Abstract
Bone marrow failure (BMF) is a rare but life-threatening disorder that usually manifests as (pan)cytopenia. BMF can be caused by a variety of diseases, but inherited BMF (IBMF) syndromes are a clinically important cause, especially in children. IBMF syndromes are a heterogeneous group of genetic disorders characterized by BMF, physical abnormalities, and predisposition to malignancy. An accurate diagnosis is critical, as disease-specific management, surveillance, and genetic counselling are required for each patient. The major differential diagnoses of IBMF syndromes are acquired aplastic anemia (AA) and refractory cytopenia of childhood (RCC). These diseases have overlapping features, such as BM hypocellularity and/or dysplastic changes, which make the differential diagnosis challenging. RCC has been defined as a histomorphologically distinct entity. Therefore, understanding the BM histopathology of these diseases is essential for the differential diagnosis. However, the BM histopathological features have not been characterized in detail, as descriptions of BM histopathology are very limited due to the rarity of the diseases. This review provides a detailed description of the BM histopathology in cases of RCC, AA, and the four most common IBMF syndromes: Fanconi anemia (FA), dysketatosis congenita (DC), Diamond-Blackfan anemia (DBA), and Shwachman-Diamond syndrome (SDS). An overview, including the clinical features and diagnosis, is also provided.
Keywords: aplastic anemia, bone marrow failure syndromes, children, histopathology, refractory cytopenia of childhood
INTRODUCTION
Bone marrow failure (BMF) is a rare but life-threatening disorder caused by ineffective/defective hematopoiesis of the bone marrow (BM) leading to (pan)cytopenia in the peripheral blood (PB).1,2 The presence of BMF is an important indication for BM biopsy. In both children and adults, BMF can be attributed to a variety of causes, either acquired or inherited. However, inherited BMF (IBMF) syndromes are more frequent in children.1,2 IBMF syndromes are a heterogeneous group of genetic diseases characterized by BMF, physical abnormalities, and predisposition to malignancy.1,3 An accurate diagnosis is essential, as patients with IBMF syndromes need disease-specific management, surveillance, and genetic counselling.
In the practical setting, differentiating IBMF syndromes from acquired aplastic anemia (AA) and refractory cytopenia of childhood (RCC) is the most important step.4 BM in IBMF syndromes usually exhibits hypocellularity with or without dysplastic changes. Overlapping features, such as BM hypocellularity and/or dysplastic changes, make the distinction from AA and RCC challenging. Furthermore, depending on the disease stage, the BM in the IBMF syndromes may demonstrate progression to secondary myelodysplastic syndrome (MDS), which should also be differentiated from primary MDS, including RCC. Thus, concurrent assessment along with clinical features, physical anomalies, and laboratory/genetic findings is essential for BM evaluation.
RCC is a low-grade MDS in children and adolescents that has been proposed as a provisional entity.4,5 It has been defined as a histomorphologically distinct entity and, for its diagnosis, BM histological evaluation is mandatory.5,6 Therefore, understanding the BM histopathology in the above-mentioned diseases causing BMF is critical and can aid in the differential diagnosis. However, detailed BM histopathological features have not been characterized, as descriptions of BM histopathology are very limited due to the rarity of the diseases.
In this review, the histopathological features of BM in RCC, AA, and the four most common IBMF syndromes: Fanconi anemia (FA), dysketatosis congenita (DC), Diamond-Blackfan anemia (DBA), and Shwachman-Diamond syndrome (SDS) are described in detail, partly according to the author’s experience. An overview, including the clinical features and diagnosis, is also provided.
REFRACTORY CYTOPENIA OF CHILDHOOD
Clinical features
RCC was first cited as a provisional MDS entity in the 2008 WHO classification5 and is still included as a provisional entity in the revised edition as well.4 This entity was proposed by the pathologists of the European Working Group of MDS of Childhood (EWOG-MDS) in order to discriminate MDS patients from AA patients.5,6 AA is considered to be an autoimmune-mediated disorder, whereas MDS is caused by a clonal stem cell defect and has the potential to progress to an advanced disease.
BM biopsy specimens for AA characteristically exhibit either severe hypoplasia or aplasia of hematopoietic cells. However, patchy foci of erythroid precursors in the fatty BM of AA patients may be observed. Historically, such erythropoietic foci and/or dyserythropoiesis were accepted as features of AA, and only the presence of micromegakaryocytes was considered to be a hallmark of MDS.6 However, some patients with AA may be less responsive than others to immunosuppressive therapy (IST) and develop clonal progression. This suggests that there is a group of MDS patients with an underlying clonal stem cell defect among those previously diagnosed with AA. Thus, in an attempt to discriminate MDS from AA, the EWOG-MDS proposed RCC.5,6 According to the proposed criteria, patchy foci of erythroid precursors in an otherwise hypoplastic BM should not be present in AA, but are in RCC.
RCC is a low-grade MDS in children and adolescents, and the most common subtype of MDS in that age group, accounting for 50%-90% of all MDS cases in children.4,7 The annual incidence of de novo MDS in a Japanese prospective registry study was 78 cases, more than 90% of which were RCC.7
The clinical symptoms are usually related to cytopenia such as anemia, bleeding tendency, and infection. Approximately 20% of patients have no clinical symptoms or signs.8 In contrast to the disease in adults, pancytopenia is more frequently present. The severity of cytopenia is often milder in RCC than in AA.7 The mean capsular volume and hemoglobin F (HbF) are usually elevated.9 Moreover, congenital abnormalities are observed in some patients.8
Monosomy 7 is the most frequent cytogenetic abnormality, being observed in 8%-48% of RCC patients, followed by trisomy 8 and other abnormalities, including complex karyotypes.8-10 Unlike in adults, del(5q) is exceedingly rare in children.9 Disease progression occurs in 12%-32% of RCC patients.8,10 Patients with monosomy 7 are more likely to develop disease progression than those with other chromosomal abnormalities or with a normal karyotype.8,10 The overall survival is 82% at 5 years10 and 48% at 10 years.8 The causes of death include complications of pancytopenia, progression to advanced clonal disease, hemophagocytic syndrome after chemotherapy, and transplantation-related causes.8,10
Diagnosis
RCC has been defined as persistent cytopenia with <2% blasts in the PB, <5% blasts in the BM, and dysplastic changes in ≥2 cell lineages or ≥10% dysplastic change within one cell lineage on BM aspirate smears.4,5 The presence of dysplasia without an increase in the blast count is a characteristic feature and required for the diagnosis of RCC, but this is only one morphological aspect of RCC and is not sufficient for a diagnosis. Histomorphological evaluation of an adequate BM trephine biopsy specimen is mandatory for a diagnosis. The minimal diagnostic criteria for RCC have been provided (Table 1A).4,5 Furthermore, differential diagnosis between AA and hypoplastic RCC has been described in detail, as approximately 80% of children with RCC have BM hypocellularity (Table 1B).5,9
Table 1A. Bone marrow minimal histological criteria for refractory cytopenia of childhood.4,5 Refractory cytopenia of childhood is defined as persistent cytopenia with <5% blasts in bone marrow and <2% blasts in peripheral blood. The criteria of dysplasia must be fulfilled in ≥2 cell lineages or ≥10% of cells within one cell lineage on bone marrow aspirate smears.
| Cellularity | Erythropoiesis | Granulopoiesis | Megakaryopoiesis |
|---|---|---|---|
| Variable | A few clusters of ≥20 erythroid precursors. Arrest in maturation, with increased number of proerythroblasts. Increased number of mitoses. |
No minimal diagnostic criteria | Unequivocal
micromegakaryocytes; immunohistochemistry is obligatory (CD61, CD41, CD42b); other dysplastic changes in variable numbers |
Table 1B. Comparison of the bone marrow histological features for hypoplastic refractory cytopenia of childhood and aplastic anemia in children.4-6,23.
| Hypoplastic refractory cytopenia of childhood | Aplastic anemia in children | |
|---|---|---|
| Cellularity | Hypocellular | > 95% fatty marrow |
| Dysplastic changea | ≥2 cell lineages or ≥10% of cells within one cell lineage | None |
| Blastb | <5% | No increase |
| Erythropoiesis | Patchy distribution Left shift Increased mitoses Abnormal localizationc |
Absent or single small focus; <10 cells with maturation No abnormal localizationc |
| Granulopoiesis | Marked decrease Left shift |
Absent or markedly decreased, with very few small foci with maturation |
| Megakaryopoiesis | Marked decrease or aplasia Dysplastic changes Micromegakaryocytes Abnormal localizationc |
Absent or very few non-dysplastic megakaryocytes No abnormal localizationc |
| Lymphocytes | May be increased focally or dispersed |
May be increased focally or dispersed |
| CD34+precursor cells |
No increase No clusters |
No increase No clusters |
| KIT+ (CD117+) precursor cells |
No increase | No increase |
| KIT+ (CD117+) mast cells |
Slightly increased | Slightly increased |
a. The number of lineages and percentage of dysplastic change are evaluated on bone marrow aspirate smears.
b. The blast percentage is evaluated in bone marrow aspirates and biopsy.
c. Abnormal localization indicates the presence of erythopoiesis or megakaryopoiesis in the paratrabecular region.
In addition to differentiation from AA, RCC must also be discriminated from other disorders, including IBMF syndromes, infection, nutritional deficiencies, and metabolic diseases.5 These disorders have overlapping features with RCC, such as a hypocellular BM and/or dysplastic changes, and are indistinguishable from each other by morphological features alone. The clinical course, family history, physical examination, and laboratory and genetic testing results are also required before a diagnosis of RCC can be concluded. Down syndrome patients are excluded from this entity.
Some patients with RCC also meet the criteria for refractory cytopenia with multilineage dysplasia (RCMD), which has been defined as low-grade MDS in adults, synonymous for MDS with multilineage dysplasia in the 2017 WHO classification.11 It is presently recommended that cases that fit the criteria for RCMD be considered RCC until the prognostic significance of multilineage dysplasia can be fully evaluated.4,7
Histopathology
The minimal histological criteria for RCC4,5 are shown in Table 1A. The BM of RCC demonstrates hypo-, normo-, or hypercellularity, although approximately 80% of patients have BM hypocellularity (Fig. 1).9 The distribution of hematopoiesis depends on the BM cellularity. A patchy distribution is typically seen in hypocellular BM specimens, and as the BM cellularity increases, the diffuse area becomes more prominent (Fig. 1).
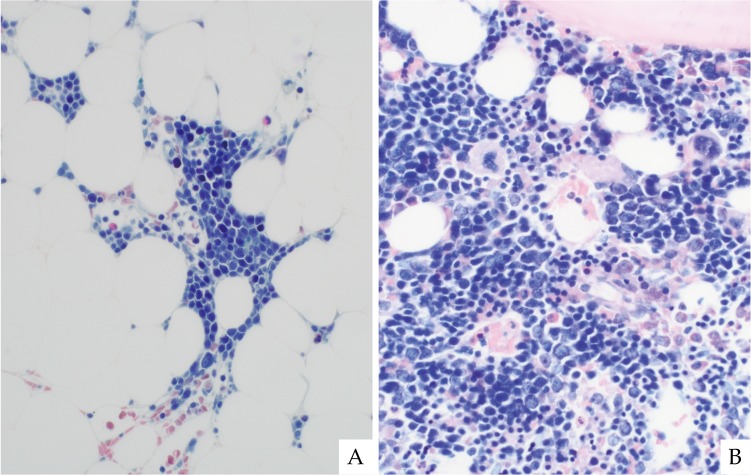
Fig. 1.
