Abstract
Leea indica (Vitaceae) is a Southeast Asian medicinal plant. In this study, an ethyl acetate fraction of L. indica leaves was studied for its phytoconstituents using high-performance liquid chromatography-electrospray ionization-mass spectrometry (HPLC-ESI-microTOF-Q-MS/MS) analysis. A total of 31 compounds of different classes, including benzoic acid derivatives, phenolics, flavonoids, catechins, dihydrochalcones, coumarins, megastigmanes, and oxylipins were identified using LC-MS/MS. Among them, six compounds including gallic acid, methyl gallate, (−)-epigallocatechin-3-O-gallate, myricetin-3-O-rhamnoside, quercetin-3-O-rhamnoside, and 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-β-d-glucopyranoside were isolated and identified by NMR analysis. The LC-MS/MS analysis led to the tentative identification of three novel dihydrochalcones namely 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-rutinoside, 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-glucosylpentoside and 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-(3″-O-galloyl)-β-d-glucopyranoside. The structural identification of novel dihydrochalcones was based on the basic skeleton of the isolated dihydrochalcone, 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-β-d-glucopyranoside and characteristic LC-MS/MS fragmentation patterns. This is the first comprehensive analysis for the identification of compounds from L. indica using LC-MS. A total 24 compounds including three new dihydrochalcones were identified for the first time from the genus Leea.
Keywords: Leea indica, HPLC-ESI-microTOF-Q-MS/MS, phenolics, dihydrochalcones
1. Introduction
Leea indica (Burm. f.) Merr. (Vitaceae), commonly known as Bandicoot berry, is an evergreen perennial shrub or a small tree of 2 to 16 m in height. It is distributed throughout Bangladesh, China, India, Malaysia, Singapore, North Australia, Thailand, and Vietnam [1,2,3]. Traditionally, L. indica is used as a remedy during pregnancy, for birth control, body pain, skin problems, and relief from dizziness [4,5]. L. indica is reported to possess various pharmacological activities, e.g., analgesic, anti-angiogenesis, anti-oxidant, anti-inflammatory, anti-microbial, anti-proliferative, hepatoprotective, sedative, and anxiolytic activities [3,5,6,7,8,9,10,11,12]. The plant contains different classes of compounds including phenolics, terpenoids, phthalic acid derivatives, and steroids [13,14,15]. Currently, there are very few reports available on the phytochemistry of L. indica.
The objective of the present study was to isolate and identify chemical constituents from an ethyl acetate fraction of L. indica leaves. The comprehensive chemical identification was carried out by high performance liquid chromatography coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry (HPLC-ESI-microTOF-Q-MS) analysis along with the isolation of compounds 1, 5, 10, 14, 18, and 27 from ethyl acetate fraction. The structures of the isolated compounds were identified using NMR and MS analyses. A total of 31 compounds belonging to different classes including benzoic acid derivatives, flavonoids, coumarins, megastigmanes, catechins, dihydrochalcones, and oxylipins were identified. Here we report the identification of three novel dihydrochalcones along with 28 known compounds from the ethyl acetate fraction of L. indica leaves. In total, 24 compounds, including three novel dihydrochalcones, are reported for the first time in the genus Leea.
2. Results and Discussion
2.1. Isolation and Identification of Compounds
The methanolic extract of L. indica leaves was fractionated with hexane, dichloromethane and ethyl acetate. The dried yields were 0.005%, 0.027% and 1.32% respectively. Purification of the major organic ethyl acetate fraction by repeated column chromatography led to the isolation of compounds 1, 5, 10, 14, 18, and 27. The compounds were identified as gallic acid (1) [16], methyl gallate (5) [17], epigallocatechin-3-O-gallate (10) [18], myricetin-3-O-rhamnoside (14) [19], quercetin-3-O-rhamnoside (18), [19] and 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-β-d-glucopyranoside (27) [20] by comparing their analytical data (1H, 13C and 2D-NMR, and LC-MS) with those reported in the literature [16,17,18,19,20].
