Abstract
Diet is a key modifiable factor in the management of malnutrition and age-related diseases such as sarcopenia, an important issue in long-term care homes. The objectives of this study were to evaluate the dietary intake of residents, define dietary patterns, and analyze their association with sex, diet texture, nutritional status, and the presence of sarcopenia. Intake was assessed by the precise weighing method, dietary patterns were defined a posteriori by cluster analysis, and nutritional status and sarcopenia were evaluated by applying the MNA-SF test and EWGSOP algorithm, respectively. A regular diet was consumed by 63% of participants; 56% were at risk of malnutrition and 63% were diagnosed with sarcopenia. Intake of potassium, magnesium, zinc, iodine, vitamin D, E, folic acid, and fiber was low in >80% of participants. Protein intake was <1 g/kg/day in 56% of participants and <25 g/meal in 100%. Two dietary patterns were identified, but neither fully met recommendations. The risk of a poorer diet was higher in females and residents with sarcopenia and was lower in those consuming regular diets. In conclusion, action is required to improve the inadequate nutritional intake of long-term care residents.
Keywords: nursing homes, older adults, dietary intake, nutrients, protein, sarcopenia, nutrition assessment
1. Introduction
The improvement of nutrition in long-term care (LTC) homes is an important research priority [1]. Diet is a key modifiable factor in the management of malnutrition [2,3] and age-related diseases, such as sarcopenia, an important issue in long-term homes [4]. Sarcopenia, the loss of muscle mass and muscle function associated with age [5], is associated with a low intake of energy and nutrients (e.g., protein, specific micronutrients), which appears to influence muscle mass (muscle quantity and quality) and function (strength and walking speed) [6,7,8,9,10]. Given that nutritional deficiencies do not generate clinical manifestations during early stages, monitoring of the dietary intake of residents is recommended to prevent severe deficiencies [11]. Various dietary assessment methods have been used to monitor dietary intake in institutionalized elderly people, but the precise weighing technique is established as the gold standard approach [12]. Few studies have undertaken the weighing of food because of the considerable effort and time required [13]; however, the relationship between dietary intake and health is complex, and accurate knowledge of the whole diet allows exploration of the health effects of interactions among food components [14]. Available statistical approaches to identify dietary patterns from intake data include principal component analysis, exploratory factor analysis, and cluster analysis. Cluster analysis defines patterns a posteriori, establishing subgroups of individuals with similar mean dietary intakes [15].
Malnutrition and sarcopenia are known to be common problems in LTC homes; however, there has been inadequate detailed research on the dietary intake of the residents, and the aim of the present study was to contribute further evidence in this regard. Therefore, the objectives of this study were: (1) to evaluate the dietary intake of institutionalized elderly people by the precise weighing method, (2) to characterize the study population according to dietary patterns defined by cluster analysis, and (3) to analyze the association of dietary patterns with sex, texture of diet, nutritional status, and the presence of sarcopenia.
2. Materials and Methods
2.1. Study Design and Recruitment
This research was part of a cross-sectional study called the Granada Sarcopenia Study, which included a representative sample of permanent residents in three randomly selected LTC homes for older adults in Granada province (Southeast Spain). The recruitment and assessment procedures have been described elsewhere [16]. Inclusion criteria were: age ≥ 70 years, residence in the home for ≥3 months, stable medical condition, and written informed consent to participation from the resident or surrogate decision-maker. Exclusion criteria were: wearing a pacemaker, terminal state, receipt of palliative care, difficult or dangerous behavior, or the presence of medical or other problems preventing participation. Agreement to participation was obtained from directors of the LTC homes, and the study protocol was approved by the Ethics Committee of the University of Granada (Spain).
2.2. Data Collection
Data were gathered by a qualified and previously trained dietician-nutritionist and level I anthropometrist (A.R-R.) certified by the International Society for the Advancement of Kinanthropometry (ISAK). Information was collected from LTC home records on sex, age, schooling, monthly income, admission date, and medical history. Participants were weighed using chair-scales (Seca 952; 0.1 kg), and their height was measured with their back against a wall or, when this was not possible, it was estimated from a standard formula based on knee height [17]. The body mass index (BMI) was calculated as weight/height2. A caliper (Innovare, Cescorf, Spain; 1 mm) was used to measure tricipital skinfold thickness (TST) and a flexible measuring tape (Cescorf, Spain; 1 mm) to measure mid-upper arm circumference (MUAC) and calf circumference (CC), calculating the mid-upper arm muscle circumference (MUAMC) as follows: MUAMC = MUAC (in cm) − (0.314 x TST [in mm]). The Barthel Index was used for the evaluation of daily living activities [18], the Lawton and Brody test for instrumental activities of daily living [19], the Functional Ambulation Classification (FAC) for the need for ambulation assistance [20], and the Pfeiffer test for cognitive status [21]. The mini-nutritional assessment short form test (MNA-SF) was used to assess nutritional status [22], considering a score of 12–14 points to indicate normal nutritional status, 8–11 points a risk of malnutrition, and <8 points malnutrition. Sarcopenia was diagnosed according to criteria of the European Working Group on Sarcopenia in Older People (EWGSOP) criteria [5], measuring muscle mass with an impedance meter, muscle strength using a Grip-D hand grip dynamometer, and gait speed (m/s) along a 4-meter course.
2.3. Dietary Assessment
Food intake data were collected for seven consecutive days (including a weekend) by the precise weighing method, weighing every portion served to individuals and the amount left on the plates at each meal [12]. The Nutrire® computer program was used for nutritional evaluation of the food consumed by each participant and for assessment of the nutritional composition of the dishes offered in different menus [23]. Results were compared with Dietary Reference Intakes (DRIs) for ≥70-year-olds [24,25], considering the Estimated Average Requirement (EAR) or, when EAR was not available, the Adequate Intake (AI). The Recommended Dietary Allowance (RDA) was also used for protein intake evaluation, alongside recommendations of the European Society for Clinical Nutrition and Metabolism (ESPEN) and PROT-AGE group [26,27]. The PROT-AGE group makes recommendations not only for the total daily protein intake but also for the minimum protein content of each main meal. The diet of each resident was classified according to its texture as regular (normal-texture), puréed, or mixed, assigning non-puréed foods with modified texture (soft or between soft and regular) to the “mixed” category.
2.4. Statistical Analysis
In a descriptive analysis of participants’ characteristics, categorical variables were expressed as frequencies and percentages and continuous variables as means with standard deviations. Results were stratified by sex.
