Abstract
Background
Multiple pathway models of ADHD suggest that multiple, separable biological pathways may lead to symptoms of the disorder. If this is the case, it should be possible to identify subgroups of children with ADHD based on distinct patterns of brain activity. Previous studies have used latent class analysis (LCA) to define subgroups at the behavioral and cognitive level and to then test whether they differ at the neurobiological level. In this proof of concept study, we took a reverse approach. We applied LCA to functional imaging data from two previously published studies to explore whether we could identify subgroups of children with ADHD symptoms at the neurobiological level with a meaningful relation to behavior or neuropsychology.
Methods
Fifty-six children with symptoms of ADHD (27 children with ADHD and 29 children with ASD and ADHD symptoms) and 31 typically developing children performed two neuropsychological tasks assessing reward sensitivity and temporal expectancy during functional magnetic resonance imaging. LCA was used to identify subgroups with similar patterns of brain activity separately for children with ADHD-symptoms and typically developing children. Behavioral and neuropsychological differences between subgroups were subsequently investigated.
Results
For typically developing children, a one-subgroup model gave the most parsimonious fit, whereas for children with ADHD-symptoms a two-subgroup model best fits the data. The first ADHD subgroup (n = 49) showed attenuated brain activity compared to the second subgroup (n = 7) and to typically developing children (n = 31). Notably, the ADHD subgroup with attenuated brain activity showed less behavioral problems in everyday life.
Conclusions
In this proof of concept study, we showed that we could identify distinct subgroups of children with ADHD-symptoms based on their brain activity profiles. Generalizability was limited due to the small sample size, but ultimately such neurobiological profiles could improve insight in individual prognosis and treatment options.
Highlights
-
•
We identified distinct subgroups of children with ADHD-symptoms based on brain activity profiles.
-
•
These subgroups differed from each other on parent-rated attention problems and reward sensitivity.
-
•
The ADHD-subgroup with most difficulties in daily life, had less attenuated brain activity.
1. Introduction
Much of what we know about ADHD comes from studies that have compared groups of children with ADHD to groups of typically developing children (Costa Dias et al., 2013). However, multiple pathway theories argue that changes in multiple neurobiological pathways could lead to symptoms of ADHD independently of one another (Nigg et al., 2005; Sonuga-Barke, 2005). If neurobiological pathways to ADHD are truly separable, then it should be possible to identify subgroups of children with ADHD-symptoms based on distinct patterns of brain activity. Moreover, neurobiological subgroups will most likely not align with current behavior-based subgroups (e.g. DSM-5 presentations of ADHD) or we would long have identified neurobiological markers of these subgroups. The present study aims to provide a proof of concept by taking a reverse approach to most studies of ADHD neurobiology: instead of subgrouping children with ADHD on the basis of their behavior and subsequently testing for differences in neurobiology, we tested whether distinct subgroups of children with ADHD-symptoms can be identified based on their brain activity profiles. In a second step, we tested for behavioral differences between these neurobiology-based subgroups.
This approach is related to the Research Domain Criteria (RDoc) rationale, which aims to “develop new ways of classifying mental disorders based on dimensions of observable behavior and neurobiological measures” (Insel et al., 2010). If we can identify meaningful neurobiological profiles within ADHD that are associated with behavioral differences, this could improve our understanding of children with ADHD symptoms. Our assessment of behavior could then be informed by neurobiological knowledge, without the need to first scan every child. Ultimately, this could lead to improved treatment options and align them closer with the needs of an individual child.
In this study, we focused on two neuropsychological domains: reward processing and temporal expectancy. As a group, children with ADHD show changes in reward processing: they tend to respond more impulsively to reward, favoring smaller immediate rewards over larger delayed ones. Furthermore, they show greater improvement in task performance following reward than typically developing children (Luman et al., 2005). Both, the dynamic developmental theory (DDT) and dopamine transfer deficit theory (DTD) propose that such sensitivity to reward may be the result of changes in dopamine signaling (Sagvolden et al., 2005; Tripp and Wickens, 2008). Activity in ventral striatum, as assessed with fMRI, can be used as proxy for local dopamine activity (Delgado et al., 2005; Knutson and Gibbs, 2007). In addition to changes in reward processing, changes in temporal expectancy have been suggested in children with ADHD (Durston et al., 2007; Nigg and Casey, 2005; Rubia et al., 2003). Attenuated temporal expectancy may be related to reduced anticipatory brain activity in fronto-striatal networks (Durston et al., 2007; Ghajar and Ivry, 2009; McClure et al., 2003), which in turn may also be related to reduced dopamine signaling (Tripp and Wickens, 2008, Tripp and Wickens, 2009).
