Abstract
The use of natural antimicrobial compounds in crop production has gained much attention from consumers and the agricultural industry. Consequently, interest in more natural, non-synthetic antimicrobials as potential alternatives to conventional chemical pesticides to combat phytopathogens has heightened. Tea polyphenol (TP), a unique and highly important functional component of tea plants, has been reported to possess antimicrobial properties against a wide spectrum of plant pathogens. The aim of this review is to discuss the emerging findings on the mechanisms of antimicrobial action, and the antimicrobial properties of TP, including their major components, effectiveness, and synergistic effects. More studies, particularly field studies, are still necessary to establish conclusive evidence for the effectiveness of TP against phytopathogens. However, the basic conclusion from existing studies suggests that TP is a potential antimicrobial agent for pesticide reduction in agricultural systems.
Keywords: tea polyphenol, antimicrobial activities, phytopathogens
1. Introduction
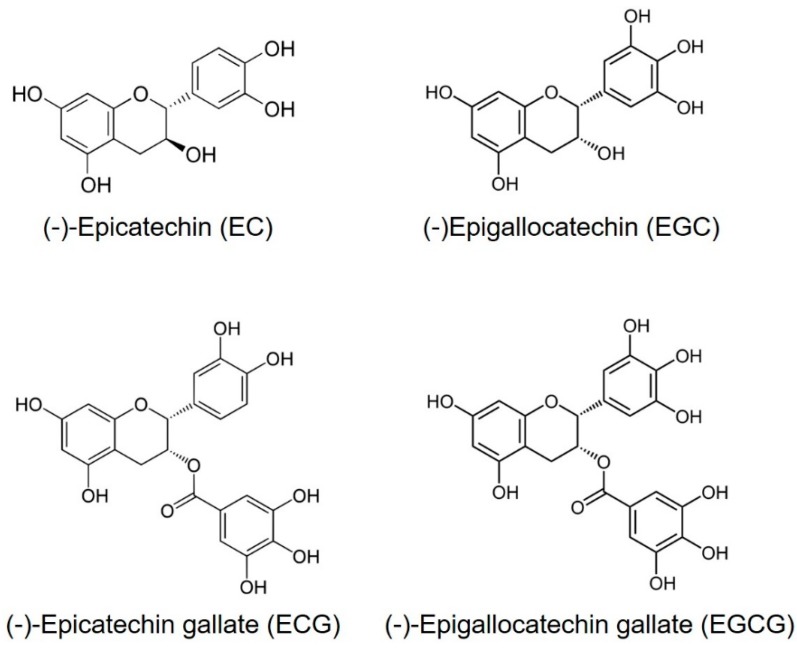
Tea (Camellia sinensis) is the most consumed beverage in the world, aside from water, and is widely believed to have positive effects on human health [1]. The Chinese have known about the medicinal benefits of green tea since around 2,737 B.C. [2]. One important aspect of green tea is the fact that it is rich in polyphenols. The major polyphenols in green tea are flavonoids. The four major flavonoids in green tea are the catechins: epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG) and epigallocatechin gallate (EGCG) (Figure 1) [3]. Among them, EGCG is the most abundant component of catechins in tea leaves, followed by EGC, EC and ECG [4]. Notably, EGCG is not found in other plants [5]. The usual concentration of TPs in dried green tea leaves is up to 30% by weight [6].
Figure 1.
Chemical structures of major polyphenols present in green tea.
Numerous studies have shown that TP components have various positive effects on human health [7,8,9]. In addition, evidence shows that TP have antimicrobial effects against a variety of human pathogens [10]. However, fewer studies have examined the antimicrobial activities of TP on phytopathogens. With more awareness of the potential health risks and environmental hazards caused by chemical pesticides, the development of botanical pesticides has aroused great interest among researchers throughout the world [11]. Phenolic compounds extracted from tea have been reported to possess antimicrobial properties against a wide spectrum of plant pathogens [12]. The main objective of this review is to unify and interpret widely scattered information on antimicrobial activities of TP against phytopathogens such as fungi, bacteria and viruses derived from existing studies. We also summarize the synergistic effects of combinations of TP and other biocontrol agents. In addition, the mechanisms of antimicrobial activities of TP are discussed. We believe that the future development and application of TP as a botanical pesticide will assist in controlling phytopathogens in a less environmentally hazardous manner than existing chemical pesticides.
