Abstract
Meniscal tears are the most common orthopaedic injuries, with chronic lesions comprising up to 56% of cases. In these situations, no benefit with surgical treatment is observed. Thus, the purpose of this study was to investigate the effectiveness and safety of percutaneous intrameniscal platelet rich plasma (PRP) application to complement repair of a chronic meniscal lesion. This single centre, prospective, randomized, double-blind, placebo-controlled study included 72 patients. All subjects underwent meniscal trephination with or without concomitant PRP injection. Meniscal non-union observed in magnetic resonance arthrography or arthroscopy were considered as failures. Patient related outcome measures (PROMs) were assessed. The failure rate was significantly higher in the control group than in the PRP augmented group (70% vs. 48%, P = 0.04). Kaplan-Meyer analysis for arthroscopy-free survival showed significant reduction in the number of performed arthroscopies in the PRP augmented group. A notably higher percentage of patients treated with PRP achieved minimal clinically significant difference in visual analogue scale (VAS) and Knee injury and Osteoarthritis Outcome Score (KOOS) symptom scores. Our trial indicates that percutaneous meniscal trephination augmented with PRP results in a significant improvement in the rate of chronic meniscal tear healing and this procedure decreases the necessity for arthroscopy in the future (8% vs. 28%, P = 0.032).
Keywords: meniscus, meniscus repair, meniscus tear, trephination, platelet-rich plasma, PRP, chronic meniscal lesion, horizontal meniscal tear
1. Introduction
The menisci are known to play a pivotal role during normal functioning of the knee joint. Their unique and complex chondral structure, as well as their biology, make treatment and repair very challenging. The menisci increase joint stability, distribute load, absorb shock and provide lubrication and nutrition to the remaining joint elements.
Meniscal tears are considered the most common orthopaedic diagnoses. For many years, arthroscopy was regarded as a “gold standard” in therapy with almost 4 million arthroscopies for meniscus pathologies performed annually all over the world, thus representing a serious socio-economic concern with relevant Health Care System costs [1]. Interestingly, more than 50% of these surgeries are conducted in patients older than 45 years with degenerative meniscal lesions [2]. This type of injury is a slowly progressing phenomenon, typically involving horizontal cleavage of the meniscal body with prevalence in the population reaching up to 56%. Interestingly, 61% of those tears have no clinical symptoms of meniscal pathology (pain, aching, stiffness or oedema) [2]. These data provided the background for studies analysing the efficacy of arthroscopy in chronic meniscal lesion therapy. Several randomized clinical trials were performed and demonstrated no additional benefit of partial meniscectomy to sham surgery [3]. These data introduced doubt into the current practice and resulted in making clinical decisions more challenging. Additionally, meniscectomy or partial meniscectomy results in rapid deterioration of articular cartilage and the development of arthritis [4]. Despite the trend of meniscus tear repair and maintaining as much vital tissue as possible [5] there is an inability amongst surgeons to restore anatomical and functional roles of the repaired meniscus. Simultaneously, osteoarthritis progressively develops. These rationales shifted the treatment protocols of chronic meniscal tears into the non-operative manner and motivated the search for new therapeutic strategies.
There are several clinical trials that have provided evidence for the use of blood or bone marrow derived products in the surgical treatment of meniscal pathology: the fibrin clot technique [6,7], platelet-rich plasma (PRP) [8,9] or the bone marrow venting procedure [10,11]. There is, however, no data in the literature evaluating the effect of blood derived products on healing of chronic meniscal tears. Thus we designed a prospective, randomized, double-blind, parallel-group, placebo-controlled study to investigate the effectiveness and safety of minimally invasive (percutaneous) intrameniscal PRP application to complement repair of a symptomatic chronic meniscal lesion. We hypothesized that intrameniscal injection of PRP with concomitant meniscal trephination would result in both an improved healing rate and better functional outcomes.
2. Results
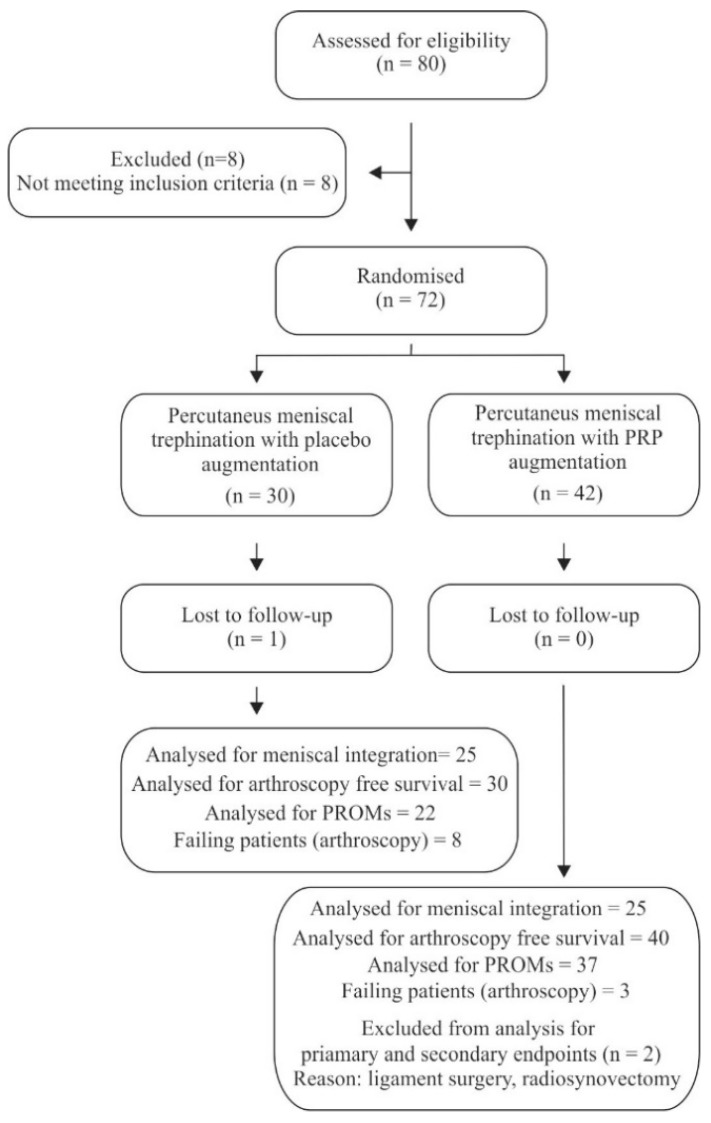
Follow-up ended on 15 January 2019. The median follow-up lasted for 92 weeks (54–157 weeks). 1 patient was lost to follow-up and 2 additional patients were excluded from analysis due to additional procedures (ligament surgery and radio synovectomy) (Figure 1). All remaining patients were functionally assessed at 3, 6, 12 months after the initial procedure. Patients undergoing arthroscopy due to unacceptable quality of life were excluded from analysis of the PROMs. There were no significant differences in baseline characteristics between the groups (Table 1).
