Graphical abstract

Keywords: Spatial learning and memory, Standardised APAE, LPS, Neuroinflammation, Cognitive deficits, MWM
Highlights
-
•
Lipopolysaccharide (LPS)-induced impairment of cognitive function.
-
•
Andrographis paniculata aqueous extract (APAE) averted LPS-induced cognitive deficit.
-
•
APAE pretreatment prevented LPS-induced hippocampal proinflammatory cytokine release.
-
•
APAE pretreatment prevented LPS-induced hippocampal oxidative stress mediator release.
-
•
Pretreatment with APAE inhibited LPS-induced hippocampal cholinesterase activity.
Abstract
Substantial evidence has shown that most cases of memory impairment are associated with increased neuroinflammation and oxidative stress. In this study, the potential of a standardised Andrographis paniculata aqueous extract (APAE) to reverse neuroinflammation and cognitive impairment induced by lipopolysaccharide (LPS) was examined in vivo. Rats were treated with APAE (50, 100, 200, and 400 mg·kg−1, p.o.) for 7 consecutive days prior to LPS (1 mg·kg−1, i.p.)-induced neuroinflammation and cognitive impairment. Spatial learning and memory were evaluated using the Morris water maze (MWM) test, while neuroinflammation and oxidative stress were assessed through the measurement of specific mediators, namely, tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β, superoxide dismutase (SOD), catalase (CAT), antioxidant glutathione (GSH), reactive oxygen species (ROS), and thiobarbituric acid reactive substance (TBARS). Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) were also evaluated. LPS caused significant memory deficits in the 2-day MWM protocol, whereas pretreatment with standardised APAE dose-dependently improved performance in the MWM test. APAE treatment also blocked the LPS-induced hippocampal increase in the concentration and expression of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and production of ROS and TBARS and enhanced the activities of AChE and BChE. Furthermore, APAE enhanced the decrease in the levels and expression of hippocampal antioxidant enzymes (SOD and CAT) following LPS-induced neuroinflammation and cognitive deficit. The findings from these studies suggested that standardised APAE improved memory and had potent neuroprotective effects against LPS-induced neurotoxicity.
Introduction
Cognitive dysfunction is a common feature primarily associated with advancing age but may also be related to a variety of neurodegenerative conditions, including Alzheimer’s disease (AD), Parkinson’s disease (PD) and stroke. The relative paucity of effective treatments for cognitive impairment illustrates that there is an unmet medical need, and consequently, the search for effective treatments of cognitive dysfunction has become a significant area of research. There is evidence that certain neurodegenerative diseases are associated with alterations in inflammatory processes in the central nervous system (CNS) [1]. Relatedly, cytokines, which are involved in inflammation, have been shown to regulate physiological functions, including learning and memory [2].
Neuroinflammation and oxidative stress play major roles in promoting neurodegeneration and subsequently affecting cognition via the production of toxic proinflammatory cytokines (PICs) and oxidative stress mediators [3]. A number of cholinesterase inhibitors have been approved for the symptomatic treatment of neurodegenerative diseases such as AD [4]. However, side effects, including syncope, bradycardia, hypertension and chronotropic effects, have been reported in patients following their prolonged use [5], [6]. Furthermore, these treatments do little to affect the underlying progression of the disease. Thus, there is a need to develop more effective alternative therapies with antiinflammatory and antioxidative properties capable of inhibiting the underlying mechanisms of neuroinflammation to promote neuroprotection and prevent or reverse cognitive impairment. One such approach is the use of medicinal plants as a source of therapeutic agents capable of targeting and preventing the toxic PICs and oxidative stress mediators associated with neurodegeneration.
Experimental studies have revealed that some traditionally used plants can enhance cognitive function [7]. Of these, Andrographis paniculata (AP) has numerous recognised activities and antiinflammatory and antioxidant properties that suggest that it may possess promising neuroprotective benefits [8]. The major active ingredients (diterpenoids) present in the aerial part of the plant are andrographolide (AGP), neoandrographolide (NAG) and 14-deoxy-11, 12-didehydroandrographolide (DDAG) (Fig. 1).
Fig. 1.
Major diterpenoids found in AP.
However, there is limited information available to date about the action of AP on the CNS, such as its effects on cognition and potential for neuroprotection. Hence, the present study examined the use of the plant as a medicinal supplement to alleviate cognitive impairment associated with inflammation and oxidative stress in a rat model of LPS-induced neuroinflammation and cognitive impairment.
Material and methods
Chemicals, reagents and kits
Lipopolysaccharide (LPS), thiobarbituric acid, trichloroacetic acid and sodium citrate used in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA). The Tanakan tablet (40 mg containing Ginkgo biloba (GB), marketed by Ipsen, France) was purchased from a local community pharmacy (Watsons, Mines Resort city, Seri Kembangan, Malaysia). Other chemicals, 5,5′-dithiobis (2-nitrobenzoic acid (DTNB), acetylthiocholine and butyrylthiocholine iodide, were supplied by Nacalai Tesque Inc. (Kyoto, Japan). Kits for the assessment of PICs and enzyme activities were purchased from Cusabio Biotech Co. Ltd, (Wuhan, China) and Cayman Chemical Company, (Ann Arbor, MI, USA), respectively.
Animals selection and care
Healthy male Wistar rats, 10–12 weeks of age and weighing between 250 and 300 g, were utilised for this study (Takrif Bistari Enterprise, Selangor, Malaysia). All rats were kept in the Faculty of Medicine animal house for a period of 10 days to adapt to laboratory conditions at an ambient temperature of 25 ± 2 °C with a 12-h light-dark cycle. The rats were maintained on standard commercial rat/mouse pellets (Specialty feeds, Glen forest, Western Australia) and water available ad libitum throughout the experiments. All experimental procedures were conducted in accordance with the principles of laboratory animal care designated and approved by the Universiti Putra Malaysia (UPM) Animal Care Use Committee, UPM/IACUC/AUP-R046/2013.