2. Antimicrobial Activities of TP on Phytopathogens
Many laboratory studies conducted over the last decades have examined that treatment of TP can inhibit the infection process of in various plant-pathogen interactions (Table 1).
Table 1.
Summary of studies of tea polyphenol against phytopathogens.
| Pathogen | Host | Disease | Reference |
|---|---|---|---|
| Fungi | |||
| Bipolaris maydis | Maize | Leaf spot | [13] |
| Colletotrichum musae | Banana | Anthracnose | [13] |
| Fusarium oxysporum | Lotus/Banana | Corruption | [13,15] |
| Pyricularia oryzae | Rice | Blast | [14] |
| Phytophthora cryptogea | Gerbera jamesonii | Root rot | [15] |
| Pestalotiopsis apiculatus | Camellia oleifera | Leaf blight | [15] |
| C. fructicola | Camellia oleifera | Anthracnose | [15] |
| C. horii | Illicium verum | Anthracnose | [15,28] |
| Rhizoctorzia solani | Rice | Sheath blight | [15] |
| Lasiodiplodia theobromae | Eucalyptus spp. | Die-back | [15,28] |
| Sclerotinia sclerotiorum | Oilseed rape | Sclerotinia rot | [15] |
| Diplodia natalensis | Citrus fruit | Stem-end rot | [16] |
| Botrytis cinerea | Grape fruit/Nectarine fruit | Gray mold | [17,18] |
| Monilinia fructicola | Nectarine fruit | Brown rot | [27] |
| Puccinia striiformis f. sp. tritici | Wheat | Stripe rust | [12] |
| Bacteria | |||
| Erwinia carotovora | Lettuce/Tomato/Eggplant/Cabbage/Radish/Potato/Cauliflower | Soft rot | [19] |
| Clavibacter michiganensis | Tomato | Canker | [19] |
| Xanthomonas campestris | Lettuce | Spot | [19] |
| Agarobacterium tumefaciens | Grape | Crown gall | [19] |
| Pseudomonas cichorii | Lettuce/Eggplant | Black leg | [19] |
| Ps. marginalis | Lettuce/Onion/Cabbage | Spring rot | [19] |
| Ps. viridiflava | Lettuce/Tomato | Black rot/Leaf rot | [19] |
| Ps. syringae pv. pisi | Bean | Halo blight | [20] |
| Ps. s. pv. phaseolicola | Bean | Halo blight | [20] |
| Virus | |||
| Tobacco mosaic virus | Tobacco | Mosaic disease | [21,22,23] |
| Cucumber mosaic virus | Cucumber | Mosaic disease | [22,23] |
2.1. Antifungal Activity of TP
Wang et al. tested the inhibitive effects of different TP concentrations on three species of plant pathogenic fungi, Bipolaris maydis, Colletotrichum musae and Fusarium oxysporum [13]. The results showed that TP significantly inhibited hyphal growth and spore germination of the three fungi, and the inhibitive effects were directly proportional to the concentration of TP solutions. Among the series concentrations, the 10 mg/mL and 5 mg/mL TP solutions showed the highest inhibitive rate against the three fungi. Of the fungi, B. maydis was inhibited most efficiently by TP; the 10 mg/mL and 5 mg/mL TP solution inhibited spore germination by 100% and caused protoplasm overflow and cell distortion. Subsequently, these researchers reported that TP had strong inhibition on hyphal growth and conidial germination of Pyricularia oryzae, which causes rice blast disease [14]. TP solutions at concentrations of 10 mg/mL and 5 mg/mL can inhibit the growth of rice blast fungi completely. Some similar results have been reported about TP antifungal activities against eight species of plant pathogenic fungi, including Phytophthora cryptogea, Pestalotiopsis apiculatus, Colletotrichum horii, Sclerotinia sclerotiorum, C. fructicola, Rhizoctorzia solani, Lasiodiplodia theobromae, and F. oxysporum [15]. The best inhibition effect was on C. horii with EC50 value of 12.84 mg/mL, followed by P. cryptogea with EC50 value of 16.01 mg/mL.
