Abstract
Temperature rising caused by global warming has imposed significant negative effects on crop qualities and yields. To get the well-known molecular mechanism upon the higher temperature, we carefully analyzed the RNA sequencing-based transcriptomic responses of two contrasting chieh-qua genotypes: A39 (heat-tolerant) and H5 (heat-sensitive). In this study, twelve cDNA libraries generated from A39 and H5 were performed with a transcriptome assay under normal and heat stress conditions, respectively. A total of 8705 differentially expressed genes (DEGs) were detected under normal conditions (3676 up-regulated and 5029 down-regulated) and 1505 genes under heat stress (914 up-regulated and 591 down-regulated), respectively. A significant positive correlation between RNA-Seq data and qRT-PCR results was identified. DEGs related to heat shock proteins (HSPs), ubiquitin-protein ligase, transcriptional factors, and pentatricopeptide repeat-containing proteins were significantly changed after heat stress. Several genes, which encoded HSPs (CL2311.Contig3 and CL6612.Contig2), cytochrome P450 (CL4517.Contig4 and CL683.Contig7), and bHLH TFs (CL914.Contig2 and CL8321.Contig1) were specifically induced after four days of heat stress. DEGs detected in our study between these two contrasting cultivars would provide a novel basis for isolating useful candidate genes of heat stress responses in chieh-qua.
Keywords: RNA-Seq, DEGs, HSPs, cytochrome P450, bHLHs, chieh-qua
1. Introduction
Heat stress due to high ambient temperatures caused by global warming has posed a serious threat to crop production worldwide and most likely the damage level will remain on an upward trend in future [1,2]. Heat stress impacts seed germination, photosynthesis, and respiration action in plants [3]. And when exposed to heat stress, plants often generated reactive oxygen species (ROS), antioxidant substances, plant hormones and other secondary metabolites [4]. In addition, continuous rising temperature leads to the change in geographical distribution and growing seasons of plants [5].
To survive in the ambient temperature conditions, plants have to evolve a multiple of internal tolerant strategies, such as the protection of heat shock proteins (HSPs), the level changes of phytohormones, and the scavenging of reactive oxygen species (ROS) by different oxidation- reduction enzymes [6,7,8]. As molecular chaperones, HSPs play crucial roles in facilitating native protein folding and preventing irreversible denatured protein aggregation, which could help plants enhance heat resistance [9,10]. In peanuts, accumulation of small HSPs could improve its resistance to heat stress [11]. Phytohormones signal transduction or content such as abscisic acid (ABA), salicylic acid (SA), and ethylene (ETH) are reported to be induced by heat stress in different plant species [12,13]. For example, ABA content levels are significantly induced when exposed to higher temperatures in pea [14,15]. Studies report that elevated levels of antioxidants are associated with plant increased thermotolerance [16]. Heat stress could induce the increase of dehydrogenase (NDH) content, which protected plants against photo-oxidation [17].
Thermotolerance studies on vegetables mainly focused on the HSPs and related enzymes. CaHSP24 in pepper is significantly induced by high temperature, leading to its thermotolerance [18]. Ectopic expression of BADH of spinach in tobacco improves the glycine betaine content together with its increased resistance to heat stress [19]. Furthermore, under high temperature stress, ABA could induce the expression of HSP70, leading to the increased heat tolerance in cucumber [20].
Transcriptome sequencing of various resistant species is becoming a suitable technique for exploring related resistant genes and searching various biological pathways involved in the different stresses [21,22]. RNA-sequencing has been widely employed in performing crucial agricultural traits such as fruit development, parthenocarpy, flower sex expression, and other plant response to abiotic stresses in cucumber [23], watermelon [24], pumpkin [25], and other Cucurbitaceae vegetables [26]. Chieh-qua (Benincasa hispida Cogn. var. Chieh-qua How) is an important vegetable in Cucurbits, which belongs to a subspecies of wax gourd (Benincasa hispida (Thunb.) Cogn.) and widely distributed all over the South China and Southeast Asian countries [27]. High temperature caused serious loss of quality and production during the chieh-qua growth especially in the open field cultivation. However, little knowledge about the molecular studies on chieh-qua of heat stress is uncovered.
In this study, we obtained two differently heat resistant chieh-qua cultivas and firstly carried out RNA-sequencing analysis to explore the transcriptional variations under normal and high temperature conditions, respectively. Different stress-responsive novel transcript isoforms were identified and genes related to heat shock proteins (HSPs), ubiquitin-protein ligase, transcriptional factors, and pentatricopeptide repeat-containing proteins were significantly changed after heat stress. Functional categorization of differentially expressed transcripts was carried out to reveal various metabolic pathways involved in heat stress responses. This study provides a theoretical basis in the regulatory mechanism on heat tolerance in chieh-qua.
2. Results
2.1. Phenotypes of A39 and H5 under Normal and Heat Conditions
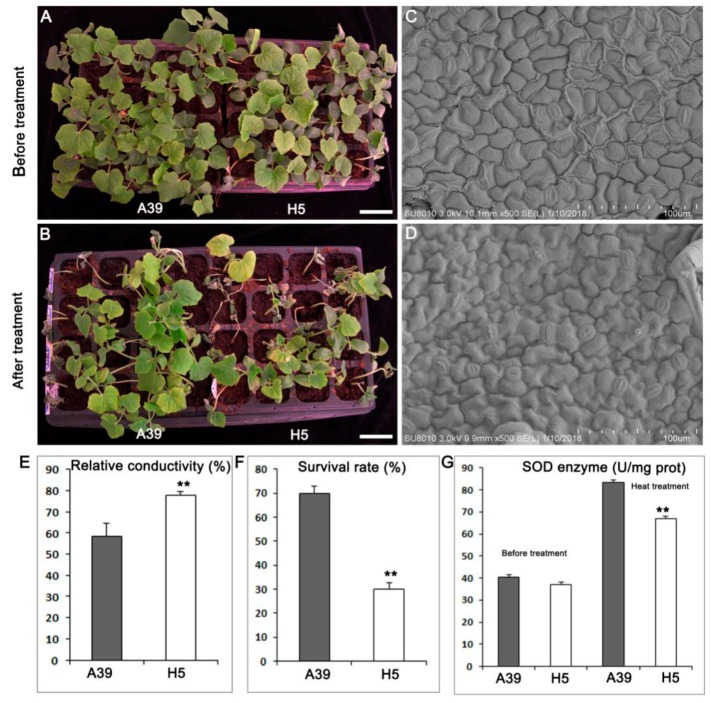
Seedlings with two true leaves of A39 and H5 (Figure 1A) were treated with a high temperature for five days and recovered for three days (Figure 1B). Both A39 and H5 showed vigorous development before heat stress. However, when treated by high temperature, A39 began to exert wilting at the top of the growth point, and its leaves turned chlorotic and yellow (Figure 1B). Using the scanning electron microscopy (SEM), we found that the number of stomas in A39 (Figure 1C) was much less than H5 (Figure 1D) in the same field size, indicating that H5 lost water more easily when encountered to heat stress. The relative conductivity of H5 was significantly higher than A39 (Figure 1E), indicating the membrane lipid showed much higher degree of damage in H5. Approximately 30% of the heat treated H5 plants survived after the subsequent three days recovery, compared with 70% of A39 plants (Figure 1F). There was no difference of enzyme superoxide dismutase (SOD) between A39 and H5 before heat, while H5 presented prominent decrease of SOD at the fourth day after heat stress (Figure 1G).
