Abstract
The meeting between Rumi and Shams, in the 13th century, was a turning point in the life of Rumi leading to a revolutionary effect in his thoughts, ideas, and poems. This was an ever-inspiring meeting with many results throughout the centuries. This meeting has created some footprints in cellular and molecular medicine: The discovery of two distinct genes in Drosophila, i.e. Rumi and Shams and their role in controlling Notch signaling, which has a critical role in cell biology. This nomination and the interactions between the two genes has led us to a number of novel studies during the last years. This article reviews the interactions between Rumi and Shams and their effects on Notch signaling in order to find potential novel drugs for pain control through drug development studies in the future.
Keywords: Notch Signaling, Pain, Shams, Rumi, Drosophila, Drug Development
1. Context
Historical introduction: Rumi or Molana (Jalal-ad-Din Mohammad Balkhi) was a 13th century poet with all of his quotes in modern Farsi (1). Many of his citations deal with pain and how to alleviate pain through love; also quoting virtual metaphors for pain alleviations including love, Sufism, wine, and opium (2). According to related stories, there was a turning point in his life after a meeting with Shams, another Persian Sufi. This meeting revolutionized his life in all mental and ideological aspects; leading to a new era in his life and its division to two epochs of his life: pre-meeting and post-meeting.
2. Cellular Aspects of Rumi and Shams Interactions
2.1. Notch Signaling and Its Importance
As a single-pass transmembrane receptor, Notch is the mediator for cell-cell interactions with an essential role in cell fate, especially throughout development. In multi-cellular organisms, Notch signaling is a matter of communication between neighboring cells in order to pave a proper developmental pathway (Figure 1); talking in brief, local cell-cell communication is the downstream result of proper Notch signaling process (3). Addition of xylose and glucose residues to Notch receptors affect Notch signaling; the former impedes and the latter enhances the process of the effects of Notch signaling and its aftermath could be described as the following:
Figure 1. Notch signaling pathway in detail; for more explanations please see the text, including genetic control of Notch signaling; please note that γ-secretase complex is the location where DAPT acts; also, NICD stand for Notch Intracellular Domain.
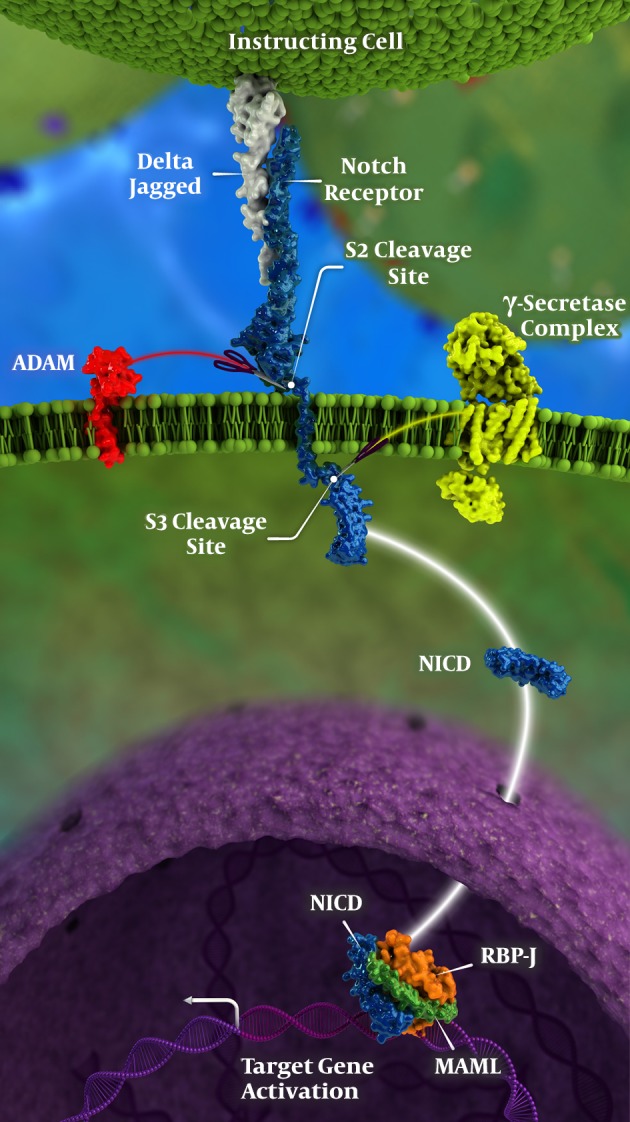
- Notch signaling pathway (including glycosylated Notch proteins) has an evolutionary conserved track, with a major role in many cell functions; including but not limited to (4-8)
(1) Final cell fate
(2) Development
(3) Vasculogenesis
(4) Tumorigenesis
(5) Immunological interactions
(6) Learning and memory
- Misregulations in Notch signaling causes a number of cell abnormalities and/or disease states.
- Using different molecules in controlling Notch signaling may be potential solutions for finding treatments in some disease states (3, 9-13).
- Mastermind-like (MAML) superfamily, which are transcriptional coactivators, are essential nuclear elements that support Notch activity; among them MAML1 is the most important one (Figure 1) (14, 15).