Figure 1.
Flow diagram of the trial.
Table 1.
Baseline characteristics of study patients in the control and PRP-treated groups.
| Control Group (n = 30) | PRP-Treated Group (n = 42) | P-Value | |
|---|---|---|---|
| Age (years) | 46 (27–68) | 44 (18–67) | P = 0.31 |
| Sex (M:F) | 19:11 | 22:20 | P = 0.25 |
| BMI (range) | 28 (21–36) | 27 (19–37) | P = 0.27 |
| Kellgren-Lawrence scale (0 grade:1 grade:2 grade) | 23:7:0 | 30:12:0 | P = 0.79 |
| PRP (PLT × 103/μL) | 732 (220–1586) | 823 (320–1659) | P = 0.16 |
| Meniscus (MM:ML) | 30:0 | 41:1 | P = 0.58 |
Data are presented as median (range) or mean ± standard error (confidence interval (CI) 95%) unless otherwise indicated. BMI, body mass index; PRP, platelet rich plasma; PLT, platelets; MM—medial meniscus; ML—lateral meniscus.
2.1. Primary Outcome
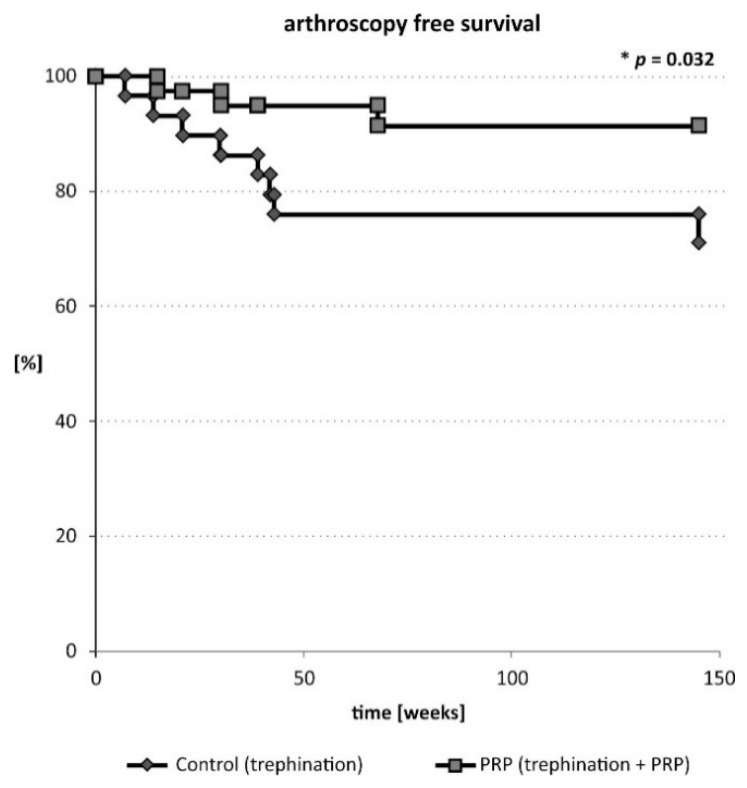
Assessment of meniscal healing on MR arthrography was performed at week 33 (13–78) in both groups (Table 2). Induction of the healing process within the meniscus was observed. The healing rate of the meniscal tear, although not significant, was superior in the PRP augmented percutaneous trephination repair group (11 fully and 4 partially healed menisci out of 25 assessed, 60%) than in the control group (7 fully and 4 partially healed menisci out of 26 assessed). When considering cumulative failure rate (arthroscopy and arthrography MRI), the success ratio was significantly better in PRP augmented percutaneous trephination group (P = 0.04) (Table 2). In case of 10 patients (8 in the control group and 2 in the PRP augmented group) subsequent arthroscopic meniscectomy or meniscal repair was performed due to unacceptable clinical symptoms. The survival of the PRP injected meniscus (arthroscopy free survival) was superior versus the control group (P = 0.032, Figure 2). No significant influence of the number of injected platelets or fold increase in the number of platelets in PRP on meniscal healing was detected.
Table 2.
Primary outcome assessment.
| Cumulative Outcome (Assessed Using MRI and Arthroscopy) (P = 0.04) | ||
|---|---|---|
| Outcome | PRP-treated group (n of menisci) | Control group (n of menisci) |
| Healed | 10 | 5 |
| Partially healed | 4 | 3 |
| Failed | 13 | 19 |
| MRI (P = 0.41) | ||
| Outcome | PRP-treated group (n of menisci) | Control group (n of menisci) |
| Healed | 11 | 7 |
| Partially healed | 4 | 4 |
| Failed | 10 | 15 |
MRI, magnetic resonance imaging; PRP, platelet-rich plasma.
Figure 2.
Arthroscopy free survival of patients undergoing trephination of the meniscus with or without PRP augmentation.
