD51A Is a Conserved DNA Repair Protein in Maize
The structure analyses on amino acid sequences and 3D model suggests that ZmRAD51A is conserved across plants to mammals. Notably, ZmRAD51A showed a close evolutionary relationship with OsRAD51A1, which can bind single and double stranded DNA, and promote homology dependent renaturation as well as strand exchange reactions [16], suggesting a similar role of ZmRAD51A in maize. Although the ZmRAD51A has been demonstrated to play important roles in meiotic recombination and DNA repair in maize, little is known about its function in disease resistance. In this study, for the first time, we isolated ZmRAD51A and functionally characterized its roles on disease resistance function in model plants.
3.2. ZmRAD51A Is Involved in SA-Signal Defense Responses
The expression patterns of ZmRAD51A showed ZmRAD51A is enriched in ears, roots, tassels, and filaments (Figure 2A), where mitosis and meiosis are more active, supporting that ZmRAD51A plays roles in the repair of DSBs. Although ZmRAD51A is usually less expressed in maize leaf, interestingly, it was significantly upregulated (up to 13-fold) within 1 h upon SA application (Figure 2B). Such a quick response was also observed in other resistance genes, including the bacterial blight disease resistance gene Xa1 in rice [37] and the Ralstonia solanacearum resistance gene AhRRS5 in peanuts [38]. SA is a well-known phytohormone signaling molecule involved in controlling the defense gene expression against disease [39]. The exogenous application of SA to a plant induces systemic acquired resistance (SAR), increases the expression of pathogeneses-related (PR) genes and enhances resistance to a broad range of pathogens [40,41,42]. Similar to those SA-dependent defense-related genes [31,43,44], our results suggested that ZmRAD51A may also involve in SA signaling pathways against pathogen infection. In addition, the transient overexpression of ZmRAD51A in N. benthamiana showed that it can induce a hypersensitive response, causing cell death and also the accumulation of H2O2 in hypersensitive responses (Figure 3), indicating that ZmRAD51A may be involved in reactive oxygen species (ROS) signaling against disease. These results together suggested that ZmRAD51A may be involved in SA-dependent cell death and disease resistance during pathogen infection.
3.3. ZmRAD51A Confers Disease Resistance in Transgenic Plants
Phenylalanine ammonia lyase (PAL) is a SA pathway-associated gene and a key enzyme that controls the biosynthesis of SA [45]. OsPAL06 knockout mutant showed increased susceptibility to M. oryzae and developed typical leaf blast symptoms, accompanied by a reduction of the SA level [45]. In this study, OsPAL06 increased significantly in ZmRAD51A-OE transgenic lines at 7 days after being inoculated with M. oryzae (Figure 5C), indicating ZmRAD51A conferred resistance to rice blast, accompanied by increasing of the SA level. Moreover, ZmRAD51A also enhanced Arabidopsis resistance to Pst DC3000, by triggering the expression of SA-dependent signal genes and defense-related genes, AtEDS1 (Enhanced Disease Susceptibility 1), AtNDR1 (Nonrace Specific Disease Resistance 1), AtRPM1 (Resistance to P. syringae 1), and AtTAO1 (Target of AvrB Operation) (Figure 6C). AtEDS1 operates in the upstream of SA-mediated defenses and requires resistance to be mediated by several R genes in Arabidopsis [32]. The increased expression level of AtEDS1 in ZmRAD51A-OE Arabidopsis indicated that ZmRAD51A is involved in SA-mediated defense pathway. It has been reported that AtNDR1 was required for disease resistance signaling mediated by members of disease resistance proteins in Arabidopsis in response to infection by P. syringae [46], while AtRPM1 and AtTAO1 were conferring disease resistance in response to Pst DC3000 [34,35]. The up-regulation of AtNDR1, AtRPM1, and AtTAO1 in ZmRAD51A-OE plants suggested that ZmRAD51A plays a positive role in resistance against Pst DC3000. Given that ZmRAD51A plays an important role in DNA recombination in maize [18], the dual roles of ZmRAD51A in disease resistant gene and DNA recombination suggest an interesting mechanistic link between defense response and DNA recombination, which is also supported by the previous reports of microbial pathogens causing DNA damage [47]. The abundance of double strand breaks is reduced by plant defense responses, suggesting that the mechanisms for activating DNA repair processes may share some similarity with the induction of PR genes [48,49]. More recently, DNA damage has also been found to be associated with SA signaling, where the increased expression of PR genes and the growth suppression of Fusarium solani were found in peas [50].
