Abstract
Current knowledge of normal developmental physiology and identification of specific cell types of the kidney at molecular levels enables us to generate various cells of the kidney. The generation of renal specialized cells in vitro with its correct molecular and functional implications is the urgent need for cellular therapy in chronic kidney diseases and for organ formation. Glomerular podocytes are one of the major renal cells lose its functionality to maintain glomerular blood filtration function. In vitro, many inductions or reprogramming methods have been established for podocytes development. In these methods transcription factors, small molecules, and growth factors play the major role to remodel stem cells into podocyte progenitors and towards mature podocytes. Micro ribonucleic acids (miRNAs) have been utilizing as another strategy to generate podocyte. In this review, current protocols for in vitro glomerular podocyte differentiation have summarized emphasizing programming methods, signaling modulation, and cytoskeletal changes. Novel ideas are also pointed out, which are required for efficient optimal glomerular podocyte generation and their functional characterization in vitro with nanoarchitecture impression of the glomerular basement membrane.
Keywords: Glomerular podocytes, differentiation, transcription factors, small molecules, growth factors, signalling, miRNA
Introduction
Kidneys are one of the vital organs for normal homeostasis of the body. Chronic kidney diseases (CKD), irrespective to their primary cause culminate in proteinuria and complete loss of kidney functions to which renal replacement therapy (RRT) is required. The current need is to replace non-functional cells from healthy cells, either providing nephron progenitors (NPs) population or to directly replace adult functional cell types.
Glomerular podocytes are one of the major renal cells associated with many renal diseases by the loss of podocytes or its function, which is to maintain glomerular blood filtration. It results in proteinuric states due to the flattening or effacement of the foot processes of podocyte. The podocytopathies include minimal change nephropathy (MCN), focal segmental glomerulosclerosis (FSGS), diffuse mesangial sclerosis, and collapsing glomerulopathy (CG). The glomerular podocytes are very specialized but incomplete epithelial cells as it demonstrates both epithelial and mesenchymal features. It carries cell polarity, low invasive capacity, anchorage dependence, and tight junction modification of epithelial features while spindle-shaped, podocyte cadherin (P-CDH) expression, neuronal cadherin (N-CDH) expression, high cell-matrix interactions, and high migration capacity of mesenchymal features [1]. Podocytes are terminally differentiated post-mitotic cells that cannot enter cell division or proliferate [2]. It expresses cyclin-dependent kinase inhibitors p27 and p57 while do not express cyclin A, Cyclin D, and Ki-67, the markers of proliferation [3]. Morphological differences of podocyte were observed during proteinuria associated nephritic diseases. In the kidney, glomerular podocyte consists of a cell body, major, secondary, and foot processes, commonly called the arborized morphology of podocyte [4]. The functional unit of the glomerular filtration barrier (GFB) is formed by inter-digitating glomerular podocytes and capillary endothelial cells. In the foot processes of podocytes, the major protein is filamentous actin (F-Actin) while at cell-cell junction multi-cell shape proteins makes a complex called as the slit diaphragm (SD). These adapter proteins include P-CDH, Podocin, Nephrin, CD2-associated protein (CD2AP), Non-catalytic region of tyrosine kinase adaptor protein 1/2 (NCK1 and NCK2), and the atypical protein kinase C-Partitioning defective 3 homolog-Partitioning defective 6 homolog complex (aPKC-PAR3-PAR6), Protein fat homolog (FAT-1), Zona occludens-1 (ZO-1)/Tight junction protein-1 (TJP1), and actin-related proteins (Arp2 and Arp3) [5].
In vitro generations of functional cells are required for treating renal disease or for the drug targets of patient-specific cells in personalized medicine. Podocyte can be generated by direct reprogramming via generation of NPs or directed reprogramming via podocyte progenitors or terminally differentiated podocytes [6]. Efforts for potential generation of podocyte progenitors and mature glomerular podocytes in vitro have been discussed and summarized in Figure 1. In this review, the term “Podocyte Progenitor,” reflects those NPs that expressed nephron lineage and specific podocyte markers. The podocyte in kidney organoid formation has not been emphasized here. The kidney organoid structure required multiple cell type generations through the use of the different scaffolds which organized in renal epithelial cells likely in the complex architecture of the kidney for which a separate in-depth discussion is required.
Figure 1.
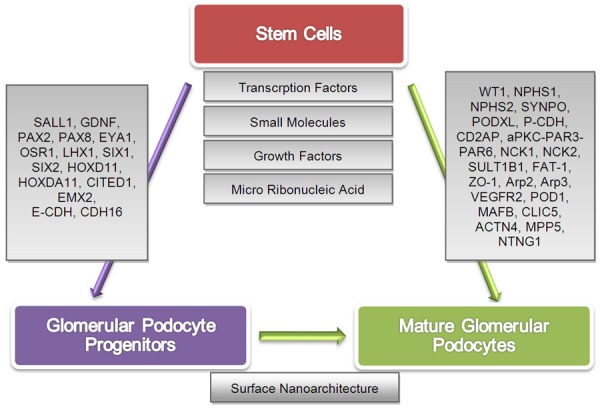
Stem cells can generate glomerular podocyte progenitors and mature glomerular podocytes in vitro. Stem cells are sensitive to environmental cues in culture conditions and affected by specific transcription factors, small molecules, growth factors, and micro ribonucleic acid individually or in combination to generate glomerular podocytes. They expressed protein markers that signify their status of being the glomerular podocyte progenitor or fully functional glomerular podocyte. Podocyte progenitor cells expressed nephron progenitor markers in combination with specific podocyte markers. Podocyte differentiation influenced by surface nanoarchitecture through respective gene expression (grey boxes on the arrows) in podocyte progenitors and mature podocytes. [SALL1, Spalt like transcription factor 1; GDNF, Glial derived nerve growth factor; PAX2, Paired box 2; PAX8, Paired box 8; EYA1, Eyes absent (Drosophila) Homolog 1; OSR1, Odd-skipped related 1; LHX1, LIM homeobox protein 1; SIX1, Sine oculis homeobox homolog 1; SIX2, Sine oculis homeobox homolog 2; HOXD11, Homeobox D11; HOXDA11, Homeobox DA11; CITED1, Cbp/P300 interacting transactivator with Glu/Asp rich carboxy-terminal domain 1; EMX2, Empty spiracles homeobox 2; E-CDH, Epithelial cadherin; CDH16, Cadherin 16; WT1, William’s tumor 1; NPHS1, Nephrin; NPHS2, Podocin; SYNPO, Synaptopodin; PODXL, Podocalyxin-like protein 1; P-CDH, Podocyte cadherin; CD2AP, CD2-associated protein; aPKC-PAR3-PAR6, Atypical protein kinase C-Partitioning defective 3 homolog-Partitioning defective 6 homolog complex; NCK1, Non-catalytic region of tyrosine kinase adaptor protein 1; NCK2, Non-catalytic region of tyrosine kinase adaptor protein 2, SULT1B1, Sulfotransferase family 1B member 1; FAT-1, Protein fat homolog; ZO-1, Zona occludens-1; Arp2, Actin-related protein 2; Arp3, Actin-related protein 3; VEGFR2, Vascular endothelial growth factor 2; POD1, Podocyte expressed 1; MAFB, Kreisler (Mouse) Maf-related leucine zipper homolog; CLIC5, Chloride intracellular channel 5; ACTN4, Actinin alpha 4; MPP5, Membrane protein, palmitoylated 5; NTNG1, Netrin G1].