In this proof of concept study, we used latent class analysis (LCA) to identify subgroups of children with ADHD-symptoms based on their individual patterns of brain activity, rather than grouping them together based on predefined criteria of symptomatic or cognitive markers. To do so, we used brain activity data from two earlier fMRI-studies (B.M. van Hulst et al., 2017a, van Hulst et al., 2017b). In addition, to be able to describe these subgroups in terms of behavior and cognition, we used questionnaire and task performance data collected in these same two fMRI-studies. In these studies, we tested reward processing and timing in children (aged 8–12) with a primary diagnosis of ADHD, as well as children with a primary diagnosis in the autism spectrum and symptoms of ADHD (ASD+). We included this group because until now it is unclear whether differences in reward processing and temporal expectancy are specific to ADHD or relate to ADHD-symptoms in a more trans-diagnostic way. We hypothesized that we would be able to identify subgroups of children with symptoms of ADHD, based on their patterns of brain activity, and that these subgroups would show meaningful differences in behavior or neuropsychology.
2. Methods and materials
2.1. Sample
We included data from all participants that were included in the main analyses of the two original fMRI-studies. In these studies, data was first screened to exclude participants with excessive head movement or low scan quality from the main analyses (for details and numbers see Supplementary text S1). This resulted in the inclusion of high quality datasets from 87 right-handed boys between the ages 8–12; 56 boys with symptoms of ADHD (27 with a primary diagnosis of ADHD and 29 with a primary diagnosis of ASD and ADHD symptoms (ASD+), Mage = 10.94, SD = 1.24) and 31 typically developing children (Mage = 10.28, SD = 1.07).
There was a difference in age between the group of children with ADHD symptoms and typically developing children (t(85) = −2.49, p = 0.014). To address this, we included age as a covariate in all comparisons between typically developing children and both ADHD groups. If age was related to an outcome measure, analyses were run both with and without the covariate. If not, age was left out of the final model. 19 Children with ADHD and 19 with ASD+ were using short-acting psychostimulants (e.g. methylphenidate). All participants were asked not to take medication 24 h prior to scanning (van Hulst et al., 2017a, van Hulst et al., 2017b).
Inclusion criteria for children with ADHD included a diagnosis of ADHD based on DSM-IV-TR criteria (Association, 2000). The diagnosis ADHD was confirmed using the Diagnostic Interview Schedule for Children (DISC-IV; (Shaffer et al., 2000)). Children with ASD and symptoms of ADHD were included if they met two criteria: The first criterion was a DSM-IV-TR diagnosis of ASD from an expert child and adolescent psychiatrist. The second criterion was the presence of attention problems as defined by a clinical or subclinical score (above the age appropriate threshold) on the attention subscale of the Child Behavior Checklist (CBCL) (Verhulst et al., 1996). Inclusion criteria for typically developing children included the absence of psychiatric disorders based on the DISC-IV (except for specific phobia and enuresis). General exclusion criteria included IQ lower than 70 as assessed using a four-subtest shortened Wechsler Intelligence Scale for Children (WISC-III; (Wechsler, 1991)), major illness of the cardiovascular, endocrine, pulmonary or gastrointestinal system, the presence of interfering metal object in or around the body, and a history of or present neurological disorder.
2.2. Procedure
The study and its procedures were approved by the institutional review board of the UMC Utrecht. Children with ADHD symptoms were recruited through the UMCU outpatient clinic for developmental disorders and schools for special education. Typically developing children were recruited through local schools. After the purpose and procedure of the study had been explained, informed consent was obtained from parents and verbal assent was obtained from children.
In the original fMRI studies, data was collected during two visits. During the first visit, the DISC-IV (Shaffer et al., 2000) was administered to one or both parents while children participated in a shortened WISC-III (Wechsler, 1991) IQ assessment. Afterwards, children participated in a mock-scanner session to prepare for the fMRI scan. Prior to the visit, parents completed the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ-C; (Luman et al., 2012)), and the Strengths and Weaknesses of Attention Deficit/Hyperactivity Disorder Symptoms and Normal Behavioral Scale (SWAN; (Swanson et al., 2001)) at home. Three composite scales were computed: SWAN-attention, SWAN-hyperactivity, SPSRQC-reward. During the second visit, the mock-scanner session was repeated. Afterwards, an fMRI session was run in two parts with a break in the middle. During the fMRI session, children performed two neurocognitive tasks, a child-friendly version of the monetary incentive delay (MID) task and a timing manipulated go/nogo task. Task performance and brain activity data were collected for both tasks. A detailed description of the tasks can be found in the supplementary text and earlier papers (De Zeeuw et al., 2012). Both are briefly described below. Task order was randomized across subjects.