Refractory cytopenia of childhood (RCC) with variable bone marrow cellularity. Most patients exhibit hypocellular bone marrow for their age (A). In some patients, hypercellular areas are present in the marrow (B). The distribution of hematopoiesis depends on the marrow cellularity. A patchy distribution is likely seen in the hypocellular marrow (C), whereas a diffuse distribution is often present in hypercellular marrow (D). Naphthol ASD chloroacetate esterase plus Giemsa double-staining (ASD-Giemsa). (A, B) ×40; (C, D) ×400.
As described in the criteria (Table 1A), erythroid cells with impaired maturation are essential for the diagnosis of RCC and invariably identified, regardless of BM cellularity.9 In hypoplastic RCC, clusters of at least 20 erythroid precursors with increased proerythroblasts are distributed in a patchy pattern (Fig. 2).5,9 In contrast, in normo- or hypercellular BM of RCC, clusters of erythroid precursors are more numerous and larger in size (Fig. 2). Abnormal localization of erythropoiesis in the paratrabecular region is often observed (Fig. 3), with the erythroid cells being occasionally positive for HbF on immunostaining.
Fig. 2.
Erythropoiesis in RCC. Erythroid clusters with arrested maturation, mainly composed of proerythroblasts, are invariably present in a patchy distribution (A). In diffuse areas, erythroid clusters are often larger in size and fused with each other, intermingled with granulocytes and megakaryocytes (B). ASD-Giemsa staining. (A, B) ×400.
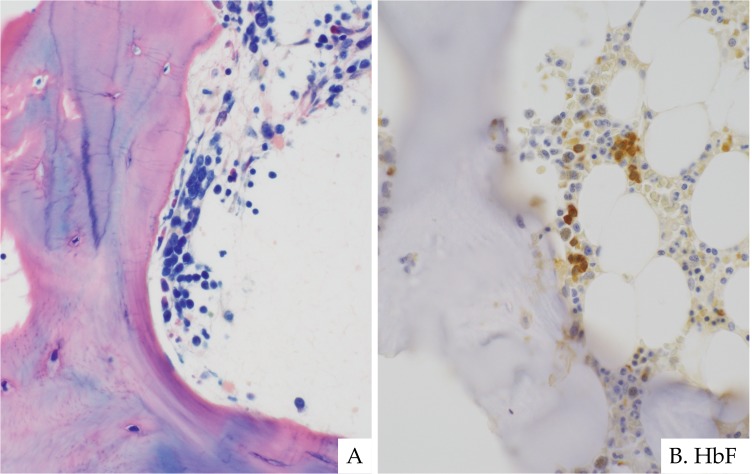
Fig. 3.
The abnormal localization of erythropoiesis in RCC. Erythropoiesis is often present in the paratrabecular region, which indicates dyserythropoiesis (A). Erythroid cells are occasionally marked by hemoglobin F immunostaining (B). ASD-Giemsa staining. (A) ×400. Immunohistochemistry. (B) ×400.
Granulopoiesis is usually decreased in RCC, although no minimal histological criteria have been established.4 In hypoplastic RCC, granulopoiesis is almost always decreased and sparsely distributed. In RCC cases with normo- or hypercellular BM, granulopoiesis may be slightly decreased and is often intermingled with erythroid clusters (Fig. 4). Regardless of the BM cellularity, left-shifted granulopoiesis is often observed. Left-shifted granulopoiesis is easily identified in BM aspirate smears, but it can be observed even in BM biopsy specimens, where myeloid precursors are more prominent than banded or segmented myeloid matured cells (Fig. 4). Clusters of myeloblasts are not found in RCC,4 unlike in advanced MDS/acute myeloid leukemia (AML).12
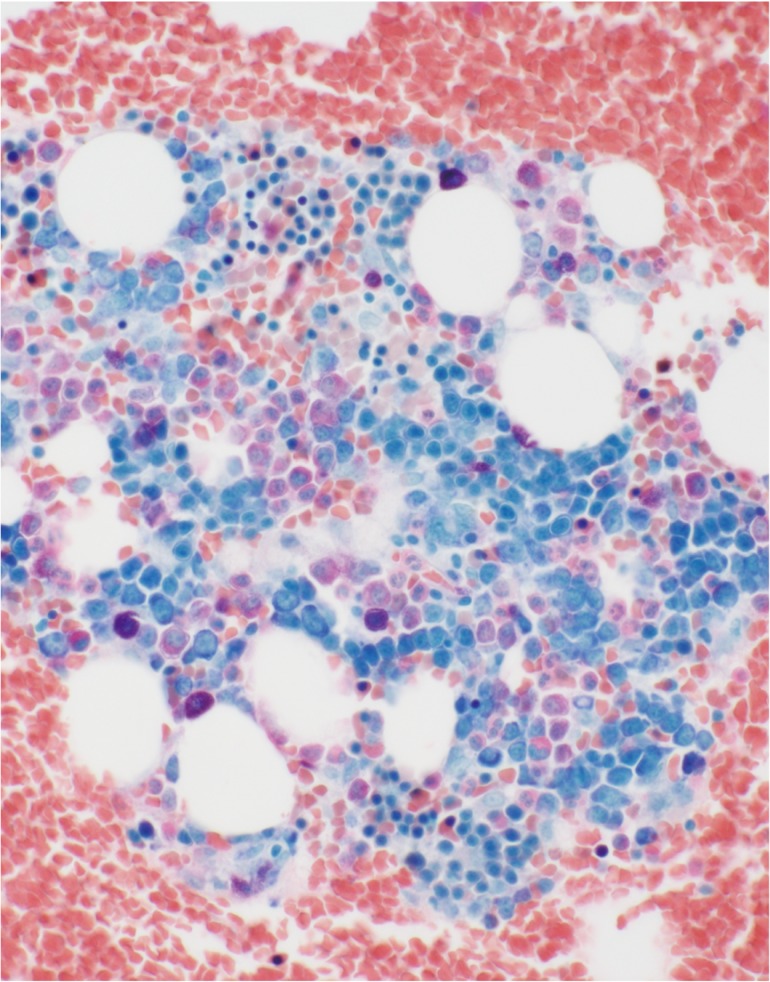
Fig. 4.
Granulopoiesis in RCC. Granulopoiesis is moderately decreased and intermingled with erythroid clusters. Left-shifted granulopoiesis is often present where myeloid precursors are prominent. Mast cells may be increased in the background. Clot section with ASD-Giemsa staining ×400.
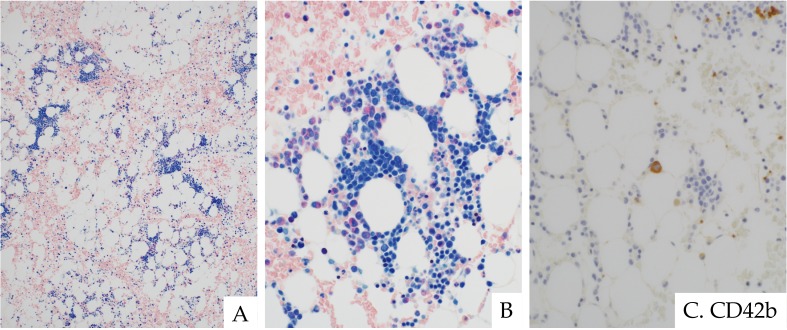
Megakaryocytes may be variable in number but are often markedly reduced or absent, especially in BM of hypoplastic RCC.5,9 The presence of micromegakaryocytes is not mandatory, but it is a strong indicator for RCC (Fig. 5). Abnormal localization of megakaryopoiesis in the paratrabecular region may be also observed. Immunohistochemistry (IHC) for megakaryocyte-reactive antibodies, such as CD61, CD41, or CD42b, is an indispensable method for diagnosing RCC.6
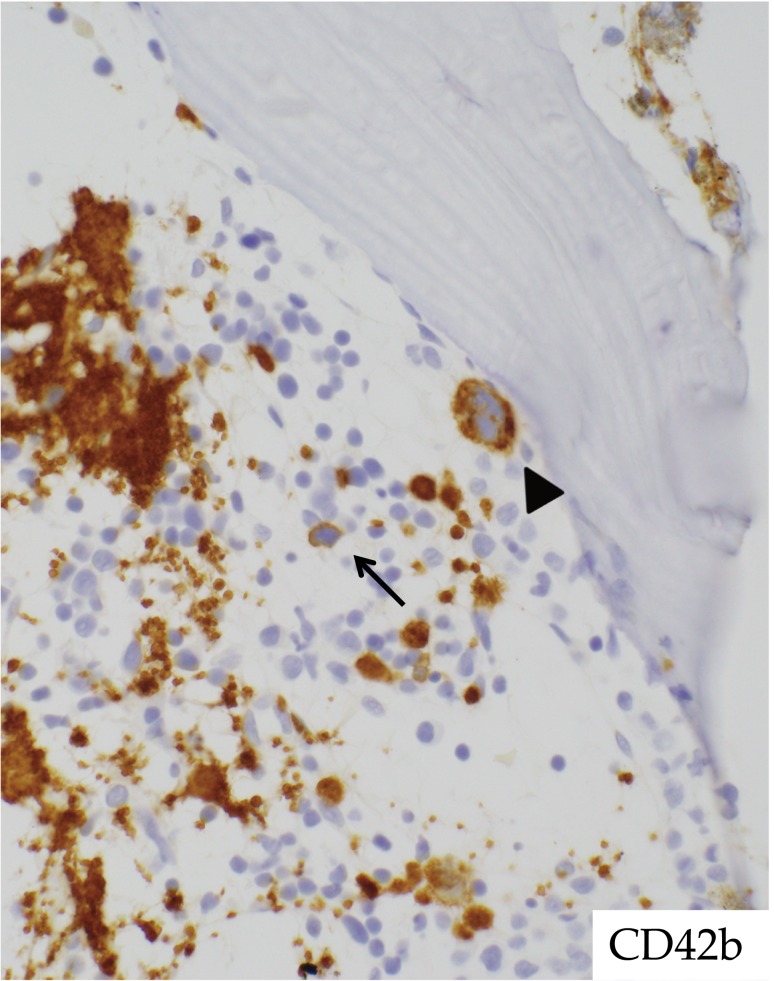
Fig. 5.
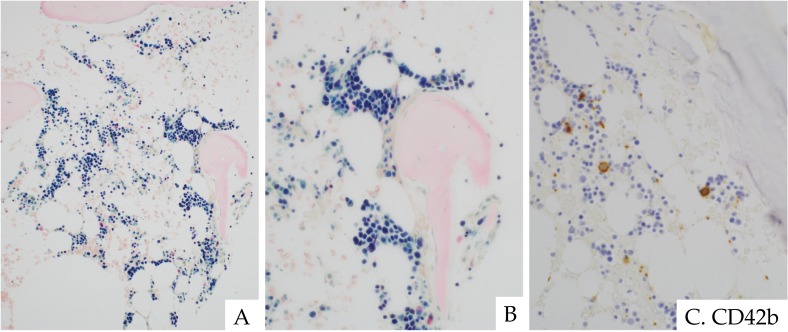
Megakaryopoiesis in RCC. Immunohistochemistry is useful for identifying megakaryocytes. CD42b immunostaining clearly indicates a micromegakaryocyte (center, arrow) and a megakaryocyte with abnormal localization (upper right, arrowhead). Immunohistochemistry ×400.
Lymphocytes, plasma cells, and mast cells may be increased in number. However, no increase in reticulin fibers is seen.
