Abstract
Recent progress in anti-tumor immunotherapy has focused on the significance of the tumor microenvironment in tumor progression and resistance to chemo/radio-therapy. Myeloid cells such as macrophages are predominant stromal components in hematological malignancies. In the present study, we investigated the regulation of programmed death-1 (PD-1) ligand expression in primary central nervous system lymphoma (PCNSL) using PCNSL cell lines and human monocyte-derived macrophages. TK PCNSL cell line-derived soluble factors induced overexpression of PD-1 ligands, indoleamine 2,3-dioxygenase (IDO1), and several other cytokines in macrophages. The expression of PD-1 ligands was dependent on the activation of signal transducer and activator of transcription 3. PD-L1 and IDO1 were overexpressed by macrophage/microglia in PCNSL tissues, and gene expression profiling indicated that IDO1 expression was positively correlated with the expression of macrophage and lymphocyte markers. Macrophage-derived factors did not influence the proliferation or chemo-sensitivity of cell lines. These data suggest that the expression of immunosuppressive molecules, including PD-1 ligands and IDO1, by macrophage/microglia may be involved in immune evasion of lymphoma cells.
Keywords: PD-L1, PD-L2, IDO1, lymphoma, PCNSL
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) accounts for approximately 3-4% of newly diagnosed central nervous sytem tumors, and the majority of PCNSL cases are highly aggressive diffuse large B-cell lymphoma.1,2 Although most cases of PCNSL occur sporadically, a prominent risk factor is immunodeficiency. Methotrexate (MTX) is a well-known antifolate-type cytotoxic agent, and combination therapy using radiotherapy and high-dose MTX-containing chemotherapy has been used as first line therapy, with a 5-year survival rate of less than 40%.3,4 Bruton’s tyrosine kinase is an enzyme associated with B cell maturation, and ibrutinib, an inhibitor of this kinase, is highly effective for the treatment of PCNSL.5 In addition, nivolumab, an antagonistic antibody for programmed cell death protein 1 (PD-1), induces a significantly higher rate of complete remission for refractory PCNSL.6 Potentially, these new treatments may improve the efficacy of chemo/radio-therapy against PCNSL.
Recent studies have highlighted the significant roles of the tumor microenvironment in driving tumor progression and the development of chemo/radio-resistance. Tumor-associated macrophages/microglia (TAMs) are a major stromal cell component in PCNSL.7,8 A high density of TAMs is reported to correlate with a poor clinical course in PCNSL, and is strongly associated with elevated interleukin (IL)-10 concentrations in cerebrospinal fluid.9 This latter phenomenon has been linked to poor prognosis in PCNSL by several groups.10-12 In a previous study, we found that PCNSL-associated TAMs preferentially express PD-1 ligand 1 (PD-L1) and that high expression of PD-L1 by TAMs shows a trend towards a correlation with a poor outcome.13 In the present study, we investigated the regulation of expression of PD-L1 and other immunosuppressive molecules by TAMs. We also investigated the functional significance of PCNSL cell line/macrophage cell-cell interactions using an in vitro co-culture system.
MATERIALS AND METHODS
Samples
Paraffin-embedded samples were prepared from specimens obtained from five patients diagnosed with PCNSL between 2014 and 2016 at Kumamoto University Hospital. Written informed consent was obtained from all patients in accordance with protocols approved by the Kumamoto University Review Board (#1174).
Immunohistochemistry
A rabbit monoclonal antibody against PD-L1 (clone E1L3N, Cell Signaling Technology, Danvers, MA, USA), rabbit polyclonal antibody against Iba1 (WAKO, Tokyo, Japan), mouse monoclonal antibody against indoleamine 2,3-dioxygenase (IDO1, clone 10.1, Merck, Kenilworth, NJ), and mouse monoclonal antibody against CD68 (clone PG-M1, DAKO, Glostrup, Denmark) were used as primary antibodies for immunostaining as described in a previous paper.14 Briefly, after samples were screened with primary antibodies, they were incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibodies (#424131, #424141, Nichirei, Tokyo, Japan). Can Get Signal Solution (#NKB-501, TOYOBO, Tokyo, Japan) was used to dilute the antibodies to enhance the immunoreaction of PD-L1. Diaminobenzidine (brown color) and HistoGreen (green color) substrate (#AYS-E109, Cosmo Bio, Tokyo, Japan) were used for visualization of positive signals. For immunofluorescence, Alexa 488 anti-mouse IgG antibody and Alexa 546 anti-rabbit IgG antibody (#A32723/A11010, Molecular Probes, Eugene, OR, USA) were used as secondary antibodies.