- Recombination signal binding protein for immunoglobulin kappa J region (i.e. known as an abbreviation: RBP-J) is "A major transcriptional effector of Notch signaling" with a great control on the whole Notch signaling pathway, especially during cell differentiation and neuronal maturation (16-18) (Figure 1).
2.2. Notch Receptor and Its Structure
In mammals Notch receptor is composed of four subtypes: Notch 1, Notch 2, Notch 3, and Notch 4, while canonical ligands of Notch receptors are five transmembrane proteins (Delta-like1,3,4 and Jagged 1 and 2); however, in Drosophila, Notch is encoded as a single transmembrane receptor (19-22).
As demonstrated in Figure 1, Notch receptors consist of these segments (23):
- An extracellular component consisted of 29 - 36 epidermal growth factor (EGF) repeats, three cysteine rich LIN repeats, and a heterodimerization domain (HD) that attached to the next segment non-covalently at the S2 cleavage site; ADAM (standing for "A Disintegrin and Metalloproteinase") could cut the S2 cleavage site.
- An integral transmembrane protein with a short extracellular part (HD) extended throughout the cell membrane towards the intracellular area.
2.2.1. Rumi
POGLUT1 also known as Rumi is a protein O-glucosyltransferase and is an abbreviation for "protein O-glucosyltransferase 1" [Homo sapiens (human)]; mutations in Drosophila Rumi have a temperature-sensitive Notch phenotype, its target is the extracellular domain of Notch and has "18 target sites on Notch for Notch signaling". Most importantly, Rumi is an important gene in controlling Notch signaling through O-glucosylation of Notch epidermal growth factor-like (EGF) domains (4, 24, 25). This means that Rumi encodes an O-glucosyltransferase while the latter attaches glucose sugars to serine residues in EGF domains of the extracellular region of Notch; this effect by Rumi modifies Notch signaling (20). O-glycosylation might be the modulator of stability in EGF repeats and is essential for Notch activity, through "transferring glucose and xylose to the EGF domains of Notch and other signaling receptors". Extracellular domain of Notch is "The target of Rumi" (Figure 1) (24). In Drosophila, "multiple O-glucose residues serve as a buffer against temperature-dependent loss of Notch signaling" (26); in this way, POGLUT1 regulates Notch signaling and cellular Notch trafficking (11, 12, 19). To explain more, O-glucose residues promote Notch signaling, with xylosylation, which is an important step in completion of this process. These events happen in the secretory pathway of the cell. O-glucose and O-fucose are added to target proteins like Notch inside the endoplasmic reticulum (ER).
Loss of Rumi results in temperature-sensitive loss of Notch signaling in flies; in other words, in flies, Rumi is considered as a temperature-sensitive regulator of Notch signaling (27). It is known that regulation of Notch activity is controlled by tissue-specific alterations in the glycan structures (19, 28-32).
2.2.2. Shams
In Drosophila, glucoside xylosyltransferase Shams has been shown to be responsible for xylosylation EGF-like repeats (9, 11, 25). Lee et al. demonstrated that in Drosophila, glucoside xylosyltransferase Shams affects the xylosylation leading to selective impediment of binding of Notch receptor with trans-Delta, while cis-ligand is unaffected; thus, Shams has important roles in developmental aspects of Drosophila (33). However, xylose residues have negative regulatory effects on surface expression of EGF 16 - 20 regulation (9). Xylosylation occurs in the Golgi and then Notch goes to the cell surface.
2.3. Meeting Between Rumi and Shams
Rumi and Shams encode two Drosophila enzymes that involved the regulation of the Notch signaling pathway in flies. It has been well shown that Rumi homologs in mice and humans (called POGLUT1) are both involved in the regulation of Notch signaling as well, although the details of how the mammalian homologs regulate signaling are not necessarily the same as Drosophila. In addition, studies have shown that if you overexpress one of the human Shams homologs (GXYLT1) and its downstream enzyme UDP-xylose:α-xyloside α1,3-xylosyltransferase (XXYLT1) in flies, phenotypes compatible with loss of Notch signaling will be observed (34-39). However, evidence from the mammalian system has not been published on these enzymes yet. Of note, human XXYLT1 shows gene amplification in a number of cancers, some of which are associated with decreased Notch signaling (9). Therefore, it is possible that in agreement with the currently available Drosophila data, human XXYLT1 overexpression in humans decreases Notch signaling and thereby promote cancer formation or some of the developmental disorders (37, 38, 40, 41). It seems that the role of Rumi, as a temperature-sensitive regulator of Notch signaling, is exerted after its glycosylation and extended through Shams, and therefore, modulating the latter effects of Shams in Notch signaling.
3. Clinical Aspects of the Meeting Between Rumi and Shams
Given the roles identified for Rumi and Shams in regulation of Notch signaling in animal models, mutations in these genes might contribute to human diseases involving altered Notch signaling; meanwhile, mutations in Notch receptors and other components of the Notch signaling pathway may lead to a number of diseases (42, 43). On the other hand, there may be hopes to treat some diseases by blocking Notch receptors. However, due to widespread effects of Notch signaling, a thorough consideration of its effects on different organ systems is needed before assessment of its effects on pain management.