The presence of dyshematopoiesis is critical for the diagnosis of RCC. Dysplastic changes are generally assessed in BM aspirate smears, but may even be histologically recognized in BM biopsy specimens.12,13 For example, micromegakaryocytes, a manifestation of dysmegakaryopoiesis, can be easily detected in BM biopsies using IHC. Furthermore, erythroid hyperplasia with clusters of precursors, left-shifted granulopoiesis, and the abnormal localization of erythroid cells and megakaryocytes have been regarded as histological indicators of dyshematopoiesis,12,13 all of which are detectable on BM biopsy. All of these findings observed in the BM of RCC with variable frequency support a diagnosis of RCC. In the author’s experience, these are noted more prominently in patients with RCC with hypercellular BM or RCC with multilineage dysplasia, some cases of which correspond to RCMD.
IHC targeting cells other than megakaryocytes is also useful for the diagnosis of RCC.4 CD34 staining is helpful for detecting myeloblasts.12 CD34-positive blasts are not increased and should account for <5% of the BM cells in RCC. Detecting ≥5% blasts or clusters of blasts may indicate progression to advanced MDS/AML. When evaluating BM blasts using CD34 staining, one should distinguish hematogones, which are also positive for CD34, from myeloid blast proliferation.4 KIT (CD117) is also useful for detecting myeloblasts.4 KIT-positive myeloblasts should be carefully distinguished from mast cells, which are also KIT-positive and may be increased in the BM of numerous conditions causing BMF.14 The reason for the increase in mast cells is unknown, but it may involve the KIT-KIT ligand pathway, which is essential for the development of mast cells.15 This pathway can be activated under certain BMF conditions where the production of the KIT ligand by BM stromal cells is elevated.16
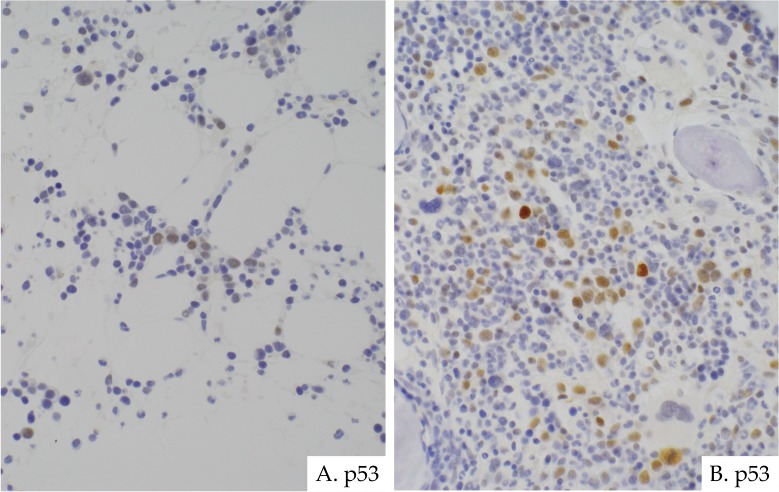
The significance of P53 IHC staining in RCC is unclear. In adults, P53 may be involved in the disease progression of MDS.17 P53-positive cells have been reported to be more frequently observed in high-risk MDS than in low-risk MDS, and are associated with p53 mutation.17,18 Furthermore, p53-positive cells have been identified in the BM of RCC. In the author’s experience, RCC generally has few p53-positive cells, but cell numbers are slightly increased in the hypercellular BM of RCC and/or in patients with prominent dysplastic changes corresponding to RCMD (Fig. 6). The association of p53 IHC with disease progression and the RCC pathogenesis remains unknown.
Fig. 6.
P53 immunostaining in RCC. In most cases, p53-positive cells are few (A). However, they often increase in cases of hypercellular marrow and/or those with prominent dysplasia corresponding to refractory cytopenia with multilineage dysplasia (RCMD) (B). Immunohistochemistry. (A, B) ×400.
Cytogenetic abnormalities can also aid in the diagnosis of RCC.9 A normal karyotype is observed in more than half of RCC cases,9,10 which may make it challenging to differentiate RCC from other non-neoplastic conditions. However, BM histology can provide clues supporting an RCC diagnosis, as BM hypercellularity and/or the presence of prominent dysplastic changes are likely involved in cytogenetic abnormalities.9,10
APLASTIC ANEMIA
Clinical features
Acquired aplastic anemia (AA) is a rare disorder characterized by pancytopenia with hypocellular BM. The incidence of AA is higher in Asia than in the West. Indeed, the incidence in Japan is 4.79/million/year,19 whereas that in Europe and North America is 2.0/million/year.20 Although the reason for the higher incidence in Asia is unknown, the involvement of genetic factors, such as genetic polymorphism of HLA types and/or cytokine genes, rather than environmental factors is suspected.21
Most patients are diagnosed between 10 and 25 years of age.22 The clinical signs and symptoms are related to pancytopenia. Children typically present with bleeding tendency, fatigue, and infection. Anemia and thrombocytopenia are the most frequent manifestations, but neutropenia is less common. Some patients are diagnosed by incidental laboratory findings.23 Hepatosplenomegaly and lymphadenopathy are usually absent.22 A history of jaundice, single-lineage cytopenia, or seronegative hepatitis may lead to the diagnosis of hepatitis-associated AA.22,23
Clonal hematopoiesis (CH), including paroxysmal nocturnal hemoglobinuria (PNH) and MDS/AML, frequently develops during the course of AA, which is the most problematic event.22 PNH clones may be observed in 41% of patients at diagnosis and 48% during IST.24 CH with acquisition of chromosomal abnormalities develops in 2.4%-8.2% patients with AA.6,25
The overall survival is excellent, reaching over 90%, due to improvements in IST and hematopoietic stem cell transplantation (HSCT).22 Relapse, CH, autoimmunity, and cancer are of special concern as late manifestations of the disease.23
Diagnosis
AA is defined as pancytopenia associated with persistent hypocellular BM, without major dysplastic signs or marrow fibrosis.22 Depending on the degree of cytopenia in PB, AA can be classified into three forms: moderate or non-severe, severe, and very severe AA.23
A diagnosis of AA is required to exclude other possible diseases or conditions causing pancytopenia with hypocellular BM. The clinical course, family history, exposure to toxins/drugs, infectious agents, physical abnormalities, and laboratory tests should be carefully evaluated. IBMF syndromes, if suspected, must be excluded and diagnosed by an appropriate molecular analysis.26 Comprehensive targeted genetic screening has identified germline mutations of known IBMF syndromes in 5.1% of AA patients, some of whom did not have physical anomalies or a family history associated with the diseases.27
Cytogenetic abnormalities in AA are absent or transient.23 Persistent abnormalities of chromosome 7 and 8, or complex cytogenetic abnormalities generally indicate MDS, not AA.23 The clinical significance of other isolated nonspecific abnormalities remains unclear.28
BM biopsy evaluation is indispensable for an AA diagnosis,26 allowing for correct evaluation of BM cellularity and histological discrimination of AA from RCC.4 The BM histological criteria for AA and hypoplastic RCC are compared in Table 1B. Detailed descriptions are given in the following sections.
Histopathology
BM biopsy specimens of AA typically demonstrate complete aplasia or marked hypoplasia of three hematological cell lineages with replacement of fat cells (>95% fatty marrow) (Fig. 7).6,9 BM cellularity must be assessed by biopsy, not aspirate smears. Lymphocytes, plasma cells, and mast cells may be focally increased or scattered.9 These cells should be excluded in the assessment for BM cellularity.
Fig. 7.
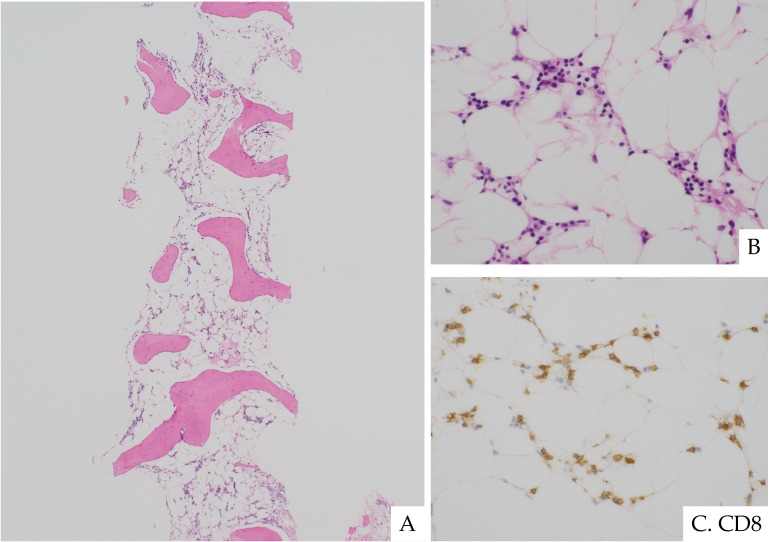
Aplastic anemia in an 8-year-old boy with pancytopenia. He had severely hypoplastic bone marrow with replacement of fat cells (A). Lymphocytes and plasma cells were scattered. Megakaryocytes and granulocytes were not present (B). CD8-positve cytotoxic T cells were increased (C). H&E staining. (A) ×40; (B) ×400. Immunohistochemistry. (C) ×400.
Differential diagnosis between AA and hypoplastic RCC is challenging. However, the vast majority of AA and RCC cases can be reliably differentiated by histology alone.6 The histological BM features are compared in Table 1B. The following sections focus on the differentiation of these two diseases.
A patchy pattern of erythropoiesis with immature erythroid precursors, characteristic of RCC, is not present in AA, although a single small cluster with <10 cells with maturation is acceptable.4,6 Abnormal localization of erythropoiesis is not observed in AA.
Granulopoiesis is absent or markedly reduced in AA. Very few small foci may be present, but they rarely represent mature differentiation of granulocytes such as banded and/or segmented forms. In contrast to AA, matured granulocytes are not uncommon in RCC.
Megakaryopoiesis is typically absent in AA. It may be rarely observed, but matured megakaryocytes are not present. Dysplastic megakaryocytes and abnormal localization of megakaryopoiesis are not found in AA. The presence of micromegakaryocytes is considered characteristic of RCC and excludes the diagnosis of AA.6 As with RCC, IHC is mandatory for detecting micromegakaryocytes as well as the abnormal localization of megakaryopoiesis.
Lymphocytes may be increased in the BM in both AA and RCC.23 In the author’s experience, CD8-positive T cells are often increased in the BM in AA (Fig. 7). The increase in CD8-positive T cells is notable, given the assumed immune-mediated mechanism of AA,23 wherein the expansion of oligoclonal CD8-positive cytotoxic T cells is observed.29 The clinical relevance of IHC staining targeting lymphocytes and its impact on the differential diagnosis are still unknown, and should be explored in future studies.
The usefulness of IHC for differentiation between AA and RCC is unclear. CD34-positive myeloblasts are not increased in either AA or RCC. One study targeting cases mainly in adults found that the number of CD34-positive BM cells was significantly higher in hypoplastic MDS than in AA.30 The positivity rate in cases with p53 IHC staining was reported to be significantly higher in RCC than in AA,31 as previously reported for adult cases.17,32 These results imply that IHC provides useful information for differentiation between these two diseases. However, the EWOG-MDS stated that IHC (CD34, CD117, CD3, and CD20) does not support differentiation.6 Given the small number of examined cases and limited studies, further investigation is needed.
