Abstract
Integrated enzyme production in the biorefinery can significantly reduce the cost of the entire process. The purpose of the present study is to evaluate the production of two hydrolyzing enzymes (amylase and xylanase) by an edible fungus used in the biorefinery, Neurospora intermedia. The enzyme production was explored through submerged fermentation of synthetic media and a wheat-based waste stream (thin stillage and wheat bran). The influence of a nitrogen source on N. intermedia was investigated and a combination of NaNO3 and yeast extract has been identified as the best nitrogen source for extracellular enzyme production. N. intermedia enzymes showed maximum activity at 65 °C and pH around 5. Under these conditions, the maximum velocity of amylase and xylanase for starch and xylan hydrolysis was found to be 3.25 U mL−1 and 14.77 U mL−1, respectively. Cultivation of N. intermedia in thin stillage and wheat bran medium resulted in relatively high amylase (8.86 ± 0.41 U mL−1, 4.68 ± 0.23) and xylanase (5.48 ± 0.21, 2.58 ± 0.07 U mL−1) production, respectively, which makes this fungus promising for enzyme production through a wheat-based biorefinery.
Keywords: amylase, xylanase, Neurospora intermedia, submerged fermentation, wheat-based biorefinery
1. Introduction
Amylases and xylanases are hydrolytic enzymes that contribute in saccharification processes to assist the hydrolysis of starch and xylan, respectively. Starch is the most common carbohydrate in the human and animal diet [1]. Xylan is also the second most abundant natural polysaccharide and the major structural component of plant cell walls [2]. Considering the vast application of starch and xylan in food, feed, textile, pulp and paper, brewing, juice, and wine industries, amylases and xylanases have received a great deal of attention, especially in these industries [1,3].
These enzymes can be obtained from animals, plants, and microorganisms. However, enzymes produced by microorganisms are more preferable to plant and animal based ones owing to their high yields and reliability, higher stability, possibility of product modification and optimization, economic feasibility, regular supply because of the absence of seasonal fluctuations, a rapid growth of microbes on low cost media, ease of cultivation in large fermenters, and greater catalytic activity [4]. In addition, enzyme production using microorganisms creates the possibility of on-site enzyme production by utilization of residual streams resulting in the biorefinery, which reduces the cost of the process.
A number of microbial sources including bacteria and fungi have been reported for amylase and xylanase production under the fermentation process [5,6]. Nevertheless, fungi-derived enzymes have obtained considerable attention due to the ease of cultivation, high production yields, and simultaneous biomass production [7]. Among fungi, specific interest has been expressed in a sort of edible fungi that can naturally synthesize and secrete enzymes. The application of edible fungi in enzyme production enhances the chance of the enzymes and biomass being free from contamination from mycotoxins (toxins of fungal origin [8]).
The ascomycete filamentous fungi Neurospora intermedia has conventionally been used in an indigenous Indonesian food—oncom—and categorized as ‘edible’ fungi. Neurospora intermedia is among the fastest growing of all filamentous fungi and has shown considerable growth in solutions containing starch and xylan [9,10]. This fungus has been used for the production of ethanol [10,11], protein-rich biomass [9], and pigment [8,12]. Nevertheless, research using N. intermedia for enzyme production is limited in the literature. The primary purpose of this study was to evaluate the potential of Neurospora intermedia to produce amylase and xylanase in liquid fermentation. The study is further extended to the characterization of these enzymes to explore their suitability for application in industry. To the best of our knowledge, this is the first report on amylase and xylanase production from N. intermedia in submerged fermentation.
2. Results and Discussion
N. intermedia is an edible fungus which grows fast and produces a high amount of protein-rich biomass. Considering these features, several investigations have been performed on this fungus for various purposes. Valorization of numerous industrial waste streams, such as straw and bran (lignocellulosic waste) [13,14,15], whole and thin stillage (starch-to-ethanol process waste) [9,10,11,16], vinasse (sugar-to-ethanol process waste) [17], and cream, cheese-whey, yoghurt and milk (dairy waste products) [18,19], along with production of ethanol [10,11], protein-rich fungal biomass for feed applications [10,11], and pigments [8,12], are samples of N. intermedia applications in various fields. Despite the great potential of this fungus for industrial applications, particularly in the biorefinery, the enzyme production potential of this fungus has not yet been investigated thoroughly. However, simultaneous enzyme production by this fungus can improve the yield and reduce the cost of the biorefinery. In this study, for the first time, production of two useful biorefinery enzymes—amylase and xylanase—by N. intermedia in submerged fermentation were investigated. The results are presented as follows.
2.1. Optimization of Enzyme Production in a Shake-Flask System
The microbial production of enzymes is significantly influenced by the components of the culture medium, especially the carbon and nitrogen sources. In addition, the nitrogen source can influence the pH of the medium, which may affect the enzyme activity and stability [20]. In order to investigate the influence of a nitrogen source on N. intermedia enzyme production, amylase and xylanase were produced in shake flasks containing medium supplemented with different nitrogen sources. No intracellular enzyme activity was detected. However, in the literature, intracellular amylase and xylanase production, by Aspergillus niger [21] and Neurospora crassa [22], respectively, were observed.
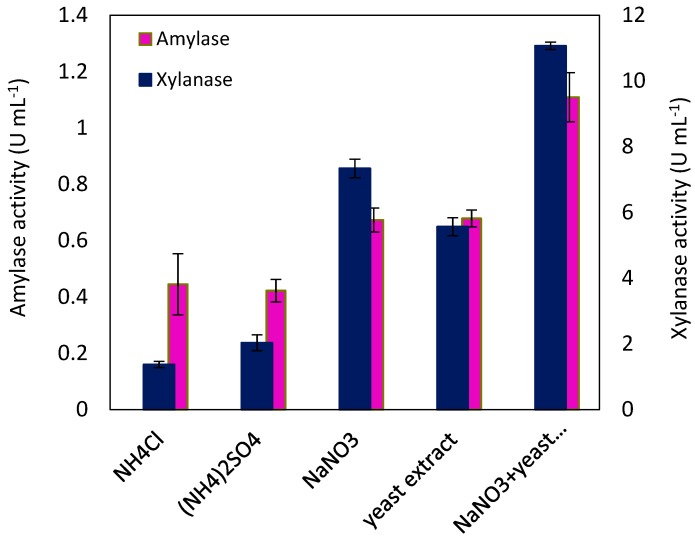
The maximum activity of extracellular enzymes in each medium was obtained after 48 h, as shown in Figure 1. An addition of NaNO3 to the basal medium resulted in the higher amylase and xylanase activity in comparison with the supplementation of the basal medium with NH4Cl or (NH4)2SO4. Higher enzyme activity observed in the medium containing NaNO3 may be rooted in two facts: either the amount of amylase and xylanase produced in this medium was higher than that in the media containing NH4Cl or (NH4)2SO4, or the enzymes produced in this medium had higher activity, which lies in the action of available ions as the enzyme cofactor.