Figure 1.
Phenotypes of A39 and H5 after four days heat stress. (A) A39 and H5 plants were grown under normal condition for 14 days. (B) Seedlings were watered and grew under normal conditions for three days. (C,D) Scanning electron microscopy (SEM) images of leaves in A39 (C) and H5 (D). (E) Detection of relative conductivity of A39 and H5. (F) The survival rate of plants of A39 and H5 following the four days heat treatment. (G) Measurement of superoxide dismutase (SOD) enzyme activity under normal condition and four days after heat stress. Data are presented as the mean ± standard deviation (n = 9). ** p < 0.01; Student’s t-test.
2.2. Transcripts Assembly of A39 and H5
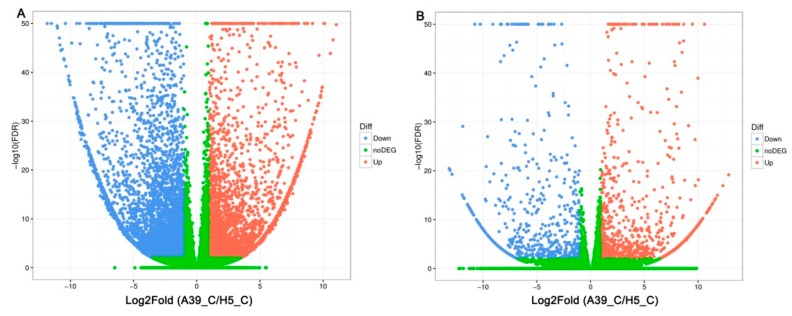
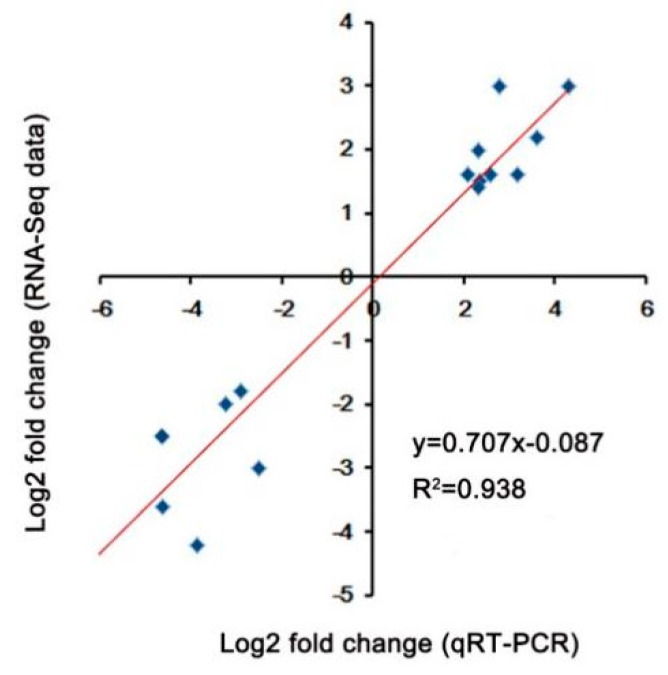
In order to explore the transcriptional variations between A39 and H5 under normal and heat condition, respectively, the RNA-sequencing assay was carried out. A total of about 45–55 million clean reads were obtained per sample (Table 1) after removing low-quality and adaptor-containing raw reads. Finally, we identified 8705 (Table S1) and 1505 (Table S2) differentially expressed genes (DEGs) in the comparison of A39 versus H5 under normal conditions and heat stress, respectively. Among them, under normal conditions, 3676 genes were up-regulated and 5029 down-regulated (gene expression in H5 compared with that in A39) (Figure 2A). Additionally, 914 up-regulated and 591 down-regulated genes were identified at the fourth day after heat treatment (Figure 2B). All the original data have been uploaded in the NCBI SRA data (SRP180414). Furthermore, in order to identify the accuracy of the RNA-sequencing data, we randomly selected 16 DEGs and performed a qRT-PCR assay. The data demonstrated that there existed a strong positive correlation (two tailed, R2 = 0.938) between the RNA-seq data and qRT-PCR results (Figure 3), indicating the accuracy of the RNA-Seq results.
Table 1.
Sample filtering statistics of A39 and H5
| Type | A39_C | H5_C | A39_H | H5_H |
|---|---|---|---|---|
| Read Length | 150 | 150 | 150 | 150 |
| Total Raw Reads | 45,345,693.33 | 54,886,327.3 | 47,159,128 | 50,066,083.33 |
| Total Raw Bases | 6,801,854,000 | 8,232,949,100 | 7,073,869,200 | 7,509,912,500 |
| Total Clean Reads | 45,233,028 | 54,676,526.67 | 47,003,104 | 49,889,418 |
| Total Clean Reads Ratio (%) | 99.75 | 99.63 | 99.67 | 99.65 |
| Total Clean Bases | 6,784,954,200 | 8,201,479,000 | 7,050,465,600 | 7,483,412,700 |
| Total Clean Bases Ratio (%) | 99.75 | 99.63 | 99.67 | 99.65 |
| Total Adapter Reads | 89,349.3 | 181,258.67 | 126,836.7 | 150,814 |
| Total Adapter Reads Ratio (%) | 0.2 | 0.32 | 0.27 | 0.3 |
| Total Low-Quality Reads | 23,316 | 28,542 | 29,187.3 | 25,851.33 |
| Total Low-Quality Reads Ratio (%) | 0.05 | 0.05 | 0.06 | 0.05 |
| Clean Reads GC (%) | 45.49 | 44.67 | 44.82 | 44.29 |
| Clean Reads Q20 (%) | 97.17 | 97.25 | 97.02 | 97.21 |
| Clean Reads Q30 (%) | 93.15 | 93.31 | 92.89 | 93.27 |
Figure 2.
