Abstract
Curcumin—a rhizomal phytochemical from the plant Curcuma longa—is well known to inhibit cell proliferation and to induce apoptosis in a broad range of cell lines. In previous studies we showed that combining low curcumin concentrations and subsequent ultraviolet A radiation (UVA) or VIS irradiation induced anti-proliferative and pro-apoptotic effects. There is still debate whether curcumin induces apoptosis via the extrinsic or the intrinsic pathway. To address this question, we investigated in three epithelial cell lines (HaCaT, A431, A549) whether the death receptors CD95, tumor necrosis factor (TNF)-receptor I and II are involved in apoptosis induced by light and curcumin. Cells were incubated with 0.25–0.5 µg/mL curcumin followed by irradiation with 1 J/cm2 UVA. This treatment was combined with inhibitors specific for distinct membrane-bound death receptors. After 24 h apoptosis induction was monitored by quantitative determination of cytoplasmic histone-associated-DNA-fragments. Validation of our test system showed that apoptosis induced by CH11 and TNF-α could be completely inhibited by their respective antagonists. Interestingly, apoptosis induced by curcumin/light treatment was reversed by none of the herein examined death receptor antagonists. These results indicate a mechanism of action independent from classical death receptors speaking for intrinsic activation of apoptosis. It could be speculated that a shift in cellular redox balance might prompt the pro-apoptotic processes.
Keywords: curcumin, death receptor, apoptosis
1. Introduction
Phytochemicals have a crucial role in drug discovery and development [1,2]. Curcumin has been a part of traditional Asian medicine for thousands of years due to its extensive effects on cell physiology. It can be isolated from the rhizome of the ginger plant Curcuma longa. Curcumin is known for its anti-inflammatory, anti-oxidative, as well as its pro-apoptotic potential [3,4,5,6,7]. Taking into account the hardly existing toxicity of curcumin, it is predestined for the development of anti-tumorigenic therapeutic strategies. Targeting the low bioavailability of curcumin [8,9] strategies including encapsulation, inhibition of metabolic degradation and development of photodynamic therapies [6,10,11,12,13,14,15,16,17,18,19,20] have been developed. Reducing the proliferative potential of neoblastic cells as well as inducing pro-apoptotic effects is the mode of choice to target cancer cells [6,18]. These two criteria can be addressed by curcumin. Presently, there is debate whether curcumin induces apoptosis via the extrinsic or the intrinsic pathway. Treatment with high curcumin concentrations has been described to induce apoptosis depending on the cell type and tissue via the extrinsic as well as via the intrinsic pathway [21,22]. Characteristic of apoptosis induction via the extrinsic pathway is the binding of extracellular ligands to transmembrane death receptors, e.g., CD95 or tumor necrosis factor (TNF)-α receptors. Receptor clustering, binding with homologous trimeric ligands and recruitment of cytoplasmic adaptor proteins ultimately leads to auto-catalytic activation of pro-caspase-8 [23,24].