Figure 1.
Effect of nitrogen sources on amylase and xylanase production by Neurospora intermedia, 10 g L−1 of single nitrogen sources, and 8 g L−1 NaNO3 and 2 g L−1 yeast extract in the combination of NaNO3 and yeast extract.
While yeast extract resulted in excessive growth of N. intermedia, it also resulted in lower enzyme activity, especially xylanase, which can probably be attributed to the presence of simple sugars in the yeast extract. However, when 8 g L−1 NaNO3 and 2 g L−1 yeast extract were combined, the amylase and xylanase activity increased 2.52- and 8.07-fold compared to an addition of NH4Cl. Furthermore, a comparison between the variation of amylase and xylanase activity in the media containing different nitrogen sources revealed a higher sensitivity of xylanase toward the nitrogen source. Similarly, NaNO3 was identified as the best nitrogen source, among different single nitrogen sources, in the production of amylase by N. crassa [23]. NaNO3 has also been referred to in the literature as a good nitrogen source for xylanase production in different microorganisms, especially filamentous fungus [20].
2.2. Enzyme Production in a Bubble Column Reactor
In order to investigate the effect of agitation and aeration on N. intermedia enzyme production, the fungus was cultivated in the optimum medium, identified for each enzyme in the shake-flask system, and cultivation was conducted in a bubble column bioreactor. Due to the high growth of N. intermedia in the solution, resulting in high viscosity, agitation and aeration are required during fungal growth and enzyme production. In contrast with a stirred tank reactor, pneumatically agitated bioreactors, such as bubble column bioreactors, cause lower shear stress and better mass transfer [24]. N. intermedia showed an acceptable growth in both starch (5.07 ± 0.36 g dry biomass L−1) and xylan (7.74 ± 0.68 g dry biomass L−1) medium.
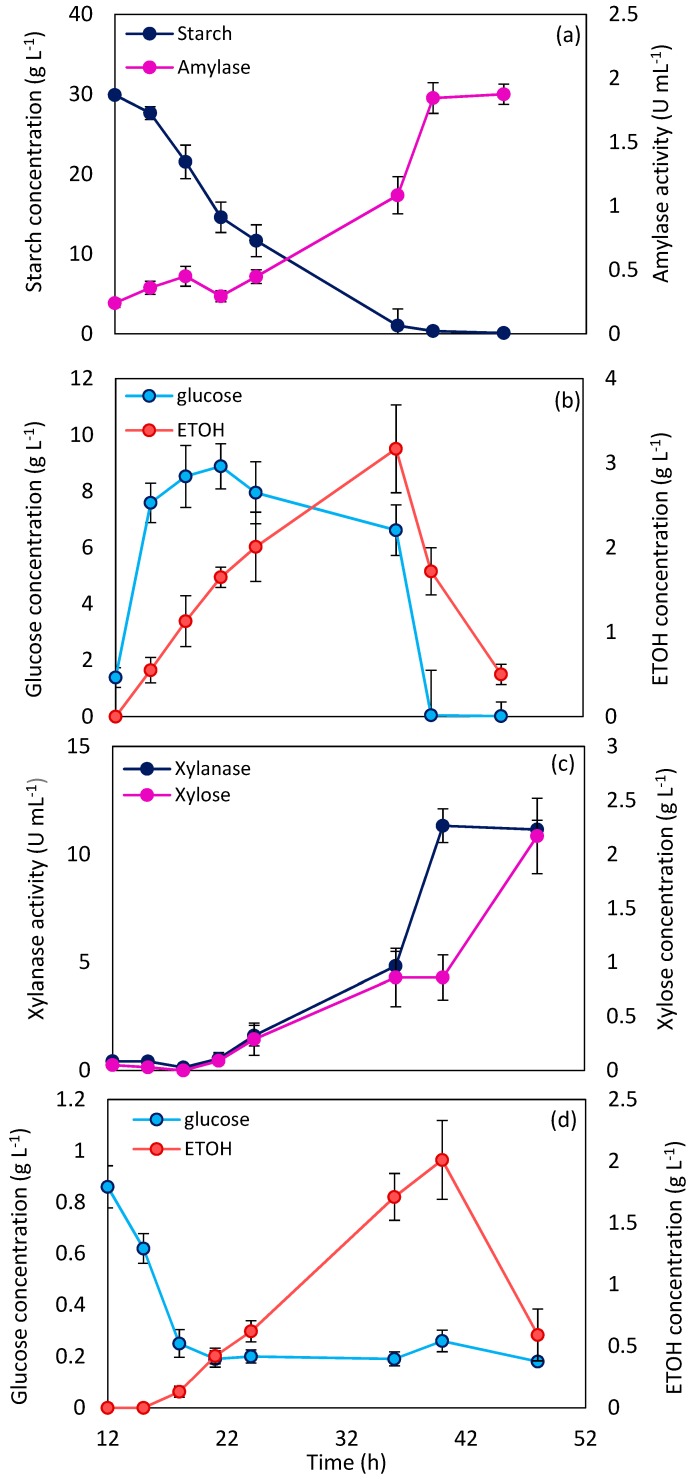
Amylase production began after 12 h, which can be considered as the N. intermedia lag phase, as shown in Figure 2a. During the cultivation process, the starch content of the medium decreased by the increase in amylase activity, resulting in 98.8% starch consumption after 39 h of fermentation. However, an unexpected drop in amylase activity was observed around 20 h after cultivation, which can be attributed to a change in the pH of the culture medium. Starch hydrolysis by N. intermedia amylase led to the release of glucose, which was consumed by fungus, and resulted in up to 3.17 ± 0.52 g L−1 ethanol, as shown in Figure 2b.
Figure 2.
Starch and amylase concentrations (a), glucose and ethanol (ETOH) concentrations (b) in the starch medium, and xylanase production and xylose release (c), glucose and ethanol (ETOH) concentrations (d) in the xylan medium during cultivation of N. intermedia at 35 °C in the bubble column bioreactor.
A similar trend was observed for xylan and xylanase. Xylanase activity reached 11.32 ± 0.78 U mL−1 after 40 h of cultivation and did not show any significant changes after that. As a consequence of xylanase function, xylan was hydrolyzed and xylose was released (up to 2.17 ± 0.35 g L−1), as shown in Figure 2c. The trend of glucose changes during fermentation; Figure 2d shows consumption of the glucose inducer by N. intermedia, in the beginning of fermentation, after which it remains almost constant. The constant amount of glucose, along with an increasing trend of ethanol concentration, confirmed the consumption of xylose by N. intermedia at this stage. A similar report of xylose consumption by N. intermedia has been presented by Batori et al. [9].