Mixed linear regression models were used to analyze nutrient data gathered for each meal consumed during 7 days, obtaining the marginal means adjusted for sex and the statistical significance of differences [28,29]. We also calculated the percentage of participants with intakes of micronutrients below the corresponding DRIs.
The dietary intake of the population was characterized by cluster analysis, using two-stage clustering [30,31] to identify dietary patterns. Cluster selection was based on the Bayesian information criterion (BIC) and considering a silhouette coefficient > 0.4 in order to maximize the quality and the validity of the consistency of selected clusters [32].
Next, logistic multivariate analysis was conducted for the association of sex, texture of diet, nutritional status, and presence of sarcopenia with dietary patterns, verifying the goodness-of-fit of the model by using the BIC, residual analysis, and a receiver operating characteristics (ROC) curve for the estimated predictions [33,34].
SPSS version 25 was used for statistical analyses, considering a 5% significance level in all tests.
3. Results
Table 1 displays the characteristics of the 249 participants (187 females and 62 males) who met eligibility criteria and were included in the study. The mean ± SD age was 84.9 ± 6.7 years, the socioeconomic level was predominantly low, and the majority suffered from moderate or severe functional and cognitive impairment. A regular (normal-texture) diet was consumed by 63% of participants; 56% of participants were at risk of malnutrition), and 63% were diagnosed with sarcopenia.
Table 1.
Characteristics of the long-term care home residents.
| Total (n = 249) X ± SD or N (%) |
Female (n = 187) X ± SD or N (%) |
Male (n = 62) X ± SD or N (%) |
|
|---|---|---|---|
| Age (years) | 84.9 ± 6.7 | 85.4 ± 6.6 | 83.3 ± 7.0 |
| Level of education | |||
| Illiterate | 38 (15) | 32 (17) | 6 (10) |
| Writing and reading ability | 139 (56) | 107 (57) | 32 (52) |
| At least primary schooling | 64 (26) | 44 (24) | 20 (32) |
| University degree | 8 (3) | 4 (2) | 4 (6) |
| Level of income (€/month) | |||
| <500 | 9 (4) | 7 (4) | 2 (3) |
| 500–1000 | 184 (74) | 151 (81) | 33 (53) |
| 1000–1500 | 46 (18) | 26 (14) | 20 (32) |
| >1500 | 10 (4) | 3 (2) | 7 (12) |
| Weight (kg) | 62.2 ± 14.4 | 59.5 ± 14.1 | 70.2 ± 12.3 |
| BMI (kg/m2) | 26.3 ± 5.4 | 26.3 ± 5.8 | 26.4 ± 4.2 |
| CC (cm) | 32.1 ± 4.9 | 32.0 ± 5.3 | 32.7 ± 3.2 |
| MUAMC (cm) | 21.8 ± 2.9 | 21.3 ± 2.8 | 23.4 ± 2.5 |
| Barthel test | |||
| Independent | 8 (3) | 3 (2) | 5 (8) |
| Mild | 10 (4) | 5 (3) | 5 (8) |
| Moderate | 65 (26) | 44 (23) | 21 (34) |
| Severe | 78 (32) | 63 (34) | 15 (24) |
| Total | 87 (35) | 71 (38) | 16 (26) |
| Lawton and Brody test | |||
| Moderate | 9 (4) | 7 (4) | 2 (3) |
| Severe | 51 (21) | 33 (18) | 18 (29) |
| Total | 187 (75) | 145 (78) | 42 (68) |
| FAC | 1.2 ± 1.6 | 1.0 ± 1.4 | 1.8 ± 1.9 |
| Pfeiffer test | |||
| Intact | 50 (22) | 31 (18) | 19 (37) |
| Mild | 33 (14) | 25 (14) | 8 (15) |
| Moderate | 50 (22) | 36 (20) | 14 (27) |
| Severe | 95 (42) | 84 (48) | 11 (21) |
| Texture of diet | |||
| Regular | 128 (63) | 91 (59) | 37 (72) |
| Puréed | 27 (13) | 23 (15) | 4 (8) |
| Mixed | 50 (24) | 40 (26) | 10 (20) |
| MNA-SF | |||
| Normal nutritional status | 67 (27) | 40 (21) | 27 (44) |
| At risk of malnutrition | 139 (56) | 113 (61) | 26 (42) |
| Malnourished | 43 (17) | 34 (18) | 9 (14) |
| Sarcopenia | |||
| Yes | 143 (63) | 119 (68) | 24 (46) |
| No | 84 (37) | 56 (32) | 28 (54) |
Notes: Results are expressed as means (X) with standard deviation (SD) or number of participants (N) with percentage (%). There was one loss to follow-up for the main variable and for some secondary variables due to the characteristics of participants, but less than 10% of data were lost for any variable. Abbreviations: BMI, body mass index; CC, calf circumference; FAC, Functional Ambulation Classification; MNA-SF, Mini Nutritional Assessment-Short Form; MUAMC, mid-upper arm muscle circumference.
Table 2 exhibits the mean dietary intakes of participants over a 7-day period by sex as measured by the precise weighing method and compares them with DRIs. In both sexes, mean intakes were below dietary recommendations for fiber, potassium, calcium, magnesium, zinc, iodine, vitamins D, E, B3, and B6, and folic acid. Intakes below dietary recommendations were also observed for selenium in females and for vitamin B1 in males.
Table 2.
Nutrient intake in care home participants.