Comparison of different genes expression (DEGs) in leaves between A39 and H5 under normal conditions (A) and four days after heat stress (B). X- and y-axes represent log2 values of gene expression. Red, green, and blue correspond to up-regulated, unaltered, and down-regulated gene expression, respectively.
Figure 3.
Validation of DEGs under heat stress. Correlation between the fold change analyzed by RNA-seq (y-axis) and data obtained using qRT-PCR (x-axis).
2.3. Functional Classification of Heat Response Genes
The GO (gene ontology) standardized classification system for gene function was employed to analyze DEGs and understand the molecular events involved in the heat response. Three categories including “biological process (BP)”, “molecular function (MF)”, and “cellular components (CC)” were classified under normal conditions (Figure S1A) (Table S3) and heat stress (Figure S1B) (Table S4), respectively. Under normal conditions, the largest number of genes were mainly in the CC category (integral component of membrane), while the main category was changed to MF (ATP binding) at the four days after heat treatment, followed by the RNA binding and zinc ion binding metabolic process (Figure S1A,B).
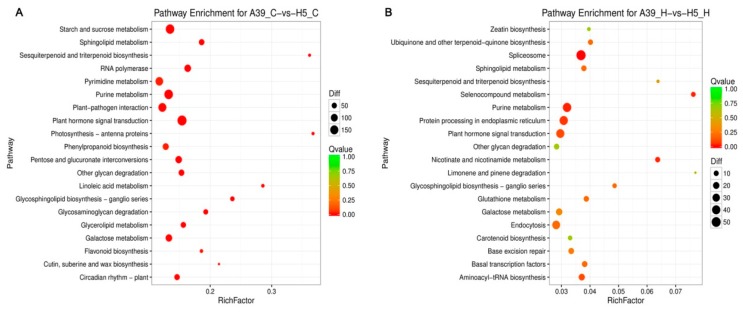
In order to examine the DEG-associated pathways, they were searched using the KEGG pathway database. The top 20 enriched pathways were shown in Figure 4. Main pathways under normal condition were “biosynthesis of secondary metabolites”, “plant hormone signal transduction”, and “purine metabolism” (Figure 4A) (Table S5). Especially, for the general metabolism pathways, DEGs were mostly enriched including the starch and sucrose, pyrimidine, purine, and galactose under control (Figure 4A). When exposed to heat stress, genes related to “splicesome”, “plant hormone signal transduction”, “purine metabolism” and “protein processing in endoplasmic reticulum” were mostly enriched (Figure 4B) (Table S6), indicating these pathways and processes possibly participated in seedling heat resistance.
Figure 4.
KEGG enrichments of annotated DEGs across three comparisons of normal condition (A) and heat stress (B). The y-axis indicates the KEGG pathway and the x-axis indicates the enrichment factor. A high q-value is represented by light blue, and a low q-value is represented by dark blue.
2.4. Expression of Genes Encoding HSPs, HSFs, and Cytochrome P450
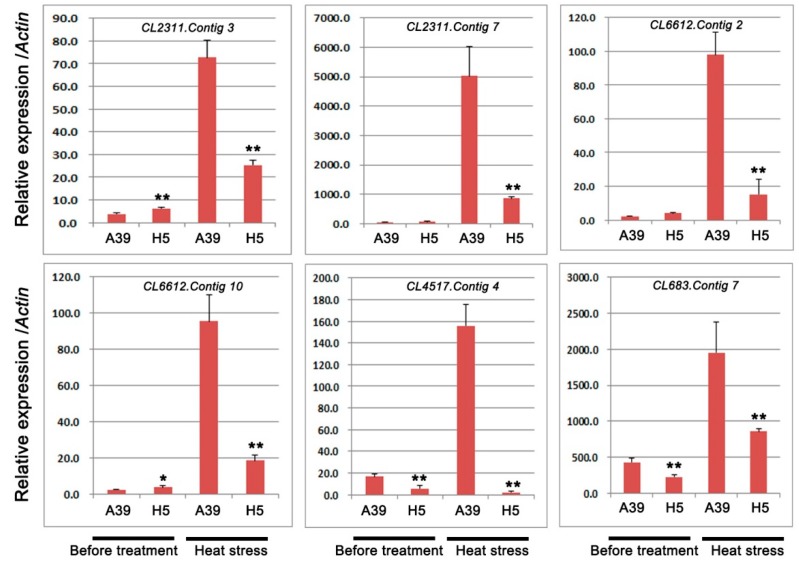
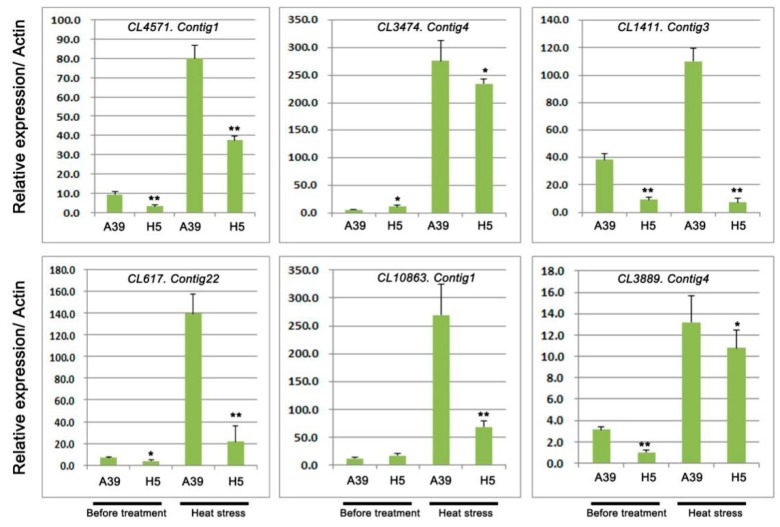
Based on the GO and KEGG analysis, several DEGs encoding heat shock proteins and ubiquitin-protein ligase were selected to perform qRT-PCR assay. A total of 6 transcripts were chosen, including four genes with heat shock proteins (HSPs) or heat stress transcription factor (HSFs) and two genes with response to water deprivation (Table 2). The qRT-PCR experiment was carried out to validate RNA-seq results under normal and heat treatment, respectively. These results showed that during heat stress, all these genes expression were induced and genes in H5 were significantly down-regulated compared with those in A39 (Figure 5). In addition, we typically selected six genes, which encoded ubiquitin-protein ligase to detect their expression changes (Table 3). The above results showed that the expression of all the six genes was observably decreased in H5 during heat stress in contrast to those in A39 (Figure 6). All these results of qRT-PCR were consistent with the RNA-sequencing data.