2.3. Task performance measures
A child-friendly modification of the MID task was used to assess reward anticipation. In brief, this is a task where children are informed on how much money they can win in the upcoming trial. The event of interest is not the trial itself, but the brain activity elicited by the anticipation of different monetary rewards. However, we did also use a measure of task performance: the shift in reaction time (RT) distribution between rewarded and unrewarded trials. This was quantified using linear regression of individual rank-ordered reaction times in the high-reward condition (i.e. 15 cents) on the individual rank-ordered reaction times in the non-rewarded conditions, as described previously (De Zeeuw et al., 2012). A regression coefficient (RegB) smaller than one indicates faster RT on rewarded than on unrewarded trials.
A timing manipulated go/nogo task was used to assess temporal expectancy. In this task children were instructed to push a button when they saw a picture of cheese (go cue) and withhold their response when they saw a cat (nogo cue). Of interest was the timing of cues which was manipulated to create a distinction between cues with expected and cues with unexpected timing. We studied brain activity differences between these expected and unexpected events. In addition, we used the difference score between mean response times on go-trials with expected timing (i.e. RTexpectedgo) and mean response times on go-trials with unexpected timing (i.e. RTunexpectedgo) divided by the standard deviation of response times, as a measure of task performance. This measure indicates whether participants' responses on expected trials were faster than on unexpected trials.
2.4. Neuroimaging measures
We used average brain activity in four regions of interest (ROIs) as input for our latent class analysis (LCA). Mean activity in these four ROIs was derived from brain activity data from two different fMRI studies that have been previously published (B.M. van Hulst et al., 2017a, van Hulst et al., 2017b). In Supplementary Text S1 we briefly describe the Methods that were used in these previous studies. The choice of ROIs was based on between-group differences found in this dataset. As such, these previous studies tested for between-group differences, where this study tests for within-group differences. Between-group differences were found in left subthalamic nucleus and left pallidum related to temporal expectancy, and bilateral nucleus accumbens related to reward anticipation. As a result, these ROIs were further explored in this study.
3. Analytic strategy
The analysis consisted of three steps: (1) identifying homogenous subgroups of children with ADHD symptoms based on patterns of brain activity during reward anticipation and temporal expectancy; (2) assessing behavioral and neuropsychological differences between these subgroups; (3) comparing neurobiological and neuropsychological measures of these subgroups to those of (subgroups of) typically developing children.
3.1. Step 1 – model selection
First, we used full information maximum likelihood (FIML) to deal with missing data. In FIML, missing values are not replaced. Instead, the model uses all available information from all variables in the model (full information likelihood) to estimate values that would most likely produce the estimates from the sample data. Then, to identify subgroups, we performed LCA on mean activity in the four ROIs listed above using Mplus, Version 7.3 (Muthén and Muthén, n.d.). Model selection followed a strict procedure that was defined a-priori. First, we determined the number of latent classes (i.e. subgroups) on the basis of the Bayesian Information Criterion (BIC). Better fitting models have a lower BIC. If model selection using BIC yielded a class with less than three subjects, the more parsimonious model was chosen (i.e. with fewer classes). Note that we determined the number of classes (i.e. subgroups) in this step, so before correlations were added to the model. Second, we tested for conditional independence using a procedure described by Vermunt and Magidson (2002). We incrementally added correlations to the model each time adding the strongest correlation. We used modification indices to check if model fit kept improving and stopped adding correlations when they yielded no further improvement in model fit. The modification index approximates the increase in chi-square of the overall model fit by freeing a parameter. By introducing possible local dependences between residuals, a more parsimonious model fit can be found compared to adding more classes (Vermunt and Magidson, 2002). Third, Wald tests were performed to characterize the differences in brain activity between subgroups.
3.2. Step 2 – differences between subgroups
Next, we analyzed the relation between the latent classes and continuous outcome variables (i.e. RegB, RTbenefit-SD, SWAN-attention, SWAN-hyperactivity, SPSRQC-R, age, TIQ) using the Bolck, Croon and Hagenaars (BCH) method in Mplus (Bolck et al., 2004). BCH is a new method for 3-step mixture modeling with continuous outcomes. It uses weighted multiple group analysis in which the subgroups correspond to the latent classes. In doing so, the subgroups are known and not susceptible to changes (Bakk and Vermunt, 2015). Categorical outcome variables (i.e. DSM-IV-TR and DISC-IV diagnosis) were analyzed using DCAT in Mplus. All results were corrected for multiple comparisons using a False Discovery Rate (FDR) correction.