Caspase-8 thereafter cleaves and activates the effector caspases-3, -6, -7, leading to the substrate proteolysis, DNA fragmentation and cell death [24,25]. To evaluate whether curcumin in our experimental set up triggers apoptosis via the extrinsic pathway death receptor, specific antagonists were used.
2. Results
2.1. Death Receptor Specific Apoptosis Induction Was Cell Species Dependent
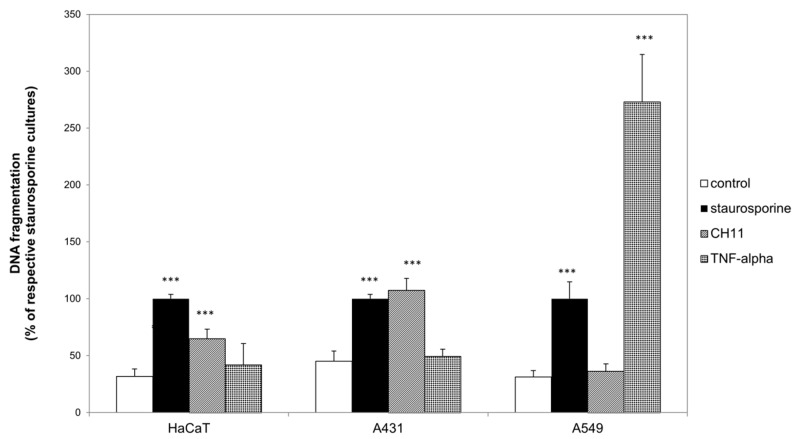
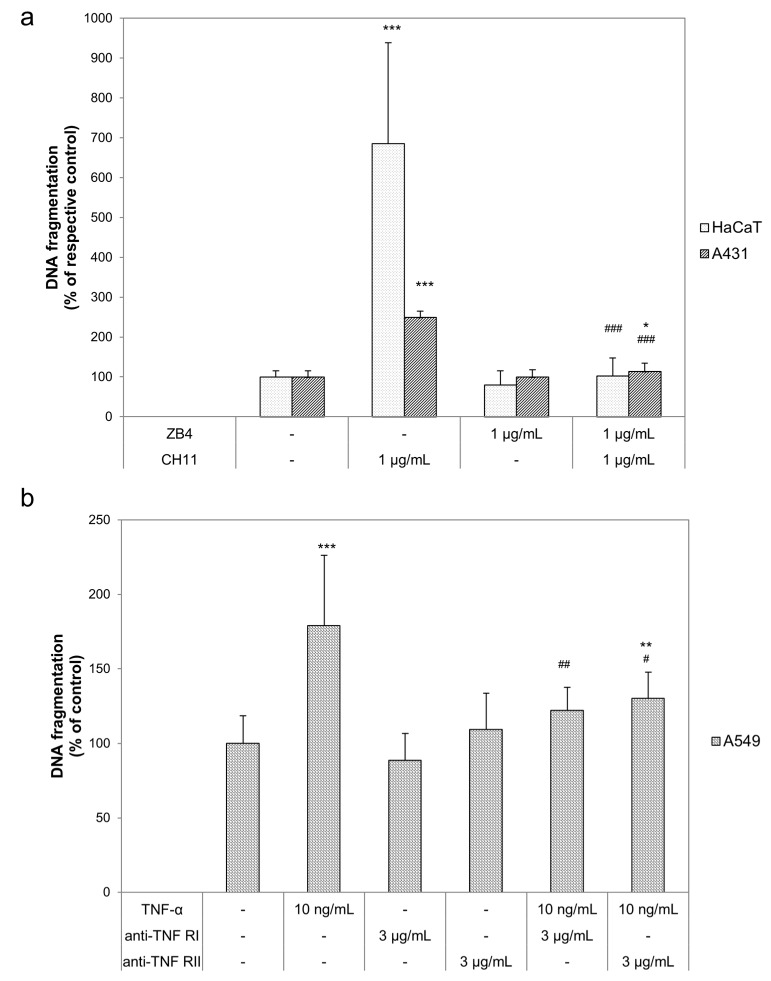
First of all, we determined to which death receptor agonist the herein investigated epidermal cell lines are susceptible. As shown in Figure 1, DNA fragmentation was induced in all three cell lines by a positive control (1 µg/mL staurosporine; black bars) which was set to 100%. Comparison of the DNA fragmentation of the respective untreated cultures (white bars) with CH11 treated cultures (striped bars), showed significantly higher DNA fragmentation in HaCaT and A431 cells. In contrast to the observed non-inducible DNA fragmentation in A549 by CH11, TNF-α (bricked bars) induced a clear increase of DNA fragmentation in comparison to the untreated control. Neither in HaCaT nor in A431 differed DNA fragmentation of the TNF-α treated cultures from the respective untreated cultures. Therefore, we further investigated CD95 related apoptosis induction in HaCaT and A431, and TNF-α related apoptosis induction in A549. Consecutively, we tested described death receptor antagonists to compensate for the pro-apoptotic stimuli. None of the used death receptor antagonists induced DNA fragmentation (Figure 2). In combination with the respective agonists all antagonists were able to reduce the pro-apoptotic impact of the agonists. In detail ZB4 completely neutralized the pro-apoptotic influence of CH11 in HaCaT (pointed bars) and A431 (striped bars; Figure 2a). The efficiency of the two investigated TNF-α antagonists varied in A549 (scaled bars; Figure 2b). Whereas anti-TNF-α RI completely neutralized the pro-apoptotic influence of TNF-α only a decreased but still significantly higher DNA fragmentation in comparison to the untreated control was observed after treatment with TNF-α and anti-TNF-α RII.
Figure 1.
Death receptor agonist specific apoptosis induction. HaCaT, A431 and A549 cells were either left untreated (white bars) or were treated with 1 µg/mL staurosporine (black bars), with 1 µg/mL CH11 (striped bars) or 10 ng/mL tumor necrosis factor (TNF)-α (bricked bars). DNA fragmentation was evaluated after 24 h. The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from quintuplicate replicates per experimental condition were calculated. *** p ≤ 0.001 versus the respective untreated control.
Figure 2.
Death receptor specific antagonists reversed apoptosis induction. (a) HaCaT (pointed bars) and A431 (striped bars) cells were treated with 1 µg/mL CH11, 1 µg/mL ZB4 or their combination to investigate the CD95 receptor. (b) A549 (scaled bars) were treated with 10 ng/mL TNF-α, 3 µg/mL anti-TNF-α RI, 3 µg/mL anti-TNF-α RII or their combinations. DNA fragmentation was evaluated after 24 h. The data displayed are representative of three experiments performed with comparable results. Average absorbance values (mean ± SD) from quintuplicate replicates per experimental condition were calculated. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001 versus the respective untreated control and # p ≤ 0.05; ## p ≤ 0.01; ### p ≤ 0.001 versus the respective death receptor agonist.