Table 2.
Genes related toHSPs, HSFs, and cytochrome P450.
| GeneID | log2 Fold | p-Value | Diff | Nr-Annotation |
|---|---|---|---|---|
| CL2311.Contig3 | −10.26 | 9.62 × 10−267 | Down | heat shock protein (HSPs) |
| CL2311.Contig7 | −3.92 | 2.92 × 10−239 | Down | heat shock protein(HSPs) |
| CL6612.Contig2 | −11.46 | 7.01 × 10−16 | Down | heat stress transcription factor(HSFs) |
| CL6612.Contig10 | −4.79 | 5.23 × 10−6 | Down | heat stress transcription factor (HSFs) |
| CL4517.Contig4 | −1.02 | 2.8121 × 10−4 | Down | cytochrome P450 |
| CL683.Contig7 | −1.10 | 3.19 × 10−16 | Down | cytochrome P450 |
Figure 5.
Relative expression of genes related to heat shock proteins (HSPs), heat stress transcription factor (HSFs), and cytochrome P450. Data are presented as the mean ± standard deviation (n = 9). * 0.01 ≤ p ≤ 0.05, ** p ≤ 0.01, Student’s t test.
Table 3.
Genes related to ubiquitin-protein ligase.
| GeneID | log2 Fold | p Value | Diff | Nr-Annotation |
|---|---|---|---|---|
| CL4571.Contig1 | −2.41 | 9.88 × 10−8 | Down | ubiquitin carboxyl-terminal hydrolase 2 |
| CL3474.Contig4 | −8.74 | 3.91 × 10−7 | Down | ubiquitin carboxyl-terminal hydrolase 14 |
| CL1411.Contig3 | −1.42 | 1.07 × 10−22 | Down | ubiquitin domain-containing protein DSK2a-like |
| CL617.Contig22 | −10.09 | 8.83 × 10−11 | Down | E3 ubiquitin-protein ligase UPL7 |
| CL10863.Contig1 | −8.26 | 3.16 × 10−6 | Down | E3 ubiquitin-protein ligase COP1 |
| CL3889.Contig4 | −8.03 | 9.51 × 10−6 | Down | ubiquitin carboxyl-terminal hydrolase 22-like |
Figure 6.
Relative expression of genes related to ubiquitin carboxyl-terminal hydrolases and E3 ubiquitin-protein ligases. Data are presented as the mean ± standard deviation (n = 9). * 0.01 ≤ p ≤ 0.05, ** p ≤ 0.01, Student’s t test.
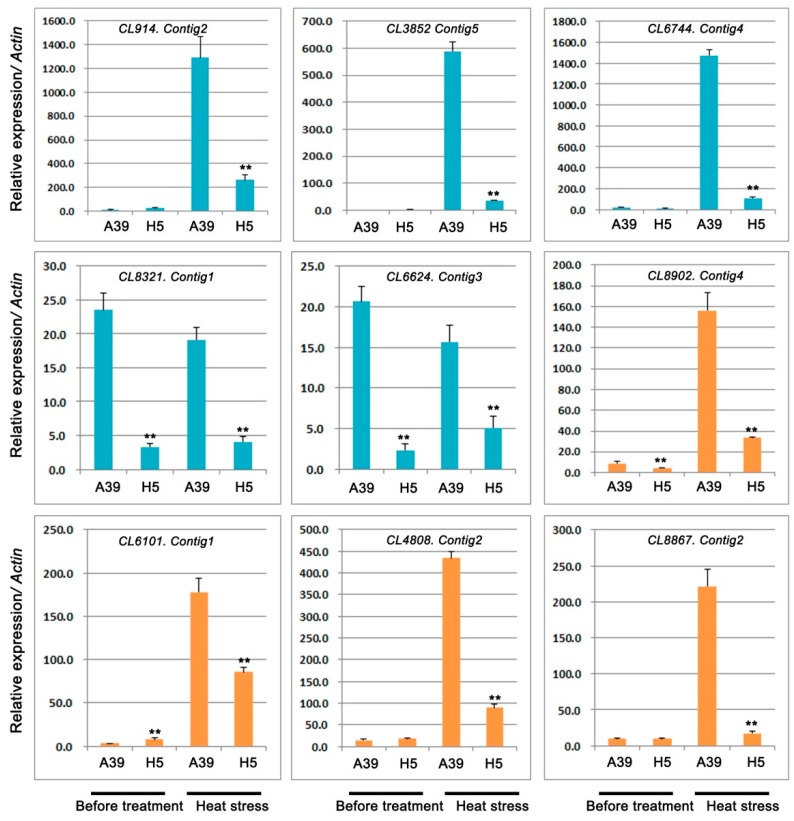
2.5. Expression Analysis of Genes Related to TFs and Pentatricopeptide Repeat-Containing Protein
In this study, five TFs were selected and performed the qRT-PCR assay to detect their expression under control and heat stress, respectively. We found that two bHLHs (bHLH128 and bHLH143), two PIF1-like, and one PCL1-like TF were significantly down-regulated in H5 under heat stress in comparison with those in A39 (Figure 7) (Table 4). Under normal conditions, three TFs showed no differences between A39 and H5, and the expression of other two TFs (bHLH143 and PIF1-like) were decreased in H5. In addition, the expression of four PPR proteins changed significantly after heat treatment (Figure 7) (Table 4). The above data demonstrated that their transcription were all prominently down-regulated in H5 compared with A39, although the differences of expression existed before treatment.
Figure 7.
Relative expression of genes related to transcription factors (bHLHs, PCL1s, PIF1s) and pentatricopeptide repeat-containing protein. Data are presented as the mean ± standard deviation (n = 9). ** p ≤0.01, Student’s t test.
Table 4.