In both starch and xylan media, after almost 40 h, ethanol concentration decreased in the course of time, which coincided with the stop in enzyme production. Ethanol consumption by N. intermedia and evaporation can be responsible for the reduction in ethanol concentration. A decrease in ethanol concentration during N. intermedia growth in different media has been reported previously [9,10,15].
Comparing the results of enzyme production in shake-flask and bubble column bioreactor systems showed that the aeration brought about a significant effect on the maximum enzyme production (39% and 2.3% increase in amylase and xylanase production, respectively). In addition, it accelerated the fermentation process and enzyme production (16.6% reduction in fermentation time). Proper aeration in the bubble column bioreactor can provide an appropriate gas–liquid interaction area followed by suitable mass transfer. Results of previous studies of N. intermedia cultivation in the airlift and bubble column bioreactor showed that this fungus has benefited from appropriate aeration [10]. An increase in enzyme production with a simultaneous decrease in fermentation time, as consequences of appropriate aeration, have been reported previously in the enzyme production by aerobic microorganisms [25].
2.3. Amylase and Xylanase Characteristics
2.3.1. Effect of Temperature on Enzyme Activity
Enzyme activity is generally defined as the amount of a certain substrate converted per unit time, which is a function of active enzyme concentration and its specific reaction rate constant [26]. In accordance with Arrhenius law (Equation (1)), the reaction rate constant (k) increases with increasing temperature (T).
| (1) |
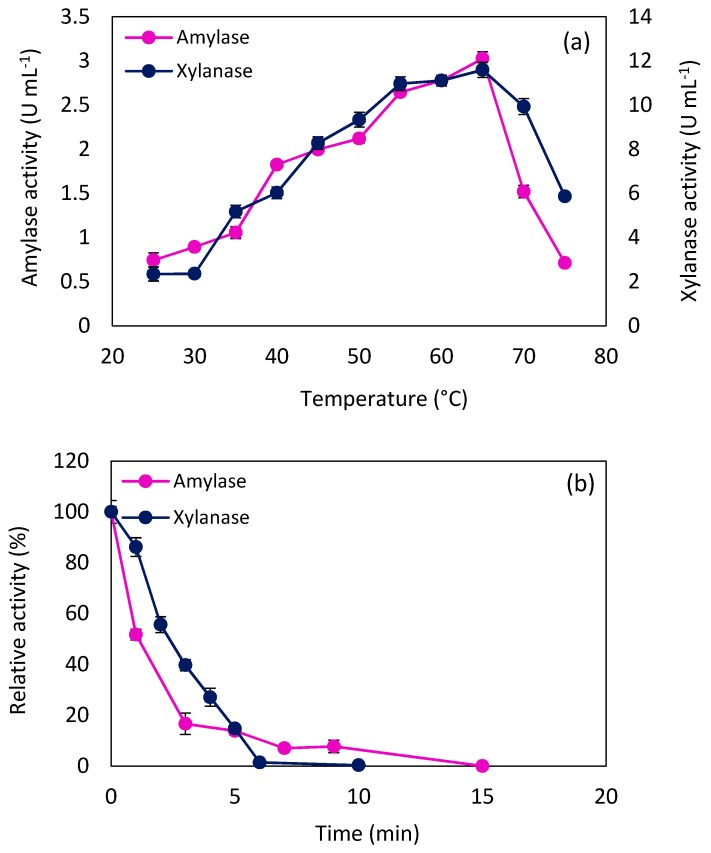
Consequently, it would be expected to observe an increasing trend in enzyme activity along with increasing temperature. However, at high temperatures, enzymes start to deactivate and lose their activity partially or entirely. Under these circumstances, enzyme stability turns out to be important and considerable. The experimental results, as shown in Figure 3a, clearly show that both amylase and xylanase activity increased sharply until 65 °C, at which point the maximum activity of amylase (3.02 ± 0.08 U mL−1) and xylanase (11.59 ± 0.34 U mL−1) were reached. Beyond this point, a significant decline in both enzyme activities were observed. Wide ranges of optimum temperature have been reported for amylase and xylanase derived from various fungi and bacteria. Nevertheless, results of amylase and xylanase optimum temperature, in this study, are in agreement with earlier studies of the enzymes produced by Aspergillus [27,28].
Figure 3.
N. intermedia amylase and xylanase (a) activity in different temperatures, (b) stability at 65 °C.
The optimum reaction temperature data can be applied in the Arrhenius equation to calculate the activation energy required for each substrate hydrolysis. At the beginning of the reaction, the substrate concentration is high, and the rate of reaction is independent of the substrate concentration. Therefore, the Arrhenius equation can be written as Equation (2).
| (2) |
where, Vmax is the enzyme activity; and Ea is the substrate hydrolysis activation energy (kJ mol−1). Considering the temperature-dependent binds between the enzymes and their polymeric substrates (starch and xylan), activation energies for hydrolyzing these substrates can be regarded as the apparent activation energy. Apparent activation energies (Ea, app) for starch and xylan hydrolysis by N. intermedia enzymes, in different ranges of temperature, are reported in Table 1, which are comparable to the starch and xylan hydrolysis activation energy of Rhizopus microsporus amylase (34.3 kJ mol−1) [29] and Fusarium sp. xylanase (37.15 kJ mol−1) [30], respectively.
Table 1.
Apparent activation energies for starch and xylan hydrolysis by N. intermedia amylase and xylanase, respectively.
| Temperature Range (°C) | Ea, app (kJ mol−1) | |
|---|---|---|
| Amylase | Xylanase | |
| 25–65 | 30.97 | 36.65 |
| 65–75 | −141.570 | −66.52 |
Different conformation of the enzyme would result in changes in the slope of the Arrhenius plot and the enzyme activation energy [26]. The results in Table 1 show that both amylase and xylanase have single conformation up to the optimum temperature (65 °C). Positive values of Ea in the range of 25 to 65 °C, revealed that catalysis reactions prevail over enzymatic deactivation below the optimum point. Furthermore, the higher values of xylanase Ea in comparison with amylase Ea may propose that the required energy for formation of the activated complex in starch hydrolysis is less than that in hydrolysis of xylan. Above the optimum point, both amylase and xylanase showed less activity towards the hydrolysis of their substrates, resulting from the enzyme’s significant denaturation above the optimum reaction temperature.