Cell lines
The human primary central nervous system lymphoma cell lines, HKBML and TK, were purchased from the RIKEN Cell Bank (WAKO) and JCRB Call Bank (Osaka, Japan), respectively. Cells were maintained in DMEM/Ham F12 supplemented with 20% or 10% fetal bovine serum. Mycoplasma testing was performed using a polymerase chain reaction (PCR) detection kit (Takara Bio Inc., Otsu, Japan). Conditioned medium (CM) from cell lines was obtained as described previously.15
Macrophage culture
Peripheral blood mononuclear cells were obtained from healthy volunteer donors who had all provided written informed consent for the use of their cells in accordance with the study protocols approved by the Kumamoto University Hospital Review Board (#1169). CD14+ monocytes were isolated using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The monocytes were plated in UpCELL 12-well culture plates (2×105 cells/well; CellSeed, Tokyo, Japan) and cultured in 2% human serum, 1 ng/mL granulocyte macrophage-colony stimulating factor (WAKO), and 50 ng/mL macrophage-colony stimulating factor (WAKO) for 7 days to induce macrophage differentiation. In some experiments, WP1066 (CAS 857064-38-1, Santa Cruz Biotech., Santa Cruz, CA, USA), an inhibitor of signal transducer and activator of transcription 3 (Stat3), was dissolved in DMSO and added at a final concentration of 20 μM.
Flow cytometry
Cells were detached using 5 μM EDTA/PBS, and stained using a PE-labeled anti-PD-L1 antibody and an APC-labeled anti-PD-L2 antibody (BioLegend, San Diego, CA, USA) with Fc receptor blocking solution (BioLegend). Isotype-matched antibodies (BioLegend) were used as controls. The stained cell samples were analyzed using flow cytometry as described previously.15
Quantitative real-time PCR (qPCR)
Total RNA was isolated using RNAiso Plus (Takara Bio Inc.) and reverse-transcribed using a PrimeScript RT Reagent Kit (Takara Bio Inc.). qPCR was performed using TaqMan polymerase with SYBR Green Fluorescence (Takara Bio Inc.) and an ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA). All primer sets were purchased from TAKARA Bio Inc.
Gene expression analysis
Affymetrix microarray gene expression values for IDO1 (210029_at), CD4 (203547_at), CD8A (205758_at), IBA1 (209901_x_at), and CD163 (215049_x_at) were derived from publicly accessible PCNSL patient data (GDS4464) available online at GEO Profiles (https://www.ncbi.nlm.nih.gov/geoprofiles) as described previously.16
Co-culture assay
Cells were labeled with CFSE (#C309, Dojindo, Kumamoto, Japan) and co-cultured with macrophages (100,000 cells/well) in 6-well culture plate for 2 days. CFSE fluorescence signals were analyzed by flow cytometry. Cells (10,000 cells/well) were cultured in 96-well culture plates with CM for 4 days, and cell numbers were evaluated by cell counting or a WST assay (#CK-04, Dojindo).
Statistics
Student’s t-test was used for statistical analysis of in vitro studies. A non-parametric rank correlation analysis was performed by means of Kendall’s tau (τ) rank correlation coefficient analysis with JMP v10 built-in modules (SAS Institute Inc., Tokyo, Japan). A P value <0.05 was considered to be statistically significant. All data from cell culture studies are representative of at least two independent experiments. All error bars indicate the standard deviation.