Experimental design
The rats were randomly assigned to seven separate groups with 10 rats in each group.
-
•
Group 1 (normal control (NC) group): These rats were orally (p.o.) treated with vehicle, namely, the equivalent volume of ultrapure water produced using an ultrafilter machine (Millipore Direct-Q, SAS, Molsheim, France) as used for the administration of Andrographis paniculata aqueous extract (APAE) and GB.
-
•
Group 2 (LPS group): The rats were given ultrapure water for 7 days and LPS (1 mg·kg−1) injected intraperitoneally (i.p.) in normal saline on day 8.
-
•
Groups 3, 4, 5 and 6 (APAE + LPS groups): Standardised APAE was dissolved in ultrapure water to achieve the required doses of 50, 100, 200, and 400 mg·kg−1 and administered p.o. to rats once daily for 7 days followed by the administration of LPS (1 mg·kg−1, i.p.) in saline on day 8.
-
•
Group 7 (GB + LPS group): The rats were treated with 200 mg·kg−1 GB p.o. once daily for 7 days prior to the administration of LPS (1 mg·kg−1, i.p.) on day 8.
Preparation of standardised APAE
Andrographis paniculata Burm. Nees [9] was grown in field 2, UPM, Serdang, Selangor. A voucher specimen (No. SK965/04) was previously deposited at the Herbarium of the Laboratory of Natural Products, Institute of Bioscience, UPM. The plant leaves were harvested at 10–12 weeks post germination, washed with running tap water, sorted, and subjected to three successive changes of ultrapure water. The leaves were dried at 40 °C in an oven dryer for 3 days followed by grinding using a grinder. The powdered samples were collected in a clean bottle and stored at 4 °C until required for extraction. Twenty mL ultrapure water was added to each g of the powdered leaf sample of AP in a conical flask. Thereafter, the mixture was homogenised and heated for 4–5 h at 60 °C in a water bath prior to filtration through Whatman No. 1 filter paper. The extract was then pooled, frozen at −80 °C and freeze dried into dry powder in a freeze dryer (Labconco FreeZone 4.5, Kansas City, MO, USA). Thereafter, the extract was standardised using a Waters high-performance liquid chromatography (HPLC) system to andrographolide (AGP), neoandrographolide (NAG), and 14-deoxy-11, 12-didehydroandrographolide (DDAG) contents, and the standardised extract was termed APAE and used for the study.
Acute oral toxicity study of standardised APAE
The acute oral toxicity of the standardised APAE was assessed in accordance with the limit dose test using the up and down procedure (UDP) adopted by the Organization for Economic Cooperation and Development [10]. A total of five adult male rats randomly selected for the study were marked for identification, housed in individual cages and allowed to acclimate to the laboratory conditions for a period of 7 days prior to dosing. The rats were fasted overnight prior to doing but allowed access to water. The first rat was picked, weighed and orally administered freshly prepared APAE at a limit dose of 5000 mg·kg−1 body weight. A second rat was given the same dose of APAE, and this was continued until all 5 rats had been fed the same dose of the extract. Each animal was monitored for instant death. Then, the animals were observed over a 24-h period for the short-term outcome and for the next 14 days for any delayed toxic effects.
HPLC system for the determination of the active constituents (diterpenoid lactones) of APAE
AGP, NAG, and DDAG were quantified using the Waters HPLC system e2695 separation and 2998 photodiode array detection modules. Chromatographic separation was performed using a reverse phase Kinetex column (C18, 150 × 4.6 mm, i.d.; 5 µm, Phenomenex Inc, 411 Madrid Avenue, Torrance, CA, USA). The mobile phase consisted of acetonitrile (ACN): 5 mM phosphate buffer, (NaH2PO4) containing 0.5% triethylamine (TEA) at a ratio of 1:2 (v/v) with the pH adjusted to 3.2 with phosphoric acid. The flow rate was set at 1 mL/min (which resulted in an operating backpressure in the range of 1000–1400 psi), detector wavelengths at 225 nm and an injection volume of 20 µL. Standard stock solutions of AGP, NAG, and DDAG in methanol were prepared and then mixed at the appropriate volume to provide a stock solution of 100 µg/mL. Thereafter, two-fold (2×) serial dilutions were conducted using the mobile phase to achieve eleven different concentrations in the range of 100–0.0488 µg/mL for the preparation of a calibration graph. A 20-µL volume of standard concentration solution was injected in triplicate into the column to obtain its chromatogram. The calibration curve was then plotted between peak areas against various concentrations of each of the standard diterpenoid lactone compounds.
Method validation
The validation of the developed HPLC method was determined in terms of linearity, accuracy, intra-day precision, inter-day precision and recovery. The limits of quantitation (LOQ) and detection (LOD) were also determined for AGP, NAG, and DDAG. Both intra-day and inter-day precision tests were conducted by analysing standards of AGP, NAG, and DDAG in varied concentrations ranging from 0.04488 to 100 µg/mL on 5 occasions in the morning and afternoon of the same day, while inter-day precision was assessed by analysing the same concentrations of the standards 5 times on 2/3 consecutive days. Percentages (%) and coefficients of variation (CVs) were calculated in both cases.