3.3. Step 3 – comparison with typically developing children
In a third step, we tested for differences on the distal outcome variables between the ADHD-symptom subgroups and control subgroups using multiple independent sample t-tests in SPSS. All results were corrected for multiple comparisons using a False Discovery Rate (FDR) correction. To this end, we performed a separate LCA-analysis on brain activity data from typically developing children. We chose to perform two separate analyses, as opposed to a combined analysis on data from all participants, as subgroups across typically developing and ADHD children may differ in both qualitative (i.e. a different ratio between activity in different brain areas) and quantitative (i.e. the same ratio between activity in different brain areas, but an overall brain activity difference) ways. As such, running a single combined analysis in a relatively small sample would have risked lumping together subgroups into larger non-specific subgroups. In this, we followed the method used by Fair and colleagues in their study of heterogeneity in ADHD (Fair et al., 2012).
4. Post-hoc analyses
As head motion can be a confounding factor in imaging analyses, we tested for differences in head motion between subgroups. Framewise displacement (FD) was calculated as a measure of between-scan head motion. FD averages the absolute values of the differentiated realignment estimates (Power et al., 2012). We used a one-way ANOVA with mean FD during both tasks as dependent variable and subgroup (ADHD-1, ADHD-2 or control) as factor; and followed up with Tukey's honestly significant difference post hoc test.
5. Results
5.1. Step 1 – model selection
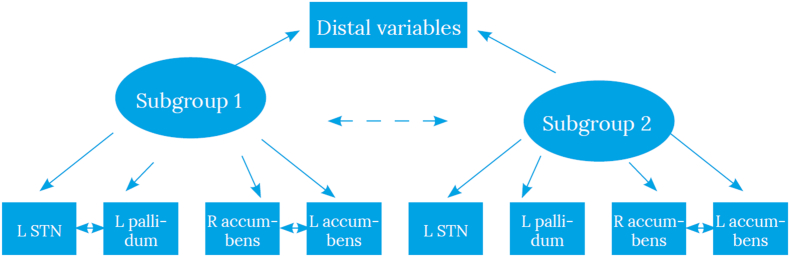
Global maximum was achieved in all models on the data from children with ADHD symptoms. The lowest BIC was found for the three-class model. However, the three and four class models each included a subgroup with only two participants. Therefore, we carried forward the two-class model that had a better model fit (BIC) than the single class model. We then tested for conditional independence by incrementally adding correlations to the model, each time adding the strongest correlation and using modification indices to check if model fit kept improving (Vermunt and Magidson, 2002). We stopped adding correlations when they yielded no further improvement in model fit. This procedure resulted in including a correlation between activity in left and right nucleus accumbens for the entire ADHD symptom group, and including a correlation between left subthalamic nucleus and left pallidum for subgroup 1. This resulted in a final model as depicted in Fig. 1, with model fit statistics shown in Table 1.
Fig. 1.
Final model for children with ADHD-symptoms.
Note. The final model includes the correlation between left and right nucleus accumbens in both subgroups, and a correlation between left subthalamic nucleus and left pallidum only for subgroup ADHD-1.
Table 1.
Model fit statistics for the one to four class models for children with ADHD-symptoms.
| 1-Class Model |
2-Class Model |
3-Class Model |
4-Class Model |
|||||
|---|---|---|---|---|---|---|---|---|
| BIC | Entropy | BIC | Entropy | BIC | Entropy | BIC | Entropy | |
| Without correlation | 364.0 | 1.00 | 352.6 | 0.88 | 339.5 | 0.86 | 339.7 | 0.88 |
| Overall correlation | 297.1 | 1.00 | 302.7 | 0.72 | 308.8 | 0.90 | 318.5 | 0.78 |
| Correlation in subgroup ADHD-1 | 287.2 | 0.85 | ||||||
BIC, bayesian information criterion; LCA, latent class analysis.
Note.Table 1 shows the different steps taken in fitting the LCA model. First, the number of latent classes was determined based on BIC-values. The 3-class model had the lowest BIC-values but included a subgroup with only two participants. In view of parsimony and interpretability, the 2-class model was carried forward. In subsequent steps, we included direct effects in the model: first, the overall correlation between left and right nucleus accumbens; then the correlation between left pallidum and left subthalamic nucleus for subgroup ADHD-1.