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Nutrients | EAR | X (SE) | 95% IC (lower bound; upper bound) |
X (SE) | 95% IC (lower bound; upper bound) |
P |
| Total energy (kcal/day) | 1542.86 (19.06) | 1505.28; 1580.43 | 1706.38 (33.01) | 1641.29; 1771.46 | <0.001 | |
| Protein (g/day) | 57.55 (0.76) | 56.04; 59.06 | 62.05 (1.32) | 59.44; 64.67 | 0.004 | |
| Protein, g/kg of BW | 0.66 | 1.00 (0.02) | 0.96; 1.03 | 0.92 (0.03) | 0.85; 0.98 | 0.035 |
| Carbohydrates (g/day) | 100 | 208.98 (2.75) | 203.55; 214.41 | 229.71 (4.77) | 220.31; 239.11 | <0.001 |
| Fiber (g/day) | 21F/30 M ǂ | 14.60 (0.35) | 13.91; 15.29 | 15.00 (0.60) | 13.81; 16.19 | 0.566 |
| Lipids (g/day) | 52.98 (1.17) | 50.68; 55.28 | 59.92 (2.02) | 55.94; 63.91 | 0.003 | |
| SFA (g/day) | 14.34 (0.43) | 13.50; 15.18 | 15.86(0.74) | 14.41; 17.31 | 0.075 | |
| MUFA (g/day) | 17.56(0.58) | 16.42; 18.70 | 20.61 (1.00) | 18.64; 22.58 | 0.009 | |
| MUFA C16:1 (g/day) | 1.19 (0.04) | 1.11; 1.27 | 1.32 (0.07) | 1.18; 1.46 | 0.122 | |
| MUFA C18:1(g/day) | 15.16 (0.53) | 14.12; 16.21 | 18.11 (0.92) | 16.30; 19.92 | 0.006 | |
| PUFA (g/day) | 5.89 (0.24) | 5.41; 6.37 | 6.60 (0.42) | 5.78; 7.44 | 0.142 | |
| PUFA C18:2 (g/day) | 4.90 (0.23) | 4.46; 5.35 | 5.62 (0.39) | 4.85; 6.39 | 0.113 | |
| PUFA C18:3 (g/day) | 0.25 (0.01) | 0.23; 0.27 | 0.29 (0.02) | 0.26; 0.33 | 0.048 | |
| Cholesterol (mg/day) | 203.83 (5.23) | 193.51; 214.14 | 232.83 (9.07) | 214.96; 250.71 | 0.006 | |
| Potassium (mg/day) | 4700 ǂ | 1805.22 (27.33) | 1751.34; 1859.09 | 1953.31 (47.34) | 1859.98 ; 2046.64 | 0.007 |
| Calcium (mg/day) | 1000 | 927.86 (9.98) | 908.19; 947.52 | 997.26 (17.28) | 963.20; 1031.33 | 0.001 |
| Phosphorus (mg/day) | 580 | 1013.41 (13.04) | 987.70; 1039.12 | 1093.64 (22.59) | 1049.11 ; 1138.18 | 0.002 |
| Magnesium (mg/day) | 265 F/350 M | 196.41 (2.95) | 190.60; 202.22 | 208.18 (5.10) | 198.11; 218.24 | 0.047 |
| Iron (mg/day) | 5 F/6 M | 7.27 (0.13) | 7.02; 7.52 | 8.00 (0.22) | 7.56; 8.44 | 0.005 |
| Zinc (mg/day) | 6.8 F/9.4 M | 5.64 (0.13) | 5.38; 5.91 | 6.35 (0.23) | 5.90; 6.81 | 0.009 |
| Selenium (µg/day) | 45 | 44.27 (1.48) | 41.36; 47.18 | 51.78 (2.56) | 46.74; 56.82 | 0.012 |
| Iodine (µg/day) | 95 | 29.89 (2.10) | 25.75; 34.03 | 32.66 (3.64) | 25.49; 39.83 | 0.511 |
| Copper (µg/day) | 700 | 775.43 (17.18) | 741.56; 809.31 | 801.35 (29.77) | 742.66; 860.05 | 0.452 |
| Vitamin A (µg/day) | 500 F/625 M | 1306.26 (30.64) | 1245.84; 1366.68 | 1413.06 (53.12) | 1308.33 ; 1517.79 | 0.083 |
| Vitamin D (µg/day) | 10 | 1.37 (0.06) | 1.25; 1.50 | 1.57 (0.11) | 1.35; 1.78 | 0.126 |
| Vitamin E (mg/day) | 12 | 4.60 (0.17) | 4.26; 4.94 | 5.61 (0.30) | 5.02; 6.20 | 0.004 |
| Vitamin C (mg/day) | 60 F/75 M | 71.85 (2.28) | 67.36; 76.35 | 80.80 (3.95) | 73.02; 88.59 | 0.051 |
| Vitamin B1 (mg/day) | 0.9 F/1 M | 0.93 (0.02) | 0.89; 0.96 | 0.98 (0.03) | 0.91; 1.05 | 0.172 |
| Vitamin B2 (mg/day) | 0.9 F/1.1 M | 1.30 (0.02) | 1.27; 1.33 | 1.38 (0.03) | 1.32; 1.44 | 0.017 |
| Vitamin B3 (mg/day) | 11 F/12 M | 9.14 (0.21) | 8.72; 9.56 | 9.66 (0.37) | 8.94; 10.40 | 0.217 |
| Vitamin B6 (mg/day) | 1.3 F/1.4 M | 1.16 (0.02) | 1.11; 1.20 | 1.20 (0.04) | 1.13; 1.27 | 0.316 |
| Vitamin B12 (µg/day) | 2 | 3.09 (0.06) | 2.98; 3.20 | 3.48 (0.10) | 3.28; 3.67 | 0.001 |
| Folic acid (µg/day) | 320 | 128.53 (2.54) | 123.53; 133.53 | 142.79 (4.39) | 134.12; 151.45 | 0.005 |
Note: Results are expressed as means (X) with standard error (SE) and 95% confidence interval (CI). Marginal means resulting from the mixed linear regression models are shown. Abbreviations: AI, adequate intake; BW, body weight; EAR, estimated average requirement; F, female; M, male; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids. ǂ AI instead of EAR.
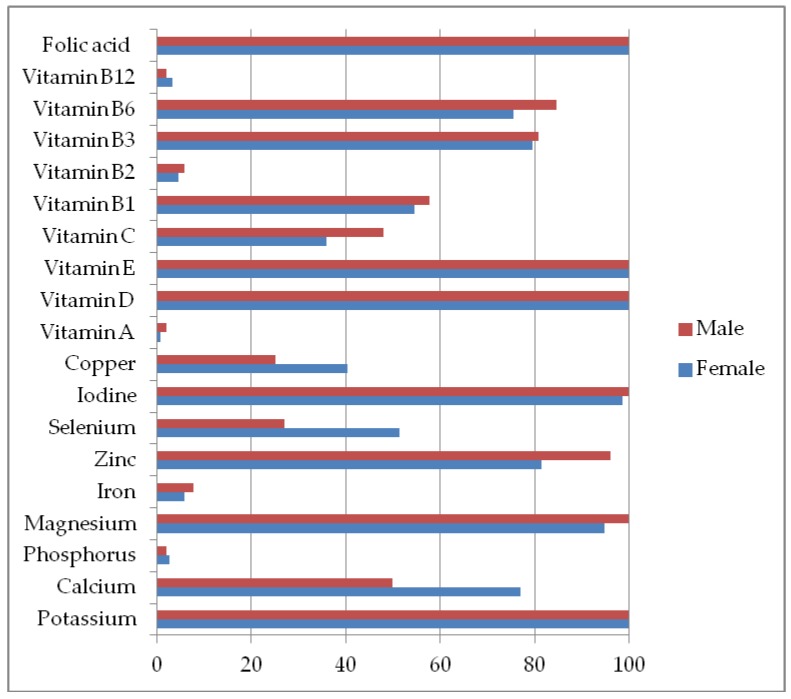
As shown in Figure 1, the intake of potassium, magnesium, zinc, iodine, vitamin D, E, and folic acid was low in >80 % of the females and males, the intake of vitamins B6 and B3 was low in >80 % of the males and the intake of fiber was low in 89% of the females and 100% of the males.