Inadequate enzyme stability can give rise to economic losses when the enzyme is to be utilized. Therefore, in terms of the bioprocess design and economy perspective, it is crucial to investigate the thermal deactivation and realize the stability of the enzyme under specific operating conditions. In this regard, N. intermedia amylase and xylanase’s thermal deactivation were investigated by measuring the residual activity of enzymes at their optimum temperature, 65 °C, as shown in Figure 3b. Data obtained from the experimental study were fitted to the equation of thermal deactivation of enzymes (Equation (3)) as follows:
| (3) |
where, t is time (min); E0 is the initial enzyme activity (U mL−1); E is the enzyme activity (U mL−1) at time t; and kd is the deactivation rate constant (min−1). Half-life values of the enzymes were also calculated by replacing E with E0/2 in Equation (3), as shown in Equation (4).
| (4) |
The comparison between the deactivation rate constant and half-life of N. intermedia enzymes, as shown in (Table 2, showed that amylase was the most stable at 65 °C (lower deactivation rate constant) and required 2.42 min to lose half of its activity, while xylanase lost half of its activity after 2.04 min.
Table 2.
Deactivation rate constant and half-life of N. intermedia enzymes at 65 °C.
| Enzyme | kd (min−1) | (min) |
|---|---|---|
| Amylase | 0.2862 | 2.42 |
| Xylanase | 0.3390 | 2.04 |
Generally, enzyme thermostability is an inherent feature, with variation based on the enzyme source. However, it has been observed that some microorganisms produce enzymes with different thermal stability regarding their special growth temperature and conditions [31]. There are several reports of amylase and xylanase thermostability investigation in the literature, which shows different half-life values of amylase [32,33] and xylanase [34,35] at 65 °C.
2.3.2. Effect of pH on Enzyme Activity
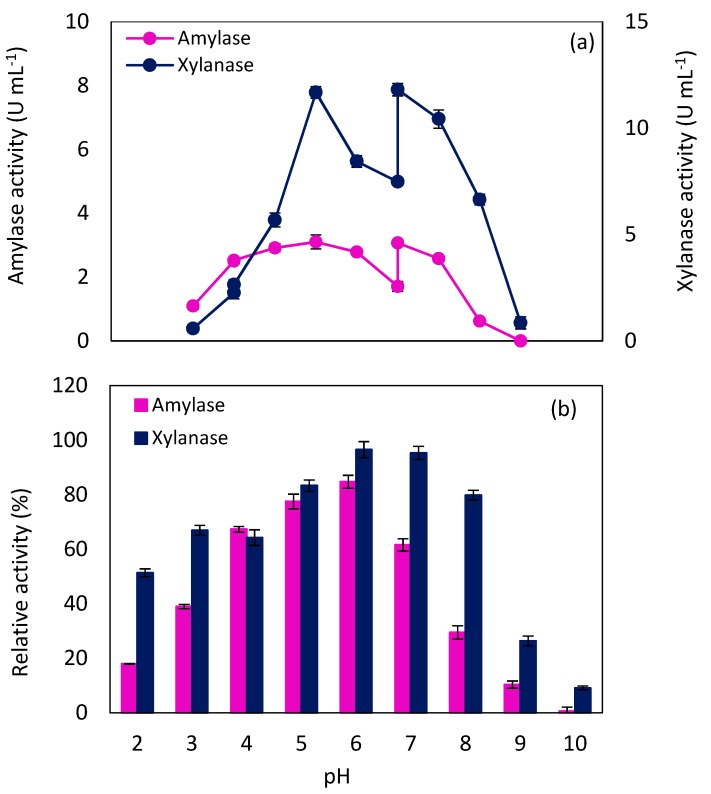
The other critical factor which affects the enzyme activity is pH. Each enzyme has its own optimum pH in which the active site can bind to the substrate tightly and catalyze the reaction favorably. In order to determine the optimum pH of amylase and xylanase produced by N. intermedia, their activity was examined in the pH range of 2.0 to 10.0, at 65 °C. Potential interaction by the buffer system was also investigated by measuring activity twice during buffer overlaps. The results shown in Figure 4a indicated that the maximum activity of amylase (3.10 ± 0.22 U mL−1) was obtained at pH 4.0–5.0, while xylanase activity reached the peak of (11.67 ± 0.28 U mL−1) at pH 5.0. As can be observed in Figure 4a, at pH 3.0, the change in buffer had no effect, whereas at pH 7.0, change in buffer type resulted in a dramatic transformation. This can probably be attributed to a presence of sodium in the sodium tetraborate buffer, which acted as a cofactor for both enzymes and enhanced the enzyme activity at pH 7.0. Similarly, optimum pH of amylase and xylanase in the pH range of 4–6 has been reported in previous studies [5,27].
Figure 4.
N. intermedia amylase and xylanase (a) activity and (b) stability in different pH at 65 °C after 4 h.
The effect of pH on enzyme stability was also determined over the pH range of 2.0 to 10.0 for 4 h, as shown in Figure 4b. Xylanase retained more than 80% of its initial activity within a pH range of 5.0 to 8.0, while in the case of amylase, pH 6.0 created a situation in which more than 80% of initial activity was retained. For both enzymes, the activity decreased dramatically at pH values higher than 8.0.
2.3.3. Kinetic Characteristics of Produced Enzymes
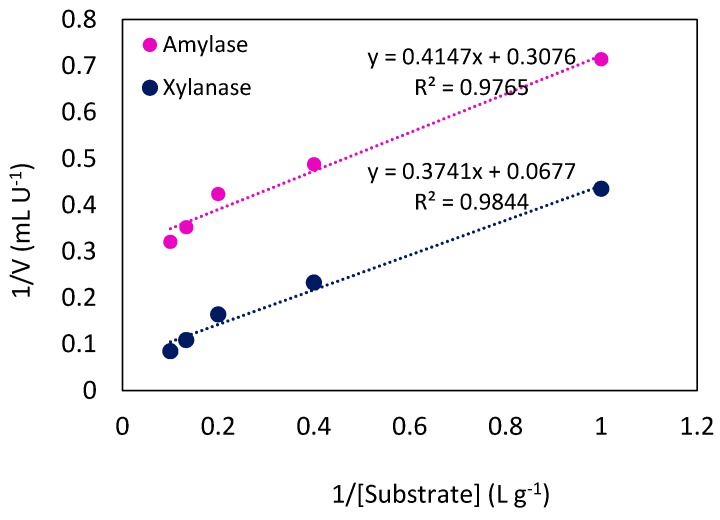
Kinetic studies on enzymes, by measuring the rate of catalyzed reaction under various conditions, can reveal the catalytic mechanism of the enzyme and the affinity with which the enzyme binds to its specific substrate. The kinetic of starch and beechwood xylan hydrolysis, using the Lineweaver–Burk plot, is depicted in Figure 5. Furthermore, the values of Km, app and Vmax, app are shown in Table 3.