Determination of the active constituents of APAE
Standardised APAE (1 mg) was mixed with 1 mL mobile phase to achieve a 1 mg/mL concentration. The mixture was then sonicated and filtered before being transferred into a 1.5 mL HPLC vial and finally loaded into the HPLC tray. Acquisition was performed with 20 µL per sample solution in replicates, and the phytochemical content of the extract was calculated from each of the standard curves obtained from the diterpenoid lactone compound mixture (AGP, NAG, and DDAG).
Morris water maze test
The Morris water maze (MWM) test is a well-established behavioural task for studying spatial learning and memory in animals [11]. The primary measure in the MWM test is escape latency and refers to the time the test animal takes to find the platform after being released into the maze. The escape latency is a relative measure of the cognitive abilities of the animal to learn and remember the platform location. In the present study, rats were tested for cognitive function using a 2-day protocol [12] with a slight modification with the addition of a probe trial. In brief, following seven days pretreatment with APAE, GB or vehicle, the animals were trained in the water maze with extra-maze cues and a visible platform, which was positioned approximately 2 cm above the water surface. The training consisted of four trials on the first day, assigned D1V1-4 (day 1, visible platform trial 1–4). During the training trials, the animals are expected to learn to find the platform within 120 sec (escape). Animals that failed to escape within the 120 sec were guided manually to the platform, where they were allowed to stay for 10 sec before being removed to a separate cage to dry. Thereafter, the experimental animals were injected with LPS. After 24 h, the animals were sequentially evaluated in three hidden platform tests (H1–H3) followed by a probe trial, consisting of a single 30-sec trial performed another 24 h later in a pool not containing the platform. During the trials, swim latency, path length, swimming speed and frequency of entry to the target area (in the probe trial) were recorded using the ANY-maze Video Tracking System (Stoelting, Wood Dale, IL, USA). All data were thereafter used to assess performance in the water maze task.
Sample collection
Following the behavioural experiment (day 3), rats were sacrificed using CO2, and the brain was removed and placed an inverted petri dish on ice. The hippocampus was then dissected, snap frozen in liquid nitrogen, and then stored at −80 °C until required for processing.
Tissue preparation
Frozen hippocampal tissue sections were homogenised in 5-times (w/v) ice-cold phosphate buffer 0.1 M (pH 7.4) containing protease inhibitor (cocktail) and further allowed to lyse for 20 min on ice prior to centrifugation at 10,000g for 20 min to obtain the supernatant. Part of the supernatant was centrifuged (1200g for 20 min) to acquire the postmitochondrial supernatant. The former was used for the determination of levels of PICs, including tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, malondialdehyde (MDA), reactive oxygen species (ROS), and cholinesterase activities, while the latter was used for the quantitative determination of superoxide dismutase (SOD) and catalase (CAT) activities in addition to glutathione (GSH) level.
Determination of the level of PICs
LPS disrupts and compromises the integrity of the blood-brain barrier by triggering neuroinflammation and oxidative stress processes [13]. An imbalance between pro- and antiinflammatory cytokines has been shown to be crucial in the pathogenesis of neurodegenerative disorders; thus, the levels of PICs were measured in this study. The cytosolic supernatant of the hippocampus was analysed for the presence of immunoreactive, TNF-α, IL-1β, and IL-6 using commercial ELISA kits (Cusabio, Wuhan, China) following the manufacturer’s instructions.
Measurement of oxidative stress markers
Determination of intracellular ROS level
Oxidative stress following stimulation with a toxicant results in the generation of excessive free radicals in cells or tissue, resulting in inflammation. The generation of intracellular ROS in hippocampal section lysates was investigated using 2,7-dichlorofluorescein diacetate (DCF-DA) as previously described [14] with a slight modification. ROS generation was reported as a fold change compared with control.
Determination of lipid peroxidation
The lipid peroxidation level was estimated according to the protocol by Draper and Hadley [15] based on the MDA index using the thiobarbituric acid reactive substance (TBARS) assay with slight modifications and expressed as a fold change compared with control. The optical density (OD) was measured at 532 nm using a microplate reader (VersaMax, Molecular devices, USA).
Measurement of GSH
Oxidative stress following stimulation with a toxicant results in the generation of excessive free radicals or ROS in cells or tissue. ROS accumulation causes the depletion of natural antioxidants (reduced GSH), leading to diminished defence mechanisms against free radical overload resulting in inflammation. Total GSH was estimated as previously described with slight modifications [16]. The colour generated was read at 412 nm and compared with that in the control.
Total RNA extraction and cDNA synthesis
Total RNA was isolated using the Total RNA Isolation kit (RBC Bioscience Corp., Taipei, Taiwan) following the manufacturer’s instructions. RNA was quantified spectrophotometrically by absorption measurements at 260 and 280 nm using the NanoDrop system (NanoDrop Technologies Inc., Wilmington, DE), and the quality was examined by separation using gel electrophoresis. Reverse transcription was performed to synthesise single-stranded cDNA using a ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs, County Road, Ipswich, Massachusetts, USA), which was then subjected to amplification using specific primers for TNF-α, IL-1β, IL-6, SOD, and CAT by polymerase chain reaction (PCR). The results were normalised to the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Gene expression analysis
The primers shown in Table 1 were from previously published studies [17], [18], [19] and provided by First Base (Selangor, Malaysia). Each of the primers (forward and backward) was reconstituted to obtain 100 µM stock solutions.
Table 1.