Genes related to transcription factors and pentatricopeptide repeat-containing protein.
| GeneID | log2 Fold | p Value | Diff | Nr-Annotation |
|---|---|---|---|---|
| CL914.Contig2 | −3.97 | 8.54 × 10−5 | Down | transcription factor bHLH128 |
| CL3852.Contig5 | −8.00 | 1.09 × 10−4 | Down | transcription factor PCL1-like |
| CL6744.Contig4 | −5.72 | 2.52 × 10−11 | Down | transcription factor PIF1-like |
| CL8321.Contig1 | −7.73 | 4.47 × 10−100 | Down | transcription factor bHLH143-like |
| CL6624.Contig3 | −8.54 | 5.01 × 10−17 | Down | transcription factor PIF1-like |
| CL8902.Contig4 | −8.49 | 1.33 × 10−5 | Down | pentatricopeptide repeat-containing protein |
| CL6101.Contig1 | −7.47 | 6.24 × 10−8 | Down | pentatricopeptide repeat-containing protein |
| CL4808.Contig2 | −3.06 | 7.6 × 10−12 | Down | pentatricopeptide repeat-containing protein |
| CL8867.Contig2 | −9.98 | 1.95 × 10−10 | Down | pentatricopeptide repeat-containing protein |
3. Discussion
Previous studies demonstrated that the analysis and availability of diverse genetic resources could provide crucial information for understanding the molecular basis of variability in their response to abiotic stresses [21]. In the present study, we characterized and analyzed two chieh-qua genotypes (A39 and H5) for their significantly different responses to heat stress. H5 showed heat sensibility in a higher temperature condition with increased relative conductivity and decreased survival rate, as well as SOD enzyme activity. Combing the analysis of RNA-seq, multiple of DEGs were up-regulated and down-regulated significantly under heat stress. Among them, several DEGs related to heat shock proteins, ubiquitin-protein ligase, and some TFs were possibly involved in the heat response tolerance with prominent expression changes between the two chieh-qua materials.
3.1. H5 Has More Stomas in the Leaf Than A39
The regulation of stomatal opening and closure is crucial to the normal transpiration of water loss and plays crucial roles in the stress resistances [28]. Rice am1 mutant have high percentage of completely closed stomata, leading to the increased drought resistance compared with the wild type [29]. Dca1 mutant with drought tolerance has less number of stomata than the control [30]. Although stomata are mostly reported in the drought resistance studies, here, we found that the number of stomas in A39 was less than that in H5 in the same field size, indicating that the global differences in morphogenetic response between A39 and H5 might serve as a reason of different heat resistance.
3.2. Analysis of HSPs, HSFs During Heat Stress
HSPs comprising several evolutionarily conserved protein families, exert crucial roles in heat stress and could be immediately induced by high temperatures [13]. In Arabidopsis, HSP101 plays a pivotal role in heat tolerance [31]. Overexpressing of a small heat shock protein, sHSP17.7, increases the heat tolerance of transgenic rice [32]. The A1 class of heat shock factors (HSFA1s) is crucial to heat and other stresses in Arabidopsis [33]. For example, HSFA3 functions in both heat stress and water-deficit stress responses in Arabidopsis [34]. In our study, we found that the expression of two respective HSPs and HSFs were significantly decreased in H5 in comparison with A39 at the fourth day of heat stress, which indicated that high expression of these genes in A39 might contribute to its tolerance to heat stress to some extent.
3.3. Analysis of Ubiquitin-Protein Ligase During Heat Stress
The ubiquitin/26S proteasome system (UPS) is a critical regulatory mechanism that controls plants response to biotic and abiotic stresses [35,36]. The UPS consists of three key enzymes, ubiquitin activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3) [37,38]. AtCHIP, encoding a u-box-containing E3 ubiquitin ligase, is up-regulated by several stresses such as the high temperature in Arabidopsis [39]. Overexpressing the RING E3 ligase gene SDIR1 enhances drought tolerance by positively regulating the ABA signaling pathway in Arabidopsis [40]. Our study detected six ubiquitin-protein ligase genes and the expression of them was significantly decreased in H5 when compared with A39 after four days of heat stress, especially the CL1411.Contig3, CL617.Contig22, and CL10863.Contig1, suggesting that low expression might be the reason for the sensitivity of H5 to high temperature.
3.4. Analysis of TFs During Heat Stress
Previous studies had reported that most types of transcription factors (TFs), such as bHLH and phytochrome-interacting factors (PIF) exerted crucial roles when plants exposed to abiotic stresses such as drought, cold, salt stress, and others mostly by mediating anthocyanin biosynthesis and plant hormone signaling [41,42]. Several studies in rice reported that bHLH played important roles in response to different stresses, for example OsbHLH1 in cold response [43], RERJ1 in drought response [44], OsPTF1 in tolerance to phosphate starvation [45], and OrbHLH2 in tolerance to salt and osmotic stress [46]. In our present study, we also found the significant expression changes of these TFs between A39 and H5 during heat stress, indicating the chieh-qua bHLHs were involved in the resistance on high temperatures.
Phytochrome-interacting factors (PIFs), as a subfamily of basic helix-loop-helix (bHLH) transcription factors, played important roles in regulating plant responses to stresses. Ectopic over expression of maize ZmPIF3 improved the resistance on drought and salt in rice [47,48]. Overexpression PIF improved drought stress tolerance in transgenic rice. Two PIFs (CL6744.Contig4 and CL6624.Contig3) were prominently decreased in H5 in contrast to A39, implying high expression of PIFs in A39 might increase the resistance on heat stress.
3.5. Analysis of PPRs During Heat Stress
Pentatricopeptide repeat-containing proteins (PPRs), defined by a tandem array of a PPR motif consisting of 35 amino acids, were most involved in regulating RNA splicing, editing, cleavage, stability, and translation during plant development and growth [49,50]. In addition, PPRs also participated in the plant stress resistance. For example, WSL5 (pentatricopeptide repeat protein) is essential for plant cold tolerance by regulating chloroplast biogenesis [51]. In our study, four PPRs were significantly down-regulated in H5 when compared with A39 at the four days after heat stress, indicating high expression of PPRs might contribute to A39′s resistance to high temperature.
3.6. Differences of Genes Expression under Control
Under control conditions, multiple DEGs were detected, which were mostly involved in the metabolism pathways such as the starch and sucrose, pyrimidine, purine, and galactose. Take the starch and sucrose metabolism for example, previous studies reported that this metabolism pathway played important role in the responses to various stresses such as salt [52], water [53,54], and drought [55]. Our study identified that the chieh-qua cultivars with heat-resistant differences exerted significantly differential expression on starch and sucrose metabolism, indicating this DEGs might contribute to their resistant differences on heat when exposed to high temperature condition.