2.2. Identification of Dihydrochalcones by LC-ESI-MS/MS Analysis
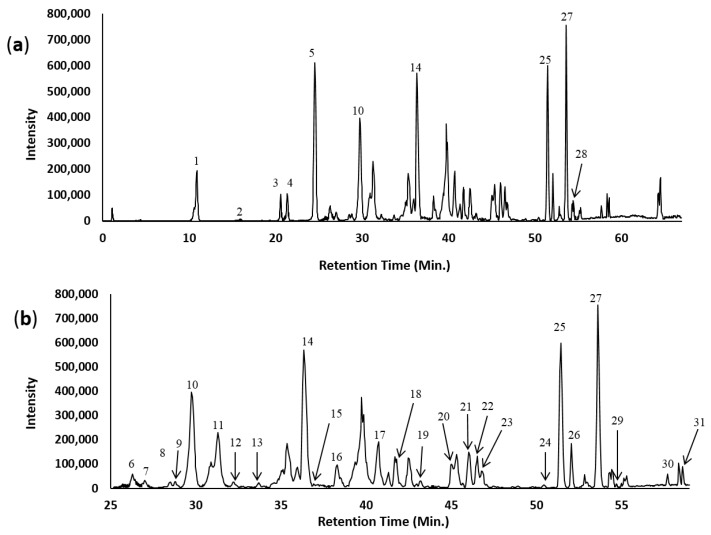
The ethyl acetate fraction of L. indica leaves was analyzed by the LC-ESI-MS/MS method. Figure 1 shows the base peak chromatogram (BPC) of the ethyl acetate fraction of L. indica leaves at 254 nm. Figure 2 shows the structures of the 31 compounds identified. In total, 31 compounds were identified of which ten compounds (1, 4, 5, 8, 9, 10, 14, 15, 18 and 21) were verified by comparison with reference standards. Seven compounds were tentatively identified as dihydrochalcone derivatives: 3-hydroxyphloridzin 17, phloridzin 21, 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-rutinoside 25 (m/z 595), 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-glucosyl pentoside 26 (m/z 581), 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-β-d-glucopyranoside 27, 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-(6″-O-galloyl)-β-d-glucopyranoside 29 (m/z 601) and 2′,4′,6′-trihydroxy-4-methoxydihydrochalcone (3-methylphloretin) 31. Compounds 25, 26 and 29 are reported for the first time. While dihydrochalcone phloridzin has been previously reported in L. indica [13], the other six dihydrochalcone derivatives have not been previously reported in the same plant species. The observed MS peaks including retention time, observed mass, calculated mass, molecular formula, ppm error, and MS/MS data are presented in Table 1.
Figure 1.
(a) Base peak chromatogram (BPC) of L. indica ethyl acetate fraction by HPLC-ESI-MS in negative ionization mode; (b) Expanded BPC. Peak labeling represents the compounds identified.
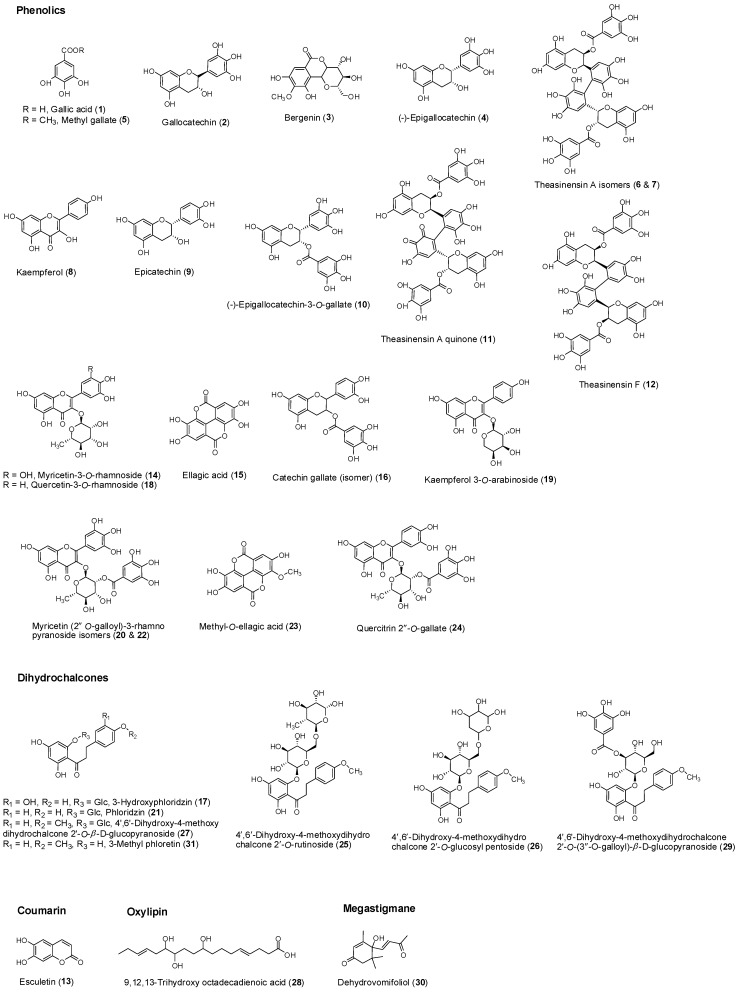
Figure 2.
Structures of compounds identified in L. indica according to their chemical classes.
Table 1.