RESULTS
PD-L1 is expressed by TAMs in PCNSL
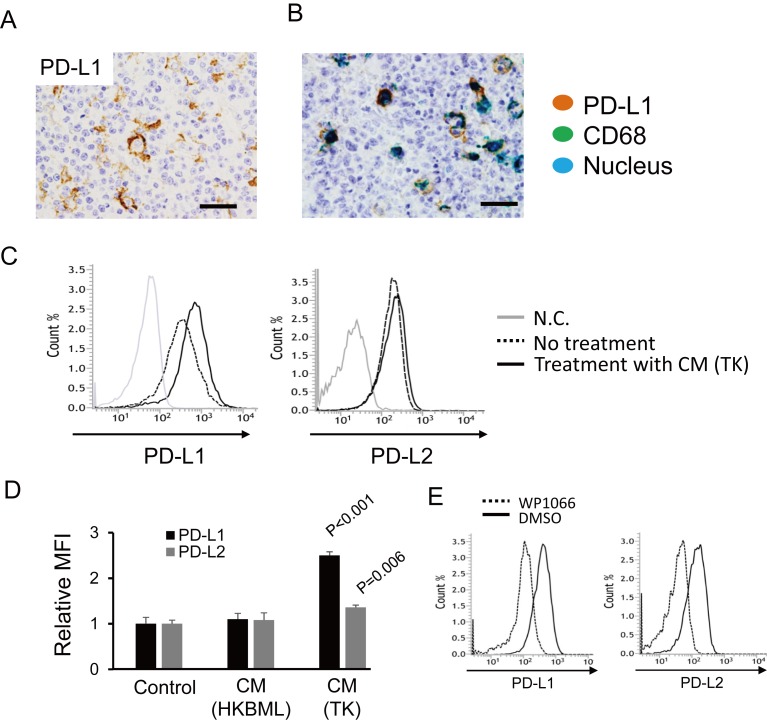
Although no PD-L1 expression was detected by immunohistochemistry on lymphoma cells, PD-L1 expression on TAMs was seen in three of five cases (60%) (Figure 1A and 1B). This observation is consistent with our previous report.13 We then investigated the mechanisms underlying PD-L1 overexpression in TAMs using human monocyte-derived macrophages and PCNSL cell lines. We found that CM from the TK cell line, but not the HKBML cell line, induced overexpression of PD-L1 on macrophages (Figure 1C). The expression of PD-L2 was also elevated by TK CM, although the difference was small (Figure 1D). The overexpression of PD-1 ligands was abrogated by treatment with a Stat3 inhibitor (Figure 1E).
Fig. 1.
The expression of PD-1 ligands on macrophages. (A) Immunohistochemistry for PD-L1 in PCNSL. Scale bar: 50 μm. (B) Double immunohistochemistry for CD68 (green) and PD-L1 (brown) was performed, and a representative photomicrograph is presented. Scale bar: 50 μm. (C) The expression of PD-L1 and PD-L2 was examined with flow cytometry after macrophages were stimulated with CM from the TK and HKBML cell lines. (D) Mean fluorescence intensities (MFI, n = 3 for each group) were compared. Significance was determined with the Student’s t-test. (E) Macrophages were stimulated by CM from TK cells with or without a Stat3 inhibitor (WP1066) for 24 hours, and PD-L1 and PD-L2 expression was assessed with flow cytometry.