Figure 5.
Lineweaver–Burk plot to calculate Km, app and Vmax, app of amylase and xylanase in hydrolysis of starch and xylan, respectively (under optimum conditions).
Table 3.
Apparent kinetic constants for hydrolysis of starch and beechwood xylan by N. intermedia amylase and xylanase, respectively.
| Enzyme | Km, app (g L−1) | Vmax, app (U mL−1) |
|---|---|---|
| Amylase | 1.35 | 3.25 |
| Xylanase | 5.52 | 14.77 |
The Km value reflects the affinity of the enzyme towards its specific substrate, with smaller values representing greater affinity for its substrate [36]. Although it is not easy to compare the Km and Vmax values of different enzymes, as they depend on type of the substrate and conditions of the reaction, the values of Km are in agreement with the previous reported range of Km values for microbial xylanases (0.27–14 g L−1) [37] and amylases (0.19–29 g L−1) [6].
2.4. Enzyme Production by N. Intermedia through Wheat-Based Biorefinery
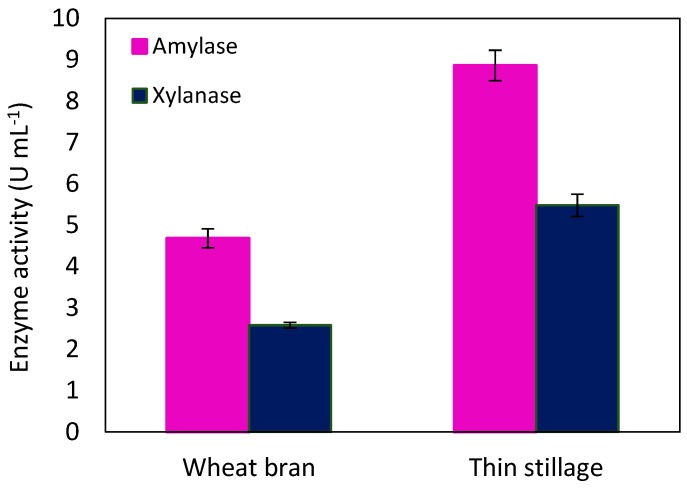
Restructuring the conventional fermentation industry into possible biorefineries for the production of enzymes brings considerable benefits, including reducing the cost and energy of the enzyme production, performing substrate hydrolysis during on-site enzyme production, and addressing the issue of waste stream disposal. Considering the tremendous consumption of wheat and its derivatives like wheat bran in the food and feed industry, wheat-based biorefineries have attracted much interest throughout the world. That is why this study was continued by cultivation of N. intermedia in thin stillage, a residual stream of the whole-wheat ethanol process, and a medium containing wheat bran, a byproduct of the wheat milling industry, to investigate enzyme production in a wheat-based biorefinery. The results in Figure 6 show that N. intermedia represented a significant potential in enzyme production from both media. However, lower starch (5.1 ± 0.048 g L−1) and xylan (4.68 ± 0.57 g L−1) content of wheat bran medium resulted in lower amylase (917.64 ± 45.09 U g starch−1) and xylanase (551.2 ± 14.95 U g xylan−1) production yield in this medium. Furthermore, the presence of pretreated wheat along with the relevant amount of mineral, carbon, and nitrogen sources in thin stillage can be the other reason for higher amylase (1004.53 ± 41.95 U g starch−1) and xylanase (1022.38 ± 50.37 U g xylan−1) production yield in thin stillage [10].
Figure 6.
Enzyme production by N. intermedia cultivation in wheat bran and thin stillage media at 35 °C and pH = 5.5, after 48 h.
Amylase production from thin stillage has been also investigated by cultivation of Aspergillus oryzae, Penicillium purpurogenum, Rhizopus, Mucor, and Monilia. Maximum amylolytic activity was reported as 3.3 U mL−1 resulting from cultivation of Aspergillus oryzae [38], which was 62% less than activity observed from cultivation of N. intermedia in the current research.
Furthermore, in the literature there are other reports of integrated enzyme production by wheat-based biorefineries in solid or submerged fermentation. As an illustration, cultivation of Aspergillus awamori in a crude broth of wheat-flour resulted in the production of glucoamylase, protease, and phosphatase, and simultaneous hydrolysis of wheat starch, protein, and phytic acid content [39]. However, this is the first report of enzyme production through the wheat-based biorefinery by edible fungus Neurospora intermedia, which can be further beneficial in protein-rich biomass production for feed applications.
3. Materials and Methods
3.1. Microorganism
The edible fungus, originally from Indonesia, Neurospora intermedia CBS 131.92 (Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands) was used to produce the enzymes in this study. The fungus was maintained on potato dextrose agar or PDA (composed of 20 g L−1 glucose, 4 g L−1 potato infusion, and 15 g L−1 agar) at 30 °C for 3–5 days followed by storage at 4 °C. The inoculum was prepared aseptically by adding 10 mL sterile distilled water to the sporulated plates and gently agitating the mycelia. The total spore count in the inoculum was determined by the Neubauer counting method. All liquid cultures were inoculated with 10 mL L−1 of spore solution containing 6.5 ± 0.4 × 105 spores mL−1.
3.2. Substrate Media
Amylase and xylanase production by N. intermedia were investigated in synthetic and semisynthetic media and an industrial residue stream. Synthetic media were prepared using 30 g L−1 of starch or xylan, as a carbon source for amylase and xylanase production, respectively. This was supplemented with 1 g L−1 glucose (as an inducer), required nutrients (2.25 g L−1 MgSO4∙7H2O, 3.5 g L−1 KH2PO4, 1 g L−1 CaCl2∙2H2O, 10 mL L−1 trace metal containing 3 g L−1 EDTA, 0.9 g L−1 ZnSO4∙7H2O, 0.6 g L−1 FeSO4∙7H2O, 0.2 g L−1 H3BO3, 0.19 g L−1 MnCl2 4H2O, 0.8 g L−1 Na2MoO4 2H2O, 0.6 g L−1 CoCl2 2H2O, 0.6 g L−1 CuSO4 5H2O, 0.02 g L−1 KI) and 10 g L−1 of a nitrogen source, which is explained in Section 3.3. In the semisynthetic media, 30 g L−1 of wheat bran was used as a carbon source and the other components were the same as which were utilized in synthetic media. The thin stillage, from a bioethanol production process based mainly on wheat, was provided by Lantmännen Agroetanol (Norrköping, Sweden). Compositions of the wheat bran and thin stillage are shown in Table 4. All media were adjusted to pH 5.5 with 10 M NaOH and 6 M HCl.