2.2. Secondary Outcomes-Pain
Baseline pain characteristics (VAS and KOOS-pain) of the patients did not differ significantly between groups (Table 3). All patients presented an improvement in pain scores. The changes in VAS and KOOS-pain exceeded minimal clinically important difference (MCID) value in majority of patients (Table 4). We detected a significant difference level in the percentage of patients who benefited by at least MCID in VAS score (39% vs. 65%, P = 0.046). No other significant changes were detected.
Table 3.
Patient-reported outcome measures (pain: VAS and KOOS-pain; function: IKDC, WOMAC, KOOS: symptom, ADL, sport/recreation and QOL).
| Control Group | PRP Group | ||||
|---|---|---|---|---|---|
| PROM | Pre-Procedure | Post Trephination | Pre-Procedure | Post Trephination | P a |
| VAS | 4.40 ± 0.07 (3.55–5.25) | 2.05 ± 0.08 (1.27–2.82) | 5.38 ± 0.05 (4.77–5.99) | 1.97 ± 0.05 (1.40–2.55) | 0.39 |
| IKDC | 54.92 ± 0.54 (49.08–60.77) | 88.12 ± 0.89 (79.97–96.28) | 51.99 ± 0.34 (47.62–56.36) | 85.98 ± 0.52 (79.79–92.16) | 0.36 |
| WOMAC | 28.93 ± 0.61 (22.42–35.45) | 7.50 ± 0.59 (2.06–12.94) | 34.36 ± 0.35 (29.90–38.82) | 9.72 ± 0.32 (5.95–13.48) | 0.21 |
| KOOS | |||||
| Pain | 65.30 ± 0.54 (59.51–71.10) | 89.00 ± 0.63 (83.19–94.81) | 57.48 ± 0.30 (57.18–57.78) | 87.24 ± 0.36 (82.99–91.48) | 0.22 |
| Symptoms | 69.86 ± 0.62 (63.18–76.54) | 90.42 ± 0.56 (85.26–95.58) | 63.53 ± 0.39 (63.23–63.83) | 92.03 ± 0.27 (88.80–95.26) | 0.27 |
| ADL | 68.42 ± 0.66 (61.33–75.50) | 92.38 ± 0.61 (86.80–97.95) | 63.70 ± 0.37 (63.40–64.00) | 89.36 ± 0.36 (85.07–93.64) | 0.25 |
| S/R | 33.50 ± 0.62 (26.84–40.16) | 78.98 ± 1.10 (68.83–89.12) | 35.83 ± 0.51 (35.53–36.14) | 69.52 ± 0.77 (60.29–78.74) | 0.11 |
| QoL | 35.00 ± 0.49 (29.73–40.27) | 68.18 ± 1.08 (58.28–78.08) | 37.90 ± 0.26 (37.59–38.20) | 67.06 – 0.55 (60.56–73.56) | 0.42 |
a For the control group vs. PRP group; Data are presented as mean ± standard error (CI 95%) unless otherwise indicated. PROM, patient related outcome measures; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; ADL, activities of daily living; S/R, sport/recreation; QOL, quality of life.
Table 4.
Patient-reported outcome measures (pain: VAS and KOOS-pain; function: IKDC, WOMAC, KOOS: symptom, ADL, sport/recreation and QOL).
| Control Group | PRP Group | ||||||
|---|---|---|---|---|---|---|---|
| PROM | MCID | Mean Change | Improved by at Least MCID [%] | Mean Change | Improved by at Least MCID [%] | P a | P b |
| VAS | 2 [12] | 2.36 ± 0.0.09 (3.86–5.20) | 39 | 3.62 ± 0.07 (2.82–4.43) | 65 | 0.027 | 0.046 |
| IKDC | 16.7 [13] | 33.66 ± 0.84 (25.95–41.36) | 83 | 34.74 ± 0.55 (28.17–41.31) | 78 | 0.48 | 0.48 |
| WOMAC | 11.5 [14] | 21.77 ± 0.67 (15.65–27.90) | 65 | 24.77 ± 0.37 (20.40–29.14) | 86 | 0.16 | 0.053 |
| KOOS | |||||||
| Pain | 16.7 [13] | 24.95 ± 0.62 (19.24–30.66) | 65 | 29.50 ± 0.45 (24.18–34.81) | 73 | 0.17 | 0.36 |
| Symptoms | 17.4 [13] | 18.38 ± 0.82 (10.81–25.95) | 48 | 27.93 ± 0.42 (22.89–32.96) | 76 | 0.016 | 0.028 |
| ADL | 18.4 [13] | 24.61 ± 0.74 (17.79–31.43) | 57 | 26.27 ± 0.39 (21.67–30.87) | 76 | 0.18 | 0.1 |
| S/R | 12.5 [13] | 43.75 ± 1.12 (33.43–54.07) | 83 | 34.65 ± 0.76 (25.57–43.74) | 70 | 0.12 | 0.22 |
| QoL | 15.6 [13] | 32.67 ± 1.06 (22.93–42.41) | 70 | 28.43 ± 0.52 (22.23–34.64) | 76 | 0.29 | 0.41 |
a For mean changes; b for % of patients improved by at least MCID. Data are presented as mean ± standard error (CI 95%) unless otherwise indicated. PROM, patient related outcome measures; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; ADL, activities of daily living; S/R, sport/recreation; QOL, quality of life; MCID, Minimal Clinically Important Difference.
2.3. Secondary Outcomes-Function
Functional outcomes were measured with the IKDC subjective scale, WOMAC and the KOOS subscales (symptoms, function in daily living [ADL], sport/recreation and knee related quality of life [QOL]). Each parameter improved over time in both groups, exceeding the MCID values in vast majority of patients. A significant difference in the percentage of patients who benefited by at least the MCID value in the KOOS Symptoms subscale was detected (48% vs. 76%, P = 0.028). We noted that the remaining KOOS subscales, IKDC score and WOMAC score were improved in both groups (Table 3 and Table 4).