4. Materials and Methods
4.1. Plant Materials and Treatments
Maize B73 plants were grown in a greenhouse at 28 °C under a 16 h light/8 h dark photoperiod. Healthy maize seedlings at the three-leaf stage were sprayed with 1 × 10–3 M salicylic acid (SA). After 0h, 1 h, 3 h, 12 h, and 24 h, leaves were harvested and stored at –80 °C for subsequent RNA extraction. Other plants were further cultured and harvested roots, leaves, stems, tassels, filaments, husks, and ears were stored at –80 °C for subsequent RNA extraction.
4.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA from maize, rice, and Arabidopsis tissues was extracted by TRIzol (Thermo Fisher Scientific, Waltham, MA, USA). The reverse transcription kit (Roche Molecular Systems, Inc., Pleasanton, CA, USA) was used for cDNA synthesize. The SYBR green PCR master mix (Roche Molecular Systems, Inc., Pleasanton, CA, USA) was used for qRT-PCR reaction. The qRT-PCR was performed by Applied Biosystems 7300 (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instruction. The qRT-PCR primers used in this study are listed in Table S1. The relative expression levels of each gene were calculated by formula 2−ΔΔCt [38].
4.3. Bioinformatics Analysis
The ZmRAD51A gene structure was analyzed by GSDS (Gene Structure Display Server; http://gsds.cbi.pku.edu.cn/) [51]. The 3D structure of ZmRAD51A was assembled by SWISS-MODEL (https://swissmodel.expasy.org/) according to homology modeling, using RAD51 from humans as a template [52]. The PDB IDs of RAD51 is 5jzc.1. A. Proteins sequences between ZmRAD51A and other RAD51 were aligned by MEGA6 [53]. A phylogenetic tree was then constructed by the neighbor-joining method (bootstrap = 1000) [54]. Gene transcription data of ZmRAD51A in various growth time and tissues was used to draw a heat map by R/Bioconductor (http://www.bioconductor.org/) [55]. Promoters were analyzed by RSAT (http://floresta.eead.csic.es/rsat/) [56].
4.4. Full-Length cDNA Cloning and Vector Construction
The full-length cDNA of ZmRAD51A was isolated from maize leaves cDNA by using PCR with specific primers (Forward ATGGCAGAAGCTGTGGTGTT and reverse CTATATGCGCAACTCCAGACC) and PrimeSTAR Max DNA Polymerase (TaKaRa Bio Inc., Kusatsu, Shiga, Japan). Then, the products were cloned and sequenced. The full-length cDNA of ZmRAD51A was constructed into the pCAMBI1301vector to obtain a p35S:ZmRAD51A fusion gene.
4.5. Cell Death Assays in Nicotiana Benthamiana
Agrobacterium tumefaciens strain GV3101 containing 35S:ZmRAD51A and pCAMBI1301 (control) constructs were respectively injected into N. benthamiana leaves by syringe, which contained the volume of about 100 μL as described by Stella et al. [57]. Photos of N. benthamiana leaves phenotype were taken 48 h after Agrobacterium infection. DAB and trypan blue staining were performed as previously described [38,58]. N. benthamiana leaves were treated with DAB (3′-Diaminobenzidine, Sigma, St. Louis, MO, USA) solution (1 mg mL−1) overnight and then cleared with 95% ethanol. For trypan blue staining, the leaves were boiled in lactophenol-trypan blue solution (1 mg mL−1) for 5 min and destained overnight in chloral hydrate (2.5 g mL−1). The DAB and trypan blue staining leaves were observed under a microscope (Leica DM5000 B, Leica Microsystems Ltd., Heerbrugg, Switzerland).