5.2. Step 2 – differences between subgroups
As per definition, we found differences in brain activity between the two subgroups, with higher ROI activity in ADHD subgroup 2 (ADHD-2, n = 7) than in ADHD subgroup 1 (ADHD-1, n = 49) (Wald (3) = 144.141, p < 0.001). Univariate tests of individual ROIs did not reach significance ((WaldleftPalidum (1) = 2.124, p = 0.145), (WaldLeSTN (1) = 0.021, p = 0.883), (WaldReNacc (1) = 3.343, p = 0.067), (WaldlEnACC (1) = 0.087, p = 0.768). As the effect sizes were medium to large for some of the univariate tests (e.g. left pallidum d = 0.52, right nucleus accumbens d = 0.77) this may reflect limited statistical power.
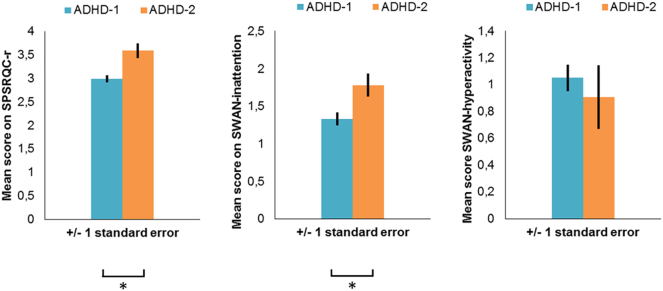
To assess whether subgroups ADHD-1 and ADHD-2 differed in demographics, task performance or behavior, we compared the subgroups using the BCH method. Subgroup ADHD-2 showed more parent-rated reward sensitivity (Wald(1) = 11.468, p < 0.001) and more parent-rated attention problems (Wald(1) = 6.059, p = 0.014) than subgroup ADHD-1 (see Fig. 3). The subgroups did not differ in age (t (55) = 0.722, p = 0.395), total IQ (t (55) = 0.383, p = 0.536), DSM-IV-TR diagnosis (χ2(1) = 0.003, p = 0.956) or DISC diagnosis (χ2 (3) = 0.687, p = 0.876).
Fig. 3.
Behavioral differences between ADHD subgroups.
Note.Fig. 3 shows differences in behavior between the two ADHD subgroups as reported by parents. Panel A shows reward sensitivity; panel B shows attention problems; panel C shows hyperactivity. Subgroup ADHD-2 showed more parent-rated reward sensitivity (Wald(1) = 11.468, p < 0.001) and more parent-rated attention problems (Wald(1) = 6.059, p = 0.014) than subgroup ADHD-1.
*Significant subgroup difference.
5.3. Step 3 – comparison with typically developing children
For the typically developing children, we selected the one-class model, as adding more classes resulted in a subgroup with only one participant. Here, the correlation between left and right nucleus accumbens was also high (r = 0.87) and was included in the final model. Conditional independence testing indicated no residual correlations. Model fit statistics are given in Table 2. Fig. 2 shows mean activity for all four ROIs for the two ADHD subgroups and the control group.
Table 2.
Model fit statistics for the one to four class models for typically developing controls.
| 1-Class Model |
2-Class Model |
3-Class Model |
4-Class Model |
|||||
|---|---|---|---|---|---|---|---|---|
| BIC | Entropy | BIC | Entropy | BIC | Entropy | BIC | Entropy | |
| Without correlation | 204.3 | 1.00 | 194.5 | 0.98 | 194.2 | 0.80 | 199.3 | 0.80 |
| Overall correlation | 172.6 | 1.00 | ||||||
BIC, bayesian information criterion; LCA, latent class analysis.
Note.Table 2 shows the different steps in fitting the LCA model for the group of typically developing children. First, the number of latent classes was determined based on BIC-values. The 2 and 3-class models had the lowest BIC-values but both included a subgroup with only one participant. Therefore the 1-class model was carried forward.
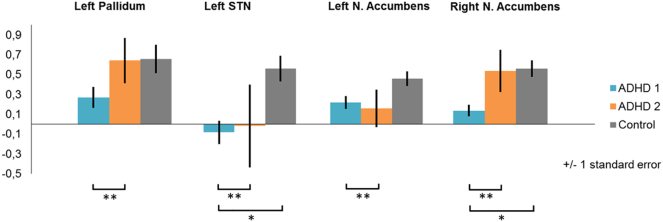
Fig. 2.
Mean activity in the four regions of interest.
Note.Fig. 2 shows mean activity per ROI for the two ADHD-symptom subgroups (ADHD-1 and ADHD-2) and typically developing children.
*Significant subgroup difference.