2.2. Curcumin and Light Induced DNA Fragmentation Independent of the First Apoptosis Signal (FAS) Ligand and the TNF-α Receptors
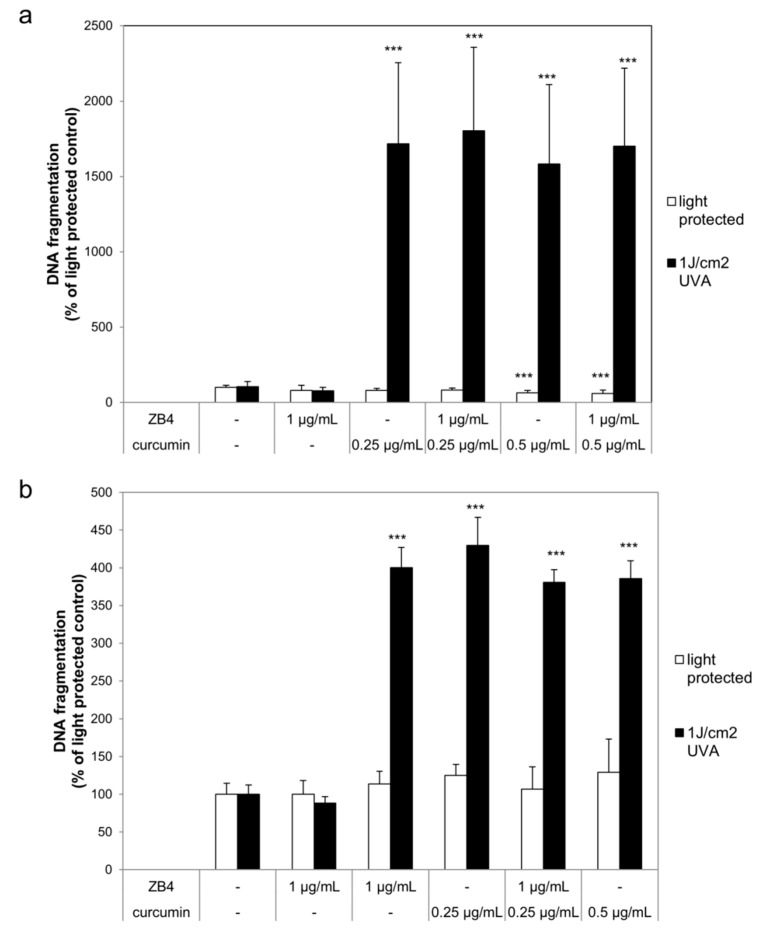
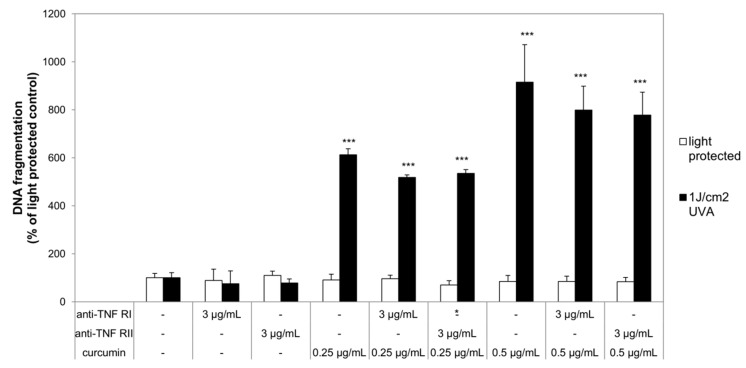
After establishing the efficiency of the herein used death receptor antagonists their ability to influence DNA fragmentation in curcumin/light treated cultures was investigated. HaCaT and A431 cells were per-incubated with or without curcumin and ZB4 whereas A549 were pre-incubated with or without curcumin and anti-TNF-α RI or anti-TNF-α RII before irradiation with 1 J/cm2 ultraviolet A radiation (UVA). Neither curcumin nor the antagonists under light-protected conditions induced DNA fragmentation (white bars; Figure 3 and Figure 4). Likewise, the herein chosen UVA irradiation regimen did not induce significant DNA fragmentation in comparison to the light-protected controls. DNA fragmentation of cell cultures treated with curcumin and light (black bars; Figure 3 and Figure 4) was significantly increased. DNA fragmentation of HaCaT after treatment with either 0.25 µg/mL or 0.5 µg/mL curcumin and UVA was 1600% higher in comparison to the light-protected control (Figure 3a). Blocking apoptosis via the first apoptosis signal (FAS) ligand by ZB4 did not change the amount of DNA fragmentation caused by the curcumin/light treatment. Comparable results were observed in A431 cells (Figure 3b). The combined treatment of curcumin and light induced a 300% higher DNA fragmentation than observed in the light-protected cultures. As observed in HaCaT cells, addition of ZB4 to A431 cells did not influence the curcumin/light induced DNA fragmentation. In A549 cells a curcumin concentration dependent increase of DNA fragmentation after irradiation was observed (Figure 4). Cultures that had been treated with 0.25 µg/mL curcumin and light showed a 500% higher DNA fragmentation compared to the light-protected control. Increasing the curcumin concentration to 0.5 µg/mL also increased the DNA fragmentation compared to the light-protected control to 800%. Independent of the applied curcumin concentration addition of anti-TNF-α RI and anti-TNF-α, RII was not able to significantly reduce the curcumin/light induced DNA fragmentation.
Figure 3.
Curcumin does not induce apoptosis via CD95 HaCaT (a) and A431 (b) cells were pre-incubated with curcumin and ZB4. Thereafter the cells were irradiated with ultraviolet A radiation (UVA) followed by ZB4 exposure. DNA fragmentation was evaluated after 24 h. The applied ZB4 or curcumin concentrations had no effect on DNA fragmentation (white bars). Combining curcumin with UVA (black bars) induced significant increase of DNA fragmentation. Addition of ZB4 did not reduce the curcumin/light induced DNA fragmentation. Data displayed are representative of four experiments performed with comparable results. Average absorbance values (mean ± SD) from quadruplicate replicates per experimental condition were calculated. *** p ≤ 0.001 versus the respective untreated control.
Figure 4.