In all, the RNA-Seq between different chieh-qua cultivars was firstly carried out to analyze the regulatory mechanism under high temperature. Several crucial genes related to heat shock proteins, ubiquitin-protein ligase, and transcriptional factors were significantly important during heat stress. This work not only provided a basis for further understanding of the molecular mechanism on heat tolerance, but also exerted valuable and useful genes involved in heat stress, which would be helpful for the genetic improvement of heat tolerance in chieh-qua breeding. Further research should focus on the functional characterization of crucial genes involved in heat response combing physiology, genetics, and molecular biology technology. Most importantly, crucial genes’ roles on heat stress should be identified by transgenic study and other methods.
4. Materials and Methods
4.1. Plant Materials and Heat Stress Treatment
A39 (heat-tolerant) was a homozygous inbred line derived from the native variety “Qixingjie” in the Guangdong province. H5 (heat-sensitive) was also a native variety of the Guangdong province. Seeds were soaked in warm water for 6–8 h in room temperature and germinated 2 days on a wet filter in a culture dish at 30–32 °C under a dark environment. Subsequently, these germinated seeds of A39 and H5 were grown in a feeding block under 14 h/10 h with 30 °C/24 °C in day/night, respectively, in a culture room (5500 lux, 60% RH). When plants were grown to two true leaves stage, they were transferred to a high temperature environment for five days (45 °C/40 °C in day/night). After that, seedlings were recovered for 3 days to normal temperature. There were a total of 120 plants for A39 and H5 seedlings, respectively. Before treatment and on the 4th day of heat stress, leaf tissue of 10 plants from each pot was sampled and pooled together from normal plants and heat stressed plants of each cultivar, respectively. Three biological replicates were applied for each cultivar. Each biological replicate contained 10 plants randomly. These samples were immediately frozen in liquid nitrogen and consistently stored at −80 °C for further analysis. Scanning electron microscopy (SEM).
Leaves of A39 and H5 under normal conditions were air-dried. Leaf abaxial epidermis was visualized under a HITACHI SU8020 variable pressure SEM (Hitachi, Tokyo, Japan) and imaged using an H-7500 transmission electron microscope (Hitachi, Japan). In addition, in view of changes along one simple leaf, we used the same leaf part and the leaves of the same sizes for management and investigation.
4.2. Measurement of SOD Activity
SOD activity of A39 and H5 was detected by the NBT method (nitroblue tetrazolium) [56,57]. In detail, the chieh-qua leaves (1 g) were homogenized in liquid nitrogen and mortar in 10 mL of 0.2 M lysate (citrate phosphate buffer (pH 6.5) with 0.5% Triton X-100). Then, the mixture was centrifuged at 10,000× g for 30 min at 4 °C and the supernatant was the enzyme source. The riboflavin was added last after the enzyme extract was finished and A560 was measured for their OD values.
4.3. Transcriptome Sequencing
A total of twelve samples (three biological replicates each of A39 and H5 at normal and heat stress, respectively) were used for RNA extraction with the TRIZOL reagent according to protocol (TaKaRa, Japan). RNA was then purified (using DNAse) and concentrated using an RNeasyMinElute clean up kit (Qiagen, Germany). Then, cDNA libraries were constructed from RNA samples for Illumina paired-end (PE) sequencing following the Illumina protocol. PE sequencing (2 × 100 bp) was performed on the IlluminaHiSeq 2000 platform (Illumina, San Diego CA, USA) at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China).
4.4. Screening and Significant Test for Differentially Expressed Genes (DEGs)
Gene expression level was calculated by quantifying reads according to RPKM (reads per kilobase per million reads) method [58]. Then the NOISeq was used to identify DEGs, which existed in normal and heat stress transcriptome libraries according to the following criteria: Fold change ≥ 2 and divergence probability ≥ 0.8. GO enrichment for these DEGs was performed using WEGO software [59]. To further understand the DEG biological functions, pathway enrichment analysis was carried out on the basis of KEGG database [60].
4.5. Quantitative Real-Time PCR Analysis
Total RNA was extracted using TRIZOL Kit (TaKaRa, Japan). First-strand cDNA was obtained from 2 μg RNA combing the QuantiTect Reverse Transcription Kit (Qiagen, Germany). qRT-PCR (20 μL volume) was performed with 0.5 μL of cDNA, 0.2 μM of primer mix, and the SYBR Premix Ex Taq Kit (TaKaRa, Japan). The endogenous chieh-qua CqActin gene (F: TCAACCCAAAGGCTAACAG; R: CTTGTCCATCAGGCAGTTC) was used as the reference genes. The experiment was carried out with three technical and biological replicates, respectively. All qRT-PCR primers are listed in Table S7.
4.6. Statistical Analysis
Heat-map and cluster analysis were carried out by R-3.4.2 and MEGA6 software. Significant differences were detected by IBM SPSS Statistics 20. Gene relative expressions were calculated using the 2−∆∆Ct method [61]. In addition, GraphPad Prism5 was used for chart preparation.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-0067/20/4/883/s1.
Author Contributions
M.W. performed most of the experiment. B.J., W.L., Y.L., and Z.L. carried out part of the experiments. M.W. wrote the paper. X.H. and Q.P. designed the experiment and edited the paper.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province (2018A030310196), the Guangdong Special Project Youth Top-notch Talent Project, (2016TQ03N529), the Presidential foundation of Guangdong Academy of Agricultural Sciences (201813), the Key Laboratory Open Fund Project of Vegetable Institution of GAAS (201701), the Natural Science Foundation of Guangdong Province (2018A030313495), the Science and Technology Planning Project of Guangdong Province (2016B020201005).
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships which could be construed as potential conflicts of interest.