Is there currently any hope to treat some disease by blocking Notch receptors? In other words, is there any means to use antagonists of Notch signaling pathway as therapeutic agents? The potential answer is yes. Several different strategies for blocking Notch signaling are being tested or developed for treating human diseases. These include small molecules that inhibit Notch receptor cleaver and/or antibodies that bind Notch and prevent its activation. There are side effects, therefore, researchers try to come up with strategies that instead of blocking the pathway in an all-or-none fashion, only inhibit specific aspects of signaling.
Here, we review in brief, the potential role of Notch signaling pathways and its interactions on pain control; a brief look at the role of Notch signaling on organ system diseases is presented in Box 1.
Box 1. A Review on the Role of Notch Signaling on Organ System Diseases.
| Review on the Role of Notch Signaling on Organ System Diseases |
|---|
| Central nervous system (CNS) |
| There are studies demonstrating that manipulating the Notch pathway leads to alterations in many aspects of CNS function, including acute and/or chronic pain. Rusanescu and Mao demonstrated that Notch3 knocked out mice have permanent changes in their nociceptive neurons resembling chronic pain states while all other aspects of their neurologic system were normal (44). |
| In Alzheimer's disease, there is a ligand-induced Notch pathway activation; which is presenilin-mediated; in this process, presenilin has a critical role process in this process; there are also potential pathways proposed to treat or to prevent Alzheimer's disease through manipulation of interactions between Notch, presenilin, and the amyloid precursor protein (APP) (45, 46). |
| Cardiovascular system disorders |
| Notch signaling is essential in lymphatic valve formation and development (47, 48). On the other hand, Notch signaling has antagonistic effects in the process of aortic valve differentiation, leading to development of bone-like cells; having a role in enhancing and accelerating of calcification, and abnormal morphogenesis of the aortic valve including in bicuspid aortic valve; in addition, in the aneurysm of the thoracic aorta and congenital Marfan syndrome (5, 48-53). |
| Musculoskeletal system |
| Paradas and colleagues recently reported a missense mutation in human POGLUT1 (Rumi) in patients with a new type of limb-girdle muscular dystrophy (54); further studies have opened new windows towards treatment of the disease through manipulating effects of Rumi on Notch signaling (30). |
| Cancer treatment |
| Rumi mutations are identified in cancer (55). Yu et al. discussed the essential role of Rumi in Notch signaling and stated that any dysregulation of Rumi is in association with several disease states in human beings; while "loss of Rumi activity" may have a role in some diseases; if these mechanisms are well recognized, Notch signaling pathway may be modulated by Rumi (55). Studies similar to the latter help us improve our pathway by modulating Notch signaling using Rumi in order to seek new treatment options for some clinical conditions including modulating cancer cells and cancer formation, especially in pancreatic, breast, lung cancer, renal cell carcinoma, and T-cell acute lymphoblastic leukemia; in addition, in acute or chronic pain management, these disease processes are involved with impaired Notch signaling pathways (40, 41, 56-58). Inhibition of ADAM 17 expression (a member of the ADAM superfamily) could inhibit Notch pathway in renal cell carcinoma with a therapeutic potential (59). Furthermore, inhibition of MAML might be a potential treatment in cancer through Notch signaling inhibition (14, 15). |
| Congenital cholangiopathy |
| Thakurdas et al. found that Rumi has a role in Alagille syndrome (an autosomal-dominant congenital cholangiopathy) through opposing Notch ligand JAG1; i.e. Rumi opposes JAG1 function in mice liver (60). |
| Retinitis pigmentosa |
| EYS produces a great number of EGF and is pressed out in retina (the photoreceptor layer), in patients with autosomal recessive retinitis pigmentosa. Rumi has specific targets on EYS and enhances the proper development of EYS shut in photoreceptor development (61-64). |
| Rare genetic disorders |
| There are some rare disorders that are the result of Rumi activity loss like Dowling-Degos disease (55). |
4. Activation of Notch Signaling Pathway and Its Implications in Pain Management: A New Hope for Treatment of Pain
During the last years, a number of studies have demonstrated the role of Notch signaling in pathogenesis and maintenance of pain. In addition, there are also complementary studies that have found new windows for modulating pain in the clinical field; these studies suggest a promise through notch signaling pathway for treatment of pain; some major controversies exist yet. A number of these studies are briefed here. These studies mainly raise questions dealing with some answers in order to find new treatments.
4.1. Role of Notch Intracellular Domain (NICD) in Pain Management
Sun et al. suggested "A pivotal role for Notch signaling pathway in development of neuropathic pain" (65); they studied the following aspects of neuropathic pain:
- Increased excitability (66)
- Decreased thresholds of primary sensory neurons (67, 68)
- Change in the processing pathway of the spinal cord synaptic functions (67)
- Impaired function of inhibitory interneurons or loss of their network (69, 70)
- Modifications of brain stem input to the spinal cord (71)
- Loss of cortical inhibition (72)
Sun et al. also found overexpression of Notch Intracellular Domain (NICD; Figure 1) in "DRG (Dorsal Root Ganglia), sciatic nerve, and spinal cord" in normal rats and suggested a therapeutic option for alleviating neuropathic pain; i.e. they used intrathecal DAPT (which is a γ-secretase inhibitor; Figure 1) and found promising results for neuropathic pain when this pain was induced by spared sciatic nerve injury. This approach could be a potential promise in treatment of neuropathic pain (65). In another somewhat similar study, Yang et al. demonstrated the effects of "minocycline combined with DAPT" in treatment of neuropathic pain (73).