Podocyte differentiation and characterization tools
Putative glomerular podocyte differentiation procedures have been developed in the last decade. The culture conditions for lineage commitment or specialized cells types conversion direct signal transduction mechanism. The capacity of stem cells potentiates this mechanism. Currently, transcription factors (TFs), small molecules, and growth factors are the three well-known factors, which boost the process for progenitor population, specialized cells formation, and kidney organoids development. Studies have been conducted to potentiate the productivity and efficiency of differentiated podocyte, for example, by utilizing micro ribonucleic acids (miRNAs) technology. Starting origin, the number of cells, duration of differentiation, detection methods, the method of characterization, and functional analysis, although varied in in vitro differentiation protocols. However, many of them showed the generation of functional podocyte according to the in vivo counterpart. In vitro, up-to-date protocols for nephron and podocyte progenitors and differentiated glomerular podocyte have been summarized in Tables 1, 2.
Table 1.
The summary of the protocols for the differentiation of stem cells into the nephron progenitor (NPs) and the podocyte progenitors
| Original cells | W9.5 ESCs (P18) derived Pax2-GFP+ mESCs | mESCs derived EBs | hiPSCs derived from human kidney mesangial cells | H6 cells (CD-1 mouse’s Pax2+ KSCs) | HK2 (Human kidney cell line-adult proximal tubule cells) |
| Number of cells | EBs from W9.5 ES cells (P18) (1 × 105/ml, 2 × 103/ml), EBs derived Pax2-GFP+ ESCs (8 × 104/ml) | - | - | - | - |
| Differentiation culture conditions (small molecules/growth factors/transcription factors/micro RNA) | Stepwise: ME: DMEM, 1% methylcellulose, BMP4 (2 ng/ml) IM: BMP2, BMP4, BMP7 each with (0.5, 5, 50 ng/ml), Chordin (1.25 µg/ml), BMP2, BMP4, BMP7 (5 ng/ml), serum & serum free conditions | Stepwise: mESCs on mouse feeder layer then on 1% agar within 2 days to form EBs then for 6 days trypsinized EBs on 0.1% gelatin, DMEM, 15% FCS, L-glutamine (2 mM), β-Mercaptoethanol (5 × 10-5 M), NEAA (1:100), RA (10-6, 10-7, 10-8), A A (1, 5, 10 ng/ml), 10th day, UB cells of gestation day 13 fetal kidney conditioned medium for further 10 days | DMEM-F12, 2.5% FBS, β-Mercaptoethanol (100 µM), 0.1% gelatin coated dishes. AA (10 ng/ml), RA (0.1 µM), BMP7 (15 ng/ml) | High glucose DMEM, 2 mM glucose, 20% FBS, No other molecules. Spontaneous differentiation | GFP-Lentivirus for 7 days + VPA (2 mM). Combinatorial screening of 15 different factors for NPs (Six1, Six2, Pax2, OSR1, CRYM, N-NYC, cMYC, HOXA11, EYA1, SNAI1, SNAI2, WT1, MEOX2, HMGA2, OCT4) |
| Endpoint duration of analysis | 16th & 19th day | 10th & 20th day | •SEM on 10th day | 4 weeks | 6 days |
| •Expension of differentiated cells + 10 days without small molecules & growth factors | |||||
| Detection methods/characterization | •ICC (Pax2+ in EBs) | •ICC IM: (Brachyury, Pax2) Renal genes: (WT1, E-CDH, POD-1, DB, Pax2) | •SEM | •ICC (Pax2, WT1, αSMA, CK8, GDNF, Musahi1, Nanog) | •ICC (10 pools were identified based on induction of CITED1. further screening identified pool 8 by qRT-PCR) |
| •qRT-PCR (α-Globin, β-h1globin, Aqp1, Brachyury, GDNF, GFP, HPRT, CDH16, LacZ, Mineralocorticoid receptor, NPHS1, OCT4, Pax2, PODXL, Pod-1, Wnt4, Wnt11, WT1) | •Flow cytometry (Brachyury, Pax2) | •ICC (Nephrin, Podocin, Pax2, WT1, SYNPO) | •RT-PCR (Pax2, WT1, GDNF, Sall1, PODXL, SYNPO, UMOD, Desmin, Megalin, AQP1, AQP2, CK8, Six2, Nanog) | •qRT-PCR (Six1, Six2, OSR1, Pax2, HOXA11, EYA1, SNAI2, CITED1, E-CDH, MMP2, MMP9) | |
| •FACS (Pax2 GFP+, PI) | •RT-PCR (Six2, CITED1, Sall1, FOXD1, GSC, FOXA2, PAX6, NANOG) | ||||
| •ICC (Pax2, CITED1) | |||||
| Functional analysis | - | - | •Cell contractility assay (AII, RFP-actin, RFP-talin) | •Alkaline phosphatase staining for proximal tubular cells | •Recombination assay E12.5 mouse kidney + GFP+ single cells, detected by |
| •Cell permeability assay {FIT-C labeled albumin (0.5 mg/ml)} | •No specific functional assay for podocytes like cells | •ICC (WT1+, Six2+, Calbindin-) | |||
| •Re-aggregation assay (mouse embryonic kidneys, E 13.5-15.5) | •Flow cytometry for stem cells markers (Sca1+, CD24+), mesenchymal markers (CD29, CD44) hematopoietic markers (CD45, CD117) | ||||
| Uni/multi progenitors | Multipotent progenitor from IM to renal lineage cells | Multipotent progenitor from IM to renal lineage cells | Unipotent, podocyte progenitors | Multipotent progenitor to podocytes, mesangial cells, & proximal tubular cells. | Nephron progenitors |
| References | [7] | [8] | [11] | [21] | [22] |
|
| |||||