References
- 1.Hall A.E. Crop Responses to Environment. CRC Press LLC; Boca Raton, FL, USA: 2001. [Google Scholar]
- 2.Ray D.K., Gerber J.S., MacDonald G.K., West P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015;6:5989. doi: 10.1038/ncomms6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahid A., Gelani S., Ashraf M., Foolad M.R. Heat tolerance in plants, an overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 4.Liu J.P., Zhang C., Wei C., Liu X., Wang M.G., Yu F., Xie Q., Tu J.M. The RING finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2-induced stomatal closure in rice. Plant Physiol. 2015;170:429. doi: 10.1104/pp.15.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter J.R. Rising temperatures are likely to reduce crop yields. Nature. 2005;436:174. doi: 10.1038/436174b. [DOI] [PubMed] [Google Scholar]
- 6.McClung C.R., Davis S.J. Ambient thermometers in plants, from physiological outputs towards mechanisms of thermal sensing. Curr. Biol. 2010;20:R1086–R1092. doi: 10.1016/j.cub.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Mittler R., Finka A., Goloubinoff P. How do plants feel the heat? Trends Biochem. Sci. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Bita C.E., Gerats T. Plant tolerance to high temperature in a changing environment, scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer M.P. Recruitment of Hsp70 chaperones, a crucial part of viral survival strategies. Rev. Physiol. Biochem. Pharmacol. 2005;153:1–46. doi: 10.1007/s10254-004-0025-5. [DOI] [PubMed] [Google Scholar]
- 10.Tkáčová J., Angelovičová M. Heat shock proteins (HSPs), a review. Lucrari Stiintifice Zootehnie Si Biotehnologii. 2012;45:349–353. [Google Scholar]
- 11.Chakraborty K., Bishi S.K., Singh A.L., Zala P.V., Mahatma M.K., Kalariya K.A., Jat R.A. Rapid induction of small heat shock proteins improves physiological adaptation to high temperature stress in peanut. J. Agron. Crop Sci. 2018;204:285–297. doi: 10.1111/jac.12260. [DOI] [Google Scholar]
- 12.Larkindale J., Mishkind M., Vierling E. Plant responses to high temperature. In: Jenks M.A., Hasegawa P.M., editors. Plant Abiotic Stress. Blackwell Publishing; Hoboken, NJ, USA: 2005. pp. 100–144. [Google Scholar]
- 13.Kotak S., Larkindale J., Lee U., von Koskull-Döring P., Vierling E., Scharf K.D. Complexity of the heat stress response in plants. Current Opinion in Plant Biol. 2007;10:310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Liu H.T., Liu Y.Y., Pan Q.H., Yang H.R., Zhan J.C., Huang W.D. Novel interrelationship between salicylic acid, abscisic acid, and pip2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J. Exp. Bot. 2006;57:3337–3347. doi: 10.1093/jxb/erl098. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima K., Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32:959–970. doi: 10.1007/s00299-013-1418-1. [DOI] [PubMed] [Google Scholar]
- 16.Yang X., Wen X., Gong H., Lu Q., Yang Z., Tang Y., Liang Z., Lu C. Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants. Planta. 2007;225:719–733. doi: 10.1007/s00425-006-0380-3. [DOI] [PubMed] [Google Scholar]
- 17.Wang P., Duan W., Takabayashi A., Endo T., Shikanai T., Ye J.Y., Mi H. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006;141:465–474. doi: 10.1104/pp.105.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W., Lu M., Gong Z., Chen R. Cloning and expression of a small heat shock protein gene CaHSP24 from pepper under abiotic stress. Afr. J. Biotechnol. 2011;1025:4968–4976. [Google Scholar]
- 19.Yang X.H., Liang Z., Lu C.M. Genetic engineering of the biosynthesis of glycinebetaine enhances photosynthesis against high temperature stress in transgenic tobacco plants. Plant Physiol. 2005;138:2299–2309. doi: 10.1104/pp.105.063164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Liu S.S., Yi C.Y., Wang F., Zhou J., Xia X.J., Shi K., Zhou Y.H., Yu J.Q. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2015;37:2768–2780. doi: 10.1111/pce.12360. [DOI] [PubMed] [Google Scholar]
- 21.Garg R., Shankar R., Thakkar B., Kudapa H., Krishnamurthy L., Mantri N., Varshney R.K., Bhatia S., Jain M. Transcriptome analyses reveal genotype-and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 2016;6:19228. doi: 10.1038/srep19228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., Yang P., Cui F., Zhang F.T., Luo X.D., Xie J.K. Transcriptome analysis of salt stress responsiveness in the seedlings of Dongxiang wild rice (Oryza rufipogon Griff.) PLoS ONE. 2016;11:e0146242. doi: 10.1371/journal.pone.0146242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawełkowicz M., Zieliński K., Zielińska D., Plader W., Yagi K., Wojcieszek M., Siedlecka E., Bartoszewski G., Skarzynska A., Przybecki Z. Next generation sequencing and omics in cucumber (Cucumissativus L.) breeding directed research. Plant Sci. Int. J. Exp. Plant Biol. 2016;242:77–88. doi: 10.1016/j.plantsci.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Grassi S., Piro G., Lee J.M., Zheng Y., Fei Z., Dalessandro G., Giovannoni J.J., Lenucci M.S. Comparative genomics reveals candidate carotenoid pathway regulators of ripening watermelon fruit. BMC Genomics. 2013;14:781. doi: 10.1186/1471-2164-14-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W.L., Chen B.H., Chen X.J., Guo Y.Y., Yang H.L., Li X.Z., Wang G.Y. Transcriptome profiling of pumpkin (CucurbitamoschataDuch.) leaves infected with powdery mildew. PLoS ONE. 2018;13:e0190175. doi: 10.1371/journal.pone.0190175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behera T.K., Rao A.R., Amarnath R., Kumar R.R. Comparative transcriptome analysis of female and hermaphrodite flower buds in bitter gourd (Momordica charantia L.) by RNA sequencing. J. Pomol. Hortic. Sci. 2016;91:250–257. doi: 10.1080/14620316.2016.1160540. [DOI] [Google Scholar]
- 27.He X.M., Xie D.S., Chen Q.H., Peng Q.W. Chieh-qua biotechnology, progress and prospects. Asia and Aust. J. Sci. Biotechnol. 2007;1:19–22. [Google Scholar]
- 28.Lim C.W., Baek W., Jung J., Kim J.H., Lee S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015;16:15251–15270. doi: 10.3390/ijms160715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng P., Tan J., Jin M., Wu F., Zhou K., Ma W., Heng Y., Wang J., Guo X., Zhang X., et al. Albino midrib 1, encoding a putative potassium efflux antiporter, affects chloroplast development and drought tolerance in rice. Plant Cell Rep. 2014;33:1581–1594. doi: 10.1007/s00299-014-1639-y. [DOI] [PubMed] [Google Scholar]
- 30.Cui L.G., Shan J.X., Shi M., Gao J.P., Lin H.X. DCA1 acts as a transcriptional co-activator of DST and contributes to drought and salt tolerance in rice. PLoS Genet. 2015;11:e1005617. doi: 10.1371/journal.pgen.1005617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Queitsch C., Hong S.W., Vierling E., Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami T., Matsuba S.H., Kawaguchi K., Saruyama H., Tanida M., Sato Y. Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed. 2004;13:165–175. doi: 10.1023/B:MOLB.0000018764.30795.c1. [DOI] [Google Scholar]