2.4. Complications
No peri- or post- procedure complications were noted among patients who participated in the final follow-up.
3. Discussion
Meniscal healing has always been a major challenge for orthopaedic surgeons. All types of meniscectomies can lead to an increase in the risk of osteoarthritis [15] and evidence comparing the results of total and partial meniscectomy provide data on the beneficial effects of meniscus preservation [16]. The rising problem in meniscal injury treatment is the substantial number of chronic meniscal lesions. Recent studies comparing non-operative and arthroscopic treatment showed no benefit of surgical treatment in large cohorts of patients [3,17]. Data provided by the European Society of Sports Traumatology, Knee Surgery and Arthroscopy [18] or the guidelines published in the British Medical Journal [19] showed no or poor clinical benefit of arthroscopy in the case of degenerative meniscal lesions. In fact, arthroscopy was titled “the last resort” of treatment and applicable due to failure of conservative management.
The most significant finding of this study was that percutaneous trephination with or without a PRP boost induced the healing response of chronic meniscus tears. The process was augmented in the PRP – treated group. Interestingly, our results also demonstrated that no full meniscal integrity is necessary to obtain a clinically important difference in respect to PROMs. Additionally, we found that the functional outcomes (KOOS Symptoms) and pain levels (VAS) scored higher in patients treated with PRP-augmentation than in the control group.
For this study we used leukocyte- and platelet-rich plasma (L-PRP). Its fluid like state enables delivery to the target site by needle injection. Once activated, L-PRP forms a gel and releases most of the growth factors in the first few hours post injection until fully dissolved within 3 days [20]. It supports growth factors to act as an assembly of platelets and leukocytes in a complex matrix. Although leukocyte and platelet rich fibrin (L-PRF), was shown to slowly release growth factors over a period of about 7 days [21] providing optimal kinetics of a release, it forms a 3D matrix that cannot be delivered via a minimally invasive way (e.g., intra-articular injection)
PRP has been shown to influence not only the process of meniscal healing in vitro and in vivo [22,23] but also the treatment of other musculoskeletal injuries [24,25]. Some evidence has been provided for the use of PRP in meniscal repair [8,9]. The authors found that clinical outcomes and healing rates were better with the introduction of PRP into the lesion at the end of surgery. Griffin et al. performed a retrospective chart review with a minimum of 2 year follow-up and failed to show any benefit of PRP augmentation [26]. However, the study was underpowered for the primary and secondary outcomes. Another Study by Strümper, R. et al. demonstrated that intra-articular autologous conditioned serum injection might be an effective treatment option for knee pain associated with meniscal lesions [27]. The authors showed that surgery was avoided during the 6-month observation period and the Oxford Knee Score improved significantly from 29.1–44.3 in 83% of patients. Interestingly, the structural findings on MRI, measured by Boston Leeds Osteoarthritis Knee Score, also showed significant improvement. The limitations of the study were its retrospective character and lack of control group analysis. We believe that an additional weak point of this study was connected to not addressing perimeniscal capillary plexus (PCP) while performing the joint injection. Trephination is a known technique usually employed during arthroscopy [28,29]. It involves the formation of vascular access channels from the meniscus periphery (PCP) to the tear. This process initiates bleeding into the meniscal lesion and subsequent tissue repair response. This simple technique was showed to increase the meniscal healing rate while applied during a surgical procedure [30], most probably by providing the injury site with both growth factors and mesenchymal stem cells.
The results of experimental studies support the hypothesis that PRP may improve meniscal healing through activation of fibrochondrocytes present within the meniscus [31]. The process also involves the activity of mesenchymal stem cells, which seem to be necessary for the repair of meniscal lesions [23]. The PRP itself releases the “cytokine cocktail” of the healing cascade [25]. The main growth factors are: platelet derived growth factor, platelet derived endothelial growth factor, vascular endothelial growth factor, insulin like growth factor, platelet derived angiogenesis factor, transforming growth factor-b, hepatocyte growth factor and others [32]. This release initiates the chemotaxis of immunocompetent cells, inflammation, angiogenesis and as a consequence the process of synthesis of the extracellular matrix and tissue remodelling. The PRP works at various levels for joint homeostasis. Studies have shown that PRP application decreases catabolism while increasing anabolic activity and observations have been made that catabolic activity in meniscus chondral tissue helps identify patients who are at risk for progression of osteoarthritis [33]. Other processes, such as chondral remodelling is promoted by PRP administration. Higher production of collagen II, matrix molecules and prostaglandin has been observed in hyaline cartilage [34,35]. On the contrary, Lee et al. showed on a rabbit model of a circular meniscal defect that PRP treatment failed to enhance the production of meniscus cartilage. Additionally, it accelerated fibrosis and increased catabolic processes [36]. However, findings from in vivo and in vitro studies cannot be directly translated to clinical practice.
Increasing data provide evidence for the necessity of mesenchymal stem cells in delivering the positive effect of PRP on healing of meniscal and hyaline cartilage defects [23,37] and the process of chondrocyte differentiation [38]. PRP has been shown to enhance proliferation of stromal stem cells [39] as well as their adhesion and migration [40]. This phenomenon is probably dependent on the release of a growth factor cocktail and triggering of synovial tissue to create a more balanced intra-articular environment. Recent studies link the synovium-derived stem cells to chondral regeneration, as they possess chondrogenic potential and encouraging results have been shown for cartilage repair purposes in experimental studies [41].
We hypothesize, that trephination, by creating multiple wounds and inducing intrameniscal bleeding, starts the process of tissue repair with activation of synovial and blood derived stem cells, which—in our study—are stimulated by addition of PRP. The combination of those two processes allows for efficient meniscal tissue regeneration.
3.1. Strengths
This is the first study to employ percutaneous trephination of a chronic meniscal lesion with or without PRP augmentation. The second strength is the study design itself, the randomized and blinded nature of this study and being adequately powered to detect differences in healing rates. Lastly, independent evaluators were used for assessing of the outcomes.