| Original cells | hESCs {HES3 (MIXL GFP/wt)} | hESCs-H9 cell line, hiPSCs-derived from CRL2097 fibroblast | hPSCs {iPSCs (Fibro-epi)}, ESC (H1) human fibroblast episomal derived | hiPSCs (SC101A-1) clone IV derived EBs | Sprague Dawly rat’s BM-MSCs, AD-MSCs |
| Number of cells | 12000-15000 cells/cm2 | - | ~300-500 cells/colonies | - | - |
| Differentiation culture conditions (small molecules/growth factors/transcription factors)/micro RNA) | Stepwise: PS: 2-3 days matrigel coated dishes, 1. BMP4 (30 ng/ml), AA (10 ng/ml), or 2. BMP4 (30 ng/ml), CHIR99021 (8 µM), serum free APEL media. IM: 4 days in FGF9 (200 ng/ml), heparin (1 µg/ml). further differentiation 4-11 (6 days) for 1. FGF9 (200 ng/ml), BMP7 (50 ng/ml), RA (0.1 µM), Heparin (1 µg/ml). 6 days For 2. FGF9 (200 ng/ml), Heparin (1 µg/ml). Cultured for further 6 days | Stepwise: Serum & feeder free system | Stepwise: IM: 2 days on growth factor reduced matrigel-coated plates, DMEM-F12, BSA (17.5 mg/ml), hInsulin (17.5 µg/ml), h holo-transferin (275 µg/ml), Monothioglycerol (450 µM), L-glutamine (2.25 mM), NEAA (2.25 mM), Penicillin (100 units/ml), Streptomycin (100 µg/ml), bFGF (50 ng/ml), hBMP4 (30 ng/ml), For further 2 days in ATRA (1 µM), hAA (10 ng/ml), BMP2 (100 ng/ml) + same medium | Stepwise: ME: 0-3 days, IM: 3-6 days, MM: 6-12 days, NPs: DMEM-F12, 5% FBS, NEAA (0.1 mM), β-Mercaptoethanol (0.1 mM), ATRA (0.1 µM), CCG1423 (1 µM), LY294002 (5 µM) till day 6; AA (10 ng/ml) for day 2-4. For 6-19 days, BMP7 (50 ng/ml), FGF2 (10 ng/ml), GDNF (15 ng/ml) | Fibronectin coated dishes, DMEM low glucose, 15% FBS, FGF2 (50 ng/ml), TGFβ2 (4 ng/ml), LIF (20 ng/ml) |
| PS: 3 days {1 day, AA (100 ng/ml), Wnt3a (100 ng/ml), IM: 2 days, BMP4 (20 ng/ml), bFGF (10 ng/ml)} IM: 6-8 days, RA (10 µM), BMP7 (50 ng/ml), bFGF (10 ng/ml). 15 days NP: BMP7 (150 ng/ml), bFGF (50 ng/ml). For all above, the medium RPMI-1640 containing 2% B27, L-glutamine (2 mM), 1% PenStrep. | |||||
| For podocytes differentiation NPs cultured on fibronectin-coated dishes in VRAD medium (DMEM-F12, RA (100 µM), 10% FBS) for 7 days | |||||
| Endpoint duration of analysis | 2-18 days | 15 days NPs. Later Podocyte formation | 1-6 days | 0, 6, 12, & 19 days | 7 days |
| Detection methods/characterization | •FACS PS: {Post 3 days, (2 × 106 Cells) MIXL1-GFP+} | •qRT-PCR PS: (T, MIXL1, EOMES) Endodermal: (SOX17, FOXA2) Ectodermal: (PAX6, SOX1) IM: (OSR1) NPs: (SIX2, WT1, GDNF, HOXDA11) Metanephric stroma/UB: (FOXD1, HOXB7. Bone, RUNX2, COL1A1) Vascular endothelium: (PCAM1, TIE2) Smooth Muscle: (MYH11, CALPONIN) Liver: (ALB, AAT) Neuron: (TUJ1, MAP2) Tubular: (SLC12A3, CD13, AQP1) Podocyte: (SYNPO, Nephrin) | •qRT-PCR IM: {T, OSR1, LHX1(LIM1), Pax2, Pax8, GATA3, OCT4, Nanog, SOX3} IM ureteric progenitor-like cells: (Six2, GDNF, WT1, Sall1, Cited1) UB: (HOXB7, RET, GFRA1) | •ICC (OSR1, WT1, Pax8, Pax2, Six2, Sall1, CD133, CD24, NCAM, Claudin1, AQP1, GGT1, SSEA4, TRA1, Nanog, T, AFP, Pax6, Nkx2.5) | •ICC (Wnt4, WT1, Pax2, Vim, Oct4, Sox2, E-CDH, ZO-1) |
| •ICC (Pax2, OSR1, LHX1, TBX6, SOX17, Six2, E-CDH, WT1, HOXD11, GATA3, JAG1) In this quantification proportion of induced cells (Pax2, LHX1, Sox17, Six2, WT1, CDH6, SYNPO) | •RT-PCR IM: (OSR1, PAX2, SALL1, EYA1, WT1) NPs: (SIX2, CITED1, OSR1, PAX2, SALL1, EYA1) | •ICC IM: (HuNu, CK8, Six2) IM ureteric progenitor-like cells: {(Six2, WT1, LHX1 (LIM1)} | •FACS (TRA-1-81) | •qRT-PCR (Pax2, Wnt4, WT1, E-CDH, ZO-1) | |
| •qRT-PCR, day 3 PS: {SOX17, Brachyury (T) MIXL1} day 6 IM: (Pax2, LHX1, FOXF1, TBX6) | •ICC (T, TRA1-81, OCT4, OSR1, PAX2, SALL1, SIX2, WT1, E-CDH, ZO1, KRT18, F-ACTIN, CD13, AQP1, MUCIN1, SYNPO, PODXL) | •qRT-PCR human (POU5F1, Nanog, DNMT3B, GABRB3, GDF3, SOX2, TDGF1, RAF1, ELF1, T, LHX1, OSR1, SIX2, PAX8, NANOG, SALL1, WT1, PAX2) | |||
| •RT-PCR (Pax2, LHX1, OSR1) | |||||
| •RT-PCR for day 0-17 PS: (MIXL1, LHX1) IM: (LHX1, Pax2, OSR1) MM: (OSR1, SIX2, WT1, GDNF, HOXD11) UE: (PAX2, CRET, HOXB7) Ectoderm: (PAX6) | |||||
| •Pellet IMF (CALB1, AQP1, AQP2, SLC3A1, HuMt, HuNu) | |||||
| Functional analysis | •3D culture (10 × 105 cells), collagen IV coated (10 µg/cm2) filter membrane (0.4 µm) | •Alkaline phosphatase staining for tubular cells | •3D organ co-culture assay | •Cisplatin-induced AKI model | - |
| •Re-aggregation assay, embryonic kidneys (12.5-13.5 dpc) collagen IV coated (10 µg/cm2) filter membrane | •In vitro tubulogenesis assay | •qRTPCR for above {(T, OSR1, HOXB7, LHX1 (LIM1), Pax2, GFRA1)} | •HC (H&E, PAS) | ||
| •No functional assay for podocytes | •ICC (CK8, HuNu, Six2, ZO1) | •IHC (HNA, hMitochondria, AQP1, WGA lectin, PNA lectin, AQP3, Ki67) | |||
| Uni/multi progenitors | Multipotent progenitors, ureteric & metanephric progenitors | Multipotent progenitor to podocytes, & tubular cells | Ureteric bud committed renal progenitor-like cells | Multipotent progenitor | Nephron progenitors |
| References | [9] | [10] | [12] | [14] | [19] |
Table 2.