Moreover, the antifungal activity of TP has been investigated against several postharvest disease microorganisms. Liu et al. reported that TP exhibited an inhibitory effect against stem-end rot (a postharvest disease caused by Diplodia natalensis) in citrus fruit at concentrations of 0.1%, 0.5% and 1.0% [16]. Then, they further confirmed the inhibitory activity of TP against gray mold, another postharvest disease of grape fruits caused by Botrytis cinerea [17]. The results showed that the spore germination of B. cinerea was significantly inhibited by TP at all concentrations, and mycelium growth was significantly inhibited by TP at 0.1% and above. Yang et al. also revealed the control efficacy of TP against nectarine gray mold decay caused by B. cinerea [18]. The in vitro experiments showed that TP inhibited the mycelial growth in a dose-dependent manner, and the in vivo experiments showed that disease incidence and lesion diameter of gray mold of inoculated fruit were significantly lowered after being treated with TP.
The beneficial effect of TP in inhibiting obligate biotrophic fungus was also observed by Yang et al. [12], who reported on the antifungal activity of TP on Puccinia striiformis f. sp. tritici (Pst), an obligate biotrophic fungus that causes severe wheat stripe rust disease. The in vitro experiments showed that, at a concentration of 1.0 mg/mL, TP significantly suppressed urediniospore germination and caused the aberrant growth of germ tubes. The in vivo experiments showed that TP reduced the incidence rate and the uredia coverage rate in a dose- and application time-dependent manner. However, the ideal TP concentration range for controlling Pst was 20–40 mg/mL, which was much higher than the effective dosage determined in other studies. This difference is mainly attributed to the fact that TP lacks the ability to effectively enter plant tissues to inhibit the growth of the infectious hyphae, due to the absence of a good systemic activity [12].
2.2. Antibacterial Activity of TP
In addition to antifungal activity, TP showed inhibitory effect on various phytopathogenic bacterial infections. Fukai et al. reported the antibacterial activity of TP measured as minimum inhibitory concentration (MIC) against phytopathogenic bacteria, including eight strains of Erwinia, 10 strains of Pseudomonas, and one strain each of Clavibacter, Xanthomonas and Agarobacterium [19]. These bacteria tend to infect commonly cultivated vegetables such as lettuce, tomatoes, eggplants, cabbage, radish, Irish potatoes, onions, and grapes. After three days incubation of the bacterial agar plates containing different concentrations of individual TPs, EGC and EGCG showed more inhibitory effect than EC and ECG against the test bacteria, and MICs were mostly below 100 ppm. However, ECG seemed to be the least effective among them, and MICs were mostly over 1000 ppm. In addition, Alstrom conducted the in vitro and greenhouse trials to demonstrate the antibacterial activity of extracts of tea extracts against phytopathogenic bacteria (P. syringae pv. pisi race 1, P. syringae pv. pisi race 2 and P. syringae pv. phaseolicola) [20]. The antibacterial activity was measured as the diameter of the inhibition zone in agar and then by periodical viable cell counts in laboratory tests. The effect on the hypersensitive reaction and the potential for disease control after leaf infiltration and seed treatment were studied on bean plants in the greenhouse. The agar diffusion test revealed that the tea extract substantially inhibited the growth of the three pathogens (5 mm diameter well filled with tea extract), whereas the inhibition of both P. syringae pv. pisi strains on agar was not observed in periodical viable cell count test. However, in vivo tests showed that tea extract could not induce a hypersensitive reaction in broad bean leaves co-inoculated with three P. syringae strains. These observations indicated that these effects varied depending on bacterial strain and the method of application.
2.3. Antiviral Activity of TP
Having noticed the antiviral effect of tea infusion on tobacco mosaic virus (TMV) [21], Okada and Furuya tested the inhibitory effect of each TP component and its own mix against TMV and cucumber mosaic virus (CMV) on tobacco leaves [22]. The aqueous solutions of TPs were injected into the soil around the base of the plants systemically infected with TMV and CMV and showed that the number of lesions on inoculated leaves on plants treated with TPs was less than that observed on untreated plants. Further study illustrated that the number of lesions was apparently decreased with the addition of ECG and ECGC, resulting in no lesion at all at 0.5% concentration of each catechin [23].
2.4. Synergistic Effect of TP Combination with Certain Bioagents
Tea saponin (TS) has been shown to have significant antifungal effect against various phytopathogenic fungi [24,25,26], prompting some researchers to focus on the antifungal activity of combinations of TP and TS. Chen et al. illustrated that both TP and TS inhibited the mycelial growth of brown rot pathogen on inoculated nectarine fruit, and their combination (TP: TS, 1:2) significantly improved the controlling effect [27]. Zou et al. also confirmed the co-toxicities of TP and TS on two kinds of forest fungal pathogens, C. horii and L. theobromae [28]. These fungi cause die-back of Eucalyptus spp. and anthracnose of Illicium verum Hook. f, respectively. The synergistic effect was significant with their combination (TP: TS, 1:1) on C. horii and L. theobromae.