4.6. Rice Transformation
The seeds of rice Zhonghua 11 were used to induce embryogenic calli. Well growth embryogenic calli were harvested for infection by A. tumefaciens GV3101 harboring the 35S:ZmRAD51A constructs and pCAMBI1301 (control) as previously described [37]. After 2 days co-cultivated with A. tumefaciens GV3101 in N6 medium containing 200 μM acetosyringone, embryogenic calli were thoroughly washed with ddH2O, and then transferred to a new N6 medium, containing 0.25 g mL−1 cefotaxime and 0.05 g mL−1 hygromycin. After several selections, the embryogenic calli propagated plants in regeneration medium.
4.7. Measurement of SA
SA extraction and quantification were performed according to the method described in the previous study [59,60]. The leaves of rice transformed of pCAMBI1301 and ZmRAD51A were excised and frozen in liquid nitrogen (N2). The SA was extracted from 0.3 g leaf powder by sequentially subjecting them to 70% and 90% methanol. The 2-methoxybenzoic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as an internal SA standard. The reverse-phase high-performance liquid chromatography (RP-HPLC) (Agilent 1200 series with a C18 column [150 mm × 4.6 mm, 5 μm], Agilent Technologies, Santa Clara, CA, USA) was used for SA measurement.
4.8. Rice Pathogens Inoculation
ZmRAD51A overexpression rice and control of rice plants with the fourth leaf fully expanded were selected for disease analysis. Rice seedlings were inoculated with Magnaporthe oryzae Guy 11 using the spraying method to assess the resistance of transgenic rice to blast [61]. Disease was scored by measuring the percent lesion area (lesion length/leaf length) at 7 days after inoculation [31].
4.9. Arabidopsis Transformation
Arabidopsis plants Col-0 were transformed by A. tumefaciens GV3101 carrying the 35S:ZmRAD51A using the floral dip method [62]. Transformed Arabidopsis seeds were screened on Murashige and Skoog plates containing 20 mg mL−1 hygromycin. DNA was extracted from the Arabidopsis plant using the CTAB (cetyl trimethyl ammonium bromide) method [63], which was used as a template for PCR to further determine positive transgene integration. The 2× Taq Master Mix (Dye Plus; Vazyme Biotech Co. Ltd., Nanjing, China) was used for the PCR reaction. After several screens, the homozygous T3 generation was used for experiments.
4.10. Arabidopsis Pathogen Inoculation
Pseudomonas syringae pv. (pathovar) tomato DC3000 (Pst DC3000) was cultured at 28 °C on King’s B (KB) medium. After 2 days, a single Pst DC3000 colony was inoculated in 5 mL KB medium for 1 day at 28 °C. One milliliter of the culture was inoculated in 100 mL KB medium for 1 day. The bacterial was harvested and suspended in a solution which included 0.01% Silwet L-77 and 10 mM MgSO4 with final OD600 = 1.0. Two transgenic Arabidopsis lines (OE-3 and OE-7) and Col-0 were sprayed with Pst DC3000 suspension. Whole Arabidopsis plants were sprayed by the bacterial suspension and then covered with plastic film for 3 days. Disease index was evaluated after 7 days post inoculation (dpi) as Niu et al. described [64]. Three biological replicates were set.
4.11. Statistical Analyses
The data were subjected to excel software 2016 with analysis of student’s test to evaluate differences between control and treatment samples. Statistical significance was set at * p < 0.05, ** p < 0.01 and *** p < 0.001.
Acknowledgments
We thank Ting Ding for providing us Magnaporthe oryzae and we thank Shanshan Xie for providing us Pst DC3000.
Abbreviations
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| SA | Salicylic acid |
| HPLC | High-performance liquid chromatography |
| PTI | PAMP-triggered immunity |
| ETI | Effector-triggered immunity |
| NPR1 | NONEXPRESSOR OF PR1 GENES |
| DSBs | DNA double strand breaks |
| SAR | systemic acquired resistance |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/4/807/s1.