INHERITED BONE MARROW FAILURE DISORDERS (TABLE 2) FANCONI ANEMIA
Table 2. Inherited bone marrow failure syndromes.1-3,34,35,41-43,47,50,55,59-61,63,78,91-93.
| Syndrome | Fanconi anemia | Dyskeratosis congenita | Diamond-Blackfan anemia | Shwachman-Diamond syndrome |
|---|---|---|---|---|
| Pathogenesis | Interstrand crosslink DNA repair | Telomere maintenance | Ribosomal biogenesis | Ribosomal biogenesis |
| Inheritance: Known genes | AR: FANCA, C, D1, D2, E, F, G, I, J, L, N, O, P, Q, S,
T XLR: FANCB |
XLR; DKC1 AD; TERT, TERC, TINF2, RTEL1 AR; TERT, WRAP53, NOP10, CTC1, NHP2, USB1, RTEL1 |
AD: RPS19, RPS17, RPS24, RPL35A, RPL5, RPL11, RPS7, RPS26,
RPS10 XLR: GATA1 |
AR: SBDS |
| Incidence | 1-3/500,000 | 1/1,000,000 | 12/1,000,000 | 1/76,000 |
| Age at the diagnosis, median (range) | 6.5 years (birth to 49 years) | 14 years (birth to 75 years) | 3 months (birth to 64 years) | 2 weeks (birth to 11years) |
| Non-hematological findings | - Short stature - Skin lesions such as café au lait spots or hypo- and hyper-pigmentation - Abnormalities of upper limbs |
- Skin lacy reticular pigmentation - Nail dystrophy - Oral leukoplakia - Pulmonary fibrosis |
- Craniofacial anomalies, hypertelorism, cleft
lip/palate - Low birth weight - Thumb anomalies - Renal and cardiac anomalies |
- Exocrine pancreatic dysfunction - Short stature - Metaphyseal dystosis - Thoracic abnormalities - Delayed development |
| Diagnostic test | - Chromosomal breakage studies - Gene sequencing |
- Telomere length measurement - Gene sequencing |
- Fetal hemoglobin and erythrocyte adenosine deaminase
activity - Gene sequencing |
- Serum trypsinogen and isoamylase - Gene sequencing |
| Cytogenetic abnormalities | 1q+, 3q+, 21q /RUNX1 (cryptic RUNX1 rearrangement), -7/7q, 11q- | Monosomy 7a, Monosomy 10 | N/A | i(7q), del(20q) |
| Hematological presentations | Pancytopenia with macrocytosis | Cytopenia, often associated with thrombocytopenia | - Macrocytic anemia with no other significant
cytopenia - Reticulocytopenia |
- Neutropenia, sometimes anemia and thrombocytopenia |
| Hematological malignancy | MDS, AML | MDS, AML | MDS, AML, ALL | MDS, AML |
| Cumulative incidence of bone marrow failure | 90% by 40 years of age | Around 45% by 40 years of age | >50% by 70 years of age | 40% by 50 years of age |
| Cumulative incidence of MDS/Leukemia | MDS: 50% by 50 years of age Leukemia: up to 5% by 30 years of age |
MDS: 20% by 50 years of age Leukemia: up to 10% by 70 years of age |
MDS: 5% by 50 years of age AML: 5% by 46 years of age |
MDS: 65% by 50 years of age AML: 5% by 20 years of age |
| Other malignancy | - Head and neck SCC - Liver tumors - Vaginal SCC - Brain tumors |
- Head and neck SCC - Gastrointestinal and anogenital tumors |
- Osteosarcoma - Colon cancer - Lung cancer - Cervical cancer |
Limited cases |
| Survival, median | 29 years | 49 years | 67 years | 41 years |
| Bone marrow histopathology | - Hypocellularity for age, or variable cellularity,
depending on the stage of the disease - Dyserythropoiesis in more than 90% of patients - Fanconi anemia is found in 14.5% of patients with histomorphological findings consistent with hypo- or normocellular RCC. - Fanconi anemia-related MDS is highly associated with increased blasts and dysgranulopoiesis. |
- Hypocellularity for age, or variable cellularity,
depending on the stage of the disease - Some degree of dysplasia in any lineage is often present. - Dyskeratosis congenita is found in 1.6%-2.5% of patients with histomorphological findings consistent with hypo- or normocellular RCC. |
- Normal marrow cellularity with a paucity of erythroid
precursors - Erythroid precursors are absent or sparsely distributed and/or in a few small clusters lacking maturing/matured erythroid cells. - Normal granulopoiesis and megakaryopoiesis - Frequent lymphocytosis |
- Hypocellularity for age, or variable cellularity,
depending on the stage of the disease - Myeloid hypoplasia, often with left-shifted maturation. - Mild dyshematopoiesis in any lineage is commonly present. |
AD, autosomal dominant; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; AR, autosomal recessive; MDS, myelodysplastic syndrome; N/A, not available; RCC, refractory cytopenia of childhood; SCC, squamous cell carcinoma; XLR, X-linked recessive
a. Secondary MDS
Clinical features
Fanconi anemia (FA) is the most common IBMF disorder, and is characterized by congenital abnormalities, progressive BMF, chromosome fragility, and predisposition to malignancy.1,33
Congenital abnormalities may be present in almost any organ. The most common are short stature, skin lesions, such as café au lait spots or hypo- and hyper-pigmentation, and abnormalities of the upper limbs.1 Approximately 60% of patients are reported to have at least one abnormality.1 Of note, some patients lack any abnormalities associated with the disease, which can delay the diagnosis.
The median age at diagnosis is 6.5 years.1 Patients with FA typically develop BMF between 5 and 15 years of age,33 and the most common presentation is pancytopenia, often accompanying macrocytosis and elevated HbF.1 The cumulative incidence of BMF is 90% by 40 years of age.34
FA is an IBMF disorder with increased risk of MDS, AML, and solid tumors. The cumulative incidence of MDS and leukemia is 50% by 50 years of age and up to 5% by 30 years of age, respectively.35 The most common solid tumors are head and neck squamous cell carcinoma (SCC), liver tumors, vaginal SCC, and brain tumors.1 The cumulative incidence of solid tumors is approximately 20% by 65 years of age.35 The median age for survival is 29 years.1 The major causes of death in FA are complications from BMF, HSCT, and malignant neoplasms.
Diagnosis
FA is a clinically and genetically heterogeneous disease. Although a correct diagnosis can be reached after careful examination of the clinical and physical features as well as the family history, the hallmark of the disease is genetic instability due to the hypersensitivity of FA cells to DNA cross-linking agents.33,36 FA is diagnosed when chromosomal breakage in lymphocytes is increased after culture with DNA cross-linkers such as diepoxybutane (DEB) or mitomycin C (MMC).33,36 The DEB/MMC assay is a reliable diagnostic test for FA, but hematopoietic reversion and somatic mosaicism, relatively common in FA, often cause ambiguous or falsely negative results.37 In patients with negative DEB/MMC assay results but a strong clinical suspicion of FA, skin fibroblasts should be tested, as there has been no evidence of such a phenomenon in these cells.37 The chromosomal breakage test with DEB/MMC can identify affected patients, but comprehensive molecular testing is required to identify specific mutations.38
Histopathology
The BM of FA patients often demonstrates hypocellularity for their age (Fig. 8),1,39 and may have variable cellularity depending on the timing of the biopsy and the stage of the disease. BM studies at the first detection of a hematologic abnormality reportedly found reduced cellularity in 75% of cases, normal or increased cellularity in 13% of cases, and MDS or AML in 12% of cases.40
Fig. 8.
Fanconi anemia in an 8-year-old boy. The bone marrow showed hypocellularity for his age. Erythroid clusters with mild megaloblastic change were distributed in a patchy pattern. Granulocytes and megakaryocytes were decreased (A). Abnormal localization of erythropoiesis (B). Micromegakaryocytes were scattered, as indicated by CD42b immunostaining (C). These findings are indistinguishable from those seen in hypoplastic RCC. ASD-Giemsa staining. (A) ×200; (B) ×400. Immunohistochemistry. (C) ×400.
In early stages, the BM may be normocellular, but it can become aplastic or hypocellular with increasing severity of BMF.2 Approximately 80% of patients with FA develop BMF by 10 years of age.34 In the aplastic phase, the BM may exhibit severe hypocellularity or fatty marrow with loss of myeloid and erythroid precursors and megakaryocytes. These histological features are indistinguishable from other forms of aplastic marrow, such as idiopathic or acquired AA, or other IBMF syndromes.2 An accurate diagnosis is therefore reached only after a comprehensive clinical assessment and, if needed, a chromosomal breakage test.
Dyserythropoiesis is usually observed, often representing immature erythroid components with megaloblastic change and/or abnormal localization of erythropoiesis (Fig. 8).2,41 Approximately 94% of BM samples with FA demonstrated dyserythropoiesis, 70% of which had dysplasia limited to the erythroid lineage.41 Dyserythropoiesis as a sole abnormality should not be regarded as evidence of progression to MDS.
Megakaryocytes are often reduced in number.39 Dysplastic changes, such as micro- or small megakaryocytes, may be present and are easily identified by IHC staining of CD61 or CD42b (Fig. 8).
Reduced granulopoiesis is also often present (Fig. 8),39 and dysplastic changes are sometimes observed.41 In general, it is difficult to detect dysgranulopoiesis on histological evaluation, but in the author’s experience, dysplasia consistently accompanies left-shifted granulopoiesis.
It is not always possible to reliably distinguish between FA and RCC by BM morphology alone (Fig. 8). A lack of increased blasts and the presence of mild dyshematopoiesis of the BM are overlapping features in both FA and RCC, making it challenging to differentiate between the two diseases. Indeed, FA was identified in 14.5% of patients with histomorphological findings consistent with hypo- or normocellular RCC.42 Notably, some of these FA patients did not have any physical anomalies or a family history. The differential diagnosis between the two diseases therefore requires a chromosomal breakage test in addition to the histomorphological evaluation.
The diagnosis of MDS secondary to FA is critical for the management of patients. RCMD is the most common subtype of FA-related MDS (Fig. 9), followed by RAEB.41 FA-related MDS often, but not always, follows a hypoplastic or aplastic phase and progresses with the emergence of abnormal cytogenetic clones.41
Fig. 9.
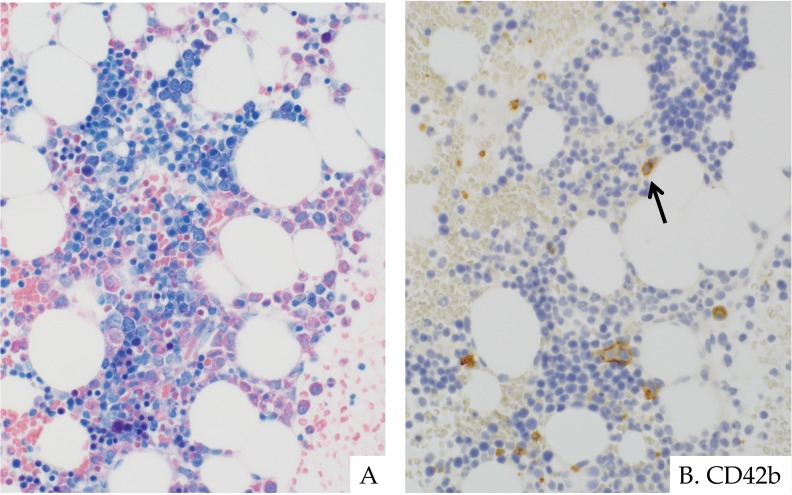
RCMD developing in a 7-year-old girl with Fanconi anemia. She developed thrombocytopenia and her pancytopenia progressed with age. Bone marrow evaluation revealed a novel chromosomal abnormality. Normocellular bone marrow with dyshematopoiesis (A). Erythroid clusters with mild megaloblastic change were seen. Granulocytes were slightly decreased in number and left-shifted. Blast cells were not increased. Scattered mast cells were also seen (A). Megakaryocytes were decreased, and a micromegakaryocyte was noted on CD42b immunostaining (arrow, B). ASD-Giemsa staining. (A) ×400. Immunohistochemistry. (B) ×400.