On the other hand, "proteolytic processing of Notch receptors and their ligands" is among the main roles of ADAM superfamily (ADAM standing for "A Disintegrin And Metalloproteinase") (74). Inhibition of ADAM 17 expression (a member of the ADAM superfamily) could inhibit Notch pathway even more effectively than γ-secretase inhibitors in renal cell carcinoma; this pathway might also serve as a potential window for pain control (Figure 1) (59).
In addition,, Xie et al. found that if notch signaling pathway was activated in rats, mechanical allodynia was induced and maintained more severely; they concluded suppression of this pathway could be a promise for treatment of neuropathic pain (75). In another study on lumbar spinal dorsal horn, Xie et al. found very similar results where they administered intrathecal DAPT (notch signaling inhibitor) before nerve injury and found decreased occurrence of mechanical pain after injury; in addition, they found intrathecal Jagged-1 (JAG-1) peptide effective for treatment of chronic pain after sciatic nerve injury (Figure 1). Therefore, they concluded their results as a promising window and a new target in treatment of neuropathic pain (76).
In summary, according to a number of studies, including the above, the following findings could be among the potential mechanisms for future works finding possible treatments of pain by using Notch signaling pathways (65, 73, 75, 76):
(1) Overexpression of Notch Intracellular Domain (NICD) occurs in nerve roots and spinal cord through the role of intrathecal DAPT (γ-secretase inhibitor) after nerve injury; there may be the potential that overexpression of NICD could serve as a means for treatment of neuropathy.
(2) The effect mechanism of some agents (e.g. minocycline) combined with DAPT may be helpful in treatment of neuropathic pain.
(3) Intrathecal Jagged-1 (JAG-1) peptide may be an effective alternative for treatment of chronic pain.
(4) The role of members of the ADAM superfamily in inhibition of Notch pathway.
4.2. Notch Signaling and Progranulin
Progranulin is a secretory protein and a growth factor, which is cysteine-rich and consists of "seven-and-a-half tandem repeats of the granulin/epithelin module". It has a number of significant functions in many normal and pathological processes; for example, it has been mainly studied in pathophysiology of neurodegenerative diseases of CNS (77-80). Lim et al. found that after neuronal injury, progranulin up-regulation could attenuate neuropathic pain while motor function recovery would be enhanced; they confessed that improvement in survival of damaged neurons and/or augmented regrowth of neuronal cells might be the underlying mechanism for their finding (81).
On the other hand, Altmann et al. demonstrated that inducible progranulin overexpression leads to a number of nerve recovery after nerve injury for example, axonal growth and sensory and motor blocks. They concluded that progranulin-induced activation of Notch signaling pathway could enhance the recovery process of partially injured neurons, leading to increased neuronal regenerative capacity, a finding in support of the progranulin-Notch crosstalk hypothesis; they also discovered progranulin as a requirement for perpetuation of Notch1 expression and as an activator for Notch receptors. Meanwhile, they found that increased levels of progranulin result in "enhanced Notch-dependent gene transcription". Finally, based on their study, Notch signaling pathway could be a promising therapeutic pathway in future of neuropathic pain management (82). Hardt et al. suggested that progranulin deficiency may contribute to chronic neuropathic pain (83). Some evidence suggest a promise in acute or chronic pain management (65, 82-86), while Notch signaling is considered as functional receptors for progranulin (GRN) and involved in neurotrophic effects of GRN; progranulin is a functional ligand of Notch (85, 87). In summary, the role of progranulin in Notch signaling pathway is a potential route for treatment of pain.
4.3. Notch Signaling, Immune System, and Pain
Notch signaling has a key role in cell development and cell fate in many subtypes of the immune system cells and has applications in treatment of the immunological disorders; however, many of these cells affect the pathogenesis, induction, and maintenance of neuropathic pain and this window could be highly promising as the new therapeutic model for treatment of pain (22, 88-91). However, Notch signaling has a dynamic response towards neuronal activity. Alberi et al. found that Notch regulation of "neuronal morphology, synaptic plasticity, learning, and memory" are all affected by Notch signaling (92). In addition, Wang et al. demonstrated "increased expression of selective Notch receptors (Notch 1 and 2), ligand (JAGGED2), and target genes" in nucleus pulposus of the intervertebral discs treated with specific inflammatory cytokines. In addition, they found that Notch genes have "dysregulated expression in degenerative disc disease" and they conclude that controlling inflammatory cytokines affecting Notch signaling pathway controls disc disease (93).
5. Conclusions
Shams modulates Rumi in cellular interactions leading to regulation of Notch signaling; however; this process has a great number of opportunities for potential future therapeutic approaches in management of neuropathic pain. Here, we reviewed the many different segments in the Notch signaling process as potential therapeutic options in pain management; future studies considering "drug development and drug delivery" could help us use these proposed segments in creating novel drugs.