Curcumin does not induce apoptosis via TNF-α receptor I and II. A549 cells were pre-incubated with curcumin and anti-TNF-α RI or anti-TNF-α RII. Thereafter the cells were irradiated with UVA followed by anti-TNF-α RI or anti-TNF-α RII exposure. DNA fragmentation was evaluated after 24 h. The applied TNF-α receptor antagonists or curcumin concentrations had no effect on DNA fragmentation (white bars). Combining curcumin with UVA (black bars) induced a significant increase of DNA fragmentation. Neither anti-TNF-α RI nor anti-TNF-α RII reduced the curcumin/light induced DNA fragmentation. Data displayed are representative of four experiments performed with comparable results. Average absorbance values (mean ± SD) from quadruplicate replicates per experimental condition were calculated. * p ≤ 0.05; *** p ≤ 0.001 versus the respective untreated control.
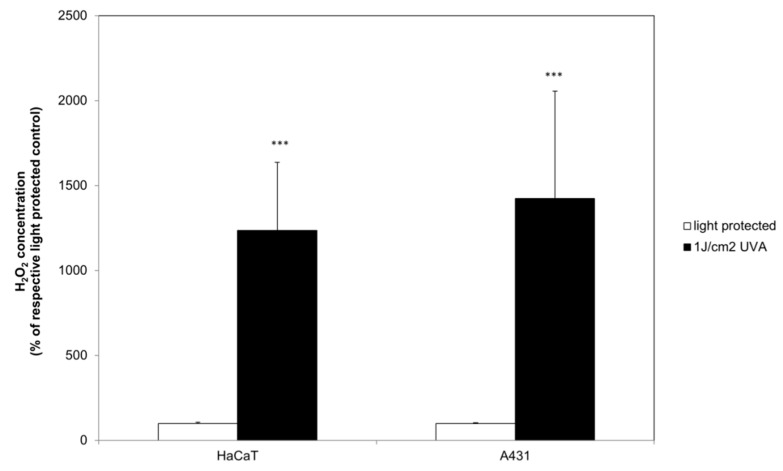
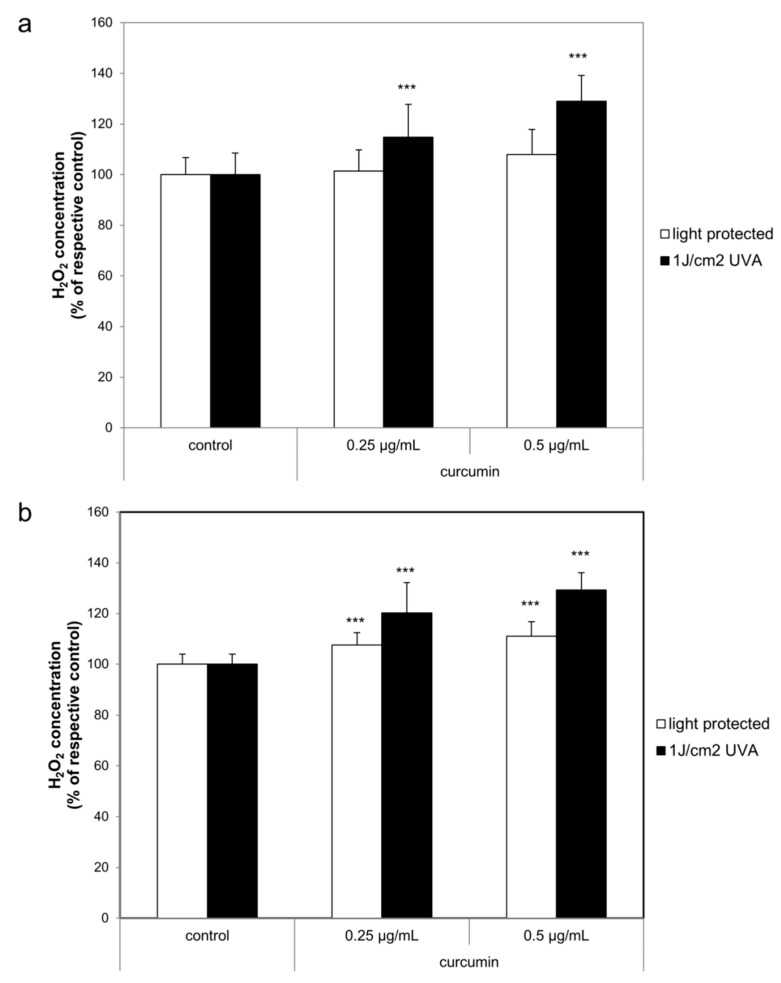
2.3. Curcumin Increased the UVA Triggered H2O2 Generation
After establishing that curcumin in the herein described treatment regimen does not induce apoptosis through the classical death receptors, we were interested to monitor whether the combinatorial curcumin/light treatment induces a shift of the cellular redox balance. The H2O2 concentration was measured 1 h after the treatment. Irradiation with 1 J/cm2 UVA induced a significant H2O2 generation increase of 1200% to 1400% in HaCaT and A431 cells (black bars, Figure 5). Under light-protected conditions (white bars) curcumin did not influence H2O2 generation in HaCaT cells (Figure 6a). Light treatment of A431 cells (Figure 6b) with curcumin induced an H2O2 increase of 7–11% in comparison to the respective controls. Comparing the UVA (black bars) induced H2O2 generation with the H2O2 generation of curcumin/light treated cultures revealed that in both cell species the H2O2 concentration was curcumin dependently increased. In HaCaT cultures treatment with 0.25 µg/mL curcumin induced a 15% higher H2O2 concentration, and treatment with 0.5 µg/mL curcumin a 29% higher H2O2 concentration than observed in the respective controls. Curcumin/light treatment of A431 cells showed a comparable influence on H2O2 generation. Treatment with 0.25 µg/mL curcumin induced a 20% increase whereas treatment with 0.5 µg/mL curcumin induced a 29% higher H2O2 concentration in comparison to the respective light-treated cultures.