3.2. Limitations
We acknowledge some limitations in this study. The study group was small, increasing the risk of type II error. Additionally, some patients refused MRI arthrography due to its interventional character, still their comfort of life improved significantly. Also, calculation of the primary outcome might have been influenced by factors that could affect MRI images and their interpretation. There is also the issue of heterogeneity within groups. Localization of the tear in medial or lateral compartments may influence the primary outcome, as the biology of those menisci might differ. We find no statistically significant differences between these groups but in the literature the results are mixed [42]. Additionally, PROMs data have partially overlapping 95% confidence intervals, increasing the risk of type II error. Moreover, it is still unknown which of the factors are solely responsible for the improved outcomes in the PRP group. The rehabilitation protocol was uniform in all patients but we could not control those differences that might have occurred in patients being treated in multiple outpatient centres. Lastly, the observation period in this study allowed only for a short-term analysis.
4. Materials and Methods
4.1. Trial Design and Informed Consent
This was a parallel-group, superiority trial with equal randomization. The study protocol was approved by an appropriate Institutional Review Board and was publicly accessible before enrolment of the first patient. We performed the study in accordance with the ethical standards outlined in the 2013 revision of 1975 Declaration of Helsinki and we report the results according to the 2010 CONSORT statement. The potential benefits and risks of meniscal trephination, PRP injection and follow-up were explained to each study patient. All patients provided written informed consent for participation in this study and no patient declined to participate. Clinical Trial Registration: The study protocol was approved by Bioethics Committee at Centre of Postgraduate Medical Education (36/PB/2013 approved on 29.05.2013) and was publicly accessible before enrolment of the first participant. The clinical trial databases at cmkp.edu.pl-36/PB/2013, clinicaltrials.gov-NCT03066583.
4.2. Eligibility Criteria
Patients were recruited from a single public knee clinic at a tertiary care, university health centre between 2016 and 2018 (Figure 1). 72 patients with chronic (horizontal) meniscal lesions were enrolled: 30 were randomized to undergo percutaneous trephination (control group) and 42 were randomized to undergo percutaneous trephination with PRP injection at the repair site. Detailed inclusion and exclusion criteria are presented in Table 5.
Table 5.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| skeletally mature patients aged 18–70 years chronic horizontal tears on MRI tear located in the vascular/avascular portion of the meniscus single tear of the medial and/or lateral meniscus |
arthritic changes (Kellgren-Lawrence scale >2) discoid meniscus axial leg deformity (valgus > 6 deg)- concomitant chondral defects (> 2 ICRS) Inflammatory diseases (rheumatoid arthritis) chondral defects above ICRS 2 on MRI |
MRI, magnetic resonance imaging.
4.3. PRP and Thrombin Preparation
PRP and its activator (thrombin) was prepared as in Reference [9]. In this study we used Red-l-PRPIIB-1 according to the new classification system [43]. PRP was prepared by a dedicated laboratory assistant in the BL2 facility. Briefly, the PRP preparation procedure involved drawing of 120 mL of venous blood and centrifuging the blood using a refrigerated centrifuge in a two-step process. First, the PRP layer was isolated, including a “buffy coat” and a small fraction of underlying red blood cells (900 rpm × 9 min). Additional centrifugation and isolation of PRP was then applied (3200 rpm × 15 min). The preparation was packed into sterile vials labelled with the patient ID. In the study group, 6–8 mL of PRP solution was used, while in the control group, 6–8 mL of sterile 0.9% saline was applied. Right before application, PRP was activated using 20 mM CaCl2 (Teva, Basel, Israel) and 25 IU/mL autologous thrombin. It was then injected into the tear site of the meniscus with a double chamber syringe. Platelets and leukocyte concentration were assessed for each sample.
4.4. Procedures
All procedures were performed by the same senior orthopaedic surgeon under ultrasound guidance (R.K.) in the outpatient department. In brief, the PRP or control solution was prepared as described above. Local anaesthetic was used. After identification of a horizontal tear via ultrasound, the needle was introduced into the tear lesion (passing through the PCP, red zone, red-white zone and white zone) with continuous injection of studied solutions (starting while in the PCP). 5–10 separate needle introductions through all layers were performed. After discharge, patients were referred to outpatient physiotherapy units and encouraged to follow a unified rehabilitation protocol. In short, all patients wore a hinged knee brace for 4 weeks. Exercises with a range of motion from 0 to 90 degrees for 6 weeks were encouraged. Weight bearing as tolerated was allowed - beginning from day 1. Early quadricep muscle activation was initiated. At 6 weeks post procedure, a low-resistance stationary bicycle and one-quarter body weight leg presses were initiated. Additional increases in low-impact knee exercises were permitted as tolerated starting at 12 weeks post procedure.
4.5. Outcomes
The primary outcome was meniscus healing assessed using 1.5T magnetic resonance imaging (MRI) arthrography with a dedicated knee coil (Siemens, Erlangen, Germany). Meniscus healing was evaluated by two independent attending radiology consultants, who were blinded to the patient allocation. We did not notice any intra-observer bias. Complete healing was considered when full meniscus integrity was noted during MR arthrography (no intrameniscal contrast media). Partial healing was considered with contrast media filling a defect between 1–3 mm. Healing failure was considered when contrast media was detected within the meniscal body. Additionally, failure was defined as performing arthroscopic meniscectomy or meniscal repair. Arthroscopy free survival was analysed.
Secondary outcomes (patient reported outcome measures–PROMS) included pain assessment with the visual analogue scale (VAS) and functional outcome assessment with the Knee injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and International Knee Documentation Committee Subjective Knee Evaluation (IKDC) [12,44,45]. All secondary outcomes were assessed before the procedure and at 3, 6, 12, 24 months post injection. Minimally clinical important difference (MCID) was assessed for PROMs [13,14,46,47]. Patients were closely monitored for complications. There were no changes to the protocol during study duration.