Is there a chance that we could use "the meeting between Shams and Rumi" as a new era in pain management; the same as meeting between Rumi and Shams created a new era in Rumi's life, dividing "pain management history" into two epochs as the meeting divided Rumi's life into two completely different epochs: pre-meeting and post-meeting? A question to be answered yet.
Acknowledgments
We appreciate Dr Hamed Jafar-Nejad, MD, Associate Professor, Molecular and Human Genetics, Baylor College of Medicine, TX, USA for his help and inspiring comments on the manuscript.
Footnotes
Authors' Contribution:Samira Rajaei helped design the study and write the manuscript. Yousef Fatahi helped write the manuscript and create the figure. Ali Dabbagh helped create the idea, search the literature, performe the study, and write the manuscript.
Conflicts of Interests:None declared.
Funding/Support:None declared.
Contributor Information
Samira Rajaei, Email: samirarajaei@yahoo.com.
Yousef Fatahi, Email: youseffatahi@gmail.com.
Ali Dabbagh, Email: alidabbagh@yahoo.com.
References
- 1.Lewis FD. Rumi-past and present, east and west: The life, teachings, and poetry of Jalâl Al-Din Rumi. Oneworld Publications; 2014. [Google Scholar]
- 2.Dabbagh A, Rajaei S, Golzari SE. History of anesthesia and pain in old Iranian texts. Anesth Pain Med. 2014;4(3):e15363. doi: 10.5812/aapm.15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovall RA, Gebelein B, Sprinzak D, Kopan R. The canonical Notch signaling pathway: Structural and biochemical insights into shape, sugar, and force. Dev Cell. 2017;41(3):228–41. doi: 10.1016/j.devcel.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley P. Glucose: A novel regulator of Notch signaling. ACS Chem Biol. 2008;3(4):210–3. doi: 10.1021/cb800073x. [DOI] [PubMed] [Google Scholar]
- 5.McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt T3. Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134(2):290–6. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(2):93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 7.Pakkiriswami S, Couto A, Nagarajan U, Georgiou M. Glycosylated Notch and cancer. Front Oncol. 2016;6:37. doi: 10.3389/fonc.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M, Biswas S, Qin X, Gong W, Deng W, Yu H. Does Notch play a tumor suppressor role across diverse squamous cell carcinomas? Cancer Med. 2016;5(8):2048–60. doi: 10.1002/cam4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TV, Sethi MK, Leonardi J, Rana NA, Buettner FF, Haltiwanger RS, et al. Negative regulation of Notch signaling by xylose. PLoS Genet. 2013;9(6):e1003547. doi: 10.1371/journal.pgen.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker H, Gerardy-Schahn R. A sweet development in Notch regulation. J Biol Chem. 2017;292(38):15974–5. doi: 10.1074/jbc.H117.800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Fischer M, Satkunarajah M, Zhou D, Withers SG, Rini JM. Structural basis of Notch O-glucosylation and O-xylosylation by mammalian protein-O-glucosyltransferase 1 (POGLUT1). Nat Commun. 2017;8(1):185. doi: 10.1038/s41467-017-00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi H, Yu H, Hao H, Takeuchi M, Ito A, Li H, et al. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J Biol Chem. 2017;292(38):15964–73. doi: 10.1074/jbc.M117.800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawaguchi S, Varshney S, Ogawa M, Sakaidani Y, Yagi H, Takeshita K, et al. O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. Elife. 2017;6 doi: 10.7554/eLife.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saint Just Ribeiro M, Wallberg AE. Transcriptional mechanisms by the coregulator MAML1. Curr Protein Pept Sci. 2009;10(6):570–6. doi: 10.2174/138920309789630543. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa M. Notch signalling in the nucleus: Roles of Mastermind-like (MAML) transcriptional coactivators. J Biochem. 2016;159(3):287–94. doi: 10.1093/jb/mvv123. [DOI] [PubMed] [Google Scholar]
- 16.Plos Genetics Staff Correction: RBPJ, the major transcriptional effector of Notch signaling, remains associated with chromatin throughout mitosis, suggesting a role in mitotic bookmarking. PLoS Genet. 2016;12(7):e1006209. doi: 10.1371/journal.pgen.1006209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lake RJ, Tsai PF, Choi I, Won KJ, Fan HY. RBPJ, the major transcriptional effector of Notch signaling, remains associated with chromatin throughout mitosis, suggesting a role in mitotic bookmarking. PLoS Genet. 2014;10(3):e1004204. doi: 10.1371/journal.pgen.1004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanigaki K, Honjo T. Two opposing roles of RBP-J in Notch signaling. Curr Top Dev Biol. 2010;92:231–52. doi: 10.1016/S0070-2153(10)92007-3. [DOI] [PubMed] [Google Scholar]