Figure 1.
Percentage of participants not reaching the Dietary Reference Intakes for micronutrients.
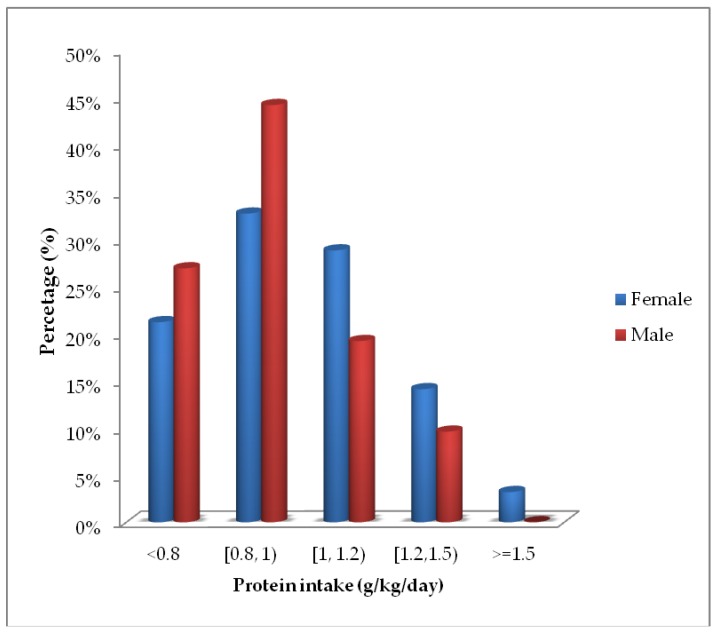
The mean daily intake of protein per kg of body weight was 1.00 (95% CI: 0.96, 1.03) g in females and 0.92 (95% CI: 0.85, 0.98) g in males. Figure 2 depicts the percentage of residents of each sex that did not reach the RDA for protein (0.8 g/kg/day) and the percentage that met recommendations of the ESPEN and PROT-AGE group (1–1.2 g/kg/day and 1.2–1.5 g/kg/day). Protein intake was <0.8 g/kg/day in 23% of participants, 0.8–1 g/kg/day by 36%, 1–1.2 g/kg/day in 26%, 1.2–1.5 g/kg/day in 13%, and >1.5 g/kg/day in 2%.
Figure 2.
Percentage of female and male residents who did not reach the Recommended Dietary Allowance (RDA) for protein and the percentage that met ESPEN and PROT-AGE group recommendations in g/kg weight/day.
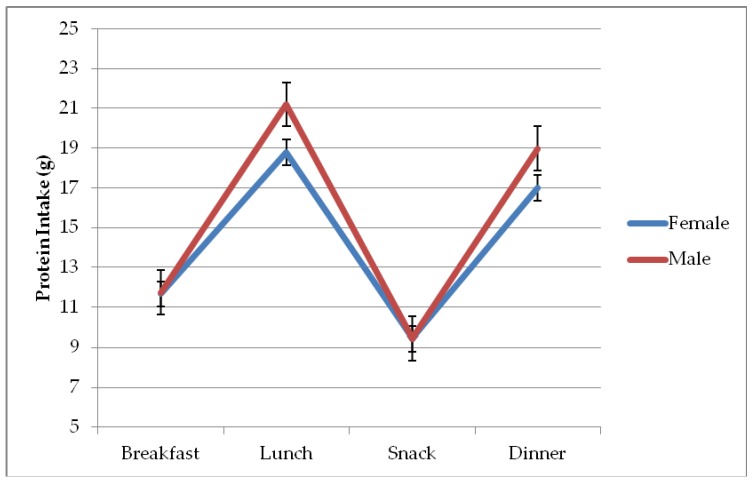
Figure 3 depicts the differences in mean protein intake among the different meals consumed in a day, being 11.66 (95% CI: 11.02, 12.29) g for females and 11.73 (95% CI: 10.63, 12.84) g for males at breakfast, 18.80 (95% CI: 18.16, 19.43) g for females and 21.20 (95% CI: 20.09, 22.30) g for males at lunch; 9.42 (95% CI: 8.78, 10.06) g for females and 9.44 (95% CI: 8.33,10.54) g for males at afternoon snack; and 17.00 (95% CI: 16.36, 17.64) g for females and 18.98 (95% CI: 17.87, 20.08) g for males at evening meal.
Figure 3.
Protein intake of participants in each main meal by sex.
The cluster analysis derived in two dietary patterns, with one (designated "poorer diet") characterized by lower intakes in comparison to the other (designated "better diet"). Table 3 lists the intake variables in order of their importance for the identification of dietary patterns and gives the mean value for these variables in each of the two patterns identified. Although the nutritional quality was better in one dietary pattern than in the other, the mean intake of folic acid, potassium, energy, iron, lipids, monounsaturated fatty acids (MUFA), protein, MUFA C18:1, cholesterol, vitamin B2, phosphorous, zinc, vitamin B12, vitamin E, and magnesium was below recommendations in both of them.
Table 3.