Table 4.
Compositions of the wheat bran and thin stillage used in this study.
| Component | g g−1 Wheat Bran (dry basis) [40] | g L−1 Thin Stillage [10] |
|---|---|---|
| Solid fraction | ||
| Arabinan | 0.088 ± 0.001 | 0.242 ± 0.140 |
| Crude protein | 0.147 ± 0.001 | 27.5 ± 1.25 |
| Galactan | <0.001 | 0.175 ± 0.0420 |
| Glucan | 0.204 ± 0.02 | 3.182 ± 0.383 |
| Mannan | <0.001 | 0.488 ± 0.234 |
| Starch | 0.17 ± 0.0016 | <0.001 |
| Xylan | 0.156 ± 0.019 | 0.907 ± 0.419 |
| Dissolved saccharides | ||
| Arabinos | 4.4 ± 0.2 | |
| Galactose | 1.6 ± 0.2 | |
| Glucose | 9.8 ± 0.7 | |
| Mannose | 1.4 ± 0.2 | |
| Xylose | 6.1 ± 0.4 | |
| Starch (using glucose as an indicator) | 8.82 ± 0.63 | |
| Xylan (using xylose as an indicator) | 5.36 ± 0.35 |
3.3. Enzyme Production in Shake Flasks and Nitrogen Source
Amylase and xylanase production were investigated in 250 mL shake flasks. The flasks containing 100 mL of cultivation medium were sterilized (at 121 °C for 20 min), and after cooling, inoculated with the spore suspension followed by incubation at 35 °C on a rotary shaker (150 rpm). From incubated cultures sampling was carried out at regular time intervals. The culture broths were clarified by centrifugation at 8000× g for 15 min and the supernatant was used as a crude enzyme extract to determine the activity of enzymes.
In order to investigate the effect of a nitrogen source on enzyme production, the basal synthetic medium of each enzyme was supplemented with 10 g L−1 of different nitrogen sources (NH4Cl, (NH4)2SO4, NaNO3, yeast extract, and a combination of NaNO3 and yeast extract), in separated runs. Submerge fermentation was carried out as explained in Section 3.3.
3.4. Enzyme Production in a Bubble Column Bioreactor
Fungal cultivations were performed in 4.5 L bench bubble column bioreactors (Belach Bioteknik, Stockholm, Sweden). The bubble column bioreactor was filled with 3 L of the optimum medium for each enzyme, described in Section 3.2 and Section 3.3, in separate runs. The bioreactor containing medium was sterilized (at 120 °C for 3 h), and after cooling, spore suspension and antifoam were added to the cultivation medium. The solution pH was maintained at 5.5 (by 2 M NaOH), temperature was set at 35 °C and aeration, at a rate of 1 vvm (volume of air per volume of reactor per min), was fulfilled by passing the inlet air through a 0.2 μm pore size sterile polytetrafluoroethylene filter followed by a 90 μm pore size stainless steel sparger. Cultivation was performed for 48 h and samples were taken periodically, centrifuged, and analyzed. At the end of each cultivation, the fungal biomass was removed from the cultivation medium by filtration through a metal sieve. The mycelia were washed with distilled water, dried, and measured gravimetrically and the medium, as an enzyme crude extract, was stored at 4 °C for further analysis.
3.5. Enzyme Characteristics
3.5.1. Effect of pH and Temperature on Enzyme Activity
The optimum pH of amylase and xylanase was investigated in a pH range of 2.0 to 10.0. To evaluate the pH stability, enzymes were preserved in different buffers at room temperature for 4 h and the remaining enzyme activity was measured. The applied buffer systems were citric acid buffer for pH 2.0–3.0, phosphate-citrate buffer for pH 3.0–7.0, and sodium tetraborate buffer for pH 7.0–10.0. To investigate the influence of temperature and determine the best temperature for enzyme operation, amylase and xylanase activity, separately, were tested at different temperatures (25–75 °C). Thermal stability was investigated by determination of enzyme activity after different pre-incubation times (0–30 min) at the optimum temperature of enzyme operation.
3.5.2. Kinetic Properties of the Enzyme-Substrate System
The effect of substrate concentration on enzyme–substrate reaction kinetics was investigated using starch and beechwood xylan (1 to 10 g L−1) under optimum assay conditions of amylase and xylanase, respectively. Apparent Michaelis-constant (Km, app) and maximum velocity (Vmax, app) values of the enzymes were calculated from the slope and intercept of the straight-line plot of V−1 vs. S−1 (Lineweaver–Burk plot) as follows:
| (5) |
3.6. Analytical Methods
The amylolytic and xylanolytic activity of the N. intermedia crude enzyme extract was determined spectrophotometrically based on the measurement of the released reducing sugar by the dinitrosalicylic acid reagent [41]. An amylolytic activity assay was carried out based on the hydrolysis of 1% starch (w/v) at 40 °C in 0.2 M sodium acetate buffer (pH 4.8). Xylanolytic activity was measured by the hydrolysis of 1% beechwood xylan (w/v) at 50 °C in 0.05 M sodium citrate buffer (pH 5.3) [42]. One unit of enzyme activity was defined as the amount of enzyme required to liberate one μmole of reduced sugar, maltose and xylose in the case of amylase and xylanase, respectively, per min under assay conditions. The enzyme assay conditions were as stated above except where otherwise specified.
Investigation of the intracellular enzyme production was carried out using two methods of cell disruption [43]. At the end of fermentation, N. intermedia mycelia (10% w/v) were suspended in 10 mL of cold citrate buffer (pH 5 at 4 °C) and placed in a salt-iced water bath ultrasonic homogenizer (2510 Branson from Branson Ultrasonics Corporation, Danbury, CT, USA) at 42 kHz. In order to prevent enzyme thermal denaturation, sonication was carried out in 30 s intervals for a total time of 10 min. As the second method, mycelia were suspended in 0.2% (v/v) aqueous solutions of Triton X-100 to give a working concentration of 10% cell (w/v) with shaking (200 rpm) for three different durations (30, 60, and 120 min). The obtained suspensions from each method were centrifuged at 14,000× g at 4 °C for 10 min and the subsequent supernatant was examined for enzyme activity.
Starch contents of solutions, in the case of amylase production, were measured based on the color development that resulted from iodine binding to the starch polymers [44]. Glucose, ethanol, and total sugar were analyzed using a high performance liquid chromatography (HPLC) system (Waters 2695, Waters Corporation, Milford, MA, USA) [10].