A previous study showed that Hanseniaspora uvarum, one of the most abundant yeast species isolated from the surface of grape, was an effective antagonist against B. cinerea [29]. The investigators further confirmed that TP at concentrations of 0.5% and 1.0% in combination with H. uvarum (1 × 106 CFU/mL) exhibited an inhibitory effect against B. cinerea [17]. In addition, the synergistic effect of TP and Candida ernobii against postharvest disease (D. natalensis) was investigated [16]. The results suggested that TP at 0.1%, 0.5% and 1.0% in combination with C. ernobii (1 × 106 CFU/mL) showed a lower infection rate of stem-end rot compared to C. ernobii alone. These findings suggested that the combination of TP and other biocontrol agents could effectively improve the biocontrol efficacy on phytopathogenic fungi.
3. Mechanisms of Antimicrobial Activity of TP
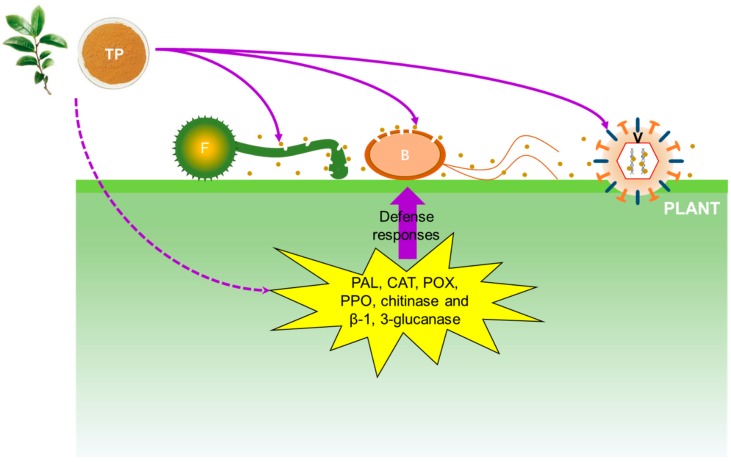
Several studies have revealed that TP shows antimicrobial effects against pathogenic organisms via several antimicrobial mechanisms (Figure 2).
Figure 2.
Schematic representation of the antimicrobial mechanisms of tea polyphenol. Brown dots represent tea polyphenol particles. TP: tea polyphenol; F: fungi; B: bacteria; V: virus; PAL: phenylalanine ammonia-lyase; CAT: catalase; POX: peroxidase; PPO: polyphenoloxidase.
3.1. Mechanism of Antifungal Activity of TP
Changes in cell membranes and structure affect membrane permeability, which can be indirectly detected by measuring the electrolyte leakage percentage [30]. A previous study revealed that the electrolyte leakage percentage of rice blast fungus treated with various concentrations of TP changed sharply, indicating that TP treatment changed the membrane permeability of rice blast fungus. Since the main component of cell membrane is a lipid bilayer containing a hydrophilic end and a hydrophobic end, the phenolic hydroxyl groups can bind the hydrophilic end of the lipid bilayer to agglomerate the membrane lipid, thereby destroying the cell membrane [14]. In parallel, TP induced the activities of several enzymes in inoculated plants or fruits, such as phenylalanine ammonia-lyase, catalase, peroxidase, polyphenoloxidase, chitinase and β-1, 3-glucanase [14,27]. All of these enzymes play important roles in plant defenses against fungal infection.
3.2. Mechanism of Antibacterial Activity of TP
The antibacterial activity of TP was also attributed to membrane perturbation. Strong bactericidal dosages of EGCG damaged the liposome membrane of E. coli and S. aureus, leading to the leakage of intramembranous materials and aggregation of the liposomes [31]. These results were in agreement with the findings that TP inhibits Pseudomonas aeruginosa through damage to the cell membrane with the release of small cellular molecules [32]. In addition, EGCG and ECG strongly inhibit biofilm formation of commensal and pathogenic E. coli strains, thereby reduce the expression of CsgD - a crucial activator of curli and cellulose biosynthesis [33].