Characterization of dietary patterns as a function of intake variables in order of importance.
| Poorer diet (n = 112) | Better Diet (n = 96) | ||||
|---|---|---|---|---|---|
| X (SD) | 95% CI | X (SD) | 95% CI | ||
| Folic acid | 1 | 110.66 (20.74) | (106.78–114.54) | 157.01 (24.31) | (152.08–161.93) |
| Potassium | 1 | 1611.79 (258.82) | (1563.33–1660.25) | 2110.48 (221.04) | (2065.7–2155.27) |
| Energy | 0.99 | 1419.63 (172) | (1387.42–1451.83) | 1774.76 (174.96) | (1739.31–1810.21) |
| Iron | 0.97 | 6.37 (1.22) | (6.14–6.6) | 8.71 (1.08) | (8.49–8.93) |
| Lipid | 0.97 | 44.95 (10.82) | (42.93–46.98) | 66.07 (10.06) | (64.03–68.11) |
| Vitamin B1 | 0.91 | 0.78 (0.16) | (0.75–0.81) | 1.12 (0.19) | (1.08–1.16) |
| MUFA | 0.87 | 13.69 (5.33) | (12.69–14.68) | 23.72 (5.38) | (22.63–24.81) |
| Protein | 0.83 | 52.6 (7.44) | (51.2–53.99) | 65.73 (7) | (64.31–67.14) |
| MUFA C18:1 | 0.83 | 11.73 (5) | (10.79–12.66) | 20.76 (5.01) | (19.74–21.77) |
| Cholesterol | 0.79 | 170.32 (53.34) | (160.33–180.3) | 258.07 (46.06) | (248.73–267.4) |
| Vitamin B2 | 0.74 | 1.2 (0.16) | (1.17–1.23) | 1.46 (0.16) | (1.43–1.5) |
| Phosphorous | 0.73 | 934.98 (133.16) | (910.05–959.92) | 1147.99 (122.66) | (1123.13–1172.84) |
| Zinc | 0.7 | 4.84 (1.14) | (4.62–5.05) | 6.97 (1.52) | (6.66–7.28) |
| Vitamin B12 | 0.68 | 2.76 (0.59) | (2.65–2.87) | 3.67 (0.56) | (3.56–3.78) |
| Vitamin E | 0.62 | 3.64 (1.62) | (3.33–3.94) | 6.27 (1.91) | (5.88–6.66) |
| Magnesium | 0.62 | 178.9 (29.35) | (173.4–184.39) | 223.19 (30.47) | (217.01–229.36) |
| PUFA | 0.57 | 4.44 (1.99) | (4.07–4.82) | 7.96 (2.97) | (7.35–8.56) |
| Selenium | 0.55 | 36.32 (15.14) | (33.49–39.16) | 57.6 (15.81) | (54.39–60.8) |
| MUFA C16:1 | 0.53 | 0.96 (0.42) | (0.88–1.03) | 1.53 (0.44) | (1.44–1.62) |
| PUFA C18:2 | 0.52 | 3.63 (1.92) | (3.27–3.98) | 6.77 (2.77) | (6.21–7.33) |
| Vitamin C | 0.5 | 59.55 (22.47) | (55.34–63.76) | 90.98 (25.83) | (85.75–96.21) |
| Calcium | 0.46 | 882.98 (108.08) | (862.74–903.22) | 1017.72 (109.92) | (995.45–1039.99) |
| Vitamin B6 | 0.44 | 1.04 (0.24) | (0.99–1.08) | 1.32 (0.22) | (1.27–1.36) |
| PUFA C18:3 | 0.43 | 0.2 (0.1) | (0.18–0.22) | 0.33 (0.12) | (0.31–0.35) |
| Vitamin A | 0.41 | 1152.95 (319.78) | (1093.08–1212.83) | 1539.86 (349.54) | (1469.03–1610.68) |
| Vitamin B3 | 0.4 | 8.04 (2.24) | (7.62–8.46) | 10.69 (2.45) | (10.2–11.19) |
| Fiber | 0.38 | 12.75 (3.38) | (12.12–13.38) | 16.97 (4.27) | (16.1–17.84) |
| SFA | 0.36 | 12.41 (4.06) | (11.65–13.17) | 17.4 (5.4) | (16.31–18.49) |
| Copper | 0.35 | 689.34 (216.88) | (648.73–729.95) | 888.03 (155.15) | (856.6–919.47) |
| Carbohydrates | 0.25 | 201.17 (29.37) | (195.67–206.67) | 229.31 (36.01) | (222.01–236.6) |
| Vitamin D | 0.24 | 1.14 (0.67) | (1.01–1.26) | 1.75 (0.81) | (1.58–1.91) |
| Iodine | 0.17 | 22.8 (19.87) | (19.08–26.52) | 39.64 (29.63) | (33.64–45.65) |
Note: Results are expressed as means (X) with standard deviation (SD) and 95% confidence interval (CI). Abbreviations: MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
As it is shown in Table 4, the results of the predictive logistic analysis revealed significant effects on dietary pattern (dependent variable: poorer vs. better diet) of sex, texture of diet, and sarcopenia (independent variables). The risk of a poorer diet was 3.2-fold higher for the females than for the males (p = 0.005), 4.6-fold lower for those consuming a regular versus mixed diet (p < 0.001) and 2.79-fold higher in participants with versus without sarcopenia (p = 0.004). There was no significant difference in risk between puréed and mixed diets or between regular and puréed diets. Likewise, no significant effect was found for nutritional status.
Table 4.
Parameter estimates from the multivariate logistic regression model fitted to the dietary patterns.
| Parameter Estimates | |||||||
|---|---|---|---|---|---|---|---|
| Poorer diet vs. Better diet | 95% Wald Confidence interval for Exp(B) | Hypothesis Test | |||||
| Parameter | B | Exp(B) | Lower | Upper | Wald Chi-Square | Df | Sig. |
| Female vs. Male | 1.165 | 3.205 | 1.416 | 7.253 | 7.808 | 1 | 0.005 |
| Regular diet vs. Mix diet | −1.541 | 0.214 | 0.090 | 0.508 | 12.245 | 1 | 0.000 |
| Puréed diet vs. Mix diet | −1.079 | 0.340 | 0.100 | 1.153 | 3.000 | 1 | 0.083 |
| MNA-SF Normal vs. Malnourished | −1.045 | 0.352 | 0.111 | 1.113 | 3.161 | 1 | 0.075 |
| MNA-SF At risk vs. Malnourished | −0.276 | 0.759 | 0.271 | 2.123 | 0.276 | 1 | 0.599 |
| Sarcopenia vs. No sarcopenia | 1.026 | 2.790 | 1.394 | 5.584 | 8.401 | 1 | 0.004 |
Note: Age was not a significant factor and did not improve the goodness of fit of the model (p > 0.1). MNA-SF: Mini Nutritional Assessment-Short Form.
The results of predictive classification as a function of the variables included in the model show that 74.5% of patients were correctly classified in the corresponding pattern. The area under the ROC curve was 0.79 (95 % CI: 0.73, 0.86), indicating the good fit of the predictive model.