Cioc et al. reported an association between BM morphology and cytogenetic abnormalities in FA-related MDS.41 The presence of MDS was highly associated with cytogenetic abnormalities. The association between MDS and cytogenetic abnormalities differed according to the morphological findings: 100% of patients with increased blasts or any degree of dysgranulopoiesis had cytogenetic abnormalities, compared with 83% of patients with dysmegakaryopoiesis and 33% of patients with dyserythropoiesis. They concluded that the most reliable criteria for the diagnosis of FA-related MDS were increased blasts and dysgranulopoiesis, followed by dysmegakaryopoiesis.41
The FA BM stage with progression to MDS/AML is associated with a specific pattern of chromosomal and genomic abnormalities.43 Abnormalities in MDS/AML include 1q+, 3q+, 21q/RUNX1 (cryptic RUNX1 rearrangement), -7/7q, and 11q-.43 Of these, 3q+, -7/7q, and 21q/RUNX1 are observed only in the MDS/AML stage, whereas 1q+ can be in all stages, including non-MDS normocellular/hypocellular BM of FA.43 FA patients with 1q+ as a sole abnormality may go years without their disease progressing to MDS/AML, suggesting that 1q+ can rescue FA cells without MDS/AML transformation.43 The sole abnormality of 1q+ may not predict progression to MDS/AML, and careful observation throughout the following years is essential.33
P53 activation related to genomic instability may be involved in the pathogenesis of FA,44 supporting the hypothesis that p53 is expressed immunohistochemically in the FA BM cells. Studies evaluating p53 IHC staining in FA are limited, but one found that the percentage of p53-positive cells in the BM of FA was similar with that in FA-non-related MDS/AML but higher than that in AA and a normal control.45 As the overexpression of p53 protein was previously reported in the BM of MDS/AML,46 a common mechanism between FA and FA-non-related MDS/AML responsible for the preleukemia/leukemia processes may exist.45 However, whether p53 immunostaining can distinguish FA from other BMF conditions or be useful as a predictor of disease progression remains unclear.45
DYSKETATOSIS CONGENITA
Clinical features
Dysketatosis congenita (DC) represents a spectrum of diseases caused by defective telomere maintenance.47 Patients usually have very short telomeres resulting from inherited mutations in telomere maintenance genes.
There is a wide spectrum of diseases associated with defective telomere biology, known collectively as telomere diseases.48 Classic DC is the prototype of these diseases. It is characterized by the mucocutaneous triad (skin lacy reticular pigmentation, nail dystrophy, and oral leukoplakia), BMF, and predisposition to malignancy.1,47 Approximately 75% of patients present with at least one physical abnormality.1 Physical abnormalities in DC patients are usually age-dependent; therefore, their absence in young individuals does not eliminate DC from the list of differential diagnoses.1
Distinct forms of diseases other than classic DC have been recognized. Hoyeraal-Hreidarsson syndrome, a severe form of DC, affects multisystem organs, and is characterized by intrauterine growth retardation, microcephaly, cerebellar hypoplasia, severe aplastic anemia, and immunodeficiency.47 The other severe form, Reversz syndrome, causes bilateral exudative retinopathy in addition to classic DC features.49
The median age at diagnosis is 14 years.1 Abnormal skin pigmentation and nail lesions usually develop in childhood; however, the onset of cytopenia can occur before the appearance of such physical abnormalities. Cytopenia, mostly associated with thrombocytopenia as the initial presentation,50 usually occurs within the first decade of life, and pancytopenia may progress before 20 years of age.47 The cumulative incidence of BMF is approximately 45% by 40 years of age.35
DC predisposes patients to hematological and solid malignancies. The cumulative incidence of MDS and leukemia is 20% by 50 years of age and up to 10% by 70 years of age, respectively.35 The most common solid tumors are similar with those in FA, i.e. head and neck SCC, and other tumors involving the gastrointestinal and anogenital regions.1 The cumulative incidence of solid tumors is approximately 20% by 65 years of age.35
The median age for the survival is 49 years.1 The main causes of mortality in DC are BMF, pulmonary disease, and malignancy.47 Pulmonary fibrosis, documented as a rare manifestation associated with DC, is a cause of death unique to this disease.1
Diagnosis
Classic DC is usually characterized by the mucocutaneous triad and BMF. The minimal clinical criteria for DC diagnosis include the presence of two of four major features: abnormal skin pigmentation, nail dystrophy, leukoplakia, and BMF, and at least two other somatic features.47 However, patients with DC may have highly variable manifestations with respect to the age of onset, disease severity, and modes of inheritance, which make the diagnosis challenging.
The hallmark of DC pathogenesis and its clinical variants is defective telomere biology, which manifests as telomere shortening. Telomere length <1st percentile compared with normal controls in lymphocyte subsets detected by flow fluorescence in situ hybridization analysis is highly sensitive and specific.51 The telomere length in patients with non-DC IBMF syndromes, such as FA, DBA, and SDS, is also usually shorter than that in unaffected individuals, but not as short as that in DC patients.52 Furthermore, short telomeres are significantly correlated with the severity of BMF in DC, but not that in FA, DBA, or SDS.52
Histopathology
As in FA, the BM of patients with DC can demonstrate variable cellularity according to the biopsy timing and the stage of the disease. The BM usually exhibits hypocellularity (Fig. 10), but can have hypercellularity in the early stage. It may become gradually or progressively hypocellular as the severity of BMF increases53 until it is ultimately indistinguishable from AA. A shortened telomere length has been correlated with the severity of PB cytopenia,54 but the relationship between the degree of aplasia in the BM and telomere length remains unclear.
Fig. 10.
Dyskeratosis congenita in a 15-year-old boy. BM exhibited hypocellularity in a patchy distribution (A). Erythroid clusters with megaloblastic change were present, intermingled with decreased granulopoiesis and megakaryopoiesis (B). CD42b immunostaining demonstrated micromegakaryocytes (C). These findings are indistinguishable from those seen in hypoplastic RCC. Clot section with ASD-Giemsa staining. (A) ×100; (B) ×400. Immunohistochemistry. (C) ×400.
Some degree of dysplasia in any lineage is often present in the BM of DC patients (Fig. 10).47,50 Immature erythroid clusters with megaloblastic changes may be observed, often distributed in a patchy pattern. Furthermore, abnormal localization of erythroid cells may be found. Granulopoiesis may range from reduced or normal to increased, possibly accompanying left-shifted maturation. Megakaryopoiesis is usually reduced.1,39 Micromegakaryocytes and abnormal localization of megakaryopoiesis are often observed, and are easily detected by IHC (Fig. 10).
It is not always possible to reliably distinguish between DC and RCC by morphology alone, similar with FA. DC shares morphological features with RCC, including a lack of increased blasts and the presence of some degree of dysplasia. DC was identified in 1.6%-2.5% of patients with histomorphological findings consistent with hypo- or normocellular RCC.42,55 In the author’s experience, several cases had BM findings indistinguishable from those of RCC (Fig. 10). The differential diagnosis between the two diseases therefore requires measurement of the PB telomere length in addition to careful clinical examination.
The diagnosis of MDS secondary to DC is challenging. Some degree of dysplasia is commonly seen in the BM of DC patients. Abnormal morphology alone does not necessarily indicate progression to DC-related MDS.50 The diagnosis of secondary MDS requires the development of morphological abnormalities including a degree of dysplasia, and an increase in the number of blast cells or BM cellularity compared with the baseline BM.56 The simultaneous acquisition of well-known chromosomal abnormalities associated with sporadic MDS/AML, such as monosomy 7, or multiple chromosomal abnormalities has been regarded as an indication of the development of malignancy,50,57 although chromosomal abnormalities specific to either non-MDS-related or MDS/AML-related DC have not been elucidated.58 Only a few cases with monosomy 7 or monosomy 10 have been reported.59,60
Studies evaluating p53 IHC staining in DC are limited, aside from the above-mentioned study demonstrating that the percentage of p53-positive cells in the BM of DC was higher than that in other IBMF disorders, AA, and normal controls.45 In contrast to other IBMF disorders, DC presented a negative correlation between p53 expression and the values of Ki-67 or survivin, an inhibitor of apoptosis proteins.45 The reason for this is unknown, but these findings suggest that the p53-mediated pathway is activated in DC, whereas the compensatory expression of Ki-67 and survivin against apoptosis is impaired. Defects in the rescue of such vulnerable DC cells may be involved in the increased incidence of BMF in DC.
DIAMOND-BLACKFAN ANEMIA
Clinical features
Diamond-Blackfan anemia (DBA) is a rare inherited BMF disorder characterized by red cell aplasia, physical anomalies, and predisposition to malignancy.61 A growing number of gene mutations or gene deletions of ribosomal proteins have been discovered, leading to DBA being considered a disorder of ribosomal biogenesis or function, collectively known as ribosomopathy.62
Approximately 25% of patients have at least one physical abnormality.1,61 The most common are craniofacial anomalies, including hypertelorism and cleft lip/palate, followed by a low birth weight, thumb anomalies, and renal and cardiac anomalies.61 These anomalies are milder than those in FA or DC patients.1
The median age at diagnosis is three months.1 More than 98% of patients are diagnosed at less than 1 year of age.1 Patients with DBA typically present with macrocytic anemia and reticulocytopenia, often with elevated HbF as well as erythrocyte adenosine deaminase (eADA) activity and decreased or absent erythroid precursors in the BM.1,61
The risk for BMF is low, and it often occurs late in life. The cumulative incidence of BMF was reported to be more than 50% by 70 years of age.35 The cumulative incidence of solid tumors is 50% by 70 years of age.35 The tumor types were reported to be osteosarcoma, colon cancer, lung cancer, and cervical cancer.35,63 These tumor types are specific to DBA, in contrast to head and neck SCC, which is the most common tumor type in FA or DC patients, implying that the underlying tumorigenesis of the diseases is different. The cumulative incidence of MDS and AML is 5% by 50 years of age35 and 5% by 46 years of age,63 respectively, both of which are lower than in FA.35
The median age for survival is 67 years.35 The major causes of death in DBA are treatment-related, infection, and complications of iron overload or HSCT.61 Survival is longer than in patients with FA or DC due to the lower risk of death from BMF or solid tumors.35
Diagnosis
The diagnostic criteria for DBA are: age less than 1 year; macrocytic anemia with no other significant cytopenia; reticulocytopenia; and normal marrow cellularity with a paucity of erythroid precursors.61 These criteria define “classical” DBA, and individuals with “non-classical” DBA may not meet all of the criteria above. In order to diagnose non-classical DBA patients, the following supporting criteria should also be considered: the presence of gene mutations described for classical DBA, a positive family history, elevated eADA activity, congenital anomalies described for classical DBA, elevated HbF, and no evidence of other IBMF disorders.61 Of those criteria, the BM findings are the most important for diagnosis. BM evaluation should be repeated if there is no erythroid hypoplasia in the first BM biopsy, as anemia and reticulocytopenia can precede the pathological changes in the BM.