Interestingly, some studies suggested that EGCG might directly bind peptidoglycan, a major component of the cell wall of bacteria, thereby caused the cross-linking bridges of the peptidoglycan layer to be broken, resulting in its degradation [34]. Moreover, EGCG involved into the inhibition of the lipopolysaccharide-induced TLR4 signaling and peptidoglycan-induced TLR2 signaling pathways [35,36,37]. TLRs (toll-like receptors) are a family of membrane proteins that trigger innate immune responses and are pathogen recognition proteins that have important roles in detecting microbes [38]. Therefore, it is likely that EGCG also perturb the barrier function of both cell wall and membrane possibly by disrupting the signaling pathways of bacteria. Although these findings were obtained from the pathogenic bacteria on mammals, the mechanism of antibacterial activity of TP against plant pathogens is likely to be consistent.
3.3. Mechanism of Antiviral Activity of TP
Unlike fungi and bacteria, TP showed different mechanisms against virus. Okada et al. identified that theaflavins, dimers of tea catechins, inhibit the multiplication of TMV by direct binding to viral RNA but not to RNA protein [39]. They presumed that the interaction of theaflavins with nucleic acids leads to the inhibition of TMV replication. This result is consistent with the inhibitory effect on human immunodeficiency virus (HIV), in which catechins inhibit HIV-1 replication by targeting several steps in HIV-1 life cycle [40]. Another study showed EGCG potently inhibited Cell-culture–derived Hepatitis C virus (HCV) entry into hepatoma cell lines as well as primary human hepatocytes and could be part of an antiviral strategy aimed at the prevention of HCV reinfection after liver transplantation [41]. These results indicated that TP showed multiple mechanisms of antiviral activity with different components.
4. Conclusions and Prospects
The findings mentioned in this review indicate that TP may have the potential to control various phytopathogens. In addition, TP has the characteristics of abundant resources, simple extraction process and low production cost [14]. Therefore, the employment of TP as a botanical pesticide may play a vital role in meeting the demand for organically produced plants in particular and in alleviating some environmental problems caused by the use of chemical pesticides. However, most of these conclusions were based on data from in vitro studies. To elucidate the confused inhibition mechanisms of TP, and study in vivo activity, toxicity and bioavailability are the main research directions in the future [42]. Moreover, the field utilization of TP is greatly limited by its poor permeability [12]. Recent studies reported that the encapsulation of TP using nanoemulsions improved the systemic bioavailability and efficacy [43,44,45], which may be a good way to improve TP’s permeability and the biocontrol effect for the management of phytopathogens.
Acknowledgments
We thank Bruce J Levine of the University of Maryland College Park for assistance in writing the manuscript, and anonymous reviewers for their kind suggestions.
Author Contributions
Data collection, Y.Y. and T.Z.; writing and editing, Y.Y. and T.Z.; funding acquisition, T.Z.
Funding
This research was funded by the National Key R&D Program of China (2018YFD0200500), the National Natural Science Foundation of China (31801719), the Fundamental and Advanced Research Projects of Chongqing City (cstc2016jcyjA0316), and the Fundamental Research Funds for the Central Universities (SWU116048, XDJK2017C085).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kris-Etherton P.M., Keen C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Vuong Q.V. Epidemiological Evidence Linking Tea Consumption to Human Health: A Review. Crit. Rev. Food Sci. 2014;54:523–536. doi: 10.1080/10408398.2011.594184. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera C., Gimenez R., Lopez M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003;51:4427–4435. doi: 10.1021/jf0300801. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda Y., Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat. Res.