Identification of compounds from ethyl acetate fraction of L. indica by HPLC-ESI-microTOF-Q-MS/MS at 254 nm in negative ionization mode.
| Peak no. | RT (min) | Observed [M − H]− | Calculated [M − H]− | Error (ppm) | Molecular Formula | Fragment Ions (m/z) | Identified Compound |
|---|---|---|---|---|---|---|---|
| 1 | 10.9 | 169.0146 | 169.0142 | −2.2 | C7H6O5 | 125.0444 | Gallic acid |
| 2 | 15.9 | 305.0668 | 305.0667 | −0.4 | C15H14O7 | 261.0623, 219.0682, 179.0279, 167.0371, 165.0179, 151.1024 | Gallocatechin † |
| 3 | 20.6 | 327.0726 | 327.0722 | −1.4 | C14H16O9 | 312.0487, 234.0173, 207.0298, 206.0222, 192.0079 | Bergenin |
| 4 | 21.4 | 305.0668 | 305.0667 | −0.4 | C15H14O7 | 287.059, 261.076, 219.0694, 221.0473, 179.0362, 167.0387, 165.0199 | Epigallocatechin † |
| 5 | 24.5 | 183.0304 | 183.0299 | −2.7 | C8H8O5 | 169.0107 | Methyl gallate † |
| 6 | 26.3 | 913.1455 | 913.1469 | 1.6 | C44H34O22 | 761.1369, 743.1264, 609.1287, 591.1153, 573.1038, 447.0733, 423.0709, 285.0410, 169.0143 | Theasinensin A (isomer 1) † |
| 7 | 27.0 | 913.1471 | 913.1469 | −0.2 | C44H34O22 | 761.131, 743.1255, 609.1205, 591.1148, 573.1104, 447.0721, 423.0752, 285.0422, 169.0178 | Theasinensin A (isomer 2) † |
| 8 | 28.5 | 285.0399 | 285.0405 | 2.1 | C15H10O6 | 243.0291, 217.0528, 199.0420, 175.047 | Kaempferol |
| 9 | 28.8 | 289.0721 | 289.0718 | −1.0 | C15H14O6 | 221.0795, 203.0724, 175.0323 | Epicatechin |
| 10 | 29.8 | 457.0784 | 457.0776 | −1.6 | C22H18O11 | 305.0660, 261.0803, 219.0637, 169.0142 | Epigallocatechin-3-O-gallate † |
| 11 | 31.0 | 911.1315 | 911.1312 | −0.2 | C44H32O22 | 759.1258, 741.1135, 589.1027, 571.0861, 441.0556, 423.0727, 305.0618, 301.0453, 285.0431, 169.0135 | Theasinensin A quinone † |
| 12 | 32.2 | 897.1515 | 897.1520 | 0.5 | C44H34O21 | 745.1526, 727.1485, 575.1195, 557.1, 449.0938, 423.0693, 287.0576, 269.0482, 169.0127 | Theasinensin F † |
| 13 | 33.7 | 177.0191 | 177.0193 | 1.2 | C9H6O4 | 148.9428, 132.9003, 105.9031 | Esculetin † |
| 14 | 36.4 | 463.0886 | 463.0882 | −0.8 | C21H20O12 | 317.029, 316.0226, 287.0199, 271.0247, 179.0012, 135.8248 | Myricetin 3-O-rhamnoside (myricitrin) |
| 15 | 36.9 | 300.9989 | 300.9990 | 0.2 | C14H6O8 | 283.9927, 245.0151, 229.0091, 201.0309, 200.0171, 173.0194 | Ellagic acid † |
| 16 | 38.3 | 441.0831 | 441.0827 | −0.9 | C22H18O10 | 289.0701, 271.06, 245.9752, 169.0132 | Catechin gallate (isomer) † |
| 17 | 41.2 | 451.1254 | 451.1246 | −1.7 | C21H24O11 | 289.0724, 271.1548, 167.0353 | 3-Hydroxyphloridzin † |
| 18 | 41.7 | 447.0931 | 447.0933 | 0.4 | C21H20O11 | 301.0325, 300.0271, 255.0296, 179.0009 | Quercetin 3-O-rhamnoside (Quercitrin) |
| 19 | 43.2 | 417.0833 | 417.0827 | −0.6 | C20H18O10 | 284.0316, 257.0446, 255.0304, 227.0339 | Kaempferol 3-O-arabinoside † |
| 20 | 45.0 | 615.1001 | 615.0992 | −1.5 | C28H24O16 | 463.0903, 317.0319, 297.0616, 178.9989, 169.0188 | Myricetin-O-(O-galloyl)-3-rhamnopyranoside (isomer 1) † |
| 21 | 46.0 | 435.1299 | 435.1297 | −0.5 | C21H24O10 | 273.0758, 167.0349 | Phloridzin |