Gene and primer sequences used in the gene expression study.
| Gene name | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| TNF-α | CACCACGCTCTTCTGTCTACTGAAC | CCGGACTGCGTGATGTCTAAGTACT |
| IL-1β | GAAGCTGTGGCAGCTACCTATGTCT | CTCTGCTTGAGAGGTGCTGATGTAC |
| IL-6 | TGATGTTGTTGACAGCCA | TAGCCACTCCTTCTGTGACTCTAACT |
| Catalase | GCGAATGGAGAGGCAGTGTAC | GAGTGACGTTGTCTTCATTAGCACTG |
| SOD | GCAGAAGGCAAGCGGTGAAC | TAGCAGGACAGCAGATGAGT |
| GAPDH* | TACCAGCCGGGGGACCAC | CGAGCTGACAGAGTAGTA |
Tumour necrosis factor (TNF)-α; interleukin (IL)-1β; SOD (superoxide dismutase); GAPDH* (Glyceraldehyde-3-phosphate dehydrogenase).
PCR was performed according to the One Taq™ 2X master mix with a standard buffer kit (New England Biolabs, Ipswich, Massachusetts, USA) in an Eppendorf Gradient Mastercycler (Thermo Fisher Scientific, Pittsburgh, PA, USA) using specific primers for TNF-α, IL-1β, IL-6, SOD and CAT. A portion of the PCR products was finally electrophoresed using a 1% agarose gel containing 1 µL ethidium bromide (0.5 µg/mL) and viewed via gel doc (Bio Rad, St. Louis, MO, USA). Images on the gels were scanned, and the mRNA expression levels for TNF-α, IL-1β, IL-6, SOD and CAT were normalised to GADPH gene expression. All testing was conducted in duplicate.
Measurement of the level of antioxidant enzyme activities in the hippocampal tissue
SOD and CAT assays were performed using ELISA kits (Cayman Chemical, Ann Arbor, MI, USA) in accordance with the manufacturer’s instructions. The OD was read at 540 nm and 450 nm, respectively, in a microplate reader (VersaMax, Molecular devices, US).
Measurement of cholinesterase activities
Acetylcholinesterase (AChE) activity
AChE functions by regulating the concentration of acetylcholine (Ach) in cholinergic synapse. Thus, improving brain ACh level with AChE inhibitors are a major therapeutic strategy for the treatment of most degenerative disorders, such as AD. In the present study, AChE activity was determined as described earlier [14] with a minor modification. The difference in OD at 412 nm was observed over 5 min spectrophotometrically.
Butyrylcholinesterase (BChE) activity
For BChE activity, butyrylthiocholine iodide was used as a substrate. All other reagents and conditions were the same as those for the AChE assay stated above.
Statistical analysis
The results were expressed as the mean ± standard deviation (SD) following analysis via one-way analysis of variance (ANOVA) to assess significant differences between groups, followed by Tukey’s post hoc test to examine significant differences (P ≤ 0.05).
Results
Standardised APAE
To quantitatively determine the bioactive ingredients (diterpenoid lactones) in the leaf APAE, HPLC analysis was conducted. The standardisation of APAE was chemically illustrated by means of marker compounds. The typical chromatograms of the marker compounds and standardised natural product (APAE) are shown in Fig. 2A and B, respectively.
Fig. 2.
HPLC of chromatogram of (A) standard marker compounds (AGP, NAG, and DDAG, 0.1 mg/mL) found in AP and (B) standardised APAE.
The amounts of the known active ingredients, AGP, NAG, and DDAG, in standardised APAE as determined by HPLC are presented in Table 2.
Table 2.
Quantity of the active ingredients in standardised APAE.
| AGP (%) | NAG (%) | DDAG (%) |
|---|---|---|
| 2.96 ± 0.36 | 1.81 ± 0.2 | 0.11 ± 0.02 |
Values are the mean ± SD (n = 3).
Acute oral toxicity of APAE
The oral administration of APAE at a limit dose of 5000 mg·kg−1 body weight did not produce any sign of acute toxicity or instant death in any of the rats tested. Similarly, no deaths were recorded in rats within the short- or long-term outcome of the limit dose test using the UDP (Table 3). Therefore, the medium lethal dose (LD50) was estimated to be greater than 5000 mg·kg−1 body weight via oral administration.
Table 3.
Result of the limit dose test of standardised APAE in rats.
| Test sequence | Animal identification | Dose (mg·kg−1) | Short-term outcome (24 h) | Delayed outcome (14 days) |
|---|---|---|---|---|
| 01 | I | 5000 | Survival | Survival |
| 02 | II | 5000 | Survival | Survival |
| 03 | III | 5000 | Survival | Survival |
| 04 | IV | 5000 | Survival | Survival |
| 05 | V | 5000 | Survival | Survival |
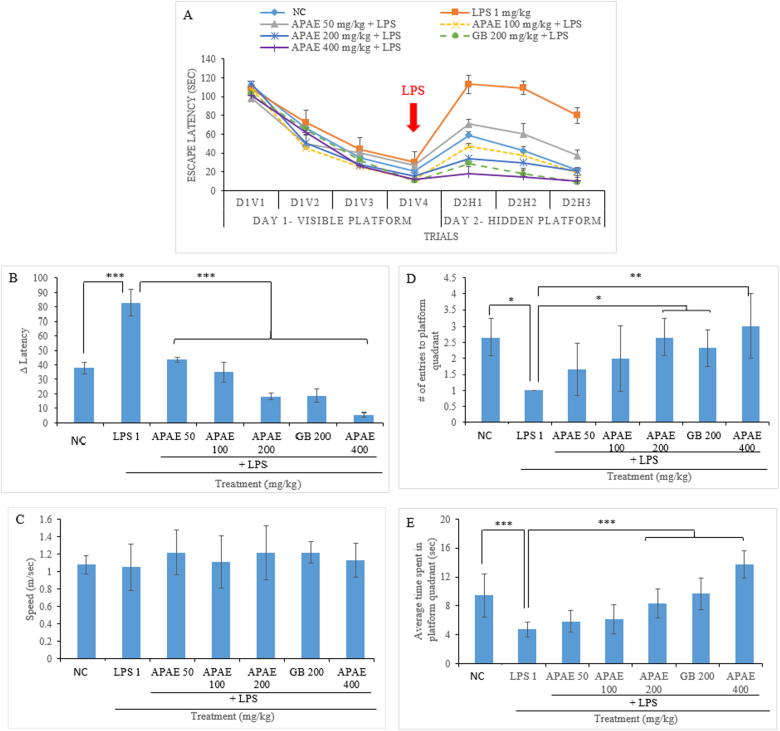
Standardised APAE pretreatment prior to LPS administration improves performance in the MWM task demonstrated by decreased latency to reach the submerged platform and prevents LPS-induced cognitive impairment