IDO1 is also expressed by TAMs
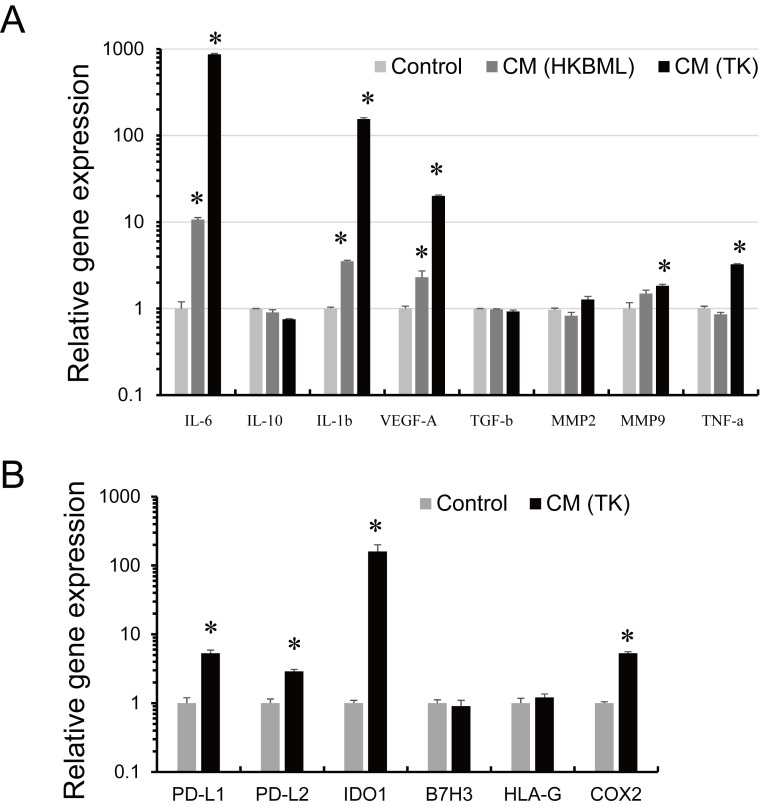
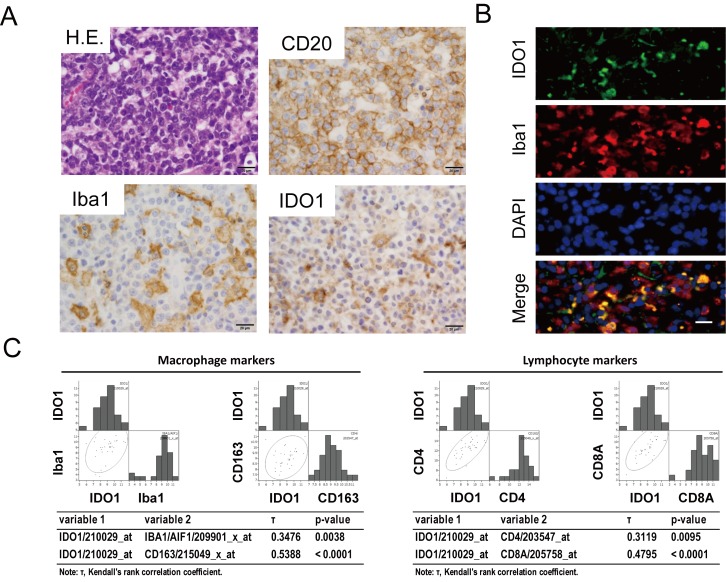
qPCR analyses revealed that TK CM significantly induced the up-regulation of expression of IL-6, IL-1β, vascular endothelial growth factor (VEGF)-A, matrix metalloproteinase (MMP)9, tumor necrosis factor (TNF)-α, PD-L1, PD-L2, and IDO1 (Figure 2A and 2B). We focused on IDO1 because, along with PD-1 ligands, it is a critical immunosuppressive molecule. Immunohistochemistry showed that IDO1 expression was strongly positive in TAMs and weakly positive in lymphoma cells (Figure 3A and 3B). Analysis of the GEO gene expression data revealed that IDO1 mRNA expression was positively correlated with mRNA expression of macrophage markers (Iba1 and CD163) and lymphocyte markers (CD4 and CD8A) (Figure 3C).
Fig. 2.
Gene expression in macrophages. mRNA was isolated from macrophages after stimulation with CM from TK cells or CM from HKBML cells for 24 hours. qPCR analysis of cytokine expression (A) and the expression of immunosuppressive molecules (B). *p < 0.05, compared with control.
Fig. 3.