-Rev. Mutat. Res. 1999;436:69–97. doi: 10.1016/S1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 5.Hamiltonmiller J.M.T. Antimicrobial properties of tea (Camellia sinensis L.) Antimicrob. Agents Chemother. 1995;39:2375–2377. doi: 10.1128/AAC.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chacko S.M., Thambi P.T., Kuttan R., Nishigaki I. Beneficial effects of green tea: A literature review. Chin. Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 8.Caruana M., Vassallo N. Tea Polyphenols in Parkinson’s Disease. Adv. Exp. Med. Biol. 2015;863:117–137. doi: 10.1007/978-3-319-18365-7_6. [DOI] [PubMed] [Google Scholar]
- 9.Afzal M., Safer A.M., Menon M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology. 2015;23:151–161. doi: 10.1007/s10787-015-0236-1. [DOI] [PubMed] [Google Scholar]
- 10.Reygaert W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014;5:434. doi: 10.3389/fmicb.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X., Lv Z., Chen J., Xu H., Zheng X., Chen L., Zhang J., Shentu X. Review on research and development of botanical pesticides in China. Acta Agric. Zhejiangensis. 2005;17:42–48. (In Chinese) [Google Scholar]
- 12.Yang Y.H., Chen Y.J., Chen F.J., Yu Y., Bi C.W. Tea polyphenol is a potential antifungal agent for the control of obligate biotrophic fungus in plants. J. Phytopathol. 2017;165:547–553. doi: 10.1111/jph.12591. [DOI] [Google Scholar]
- 13.Wang J., Qiu Y., Chen H., Lu H., Lin X., Ma L. Inhibitive Action of Tea-polyphenol on Some Plant Pathogenic Fungi. Nat. Prod. Res. Dev. 2008;20:690–694. (In Chinese) [Google Scholar]
- 14.Wang J., Qiu Y., Fu J., Han J., Feng W. Inhibitive Effect of Tea-polyphenols on Pyricularia oryzae and Its Mechanism. Nat. Prod. Res. Dev. 2011;23:918–922. (In Chinese) [Google Scholar]
- 15.Zou D., Liao W., Huang H., Jiang X. The Inhibition Effect of Tea Polyphenol on 8 kinds of Plant Pathogenic Fungi. Guangxi For. Sci. 2017;46:412–415. (In Chinese) [Google Scholar]
- 16.Liu H.M., Guo J.H., Liu P., Cheng Y.J., Wang B.Q., Long C.A., Deng B.X. Inhibitory activity of tea polyphenol and Candida ernobii against Diplodia natalensis infections. J. Appl. Microbiol. 2010;108:1066–1072. doi: 10.1111/j.1365-2672.2009.04511.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu H.M., Guo J.H., Cheng Y.J., Liu P., Long C.A., Deng B.X. Inhibitory activity of tea polyphenol and Hanseniaspora uvarum against Botrytis cinerea infections. Lett. Appl. Microbiol. 2010;51:258–263. doi: 10.1111/j.1472-765X.2010.02888.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang X.P., Jiang X.D., Chen J.J., Zhang S.S. Control of postharvest grey mould decay of nectarine by tea polyphenol combined with tea saponin. Lett. Appl. Microbiol. 2013;57:502–509. doi: 10.1111/lam.12139. [DOI] [PubMed] [Google Scholar]
- 19.Fukai K., Ishigami T., Hara Y. Antibacterial Activity of Tea Polyphenols against Phytopathogenic Bacteria. Agr. Biol. Chem. Tokyo. 1991;55:1895–1897. [Google Scholar]
- 20.Alstrom S. Antibacterial Activity of Tea and Coffee Wastes against Some Plant Pathogenic Pseudomonas-Syringae Strains. J. Phytopathol. 1992;136:329–334. doi: 10.1111/j.1439-0434.1992.tb01315.x. [DOI] [Google Scholar]
- 21.Thornberry H.H. Effect of tannic acid on the infectivity of tobacco-mosaic virus. Phytopathology. 1935;25:931–937. [Google Scholar]
- 22.Okada F., Furuya K. Inhibitory effect of tea catechins on some plant virus diseases. Chagyo Kenkyu Hokoku. 1971;1971:69–76. doi: 10.5979/cha.1971.35_69. (In Japanese) [DOI] [Google Scholar]
- 23.Okada F. Antiviral effects of tea catechins and black tea theaflavins on plant viruses. JARQ. 1978;12:27–32. [Google Scholar]
- 24.Hao W.N., Zhong G.H., Hu M.Y., Luo J.J., Weng Q.F., Rizwan-ul-Haq M. Control of citrus postharvest green and blue mold and sour rot by tea saponin combined with imazalil and prochloraz. Postharvest Biol. Tec. 2010;56:39–43. doi: 10.1016/j.postharvbio.2009.10.003. [DOI] [Google Scholar]
- 25.Nas M.N. In vitro studies on some natural beverages as botanical pesticides against Erwinia amylovora and Curtobacterium flaccumfaciensis subsp. Poinsettiae. Turkish J. Agric. For. 2004;28:57–61. [Google Scholar]