4.6. Randomization
The randomization list for allocating patients to the study groups was generated using the “simple randomization” function on the StatSoft GraphPad QuickCalcs web site (http://www.graphpad.com/quickcalcs) [48]. We used sequentially numbered, opaque, sealed envelopes to conceal the allocation. Patients were consecutively enrolled and assigned to the study groups. Intervention assignment was performed during PRP preparation.
4.7. Blinding
The patients, the data collectors and the assessors were blinded to the intervention type.
4.8. Statistical Analysis
We used the R statistical package (www.rproject.org) for statistical analyses [49]. Differences in meniscus healing rates were assessed through analysis of a contingency table using Fisher’s exact test. All categorical data were analysed using Fisher’s exact test. The VAS score, KOOS, WOMAC and IKDC score were analysed using the two-tailed Mann-Whitney U test or unpaired t-test (after assessment for parametric or non-parametric distribution using the Shapiro-Wilk test) [50]. Arthroscopy-free survival was analysed using Kaplan Meyer plot and log-rank testing for statistical significance. Results were considered statistically significant at a P-value < 0.05. Sample size was calculated for the primary outcome (meniscus healing), with a two-tailed significance level at alpha = 0.05 and beta = 0.8, assuming a difference in the meniscus healing rate of 15% between the study groups according to the method described in Reference [51] and based on previous studies [52,53]. Minimum recruitment level was estimated to be 28 patients per group. Assuming an attrition or non-compliance rate of 10% during the study, we aimed to recruit at least 30 patients per group.
5. Conclusions
Our blinded, prospective, randomized, controlled trial on the role of PRP and percutaneous trephination of the chronically torn meniscal tissue indicates that percutaneous trephination of the meniscal tissue is an effective technique improving meniscal integrity as well as PROMs. The augmentation of this technique with PRP results in a significant improvement in the rate of meniscal healing (52% vs. 30%, P = 0.04). Importantly, this simple procedure seems to decrease the necessity for arthroscopy in the future (8% vs. 28%, P = 0.032). This study showed that PRP augmentation could provide significant and clinically important benefits. Further studies in this field are encouraged. The risk of adverse events related to percutaneous trephination with augmentation with PRP is very low.
Author Contributions
Conceptualization, R.K. and K.K.-K.; Data curation, R.K. and K.K.-K.; Formal analysis, R.K. and S.P.; Funding acquisition, R.K. and S.P.; Investigation, R.K., M.M.-W., K.K. and A.D.-T.; Methodology, R.K., K.K.-K., A.D.-T. and S.P.; Project administration, M.M.-W.; Resources, R.K., M.M.-W. and A.D.-T.; Software, R.K.; Supervision, R.K.; Validation, R.K., M.M.-W., K.K.-K. and A.D.-T.; Visualization, R.K.; Writing—original draft, R.K. and K.K.-K.; Writing—review & editing, R.K., M.M.-W., K.K., K.K.-K. and S.P.
Funding
This research was funded by Postgraduate Center for Medical Education Grant, grant number 501–1–07–18–14.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hawker G. Knee Arthroscopy in England and Ontario: Patterns of Use, Changes Over Time and Relationship to Total Knee Replacement. J. Bone Jt. Surg. Am. 2008;90:2337. doi: 10.2106/JBJS.G.01671. [DOI] [PubMed] [Google Scholar]
- 2.Englund M., Guermazi A., Gale D., Hunter D.J., Aliabadi P., Clancy M., Felson D.T. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N. Engl. J. Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sihvonen R., Paavola M., Malmivaara A., Itälä A., Joukainen A., Nurmi H., Kalske J., Järvinen T.L.N. Arthroscopic Partial Meniscectomy versus Sham Surgery for a Degenerative Meniscal Tear. N. Engl. J. Med. 2013;369:2515–2524. doi: 10.1056/NEJMoa1305189. [DOI] [PubMed] [Google Scholar]
- 4.Fairbank T.J. Knee joint changes after meniscectomy. J. Bone Jt.Surg. Br. 1948;30B:664–670. doi: 10.1302/0301-620X.30B4.664. [DOI] [PubMed] [Google Scholar]
- 5.Noyes F.R., Barber-Westin S.D. Repair of complex and avascular meniscal tears and meniscal transplantation. J. Bone Jt.Surg. Am. 2010;92:1012–1029. [PubMed] [Google Scholar]
- 6.Henning C.E., Lynch M.A., Yearout K.M., Vequist S.W., Stallbaumer R.J., Decker K.A. Arthroscopic meniscal repair using an exogenous fibrin clot. Clin. Orthop. 1990:64–72. doi: 10.1097/00003086-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Van Trommel M.F., Simonian P.T., Potter H.G., Wickiewicz T.L. Arthroscopic meniscal repair with fibrin clot of complete radial tears of the lateral meniscus in the avascular zone. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 1998;14:360–365. doi: 10.1016/S0749-8063(98)70002-7. [DOI] [PubMed] [Google Scholar]
- 8.Pujol N., Tardy N., Boisrenoult P., Beaufils P. Long-term outcomes of all-inside meniscal repair. Knee Surg. Sports Traumatol. Arthrosc. 2015;23:219–224. doi: 10.1007/s00167-013-2553-5. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski R., Kulinski K., Kozar-Kaminska K., Wielgus M., Langner M., Wasko M.K., Kowalczewski J., Pomianowski S. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Evaluating Meniscal Healing, Clinical Outcomes and Safety in Patients Undergoing Meniscal Repair of Unstable, Complete Vertical Meniscal Tears (Bucket Handle) Augmented with Platelet-Rich Plasma. BioMed Res. Int. 2018;2018:9315815. doi: 10.1155/2018/9315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminski R., Kulinski K., Kozar-Kaminska K., Wasko M.K., Langner M., Pomianowski S. Repair augmentation of unstable, complete vertical meniscal tears with bone marrow venting procedure. A prospective, randomized, double-blind, parallel-group, placebo-controlled study. Arthrosc. J. Arthrosc. Relat. Surg. 2018;2018:9315815. [Google Scholar]