The latency to locate the platform (Fig. 3A) in addition to the latency difference (Fig. 3B: D2H1-D1V4; long-term memory) was significantly (P ≤ 0.001) longer in the LPS group than in the vehicle-treated control animals. Similarly, in the probe trial, the number of entries (Fig. 3D) and the time spent (Fig. 3E) in the target quadrant were significantly lower in the LPS-treated animals than in the control animals. However, APAE pretreatment 24 h prior to LPS administration dose-dependently improved performance in the MWM task, as illustrated by a shorter latency to reach the submerged platform (Fig. 3A) and a smaller latency difference than in the group treated with LPS alone. The result revealed significantly (P ≤ 0.001) distinct learning abilities illustrated by a smaller latency difference in the treated groups than in the LPS control group. Importantly, GB displayed a similar effect as APAE. Both agents produced similar effects at 200 mg·kg−1, and the GB (200 mg·kg−1) and APAE (200 and 400 mg·kg−1)-treated groups were significantly different from the NC group. Furthermore, examination of the number of entries into the target quadrant (probe trial) the time spent in the platform quadrant revealed that the APAE-pretreated groups displayed significantly (P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001) higher numbers and times than the LPS control group, illustrating recall of the previously learned task.
Fig. 3.
Evaluation of the effect of APAE on the prevention of cognitive impairment in rats using the MWM task. (A) Escape latency, (B) latency difference (D2H1-D1V4), (C) speed, (D) number of entries into target quadrant, and (E) time spent in the target quadrant. Values are expressed as the mean ± SD (n = 10). *P ≤ 0.05, **P ≤ 0.01,***P ≤ 0.001 compared with the LPS group.
Standardised APAE prevents the LPS-induced hippocampal production of PICs
LPS-treated rats exhibited significantly elevated TNF-α (Fig. 4A), IL-6 (Fig. 4B) and IL-1β (Fig. 4C) production in the hippocampus compared to NC rats. However, compared with LPS alone, pretreatment with increasing doses of APAE significantly suppressed the production of all measured PICs in a dose-dependent manner (Fig. 4A-C).
Fig. 4.
Effect of standardised APAE on LPS-induced production of PICs. (A) TNF-α, (B) IL-6, and (C) IL-1β. Values are expressed as the mean ± SD (n = 10). *P ≤ 0.05, ***P ≤ 0.001 compared with the LPS group.
Standardised APAE attenuates the LPS-induced hippocampal production of oxidative stress markers in rats
LPS-treated animals exhibited significantly (P ≤ 0.001) elevated intracellular ROS and TBARS levels in the hippocampal region when compared with NC animals. APAE or GB pretreatment significantly (P ≤ 0.001) decreased the central ROS and MDA levels compared with LPS treatment alone (Fig. 5A and B).
Fig. 5.
Effect of standardised APAE and GB on LPS-induced production of oxidative stress markers. (A) ROS and (B) TBARS. Values are expressed as the mean ± SD (n = 10). ***P ≤ 0.001 compared with the LPS group.
Standardised APAE enhances hippocampal antioxidant enzyme activities and level
LPS significantly (P ≤ 0.001) decreased the activities of SOD and CAT and significantly depleted (P ≤ 0.001) GSH levels within the rat hippocampal region, indicating increased oxidative stress processes (Fig. 6A-C). APAE pretreatment produced a significant dose-dependent (P ≤ 0.001) increase in enzyme activities and antioxidant levels (Fig. 6A, B, and C), reaching normal levels at doses of 200 mg·kg−1 above. This effect was also observed with GB at 200 mg·kg−1.
Fig. 6.
Effect of pretreatment with standardised APAE or GB on the LPS-induced decrease in hippocampal antioxidant enzyme (A) SOD (B) CAT and (C) GSH activities and levels. Values are expressed as the mean ± SD (n = 10). ***P ≤ 0.001 compared with the LPS group.
Standardised APAE inhibits the hippocampal cholinesterase activities induced by LPS
LPS significantly (P ≤ 0.01) increased AChE and BChE activities in the rat hippocampus, and this effect was significantly and dose-dependently attenuated (P ≤ 0.001 for 100, 200, and 400 mg·kg−1 APAE; P ≤ 0.05 for 50 mg·kg−1) by pretreatment with standardised APAE (Fig. 7A and B). GB (200 mg·kg−1) produced a similar effect as that of 200 mg·kg−1 APAE. The AChE and BChE activities were restored to normal levels with 400 mg·kg−1 APAE.
Fig. 7.
Effect of standardised APAE on the LPS-induced upregulation of cholinesterase activities in the hippocampus. (A) AChE and (B) BChE. Values are expressed as the mean ± SD (n = 10). *P ≤ 0.05, ***P ≤ 0.001 compared with the LPS group.