**Multivariate subgroup difference; univariate tests of individual ROIs did not reach significance.
We compared the two subgroups of children with ADHD symptoms to the typically developing group on the same measures as above and found differences in brain activity (see Fig. 2). Subgroup ADHD-1 had less activation than typically developing controls in left subthalamic nucleus and right nucleus accumbens at a statistically significant level (see supplementary Table 1). In addition, at the descriptive level, all four ROIs appeared less active in subgroup ADHD-1, but not subgroup ADHD-2, compared to the typically developing group. As expected, both subgroups of children with ADHD showed more parent-rated reward sensitivity, attention problems and hyperactivity in everyday live than typically developing children (see supplementary Table 2). There were no differences in task performance. Age was not significant as a covariate in any of the analyses above and was consequently left out of the final analyses.
5.4. Post-hoc analyses
We found an overall difference in head motion (i.e. framewise displacement) between the three subgroups (F(2,83) = 5.863, p = 0.004). A posteriori testing indicated no difference (p = 0.242) in head motion between children in subgroup ADHD-1 (M = 0.29, SD = 0.15) and children in subgroup ADHD-2 (M = 0.38, SD = 0.16). While both subgroup ADHD-1 (p = 0.031) and subgroup ADHD-2 (p = 0.010) differed from typically developing children (M = 0.42, SD = 0.22).
6. Discussion
The goal of this proof of concept study was to explore whether we could identify latent neurobiological subgroups among children with symptoms of ADHD, based on their brain activity. Previous studies have used latent class analysis (LCA) to define subgroups at the behavioral and cognitive level and have subsequently investigated neurobiological differences between these subgroups. We took a reverse approach and used LCA to directly identify neurobiological subgroups based on their functional imaging data. Two subgroups of children with ADHD-symptoms were identified. The largest ADHD subgroup (ADHD-1) had lower brain activity in the selected ROIs than the second subgroup (ADHD-2). However, subgroup ADHD-2 exhibited more ADHD-related symptoms as reported by parents.
Children in this study were classified based on their brain activity in left pallidum, left subthalamic nucleus and bilateral nucleus accumbens. This permitted us to identify two subgroups of children with ADHD symptoms: subgroup ADHD-1 was characterized by attenuated brain activity across all four ROIs compared to subgroup ADHD-2. In part, this is in keeping with the multiple pathway hypothesis that proposes that children with ADHD can be distinguished based on separable brain activity profiles while exhibiting the same, or similar, behavioral problems (Sonuga-Barke, 2005). On the other hand, multiple pathway models could be taken to suggest a more differentiated profile, where one subgroup might be affected in reward sensitivity but not timing and another subgroup vice versa (De Zeeuw et al., 2012; Sonuga-Barke et al., 2010).
As a group, typically developing children showed a more homogenous pattern of brain activity and the 1-class model gave the most parsimonious fit. Interestingly, brain activity of subgroup ADHD-2 resembled that of typically developing children more than subgroup ADHD-1, but subgroup ADHD-2 simultaneously exhibited more symptoms of ADHD.
We investigated whether (sub-) groups defined on functional imaging data differed at the cognitive and behavioral levels. No differences between the two ADHD subgroups were found on task performance measures. There were no differences between the ADHD subgroups on DSM-IV-TR (sub-)diagnosis, which is consistent with previous studies that have reported that multiple etiological pathways to ADHD do not appear to correspond to DSM-IVs behavioral subtypes (Castellanos and Tannock, 2002; Durston et al., 2003). However, at the behavioral level, the subgroups differed from each other on measures of attention problems and reward sensitivity: the parents of children in subgroup ADHD-2 reported more attention problems and more reward sensitive behavior in everyday life. This is a counterintuitive result, as ADHD subgroup 2 showed most difficulties in daily life, but had a profile of brain activity more similar to typically developing children than ADHD subgroup 1. One explanation could be that children in ADHD subgroup 2 seek more external stimulation to compensate for reduced brain activity. This fits with existing theories that have proposed that an organism will work to maintain optimal levels of arousal (Geissler et al., 2014; Zentall and Zentall, 1983). According to this theory, impulsive and reward seeking behavior represents an auto-regulatory mechanism to attain more optimal levels of brain activity (Geissler et al., 2014). If this is true, it suggests that forms of therapy that directly target arousal levels may be effective. Indeed, some studies have shown that introducing environmental noise can improve ADHD symptoms for some children (SöDerlund et al., 2007). Furthermore, one interpretation for the mechanism by which stimulants are effective is that they increase dopamine levels and may therefore lead to more optimal levels of arousal, and thus ameliorate behavior (Bresnahan et al., 2006). Lastly, it is noteworthy that differences in reward sensitivity between these neurobiology-based subgroups were found in parent-rated reward sensitivity but not in the task performance proxy of reward sensitivity. As such, it seems that these two measures of reward sensitivity may not relate to the same underlying mechanisms.