The summary of the protocols for the differentiation of stem cells into the glomerular podocytes
| Original cells | hiPSCs | hiPSCs (Episomal iPS cell lines) | PGP1 hiPSCs cell line | CD34+ hHSCs | hAD-MSCs |
| Number of cells | NPHS1-GFP+ iPSCs (201B7) | 30,000/50,000 cells/cm2 | 4 × 104 cells/cm2 | 1 × 103 cells/cm2 | 5 × 105 cells/well of 6 well plate |
| Differentiation culture conditions (small molecules/growth factors/transcription factors/micro RNA) | iNPs aggregates, 0.8 µM polycarbonate filter, DMEM, 10% FCS, mouse embryonic spinal cord (E12.5), clone 3 on feeder free condition | Stepwise: ME, IM, NP, Podocytes. ME: Growth factor reduced matrigel coated dishes, For 3 days DMEM-F12, 2.5% FBS, GlutaMax (1:1), neurobasal media + N2B27 + CP21R7 (1 µM), BMP4 (25 ng/ml). IM: first medium replaced by STEMdiff APEL medium {RA (100 nM), BMP7 (50 ng/ml), FGF9 (200 ng/ml) for 2 days (total 5 days)}. NPs: 6th day for 7 days passaged by Accutase plated on type1 collagen-coated plates at a density of 20,000/40,000 cell/cm2 in VRAD medium {DMEM-F12 plus GlutaMax, 10% FBS, RA (80-100 µM), Vitamin D3 (100 nM)} | Stepwise: ME, IM, mature podocytes. On ECM (laminin 511-E8-coated plates). For 2 days in ME medium i.e. DMEM-F12 + GlutaMax, AA (100 ng/ml), CHIR99021 (3 µM), Y27632 (10 µM), 1X B27 serum free supplement. IM medium for 14 days DMEM-F12 + GlutaMax, BMP7 (100 ng/ml), CHIR99021 (3 µM), 1X B27 serum free supplement. Split cells 1:4 on ECM for 4-5 days in podocyte medium . DMEM-F12 + GlutaMax, BMP7 (100 ng/ml), CHIR99021 (3 µM), BMP7 (100 ng/ml), AA (100 ng/ml), VEGF (50 ng/ml), RA (0.1 µM), 1X B27 serum free supplement | Stepwise: For 5 days AA (10 ng/ml), RA (2.5-10 ng/ml, optimum 7.5 ng/ml), BMP7 (2.5-10 ng/ml, optimum 5 ng/ml) resulted OSR1+ cells. These cells for 9 days AA (10 ng/ml), RA (7.5 ng/ml), BMP7 (5 ng/ml), EGF (20 ng/ml), bFGF (20 ng/ml) | Stepwise: IM: for 3 days DMEM-F12, 2% FBS, AA (10 ng/ml), RA (10 µM). Three types of culture conditions + same basal medium 1. AA (10 ng/ml), RA (0.1 µM), BMP7 (20 ng/ml) 2. AA (10 ng/ml), RA (0.1 µM), GDNF (20 ng/ml) 3. AA (10 ng/ml), RA (0.1 µM), Wnt4 (50 ng/ml) |
| •Transfection by lipofectamine2000 | |||||
| •mi-RNA selection, miRNA-498 by TargetScan & Pictar algorithm | |||||
| Endpoint duration of analysis | Day 9 | ME day 2, IM day 4, NP day 6, mature podocytes day 13 | 21 days | 14 days | Day 9 |
| Detection methods/characterization | •ICC (WT1, E-CDH, CDH-6) (NPHS1, WT1, PODXL) | •ICC ME: (Oct4, T) IM: (Pax2, OSR1, LHX1) NP: (Pax2, Six2, WT1) | •Flow cytometry (Oct4, WT1, Nephrin) | •ICC (Podocin, SYNPO, GLEPP1), post 3 days (Pax2, WT1) post 9 days (Pax2, NPHS1, SULT1B1, NPHS2, SYNPO) | •ICC (OSR1, WT1, Pax2, Podocin, Nephrin, SYNPO, Laminin, HNA) |
| •IHC (H&E) Day 9 (Nephrin, GFP, WT1, Type IV collagen, E-CDH, CDH6, PODXL, CD31, human nuclear antibody) | •PCR ME: (T, Nanog) IM: (Pax2, OSR1, LHX1) NPs: (WT1, SYNPO, NPHS1, ACTN4, CD2AP, VEGF-A) | •ICC (Nephrin, WT1, Pax2, Podocin, Oct4, OSR1, EdU, PKCλ/I, Collagen type IV, FcRn receptor for albumin & IgG transport) | •Leishman’s staining | •Flow cytometry (OSR1) | |
| •Flow cytometry (Nephrin, PODXL) | •qRT-PCR (Six2, ACTN4, HPRT) | •qRT-PCR (POU5F1, Pax2, WT1, NPHS1, NPHS2) | •Western blot (Podocin, SYNPO) | •qRT-PCR (miR-498) | |
| •qRT-PCR (WT1, NPHS1, NPHS2, SYNPO, PODXL) | •SEM, day 6 | •Western blot (PKCλ/I) | •Flow cytometry (CD45, CD34) | •Western blot (Podocin, Nephrin, SYNPO, WT1) | |
| •Microarray | •SEM | •SEM | |||
| •SEM | |||||
| •TEM | |||||
| Functional analysis | •Transplantation of cells using solid agarose rods under NOD/SCID/JAK3 null mice kidney capsule, post 20 days characterized by | •Cell proliferation assay (differentiated vs. undifferentiated) | •ICC EdU-incorporation assay | •Tyrosine kinase assay | •Re-aggregation assay (E12.5) by HC, ICC (WT1, Laminin, Nephrin, HNA, Podocin) |
| •HC (H&E) | •Cytoskeleton rearrangement evaluation (peripheral localization of F-actin) | •Albumin uptake assay (confocal imaging & quantification of albumin positive cells) | •Scratch assay (1 × 106 cells on 0.8% agarose molds) | •Estimation of urinary protein excretion (Adriamycin induced-model in Balb/c mice) | |
| •IHC (WT1, CD31) | •Albumin uptake assay | •SEM | |||
| •Chimeric organoid cultures (E12.5 CD1 mouse) | |||||
| •ICC (E-CDH, HNA, WT1) | |||||
| References | [13] | [15] | [16] | [18] | [20] |
NPs and podocytes are derived from different sources like embryonic stem cells (ESCs) [7-10], induced pluripotent stem cells (iPSCs) [11-17], hematopoietic stem cells (HSCs) [18], adipose-derived mesenchymal stem cells (AD-MSCs) [19,20], kidney-derived stem cells (KSCs) [21], and kidney cell line [22]. Almost all protocols used immunofluorescence analysis by immunocytochemistry (ICC), flow cytometry; polymerase chain reaction (PCR), and quantitative real time polymerase chain reaction (qRT-PCR) for the characterization of generated cells. Functional analysis of these protocols includes cell contractility via Angiotensin II (AII), cell permeability assay or albumin uptake assay [11,15,16] for the perinuclear accumulation of albumin [15], and scratch assay for the migration of cells [18]. The re-aggregation assay or recombination assay or chimeric organoid cultures utilized embryonic Kidney’s cells of 12.5-13.5 days post coitus (dpc) [9,11,20,22]. Three dimensional (3D) organ co-cultures methods were utilized to grow cells similar to in vivo condition to observe organ niche integration, interaction, and the generation of the response of the cells [9,12]. In vitro generated cell transplantation was also carried out in the kidney capsule [13,20]. Urinary protein excretion levels [20], cytoskeletal examination by F-actin rearrangement [15], negative expression of the ectodermal and endodermal genes, which does not induce Pax6, NES, SOX17, ALB, ACTA2, and α-SMA, FOXD1 respectively in reprogrammed cells, further validate the results [12]. However, some of the studies did not contribute to the functional aspects of the kidney in newly developed cells [7,8,19]. Sequencing data of single cell analysis characterized the progenitor and mature podocyte by the expression of LHX1, EMX2, JAG1, and NPHS1, NPHS2, CLIC5, PODXL, SYNPO, VEGF, MPP5, TJP1, NTNG1, MAFB respectively [23].
Direct programming by transcription factors
Regulations of cellular processes are governed under coordination between target genes and proteins. Specific regulatory proteins are TFs that bind to deoxyribonucleic acid (DNA) through their DNA-binding domains (DBDs). The sequences on the DNA are termed transcription factor binding sites (TFBS) [24,25]. Remodeling of cells is associated with transcription levels driven by TFs. The direct approach for reprogramming is the forced or exogenous expression of key TFs to change the identity of cells into the desired cells. Stable transcription of glomerular podocyte specific genes can maintain the gene expression and capture the phenotype and function of podocyte. Complete TFs for cognate DNA elements and the correct combination of a few specific TFs for converting stem cells or fibroblast into podocyte are still unknown. However, some strategies have been utilized and new combinations are continuously evolving [6,22,26]. Two approaches for transporting TFs were frequently practiced that is non-integrating (chemicals, physical) and integrating (retro-lentiviral expression system) [22].