| 22 | 46.5 | 615.0988 | 615.0992 | 0.6 | C28H24O16 | 463.0817, 317.0332, 297.0677, 178.9976, 169.011 | Myricetin-O-(O-galloyl)-3-rhamnopyranoside (isomer 2) † |
| 23 | 46.8 | 315.0146 | 315.0146 | 0.1 | C15H8O8 | 299.9902, 270.9912, 243.9987, 151.0037 | Methyl-O-ellagic acid † |
| 24 | 50.4 | 599.1048 | 599.1042 | −1.0 | C28H24O15 | 447.0893, 301.0369, 169.0125, 151.8637 | Quercitrin 2″-O-gallate † |
| 25 | 51.4 | 595.2031 | 595.2032 | 0.2 | C28H36O14 | 433.1347, 329.1078, 308.2508, 287.0929, 167.0376 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-rutinoside † |
| 26 | 52.1 | 581.1889 | 581.1876 | −0.5 | C27H34O14 | 419.1210, 329.102, 311.0951, 293.0907, 287.0926, 273.0953, 243.1026, 167.0355 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-glucosylpentoside † |
| 27 | 53.6 | 449.1452 | 449.1453 | 0.4 | C22H26O10 | 329.1080, 287.0921, 273.0744, 272.0683, 243.1032, 181.017, 167.0298, 166.0275, 151.0067 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-β-d-glucopyranoside † |
| 28 | 54.5 | 327.2171 | 327.2177 | 1.8 | C18H32O5 | 309.2164, 298.9867, 291.1998, 239.1283, 229.1447, 211.1327, 183.0131, 171.103 | 9,12,13-Trihydroxy octadecadienoic acid † |
| 29 | 55.0 | 601.1595 | 601.1563 | −5.3 | C29H30O14 | 439.0901, 329.1098, 313.0559, 287.0914, 271.0502, 243.1106, 211.0199, 169.0167 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-(3″-O-galloyl)-β-d-glucopyranoside † |
| 30 | 57.7 | 221.1186 | 221.1183 | −1.4 | C13H18O3 | 149.0978 | Dehydrovomifoliol † |
| 31 | 58.6 | 287.0926 | 287.0925 | −0.2 | C16H16O5 | 243.1034, 167.037, 151.0043 | 2′,4′,6′-Trihydroxy-4-methoxy dihydrochalcone (3-Methylphloretin) † |
† Compounds identified for the first time in the genus Leea.
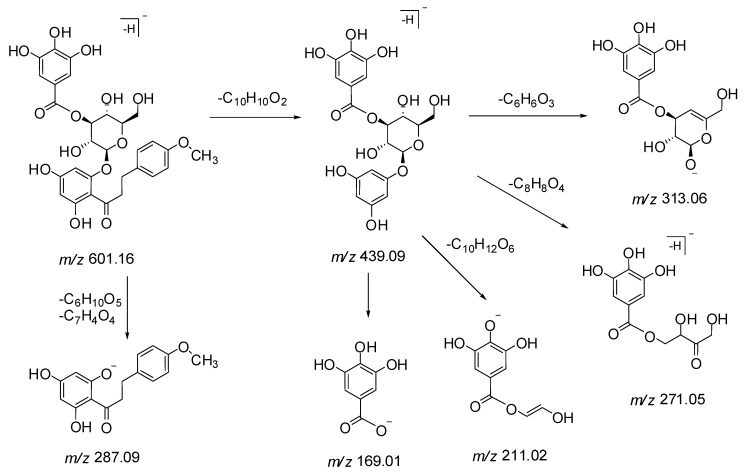
The structural identification of three new dihydrochalcones 25, 26 and 29 was based on the relevance of the LC-MS/MS fragmentation patterns with the isolated compound 4′,6′-dihydroxy-4-methoxy dihydrochalcone 2′-O-β-d-glucopyranoside 27. The MS/MS spectra of compounds 25, 26, 27 and 29, showed a common base ion peak at m/z 287 for 2′,4′,6′-trihydroxy-4-methoxydihydrochalcone, which is a characteristic ion formed by the loss of glycoside(s) and/or galloyl glycoside moieties.
In LC-MS spectra, peaks 25, 26 and 29 eluted at retention times (RT) 51.4, 52.1 and 55.0 min, and showed precursor ions [M − H]− at m/z 595.2031, 581.1889 and 601.1595, respectively. Peaks 25 (m/z 595) and 26 (m/z 581) showed a mass difference of 146 Da (rhamnose) and 132 Da (arabinose/xylose) respectively compared to the isolated dihydrochalcone 27 (m/z 449). Also, peak 26 (m/z 581) was found to be 14 Da lighter than peak 25 (m/z 595), indicating the presence of a pentose sugar. In agreement with mass analysis data, peaks 25 and 26 were tentatively characterized as 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-rutinoside (m/z 595) and 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-glucosylpentoside (m/z 581) respectively.