Author Contributions
F.L., Y.X., L.Z., A.A., H.J., S.Z. and X.L. conceived the project. F.L. performed Arabidopsis transformation and L.Z. performed rice transformation. Y.X. carried out the other experiments. Y.X. and F.L. performed the statistical analysis. F.L., Y.X., A.A., H.J., S.Z. and X.L. wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the special project of local science and technology development guided by the central government of Anhui province (No.2018080503B0015), National Natural Science Foundation of China (No. 31870415) and Graduate Innovation Fund of Anhui Agriculture University (No. 2018yjs-40).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dodds P.N., Rathjen J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010;11:539. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 2.Mishina T.E., Zeier J. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 2007;50:500–513. doi: 10.1111/j.1365-313X.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- 3.Loon L.C.V., Rep M., Pieterse C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 4.Mou Z., Fan W., Dong X. Inducers of Plant Systemic Acquired Resistance Regulate NPR1 Function through Redox Changes. Cell. 2003;113:935. doi: 10.1016/S0092-8674(03)00429-X. [DOI] [PubMed] [Google Scholar]
- 5.Spoel S.H., Mou Z., Tada Y., Spivey N.W., Genschik P., Dong X. Proteasome-Mediated Turnover of the Transcription Co-Activator NPR1 Plays Dual Roles in Regulating Plant Immunity. Cell. 2009;137:860–872. doi: 10.1016/j.cell.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrant W.E., Dong X. Systemic acquired resistance. Plant Physiol. 2013;42:627–629. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 7.Durrant W.E., Wang S., Dong X. Arabidopsis SNI1 and RAD51D Regulate Both Gene Transcription and DNA Recombination during the Defense Response. Proc. Natl. Acad. Sci. USA. 2007;104:4223. doi: 10.1073/pnas.0609357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J., Durrant W.E., Wang S., Yan S., Tan E.H., Dong X. DNA repair proteins are directly involved in regulation of gene expression during plant immune response. Cell Host Microbe. 2011;9:115–124. doi: 10.1016/j.chom.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Shinohara A., Ogawa H., Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-K. [DOI] [PubMed] [Google Scholar]
- 10.Kou Y., Chang Y., Li X., Xiao J., Wang S. The rice RAD51C gene is required for the meiosis of both female and male gametocytes and the DNA repair of somatic cells. J. Exp. Bot. 2012;63:5323. doi: 10.1093/jxb/ers190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symington L.S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 2002;66:630. doi: 10.1128/MMBR.66.4.630-670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Fedoroff N. The Arabidopsis AtRAD51 gene is dispensable for vegetative development but required for meiosis. Proc. Natl. Acad. Sci. USA. 2004;101:10596–10601. doi: 10.1073/pnas.0404110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doutriaux M.P., Couteau F., Bergounioux C., White C. Isolation and characterisation of the RAD51 and DMC1 homologs from Arabidopsis thaliana. Mol. Gen. Genet. 1998;257:283–291. doi: 10.1007/s004380050649. [DOI] [PubMed] [Google Scholar]
- 14.Abe K., Osakabe K., Nakayama S., Endo M., Tagiri A., Todoriki S., Ichikawa H., Toki S. Arabidopsis RAD51C gene is important for homologous recombination in meiosis and mitosis. Plant Physiol. 2005;139:896. doi: 10.1104/pp.105.065243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osakabe K., Abe K., Yamanouchi H., Takyuu T., Yoshioka T., Ito Y., Kato T., Tabata S., Kurei S., Yoshioka Y. Arabidopsis Rad51B is important for double-strand DNA breaks repair in somatic cells. Plant Mol. Biol. 2005;57:819–833. doi: 10.1007/s11103-005-2187-1. [DOI] [PubMed] [Google Scholar]