4. Conclusions
The results confirm that N. intermedia possess the great ability in extracellular amylase and xylanase production. At the optimum temperature of hydrolysis reactions, 65 °C, N. intermedia amylase represented more stability and greater substrate affinity. However, its maximum substrate hydrolyzing rate was less than xylanase. Both enzymes produced by N. intermedia showed their optimum activity in pH around 5 and retained more than 50% of their initial activity in the pH range of 4 to 7 after 4 h. Relatively high enzyme production through cultivation of N. intermedia in thin stillage and the medium of wheat bran makes this fungus an appropriate candidate in enzyme production through a wheat-based biorefinery.
Acknowledgments
Zohre Shahryari would like to thank Jorge Ferreira and Amir Mahboubi Suffiani for their technical advice.
Author Contributions
Formal analysis, Z.S. and P.R.L.; Investigation, Z.S. and P.R.L.; Methodology, Z.S.; Project administration, M.J.T.; Supervision, M.H.F., Y.G., P.R.L., and M.J.T.; Writing-original draft, Z.S.; Writing-review & editing, Z.S., M.H.F., Y.G., P.R.L., and M.J.T.
Funding
This study was financially supported by Biotechnology Development Council, Islamic Republic of Iran (grant No 950508), and Swedish Agency for Economic and Regional Growth (Tillväxtverket) for the financial support through a European Regional Development Fund (grant No. 20201656).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Souza P.M. Application of microbial α-amylase in industry-A review. Braz. J. Microbiol. 2010;41:850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purohit A., Rai S.K., Chownk M., Sangwan R.S., Yadav S.K. Xylanase from Acinetobacter pittii MASK 25 and developed magnetic cross-linked xylanase aggregate produce predominantly xylopentose and xylohexose from agro biomass. Bioresour. Technol. 2017;244:793–799. doi: 10.1016/j.biortech.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D., Kumar S.S., Kumar J., Kumar O., Mishra S.V., Malyan S., Kumar R. Xylanase and their industrial applications: A review. Biochem. Cell. Arch. 2017;17:353–360. [Google Scholar]
- 4.Fath M., Fazaelipoor M.H. Production of proteases in a novel trickling tray bioreactor. Waste Biomass Valoriz. 2015;6:475–480. doi: 10.1007/s12649-015-9371-6. [DOI] [Google Scholar]
- 5.Beg Q., Kapoor M., Mahajan L., Hoondal G. Microbial xylanases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R., Gigras P., Mohapatra H., Goswami V.K., Chauhan B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003;38:1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- 7.Shahryari Z., Fazaelipoor M.H., Setoodeh P., Nair R.B., Taherzadeh M.J., Ghasemi Y. Utilization of wheat straw for fungal phytase production. Int. J. Recycl. Org. Waste Agric. 2018;7:345–355. doi: 10.1007/s40093-018-0220-z. [DOI] [Google Scholar]
- 8.Gmoser R., Ferreira J.A., Lennartsson P.R., Taherzadeh M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal. Biol. Biotechnol. 2017;4:1–25. doi: 10.1186/s40694-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bátori V., Ferreira J.A., Taherzadeh M.J., Lennartsson P.R. Ethanol and protein from ethanol plant by-products using edible fungi Neurospora intermedia and Aspergillus oryzae. BioMed Res. Int. 2015:1–10. doi: 10.1155/2015/176371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira J.A., Lennartsson P.R., Taherzadeh M.J. Production of ethanol and biomass from thin stillage by neurospora intermedia: A pilot study for process diversification. Eng. Life Sci. 2015;15:751–759. doi: 10.1002/elsc.201400213. [DOI] [Google Scholar]
- 11.Ferreira J.A., Lennartsson P.R., Taherzadeh M.J. Production of ethanol and biomass from thin stillage using food-grade zygomycetes and ascomycetes filamentous fungi. Energies. 2014;7:3872–3885. doi: 10.3390/en7063872. [DOI] [Google Scholar]
- 12.Gmoser R., Ferreira J.A., Lundin M., Taherzadeh M.J., Lennartsson P.R. Pigment production by the edible filamentous fungus Neurospora intermedia. Fermentation. 2018;4:1–11. [Google Scholar]
- 13.Nair R.B., Kabir M.M., Lennartsson P.R., Taherzadeh M.J., Horváth I.S. Integrated process for ethanol, biogas, and edible filamentous fungi-based animal feed production from dilute phosphoric acid-pretreated wheat straw. Appl. Biochem. Biotechnol. 2018;184:48–62. doi: 10.1007/s12010-017-2525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair R.B., Lundin M., Brandberg T., Lennartsson P.R., Taherzadeh M.J. Dilute phosphoric acid pretreatment of wheat bran for enzymatic hydrolysis and subsequent ethanol production by edible fungi Neurospora intermedia. Ind Crops Prod. 2015;69:314–323. doi: 10.1016/j.indcrop.2015.02.038. [DOI] [Google Scholar]
- 15.Nair R.B., Lundin M., Lennartsson P.R., Taherzadeh M.J. Optimizing dilute phosphoric acid pretreatment of wheat straw in the laboratory and in a demonstration plant for ethanol and edible fungal biomass production using Neurospora intermedia. J. Chem. Technol. Biotechnol. 2017;92:1256–1265. doi: 10.1002/jctb.5119. [DOI] [Google Scholar]
- 16.Ferreira J.A., Mahboubi A., Lennartsson P.R., Taherzadeh M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016;215:334–345. doi: 10.1016/j.biortech.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Nair R.B., Taherzadeh M.J. Valorization of sugar-to-ethanol process waste vinasse: A novel biorefinery approach using edible ascomycetes filamentous fungi. Bioresour. Technol. 2016;221:469–476. doi: 10.1016/j.biortech.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 18.Mahboubi A., Ferreira J.A., Taherzadeh M.J., Lennartsson P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2017;59:518–525. doi: 10.1016/j.wasman.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Thunuguntla R., Mahboubi A., Ferreira J., Taherzadeh M. Integration of membrane bioreactors with edible filamentous fungi for valorization of expired milk. Sustainability. 2018;10:1940. doi: 10.3390/su10061940. [DOI] [Google Scholar]