Effect of APAE on LPS-induced PIC mRNA expression in the rat hippocampus
Analysis of the intensity of the bands using Image Lab software (Bio Rad, USA) revealed that LPS (1 mg·kg−1) caused a marked 10.8-, 7- and 5.47-fold upregulation of TNF-α, IL-1β and IL-6 mRNA expression in the hippocampus, respectively, 48 h after exposure and completion of the MWM test (Fig. 8A-C). Pretreatment with standardised APAE produced a significant (P ≤ 0.001) dose-dependent inhibition of this upregulation.
Fig. 8.
Standardised APAE attenuates the LPS-induced increase in the mRNA expression levels of (A) TNF-α (B) IL-1β and (C) IL-6 in the rat hippocampus. Values are expressed as relative fold change (n = 10). *P ≤ 0.05, ***P ≤ 0.001.
Effect of standardised APAE on LPS-induced rat hippocampal antioxidant enzyme expression levels
The effects of standardised APAE on CAT and SOD mRNA levels in LPS-induced rat hippocampus sections are shown in Fig. 9A and B. In the rat hippocampus, CAT and SOD mRNA expression levels were significantly (P ≤ 0.001) lower in the LPS-treated group than in the NC group (unstimulated). However, pretreatment with APAE or GB significantly (P ≤ 0.05, P ≤ 0.001) inhibited the LPS-induced downregulation of the mRNA expression levels of these antioxidant enzyme in a dose-dependent manner (Fig. 9A and B).
Fig. 9.
Standardised APAE inhibits LPS-induced downregulation of (A) CAT and (B) SOD mRNA in the rat hippocampus. Values are expressed as relative fold change (n = 10). *P ≤ 0.05, ***P ≤ 0.001.
Discussion
Safety studies on herbal products have been evaluated by conducting acute toxicity tests amongst other toxicity testing in laboratory animals [20]. In the present acute toxicity study, the oral administration of a single 5000 mg·kg−1 body weight dose of APAE did not produce any sign of acute toxicity or instant mortality in any of the rats tested (Table 3), suggesting that the extract has low toxicity and is safe when administered orally. Thus, the LD50 of the extract was considered to be greater than 5000 mg·kg−1. This result is similar to the finding of Mohammed et al. [21], who reported that up to 2000 mg·kg−1 of the ethanolic extract of aerial parts of AP is considered safe in rats.
Evidence has long shown that neuroinflammation plays a major role in the pathophysiology associated with impaired cognitive function [22]. Exposure of rats to LPS induces significant memory deficits as evidenced by an alteration in spatial learning shown in MWM test. The LPS-treated group showed higher levels of PICs in the hippocampal sections than did the NC and APAE/GB pretreated rats. This observation is in line with earlier studies demonstrating that systemic administration of LPS produces increased levels of proinflammatory mediators in the brain, including TNF-α and IL-1β, in laboratory animals [23]. Furthermore, LPS-treated rats, showing elevated levels of PICs, exhibited decreased performance in the MWM, consistent with a previous study that reported that LPS-induced neuroinflammation causes cognitive impairment [24]. In addition, the ability of the rats to locate the target quadrant and the total time spent in the target quadrant during the probe test were significantly lower (P ≤ 0.001) in the group treated with LPS alone, which exhibited signs of neuroinflammation, than in the treatment groups (Fig. 3E). Long-term memory was also evaluated in the rats by comparing the differences in the performance on D1V4 to those on D2H1 (Fig. 3B). Pretreatment with standardised APAE or GB significantly (P ≤ 0.001) reduced the escape latency in LPS-treated rats (Fig. 3A). These observations agree with those in a recent study that reported cognitive deficits after a 7-day repeated exposure to LPS [25]. Similarly, assessment of long-term memory (D2H1-D1V4) also revealed a dose-dependent amelioration of the LPS-induced cognitive deficits in the treatment groups (Fig. 3B). A previous study showed that a large D2H1-D1V4 represents poor performance, whereas a smaller D2H1-D1V4 indicates that the animals have learned the location of the visible platform during day 1 training and can remember the location of the hidden platform on day 2 [12]. Interestingly, only the group treated with the highest dose of APAE (400 mg·kg−1) showed significantly more (P ≤ 0.05) entries into the target quadrant (probe trial experiment) than the LPS group (Fig. 3C). The findings in this study are consistent with a recent study that showed an influence of LPS-induced upregulation of PICs on learning and memory [25]. Similarly, the observed effect in the GB-treated group is consistent with an earlier report of the medicinal effect of GB against stress and memory loss [26].
Proinflammatory agents, including LPS, trigger neuroinflammation and oxidative stress processes [13]. In addition, an imbalance between pro- and antiinflammatory cytokines contributes to the development of neurodegenerative disorders and impaired neurogenesis [27]. Studies have also reported deficits in learning and memory as a result of neuroinflammation affecting hippocampal function [28]. In this study, LPS caused a marked increase in the production of PICs (IL-1β, TNF-α, and IL-6) in the rat hippocampus compared to vehicle treatment. The elevated cytokine levels in this study could explain the cognitive deficit observed in the LPS control group. These findings agree with recent studies that reported LPS activation of glial cells caused upregulation of IL-1β, IL-6, and TNF-α in the hippocampus with cognitive deficits and subsequent neuroinflammatory pathologies [29]. However, compared LPS treatment alone, pretreatment with graded doses of APAE or GB significantly arrested the production of these measured cytokines in a dose-dependent manner (Fig. 4A-C). This observed effect is consistent with an earlier related finding that 8 weeks of treatment with GB extract decreased TNF-α and IL-1β expression in the hippocampus and cerebral cortex sections in atherosclerotic rats [30].