- 11.Ahn J.-H., Kwon O.-J., Nam T.-S. Arthroscopic Repair of Horizontal Meniscal Cleavage Tears With Marrow-Stimulating Technique. Arthrosc. J. Arthrosc. Relat. Surg. 2015;31:92–98. doi: 10.1016/j.arthro.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Katz N.P., Paillard F.C., Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J. Orthop. Surg. 2015;10:24. doi: 10.1186/s13018-014-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris J.D., Brand J.C., Cote M.P., Faucett S.C., Dhawan A. Research Pearls: The Significance of Statistics and Perils of Pooling. Part 1: Clinical Versus Statistical Significance. Arthrosc. J. Arthrosc. Relat. Surg. 2017;33:1102–1112. doi: 10.1016/j.arthro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Greco N.J., Anderson A.F., Mann B.J., Cole B.J., Farr J., Nissen C.W., Irrgang J.J. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System and Short Form 36 in patients with focal articular cartilage defects. Am. J. Sports Med. 2010;38:891–902. doi: 10.1177/0363546509354163. [DOI] [PubMed] [Google Scholar]
- 15.Delos D., Rodeo S.A. Enhancing meniscal repair through biology: Platelet-rich plasma as an alternative strategy. Instr. Course Lect. 2011;60:453–460. [PubMed] [Google Scholar]
- 16.Paxton E.S., Stock M.V., Brophy R.H. Meniscal Repair Versus Partial Meniscectomy: A Systematic Review Comparing Reoperation Rates and Clinical Outcomes. Arthrosc. J. Arthrosc. Relat. Surg. 2011;27:1275–1288. doi: 10.1016/j.arthro.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 17.Katz J.N., Brophy R.H., Chaisson C.E., de Chaves L., Cole B.J., Dahm D.L., Donnell-Fink L.A., Guermazi A., Haas A.K., Jones M.H., et al. Surgery versus Physical Therapy for a Meniscal Tear and Osteoarthritis. N. Engl. J. Med. 2013;368:1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaufils P., Becker R., Kopf S., Englund M., Verdonk R., Ollivier M., Seil R. Surgical management of degenerative meniscus lesions: The 2016 ESSKA meniscus consensus. Knee Surg. Sports Traumatol. Arthrosc. 2017;25:335–346. doi: 10.1007/s00167-016-4407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siemieniuk R.A.C., Harris I.A., Agoritsas T., Poolman R.W., Brignardello-Petersen R., Van de Velde S., Buchbinder R., Englund M., Lytvyn L., Quinlan C., et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: A clinical practice guideline. BMJ. 2017;357:j1982. doi: 10.1136/bmj.j1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crisci A., Crescenzo U.D., Crisci M. Platelet-rich concentrates (L-PRF, PRP) in tissue regeneration: Control of apoptosis and interactions with regenerative cells. J. Clin. Mol. Med. 2018;1:1–2. doi: 10.15761/JCMM.1000116. [DOI] [Google Scholar]
- 21.Dohan Ehrenfest D.M., Bielecki T., Jimbo R., Barbé G., Del Corso M., Inchingolo F., Sammartino G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) Curr. Pharm. Biotechnol. 2012;13:1145–1152. doi: 10.2174/138920112800624382. [DOI] [PubMed] [Google Scholar]
- 22.Ishida K., Kuroda R., Miwa M., Tabata Y., Hokugo A., Kawamoto T., Sasaki K., Doita M., Kurosaka M. The Regenerative Effects of Platelet-Rich Plasma on Meniscal Cells In Vitro and Its In Vivo Application with Biodegradable Gelatin Hydrogel. Tissue Eng. 2007;13:1103–1112. doi: 10.1089/ten.2006.0193. [DOI] [PubMed] [Google Scholar]
- 23.Zellner J., Mueller M., Berner A., Dienstknecht T., Kujat R., Nerlich M., Hennemann B., Koller M., Prantl L., Angele M., et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J. Biomed. Mater. Res. A. 2010;94:1150–1161. doi: 10.1002/jbm.a.32796. [DOI] [PubMed] [Google Scholar]
- 24.Andia I., Maffulli N. New biotechnologies for musculoskeletal injuries. Surg. J. R. Coll. Surg. Edinb. Irel. 2018 doi: 10.1016/j.surge.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Andia I., Maffulli N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regen. Med. 2018;13:717–728. doi: 10.2217/rme-2018-0042. [DOI] [PubMed] [Google Scholar]
- 26.Griffin J.W., Hadeed M.M., Werner B.C., Diduch D.R., Carson E.W., Miller M.D. Platelet-rich Plasma in Meniscal Repair: Does Augmentation Improve Surgical Outcomes? Clin. Orthop. Relat. Res. 2015;473:1665–1672. doi: 10.1007/s11999-015-4170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strümper R. Intra-Articular Injections of Autologous Conditioned Serum to Treat Pain from Meniscal Lesions. Sports Med. Int. Open. 2017;1:E200–E205. doi: 10.1055/s-0043-118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doral M.N., Bilge O., Huri G., Turhan E., Verdonk R. Modern treatment of meniscal tears. EFORT Open Rev. 2018;3:260–268. doi: 10.1302/2058-5241.3.170067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghazi Zadeh L., Chevrier A., Farr J., Rodeo S.A., Buschmann M.D. Augmentation Techniques for Meniscus Repair. J. Knee Surg. 2018;31:99–116. doi: 10.1055/s-0037-1602247. [DOI] [PubMed] [Google Scholar]