Podocytopathies are caused by genetic mutations in TFs, signaling mediators, and SD proteins. These mutations and mesenchymal to epithelial transition (MET) during development can provide clues for targeted protein expression for in vitro differentiation of podocyte. For characterization, WT1 and Nephrin are specific podocyte markers as they do not express in other nephron’s cell types. Cell adhesion proteins cadherins (CDH) are focal for specification and characterization of cells types. Mature podocytes do not have epithelial cadherin (E-CDH) but express P-CDH, while N-CDH expressed upon TGF-β1 treatment [1]. Although no reports for the kidney, in situ direct reprogramming of functional regenerative cells by delivering specific TFs have been reported in the mice models of cardiomyocytes in myocardial infarction, endocrine beta cells, neurons, and hepatocytes [6]. In situ direct programming methods, their efficiencies, and safety methods are required to optimize for the renal therapy in humans.
A major technology to examine the genome-wide binding of TFs is chromatin immunoprecipitation (ChIP) followed by deep sequencing (ChIP-seq) but only limited TFs were identified by ChIP-Seq for podocyte differentiation. Dynamic motif occupancy analysis (DynaMo) is an algorithm to accurately predict the spatiotemporal binding pattern of TFs responsible for the dynamic process. This program has been utilized for human neural differentiation [24] and other studies can also be carried out using this tool, but it exhibits no nephron related study. JASPAR (http://jaspar.genereg.net) is an open-access database of TF-binding profiles, which are stored as position frequency matrices (PFMs). A PFM summarizes experimentally determined DNA sequences bound by individual TFs [25]. Direct programming can utilize a cocktail of TFs which can yield high efficiency of a homogeneous population. Novel targeted TFs binding sites in the genome can be identified through a computational tool, protein interaction quantitation (PIQ) (http://piq.csail.mit.edu) at corresponding motifs from deoxyribonuclease I (DNase I) hypersensitive sites sequencing (DNase-Seq) experiments with accuracy comparable to ChIP-Seq. This technique utilizes DNaseI hypersensitivity profiles. It also models the magnitude and shapes of genome-wide DNase profiles to facilitate the identification of TF-binding sites. It consists of three steps: candidate site identification, the background model computation, and TF binding estimation [26]. Mogrify and CellNet, which can select candidate factors for cell fate decisions, are other computational programs and prediction methods [6].
Directed programming by small molecules and growth factors
Small molecules and growth factors combination create a synthetic niche for induction, to maintain differentiation potential for the expansion and propagation of newly developed cells. The directed or instructive signaling cues towards podocytes generation utilized chemically defined culture conditions, which are comprised of a basal medium with fetal bovine serum (FBS), rich in small molecules and growth factors. Small molecules like CHIR99021 [9,16], activin A (AA) [8-12,14,16,18], all-trans retinoic acid (ATRA) [8,11,12,14-18,20], Valproic acid (VPA) [22], CP21R7 [15], Y27632 [16], CCG1423, LY294002 [14], Chordin [7], and growth factors such as bone morphogenic protein (BMP) family (BMP2) [7,12], BMP4 [7,9,10,12,15], BMP7 [7-11,14-18,20], Fibroblast growth factor (FGF) family, FGF2 [10,12,14,18,19], FGF9 [9,15], Wingless/Integrated (Wnt) family Wnt4 [20], Wnt3a [10], Glial cell derived neurotrophic factor (GDNF) [14,16,20], Vascular endothelial growth factor (VEGF) [16], Epidermal growth factor (EGF) [18], Leukemia inhibitory factor (LIF), Transforming growth factor, beta 2 (TGFβ2) [19], have been utilized and function as the first messenger to produce signals to generate renal progenitor and glomerular podocyte. Small molecules serve as an alternative to TFs. Small molecules and growth factors provide non-integrative effects to initiate the renal development program for cell fate conversion. Chordin is a BMP antagonist whose complete function regarding podocyte differentiation is not known. RA and AA induce intermediate mesoderm (IM) and express OSR1, Pax2, and WT1. Sall 1 is expressed in metanephric mesenchyme (MM) and represent as NPs population for podocyte generation [10,12,14,21,22]. The major cellular functions of small molecules have been summarized in Table 3.
Table 3.
Small molecules and their specific roles in the podocyte differentiation
| Small molecules | Cellular functions | References |
|---|---|---|
| CHIR99021 | •1GSK3β1 inhibitor | [9,16] |
| •Wnt agonist | ||
| Activin A (AA) | •Cellular homeostasis | [8-12,14,16-18,20] |
| •Inducer of differentiation | ||
| •Activator of cell differentiation and inhibitor of cell growth and proliferation | ||
| All-trans retinoic acid (ATRA) | •Cellular homeostasis | [8,10-12,14-18,20] |
| •Inducer of differentiation | ||
| •Bind to 2CRABP2 in the nucleus | ||
| •Activate transcription of RA primary response genes | ||
| Chordin | •3BMP antagonist | [7] |
| •Development of the vertebrate gastrula | ||
| Valproic acid (VPA) | •4HDAC inhibitor | [22] |
| CP21R7 | •1GSK3β1 inhibitor | [15] |
| •Activate canonical 5Wnt signaling. | ||
| Y27632 | •6RHO/ROCK pathway inhibitor | [16,17] |
| CCG1423 | •A potent and specific inhibitor of Rho pathway signaling | [14] |
| LY294002 | •Inhibitor of 7PI3Ks | [14] |
Glycogen synthase kinase 3 beta1;
Cellular retinoic acid binding protein 2;
Bone morphogenic protein;
Histone deacetylase;
Wingless/Integrated;
Rho-associated coiled-coil forming protein serine/threonine kinase;
Phosphoinositide 3-kinases.
Growth factors control cell growth through cell proliferation, differentiation, survival, and migration. It contributes to renal metabolism and development of the kidney. It triggers the differentiation and proliferation of cells by activating specific receptors. A number of signal transduction receptors, including nuclear receptors, receptor tyrosine kinases (RTKs), and G-protein coupled receptors (GPCRs) play a crucial role to initiate this process. The outside- in signal mediates cell-matrix adhesion via Integrins [2] and regulates the differentiation of podocytes [27]. The differentiation studies showed that combinatorial programming was employed to produce nephron progenitor cells in two to three steps or induced a sequential programming as governed in the physiological program in kidney development that is starting from primitive streak (PS) formation followed to mesoderm (ME), IM, NPs like cells, and then functional glomerular podocytes [6]. Defined culture conditions may retain the inductive signals and facilitate orchestration of events of in vivo nephrogenesis. This represents the graded cues activate Nodal/Activin and Wnt signalling, which guides the differentiation process of original cells. 3D aggregates, i.e. embroid bodies (EBS) [7,14], which first expressed NPs population that were further turned to account either for renal tubules [28-34] or glomerular podocyte formation or both tubules and podocytes formation [10,21]. Ciampi et al., 2016 showed that NPs at day six whiles at day thirteen mature podocytes were generated in the STEMDIFFApel medium containing RA (100 nM), BMP7 (50 ng/ml), and FGF9 (200 ng/ml). It showed the highest expression levels of Six2 on day six of the differentiation upon testing of five different conditions comprised of RA, BMP7, FGF9, STEMDIFF Apel medium vs. DMEM-F12 [15]. Patient’s specific iPSCs derived progenitors can be utilized for clinical trials as it does not have ethical concerns like ESCs. Sharmin et al., 2015 induced iPSCs cell to podocyte and showed the overlap of human glomeruli and mouse podocyte marker expression [13]. Imberti et al., 2015 showed that up-regulated WT1 gene expression levels were also increased that can be utilized in the podocyte injury model to observe its integration into glomeruli. However, they operated NPs for renal tubule formation in the acute kidney injury model [14]. Podocyte progenitors could be very effective in ameliorating podocytopathies and can differentiate into mature functional podocytes in the niche of nephron and can replace non-functional podocytes. Similarly, functional podocytes are also considered to be essential for the urgent and the direct utilization of these cells in the cellular therapy of CKD. Matured podocytes are imperative to microfluidics/organ-on-a-chip technology for podocytes i.e. facilitates drug discovery and illuminates disease mechanism. Human clinical trials are required to observe ex vivo and in vivo potential to these cells [16,17].