- 19.Ishio A, Sasamura T, Ayukawa T, Kuroda J, Ishikawa HO, Aoyama N, et al. O-fucose monosaccharide of Drosophila Notch has a temperature-sensitive function and cooperates with O-glucose glycan in Notch transport and Notch signaling activation. J Biol Chem. 2015;290(1):505–19. doi: 10.1074/jbc.M114.616847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irvine KD. A notch sweeter. Cell. 2008;132(2):177–9. doi: 10.1016/j.cell.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, et al. A novel proteolytic cleavage involved in Notch signaling: The role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5(2):207–16. doi: 10.1016/S1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 22.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32(1):14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 24.Acar M, Jafar-Nejad H, Takeuchi H, Rajan A, Ibrani D, Rana NA, et al. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. 2008;132(2):247–58. doi: 10.1016/j.cell.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonardi J, Jafar-Nejad H. Structure-function analysis of Drosophila Notch using genomic rescue transgenes. Methods Mol Biol. 2014;1187:29–46. doi: 10.1007/978-1-4939-1139-4_3. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi J, Fernandez-Valdivia R, Li YD, Simcox AA, Jafar-Nejad H. Multiple O-glucosylation sites on Notch function as a buffer against temperature-dependent loss of signaling. Development. 2011;138(16):3569–78. doi: 10.1242/dev.068361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee TV, Takeuchi H, Jafar-Nejad H. Regulation of notch signaling via O-glucosylation insights from Drosophila studies. Methods Enzymol. 2010;480:375–98. doi: 10.1016/S0076-6879(10)80017-5. [DOI] [PubMed] [Google Scholar]
- 28.Acar M, Mettetal JT, van Oudenaarden A. Stochastic switching as a survival strategy in fluctuating environments. Nat Genet. 2008;40(4):471–5. doi: 10.1038/ng.110. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Valdivia R, Takeuchi H, Samarghandi A, Lopez M, Leonardi J, Haltiwanger RS, et al. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development. 2011;138(10):1925–34. doi: 10.1242/dev.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Hunt SD, Matthias N, Servian-Morilla E, Lo J, Jafar-Nejad H, et al. Generation of an induced pluripotent stem cell line (CSCRMi001-A) from a patient with a new type of limb-girdle muscular dystrophy (LGMD) due to a missense mutation in POGLUT1 (Rumi). Stem Cell Res. 2017;24:102–5. doi: 10.1016/j.scr.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey BM, Rana NA, Moss H, Leonardi J, Jafar-Nejad H, Haltiwanger RS. Mapping sites of O-Glycosylation and fringe elongation on drosophila Notch. J Biol Chem. 2016;291(31):16348–60. doi: 10.1074/jbc.M116.732537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana NA, Haltiwanger RS. Fringe benefits: Functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr Opin Struct Biol. 2011;21(5):583–9. doi: 10.1016/j.sbi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee TV, Pandey A, Jafar-Nejad H. Xylosylation of the Notch receptor preserves the balance between its activation by trans-Delta and inhibition by cis-ligands in Drosophila. PLoS Genet. 2017;13(4):e1006723. doi: 10.1371/journal.pgen.1006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Takeuchi M, LeBarron J, Kantharia J, London E, Bakker H, et al. Notch-modifying xylosyltransferase structures support an SNi-like retaining mechanism. Nat Chem Biol. 2015;11(11):847–54. doi: 10.1038/nchembio.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breton C, Fournel-Gigleux S, Palcic MM. Recent structures, evolution and mechanisms of glycosyltransferases. Curr Opin Struct Biol. 2012;22(5):540–9. doi: 10.1016/j.sbi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Hurtado-Guerrero R, Davies GJ. Recent structural and mechanistic insights into post-translational enzymatic glycosylation. Curr Opin Chem Biol. 2012;16(5-6):479–87. doi: 10.1016/j.cbpa.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: Structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–55. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 38.Sethi MK, Buettner FF, Ashikov A, Krylov VB, Takeuchi H, Nifantiev NE, et al. Molecular cloning of a xylosyltransferase that transfers the second xylose to O-glucosylated epidermal growth factor repeats of notch. J Biol Chem. 2012;287(4):2739–48. doi: 10.1074/jbc.M111.302406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sethi MK, Buettner FF, Krylov VB, Takeuchi H, Nifantiev NE, Haltiwanger RS, et al. Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats. J Biol Chem. 2010;285(3):1582–6. doi: 10.1074/jbc.C109.065409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefort K, Ostano P, Mello-Grand M, Calpini V, Scatolini M, Farsetti A, et al. Dual tumor suppressing and promoting function of Notch1 signaling in human prostate cancer. Oncotarget. 2016;7(30):48011–26. doi: 10.18632/oncotarget.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carvalho FL, Simons BW, Eberhart CG, Berman DM. Notch signaling in prostate cancer: A moving target. Prostate. 2014;74(9):933–45. doi: 10.1002/pros.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97(4):1235–94. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 43.Louvi A, Artavanis-Tsakonas S. Notch and disease: A growing field. Semin Cell Dev Biol. 2012;23(4):473–80. doi: 10.1016/j.semcdb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rusanescu G, Mao J. Notch3 is necessary for neuronal differentiation and maturation in the adult spinal cord. J Cell Mol Med. 2014;18(10):2103–16. doi: 10.1111/jcmm.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fortini ME. Notch and presenilin: A proteolytic mechanism emerges. Curr Opin Cell Biol. 2001;13(5):627–34. doi: 10.1016/S0955-0674(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 46.Selkoe D, Kopan R. Notch and Presenilin: Regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–97. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 47.Choi D, Park E, Jung E, Seong YJ, Yoo J, Lee E, et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J Clin Invest. 2017;127(4):1225–40. doi: 10.1172/JCI87442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng X, Cha B, Mahamud MR, Srinivasan RS. Intraluminal valves: Development, function and disease. Dis Model Mech. 2017;10(11):1273–87. doi: 10.1242/dmm.030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuda D, Aikawa M. Expanding role of delta-like 4 mediated notch signaling in cardiovascular and metabolic diseases. Circ J. 2013;77(10):2462–8. doi: 10.1253/circj.cj-13-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giusti B, Sticchi E, De Cario R, Magi A, Nistri S, Pepe G. Genetic bases of bicuspid aortic valve: The contribution of traditional and high-throughput sequencing approaches on research and diagnosis. Front Physiol. 2017;8:612. doi: 10.3389/fphys.2017.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandra R, Engeln M, Schiefer C, Patton MH, Martin JA, Werner CT, et al. Drp1 mitochondrial fission in D1 neurons mediates behavioral and cellular plasticity during early cocaine abstinence. Neuron. 2017;96(6):1327–1341 e6. doi: 10.1016/j.neuron.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acharya A, Hans CP, Koenig SN, Nichols HA, Galindo CL, Garner HR, et al. Inhibitory role of Notch1 in calcific aortic valve disease. PLoS One. 2011;6(11):e27743. doi: 10.1371/journal.pone.0027743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murtomaki A, Uh MK, Kitajewski C, Zhao J, Nagasaki T, Shawber CJ, et al. Notch signaling functions in lymphatic valve formation. Development. 2014;141(12):2446–51. doi: 10.1242/dev.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Servian-Morilla E, Takeuchi H, Lee TV, Clarimon J, Mavillard F, Area-Gomez E, et al. A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss. EMBO Mol Med. 2016;8(11):1289–309. doi: 10.15252/emmm.201505815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H, Takeuchi H, Takeuchi M, Liu Q, Kantharia J, Haltiwanger RS, et al. Structural analysis of Notch-regulating Rumi reveals basis for pathogenic mutations. Nat Chem Biol. 2016;12(9):735–40. doi: 10.1038/nchembio.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brzozowa-Zasada M, Piecuch A, Michalski M, Segiet O, Kurek J, Harabin-Slowinska M, et al. Notch and its oncogenic activity in human malignancies. Eur Surg. 2017;49(5):199–209. doi: 10.1007/s10353-017-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi A, Illendula A, Pulikkan JA, Roderick JE, Tesell J, Yu J, et al. RUNX1 is required for oncogenic Myb and Myc enhancer activity in T-cell acute lymphoblastic leukemia. Blood. 2017;130(15):1722–33. doi: 10.1182/blood-2017-03-775536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma W, Du J, Chu Q, Wang Y, Liu L, Song M, et al. hCLP46 regulates U937 cell proliferation via Notch signaling pathway. Biochem Biophys Res Commun. 2011;408(1):84–8. doi: 10.1016/j.bbrc.2011.03.124. [DOI] [PubMed] [Google Scholar]
- 59.Guo Z, Jin X, Jia H. Inhibition of ADAM-17 more effectively down-regulates the Notch pathway than that of gamma-secretase in renal carcinoma. J Exp Clin Cancer Res. 2013;32:26. doi: 10.1186/1756-9966-32-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakurdas SM, Lopez MF, Kakuda S, Fernandez-Valdivia R, Zarrin-Khameh N, Haltiwanger RS, et al. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi). Hepatology. 2016;63(2):550–65. doi: 10.1002/hep.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fahim AT, Daiger SP, Weleber RG. Nonsyndromic retinitis pigmentosa overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, editors. Gene Reviews®. Seattle: University of Washington; 1993. [PubMed] [Google Scholar]
- 62.Chen X, Liu X, Sheng X, Gao X, Zhang X, Li Z, et al. Targeted next-generation sequencing reveals novel EYS mutations in Chinese families with autosomal recessive retinitis pigmentosa. Sci Rep. 2015;5:8927. doi: 10.1038/srep08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonilha VL, Rayborn ME, Bell BA, Marino MJ, Pauer GJ, Beight CD, et al. Histopathological comparison of eyes from patients with autosomal recessive retinitis pigmentosa caused by novel EYS mutations. Graefes Arch Clin Exp Ophthalmol. 2015;253(2):295–305. doi: 10.1007/s00417-014-2868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alfano G, Kruczek PM, Shah AZ, Kramarz B, Jeffery G, Zelhof AC, et al. EYS Is a Protein Associated with the Ciliary Axoneme in Rods and Cones. PLoS One. 2016;11(11):e0166397. doi: 10.1371/journal.pone.0166397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun YY, Li L, Liu XH, Gu N, Dong HL, Xiong L. The spinal notch signaling pathway plays a pivotal role in the development of neuropathic pain. Mol Brain. 2012;5:23. doi: 10.1186/1756-6606-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: Implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8(1):1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 67.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishimura I, Thakor D, Lin A, Ruangsri S, Spigelman I. Frontiers in neuroscience molecular strategies for therapeutic targeting of primary sensory neurons in chronic pain syndromes. In: Kruger L, Light AR, editors. Translational pain research: From mouse to man. Boca Raton, FL: CRC Press/Taylor & Francis Llc.; 2010. [PubMed] [Google Scholar]