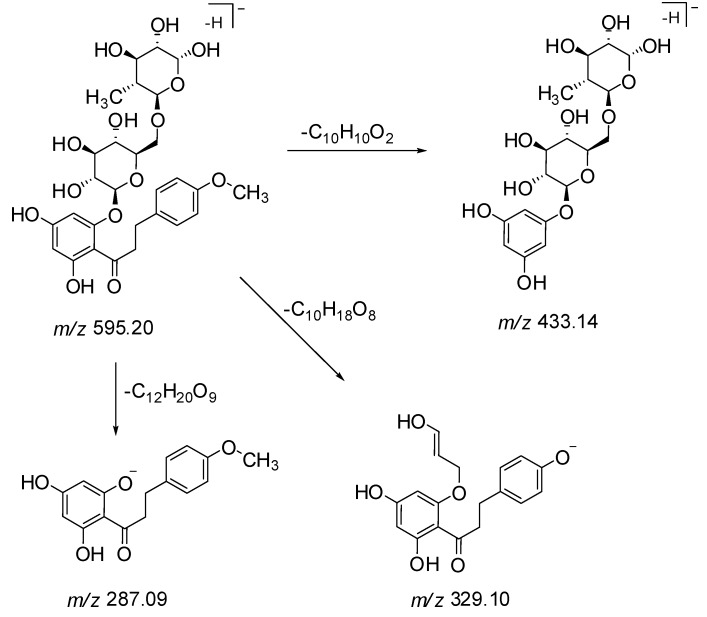
Peak 25 displayed a molecular ion [M − H]− at m/z 595.2031 (C28H36O14) and fragment ions at m/z 433, 329 and 287 (Scheme 1 and Figure S1). In the MS/MS spectrum, a characteristic fragment ion at m/z 287 as base peak suggested that this compound corresponded to a 2′,4′,6′-trihydroxy-4-methoxydihydrochalcone linked to a rutinose moiety, where the neutral loss of 308 Da is characteristic of the loss of a rutinose moiety [21]. The fragments at m/z 433 [M − C10H10O2 − H]− and 329 [M − C10H18O8 − H]− were obtained by the cleavage of the C-C bond of chalcone and sugar moiety respectively (Scheme 1). The fragment ion at m/z 329 [M − H − C9H13O7 − H2O − CH3]− was obtained by the cleavage of a glucose moiety, with the loss of a water molecule and further by losing a methyl group. Based on these deductions, peak 25 was tentatively identified as 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-rutinoside, a new dihydrochalcone.
Scheme 1.
Proposed MS/MS fragmentation of compound 25.
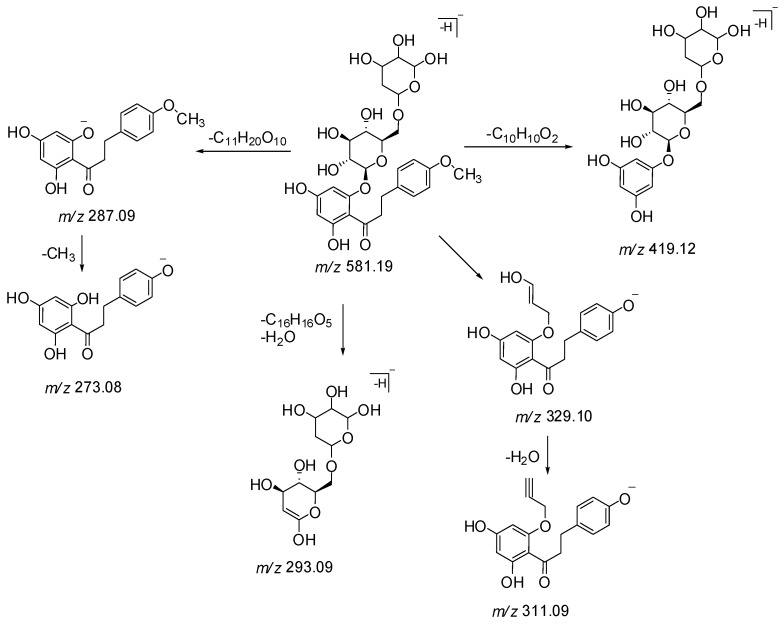
Peak 26 exhibited a precursor ion [M − H]− at m/z 581.1889 (C27H34O14) and fragment ions at m/z 419, 311, 293, and 243 (Scheme 2 and Figure S2). The MS/MS spectrum showed product ion at m/z 287 (C16H16O5) [M − H − 162 Da − 132 Da]− as base peak by the loss of a glucosylpentoside moiety, suggesting to possess a basic skeleton of isolated dihydrochalcone 27. The cleavage of a C-C bond gave a fragment ion at m/z 419 due to the loss of a C10H10O2 moiety. The neutral loss of 312 Da showed the presence of a glucosyl pentoside moiety, losing a molecule of water to generate a product ion at m/z 293 (Scheme 3). Therefore, compound 26 was plausibly identified as 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-glucosylpentoside and found as first occurrence in nature.
Scheme 2.
Proposed MS/MS fragmentation of compound 26.
Scheme 3.
Proposed MS/MS fragmentation of compound 29.