LPS challenge contributes significantly to the production of ROS and the pathogenesis of various inflammatory diseases [31]. In this study, the increased production of ROS and TBARS in LPS-treated animals was correlated with the compromised state of learning and memory, likely as a result of impaired hippocampal functioning. Thus, oxidative stress is involved in this condition. However, pretreatment with APAE or GB significantly inhibited the LPS-induced upregulation of these oxidative stress markers (Fig. 5A and B) and improved learning and memory compared to LPS treatment alone.
Evidence suggests that upregulation of free radicals and other ROS with a concomitant decrease in natural antioxidants, coupled with elevated TBARS levels, a measure of lipid peroxidation, leads to significant cellular damage in various conditions [32]. Therefore, the levels of ROS, TBARS and antioxidant enzymes such as CAT and SOD were measured in the hippocampal sections of both control and treated rats. Administration of LPS upregulated the production of oxidative stress markers and produced a significant reduction in SOD and catalase activities, which was prevented by pretreatment with graded doses of APAE or GB (Fig. 6A-C). These observations agree with an earlier report that suggested that therapies aimed at preventing the production of free radicals could be potentially effective therapies for neurodegenerative diseases [33]. The observed decrease in CAT and SOD functions in our study could be associated with increased ROS production. However, treatment of rats with standardised APAE or GB significantly (P ≤ 0.05) ameliorated the changes induced by LPS in a dose-dependent manner, consistent with recent related studies that reported an attenuation of the serum levels of these enzymes [34].
To illustrate behavioural changes in the context of biochemical alterations, the levels of enzyme activity were measured in rat hippocampal sections. AChE is an essential biological enzyme that hydrolyses ACh, a neurotransmitter considered crucial in AD pathology [35]. Increased AChE activity lowers ACh levels and facilitates inflammatory responses [22]. ACh has been reported to prevent the upregulation of PICs induced by LPS from microglia [36]. Earlier studies and reports have shown that inhibition of AChE activity enhances ACh levels, resulting in inhibition of TNF-α, IL-6 and IL-1β production via the cholinergic antiinflammatory pathway [37]. In the present study, AChE levels were measured in the hippocampal region of the brain. LPS treatment significantly increased hippocampal AChE activity in the LPS control group, signifying a decrease in cholinergic activities and supporting earlier findings [23]. However, we showed that pretreatment with varied doses of APAE or GB decreased AChE activity in a dose-dependent manner (Fig. 7A and B). Consistent with previous studies, AP extract inhibited AChE with an IC50 value of 222.41 µg/mL [38]. Thus, the observed inhibitory effect of APAE on AChE activity in this study further supported its neuroprotective effect via the cholinergic antiinflammatory pathway.
Increased hippocampal PIC mRNA expression levels have been shown to contribute to cognitive impairment [39]. In the present study, LPS injection markedly upregulated TNF-α, IL-1β and IL-6 levels (Fig. 8A-C and D) and downregulated antioxidant enzyme levels (Fig. 9AC). This finding supports earlier studies that reported increased expression of proinflammatory mediator genes following LPS induction [40]. However, consistent with previous studies, treatment with APAE showed a significant (P ≤ 0.05) neuroprotective effect via downregulation of mRNA levels of these inflammatory and oxidative stress markers, thus preventing cognitive deficits in experimental rats [40].
Conclusions
APAE exerts its anti-neuroinflammatory and memory enhancing effect through inhibition of pro-inflammatory and oxidative stress mediators production to prevent neuronal death thereby enhancing learning and memory. This study demonstrated that APAE is safe and protects against the cognitive impairment and neuroinflammation induced by LPS. The activity was shown to be more effective than that of GB, specifically EGb761 (Tanakan™), which has been shown to be clinically effective in patients with cognitive impairment. These findings illustrate the potential for AP to be used clinically and indicate that the therapeutic uses of AP should be further explored.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgements
This study was supported by the Malaysia Ministry of Agriculture and Agro-based Industry (NRGS grant, NH612D009). Dahiru Sani is grateful to the Government of Sokoto State, Nigeria for providing him with a PhD scholarship.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Dheen S.T., Kaur C., Ling E.A. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14(11):1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 2.Charlton R.A., Lamar M., Zhang A., Ren X., Ajilore O., Pandey G.N. Associations between pro-inflammatory cytokines, learning, and memory in late-life depression and healthy aging. Int J Geriatr Psychiatry. 2018;33(1):104–112. doi: 10.1002/gps.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elissa J.D., Natalie C.T. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol Learn Mem. 2014:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyde C., Peters J., Bond M., Rogers G., Hoyle M., Anderson R. Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer’s disease: systematic review and economic model. Age Ageing. 2013;42(1):14–20. doi: 10.1093/ageing/afs165. [DOI] [PubMed] [Google Scholar]
- 5.Gill S.S., Anderson G.M., Fischer H.D., Bell C.M., Li P. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169:867–873. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 6.Ahmet T.I., Pinar S., Brendon S., Marco S., Cristina B., Stefania M. Cardiovascular outcomes of cholinesterase inhibitors in individuals with dementia: a meta-analysis and systematic review. J Am Geriatr Soc. 2018;0002:8614–8618. doi: 10.1111/jgs.15415. [DOI] [PubMed] [Google Scholar]
- 7.Howes M.-J.R., Perry N.S.L., Houghton P.J. Plant with traditional uses and activities related to management of Alzheimer’s disease and other cognitive disorders. Phytother Res. 2003;17(1):1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- 8.Lim J.C.W., Chan T.K., Ng D.S.W., Sagineedu S.R., Stanslas J., Wong W.S.F. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39(3):300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 9.http://www.theplantlist.org/tpl/record/kew-2637069.