4. Discussion
In this study, the precise weighing method was used to analyze the diet of LTC residents who were generally characterized by a high age, low socioeconomic level, and frequent functional and cognitive impairment. The main finding was of an intake below dietary recommendations of protein, fiber, and certain vitamins (vitamins D, E, B3, and B6, and folic acid), and minerals (potassium, calcium, magnesium, zinc, iodine) in both sexes and of selenium in females and vitamin B1 in males.
Comparisons with findings of the few previous studies in LTC homes are hampered by differences in dietary assessment methods, dietary recommendations considered, and databases used [11,35,36,37,38]. However, although results vary among studies, the observation of inadequate intakes in institutionalized elderly people has been a frequent finding.
The protein intake of 22.6% of these residents was below the RDA (0.8 g/kg/day), and this in itself may be inadequate to maintain muscle health in the elderly [26,27,39,40]. The ESPEN and PROT-AGE group recommend 1–1.2 g/kg/day, an amount consumed by 26% of the study population, and a higher intake of 1.2–1.5 g/kg/day for those with acute or chronic disease, which was consumed by 13% of participants [26,27]. With regard to the distribution of this intake during the day, the PROT-AGE group proposed that elderly people should consume 25–30 g at each main meal (breakfast, lunch, evening) [26], and other authors recommended an intake of >30 g per main meal to maintain muscle mass while controlling fat mass [40]. These recommendations for the distribution of protein intake were not met in the present study. The largest protein intake was at lunch [18.80 [95% CI: 18.16, 19.43] g for females and 21.20 [95% CI: 20.09, 22.30] g for males). There has been limited research on this issue, with one study of older adults in a rehabilitation center finding that protein recommendations per meal were usually not met at breakfast or lunch except when certain foods were substituted by protein-enriched products [41].
The residents in this study were considered to have a “poorer” or “better” diet according to their dietary pattern as established by cluster analysis. The results of the logistic model show a significant association of sex, diet texture, and the presence of sarcopenia with dietary patterns. With respect to the texture of diet, participants with a regular diet had significantly lower risk of a poorer versus those with a mixed diet. Those who consumed a puréed diet had a higher risk of a poorer diet, although the difference was not significant. In a previous study, we found that the menus offered in these LTC homes [23] were below the EAR or AI for the minerals potassium, magnesium, zinc, iodine, calcium, and selenium and for vitamins D, E, C, B3, and folate in some or all cases. The lowest energy and macro/micro-nutrient values were observed in the puréed menus. Given these findings, the nutrient intake of the residents was expected to be inadequate. The lower energy and nutrient values in puréed than regular menus may explain the significant association observed between diet texture and dietary pattern.
Iuliano et al. [42] raised concerns about the nutritional value of menus offered by LTC homes in Australia, observing that the intake of residents was below national dietary recommendations for calcium, zinc, magnesium, potassium, folate, and dietary fiber and included excessive sodium (3-fold higher than recommendations) and sugars. In addition, many of the residents did not meet recommendations for energy or protein intake. An insufficient intake of nutrients by the elderly is frequently attributed to their lack of appetite [43], but the aforementioned study found that the inadequacy of the meals provided by the home played a more important role. With regard to the quality of the menus offered by homes, puréed diets are of special interest and concern due to the work involved in their preparation and their low nutritional content, as found in the present study. Keller et al. [2] highlighted the need to improve the nutritional quality of puréed food, Dahl et al. [44] concluded that puréed food prepared in diets in Canadian LTC homes contained inadequate levels of protein, and Vucea et al. [45] reported a significant association between the consumption of a soft or puréed diet and a higher risk of malnutrition. Importantly, the energy or nutrient requirements of individuals needing a texture-modified diet do not differ from those of people with the same age and sex except in the presence of disease [46]; therefore, a puréed menu should meet the same general dietary recommendations. The texture of the diet (e.g., puréed) was prescribed for each resident according to their needs by the physicians at the residences, who are usually responsible for designing these menus in Spain. The staff responsible for serving the food (servers or kitchen assistants) would at times take into account the appetite, preferences or aversions of residents.
The risk of a poorer diet was 2.79-fold higher in the participants diagnosed with sarcopenia than in those who were not. Given that 63 % of the residents had sarcopenia, deficiencies in the diet of residents are a matter of major concern. An optimal intake of nutrient intakes is essential for the prevention and treatment of sarcopenia [9,10,47,48]. Besides the relationship of this condition with the inadequate consumption of protein, recent studies have described the need for the intake of vitamin D, an appropriate omega 6/omega 3 ratio [49,50], selenium, calcium, and magnesium [6]. There has been less research interest in the influence of overall diet quality on sarcopenia until recently [51]. Robinson et al. [52] underlined the importance of healthier dietary patterns in elderly people to ensure an adequate intake of proteins, vitamin D, antioxidant nutrients, and omega 3 (eicosapentaenoic and docosahexaenoic acids), given their potential role in sarcopenia prevention and treatment.
In regard to nutritional status, it was previously observed that energy, protein, and micronutrient intakes are frequently low in malnourished residents or in those at risk of malnutrition [13,38]. In the present study, the intake of nutrients was not only inadequate in residents who were malnourished or at risk of malnutrition but also in those with normal nutritional status. Other authors have observed a poor energy and vitamin intake in a large proportion of elderly people classified as having normal nutritional status [53]. These results support the need to evaluate the actual dietary intake of residents as well as assessing their nutritional status with instruments such as the MNA-SF.
The strengths of this study include the use of validated methodologies by trained professionals. The precise weighing method is considered the gold standard, and data were gathered on 7 consecutive days for each participant. Cluster analysis permitted the identification of dietary patterns, enabling evaluation of the dietary intake as a whole rather than particular components.
The main limitation was the loss of some data due to the severe cognitive and functional impairment of some participants. Food in addition to the meals provided by the home was consumed by some residents, risking an underestimation of their intake; however, the extra intake was taken into account when detected by researchers or home staff.
5. Conclusions
The dietary intake of institutionalized elderly people in this study did not meet nutritional recommendations. The total amount of protein consumed per day and per meal did not meet the guidelines of the ESPEN and PROT-AGE group. Sex, texture of diet, and sarcopenia were associated with dietary intake as a function of the dietary pattern identified by cluster analysis. The risk of a poorer diet was higher in females and residents with sarcopenia and was lower in those consuming regular diets. However, this risk was not significantly affected by the nutritional status of participants, and nutritional recommendations were not fully met by any residents, whether they received a poorer or better diet. According to these findings, the nutritional intake of the residents of LTC homes, an especially vulnerable population group, remains inadequate. It is, therefore, necessary for action to be taken to ensure a sufficient food intake by each and every resident. This requires the special attention of properly trained health care professionals and the provision of meals that meet dietary recommendations, taking particular account of scientific evidence published on the supply of proteins.