Figure 5.
UVA induced H2O2 generation in HaCaT and A431. The data displayed are representative of four experiments performed with comparable results. Average luminescence values (mean ± SD) from triplicate replicates per experimental condition were calculated. *** p ≤ 0.001 versus the respective light-protected control.
Figure 6.
Curcumin enhanced the UVA triggered H2O2 generation in HaCaT (a) and A431 (b). The cells were pre-incubated with curcumin before irradiation with UVA. H2O2 generation was evaluated after 1 h. The data displayed are representative of four experiments performed with comparable results. Average luminescence values (mean ± SD) from triplicate replicates per experimental condition were calculated. *** p ≤ 0.001 versus the respective control.
3. Discussion
Investigation of natural compounds that have been used for centuries in traditional medicine by scientific means has increased during the last decades. Curcumin is one of those phytochemicals with an anti-cancer potential showing a lower risk of inducing adverse events [26,27] than described for other cytostatic drugs. Curcumin influences an extensive spectrum of signaling pathways involved in cancer and inflammatory diseases [28,29,30]. Establishing a photodynamic treatment combining low curcumin concentrations and radiation with either UVA or VIS [6,16,17,18] was our approach to address the low bioavailability of curcumin [9]. As it is described that curcumin interacts, e.g., with the epidermal growth factor (EGF) receptor [31,32,33], we hypothesized that curcumin also directly interacts with different death receptors facilitating apoptosis via the extrinsic pathway. There are contradictory observations concerning the mode of apoptosis induction by curcumin. On the one hand, intrinsic apoptosis induction was observed in mamma carcinoma cells as well as in HL-60 and kidney carcinoma cells [21,34,35,36,37]. On the other hand, apoptosis induction via the CD95 receptor was observed, e.g., in melanoma cells while TNF-related apoptosis-inducing ligand (TRAIL) receptor triggered apoptosis was observed in ovarial and prostate carcinoma cells [22,38,39]. In this study we analyzed whether curcumin induced apoptosis in our treatment regimen via either the CD95 or the TNF-α receptor according to Schon et al. [40]. First, we showed that susceptibility to apoptosis inductors differed in the epithelial cell lines used. In particular, HaCaT and A431 seemed to be resistant to the applied TNF-α whereas A549 did not respond to FAS ligand specific apoptosis induction. Death receptor resistance can be acquired by cells through a broad variety of modulatory mechanisms [41,42]. We were furthermore interested whether curcumin might be able to overcome such resistances. The observed curcumin/irradiation dependent induction of DNA fragmentation was taken as a positive indicator of apoptosis [4,43,44]. Hence, DNA fragmentation was monitored to observe whether the herein investigated death receptor antagonists were able to reduce or inhibit the previously described pro-apoptotic influence of the combinatory treatment with low curcumin concentrations and light irradiation. In contrast to others that observed apoptosis induction by curcumin via the FAS ligand pathway [22,45,46], no FAS ligand-related apoptosis was measured in our experimental set up. The FAS ligand specific antagonist ZB4 failed to inhibit or reduce the pro-apoptotic influence of curcumin/light treatment in either of the investigated cell lines. Furthermore, inhibition of the TNF-α receptor I and II by specific antagonists in A549 cells did not change the curcumin/light triggered apoptosis. These observations indicate that in our experimental set up, a mechanism of apoptosis induction independent from classical death receptors is very likely. A possible alternative mode of action can be related to the observations of Kim et al. [47]. They showed that curcumin-related inhibition of EGF receptor phosphorylation and subsequent inhibition of the downstream kinases lead to activation of the effector caspase-3. It seems that curcumin triggers apoptosis in a cell specific manner by different pathways. This characteristic makes curcumin potentially useful to target a broad range of different tumor cells that are sensitive to different pro-apoptotic triggers. It is known that triggering apoptosis in cells that are non-responsive to death receptor agonists or chemotherapeutics is challenging [48]. Therefore, it is of great interest to identify or develop active agents that overcome such resistances [49,50,51,52]. The observations showing that curcumin in combination with UVA-boosted H2O2 generation indicated that a shift in the cellular redox balance might elicit the observed pro-apoptotic processes as also observed by others [53,54,55,56,57,58]. Future studies need to address this issue. Moreover, utilizing more complex systems, e.g., tissue cultured skin equivalents [59,60,61] and long-term analysis are advised to further analyze the potential of curcumin to overcome chemotherapeutic resistances.
4. Materials and Methods
4.1. Cell Culture and Identification of Death Receptor Agonist Susceptibility
The spontaneous immortalized human keratinocyte cell line HaCaT [62] (kindly provided by Prof. Norbert Fusenig (German Cancer Research Institute, Heidelberg, Germany)) and the human epidermoid carcinoma cell lines A431 (ATCC® CRL-1555™, American Culture Type Collection, Manassas, VA, USA) and A549 (ATCC® CCL-185™, American Culture Type Collection) were cultured in Dulbecco’s Modified Eagle’s Medium (D-MEM, Gibco, Karlsruhe, Germany) with GlutaMax supplemented with 10% (v/v) fetal calf serum (FCS, PAA, Cölbe, Germany) and 1% (v/v) penicillin/streptomycin solution (Gibco) in a 7.5% CO2 atmosphere at 37 °C. The cells were either stimulated with 1 µg/mL staurosporine (Sigma-Aldrich, Traufkirchen, Germany) or with death receptor specific agonists: (anti-) Fas activating antibody (clone CH11, Merck Millipore, Darmstadt Germany) and TNF-α (R&D Systems, Wiesbaden-Nordenstadt, Germany). These death receptor agonists were combined with their respective antagonists: anti-Fas neutralizing antibody (clone ZB4, Merck Millipore) and anti-TNFα RI and anti-TNFα RII (both R&D Systems).