Peak 29 showed a precursor ion [M − H]− at m/z 601.1595 (C29H30O14) and fragment ions at m/z 439, 313, 287, 271, 211, and 169 in the MS/MS spectrum. A base ion peak at m/z 287 [M − C6H10O5 − C7H4O4 − H]− was observed due to the loss of glucose (162 Da) and galloyl (153 Da) moieties. Fragment ions at m/z 169 and m/z 313 indicate the presence of a galloyl and a galloylglucose moiety respectively. Monogalloylglucose can exist as five possible isomers namely, 1-O-galloylglucose, 2-O-galloylglucose, 3-O-galloylglucose, 4-O-galloylglucose, and 6-O-galloylglucose [22]. The characteristic fragment ions at m/z 271 and 211 suggest that the substitution of the galloyl group could be at the C-3 position of the glucose moiety (Scheme 3 and Figure S3). Product ion detected at m/z 439 suggested the cleavage of the C-C bond (loss of C10H10O2 moiety) in the MS/MS spectrum. Thus, the compound corresponding to peak 29 was tentatively identified as a new dihydrochalcone 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-(3″-O-galloyl)-β-d-glucopyranoside. It is also a gallic acid derivative of the isolated dihydrochalcone 27.
The isolated dihydrochalcone 27 exhibited a precursor ion [M − H]− at m/z 449.1452 (C22H26O10). The MS/MS spectrum showed product ions at m/z 287 [M − C6H10O5 −H]− and 273 [M − C6H10O5 − CH3 − H]− due to the loss of glucose (162 Da) and methyl groups (15 Da) (Figure S4). Compound 27 was isolated and identified as 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-β-d-glucopyranoside.
The LC-MS fragmentation patterns of the three novel dihydrochalcones (25, 26 and 29) were compared to the isolated dihydrochalcone (27), and we noted that the observed HR-MS data were in good agreement with the calculated masses. Further isolation of the peaks 25, 26 and 29 and spectroscopic analyses would be required to unambiguously confirm the proposed structures of these dihydrochalcones.
3. Materials and Methods
3.1. Plant Materials
Fresh ground leaves of L. indica were collected in Singapore. A voucher specimen (no. LI-0109) was deposited at the herbarium of the National University of Singapore (NUS) Medicinal Plant Research Group.
3.2. Chemicals and Reagents
Standards gallic acid, methyl gallate, myricitrin, quercitrin, epigallocatechin-3-O-gallate, ellagic acid, epicatechin, and kaempferol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phloridzin and epigallocatechin were purchased from TCI Co. Ltd. (Tokyo, Japan). LC-MS grade solvents (acetonitrile, methanol and formic acid) were purchased from MERCK (Darmstadt, Germany) and water used in LC analysis was obtained using Milli-Q advanced system (Millipore, Milford, MA, USA).
3.3. Extraction and Isolation
The fresh ground leaves of L. indica (2.8 kg) were macerated with 70% v/v MeOH at room temperature. The extract was filtered and concentrated under vacuum, yielding a crude methanolic extract. The dried methanolic extract was dissolved in water and partitioned with different solvents, concentrated under vacuum to give hexane (0.005%), dichloromethane (0.027%) and ethyl acetate (1.32%) fractions.
The ethyl acetate fraction (37.0 g) was chromatographed over silica gel using 25% EtOAc–hexane as eluent, yielding a white solid, which was recrystallized in CHCl3-MeOH as white needles of methyl gallate (60 mg). Fractions obtained from repeated silica gel column chromatography of EtOAc fraction using 6–10% MeOH-CHCl3 as eluent were further purified by Sephadex (LH-20) and reversed phase cartridge yielding two compounds gallic acid (140 mg) and 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-β-d-glucopyranoside (12 mg). The estimated concentration of gallic acid in the fresh leaves was 0.005–0.011% w/w. Pooled fractions obtained from silica gel column chromatography of the EtOAc fraction using 10–20% MeOH-CHCl3 were further subjected to Sephadex (LH-20) column chromatography. At an eluent concentration of 50% MeOH-water, a mixture of two compounds was obtained. It was further purified by silica gel column chromatography eluting with 8% MeOH-CHCl3 and 10–12% MeOH-CHCl3 to yield quercetin-3-O-rhamnoside (5 mg) and myricetin-3-O-rhamnoside (650 mg) respectively. Epigallocatechin-3-O-gallate (64 mg) was obtained from the silica gel column chromatography using 2–5% methanol in dichloromethane. The structures of isolated compounds 1, 5, 10, 14, 18, and 27 were confirmed by NMR and LC-MS analyses.
3.4. General Information
NMR spectra were recorded on a Bruker Avance-400 Spectrometer (Fallanden, Switzerland), 1H at 400 MHz and 13C at 100 MHz in deuterated solvents using tetramethylsilane (TMS) as an internal reference. Deuterated solvents, methanol-d4 and dimethyl sulfoxide-d6 for NMR were purchased from Sigma-Aldrich (USA).