- 16.Rajanikant C., Melzer M., Rao B.J., Sainis J.K. Homologous recombination properties of OsRad51, a recombinase from rice. Plant Mol. Biol. 2008;68:479. doi: 10.1007/s11103-008-9385-6. [DOI] [PubMed] [Google Scholar]
- 17.Byun M.Y., Kim W.T. Suppression of OsRAD51D results in defects in reproductive development in rice (Oryza sativa L.) Plant J. 2014;79:256–269. doi: 10.1111/tpj.12558. [DOI] [PubMed] [Google Scholar]
- 18.Franklin A.E., Mcelver J., Sunjevaric I., Rothstein R., Bowen B., Cande W.Z. Three-dimensional microscopy of the Rad51 recombination protein during meiotic prophase. Plant Cell. 1999;11:809–824. doi: 10.1105/tpc.11.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin A.E., Golubovskaya I.N., Bass H.W., Cande W.Z. Improper chromosome synapsis is associated with elongated RAD51 structures in the maize desynaptic2 mutant. Chromosoma. 2003;112:17–25. doi: 10.1007/s00412-003-0242-8. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski W.P., Golubovskaya I.N., Cande W.Z. Altered nuclear distribution of recombination protein RAD51 in maize mutants suggests the involvement of RAD51 in meiotic homology recognition. Plant Cell. 2003;15:1807. doi: 10.1105/tpc.012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devisetty U.K., Mayes K., Mayes S. The RAD51 and DMC1 homoeologous genes of bread wheat: Cloning, molecular characterization and expression analysis. BMC Res. Notes. 2010;3:245. doi: 10.1186/1756-0500-3-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F., Liu X., Zuo K., Zhang J., Sun X., Tang K. Molecular Cloning and Characterization of a Novel Gossypium barbadense L. RAD-Like Gene. Plant Mol. Biol. Report. 2011;29:324–333. doi: 10.1007/s11105-010-0234-9. [DOI] [Google Scholar]
- 23.Wang S., Schulze-Lefert P. Arabidopsis BRCA2 and RAD51 proteins are specifically involved in defense gene transcription during plant immune responses. Proc. Natl. Acad. Sci. USA. 2010;107:22716. doi: 10.1073/pnas.1005978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B., Lin X., Poland J., Trick H., Leach J., Hulbert S. A maize resistance gene functions against bacterial streak disease in rice. Proc. Natl. Acad. Sci. USA. 2005;102:15383–15388. doi: 10.1073/pnas.0503023102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins N., Drake J., Ayliffe M., Sun Q., Ellis J., Hulbert S., Pryor T. Molecular Characterization of the Maize Rp1-D Rust Resistance Haplotype and Its Mutants. Plant Cell. 1999;11:1365–1376. doi: 10.1105/tpc.11.7.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurni S., Scheuermann D., Krattinger S.G., Kessel B., Wicker T., Herren G., Fitze M.N., Breen J., Presterl T., Ouzunova M. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl. Acad. Sci. USA. 2015;112:8780. doi: 10.1073/pnas.1502522112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Wen T.J., Schnable P.S. Role of RAD51 in the Repair of MuDR-Induced Double-Strand Breaks in Maize (Zea mays L.) Genetics. 2008;178:57. doi: 10.1534/genetics.107.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C., Deng W., Tang N., Wang X., Fang Y., Lin D., Li Z. Overexpression of ZmAFB2, the maize homologue of AFB2 gene, enhances salt tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. 2013;112:171–179. doi: 10.1007/s11240-012-0219-5. [DOI] [Google Scholar]
- 29.Zhang H., Teng W., Liang J., Liu X., Zhang H., Zhang Z., Zheng X. MADS1, a novel MADS-box protein, is involved in the response of Nicotiana benthamiana to bacterial harpin (Xoo) J. Exp. Bot. 2016;67:131–141. doi: 10.1093/jxb/erv448. [DOI] [PubMed] [Google Scholar]
- 30.Li L.Y., Wang L., Jing J.X., Li Z.Q., Lin F., Huang L.F., Pan Q.H. The Pik m gene, conferring stable resistance to isolates of Magnaporthe oryzae, was finely mapped in a crossover-cold region on rice chromosome 11. Mol. Breed. 2007;20:179–188. doi: 10.1007/s11032-007-9118-6. [DOI] [Google Scholar]