4.2. Irradiation Regimen
Curcumin (Sigma-Aldrich) was dissolved and applied as previously described [16,17]. Briefly, cells were incubated for 1 h in a medium containing 0.25–0.5 µg/mL curcumin, with or without the above mentioned death receptor antagonists. After replacement of the culture medium with PBS++ (Gibco) the cells were either kept light, and/or were irradiated with 1 J/cm2 ultraviolet A (UVA, Waldmann, Villingen-Schwenningen, Germany). After irradiation PBS++ was replaced with culture medium with and without the above mentioned death receptor antagonists.
4.3. DNA Fragmentation
DNA fragmentation, the chosen apoptosis indicator, was quantified after 24 h. The adherent cells were lysed and DNA fragmentation was analyzed with the Cell Death Detection (CDD; Roche, Mannheim, Germany) enzyme-linked immunosorbent assay (ELISA), as described [16].
4.4. Monitoring of the Cellular Redox Balence
The generation of H2O2—monitored with the ROS-Glo™-H2O2 Assay (Promega, Mannheim, Germany) was chosen as the indicator for oxidative stress in relation to the herein described treatment regimen. Briefly, cells were pre-incubated for 1 h with PBS++ containing 0.25–0.5 µg/mL curcumin and were subsequently irradiated with 1 J/cm2 UVA. After 1 h the assay was conducted as recommended by the manufacturer. The luminescence was recorded using a microplate luminometer (CentroPro LB962, Berthold Technologies, Bad Wildbach, Germany).
4.5. Presentation of Data and Statistical Analysis
All data are presented as mean values ± standard deviation. Statistical significance of the data was evaluated by the Wilcoxon-Mann-Whitney U-test (BiAS, version 11.06, epsilon-Verlag, Frankfurt, Germany). Each set of data was related to the referring untreated control (*) or the respective agonist (#). Differences were considered significant at *,# p ≤ 0.05; **,## p ≤ 0.01; ***,### p ≤ 0.001.
Abbreviations
| TNF-α | tumor necrosis factor α |
| UVA | ultraviolet A |
| EGF | endothelial growth factor |
Author Contributions
Conceptualization, S.K. and A.B.; methodology, V.L and N.Z.; validation, S.K., A.B. and N.Z.; formal analysis, V.L. and N.Z.; investigation, V.L.; resources, S.K. and A.B.; writing—original draft preparation, N.Z.; review and editing, N.Z.; visualization, N.Z.; supervision, S.K.; project administration, A.B. and R.K.; funding acquisition, A.B., S.K. and R.K.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kadioglu O., Cao J., Saeed M.E., Greten H.J., Efferth T. Targeting epidermal growth factor receptors and downstream signaling pathways in cancer by phytochemicals. Target. Oncol. 2015;10:337–353. doi: 10.1007/s11523-014-0339-4. [DOI] [PubMed] [Google Scholar]
- 2.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y.X., Pindolia K.R., Janakiraman N., Noth C.J., Chapman R.A., Gautam S.C. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 1997;25:413–422. [PubMed] [Google Scholar]
- 4.Zhu L., Han M.B., Gao Y., Wang H., Dai L., Wen Y., Na L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015;12:1151–1156. doi: 10.3892/mmr.2015.3450. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C., Li B., Zhang X., Hazarika P., Aggarwal B.B., Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: Potential role for STAT-3 and NF-kappaB signaling. J. Investig. Dermatol. 2010;130:2110–2119. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 6.Dujic J., Kippenberger S., Ramirez-Bosca A., Diaz-Alperi J., Bereiter-Hahn J., Kaufmann R., Bernd A., Hofmann M. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. Int. J. Cancer. 2009;124:1422–1428. doi: 10.1002/ijc.23997. [DOI] [PubMed] [Google Scholar]
- 7.Calaf G.M., Ponce-Cusi R., Carrion F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018;40:2381–2388. doi: 10.3892/or.2018.6603. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.J., Pan M.H., Cheng A.L., Lin L.I., Ho Y.S., Hsieh C.Y., Lin J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15:1867–1876. doi: 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 9.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 10.Schiborr C., Kocher A., Behnam D., Jandasek J., Toelstede S., Frank J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutrit. Food Res. 2014;58:516–527. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 11.Hegge A.B., Bruzell E., Kristensen S., Tonnesen H.H. Photoinactivation of Staphylococcus epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin--effect of selected nanocarrier: Studies on curcumin and curcuminoides XLVII. Eur. J. Pharm. Sci. 2012;47:65–74. doi: 10.1016/j.ejps.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang W., Zhu R., Xie Q., Li A., Xiao Y., Li K., Liu H., Cui D., Chen Y., Wang S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012;7:3667–3677. doi: 10.2147/IJN.S30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H., Teh C., Sreejith S., Zhu L., Kwok A., Fang W., Ma X., Nguyen K.T., Korzh V., Zhao Y. Functional mesoporous silica nanoparticles for photothermal-controlled drug delivery in vivo. Angew. Chem. Int. Ed. Engl. 2012;51:8373–8377. doi: 10.1002/anie.201203993. [DOI] [PubMed] [Google Scholar]