- 30.Fox J.M., Rintz K.G., Ferkel R.D. Trephination of incomplete meniscal tears. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 1993;9:451–455. doi: 10.1016/S0749-8063(05)80321-4. [DOI] [PubMed] [Google Scholar]
- 31.Tumia N.S., Johnstone A.J. Platelet derived growth factor-AB enhances knee meniscal cell activity in vitro. The Knee. 2009;16:73–76. doi: 10.1016/j.knee.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Cozma C.N., Raducu L., Jecan C.R. Platelet Rich Plasma- mechanism of action and clinical applications. J. Clin. Investig. Surg. 2016;1:41–46. doi: 10.25083/2559.5555.12.16. [DOI] [Google Scholar]
- 33.Brophy R.H., Rai M.F., Zhang Z., Torgomyan A., Sandell L.J. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J. Bone Jt.Surg. Am. 2012;94:385–393. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon M.S., Patel S., John R. PRP in OA knee—Update, current confusions and future options. SICOT-J. 2017;3:27. doi: 10.1051/sicotj/2017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira R.C., Scaranari M., Benelli R., Strada P., Reis R.L., Cancedda R., Gentili C. Dual Effect of Platelet Lysate on Human Articular Cartilage: A Maintenance of Chondrogenic Potential and a Transient Proinflammatory Activity Followed by an Inflammation Resolution. Tissue Eng. Part A. 2013;19:1476–1488. doi: 10.1089/ten.tea.2012.0225. [DOI] [PubMed] [Google Scholar]
- 36.Lee H.-R., Shon O.-J., Park S.-I., Kim H.-J., Kim S., Ahn M.-W., Do S.H. Platelet-Rich Plasma Increases the Levels of Catabolic Molecules and Cellular Dedifferentiation in the Meniscus of a Rabbit Model. Int. J. Mol. Sci. 2016;17:120. doi: 10.3390/ijms17010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haleem A.M., Singergy A.A.E., Sabry D., Atta H.M., Rashed L.A., Chu C.R., Shewy M.T.E., Azzam A., Aziz M.T.A. The Clinical Use of Human Culture–Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. CARTILAGE. 2010;1:253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeyakumar V., Niculescu-Morzsa E., Bauer C., Lacza Z., Nehrer S. Redifferentiation of Articular Chondrocytes by Hyperacute Serum and Platelet Rich Plasma in Collagen Type I Hydrogels. Int. J. Mol. Sci. 2019;20:316. doi: 10.3390/ijms20020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucarelli E., Beccheroni A., Donati D., Sangiorgi L., Cenacchi A., Del Vento A.M., Meotti C., Bertoja A.Z., Giardino R., Fornasari P.M., et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. doi: 10.1016/S0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 40.Rubio-Azpeitia E., Sánchez P., Delgado D., Andia I. Adult Cells Combined with Platelet-Rich Plasma for Tendon Healing: Cell Source Options. Orthop. J. Sports Med. 2017;5:232596711769084. doi: 10.1177/2325967117690846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubosch E.J., Lang G., Furst D., Kubosch D., Izadpanah K., Rolauffs B., Sudkamp N.P., Schmal H. The Potential for Synovium-derived Stem Cells in Cartilage Repair. Curr. Stem Cell Res. Ther. 2018;13:174–184. doi: 10.2174/1574888X12666171002111026. [DOI] [PubMed] [Google Scholar]
- 42.Dean C.S., Chahla J., Matheny L.M., Mitchell J.J., LaPrade R.F. Outcomes After Biologically Augmented Isolated Meniscal Repair with Marrow Venting Are Comparable with Those After Meniscal Repair With Concomitant Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2017;45:1341–1348. doi: 10.1177/0363546516686968. [DOI] [PubMed] [Google Scholar]
- 43.Harrison P. The Subcommittee on Platelet Physiology The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018;16:1895–1900. doi: 10.1111/jth.14223. [DOI] [PubMed] [Google Scholar]
- 44.Roos E.M., Roos H.P., Lohmander L.S., Ekdahl C., Beynnon B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 45.Bellamy N. WOMAC: A 20-year experiential review of a patient-centered self-reported health status questionnaire. J. Rheumatol. 2002;29:2473–2476. [PubMed] [Google Scholar]
- 46.Crawford K., Briggs K.K., Rodkey W.G., Steadman J.R. Reliability, validity and responsiveness of the IKDC score for meniscus injuries of the knee. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2007;23:839–844. doi: 10.1016/j.arthro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Roos E.M., Lohmander L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011;4:8. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- 50.Malavolta E.A., Gracitelli M.E.C., Ferreira Neto A.A., Assuncao J.H., Bordalo-Rodrigues M., de Camargo O.P. Platelet-Rich Plasma in Rotator Cuff Repair: A Prospective Randomized Study. Am. J. Sports Med. 2014;42:2446–2454. doi: 10.1177/0363546514541777. [DOI] [PubMed] [Google Scholar]
- 51.Van der Tweel I., Askie L., Vandermeer B., Ellenberg S., Fernandes R.M., Saloojee H., Bassler D., Altman D.G., Offringa M., van der Lee J.H., et al. Standard 4: Determining adequate sample sizes. Pediatrics. 2012;129(Suppl. S3):S138–S145. doi: 10.1542/peds.2012-0055G. [DOI] [PubMed] [Google Scholar]
- 52.Stein T., Mehling A.P., Welsch F., von Eisenhart-Rothe R., Jäger A. Long-term outcome after arthroscopic meniscal repair versus arthroscopic partial meniscectomy for traumatic meniscal tears. Am. J. Sports Med. 2010;38:1542–1548. doi: 10.1177/0363546510364052. [DOI] [PubMed] [Google Scholar]
- 53.Järvelä S., Sihvonen R., Sirkeoja H., Järvelä T. All-Inside Meniscal Repair with Bioabsorbable Meniscal Screws or with Bioabsorbable Meniscus Arrows: A Prospective, Randomized Clinical Study with 2-Year Results. Am. J. Sports Med. 2010;38:2211–2217. doi: 10.1177/0363546510374592. [DOI] [PubMed] [Google Scholar]