Acknowledgments
The authors thank Llenalia Garcia and Ángela Hernández for their assistance with the statistical analysis, Richard and Layla Davies for their assistance with the English version, and the staff and residents from each LTC home for their support. They are also grateful to Nutrition and Dietetics students for their collaboration in the research. This study formed part of the doctoral thesis by Ana Rodríguez-Rejón, carried out under the “Human Nutrition Program” at the University of Granada. She was supported by a Research Fellowship from the Government of Spain.
Author Contributions
Conceptualization, A.I.R.-R., M.D.R.-L. and R.A.; Data curation, A.I.R.-R. Formal analysis, A.I.R.-R. Investigation, A.I.R.-R., M.D.R.-L. and R.A.; Methodology, A.I.R.-R., M.D.R.-L. and R.A. Supervision, M.D.R.-L. and R.A. Writing—original draft, A.I.R.-R. Writing—review & editing, A.I.R.-R., M.D.R.-L. and R.A.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Morley J.E., Caplan G., Cesari M., Dong B., Flaherty J.H., Grossberg G.T., Holmerova I., Katz P.R., Koopmans R., Little M.O., et al. International survey of nursing home research priorities. J. Am. Med. Dir. Assoc. 2014;15:309–312. doi: 10.1016/j.jamda.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Keller H.H., Carrier N., Slaughter S.E., Lengyel C., Steele C.M., Duizer L., Morrison J., Brown K.S., Chaudhury H., Yoon M.N., et al. Prevalence and Determinants of Poor Food Intake of Residents Living in Long-Term Care. J. Am. Med. Dir. Assoc. 2017;18:941–947. doi: 10.1016/j.jamda.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Granic A., Mendonça N., Hill T.R., Jagger C., Stevenson E.J., Mathers J.C., Sayer A.A. Nutrition in the very old. Nutrients. 2018;10:269. doi: 10.3390/nu10030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Rejón A.I., Ruiz-López M.D., Wanden-Berghe C., Artacho R. Prevalence and Diagnosis of Sarcopenia in Residential Facilities: A Systematic Review. Adv. Nut. 2018 doi: 10.1093/advances/nmy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dronkelaar C., van Velzen A., Abdelrazek M., van der Steen A., Weijs P.J.M., Tieland M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018;19:6–11. doi: 10.1016/j.jamda.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Lappe J.M., Binkley N. Vitamin D and Sarcopenia/Falls. J. Clin. Densitom. 2015;18:478–482. doi: 10.1016/j.jocd.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Ates Bulut E., Soysal P., Ekrem A., Dokuzlar O., Emre S., Turan A. Vitamin B12 deficiency might be related to sarcopenia in older adults. Exp. Gerontol. 2017;95:136–140. doi: 10.1016/j.exger.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Marzetti E., Calvani R., Tosato M., Cesari M., Di Bari M., Cherubini A., Collamati A., D’Angelo E., Pahor M., Bernabei R., et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017;29:11–17. doi: 10.1007/s40520-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 10.Dennison E.M., Sayer A.A., Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat. Rev. Rheumatol. 2017;13:340–347. doi: 10.1038/nrrheum.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assis B.S., Jairza J.M.B., Lopes J.A., Roriz A.K.C., Melo A.L., Previdell A., Aquino R.C., Ramos L.B. Micronutrient intake in elderly living in nursing homes. Nutr. Hosp. 2018;35:59–64. doi: 10.20960/nh.1348. [DOI] [PubMed] [Google Scholar]
- 12.Yago Torregrosa M.D., Martínez de Victoria E., Mañas Almendros M. Evaluación del estado nutricional: Valoración dietética. In: Gil-Campos M., Martínez de Victoria E., Maldonado J., editors. Tratado de Nutrición. Tomo IV. Nutrición Humana en el Estado de Salud. 3rd ed. Panamericana, S.A.; Madrid, España: 2017. [Google Scholar]
- 13.Buckinx F., Allepaerts S., Paquot N., Reginster J.Y., de Cock C., Petermans J., Bruyère O. Energy and nutrient content of food served and consumed by nursing home residents. J. Nutr. Health Aging. 2017;21:727–732. doi: 10.1007/s12603-016-0782-2. [DOI] [PubMed] [Google Scholar]
- 14.Ocké M.C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013;72:191–199. doi: 10.1017/S0029665113000013. [DOI] [PubMed] [Google Scholar]
- 15.Newby P.K., Tucker K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004;62:177–203. doi: 10.1111/j.1753-4887.2004.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Rejón A.I., Artacho R., Puerta A., Zuñiga A., Ruiz-Lopez M.D. Diagnosis of Sarcopenia in Long-Term Care Homes for the Elderly: The Sensitivity and Specificity of Two Simplified Algorithms with Respect to the EWGSOP Consensus. J. Nutr. Health Aging. 2018;22:796–801. doi: 10.1007/s12603-018-1004-x. [DOI] [PubMed] [Google Scholar]
- 17.Berral de la Rosa F., Del Águila-Quirós D. Nutritional/anthropometric assessment of patients staying in bed or at hospital. Arch. Med. Deporte. 2002;19:129–135. [Google Scholar]
- 18.Mahoney F., Barthel D. Functional evaluation: The Barthel Index. MD State Med. J. 1965;71:61–65. [PubMed] [Google Scholar]
- 19.Lawton M.P., Brody E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 20.Holden M.K., Gill K.M., Magliozzi M.R., Nathan J., Piehl-Baker L. Clinical Gait Assessment in the Neurologically Impaired: Reliability and Meaningfulness. Phys. Ther. 1984;64:35–40. doi: 10.1093/ptj/64.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser M., Bauer J., Ramsch C., Uter W., Guigoz Y., Cederholm T., Thomas D.R., Anthony P., Charlton K.E., Maggio M., et al. Validation of the Mini Nutritional Assessment Short-Form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging. 2009;13:782–788. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Rejón A.I., Ruiz-López M.D., Malafarina V., Puerta A., Zuñiga A., Artacho R. Menus offered in long-term care homes: Quality of meal service and nutritional analysis. Nutr. Hosp. 2017;34:584–592. doi: 10.20960/nh.941. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine . Dietary Reference Intake: Applications in Dietary Assessment. National Academy Press; Washington, DC, USA: 2000. [Google Scholar]
- 25.Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin, D. National Academy Press; Washington, DC, USA: 2011. [Google Scholar]