IDO1 expression in TAMs. Immunohistochemistry for CD20, Iba1, and IDO1 is presented (A). Double immunofluorescence for Iba1 and IDO1 (B). Scale bar: 20 μm. (C) A non-parametric rank correlation analysis was performed to measure the strength of the relationship between two variables. Kendall’s tau (τ) rank correlation coefficient analysis assessed statistical associations based on the ranks of the data. P < 0.05 was considered statistically significant.
Macrophages do not contribute to driving cell growth or the development of chemo/radio-resistance in PCNSL cell lines
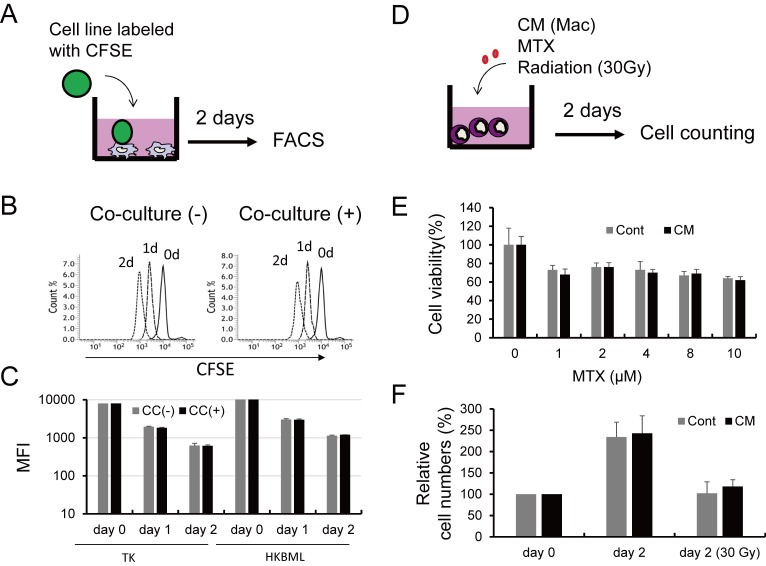
Finally, we tested if TAMs affect lymphoma cell growth and chemo/radio-resistance by performing co-culture studies. CFSE-labeled TK or HKBML cells were directly co-cultured with macrophages, and cell proliferation was examined with flow cytometry (Figure 4A). However, no significant differences were seen between the mono-culture group and co-culture group (Figure 4B and 4C). Similarly, when TK cells were treated with MTX or radiation with or without CM from macrophages (Figure 4D), no significant differences in viability were detected (Figure 4E and 4F).
Fig. 4.
Cell-cell interactions between PCNSL cells and macrophages. Lymphoma cells labeled with CFSE were co-cultured with macrophages for 2 days (A), fluorescence signals were evaluated by flow cytometry (B), and then mean fluorescence intensities were compared (C). Lymphoma cells were co-cultured with CM from macrophages and then treated with MTX and radiation (30 Gy) (D). Cell numbers were evaluated with the WST assay following treatment with MTX at various concentrations for 2 days (E). Cells were treated with radiation with or without CM, and cell numbers were assessed with the WST assay following culture for 2 days (F).
DISCUSSION
In the present study, we found that the overexpression of PD-1 ligands and IDO1 in TAMs was induced by soluble factors derived from lymphoma cells. Soluble factors produced by lymphoma cells may induce TAM activation and the production of not only PD-1 ligands but also several molecules related to lymphoma cell activation and angiogenesis such as IL-6, IL-1β, TNF-α, VEGF-A, and MMP9. PD-1 ligands are critical immunosuppressive molecules, and the clinical prognosis of patients with PCNSL who show overexpression of PD-L1 in TAMs tends to be worse.13 CD163- and CD204-positive TAMs in oral cancer preferentially express high levels of PD-L1, and an in vitro study demonstrated that PD-L1 expressed on macrophages induces apoptosis of activated lymphocytes.17 The significance of macrophage-expressed PD-L1 in immunosuppression was shown with an in vivo study.18 PD-L1 expression on TAMs is associated with the efficacy of anti-PD-1 therapy in patients with melanoma and ovarian cancer.19 Macrophages suppress natural killer cells in lymphoma via the PD-1-PD-L1 pathway.20 IL-6 and TNF-α promote lymphoma cell growth,21 and VEGF-A and MMP9 are potent angiogenic factors.22 These data suggest that TAMs are involved in lymphoma progression by inducing angiogenesis, immunosuppression, and lymphoma cell proliferation.