The identification of gene mutations strongly supports the diagnosis, but approximately 50% of DBA patients lack identifiable mutations.1
Histopathology
The BM of patients with DBA is usually normocellular for age, unlike in other IBMF disorders.39
By definition, erythroid hypoplasia is always seen regardless of normal BM cellularity (Fig. 11).61 Erythroid precursors are decreased or absent. In most cases, erythroid precursors are sparsely distributed or, less frequently, in a few small clusters. Such clusters almost always consist of proerythroblasts, lacking maturing/matured erythroid cells. CD71 IHC is a useful tool for identifying erythroid precursors and their distribution (Fig. 11).64 Erythroid precursors may exhibit mild megaloblastic changes, sometimes mimicking the enlarged proerythroblasts characteristically seen in Parvovirus B19 infection.2
Fig. 11.
Diamond-Blackfan anemia in a 3-month-old girl. BM showed normal cellularity with erythroid hypoplasia. Erythroid hypoplasia with few proerythroblasts scattered and intermingled with increased lymphocytes. Granulopoiesis and megakaryopoiesis were normal (A). CD71-positive erythroid precursors were scattered but not present in clusters (B). P53 was also positive in some erythroid precursors (C). Clot section with ASD-Giemsa staining. (A) ×400. Immunohistochemistry. (B, C) ×400.
Granulopoiesis and megakaryopoiesis are usually normal (Fig. 11), but mild dysplastic changes are not uncommon. Micro- or small megakaryocytes, easily detected by IHC, are often observed. However, the presence of small-sized megakaryocytes is a normal finding during infancy65 and does not necessarily indicate pathognomonic features. In non-neoplastic settings, megakaryocytes of varying size are usually observed in the background, reflecting the physiological differentiation in megakaryopoiesis.
Lymphocytes are frequently increased (Fig. 11), which may represent a physiological feature during infancy.66 The increase of normal lymphoid progenitors, known as hematogones, can be present.53 CD34- or TdT-positive immature cells on IHC are increased under such conditions.
Nuclear P53 IHC staining in selective erythroid progenitor cells in BM biopsies from DBA patients has been reported (Fig. 11).67 These results are notable considering the implication of p53 in the pathogenesis of DBA.67-71 The staining pattern varies among patients, likely depending on mutations as well as the disease status.67 The diagnostic utility of p53 IHC should be evaluated in a larger sample size.
The main differential diagnosis is transient erythroblastocytopenia of childhood (TEC). TEC is an uncommon, self-limiting disorder characterized by reduced or absent erythroid precursors in otherwise normocellular BM.72 TEC typically occurs between 3 months and 4 years,72 and usually persists for about a month.73 Its etiology is unknown, but a transient autoimmune mechanism triggered by infection or unknown environmental factor in genetically susceptible individuals is suspected.72-74 Overlapping morphological features between TEC and DBA make differentiation challenging. Both disorders present erythroid hypoplasia, absent or reduced erythroid precursors, and normal granulo- and megakaryopoiesis in the BM. However, the BM of DBA patients often has higher cellularity than that in TEC patients, probably because BM evaluations are performed at an earlier age for DBA. The BM of TEC patients can have pronounced lymphocytosis75 or erythroid hyperplasia76 in the recovery phase, which is less obvious in DBA.
The diagnosis of MDS/AML secondary to DBA should be carefully decided, although this entity has a low incidence and occurs late in life.35,63 BM during the neonate-infant period, when BM biopsies are frequently performed for DBA diagnosis, often exhibits histomorphological features mimicking clonal evolution: BM hypercellularity, increased blast cells, and mild dyshematopoiesis. Histomorphological evaluations with comparisons with the baseline BM and concurrent chromosomal studies are mandatory for accurate diagnosis.61 The acquisition of well-known chromosomal abnormalities associated with sporadic MDS/AML or multiple chromosomal abnormalities is considered a warning finding, although routine chromosomal analyses are usually normal, and abnormalities specific for DBA patients have not been described.57,58,61
SHWACHMAN-DIAMOND SYNDROME
Clinical features
Shwachman-Diamond syndrome (SDS) is a rare autosomal recessive disorder characterized by exocrine pancreatic dysfunction, BMF, and predisposition to MDS/AML.1,77,78 Most patients with SDS have biallelic mutations in the SBDS gene, which functions in ribosome biogenesis.79
SDS is also a multisystem disease that can affect the skeletal, cardiac, neurocognitive, gastrointestinal, and immune systems. Physical abnormalities are present in 55% of patients with SDS.1 The most common is short stature, followed by metaphyseal dystosis, thoracic abnormalities, and delayed development.1
Variable exocrine pancreatic dysfunction with or without malabsorption is a hallmark of SDS.78 Over 90% of patients with SDS are diagnosed with pancreatic dysfunction, which usually presents as steatorrhea within the first 6 months of life.77,78 The median age at which malabsorption occurs is 2 weeks, ranging from birth to 11 years.1 Pancreatic ductular fluid secretion remains normal, but secretion of proteolytic enzymes is decreased, leading to steatorrhea.78 Histological examination of the pancreas can reveal the fatty replacement of acinar cells, an increase in the interstitial connective tissue, and dilated ductules.80 Approximately 50% of patients with SDS demonstrate age-related improvement in pancreatic function, although the reason remains unclear.81 Although fat absorption is normal, patients are persistently deficient in enzyme secretion.
Neutropenia is the most common hematological manifestation and occurs in almost all patients.78 It often develops slightly later than malabsorption, but may be observed in the neonatal period.1 Neutropenia can be either intermittent or persistent with variable severity.77 Anemia and thrombocytopenia are also present in most patients.77 Anemia is usually asymptomatic, and associated with elevated HbF and macrocytosis.78
Neutropenia may progress beyond anemia and thrombocytopenia to tri-lineage cytopenia.77 The cumulative incidence of BMF was reported to be 40% by 50 years of age.35 The cumulative incidence of MDS and AML is 65% by 50 years of age and 5% by 20 of age, respectively.35 Limited cases of solid tumors in SDS patients have been reported.82,83 SDS may be less cancer-prone, but cohort sizes have been too small for a thorough risk assessment. In a recent review, the cumulative incidence of solid tumors was reported to be approximately 40% by 45 years of age.35
The median age for survival is 41 years.35 The major causes of death are infection (sepsis or pneumonia), AML, and myocardial necrosis.1
Diagnosis
The diagnosis of SDS is based on clinical findings, particularly exocrine pancreatic dysfunction and hematological manifestations, and is confirmed by SBDS genetic testing.78 The clinical diagnosis is usually made during infancy, but it may be established in older children and even adults. Classic neutropenia associated with diarrhea is observed in approximately half of all patients.84
The serum trypsinogen and pancreatic isoamylase levels adjusted for age are sensitive tests for pancreatic dysfunction.78 Fatty replacement of the pancreas on imaging and a low concentration of fecal pancreatic enzyme also indicate pancreatic dysfunction, and support the diagnosis.
Not all patients present with typical clinical features. It is therefore advisable to test all suspected cases for mutations in the SBDS gene. Approximately 90% of SDS patients are identified by biallelic mutations in the SBDS gene.79 As approximately 10% of patients lack such abnormalities, mutations of genes other than SDSB have been assumed to cause the disorder. Recently, biallelic mutations in DNAJC21, the 60S ribosome assembly factor, were identified in a subset of SDS patients.85
Histopathology
The BM cellularity in SDS patients is usually hypocellular for their age (Fig. 12) but may be normo- or hypercellular.2,78 BM cellularity may be normocellular early in life but eventually becomes hypocellular, typically during childhood,28 although the degree of cellularity is poorly correlated with the severity of peripheral cytopenia.86
Fig. 12.
Shwachman-Diamond syndrome in a 7-year-old boy. BM showed hypocellularity with a patchy distribution (A). Erythroid clusters with mild megaloblastic change, and decreased granulopoiesis and megakaryopoiesis (B). These findings are indistinguishable from those seen in hypoplastic RCC. ASD-Giemsa staining. (A) ×100; (B) ×400.
BM hypocellularity in SDS is usually due to decreased granulopoiesis, but decreased erythropoiesis and/or megakaryopoiesis is not uncommon.39,78 Hypocellular BM in SDS often has patchy distribution of hematopoiesis, as seen in RCC (Fig. 12). Mild dyshematopoiesis is commonly observed and can be transient.2,78 Dysgranulopoiesis is often present as left-shifted, arrested maturation, or reduced matured granulocytes. Dyserythropoiesis usually presents with megaloblastic changes or increased proerythroblasts and abnormal localization. Dysmegakaryopoiesis is typically based on the presence of micro- or small-sized megakaryocytes and abnormal localization. Prominent multilineage dysplasia is less common and may indicate malignant transformation if it occurs.78
As with FA and DC, the distinction between SDS and RCC by morphological features alone is challenging (Fig. 12). Patients diagnosed with RCC by BM morphological examination may incorrectly include those with SDS. However, one study screening for SBDS mutations in 120 RCC patients found that no SDS patients had been misdiagnosed with RCC.87 They concluded that mutational SBDS screening for RCC patients lacking clinical features of SDS is not recommended.
The diagnosis of MDS/AML secondary to SDS is critical for patients, as they are the main hematological complications and impact survival.78 MDS secondary to SDS includes refractory cytopenia with dysplasia, RCMD, RAEB, and RAEB in transformation.78,88 Secondary AML commonly presents as AML with MDS-related changes (AML-M2, AML-M4, AML-M6, AML-M0).88 AML-M6 frequently occurs in SDS patients, accounting for approximately 30% of AML cases.88
The diagnosis of MDS secondary to SDS is challenging. Mild dysplastic changes are common and can be transient in SDS patients. The presence of mild dyshematopoiesis alone does not necessarily indicate MDS. Furthermore, not all cytogenetic abnormalities indicate malignant clonal progression. Cytogenetic abnormalities, such as i(7q) and del(20q), may persist in SDS patients without progression to leukemia and can regress spontaneously.78
To establish the diagnosis of SDS-related MDS/AML, cytogenetic abnormalities should be interpreted in the context of the BM histomorphology.78 Deterioration of histomorphological changes compared with the baseline BM, concurrent with acquisition of well-known cytogenetic aberrations associated with MDS/AML, such as monosomy 7, or multiple cytogenetic abnormalities should be considered warning features (Fig. 13).57,88 The definitive histomorphological risk for hematological malignancy remains unknown, although blood counts at diagnosis and the age of onset of the first symptoms have been correlated with such complications.88
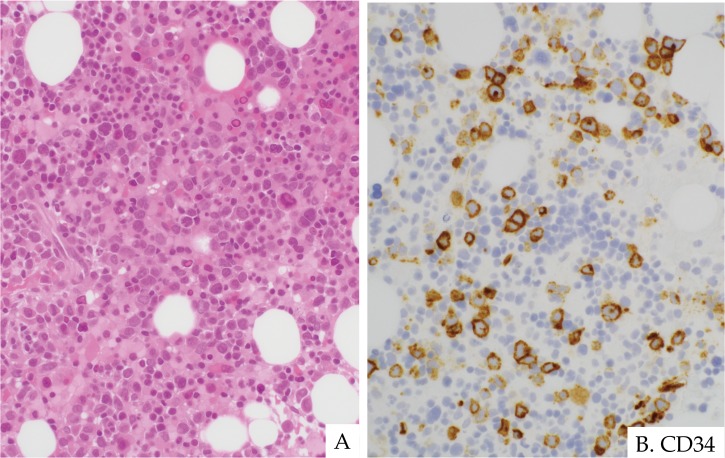
Fig. 13.