Silica-gel (60–120, 100–200, 70–230 mesh; Merck, Germany), Sephadex LH-20 (Sigma, Uppsala, Sweden) and reversed phase C18 (77.9 μm) cartridge column from Waters (Ireland) were used for chromatographic separation. Thin layer chromatography was performed on pre-coated Si-gel 60 F254 plates (Merck, Germany) using a visualizing reagent.
The LC-MS analysis was carried out using a Dionex Ultimate 3000 VWD system coupled with a VWD and a micro-TOF-Q mass detector (Bruker Daltonics Inc., Billerica, MA, USA). Chromatographic separation was performed on an RP-C18 column (3.0 × 150 mm; particle size 2.7 μM; Agilent Poroshell 120, New Castle, DE, USA), operated at 25 °C. Analysis was carried out using a gradient elution program of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) as a mobile phase at a flow rate of 0.5 mL/min. The following gradient system was used: 0–45 min, 5–30% B; 45–60 min 30–100% B and 60–65 min 100% B. UV detection was performed by scanning the samples at 210, 254, 280, and 360 nm. Electrospray ionization mass spectra (ESI-MS) were recorded in negative ionization mode. The mass range of m/z 50–2000 was scanned. For MS/MS analysis, collision energies were set automatically.
4. Conclusions
This study presents the comprehensive identification of chemical constituents of an ethyl acetate fraction of L. indica leaves using HPLC-ESI-microTOF-Q-MS/MS analysis. Here we identified 31 compounds, among them six phenolic compounds were isolated by column chromatography. Three novel dihydrochalcones derivatives were tentatively identified as 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-rutinoside, 4′,6′-dihydroxy-4-methoxydihydro chalcone 2′-O-glucosylpentoside and 4′,6′-dihydroxy-4-methoxydihydrochalcone 2′-O-(3″-O-galloyl)-β-D-glucopyranoside. A total of 24 compounds are reported for the first time in the genus Leea. Our results indicated that L. indica is a good source of diverse phenolic contents including phenolic acids (gallic acid and methyl gallate), polyphenolic (ellagic acid), flavan-3-ols (gallocatechin, epigallocatechin and epigallocatechin-3-O-gallate), flavonoids/flavonoid glycosides (kaempferol, quercitrin, myricitrin), dihydrochalcones (phloridzin and its derivatives), and dimeric catechins (theasinensin A dimers and theasinensin F). The wide range of potential bioactive compounds supports the diverse pharmacological activities of L. indica. Further research to identify and develop useful therapeutics and health supplements from L. indica is warranted.
Acknowledgments
The authors are grateful to Steven Yuan Cheng Hui, Dept. of Chemistry, NUS for technical support and Kim-Chuan Ng of the Nanyang Technological University Community Herb Garden, Singapore for his kind support.
Supplementary Materials
Supplementary materials are available online. Figure S1: MS2 spectrum and proposed fragmentation pattern of compound 25; Figure S2: MS2 spectrum and proposed fragmentation pattern of compound 26; Figure S3: MS2 spectrum and proposed fragmentation pattern 29; Figure S4: MS2 spectrum and proposed fragmentation pattern of compound 27.
Author Contributions
D.S.: isolation and identification of compounds, data analysis, preparation and correction of manuscript; Y.-Y.S., T.-I.C.: extraction of plant materials, isolation and identification of compounds; H.-C.Y., S.S.-W.H., C.S.E.-S.L. and W.-X.T.: extraction of plant materials; S.-Y.N.: correction of manuscript; H.-L.K.: conception of study and correction of manuscript.
Funding
This work is supported by the National University of Singapore-Leeward Pacific Pte Ltd research collaboration grant (R-148-000-172-592) to H.-L.K. and the National University of Singapore Provost Industrial PhD Programme Research Scholarship to Y.-Y.S.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples are not available from the authors.