Signaling pathways of podocyte differentiation
BMP signaling is established during development. The BMP family members; BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, and BMP-7/Osteogenic protein1 are present in the kidney. Among all the BMP family members, BMP-7 is most abundant in human fetal and adult kidney [35]. BMP4 promote PS genes like Brachyury (T), Mixli, Tbx6, Flk1, and IM genes [7]. In the postnatal stage in mice, BMP4 expression was observed, but Bmp2 and BMP7 were decreased [36].
Wnt/β catenin signaling is crucial for nephron development. Wnt can activate three intracellular pathways: First, the canonical pathway; T-Cell factor (Tcf)/β-catenin, second the non-canonical (planar cell polarity), and third the Wnt/Ca2+ pathway. However, in adult kidney Wnt signaling turns to be silenced. The canonical pathway regulates β-catenin through glycogen synthase kinase-3β (Gsk/3β)/Wnt signaling. β-catenin with P-CDH maintains podocyte integrity by stabilizing cell adherens junctions [37]. Phosphorylated Nephrin is critical for podocyte function i.e. maintains its morphology [38]. Phosphoinositide 3-kinase (PI3K)/Akt play role in cell survival and regulation of integrity of actin stress fibers. The SD proteins, including Nephrin, Podocin, and CD2AP complex facilitate the maintenance of Akt phosphorylation by interacting PI3K [37,38]. Insulin-like growth factor 1 (IGF-1), although did not utilize in any of the in vitro protocol discussed here, it has a protective effect in fetal podocytes through IGF-1 receptor (IGF-R) stimulation form insulin receptor substrate (IRS)-1-p85 complex, an increase in PI3K activity, protein kinase B (PKB/AKT), and reduced apoptotic protein Bad. It confers survival and maintenance of podocytes in vitro. Both Nephrin and CD2AP interact with the p85 regulatory subunit of PI3K and it stimulate AKT signaling with Podocin [39]. Hence, IGF-1 could be a contributing factor for podocyte differentiated cells for optimal survival in vitro.
The cyclic adenosine monophosphate (cAMP) pathway is involved in podocyte differentiation. RA induction stimulate Kruppel like factor 15 (KLF15) expression and binds to the promoter regions of Nephrin and Podocin, two critical differentiation markers. With the activation of protein kinase A (PKA) and cAMP-response element binding protein (CREB), RA attenuates podocyte dedifferentiation. GDNF, which is implicated in podocyte differentiation, is also CREB target and highly regulated by RA [40].
All kidney cells required the fibroblast growth factor receptor (FGFR) signaling, mainly for growth and patterning. Mature podocyte expresses several FGF molecules, including, FGF1, FGF2, FGF7, and FGF10 but FGF2 has mitogenic effects on podocyte by autocrine signaling and paracrine signaling through mesangial and endothelial cells. During podocyte differentiation, FGF2 proteins remain highly expressed in functional podocyte. It maintains cells in the induced state in absence of inductive signals [2]. FGF2 and BMP7 inhibit tubulogenesis and both promote stromal progenitor cell population during the differentiation process. FGF effect is enhanced by BMP7, and it prevents apoptosis and also up-regulate expression of WT1 in MM culture [41]. FGF signaling is critical for podocyte foot processes formation in differentiation-induced cytoskeletal reorganization via F-actin. In this process expression of slug and vimentin (VIM) evoke epithelial to mesenchymal (EMT) changes, which are necessary for terminal differentiation [42].
Adult kidneys do not express Notch signals. Notch pathway components are expressed and up-regulated in renal and podocyte progenitors throughout nephrogenesis and in glomerular diseases. Notch establishes the proximo-distal axis of a nephron. Notch stimulates S-phase entry and cell division in renal progenitors whiles its downregulation facilitate differentiation of podocyte. It may start abnormal mitosis (mitotic catastrophe) in podocyte. There are four single-pass transmembrane Notch receptors (Notch 1-4), and five ligands, Delta-like (Dll 1, 3, 4), Jagged 1 (Jag 1, 2) members of this family [43,44]. Ligand binding results in Notch intracellular domain (NICD) activation by cleavage through c-Secretases and NICD nuclear translocation. NICD binds with recombination signal-binding protein-J (RBP-J) and activates downstream transcriptional target genes the Hairy enhancer of split (Hes) factors and its related repressor proteins (Hey). Hes control tissue-specific differentiation genes [43]. Podocyte progenitors express Notch 1, Notch 2, and downstream transcriptional targets Hes1, and Hey1 in the S-shaped body stage of glomerulogenesis. Gradually during terminal podocyte differentiation, which includes tertiary foot process assembly and SD formation, is in agreement with down-regulation of Notch pathway components. The deletion of Notch processing gene PSEN that encodes presenilin in murine kidneys, results in loss of podocytes and proximal tubules [45]. During terminal podocyte differentiation, Notch signals also regulate autophagy for podocyte differentiation as Notch 1 and autophagy increased simultaneously in this processes [44].
Podocytes have autocrine VEGF regulation. Isomeric VEGF-A and VEGF-C play the autocrine role in podocyte survival. Its inhibition provoke proteinuria [46]. Podocytes have no proliferation capacity in its matured form [47]. VEGF-A signaling regulates SD proteins by inducing a dose-response Podocin up-regulation and increase its interaction with CD2AP. The data indicate that podocytes in culture have a functional autocrine VEGF-A system that is regulated by differentiation and ligand availability. VEGF-A in podocytes promote survival through VEGFR2, induce Podocin up-regulation, and increases Podocin/CD2AP interaction [48]. Additional in vitro and in vivo studies are required for defining the role of VEGF during differentiation towards podocytes.
Role of podocyte cytoskeleton
The three parts of podocytes are the apical membrane, foot process, and basal membrane. These are maintained by the cytoskeletal organization, which includes microtubules, Vimentin rich intermediate filaments, and Actin proteins. All of these components maintain cell shape, rigidity, and cell motility. The positioning of membrane organelles and signals is transmitted through microtubules via protein vesicular transport along their tracks. Precise Actin cytoskeleton organization and regulation between cell-cell contacts are conferred normal structure and foot process movement of podocytes [5,49]. The cortical Actin protein filaments are associated with many proteins as mentioned previously. Loss and gain podocyte’s function is attributed to modeling actin as it supports SD proteins [46]. Therefore, many studies showed phosphorylated Synaptopodin (SYNPO) as a characterization tool, which acts as a stabilizer of Actin cytoskeleton [9-11,15,18,20,21]. Actin network in stress fibers of podocytes is controlled by Rass homolog family member A (Rho-A) and calcium pathways [50]. Rho A protein maintain an optimal degree of podocyte motility and its reduction is associated with hypermotility. Recent advances in imaging by multiphoton and light sheet imaging showed that reduction in podocyte’s motility causes proteinuria in mice. It is produced when Rac1 and Cdc42, TRPC6 become inactive, and blockade of αvβ3 Integrin. While in disease condition hyperactivity of podocyte motility is associated with up-regulation of TRPC5, CatL in response to CD2AP deficiency. Phosphorylated SYNPO link to the Actin cytoskeleton and it binds to CD2AP and α Actinin-4, regulate Rho-A protein [5]. Up to date, there are no in vitro optimal motility functional assays available for the podocyte, which should have motility profiles as standard.