We tested for neurobiological subgroups within children with symptoms of ADHD as the search for a neurobiological profile of ADHD might have been hampered by the assumption of a homogeneous diagnostic group (Crosbie et al., 2008; Nigg et al., 2005). Findings that only hold for a subgroup might be diluted or even cancelled out when heterogeneity is disregarded. Techniques to quantify neurobiological heterogeneity should make it possible to study differential relationships between behavior and neurobiology within one clinical group (Fair et al., 2012; van Hulst et al., 2015). We showed that by temporarily disregarding our knowledge of behavioral profiles (e.g. ADHD) and behavioral subtyping (e.g. DSM-5 presentations of ADHD) and focusing on neurobiological subgroups, we could eventually study behavior from a more biological perspective. This follows the RDoc rationale (Insel et al., 2010), as it steers away from symptom-based definitions as a basis for research. In addition, we would like to argue that for any neurobiological finding to be clinically relevant, it would have to add significantly to our perspective on behavior, as opposed to simply restating what we already knew from behavioral studies.
7. Limitations and future directions
While the sample size of the current study is typical for fMRI studies, especially ones including young children with behavioral disorders, it also borders on the minimum for LCA. As such, complex models such as the ones presented here should not be generalized to the population at large. Moreover, our between subgroup analyses had limited power to detect differences and thus conferred an increased risk for type-II errors. A promising direction for future studies would be to include larger samples and to cross-validate the resulting neurobiological profiles in independent samples. However, the sample size here was sufficient for a proof of concept, as our intention was to demonstrate a bottom-up approach where we profiled at the neurobiological level and subsequently explored differences at the behavioral level. A second limitation is that our regions of interest were chosen a priori, based on previously found between-group differences in the same dataset. Accordingly, other subgroup differences might have gone undetected. Larger samples would enable analyses of a wider range of brain regions or even a whole brain approach to differences in brain activity. A third limitation is our limited data on medication use. Children using short acting psychostimulants were equally spread over the two original ADHD symptom groups (ADHD and ASD+) and all children were asked to not to take medication 24 h prior to scanning. However, since psychostimulants may have long lasting effects it would have been relevant to know how long and in which doses medication had been used (Schrantee et al., 2016). Last but not least, it would be of further interest to design longitudinal studies to investigate the development and the temporal stability of the different developmental pathways and examine inter-subject variability.
8. Conclusion
We found that we could use neuroimaging data to identify subgroups within a group of children with ADHD symptoms, but that generalizability was limited due to a small sample size. This study should be taken as an incentive for other initiatives to empirically address neurobiological heterogeneity in ADHD. The present findings suggest that insight into underlying neurobiological subgroups of children with symptoms of ADHD may enhance our perspective on behavior. Our assessment of behavior could then be informed by neurobiological knowledge, without the need to scan each individual child.
Acknowledgments
Acknowledgments
This work was supported by a grant from the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO) (S.D., grant number Vici-453-10-005). The funding source had no influence on study design, data analysis or the written report.
Financial disclosures
This work was supported by a grant from the Netherlands Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO) (S.D., grant number Vici-453-10-005). The funding source had no influence on study design, data analysis or the written report. The authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.11.011.
Contributor Information
Aleksandra Lecei, Email: aleksandra.lecei@gmail.com.
Branko M. van Hulst, Email: B.M.vanHulst@umcutrecht.nl.