- 31.Qiu D., Xiao J., Ding X., Xiong M., Cai M., Cao Y., Li X., Xu C., Wang S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant-Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- 32.Falk A., Feys B.J., Frost L.N., Jones J.D., Daniels M.J., Parker J.E. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA. 1999;96:3292. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day B., Dahlbeck D., Staskawicz B.J. NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. Plant Cell. 2006;18:2782–2791. doi: 10.1105/tpc.106.044693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z., Chung E.H., Eitas T.K., Dangl J.L. Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc. Natl. Acad. Sci. USA. 2011;108:7619–7624. doi: 10.1073/pnas.1104410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eitas T.K., Nimchuk Z.L., Dangl J.L. Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. USA. 2008;105:6475–6480. doi: 10.1073/pnas.0802157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y., Guo L., Liu R., Jiao B., Zhao X., Ling Z., Luo K. Overexpression of Poplar PtrWRKY89 in Transgenic Arabidopsis Leads to a Reduction of Disease Resistance by Regulating Defense-Related Genes in Salicylate- and Jasmonate-Dependent Signaling. PLoS ONE. 2016;11:e0149137. doi: 10.1371/journal.pone.0149137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datta K., Koukolikova-Nicola Z., Baisakh N., Oliva N., Datta S.K. Agrobacterium-mediated engineering for sheath blight resistance of indica rice cultivars from different ecosystems. Theor. Appl. Genet. 2000;100:832–839. doi: 10.1007/s001220051359. [DOI] [Google Scholar]
- 38.Zhang C., Chen H., Cai T., Deng Y., Zhuang R., Zhang N., Zeng Y., Zheng Y., Tang R., Pan R., et al. Overexpression of a novel peanut NBS-LRR gene AhRRS5 enhances disease resistance to Ralstonia solanacearum in tobacco. Plant Biotechnol. J. 2017;15:39–55. doi: 10.1111/pbi.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Divi U.K., Rahman T., Krishna P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010;10:151. doi: 10.1186/1471-2229-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D.L., Yang Y.N., He Z.H. Roles of Plant Hormones and Their Interplay in Rice Immunity. Mol. Plant. 2013;6:675–685. doi: 10.1093/mp/sst056. [DOI] [PubMed] [Google Scholar]
- 41.Novakova M., Sasek V., Dobrev P.I., Valentova O., Burketova L. Plant hormones in defense response of Brassica napus to Sclerotinia sclerotiorum—Reassessing the role of salicylic acid in the interaction with a necrotroph. Plant Physiol. Biochem. 2014;80:308–317. doi: 10.1016/j.plaphy.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Gaffney T., Friedrich L., Vernooij B., Negrotto D., Nye G., Uknes S., Ward E., Kessmann H., Ryals J. Requirement of salicylic Acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 43.Grant J.J., Chini A., Basu DLoake G.J. Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol. Plant-Microbe Interact. 2003;16:669–680. doi: 10.1094/MPMI.2003.16.8.669. [DOI] [PubMed] [Google Scholar]
- 44.Deslandes L., Olivier J., Theulieres F., Hirsch J., Feng D.X., Bittner-Eddy P., Beynon J., Marco Y. Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1-R gene, a member of a novel family of resistance genes. Proc. Natl. Acad. Sci. USA. 2002;99:2404–2409. doi: 10.1073/pnas.032485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan L., Liu H., Li X., Xiao J., Wang S. Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol. Plant. 2014;152:486. doi: 10.1111/ppl.12192. [DOI] [PubMed] [Google Scholar]