Role of micro RNA
miRNAs are single-stranded, non-coding RNAs molecules that negatively regulate or destabilized mRNAs via binding to its 3’-untranslated region. The roles of mi-RNAs have already been described for mechanisms like autophagy [51], apoptosis, proliferation, and differentiation [52]. Several mi-RNAs have been identified in physiologic and pathologic conditions of kidney [53]. miR-26a-5p levels were found to be lowered in lupus nephritis or IgA nephropathy [54,55]. FSGS can be induced by an up-regulation of miR-193a-5p, which downregulates WT1 [56]. However, limited studies have been conducted on the role of miRNAs in podocytes as a regulatory molecule in its differentiation. The miR-200 family has five members organized as two clusters, miRs-200a/b/429, and miRs-200c/141 abundantly found in the kidney and expressed in pronephros [57]. Initially, it was found to be involved in renal fibrosis and diabetic nephropathy [58]. Later miR-200 family found to promote podocyte differentiation. miR-200a, miR-200b, and miR-429 significantly up-regulated during podocyte differentiation with optimal expression of miR-200a. The miR-200 family directly inhibit radical S-adenosyl methionine domain-containing protein2 (RSAD2) also known as Viperin or Cig5, an anti-viral protein induced by interferon. The structural integrity of podocytes played a central role in maintaining the normal function of GFB [57]. miR-30a-5p and miR-193a-5p, maintain the phenotypic marker expression of podocytes [59,60]. miRNA act through several podocyte adapters and effector proteins, and linked to the Actin cytoskeleton, for example; miR-155-5p enhanced Nephrin acetylation, which attenuates renal damage in hyperglycemia-induced nephropathy [61]. miRNA-498 inhibition improves human hAD-MSCs differentiated into podocytes. These cells were used in two steps to induce podocyte, first in IM by the application of AA and high concentration of RA, and then by the low concentration of RA and BMP7. The functional characterization was analyzed by embryonic explant culture and Adriamycin-induced injury model that showed integration capacity and reduction in proteinuria respectively. This method has been summarized in Table 2 [20].
Excellence in in vitro differentiated cells
Quality measures are attributed to characterization techniques, the identification, and the use of specific gene expression compared to adult and developmental stages vs. in vitro remodeled cells. The major obstacles for cellular therapy in directed differentiation are a risk for teratogens and incomplete phenotypic resemblance of newly generated cells and its function. Incomplete characterization shows residual features of the originating population or has a non-homogenous population. The remainders of in vitro generated cells were not discussed in many published articles. After differentiation, a small number of pluripotent stem cells may produce heterogeneous cells. Therefore, the choice of original cell type is critical for in vitro differentiation. Complete native cell’s transcriptomic and epigenetic studies may further enhance the information to resolve this problem. Multiplex gene and protein data may provide efficient quality control for cell fate conversion towards podocyte.
The in vitro programming methods require attention towards nanoarchitecture of glomerular basement membrane (GBM) in 3D culture. Podocyte in healthy glomerular tissue exists with physiological substrate stiffness, i.e. native GBM of the capillary that provides mechanical support [62]. Freeze-fracture of GBM in the scanning electron microscope (SEM) shows its porous nanotopgraphy, which supports filtration mechanism [4]. This phenomenon should include in in vitro differentiation protocols of podocytes as it can effect changes in podocyte phenotype, maturation, and filtration function. Podocyte iconic gene WT1 expression was observed as a podocyte mechano responsive gene in presence of transglutaminase microbial gelatin (gelatin-mTG) in the hydrogel culturing system [62]. Various surfaces can be employed for in vitro generation of podocyte considering the geometrical nanoporous surface, specifically for podocytes mimicking the in vivo conditions. Zennaro et al., 2016 showed that porous surfaces allow cytoskeletal remodeling and formation of focal contacts of podocyte. Actin reorganization and microtubule assembly via microtubule-associated protein 2 (MAP2) and Tau stabilized podocyte structure [4]. Besides soluble cues from growth factor and cytokine-mechano-transduction mechanism can cause the change in gene expression, which ultimately direct differentiation. Allylamine (AA) and Octadiene (OD) (low AA) composed homo-and co-polymeric plasma coatings surfaces directed differentiation towards glomerular podocytes and proximal tubules by the appearance of WT1, Nephrin, and Megalin respectively [27].
Future endeavor
Studies are required in efforts to generate efficient podocyte progenitor or mature podocyte. Functional assays are of a great magnitude to observe the programmed cells. The more likely models should be designed in combination to generate podocyte with the capillary network or endothelial cells to closely observe the filtration’s mechanism. Secretion of growth factors like Notch 2 and VEGF-A, which affect capillary development as well as the differentiation of endothelial cells can be exercised as a characterization tool [63]. Purposive podocytes differentiation can be carried out such as α3ß1 Integrin expression as it is essential for the regulation of foot process assembly [12]. Insulin signaling is crucial for podocytes function. It is involved in the activation of AKT by Nephrin-dependent pro-survival cascade and the regulation of Actin cytoskeleton [2]. Advanced podocyte motility models in vitro similar to physiological conditions are lacking and required more attention not only to drive highly efficient podocytes but to observe novel pathology. Electrophysiological properties of podocyte should be monitored to control the contractile state of the foot process. Developing podocyte expressed Scribble, a protein that translocates from the lateral aspects of immature podocytes to the basal cell membrane and foot processes of mature podocytes [64]. This phenomenon can be observed in vitro in the progenitor or differentiated podocytes for validation experiments.
Concluding remarks
Highly efficient and homogeneous podocyte’s progenitor and mature podocyte generation is the first and crucial step for either in vivo direct cell replacement therapy or ex vivo functional kidney development as the final treatment strategy for CKD patients instead of RRT. The protocols for procuring podocyte are being improved by the usability of the signal targeted TFs, small molecules, growth factors, and inhibition strategies through miRNAs. Identification of a specific combination of TFs and miRNAs for in vitro differentiation of podocyte is the current need to explore a dynamic process for programming. Monitoring podocyte cytoskeletal re-organization facilitates phenotypic change and cell fate conversion. The combination of novel in vitro nanoarchitecture of GBM in 3D culture with podocyte motility analyzing methods would provide a new insight into the functional improvement of programmed differentiated podocyte from various cells sources.
All acronyms are mentioned in Supplementary Table 1.