- 33.Liu H.C., Liao H.T., Charng Y.Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34:738–751. doi: 10.1111/j.1365-3040.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- 34.Schramm F., Larkindale J., Kiehlmann E., Ganguli A., Englich G., Vierling E., von Koskull-Doring P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008;53:264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- 35.Smalle J., Vierstra R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 36.Santner A., Estelle M. The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 2010;61:1029–1040. doi: 10.1111/j.1365-313X.2010.04112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dreher K., Callis J. Ubiquitin, hormones and biotic stress in plants. Ann. Bot. (Lond.) 2007;99:787–822. doi: 10.1093/aob/mcl255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vierstra R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009;10:385–397. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- 39.Yan J., Wang J., Hwang J.R., Patterson C., Zhang H. AtCHIP, a U-Box-containing E3 ubiquitin ligase, plays a critical role in temperature stress tolerance in Arabidopsis. Plant Physiol. 2003;13:861–869. doi: 10.1104/pp.103.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Yang C., Li Y., Zheng N., Chen H., Zhao Q., Gao T., Guo H., Xie Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell. 2007;19:1912–1929. doi: 10.1105/tpc.106.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J., Kim H.Y. Functional analysis of a calcium-binding transcription factor involved in plant salt stress signaling. FEBS Lett. 2006;580:5251–5256. doi: 10.1016/j.febslet.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 42.George C., Witmer J.A., Cryer J.D. Corrigendum to “Possible roles of basic helix-loop-helix transcription factors in adaptation to drought”. Plant Sci. 2014;223:1–7. doi: 10.1016/j.plantsci.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y.J., Zhang Z.G., He X.J., Zhou H.L., Wen Y.X., Dai J.X., Zhang J.S., Chen S.Y. A rice transcription factor OsbHLH1 is involved in cold stress response. Theor. Appl. Genet. 2003;107:1402–1409. doi: 10.1007/s00122-003-1378-x. [DOI] [PubMed] [Google Scholar]
- 44.Kiribuchi K., Jikumaru Y., Kaku H., Minami E., Hasegawa M., Kodama O., Seto H., Okada K., Nojiri H., Yamane H. Involvement of the basic helix-loophelix transcription factor RERJ1 in wounding and drought stress responses in rice plants. Biosci. Biotechnol. Biochem. 2005;69:1042–1044. doi: 10.1271/bbb.69.1042. [DOI] [PubMed] [Google Scholar]
- 45.Yi K., Wu Z., Zhou J., Du L., Guo L., Wu Y., Wu P. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol. 2005;138:2087–2096. doi: 10.1104/pp.105.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J., Li F., Wang J.L., Ma Y., Chong K., Xu Y. Basic helix-loop-helix transcription factor from wild rice (OrbHLH2) improves tolerance to salt and osmotic stress in Arabidopsis. J. Plant Physiol. 2009;166:1296–1306. doi: 10.1016/j.jplph.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y., Jiang W., Dai Y., Xiao N., Zhang C., Li H., Lu Y., Wu M.Q., Tao X.Y., Deng D.X., Chen J.M. A maize phytochrome-interacting factor 3 improves drought and salt stress tolerance in rice. Plant Mol. Biol. 2015;87:413–428. doi: 10.1007/s11103-015-0288-z. [DOI] [PubMed] [Google Scholar]
- 48.Kudo M., Kidokoro S., Yoshida T., Mizoi J., Todaka D., Fernie A.R., Shinozaki K., Yamaguchi-Shinozaki K. Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants. Plant Biotechnol. J. 2016;15:458–471. doi: 10.1111/pbi.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q.B., Jiang Y., Chong K., Yang Z.N. AtECB2, a pentatricopeptide repeat protein, is required for chloroplast transcript accD RNA editing and early chloroplast biogenesis in Arabidopsis thaliana. Plant J. 2009;59:1011–1023. doi: 10.1111/j.1365-313X.2009.03930.x. [DOI] [PubMed] [Google Scholar]
- 50.Ichinose M., Tasaki E., Sugita C., Sugita M. A PPR-DYW protein is required for splicing of a group II intron of cox1 pre-mRNA in Physcomitrella patens. Plant Journal. 2012;70:271–278. doi: 10.1111/j.1365-313X.2011.04869.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu X., Lan J., Huang Y.S., Cao P.H., Zhou C.L., Ren Y., He N.Q., Liu S.J., Tian Y.L., Nguyen T., Jiang L., Wan J.M. WSL5, a pentatricopeptide repeat protein, is essential for chloroplast biogenesis in rice under cold stress. J. Exp. Bot. 2018;69:3949–3961. doi: 10.1093/jxb/ery214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliveira H., Costa A., Santos C. NaCl and Phaeomoniella chlamydospora affect differently starch and; sucrose metabolism in grapevines. Acta Sci. Agron. 2013;35:153–159. doi: 10.4025/actasciagron.v35i2.15690. [DOI] [Google Scholar]
- 53.Liao W.B., Li Y.Y., Lu C., Peng M. Expression of sucrose metabolism and transport genes in cassava petiole abscission zones in response to water stress. Biol. Plant. 2017;61:219–226. doi: 10.1007/s10535-016-0658-7. [DOI] [Google Scholar]
- 54.Chang C.W., Ryan R.D. Effects of water stress on starch and sucrose metabolism in cotton leaves. Starch. 1987;39:84–87. doi: 10.1002/star.19870390305. [DOI] [Google Scholar]
- 55.Netrphan S., Tungngoen K., Suksangpanomrung M., Boonseng O., Narangajavana J. Differential expression of genes involved in sucrose synthesis in source and sink organs of cassava plants undergoing seasonal drought stress. J. Agric. Sci. 2012;4:171–185. [Google Scholar]
- 56.Giannopolitis C.N., Ries S.K. Superoxide dismutase. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paranidharan V., Palaniswami A., Vidhyasekaran P., Velazhahan R. A host-specific toxin of Rhizoctonia solani triggers superoxide dismutase (SOD) activity in rice. Archiv für Pflanzenschutz. 2005;38:151–156. doi: 10.1080/03235400500094159. [DOI] [Google Scholar]
- 58.Li B., Dewey C.N. RSEM, Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye J., Fang L., Zheng H., Zhang Y., Chen J., Zhang Z., Wang J., Li S., Li R., Bolund L., et al. WEGO, A web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:W293. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., Katayama T., Kawashima S., Okuda S., Tokimatsu T., et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:480–484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandesompele J., de Preter K., Pattyn F., Poppe B., van Roy N., de Paepe A., Speleman F. Accuratenormalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal controlgenes. Genome Biol. 2002;3:research0034.1. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.