IDO1 is a well-known immunosuppressive molecule that is expressed ubiquitously in various tissues and cell types. IDO1 modulates lymphocyte behavior via the catabolism of tryptophan to kynurenine.23 High IDO1 expression in cancer cells or low serum tryptophan/kynurenine ratios are associated with poor clinical prognosis in several cancers.23 Recent studies have focused on the efficacy of IDO1 inhibitors, and clinical trials using some of these compounds are now underway.24 However, IDO1 inhibitor monotherapy was ineffective in animal studies and clinical trials,25-27 suggesting that combination therapy using IDO1 inhibitors and checkpoint inhibitors may be a more promising approach to targeting malignant tumors.28 High expression of IDO1 by TAMs is correlated with favorable prognosis in diffuse large B-cell lymphoma.29 In the present study, we also detected strong expression of IDO1 in TAMs in PCNSL, and we also found a significant positive correlation between IDO1 expression and the expression of TAM markers from the analysis of gene expression data. These findings suggest that TAMs are a critical source of IDO1 in several kinds of tumors. IDO1 expression was also positively correlated with the expression of lymphocyte markers, and this suggested that IDO1 expression in TAMs is mediated by inflammatory signals induced by soluble factors derived from activated lymphocytes.
In the present study, we did not investigate the types of molecules secreted from PCNSL cell lines that induced PD-L1 and IDO1 expression in macrophages. Recent studies indicated that exosomes and IL-27 derived from tumor cells induce increased expression of PD-L1 in macrophages.15,30 Further studies are necessary to address the molecular mechanisms related to PD-L1 or IDO1 overexpression in TAMs in PCNSL.
In conclusion, our data suggest that the expression of PD-1 ligands and IDO1 in TAMs is involved in immune evasion by lymphoma cells in patients with PCNSL. Because CM from macrophages did not influence the proliferation or chemo-sensitivity of cell lines, TAMs seem to be associated with immunosuppression rather than protumor function in PCNSL pathogenesis. These data provide additional support for the further investigation of TAMs as promising targets for PCNSL therapy.
ACKNOWLEDGMENTS
We thank Ms. Ikuko Miyakawa and Mr. Takenobu Nakagawa for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 16H05162 and 16K15503).
Footnotes
CONFLICT OF INTEREST: None of the authors have any conflicts of interest in association with this manuscript.
REFERENCES
- 1.Citterio G, Reni M, Gatta G, et al. Primary central nervous system lymphoma. Crit Rev Oncol Hematol. 2017; 113: 97-110. 10.1016/j.critrevonc.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka R. Management of refractory or relapsed primary central nervous system lymphoma (Review). Mol Med Rep. 2009; 02: 879-885. 10.3892/mmr_00000186 [DOI] [PubMed] [Google Scholar]
- 3.Paydas S. Primary central nervous system lymphoma: essential points in diagnosis and management. Med Oncol. 2017; 34: 61. 10.1007/s12032-017-0920-7 [DOI] [PubMed] [Google Scholar]
- 4.Makino K, Nakamura H, Yano S, et al. Pediatric primary CNS lymphoma: longterm survival after treatment with radiation monotherapy. Acta Neurochir (Wien). 2007; 149: 295-298. 10.1007/s00701-006-1094-9 [DOI] [PubMed] [Google Scholar]
- 5.Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017; 31: 833-843.e5. 10.1016/j.ccell.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017; 129: 3071-3073. 10.1182/blood-2017-01-764209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014; 105: 1-8. 10.1111/cas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komohara Y, Horlad H, Ohnishi K, et al. M2 macrophage/microglial cells induce activation of Stat3 in primary central nervous system lymphoma. J Clin Exp Hematop. 2011; 51: 93-99. 10.3960/jslrt.51.93 [DOI] [PubMed] [Google Scholar]