- 10.OECD. OECD guidelines for the testing of chemicals, acute oral toxicity- up-and-down-procedure (UDP). 425; 2008. p. 27.
- 11.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 12.Gulinello M., Gertner M., Mendoza G., Schoenfeld B.P., Oddo S., LaFerla F. Validation of a 2-day water maze protocol in mice. Behav Brain Res. 2009;196(2):220–227. doi: 10.1016/j.bbr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks W.A., Gray A.M., Erickson M.A., Salameh T.S., Damodarasamy M., Sheibani N. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation. 2015;12(1):223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinomol G.K., Muralidhara Bacopa monnieri modulates endogenous cytoplasmic and mitochondrial oxidative markers in prepubertal mice brain. Phytomedicine. 2011;18(4):317–326. doi: 10.1016/j.phymed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Draper H.H., Hadley M. Malaondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran N.S., Nithya M., Rose C., Chandra T.S. The effect of finger millet feeding on the early responses during the process of wound healing in diabetic rats. Biochim Biophys Acta. 2004;1689(3):190–201. doi: 10.1016/j.bbadis.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Limaye P.V., Raghuram N., Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003;243:147–152. doi: 10.1023/a:1021620414979. [DOI] [PubMed] [Google Scholar]
- 18.Sun J., Li F., Chen J., Xu J. Effect of ketamine on NF-kappa B activity and TNF-alpha production in endotoxin-treated rats. Ann Clin Lab Sci. 2004;34(2):181–186. [PubMed] [Google Scholar]
- 19.Won K.J., Suzuki T., Hori M., Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motility. 2006;18:53–61. doi: 10.1111/j.1365-2982.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 20.Fennell C.W., Lindsey K.L., McGaw L.J., Sparg S.G., Stafford G.I., Elgorashi E.E. Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J Ethnopharmacol. 2004;94:205–217. doi: 10.1016/j.jep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Mohammed G.M., Shakil S.S., Kavimani S. Effect of ethanolic extract of aerial parts of Andrographis paniculata on the pharmacokinetics of gliclazide in rats. Asian J Biomed Pharm Sci. 2015;5(51):21–24. [Google Scholar]
- 22.Magaki S., Mueller C., Dickson C., Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42(3):233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagi E., Agrawal R., Nath C., Shukla R. Influence of LPS-induced neuroinflammation on acetylcholinesterase activity in rat brain. J Neuroimmunol. 2008;205(1–2):51–56. doi: 10.1016/j.jneuroim.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.W., Lee Y.K., Yuk D.Y., Choi D.Y., Ban S.B., Oh K.W. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn M.S., Kranjac D., Alonzo C.A., Haase J.H., Cedillos R.O., McLinden K.A. Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav Brain Res. 2012;229(1):176–184. doi: 10.1016/j.bbr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Mohanta T.K., Tamboli Y., Zubaidha P.K. Phytochemical and medicinal importance of Ginkgo biloba L. Nat Prod Res. 2014;28(10):746–752. doi: 10.1080/14786419.2013.879303. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y.P., Lin H.I., Tzeng S.F. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054(2):152–158. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 28.Gong Q.-H., Wang Q., Pan L.-L., Liu X.-H., Huang H., Zhu Y.-Z. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a proinflammatory pathway in rats. Pharmacol Biochem Behav. 2010;96(1):52–58. doi: 10.1016/j.pbb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Fu H.Q., Yang T., Xiao W., Fan L., Wu Y., Terrando N. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS ONE. 2014;9(8):e106331. doi: 10.1371/journal.pone.0106331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao Y.B., Rui Y.C., Li T.J., Yang P.Y., Qiu Y. Expression of proinflammatory and anti-inflammatory cytokines in brain of atherosclerotic rats and effects of Ginkgo biloba extract. Acta Pharmacol Sin. 2005;26(7):835–839. doi: 10.1111/j.1745-7254.2005.00106.x. [DOI] [PubMed] [Google Scholar]
- 31.Maitra U., Singh N., Gan L., Ringwood L., Li L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J Biol Chem. 2009;284(51):35403–35411. doi: 10.1074/jbc.M109.059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markesbery W.R., Lovell M.A. Damage to lipids, proteins, DNA and RNA in mild cognitive impairment. Arch Neurol. 2008;64(7):954–956. doi: 10.1001/archneur.64.7.954. [DOI] [PubMed] [Google Scholar]
- 33.Sonia G., Andrey Y.A. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012 doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karaboduk H., Uzunhisarcikli M., Kalender Y. Protective effects of sodium selenite and vitamin E on mercuric chloride-induced cardiotoxicity in male rats. Braz Arch Biol Technol. 2015;58(2):229–238. [Google Scholar]
- 35.Prakash R., Sandhya E., Ramya N., Dhivya R., Priyadarshini M., Sakthi P.B. Neuroprotective activity of ethanolic extract of Tinospora cordifolia on LPS induced neuroinflammation. Transl Biomed. 2017;8(4):135. [Google Scholar]
- 36.Shytle R.D., Mori T., Townsend K., Vendrame M., Sun N., Zeng J. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 37.Alexander K., Clarissa von H., Marco S., Annalena T., Nadine P., Mariya K. Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS ONE. 2013;8(5):e6267. doi: 10.1371/journal.pone.0062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee P.K., Kumar V., Houghton P.J. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phytother Res. 2007;21(12):1142–1145. doi: 10.1002/ptr.2224. [DOI] [PubMed] [Google Scholar]
- 39.Belarbi K., Jopson T., Tweedie D., Arellano C., Luo W., Greig N.H. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23–25. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur H., Patro I., Tikoo K., Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]