- 14.Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 15.Singh D.V., Godbole M.M., Misra K. A plausible explanation for enhanced bioavailability of P-gp substrates in presence of piperine: Simulation for next generation of P-gp inhibitors. J. Mol. Model. 2013;19:227–238. doi: 10.1007/s00894-012-1535-8. [DOI] [PubMed] [Google Scholar]
- 16.Buss S., Dobra J., Goerg K., Hoffmann S., Kippenberger S., Kaufmann R., Hofmann M., Bernd A. Visible light is a better co-inducer of apoptosis for curcumin-treated human melanoma cells than UVA. PLoS ONE. 2013;8:e79748. doi: 10.1371/journal.pone.0079748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dujic J., Kippenberger S., Hoffmann S., Ramirez-Bosca A., Miquel J., Diaz-Alperi J., Bereiter-Hahn J., Kaufmann R., Bernd A. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J. Investig. Dermatol. 2007;127:1992–2000. doi: 10.1038/sj.jid.5700801. [DOI] [PubMed] [Google Scholar]
- 18.Beyer K., Nikfarjam F., Butting M., Meissner M., König A., Ramirez Bosca A., Kaufmann R., Heidemann D., Bernd A., Kippenberger S., et al. Photodynamic Treatment of Oral Squamous Cell Carcinoma Cells with Low Curcumin Concentrations. J. Cancer. 2017;8:1271–1283. doi: 10.7150/jca.17176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Megalathan A., Kumarage S., Dilhari A., Weerasekera M.M., Samarasinghe S., Kottegoda N. Natural curcuminoids encapsulated in layered double hydroxides: A novel antimicrobial nanohybrid. Chem. Cent. J. 2016;10:35. doi: 10.1186/s13065-016-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park K., Lee J.H. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol. Rep. 2007;17:537–540. doi: 10.3892/or.17.3.537. [DOI] [PubMed] [Google Scholar]
- 21.Kim M.S., Kang H.J., Moon A. Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Arch. Pharm. Res. 2001;24:349–354. doi: 10.1007/BF02975105. [DOI] [PubMed] [Google Scholar]
- 22.Bush J.A., Cheung K.J., Jr., Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 23.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hongmei Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. In: Ntuli T., editor. Apoptosis and Medicine. IntechOpen; London, UK: 2012. pp. 3–22. [Google Scholar]
- 25.Pfeffer C.M., Singh A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa) J. Altern. Complement. Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 28.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Anand P., Sundaram C., Jhurani S., Kunnumakkara A.B., Aggarwal B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squires M.S., Hudson E.A., Howells L., Sale S., Houghton C.E., Jones J.L., Fox L.H., Dickens M., Prigent S.A., Manson M.M. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem. Pharmacol. 2003;65:361–376. doi: 10.1016/S0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y., Zheng S., Lin J., Zhang Q.J., Chen A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARgamma in rat activated hepatic stellate cell in vitro. Lab. Investig. 2007;87:488–498. doi: 10.1038/labinvest.3700532. [DOI] [PubMed] [Google Scholar]
- 33.Dorai T., Gehani N., Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol. Urol. 2000;4:1–6. [PubMed] [Google Scholar]
- 34.Choudhuri T., Pal S., Agwarwal M.L., Das T., Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/S0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 35.Choudhuri T., Pal S., Das T., Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 36.Bielak-Mijewska A., Piwocka K., Magalska A., Sikora E. P-glycoprotein expression does not change the apoptotic pathway induced by curcumin in HL-60 cells. Cancer Chemother. Pharmacol. 2004;53:179–185. doi: 10.1007/s00280-003-0705-x. [DOI] [PubMed] [Google Scholar]
- 37.Woo J.H., Kim Y.H., Choi Y.J., Kim D.G., Lee K.S., Bae J.H., Min D.S., Chang J.S., Jeong Y.J., Lee Y.H., et al. Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 38.Wahl H., Tan L., Griffith K., Choi M., Liu J.R. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol. Oncol. 2007;105:104–112. doi: 10.1016/j.ygyno.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 39.Deeb D., Jiang H., Gao X., Al-Holou S., Danyluk A.L., Dulchavsky S.A., Gautam S.C. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J. Pharmacol. Exp. Ther. 2007;321:616–625. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 40.Schon M.P., Wienrich B.G., Drewniok C., Bong A.B., Eberle J., Geilen C.C., Gollnick H., Schon M. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J. Investig. Dermatol. 2004;122:1266–1276. doi: 10.1111/j.0022-202X.2004.22528.x. [DOI] [PubMed] [Google Scholar]
- 41.Min K.J., Woo S.M., Shahriyar S.A., Kwon T.K. Elucidation for modulation of death receptor (DR) 5 to strengthen apoptotic signals in cancer cells. Arch. Pharm. Res. 2019;42:88–100. doi: 10.1007/s12272-018-01103-y. [DOI] [PubMed] [Google Scholar]