- 20.Adhyaru D.N., Bhatt N.S., Modi H.A. Optimization of upstream and downstream process parameters for cellulase-poor-thermo-solvent-stable xylanase production and extraction by Aspergillus tubingensis fdhn1. Bioresour Bioprocess. 2015;2:1–14. doi: 10.1186/s40643-014-0029-1. [DOI] [Google Scholar]
- 21.Van der Kaaij R., Janeček Š., van der Maarel M., Dijkhuizen L. Phylogenetic and biochemical characterization of a novel cluster of intracellular fungal α-amylase enzymes. Microbiology. 2007;153:4003–4015. doi: 10.1099/mic.0.2007/008607-0. [DOI] [PubMed] [Google Scholar]
- 22.Mishra C., Keskar S., Rao M. Production and properties of extracellular endoxylanase from Neurospora crassa. Appl. Environ. Microbiol. 1984;48:224–228. doi: 10.1128/aem.48.1.224-228.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanti A. Effect of nitrogen addition on the α-amylase production by Aspergillus niger, Rhizopus oligosporus and Neurospora crassa in media contained sargassum and rice seed on solid state fermentation. J. Biolog. Indones. 2016;12:249–256. [Google Scholar]
- 24.Pino M.S., Rodríguez-Jasso R.M., Michelin M., Flores-Gallegos A.C., Morales-Rodriguez R., Teixeira J.A., Ruiz H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018;347:119–136. doi: 10.1016/j.cej.2018.04.057. [DOI] [Google Scholar]
- 25.Gangadharan D., Nampoothiri K.M., Pandey A. α-amylase production by Bacillus amyloliquefaciens using agro wastes as feed stock. Food Technol. Biotechnol. 2011;49:336–340. [Google Scholar]
- 26.Melikoglu M., Lin C.S.K., Webb C. Kinetic studies on the multi-enzyme solution produced via solid state fermentation of waste bread by Aspergillus awamori. Biochem. Eng. J. 2013;80:76–82. doi: 10.1016/j.bej.2013.09.016. [DOI] [Google Scholar]
- 27.Pasin T.M., Benassi V.M., Heinen P.R., de Lima Damasio A.R., Cereia M., Jorge J.A., de Moraes M.d.L.T. Purification and functional properties of a novel glucoamylase activated by manganese and lead produced by Aspergillus japonicus. Int. J. Biol. Macromol. 2017;102:779–788. doi: 10.1016/j.ijbiomac.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 28.de Lima Damásio A.R., Silva T.M., dos Reis Almeida F.B., Squina F.M., Ribeiro D.A., Leme A.F.P., Segato F., Prade R.A., Jorge J.A., Terenzi H.F. Heterologous expression of an Aspergillus niveus xylanase gh11 in Aspergillus nidulans and its characterization and application. Process Biochem. 2011;46:1236–1242. doi: 10.1016/j.procbio.2011.01.027. [DOI] [Google Scholar]
- 29.Shen H., Mo X., Chen X., Han D., Zhao C. Purification and enzymatic identification of an acid stable and thermostable α-amylase from Rhizopus microsporus. J. Inst. Brew. 2012;118:309–314. doi: 10.1002/jib.45. [DOI] [Google Scholar]
- 30.Sugumaran K., Srivastava S., Ponnusami V. Kinetic and thermodynamic characterization of Pleurotus eryngii mtcc 1798 xylanase and Fusarium oxysporum mtcc 3300 xylanase: A comparision. Int. J. Chem. Sci. 2012;10:1626–1636. [Google Scholar]
- 31.Ward O., Moo-Young M. Thermostable enzymes. Biotechnol. Adv. 1988;6:39–69. doi: 10.1016/0734-9750(88)90573-3. [DOI] [PubMed] [Google Scholar]
- 32.Wang C., Wang Q., Liao S., He B., Huang R. Thermal stability and activity improvements of a ca-independent α-amylase from Bacillus subtilis cn7 by c-terminal truncation and hexahistidine-tag fusion. Biotechnol. Appl. Biochem. 2014;61:93–100. doi: 10.1002/bab.1150. [DOI] [PubMed] [Google Scholar]
- 33.Negi S., Banerjee R. Characterization of amylase and protease produced by Aspergillus awamori in a single bioreactor. Food Res. Int. 2009;42:443–448. doi: 10.1016/j.foodres.2009.01.004. [DOI] [Google Scholar]
- 34.Terrasan C.R.F., Guisan J.M., Carmona E.C. Xylanase and β-xylosidase from Penicillium janczewskii: Purification, characterization and hydrolysis of substrates. Electron. J. Biotechnol. 2016;23:54–62. doi: 10.1016/j.ejbt.2016.08.001. [DOI] [Google Scholar]
- 35.Irfan M., Gonzalez C.F., Raza S., Rafiq M., Hasan F., Khan S., Shah A.A. Improvement in thermostability of xylanase from Geobacillus thermodenitrificans c5 by site directed mutagenesis. Enzyme Microb. Technol. 2018;111:38–47. doi: 10.1016/j.enzmictec.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Das R., Kayastha A.M. An antioxidant rich novel β-amylase from peanuts (arachis hypogaea): Its purification, biochemical characterization and potential applications. Int. J. Biol. Macromol. 2018;111:148–157. doi: 10.1016/j.ijbiomac.2017.12.130. [DOI] [PubMed] [Google Scholar]
- 37.Ding C., Li M., Hu Y. High-activity production of xylanase by Pichia stipitis: Purification, characterization, kinetic evaluation and xylooligosaccharides production. Int. J. Biol. Macromol. 2018;117:72–77. doi: 10.1016/j.ijbiomac.2018.05.128. [DOI] [PubMed] [Google Scholar]
- 38.Le Mense E., Corman J., Van Lanen J., Langlykke A. Production of mold amylases in submerged culture. J. Bacteriol. 1947;54:149–159. [PMC free article] [PubMed] [Google Scholar]
- 39.Koutinas A., Arifeen N., Wang R., Webb C. Cereal-based biorefinery development: Integrated enzyme production for cereal flour hydrolysis. Biotechnol. Bioeng. 2007;97:61–72. doi: 10.1002/bit.21206. [DOI] [PubMed] [Google Scholar]
- 40.Nair R.B., Kalif M., Ferreira J.A., Taherzadeh M.J., Lennartsson P.R. Mild-temperature dilute acid pretreatment for integration of first and second generation ethanol processes. Bioresour. Technol. 2017;245:145–151. doi: 10.1016/j.biortech.2017.08.125. [DOI] [PubMed] [Google Scholar]
- 41.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 42.Bailey M.J., Biely P., Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992;23:257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- 43.Yeng A.L.Y., Ab Kadir M.S., Ghazali H.M., Rahman R.N.Z.R.A., Saari N. A comparative study of extraction techniques for maximum recovery of glutamate decarboxylase (GAD) from Aspergillus oryzae nsk. BMC Res. Notes. 2013;6:1–9. doi: 10.1186/1756-0500-6-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao Z., Storms R., Tsang A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006;351:146–148. doi: 10.1016/j.ab.2006.01.036. [DOI] [PubMed] [Google Scholar]