- 26.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D., et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE study group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Deutz N.E., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A., Cederholm T., Cruz-Jentoft A., Krznariç Z., Nair K.S., et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardiner J.L., Roman L.A. Fixed effects, random effects and GEE: What are the differences? Stat. Med. 2009;28:221–239. doi: 10.1002/sim.3478. [DOI] [PubMed] [Google Scholar]
- 29.Twisk J.W.R. Applied Longitudinal Data Analysis for Epidemiology. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- 30.Rubio-Hurtado M.J., Vilà-Baños R. El análisis de conglomerados bietápico o en dos fases con SPSS. REIRE. 2017;10:118–126. [Google Scholar]
- 31.Kaufman L., Rousseeuw P.J. Finding Groups in Data: An Introduction to Cluster Analysis. John Wiley & Sons; New York, NY, USA: 1990. [Google Scholar]
- 32.Rousseeuw P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. doi: 10.1016/0377-0427(87)90125-7. [DOI] [Google Scholar]
- 33.Rawlings J.O., Pantula S.G., Dickey D. Applied Regression Analysis. 2nd ed. Springer; New York, NY, USA: 1998. [Google Scholar]
- 34.Harrel J.F. Regression Modeling Strategies. Springer; New York, NY, USA: 2001. [Google Scholar]
- 35.Milà R., Abellana R., Padró L., Farran A. Assessment of food consumption, energy and protein intake in the meals offered in four Spanish nursing homes. Nutr. Hosp. 2012;27:914–921. doi: 10.3305/nh.2012.27.3.5730. [DOI] [PubMed] [Google Scholar]
- 36.Engelheart S., Akner G. Dietary intake of energy, nutrients and water in elderly people living at home or in nursing home. J. Nutr. Health Aging. 2015;19:265–272. doi: 10.1007/s12603-015-0440-0. [DOI] [PubMed] [Google Scholar]
- 37.Rumbak I., Šatalić Z., Keser I., Krbavčić I.P., Giljević Z., Zadro Z., Barić I.C. Diet quality in elderly nursing home residents evaluated by diet quality Index Revised (DQI-R) Coll. Antropol. 2010;34:577–585. [PubMed] [Google Scholar]
- 38.Ongan D. Nutritional status and dietary intake of institutionalized elderly in Turkey: A cross-sectional, multi-center, country representative study. Arch. Gerontol. Geriatr. 2015;61:271–276. doi: 10.1016/j.archger.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Morley J.E., Argiles J.M., Evans W.J., Bhasin S., Cella D., Deutz N.E., Doehner W., Fearon K.C., Ferrucci L., Hellerstein M.K., et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2008;11:391–396. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paddon-Jones D., Leidy H. Dietary protein and muscle in older persons. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:5–11. doi: 10.1097/MCO.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Til A.J., Naumann E., Cox-Claessens I.J., Kremer S., Boelsma E., de van der Schueren M.A. Effects of the daily consumption of protein enriched bread and protein enriched drinking yoghurt on the total protein intake in older adults in a rehabilitation centre: A single blind randomised controlled trial. J. Nutr. Health Aging. 2015;19:525–530. doi: 10.1007/s12603-015-0471-6. [DOI] [PubMed] [Google Scholar]
- 42.Iuliano S., Olden A., Woods J. Meeting the nutritional needs of elderly residents in aged-care: Are we doing enough? J. Nutr. Health Aging. 2013;17:503–508. doi: 10.1007/s12603-013-0042-7. [DOI] [PubMed] [Google Scholar]
- 43.Malafarina V., Uriz-Otano F., Gil-Guerrero L., Iniesta R. The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas. 2013;74:293–302. doi: 10.1016/j.maturitas.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Dahl W.J., Whiting S.J., Tyler R.T. Protein content of pureed diets: Implications for planning. Can. J. Diet. Pract. Res. 2007;68:99–102. doi: 10.3148/68.2.2007.99. [DOI] [PubMed] [Google Scholar]
- 45.Vucea V., Keller H.H., Ducak K. Interventions for Improving Mealtime Experiences in Long-Term Care. J. Nutr. Gerontol. Geriatr. 2014;33:249–324. doi: 10.1080/21551197.2014.960339. [DOI] [PubMed] [Google Scholar]
- 46.De Luis D., Aller R., Izaola O. Modified texture diet and useful in patients with nutritional risk. Nutr. Hosp. 2014;29:751–759. doi: 10.3305/nh.2014.29.4.7003. [DOI] [PubMed] [Google Scholar]
- 47.Beaudart C., Dawson A., Shaw S.C., Harvey N.C., Kanis J.A., Binkley N., Reginster J.Y., Chapurlat R., Chan D.C., Bruyère O., et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017;28:1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martone A.M., Marzetti E., Calvani R., Picca A., Tosato M., Santoro L., Di Giorgio A., Nesci A., Sisto A., Santoliquido A., et al. Exercise and Protein Intake: A Synergistic Approach against Sarcopenia. Biomed. Res. Int. 2017 doi: 10.1155/2017/2672435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rondanelli M., Faliva M., Monteferrario F., Peroni G., Repaci E., Allieri F., Perna S. Novel insights on nutrient management of sarcopenia in elderly. Biomed. Res. Int. 2015 doi: 10.1155/2015/524948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattoa H.P., Konstantynowicz J., Laszcz N., Wojcik M., Pludowski P. Vitamin D: Musculoskeletal health. Rev. Endocr. Metab. Disord. 2016:1–9. doi: 10.1007/s11154-016-9404-x. [DOI] [PubMed] [Google Scholar]
- 51.Bloom I., Shand C., Cooper C., Robinson S., Baird J. Diet quality and sarcopenia in older adults: A systematic review. Nutrients. 2018;10:308. doi: 10.3390/nu10030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson S.M., Reginster J.Y., Rizzoli R., Shaw S.C., Kanis J.A., Bautmans I., Bischoff-Ferrari H., Bruyère O., Cesari M., Dawson-Hughes B., et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018;37:1121–1132. doi: 10.1016/j.clnu.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jyväkorpi S.K., Pitkälä K.H., Puranen T.M., Björkman M.P., Kautiainen H., Strandberg T.E., Soini H.H., Suominen M.H. High proportions of older people with normal nutritional status have poor protein intake and low diet quality. Arch. Gerontol. Geriatr. 2016;67:40–45. doi: 10.1016/j.archger.2016.06.012. [DOI] [PubMed] [Google Scholar]