- 46.Coppinger P., Repetti P.P., Day B., Dahlbeck D., Mehlert A., Staskawicz B.J. Overexpression of the plasma membrane-localized NDR1 protein results in enhanced bacterial disease resistance in Arabidopsis thaliana. Plant J. 2004;40:225–237. doi: 10.1111/j.1365-313X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 47.Song J., Bent A.F. Microbial pathogens trigger host DNA double-strand breaks whose abundance is reduced by plant defense responses. PLOS Pathog. 2014;10:e1004030. doi: 10.1371/journal.ppat.1004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasser S., Orsulic S., Brown E.J., Raulet D.H. The DNA damage pathway regulates innate immune system ligands for the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan S., Wang W., Marqués J., Mohan R., Saleh A., Durrant W.E., Song J., Dong X. Salicylic Acid Activates DNA Damage Responses to Potentiate Plant Immunity. Mol. Cell. 2013;52:602–610. doi: 10.1016/j.molcel.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka K., Hadwiger L.A. Nonhost resistance: Reactive oxygen species (ROS) signal causes DNA damage prior to the induction of PR genes and disease resistance in pea tissue. Physiol. Mol. Plant Pathol. 2017;98:18–24. [Google Scholar]
- 51.Zhang C., Zhang H., Zhao Y., Jiang H., Zhu S., Cheng B., Xiang Y. Genome-wide analysis of the CCCH zinc finger gene family in Medicago truncatula. Plant Cell Rep. 2013;32:1543–1555. doi: 10.1007/s00299-013-1466-6. [DOI] [PubMed] [Google Scholar]
- 52.Short J.M., Liu Y., Chen S., Soni N., Madhusudhan M.S., Shivji M.K.K., Venkitaraman A.R. High-resolution structure of the presynaptic RAD51 filament on single-stranded DNA by electron cryo-microscopy. Nucleic Acids Res. 2016;44:9017–9030. doi: 10.1093/nar/gkw783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu F., Xu Y.J., Jiang H.H., Jiang C.S., Du Y.B., Gong C., Wang W., Zhu S.W., Han G.M., Cheng B.J. Systematic Identification, Evolution and Expression Analysis of the Zea mays PHT1 Gene Family Reveals Several New Members Involved in Root Colonization by Arbuscular Mycorrhizal Fungi. Int. J. Mol. Sci. 2016;17:930. doi: 10.3390/ijms17060930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekhon R.S., Lin H.N., Childs K.L., Hansey C.N., Buell C.R., de Leon N., Kaeppler S.M. Genome-wide atlas of transcription during maize development. Plant J. 2011;66:553–563. doi: 10.1111/j.1365-313X.2011.04527.x. [DOI] [PubMed] [Google Scholar]
- 56.Medina-Rivera A., Defrance M., Sand O., Herrmann C., Castro-Mondragon J.A., Delerce J., Jaeger S., Blanchet C., Vincens P., Caron C., et al. RSAT 2015: Regulatory sequence analysis tools. Nucleic Acids Res. 2015;43:W50–W56. doi: 10.1093/nar/gkv362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cesari S., Kanzaki H., Fujiwara T., Bernoux M., Chalvon V., Kawano Y., Shimamoto K., Dodds P., Terauchi R., Kroj T. The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J. 2014;33:1941–1959. doi: 10.15252/embj.201487923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang I.S., Hwang B.K. The Pepper Mannose-Binding Lectin Gene CaMBL1 Is Required to Regulate Cell Death and Defense Responses to Microbial Pathogens. Plant Physiol. 2011;155:447–463. doi: 10.1104/pp.110.164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho K., Han O., Tamogami S., Shibato J., Kubo A., Agrawal G.K., Rakwal R. Quantification of jasmonic and salicylic acids in rice seedling leaves. Methods Mol. Biol. 2013;956:185–200. doi: 10.1007/978-1-62703-194-3_13. [DOI] [PubMed] [Google Scholar]
- 60.Meuwly P., Metraux J.P. Ortho-anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal. Biochem. 1993;214:500–505. doi: 10.1006/abio.1993.1529. [DOI] [PubMed] [Google Scholar]
- 61.Krattinger S.G., Sucher J., Selter L.L., Chauhan H., Zhou B., Tang M.Z., Upadhyaya N.M., Mieulet D., Guiderdoni E., Weidenbach D., et al. The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotechnol. J. 2016;14:1261–1268. doi: 10.1111/pbi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 63.Clarke J.D. Cetyltrimethyl ammonium bromide (CTAB) DNA miniprep for plant DNA isolation. CSH Protoc. 2009;2009:pdb-prot5177. doi: 10.1101/pdb.prot5177. [DOI] [PubMed] [Google Scholar]
- 64.Niu D.D., Liu H.X., Jiang C.H., Wang Y.P., Wang Q.Y., Jin H.L., Guo J.H. The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol. Plant-Microbe Interact. 2011;24:533–542. doi: 10.1094/MPMI-09-10-0213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.