Acute myeloid leukemia developing in a 10-year-old boy with SDS. He developed neutropenia and thrombocytopenia and, subsequently, blast cells were noted in the peripheral blood. Bone marrow evaluation revealed novel complex chromosomal abnormalities. BM showed hypercellularity with multilineage dysplasia and increased blast cells (A). CD34-positive blast cells were increased in a few clusters (B). H&E staining. (A) ×400. Immunohistochemistry. (B) ×400.
Approximately 80% of SDS BM biopsies were reported to be p53-positive on IHC.89 Furthermore, IHC expression levels of p53, Ki67, and survivin in SDS were found to be positively correlated.45 These findings suggest that survivin is elevated in order to overcome p53-dependent apoptosis and promotes cell proliferation, which manifests as elevated Ki67. The retained function compensating for p53-dependent apoptosis may be associated with the reduced incidence of BMF in SDS.
Bone abnormalities may be observed in SDS patients.90 Bone biopsies from SDS patients may demonstrate osteoporosis characterized by a reduced trabecular bone volume, low numbers of osteoclasts and osteoblasts, and a reduced amount of osteoid. Osteoporosis may be a primary defect in bone metabolism in SDS patients, and may be associated with BM dysfunction and neutropenia.90
CONCLUSION
RCC, AA, and DBA present distinct histomorphological features in BM, but as discussed in this review, BM hypocellularity and dyshematopoiesis are overlapping features present in RCC and other IBMF syndromes (FA, DC, and SDS), as well as in secondary MDS. Therefore, concurrent assessment of BM histology as well as the clinical features, physical anomalies, and laboratory/genetic findings, and comparison with the baseline BM are necessary for an accurate diagnosis. Obtaining more information on germline and somatic genomic abnormalities may not only aid in the differential diagnosis but also provide novel insight into the pathogenesis of BMF in children. The correlation between BM histology and the genotype should be explored in future studies.
ACKNOWLEDGMENTS
The author would like to thank all of the patients, families, and physicians who referred the patients.
Footnotes
CONFLICT OF INTEREST: None declared.
REFERENCES
- 1.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010; 24: 101-122. 10.1016/j.blre.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leguit RJ, Van Den Tweel JG. The pathology of bone marrow failure. Histopathology. 2010; 57: 655-670. 10.1111/j.1365-2559.2010.03612.x [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi H, Nakanishi K, Kojima S. Inherited bone marrow failure syndromes in 2012. Int J Hematol. 2013; 97: 20-29. 10.1007/s12185-012-1249-9 [DOI] [PubMed] [Google Scholar]
- 4.Baumann I, Niemeyer CM, Bennett JM. Childhood myelodysplastic syndrome. In: Swerdlow SH, Campo E, Harris NL, et al. (eds). World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed, Lyon, IARC Press. 2017; pp. 116-120. [Google Scholar]
- 5.Baumann I, Niemeyer CM, Bennett JM, et al. Childhood myelodysplastic syndrome. In: Swerdlow SH, Campo E, Harris NL, et al. (eds). World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed, Lyon, IARC Press. 2008; pp. 104-107. [Google Scholar]
- 6.Baumann I, Führer M, Behrendt S, et al. Morphological differentiation of severe aplastic anaemia from hypocellular refractory cytopenia of childhood: reproducibility of histopathological diagnostic criteria. Histopathology. 2012; 61: 10-17. 10.1111/j.1365-2559.2011.04156.x [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa D. The current perspective of low-grade myelodysplastic syndrome in children. Int J Hematol. 2016; 103: 360-364. 10.1007/s12185-016-1965-7 [DOI] [PubMed] [Google Scholar]
- 8.Kardos G, Baumann I, Passmore SJ, et al. Refractory anemia in childhood: a retrospective analysis of 67 patients with particular reference to monosomy 7. Blood. 2003; 102: 1997-2003. 10.1182/blood-2002-11-3444 [DOI] [PubMed] [Google Scholar]
- 9.Niemeyer CM, Baumann I. Classification of childhood aplastic anemia and myelodysplastic syndrome. Hematology. 2011; 2011: 84-89. 10.1182/asheducation-2011.1.84 [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa D, Chen X, Hirabayashi S, et al. Clinical characteristics and treatment outcome in 65 cases with refractory cytopenia of childhood defined according to the WHO 2008 classification. Br J Haematol. 2014; 166: 758-766. 10.1111/bjh.12955 [DOI] [PubMed] [Google Scholar]
- 11.Brunning RD, Bennett JM, Matutes E, et al. Myelodysplastic syndrome with multilineage dysplasia. In: Swerdlow SH, Campo E, Harris NL, et al. (eds). World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed, Lyon, IARC Press. 2017; pp. 111-113. [Google Scholar]
- 12.Orazi A. Histopathology in the diagnosis and classification of acute myeloid leukemia, myelodysplastic syndromes, and myelodysplastic/myeloproliferative diseases. Pathobiology. 2007; 74: 97-114. 10.1159/000101709 [DOI] [PubMed] [Google Scholar]
- 13.Horny HP, Sotlar K, Valent P. Diagnostic value of histology and immunohistochemistry in myelodysplastic syndromes. Leuk Res. 2007; 31: 1609-1616. 10.1016/j.leukres.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 14.Prokocimer M, Polliack A. Increased bone marrow mast cells in preleukemic syndromes, acute leukemia, and lymphoproliferative disorders. Am J Clin Pathol. 1981; 75: 34-38. 10.1093/ajcp/75.1.34 [DOI] [PubMed] [Google Scholar]
- 15.Iemura A, Tsai M, Ando A, et al. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994; 144: 321-328. [PMC free article] [PubMed] [Google Scholar]
- 16.Koijima S. Hematopoietic growth factors and marrow stroma in aplastic anemia. Int J Hematol. 1998; 68: 19-28. 10.1016/S0925-5710(98)00028-0 [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki T, Murakami M, Sugisaki C, et al. Characterization of myelodysplastic syndrome and aplastic anemia by immunostaining of p53 and hemoglobin F and karyotype analysis: differential diagnosis between refractory anemia and aplastic anemia. Pathol Int. 2008; 58: 353-360. 10.1111/j.1440-1827.2008.02236.x [DOI] [PubMed] [Google Scholar]
- 18.Saft L, Karimi M, Ghaderi M, et al. p53 protein expression independently predicts outcome in patients with lower-risk myelodysplastic syndromes with del(5q). Haematologica. 2014; 99: 1041-1049. 10.3324/haematol.2013.098103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara A. Japan national registry of aplastic anemia in children 1988-2005: clinical features and prognosis. Japanese J Pediatr Hematol. 2008; 22: 53-62. [Google Scholar]
- 20.Young NS, Kaufman DW. The epidemiology of acquired aplastic anemia. Haematologica. 2008; 93: 489-492. 10.3324/haematol.12855 [DOI] [PubMed] [Google Scholar]
- 21.Kojima S. Why is the incidence of aplastic anemia higher in Asia? Expert Rev Hematol. 2017; 10: 277-279. 10.1080/17474086.2017.1302797 [DOI] [PubMed] [Google Scholar]
- 22.Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015; 101: 527-535. 10.1007/s12185-015-1787-z [DOI] [PubMed] [Google Scholar]
- 23.Hartung HD, Olson TS, Bessler M. Acquired aplastic anemia in children. Pediatr Clin North Am. 2013; 60: 1311-1336. 10.1016/j.pcl.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timeus F, Crescenzio N, Longoni D, et al. Paroxysmal nocturnal hemoglobinuria clones in children with acquired aplastic anemia: a multicentre study. PLoS One. 2014; 9: e101948. 10.1371/journal.pone.0101948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hama A, Takahashi Y, Muramatsu H, et al. Comparison of long-term outcomes between children with aplastic anemia and refractory cytopenia of childhood who received immunosuppressive therapy with antithymocyte globulin and cyclosporine. Haematologica. 2015; 100: 1426-1433. 10.3324/haematol.2015.128553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barone A, Lucarelli A, Onofrillo D, et al. Guidelines from the Marrow Failure Study Group of the Pediatric Haemato-Oncology Italian Association (AIEOP) . Diagnosis and management of acquired aplastic anemia in childhood. Blood Cells Mol Dis. 2015; 55: 40-47. 10.1016/j.bcmd.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 27.Keel SB, Scott A, Sanchez-Bonilla M, et al. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica. 2016; 101: 1343-1350. 10.3324/haematol.2016.149476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimamura A. Aplastic anemia and clonal evolution: germ line and somatic genetics. Hematology. 2016; 2016: 74-82. 10.1182/asheducation-2016.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Risitano AM, Maciejewski JP, Green S, et al. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR β-CDR3 sequencing. Lancet. 2004; 364: 355-364. 10.1016/S0140-6736(04)16724-X [DOI] [PubMed] [Google Scholar]
- 30.Cha CH, Park CJ, Chi HS, et al. CD34 and p53 immunohistochemical stains differentiate hypocellular myelodysplastic syndrome (hMDS) from aplastic anemia and a CD34 immunohistochemical stain provides useful survival information for hMDS. Ann Lab Med. 2014; 34: 426-432. 10.3343/alm.2014.34.6.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SH, Chi HS, Cho YU, et al. Clinical Relevance of p53 Immunohistochemical Stain in the Differential Diagnosis Between Pediatric Aplastic Anemia and Refractory Cytopenia of Childhood. Ann Lab Med. 2016; 36: 174-176. 10.3343/alm.2016.36.2.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elghetany MT, Vyas S, Yuoh G. Significance of p53 overexpression in bone marrow biopsies from patients with bone marrow failure: aplastic anemia, hypocellular refractory anemia, and hypercellular refractory anemia. Ann Hematol. 1998; 77: 261-264. 10.1007/s002770050455 [DOI] [PubMed] [Google Scholar]
- 33.Soulier J. Fanconi Anemia. Hematology. 2011; 2011: 492-497. 10.1182/asheducation-2011.1.492 [DOI] [PubMed] [Google Scholar]
- 34.Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003; 101: 1249-1256. 10.1182/blood-2002-07-2170 [DOI] [PubMed] [Google Scholar]
- 35.Alter BP, Giri N, Savage SA, et al. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018; 103: 30-39. 10.3324/haematol.2017.178111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res Fundam Mol Mech Mutagen. 2009; 668: 4-10. 10.1016/j.mrfmmm.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godley LA. Inherited predisposition to acute myeloid leukemia. Semin Hematol. 2014; 51: 306-321. 10.1053/j.seminhematol.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 38.Seif AE. Pediatric leukemia predisposition syndromes: clues to understanding leukemogenesis. Cancer Genet. 2011; 204: 227-244. 10.1016/j.cancergen.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 39.Hashmi SK, Allen C, Klaassen R, et al. Comparative analysis of Shwachman-Diamond syndrome to other inherited bone marrow failure syndromes and genotype-phenotype correlation. Clin Genet. 2011; 79: 448-458. 10.1111/j.1399-0004.2010.01468.x [DOI] [PubMed] [Google Scholar]
- 40.Butturini A, Gale RP, Verlander PC, et al. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study. Blood. 1994; 84: 1650-1655. [PubMed] [Google Scholar]
- 41.Cioc AM, Wagner JE, MacMillan ML, et al. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with fanconi anemia: morphologic and cytogenetic characteristics. Am J Clin Pathol. 2010; 133: 92-100. 10.1309/AJCP7W9VMJENZOVG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimi A, Niemeyer C, Baumann I, et al. High incidence of Fanconi anaemia in patients with a morphological picture consistent with refractory cytopenia of childhood. Br J Haematol. 2013; 160: 109-111. 10.1111/bjh.12083 [DOI] [PubMed] [Google Scholar]
- 43.Quentin S, Cuccuini W, Ceccaldi R, et al. Myelodysplasia and leukemia of Fanconi anemia are associated with a specific pattern of genomic abnormalities that includes cryptic RUNX1/AML1 lesions. Blood. 2011; 117: e161-e170. 10.1182/blood-2010-09-308726 [DOI] [PubMed] [Google Scholar]
- 44.Ceccaldi R, Parmar K, Mouly E, et al. Bone marrow failure in Fanconi anemia is triggered by an exacerbated p53/p21 DNA damage response that impairs hematopoietic stem and progenitor cells. Cell Stem Cell. 2012; 11: 36-49. 10.1016/j.stem.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Rahawan MM, Alter BP, Bryant BJ, et al. Bone marrow cell cycle markers in inherited bone marrow failure syndromes. Leuk Res. 2008; 32: 1793-1799. 10.1016/j.leukres.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurotaki H, Tsushima Y, Nagai K, et al. Apoptosis, bcl-2 expression and p53 accumulation in myelodysplastic syndrome, myelodysplastic-syndrome-derived acute myelogenous leukemia and de novo acute myelogenous leukemia. Acta Haematol. 1999; 102: 115-123. 10.1159/000040984 [DOI] [PubMed] [Google Scholar]
- 47.Dokal I. Dyskeratosis Congenita. Hematology. 2011; 2011: 480-486. 10.1182/asheducation-2011.1.480 [DOI] [PubMed] [Google Scholar]
- 48.Calado RT, Young NS. Telomere Diseases. N Engl J Med. 2009; 361: 2353-2365. 10.1056/NEJMra0903373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Revesz T, Fletcher S, al-Gazali LI, et al. Bilateral retinopathy, aplastic anaemia, and central nervous system abnormalities: a new syndrome? J Med Genet. 1992; 29: 673-675. 10.1136/jmg.29.9.673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savage SA, Cook EF, eds. Dyskeratosis congenital and telomere biology disorders: Diagnosis and management guidelines. https://www.dcoutreach.org/sites/default/files/DC%20%26%20TBD%20Diagnosis%20And%20Management%20Guidelines.pdf.