- 69.Tsuda M, Koga K, Chen T, Zhuo M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J Neurochem. 2017;141(4):486–98. doi: 10.1111/jnc.14001. [DOI] [PubMed] [Google Scholar]
- 70.Shetty AK, Bates A. Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer's and Parkinson's diseases. Brain Res. 2016;1638(Pt A):74–87. doi: 10.1016/j.brainres.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsieh TH, Lee HHC, Hameed MQ, Pascual-Leone A, Hensch TK, Rotenberg A. Trajectory of parvalbumin cell impairment and loss of cortical inhibition in traumatic brain injury. Cereb Cortex. 2017;27(12):5509–24. doi: 10.1093/cercor/bhw318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang C, Gao J, Wu B, Yan N, Li H, Ren Y, et al. Minocycline attenuates the development of diabetic neuropathy by inhibiting spinal cord Notch signaling in rat. Biomed Pharmacother. 2017;94:380–5. doi: 10.1016/j.biopha.2017.07.078. [DOI] [PubMed] [Google Scholar]
- 74.Zolkiewska A. ADAM proteases: Ligand processing and modulation of the Notch pathway. Cell Mol Life Sci. 2008;65(13):2056–68. doi: 10.1007/s00018-008-7586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie K, Jia Y, Hu Y, Sun Y, Hou L, Wang G. Activation of notch signaling mediates the induction and maintenance of mechanical allodynia in a rat model of neuropathic pain. Mol Med Rep. 2015;12(1):639–44. doi: 10.3892/mmr.2015.3379. [DOI] [PubMed] [Google Scholar]
- 76.Xie K, Qiao F, Sun Y, Wang G, Hou L. Notch signaling activation is critical to the development of neuropathic pain. BMC Anesthesiol. 2015;15:41. doi: 10.1186/s12871-015-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, et al. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 2008;17(4):711–24. doi: 10.1110/ps.073295308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bateman A, Bennett HP. The granulin gene family: From cancer to dementia. Bioessays. 2009;31(11):1245–54. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- 79.He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9(2):225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 80.Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89(5):1715–9. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim HY, Albuquerque B, Haussler A, Myrczek T, Ding A, Tegeder I. Progranulin contributes to endogenous mechanisms of pain defense after nerve injury in mice. J Cell Mol Med. 2012;16(4):708–21. doi: 10.1111/j.1582-4934.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Altmann C, Vasic V, Hardt S, Heidler J, Haussler A, Wittig I, et al. Progranulin promotes peripheral nerve regeneration and reinnervation: Role of notch signaling. Mol Neurodegener. 2016;11(1):69. doi: 10.1186/s13024-016-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardt S, Heidler J, Albuquerque B, Valek L, Altmann C, Wilken-Schmitz A, et al. Loss of synaptic zinc transport in progranulin deficient mice may contribute to progranulin-associated psychopathology and chronic pain. Biochim Biophys Acta Mol Basis Dis. 2017;1863(11):2727–45. doi: 10.1016/j.bbadis.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 84.Chitramuthu BP, Bennett HPJ, Bateman A. Progranulin: A new avenue towards the understanding and treatment of neurodegenerative disease. Brain. 2017;140(12):3081–104. doi: 10.1093/brain/awx198. [DOI] [PubMed] [Google Scholar]
- 85.Beel S, Moisse M, Damme M, De Muynck L, Robberecht W, Van Den Bosch L, et al. Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Hum Mol Genet. 2017;26(15):2850–63. doi: 10.1093/hmg/ddx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vasic V, Schmidt MHH. Resilience and vulnerability to pain and inflammation in the hippocampus. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schafer MKE, Tegeder I. NG2/CSPG4 and progranulin in the posttraumatic glial scar. Matrix Biol. 2018;68-69:571–88. doi: 10.1016/j.matbio.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Shang Y, Smith S, Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell. 2016;7(3):159–74. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakano T, Fukuda D, Koga J, Aikawa M. Delta-like ligand 4-Notch signaling in macrophage activation. Arterioscler Thromb Vasc Biol. 2016;36(10):2038–47. doi: 10.1161/ATVBAHA.116.306926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseno CG, Iwata A, et al. Transcriptional control of dendritic cell development. Annu Rev Immunol. 2016;34:93–119. doi: 10.1146/annurev-immunol-032713-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meng L, Hu S, Wang J, He S, Zhang Y. DLL4(+) dendritic cells: Key regulators of Notch signaling in effector T cell responses. Pharmacol Res. 2016;113(Pt A):449–57. doi: 10.1016/j.phrs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alberi L, Liu S, Wang Y, Badie R, Smith-Hicks C, Wu J, et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69(3):437–44. doi: 10.1016/j.neuron.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H, Tian Y, Wang J, Phillips KL, Binch AL, Dunn S, et al. Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem. 2013;288(23):16761–74. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]