References
- 1.Ridsdale C.E. Flora Malesiana, Series I–Spermatophyta Flowering Plants. Volume 7. Noordhoff International Publishing; Leiden, The Netherlands: 1976. Leeaceae; pp. 755–782. [Google Scholar]
- 2.The Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families plants: APG III. Bot. J. Linn. Soc. 2009;161:105–121. doi: 10.1111/j.1095-8339.2009.00996.x. [DOI] [Google Scholar]
- 3.Siew Y.Y., Yew H.C., Neo S.Y., Seow S.V., Lew S.M., Lim S.W., Lim C.S.E.S., Ng Y.C., Seetoh W.G., Ali A., et al. Evaluation of anti-proliferative activity of medicinal plants used in Asian Traditional Medicine to treat cancer. J. Ethnopharmacol. 2019;235:75–87. doi: 10.1016/j.jep.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 4.Bourdy G., Walter A. Maternity and medicinal plants in Vanuatu I. The cycle of reproduction. J. Ethnopharmacol. 1992;37:179–196. doi: 10.1016/0378-8741(92)90033-N. [DOI] [PubMed] [Google Scholar]
- 5.Singh D., Siew Y.Y., Yew H.C., Neo S.Y., Koh H.L. Botany, phytochemistry and pharmacological activities of Leea species. In: Swamy M.K., Patra J.K., Rudramurthy G.R., editors. Medicinal Plants: Chemistry, Pharmacology, and Therapeutic Applications. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2019. in press. [Google Scholar]
- 6.Wiart C., Mogana S., Khalifah S., Mahan M., Ismail S., Buckle M., Narayana A.K., Sulaiman M. Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia. Fitoterapia. 2004;75:68–73. doi: 10.1016/j.fitote.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Raihan M.O., Habib M.R., Brishti A., Rahman M.M., Saleheen M.M., Manna M. Sedative and anxiolytic effects of the methanolic extract of Leea indica (Burm. f.) Merr. Leaf. Drug Discov. Ther. 2011;5:185–189. doi: 10.5582/ddt.2011.v5.4.185. [DOI] [PubMed] [Google Scholar]
- 8.Wong Y.H., Kadir H.A. Leea indica ethyl acetate fraction induces growth-inhibitory effect in various cancer cell lines and apoptosis in Ca Ski human cervical epidermoid carcinoma cells. [(accessed on 18 January 2019)];Evid. Based Complement Alternat. Med. 2011 doi: 10.1155/2011/293060. Available online: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy N.S., Navanesan S., Sinniah S.K., Wahab N.A., Sim K.S. Phenolic content, antioxidant effect and cytotoxic activity of Leea indica leaves. [(accessed on 18 January 2019)];BMC Complement Altern. Med. 2012 12:128. doi: 10.1186/1472-6882-12-128. Available online: http://dx.doi.org./10.1186/1472-6882-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman M.A., Imran T.B., Islam S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013;20:213–225. doi: 10.1016/j.sjbs.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avin B.R.V., Thirusangu P., Ramesh C.K., Vigneswarana V., Kumar M.V.P., Mahmood R., Prabhakar B.T. Screening for the modulation of neovessel formation in non-tumorigenic and tumorigenic conditions using three different plants native to Western ghats of India. Biomed. Aging Pathol. 2014;4:343–348. doi: 10.1016/j.biomag.2014.07.006. [DOI] [Google Scholar]
- 12.Mishra G., Khosa R.L., Singh P., Jha K.K. Hepatoprotective activity of ethanolic extract of Leea indica (Burm. f.) Merr. (Leeaceae) stem bark against paracetamol induced liver toxicity in rats. Niger. J. Exp. Clin. Biosci. 2014;2:59–63. doi: 10.4103/2348-0149.135732. [DOI] [Google Scholar]
- 13.Saha K., Shaari K., Lajis N.H. Phytochemical study on Leea indica (Burm. F.) Merr. (Leeaceae) J. Bangladesh Chem. Soc. 2007;20:139–147. [Google Scholar]
- 14.Srinivasan G.V., Ranjith C., Vijayan K.K. Identification of chemical compounds from the leaves of Leea indica. Acta Pharm. 2008;58:207–214. doi: 10.2478/v10007-008-0002-7. [DOI] [PubMed] [Google Scholar]
- 15.Wong Y.H., Kadir H.A., Ling S.K. Bioassay-guided isolation of cytotoxic cycloartane triterpenoid glycosides from the traditionally used medicinal plant Leea indica. [(accessed on 18 January 2019)];Evid. Based Complement Alternat. Med. 2012 doi: 10.1155/2012/164689. Available online: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J.X., Di D.L., Shi Y.P. Diversity of chemical constituents from Saxifraga montana H. J. Chinese Chem. Soc. 2008;55:863–870. doi: 10.1002/jccs.200800129. [DOI] [Google Scholar]
- 17.Ekaprasada M.T., Nurdin H., Ibrahim S., Dachriyanus H. Antioxidant activity of methyl gallate isolated from the leaves of Toona sureni. Indones. J. Chem. 2009;9:457–460. doi: 10.22146/ijc.21515. [DOI] [Google Scholar]
- 18.Zhong Y., Shahidi F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011;59:6526–6533. doi: 10.1021/jf201050j. [DOI] [PubMed] [Google Scholar]
- 19.Aderogba M.A., Ndhlala A.R., Rengasamy K.R.R. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules. 2013;18:12633–12644. doi: 10.3390/molecules181012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva D.H.S., Yoshida M., Kato M.J. Flavonoids from Iryanthera sagotiana. Phytochemistry. 1997;46:579–582. doi: 10.1016/S0031-9422(97)00306-3. [DOI] [Google Scholar]
- 21.Zhang M., Duan C., Zang Y., Huang Z., Liu G. The flavonoid composition of flavedo and juice from the pummel cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus paradise) Food Chem. 2011;129:1530–1536. doi: 10.1016/j.foodchem.2011.05.136. [DOI] [Google Scholar]
- 22.Fathoni A., Saepudin A., Cahyanal H., Rahayu D.U.C., Haib J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017;1862 doi: 10.1063/1.4991183. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.