- 26.Huang J., Chen X., Xu H., Wang H. Studies on inhibitory activity of tea saponin against twelve plant pathogenic fungi. J. Huazhong Agric.Univ. 2013;32:50–53. (In Chinese) [Google Scholar]
- 27.Chen J.J., Zhang S.S., Yang X.P. Control of brown rot on nectarines by tea polyphenol combined with tea saponin. Crop. Prot. 2013;45:29–35. doi: 10.1016/j.cropro.2012.11.006. [DOI] [Google Scholar]
- 28.Zou D., Liao W., Huang H., Jiang X. Co-toxicities of tea saponin and tea polyphenol on 2 plant pathogens. J. West China For. Sci. 2018;47:41–44, 58. (In Chinese) [Google Scholar]
- 29.Liu H., Guo J., Cheng Y., Luo L., Liu P., Wang B., Deng B., Long C. Control of gray mold of grape by Hanseniaspora uvarum and its effects on postharvest quality parameters. Annals Microbiol. 2010;60:31–35. doi: 10.1007/s13213-010-0018-3. [DOI] [Google Scholar]
- 30.Shadmani N., Ahmad S.H., Saari N., Ding P., Tajidin N.E. Chilling injury incidence and antioxidant enzyme activities of Carica papaya L. ‘Frangi’ as influenced by postharvest hot water treatment and storage temperature. Postharvest Biol. Tec. 2015;99:114–119. doi: 10.1016/j.postharvbio.2014.08.004. [DOI] [Google Scholar]
- 31.Ikigai H., Nakae T., Hara Y., Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-R. [DOI] [PubMed] [Google Scholar]
- 32.Yi S., Zhu J., Fu L., Li J. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int. J. Food Microbiol. 2010;144:111–117. doi: 10.1016/j.ijfoodmicro.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Serra D.O., Mika F., Richter A.M., Hengge R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σE-dependent sRNA RybB. Mol. Microbiol. 2016;101:136–151. doi: 10.1111/mmi.13379. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y., Oh Y.J., Lim J., Youn M., Lee I., Pak H.K., Park W., Jo W., Park S. AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012;29:80–87. doi: 10.1016/j.fm.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Byun E.H., Omura T., Yamada K., Tachibana H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Letters. 2011;585:814–820. doi: 10.1016/j.febslet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Byun E., Choi H., Sung N., Byun E. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor on lipopolysaccharide-stimulated dendritic cells. Biochem. Biophys. Res. Commun. 2012;426:480–485. doi: 10.1016/j.bbrc.2012.08.096. [DOI] [PubMed] [Google Scholar]
- 37.Byun E.H., Fujimura Y., Yamada K., Tachibana H. TLR4 Signaling Inhibitory Pathway Induced by Green Tea Polyphenol Epigallocatechin-3-Gallate through 67-kDa Laminin Receptor. J. Immunol. 2010;185:33–45. doi: 10.4049/jimmunol.0903742. [DOI] [PubMed] [Google Scholar]
- 38.Takeda K., Akira S. Toll-Like Receptors in Innate Immunity. Int. Immunol. 2004;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 39.Okada F., Takeo T., Okada S., Tamemasa O. Antiviral Effect of Theaflavins on Tobacco Mosaic Virus. Agr. Biol. Chem. Tokyo. 1977;41:791–794. [Google Scholar]
- 40.Liu S., Lu H., Zhao Q., He Y., Niu J., Debnath A.K., Wu S., Jiang S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta. 2005;1723:270–281. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Ciesek S., Von Hahn T., Colpitts C.C., Schang L.M., Friesland M., Steinmann J., Manns M.P., Ott M., Wedemeyer H., Meuleman P. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54:1947–1955. doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- 42.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Kumar D.H.L., Sarkar P. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018;16:59–70. doi: 10.1007/s10311-017-0663-x. [DOI] [Google Scholar]
- 44.Lante A., Friso D. Oxidative stability and rheological properties of nanoemulsions with ultrasonic extracted green tea infusion. Food Res. Int. 2013;54:269–276. doi: 10.1016/j.foodres.2013.07.009. [DOI] [Google Scholar]
- 45.Kim Y.J., Houng S.J., Kim J.H., Kim Y., Ji H.G., Lee S. Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J. Nutr. Biochem. 2012;23:186–191. doi: 10.1016/j.jnutbio.2010.11.015. [DOI] [PubMed] [Google Scholar]