- 51.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007; 110: 1439-1447. 10.1182/blood-2007-02-075598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alter BP, Giri N, Savage SA, et al. Telomere length in inherited bone marrow failure syndromes. Haematologica. 2015; 100: 49-54. 10.3324/haematol.2014.114389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foucar K, Viswanatha DS, Wilson CS. Non-Neoplastic Disorders of Bone Marrow (Atlas of Nontumor Pathology). 1st series. Washington, DC, American Registry of Pathology. 2008; pp. 221-248. [Google Scholar]
- 54.Alter BP, Rosenberg PS, Giri N, et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012; 97: 353-359. 10.3324/haematol.2011.055269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortmann CA, Niemeyer CM, Wawer A, et al. TERC mutations in children with refractory cytopenia. Haematologica. 2006; 91: 707-708. [PubMed] [Google Scholar]
- 56.Erlacher M, Strahm B. Missing Cells: Pathophysiology, Diagnosis, and Management of (Pan)Cytopenia in Childhood. Front Pediatr. 2015; 3: 64. 10.3389/fped.2015.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godley LA, Shimamura A. Genetic predisposition to hematologic malignancies: management and surveillance. Blood. 2017; 130: 424-432. 10.1182/blood-2017-02-735290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babushok DV, Bessler M, Olson TS. Genetic predisposition to myelodysplastic syndrome and acute myeloid leukemia in children and young adults. Leuk Lymphoma. 2016; 57: 520-536. 10.3109/10428194.2015.1115041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood. 2009; 113: 6549-6557. 10.1182/blood-2008-12-192880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi H, Sakaguchi H, Yoshida K, et al. Clinical and genetic features of dyskeratosis congenita, cryptic dyskeratosis congenita, and Hoyeraal-Hreidarsson syndrome in Japan. Int J Hematol. 2015; 102: 544-552. 10.1007/s12185-015-1861-6 [DOI] [PubMed] [Google Scholar]
- 61.Vlachos A, Ball S, Dahl N, et al. Participants of Sixth Annual Daniella Maria Arturi International Consensus Conference Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008; 142: 859-876. 10.1111/j.1365-2141.2008.07269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruggero D, Shimamura A. Marrow failure: a window into ribosome biology. Blood. 2014; 124: 2784-2792. 10.1182/blood-2014-04-526301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlachos A, Rosenberg PS, Atsidaftos E, et al. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012; 119: 3815-3819. 10.1182/blood-2011-08-375972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong HY, Wilkes S, Yang H. CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: a comparative immunochemical study with glycophorin A and hemoglobin A. Am J Surg Pathol. 2011; 35: 723-732. 10.1097/PAS.0b013e31821247a8 [DOI] [PubMed] [Google Scholar]
- 65.Fuchs DA, McGinn SG, Cantu CL, et al. Developmental differences in megakaryocyte size in infants and children. Am J Clin Pathol. 2012; 138: 140-145. 10.1309/AJCP4EMTJYA0VGYE [DOI] [PubMed] [Google Scholar]
- 66.Rosse C, Kraemer MJ, Dillon TL, et al. Bone marrow cell populations of normal infants; the predominance of lymphocytes. J Lab Clin Med. 1977; 89: 1225-1240. [PubMed] [Google Scholar]
- 67.Dutt S, Narla A, Lin K, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011; 117: 2567-2576. 10.1182/blood-2010-07-295238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goudarzi KM, Lindström MS. Role of ribosomal protein mutations in tumor development (Review) [Review]. Int J Oncol. 2016; 48: 1313-1324. 10.3892/ijo.2016.3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009; 16: 369-377. 10.1016/j.ccr.2009.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danilova N, Gazda HT. Ribosomopathies: how a common root can cause a tree of pathologies. Dis Model Mech. 2015; 8: 1013-1026. 10.1242/dmm.020529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raiser DM, Narla A, Ebert BL. The emerging importance of ribosomal dysfunction in the pathogenesis of hematologic disorders. Leuk Lymphoma. 2014; 55: 491-500. 10.3109/10428194.2013.812786 [DOI] [PubMed] [Google Scholar]
- 72.van den Akker M, Dror Y, Odame I. Transient erythroblastopenia of childhood is an underdiagnosed and self-limiting disease. Acta Paediatr. 2014; 103: e288-e294. [DOI] [PubMed] [Google Scholar]
- 73.Lipton JM, Ellis SR. Diamond-Blackfan anemia: diagnosis, treatment, and molecular pathogenesis. Hematol Oncol Clin North Am. 2009; 23: 261-282. 10.1016/j.hoc.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw J, Meeder R. Transient erythroblastopenia of childhood in siblings: case report and review of the literature. J Pediatr Hematol Oncol. 2007; 29: 659-660. 10.1097/MPH.0b013e31814684e9 [DOI] [PubMed] [Google Scholar]
- 75.Foot AB, Potter MN, Ropner JE, et al. Transient erythroblastopenia of childhood with CD10, TdT, and cytoplasmic mu lymphocyte positivity in bone marrow. J Clin Pathol. 1990; 43: 857-859. 10.1136/jcp.43.10.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickerman JD. Transient erythroblastopenia of childhood presenting with reticulocytosis and erythroid hyperplasia in the bone marrow. Pediatrics. 1981; 67: 562-564. [PubMed] [Google Scholar]
- 77.Myers KC, Davies SM, Shimamura A. Clinical and molecular pathophysiology of Shwachman-Diamond syndrome: an update. Hematol Oncol Clin North Am. 2013; 27: 117-128, ix. 10.1016/j.hoc.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dror Y, Donadieu J, Koglmeier J, et al. Draft consensus guidelines for diagnosis and treatment of Shwachman-Diamond syndrome. Ann N Y Acad Sci. 2011; 1242: 40-55. 10.1111/j.1749-6632.2011.06349.x [DOI] [PubMed] [Google Scholar]
- 79.Boocock GRB, Morrison JA, Popovic M, et al. Mutations in SBDS are associated with Shwachman–Diamond syndrome. Nat Genet. 2002; 33: 97-101. 10.1038/ng1062 [DOI] [PubMed] [Google Scholar]
- 80.Aggett PJ, Cavanagh NP, Matthew DJ, et al. Shwachman’s syndrome. A review of 21 cases. Arch Dis Child. 1980; 55: 331-347. 10.1136/adc.55.5.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mack DR, Forstner GG, Wilschanski M, et al. Shwachman syndrome: exocrine pancreatic dysfunction and variable phenotypic expression. Gastroenterology. 1996; 111: 1593-1602. 10.1016/S0016-5085(96)70022-7 [DOI] [PubMed] [Google Scholar]
- 82.Singh SA, Vlachos A, Morgenstern NJ, et al. Breast cancer in a case of Shwachman Diamond syndrome. Pediatr Blood Cancer. 2012; 59: 945-946. 10.1002/pbc.24052 [DOI] [PubMed] [Google Scholar]
- 83.Nakaya T, Kurata A, Hashimoto H, et al. Young-age-onset pancreatoduodenal carcinoma in Shwachman-Diamond syndrome. Pathol Int. 2014; 64: 75-80. 10.1111/pin.12133 [DOI] [PubMed] [Google Scholar]
- 84.Myers KC, Bolyard AA, Otto B, et al. Variable clinical presentation of Shwachman-Diamond syndrome: update from the North American Shwachman-Diamond Syndrome Registry. J Pediatr. 2014; 164: 866-870. 10.1016/j.jpeds.2013.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dhanraj S, Matveev A, Li H, et al. Biallelic mutations in DNAJC21 cause Shwachman-Diamond syndrome. Blood. 2017; 129: 1557-1562. 10.1182/blood-2016-08-735431 [DOI] [PubMed] [Google Scholar]
- 86.Dror Y, Freedman MH. Shwachman-Diamond syndrome: an inherited preleukemic bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. Blood. 1999; 94: 3048-3054. [PubMed] [Google Scholar]
- 87.Elghetany MT, Alter BP. p53 protein overexpression in bone marrow biopsies of patients with Shwachman-Diamond syndrome has a prevalence similar to that of patients with refractory anemia. Arch Pathol Lab Med. 2002; 126: 452-455. [DOI] [PubMed] [Google Scholar]
- 88.Donadieu J, Fenneteau O, Beaupain B, et al. Associated investigators of the French Severe Chronic Neutropenia Registry* Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica. 2012; 97: 1312-1319. 10.3324/haematol.2011.057489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elghetany MT, Alter BP. p53 protein overexpression in bone marrow biopsies of patients with Shwachman-Diamond syndrome has a prevalence similar to that of patients with refractory anemia. Arch Pathol Lab Med. 2002; 126: 452-455. [DOI] [PubMed] [Google Scholar]
- 90.Toiviainen-Salo S, Mäyränpää MK, Durie PR, et al. Shwachman–Diamond syndrome is associated with low-turnover osteoporosis. Bone. 2007; 41: 965-972. 10.1016/j.bone.2007.08.035 [DOI] [PubMed] [Google Scholar]
- 91.Khincha PP, Savage SA. Genomic characterization of the inherited bone marrow failure syndromes. Semin Hematol. 2013; 50: 333-347. 10.1053/j.seminhematol.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bogliolo M, Surrallés J. Fanconi anemia: a model disease for studies on human genetics and advanced therapeutics. Curr Opin Genet Dev. 2015; 33: 32-40. 10.1016/j.gde.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 93.Draptchinskaia N, Gustavsson P, Andersson B, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfananaemia. Nat Genet. 1999; 21: 169-175. 10.1038/5951 [DOI] [PubMed] [Google Scholar]