- 42.Twomey J.D., Zhang B. Circulating Tumor Cells Develop Resistance to TRAIL-Induced Apoptosis Through Autophagic Removal of Death Receptor 5: Evidence from an In Vitro Model. Cancers. 2019;11:94. doi: 10.3390/cancers11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang P.Y., Peng S.F., Lee C.Y., Lu C.C., Tsai S.C., Shieh T.M., Wu T.S., Tu M.G., Chen M.Y., Yang J.S. Curcumin-loaded nanoparticles induce apoptotic cell death through regulation of the function of MDR1 and reactive oxygen species in cisplatin-resistant CAR human oral cancer cells. Int. J. Oncol. 2013;43:1141–1150. doi: 10.3892/ijo.2013.2050. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Man S., Qiu H., Liu Z., Zhang M., Ma L., Gao W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016;48:31–38. doi: 10.1016/j.etap.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 45.Wang W.Z., Li L., Liu M.Y., Jin X.B., Mao J.W., Pu Q.H., Meng M.J., Chen X.G., Zhu J.Y. Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci. 2013;92:352–358. doi: 10.1016/j.lfs.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Lee H.P., Li T.M., Tsao J.Y., Fong Y.C., Tang C.H. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int. Immunopharmacol. 2012;13:163–169. doi: 10.1016/j.intimp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Kim J.H., Xu C., Keum Y.S., Reddy B., Conney A., Kong A.N. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27:475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 48.Chresta C.M., Arriola E.L., Hickman J.A. Apoptosis and cancer chemotherapy. Behring Inst. Mitt. 1996:232–240. [PubMed] [Google Scholar]
- 49.Thulasiraman P., Garriga G., Danthuluri V., McAndrews D.J., Mohiuddin I.Q. Activation of the CRABPII/RAR pathway by curcumin induces retinoic acid mediated apoptosis in retinoic acid resistant breast cancer cells. Oncol. Rep. 2017;37:2007–2015. doi: 10.3892/or.2017.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labbozzetta M., Notarbartolo M., Poma P., Maurici A., Inguglia L., Marchetti P., Rizzi M., Baruchello R., Simoni D., D’Alessandro N. Curcumin as a possible lead compound against hormone-independent, multidrug-resistant breast cancer. Ann. N. Y. Acad. Sci. 2009;1155:278–283. doi: 10.1111/j.1749-6632.2009.03699.x. [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee S.J., Pandey S. Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy. Cancer Biol. Ther. 2011;11:216–228. doi: 10.4161/cbt.11.2.13798. [DOI] [PubMed] [Google Scholar]
- 52.Shankar S., Ganapathy S., Chen Q., Srivastava R.K. Curcumin sensitizes TRAIL-resistant xenografts: Molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol. Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou D.R., Eid R., Boucher E., Miller K.A., Mandato C.A., Greenwood M.T. Stress is an agonist for the induction of programmed cell death: A review. Biochim. Biophys. Acta. 2019 doi: 10.1016/j.bbamcr.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Jiang S., Zhu R., He X., Wang J., Wang M., Qian Y., Wang S. Enhanced photocytotoxicity of curcumin delivered by solid lipid nanoparticles. Int. J. Nanomed. 2017;12:167–178. doi: 10.2147/IJN.S123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moradi-Marjaneh R., Hassanian S.M., Rahmani F., Aghaee-Bakhtiari S.H., Avan A., Khazaei M. Phytosomal curcumin elicits anti-tumor properties through suppression of angiogenesis, cell proliferation and induction of oxidative stress in colorectal cancer. Curr. Pharm. Des. 2019 doi: 10.2174/1381612825666190110145151. [DOI] [PubMed] [Google Scholar]
- 56.Jayakumar S., Patwardhan R.S., Pal D., Singh B., Sharma D., Kutala V.K., Sandur S.K. Mitochondrial targeted curcumin exhibits anticancer effects through disruption of mitochondrial redox and modulation of TrxR2 activity. Free Rad. Biol. Med. 2017;113:530–538. doi: 10.1016/j.freeradbiomed.2017.10.378. [DOI] [PubMed] [Google Scholar]
- 57.Mortezaee K., Salehi E., Mirtavoos-Mahyari H., Motevaseli E., Najafi M., Farhood B., Rosengren R.J., Sahebkar A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019 doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 58.Gopal P.K., Paul M., Paul S. Curcumin induces caspase mediated apoptosis in JURKAT cells by disrupting the redox balance. Asian Pac. J. Cancer Prev. 2014;15:93–100. doi: 10.7314/APJCP.2014.15.1.93. [DOI] [PubMed] [Google Scholar]
- 59.Zöller N., Valesky E., Butting M., Hofmann M., Kippenberger S., Bereiter-Hahn J., Bernd A., Kaufmann R. Clinical application of a tissue-cultured skin autograft: An alternative for the treatment of non-healing or slowly healing wounds? Dermatology. 2014;229:190–198. doi: 10.1159/000362927. [DOI] [PubMed] [Google Scholar]
- 60.Golinski P., Menke H., Hofmann M., Valesky E., Butting M., Kippenberger S., Bereiter-Hahn J., Bernd A., Kaufmann R., Zöller N.N. Development and Characterization of an Engraftable Tissue-Cultured Skin Autograft: Alternative Treatment for Severe Electrical Injuries. Cells Tissues Organs. 2014;200:227–239. doi: 10.1159/000433519. [DOI] [PubMed] [Google Scholar]
- 61.Zöller N.N., Kippenberger S., Thaci D., Mewes K., Spiegel M., Sattler A., Schultz M., Bereiter-Hahn J., Kaufmann R., Bernd A. Evaluation of beneficial and adverse effects of glucocorticoids on a newly developed full-thickness skin model. Toxicol. in Vitro. 2008;22:747–759. doi: 10.1016/j.tiv.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 62.Boukamp P., Petrussevska R.T., Breitkreutz D., Hornung J., Markham A., Fusenig N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]