Appendix A. Supplementary data
Supplementary material
References
- Association, A. P . 2000. Diagnostic and Statistical Manual for Mental Disorders (Author) Washington, DC. [Google Scholar]
- Bakk Z., Vermunt J.K. Robustness of stepwise latent class modeling with continuous distal outcomes. Struct. Equ. Model. Multidiscip. J. 2015;5511(December):1–12. [Google Scholar]
- Bolck A., Croon M., Hagenaars J. Estimating latent structure models with categorical variables: one-step versus three-step estimators. Polit. Anal. 2004;12(1):3–27. [Google Scholar]
- Bresnahan S.M., Barry R.J., Clarke A.R., Johnstone S.J. Quantitative EEG analysis in dexamphetamine-responsive adults with attention-deficit/hyperactivity disorder. Psychiatry Res. 2006;141(2):151–159. doi: 10.1016/j.psychres.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Costa Dias T.G., Wilson V.B., Bathula D.R., Iyer S.P., Mills K.L., Thurlow B.L.…Fair D.a. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013;23(1):33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie J., Pérusse D., Barr C.L., Schachar R.J. Validating psychiatric endophenotypes: inhibitory control and attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2008;32(1):40–55. doi: 10.1016/j.neubiorev.2007.05.002. [DOI] [PubMed] [Google Scholar]
- De Zeeuw P., Weusten J., van Dijk S., van Belle J., Durston S. Deficits in cognitive control, timing and reward sensitivity appear to be dissociable in ADHD. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Miller M.M., Inati S., Phelps E.A. An fMRI study of reward-related probability learning. NeuroImage. 2005;24(3):862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.-M., Yang Y.…Casey B.J. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Mulder M.J., Spicer J.A., Galvan A., Tottenham N.…Casey B.J. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J. Child Psychol. Psychiatry. 2007;48(9):881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Bathula D., Nikolas M.A., Nigg J.T. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. U. S. A. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler J., Romanos M., Hegerl U., Hensch T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten. Deficit Hyperactivity Disorder. 2014;6(3):159–173. doi: 10.1007/s12402-014-0144-z. [DOI] [PubMed] [Google Scholar]
- Ghajar J., Ivry R.B. The predictive brain state: asynchrony in disorders of attention? Neuroscientist. 2009;15(3):232–242. doi: 10.1177/1073858408326429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K.…Wang P. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Knutson B., Gibbs S.E.B. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191(3):813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Luman M., Oosterlaan J., Sergeant J.a. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin. Psychol. Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M., van Meel C.S., Oosterlaan J., Geurts H.M. Reward and punishment sensitivity in children with ADHD: validating the sensitivity to punishment and sensitivity to reward questionnaire for children (SPSRQ-C) J. Abnorm. Child Psychol. 2012;40(1):145–157. doi: 10.1007/s10802-011-9547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., Berns G.S., Montague P.R. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Muthén, B., & Muthén, L. (n.d.). USER ’ s Guide, (JANUARY 1995).
- Nigg J.T., Casey B.J. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Willcutt E.G., Doyle A.E., Sonuga-Barke E.J.S. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A., Brammer M., Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20(1):351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Aase H., Johansen E.B., Russell V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Res. 2005;28(OCTOBER):397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Schrantee A., Tamminga H.G.H., Bouziane C., Bottelier M.A., Bron E.E., Mutsaerts H.J.M.M.…Reneman L. Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73(9):955–962. doi: 10.1001/jamapsychiatry.2016.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- SöDerlund G., SikströM S., Smart A. Listen to the noise: noise is beneficial for cognitive performance in ADHD. J. Child Psychol. Psychiatry. 2007;48(8):840–847. doi: 10.1111/j.1469-7610.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol. Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Bitsakou P., Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(4):345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Swanson J., Schuck S., Mann M., Carlson C., Hartman K., Sergeant J.…McCleary R. 2001. The SWAN rating scale. Retrieved July, 15, 2005. [Google Scholar]
- Tripp G., Wickens J.R. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J. Child Psychol. Psychiatry. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Tripp G., Wickens J.R. Neurobiology of ADHD. Neuropharmacology. 2009;57(7–8):579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- van Hulst B.M., de Zeeuw P., Durston S. Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychol. Med. 2015;45(4):735–745. doi: 10.1017/S0033291714001792. [DOI] [PubMed] [Google Scholar]
- van Hulst B.M., de Zeeuw P., Bos D.J., Rijks Y., Neggers S.F.W., Durston S. Children with ADHD symptoms show decreased activity in ventral striatum during the anticipation of reward, irrespective of ADHD diagnosis. J. Child Psychol. Psychiatry. 2017;58(2) doi: 10.1111/jcpp.12643. [DOI] [PubMed] [Google Scholar]
- van Hulst B.M., de Zeeuw P., Rijks Y., Neggers S.F.W., Durston S. What to expect and when to expect it: an fMRI study of expectancy in children with ADHD symptoms. Eur. Child Adolesc. Psychiatry. 2017;26(5):583–590. doi: 10.1007/s00787-016-0921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst F., Van der Ende J., Koot H. Department of Child and Adolescent Psychiatry, Erasmus Academic Medical Centre; Rotterdam: 1996. Handleiding voor de CBCL/4-18 (Manual for the CBCL/4-18) [Google Scholar]
- Vermunt J., Magidson J. Applied Latent Class Analysis. 2002. Latent class cluster analysis; pp. 1–21. [Google Scholar]
- Wechsler D. 3rd ed. The Psychological Corporation; TX: 1991. Wechsler Intelligence Scale for Children. [Google Scholar]
- Zentall S.S., Zentall T.R. Optimal stimulation: a model of disordered activity and performance in normal and deviant children. Psychol. Bull. 1983;94(3):446–471. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material