- 9.Sasayama T, Tanaka K, Mizowaki T, et al. Tumor-Associated Macrophages Associate with Cerebrospinal Fluid Interleukin-10 and Survival in Primary Central Nervous System Lymphoma (PCNSL). Brain Pathol. 2016; 26: 479-487. 10.1111/bpa.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasayama T, Nakamizo S, Nishihara M, et al. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro-oncol. 2012; 14: 368-380. 10.1093/neuonc/nor203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Zhang W, Zhang L, et al. Cerebrospinal Fluid IL-10 and IL-10/IL-6 as Accurate Diagnostic Biomarkers for Primary Central Nervous System Large B-cell Lymphoma. Sci Rep. 2016; 6: 38671. 10.1038/srep38671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen-Them L, Costopoulos M, Tanguy ML, et al. French LOC Network for CNS Lymphoma The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer. 2016; 61: 69-76. 10.1016/j.ejca.2016.03.080 [DOI] [PubMed] [Google Scholar]
- 13.Hayano A, Komohara Y, Takashima Y, et al. Programmed Cell Death Ligand 1 Expression in Primary Central Nervous System Lymphomas: A Clinicopathological Study. Anticancer Res. 2017; 37: 5655-5666. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T, Ohnishi K, Kosaki Y, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. J Clin Exp Hematop. 2017; 57: 31-36. 10.3960/jslrt.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horlad H, Ma C, Yano H, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016; 107: 1696-1704. 10.1111/cas.13065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi A, Iwadate Y, Komohara Y, et al. Gene expression signature-based prognostic risk score in patients with primary central nervous system lymphoma. Clin Cancer Res. 2012; 18: 5672-5681. 10.1158/1078-0432.CCR-12-0596 [DOI] [PubMed] [Google Scholar]
- 17.Kubota K, Moriyama M, Furukawa S, et al. CD163+CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci Rep. 2017; 7: 1755. 10.1038/s41598-017-01661-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau J, Cheung J, Navarro A, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017; 8: 14572. 10.1038/ncomms14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade–mediated tumor regression. J Clin Invest. 2018; 128: 805-815. 10.1172/JCI96113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vari F, Arpon D, Keane C, et al. Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood. 2018; 131: 1809-1819. 10.1182/blood-2017-07-796342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komohara Y, Niino D, Saito Y, et al. Clinical significance of CD163 + tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013; 104: 945-951. 10.1111/cas.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017; 108: 1921-1926. 10.1111/cas.13336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. OncoImmunology. 2014; 3: e957994. 10.4161/21624011.2014.957994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. 2017; 76: 167-182. 10.1016/j.ejca.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 25.Takamatsu M, Hirata A, Ohtaki H, et al. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci. 2015; 106: 1008-1015. 10.1111/cas.12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristeleit R, Davidenko I, Shirinkin V, et al. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)–only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol. 2017; 146: 484-490. 10.1016/j.ygyno.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 27.Beatty GL, O’Dwyer PJ, Clark J, et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin Cancer Res. 2017; 23: 3269-3276. 10.1158/1078-0432.CCR-16-2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epacadostat Shows Value in Two SCCHN Trials Cancer Discov. 2017; 7: OF2. [DOI] [PubMed] [Google Scholar]
- 29.Nam SJ, Kim S, Paik JH, et al. An increase in indoleamine 2,3-dioxygenase-positive cells in the tumor microenvironment predicts favorable prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. Leuk Lymphoma. 2016; 57: 1956-1960. 10.3109/10428194.2015.1117610 [DOI] [PubMed] [Google Scholar]
- 30.Gabrusiewicz K, Li X, Wei J, et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. OncoImmunology. 2018; 7: e1412909. 10.1080/2162402X.2017.1412909 [DOI] [PMC free article] [PubMed] [Google Scholar]