Acknowledgements
This review article is supported by the grant from Sindh Institute of Urology and Transplantation (SIUT), Karachi-Pakistan.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.May CJ, Saleem M, Welsh GI. Podocyte dedifferentiation: a specialized process for a specialized cell. Front Endocrinol. 2014;5:148. doi: 10.3389/fendo.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiser J, Altintas MM. Podocytes. F1000Res. 2016:5. doi: 10.12688/f1000research.7255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang PY, He JC. Signaling in regulation of podocyte phenotypes. Nephron Physiol. 2009;111:9–15. doi: 10.1159/000191075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zennaro C, Rastaldi MP, Bakeine GJ, Delfino R, Tonon F, Farra R, Grassi G, Artero M, Tormen M, Carraro M. A nanoporous surface is essential for glomerular podocyte differentiation in three-dimensional culture. Int J Nanomedicine. 2016;11:4957. doi: 10.2147/IJN.S110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding WY, Saleem MA. Current concepts of the podocyte in nephrotic syndrome. Kidney Res Clini Pract. 2012;31:87–93. doi: 10.1016/j.krcp.2012.04.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminski MM, Tosic J, Pichler R, Arnold SJ, Lienkamp SS. Engineering kidney cells: reprogramming and directed differentiation to renal tissues. Cell Tissue Res. 2017;369:185–197. doi: 10.1007/s00441-017-2629-5. [DOI] [PubMed] [Google Scholar]
- 7.Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75:337–349. doi: 10.1111/j.1432-0436.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 8.Ren X, Zhang J, Gong X, Niu X, Zhang X, Chen P, Zhang X. Differentiation of murine embryonic stem cells toward renal lineages by conditioned medium from ureteric bud cells in vitro. Acta Biochim Biophys Sin. 2010;42:464–471. doi: 10.1093/abbs/gmq046. [DOI] [PubMed] [Google Scholar]
- 9.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2013;16:118–26. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 10.Kang M, Han YM. Differentiation of human pluripotent stem cells into nephron progenitor cells in a serum and feeder free system. PLoS One. 2014;9:e94888. doi: 10.1371/journal.pone.0094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 13.Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R. Human induced pluripotent stem cells-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol. 2016;27:1778–91. doi: 10.1681/ASN.2015010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imberti B, Tomasoni S, Ciampi O, Pezzotta A, Derosas M, Xinaris C, Rizzo P, Papadimou E, Novelli R, Benigni A. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci Rep. 2015;5:8826. doi: 10.1038/srep08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciampi O, Iacone R, Longaretti L, Benedetti V, Graf M, Magnone MC, Patsch C, Xinaris C, Remuzzi G, Benigni A. Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res. 2016;17:130–139. doi: 10.1016/j.scr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1:0069. doi: 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a glomerulus chip. Nat Protoc. 2018;13:1662–1685. doi: 10.1038/s41596-018-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunitha MM, Srikanth L, Kumar PS, Chandrasekhar C, Sarma PVGK. Down-regulation of PAX2 promotes in vitro differentiation of podocytes from human CD34+ cells. Cell Tissue Res. 2017;370:477–488. doi: 10.1007/s00441-017-2680-2. [DOI] [PubMed] [Google Scholar]
- 19.Tayyeb A, Shahzad N, Gibran A. Differentiation of mesenchymal stem cells towards nephrogenic lineage and their enhanced resistance to oxygen peroxide-induced oxidative stress. Iran J Kidney Dis. 2017;11:271. [PubMed] [Google Scholar]
- 20.Zhang L, Li K, Yan X, Liang X, Wang S, Han Q, Zhao RC. MicroRNA-498 Inhibition enhances the differentiation of human adipose-derived mesenchymal stem cells into podocyte-like cells. Stem Cells Dev. 2015;24:2841–2852. doi: 10.1089/scd.2015.0027. [DOI] [PubMed] [Google Scholar]
- 21.Fuente Mora C, Ranghini E, Bruno S, Bussolati B, Camussi G, Wilm B, Edgar D, Kenny SE, Murray P. Differentiation of podocyte and proximal tubule-like cells from a mouse kidney-derived stem cell line. Stem Cells Dev. 2012;21:296–307. doi: 10.1089/scd.2010.0470. [DOI] [PubMed] [Google Scholar]
- 22.Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, Little MH. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol. 2013;24:1424–1434. doi: 10.1681/ASN.2012121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon R, Otto EA, Kokoruda A, Zhou J, Zhang Z, Yoon E, Chen YC, Troyanskaya O, Spence JR, Kretzler M, Cebrian C. Single-cell analysis of progenitor cell dynamics and lineage specification in the human fetal kidney. Development. 2018;145 doi: 10.1242/dev.164038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang Z, Ji Z, Boeke JD, Ji H. Dynamic motif occupancy (DynaMO) analysis identifies transcription factors and their binding sites driving dynamic biological processes. Nucleic Acids Res. 2017;46:e2. doi: 10.1093/nar/gkx905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan A, Fornes O, Stigliani A, Gheorghe M, Castro-Mondragon JA, van der Lee R, Bessy A, Chèneby J, Kulkarni SR, Tan G, Baranasic D, Arenillas DJ, Sandelin A, Vandepoele K, Lenhard B, Ballester B, Wasserman WW, Parcy F, Mathellier A. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2018;46:D260–D266. doi: 10.1093/nar/gkx1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwood RI, Hashimoto T, O’Donnell CW, Lewis S, Barkal AA, Van Hoff JP, Karun V, Jaakkola T, Gifford DK. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat Biotechnol. 2014;32:171. doi: 10.1038/nbt.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacGregor-Ramiasa M, Hopp I, Bachhuka A, Murray P, Vasilev K. Surface nanotopography guides kidney-derived stem cell differentiation into podocytes. Acta Biomater. 2017;56:171–180. doi: 10.1016/j.actbio.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Tanaka H, Kuwana H, Inoshita S, Teraoka H, Sasaki S, Terada Y. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Commun. 2005;336:585–595. doi: 10.1016/j.bbrc.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Neph. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 30.Singaravelu K, Padanilam BJ. In vitro differentiation of MSC into cells with a renal tubular epithelial-like phenotype. Ren Fail. 2009;31:492–502. doi: 10.1080/08860220902928981. [DOI] [PubMed] [Google Scholar]
- 31.Guimaraes-Souza NK, Yamaleyeva LM, AbouShwareb T, Atala A, Yoo JJ. In vitro reconstitution of human kidney structures for renal cell therapy. Nephrol Dial Transplant. 2012;27:3082–3090. doi: 10.1093/ndt/gfr785. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan K, Schumacher KM, Tasnim F, Kandasamy K, Schumacher A, Ni M, Gao S, Gopalan B, Zink D, Ying JY. Human embryonic stem cells differentiate into functional renal proximal tubular like cells. Kidney Int. 2013;83:593–603. doi: 10.1038/ki.2012.442. [DOI] [PubMed] [Google Scholar]
- 33.Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–25. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadimou E, Morigi M, Iatropoulos P, Xinaris C, Tomasoni S, Benedetti V, Longaretti L, Rota C, Todeschini M, Rizzo P. Direct reprogramming of human bone marrow stromal cells into functional renal cells using cell-free extracts. Stem Cell Rep. 2015;4:685–698. doi: 10.1016/j.stemcr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopp JB. BMP receptors in kidney. Kidney Int. 2000;58:2237–2238. doi: 10.1111/j.1523-1755.2000.00402.x. [DOI] [PubMed] [Google Scholar]
- 36.Nishinakamura R, Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatr Nephrol. 2014;29:681–686. doi: 10.1007/s00467-013-2671-9. [DOI] [PubMed] [Google Scholar]
- 37.Ha TS. Roles of adaptor proteins in podocyte biology. World J Nephrol. 2013;2:1–10. doi: 10.5527/wjn.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Q, Ma Y, Liu Y, Liang W, Chen X, Ren Z, Wang H, Singhal PC, Ding G. Angiotensin II down-regulates nephrin-Akt signaling and induces podocyte injury: role of c-Abl. Mol Biol Cell. 2016;27:197–208. doi: 10.1091/mbc.E15-04-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bridgewater DJ, Ho J, Sauro V, Matsell DG. Insulin-like growth factors inhibit podocyte apoptosis through the PI3 kinase pathway. Kidney Int. 2005;67:1308–1314. doi: 10.1111/j.1523-1755.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 40.Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, Jain MK, Saleem M, D’Agati V, Klotman P. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem. 2012;287:19122–19135. doi: 10.1074/jbc.M112.345983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidson G, Dono R, Zeller R. FGF signalling is required for differentiation-induced cytoskeletal reorganisation and formation of actin-based processes by podocytes. J Cell Sci. 2001;114:3359–3366. doi: 10.1242/jcs.114.18.3359. [DOI] [PubMed] [Google Scholar]
- 43.Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2015;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Li W, Wen J, Yang Z. Autophagy is involved in mouse kidney development and podocyte differentiation regulated by Notch signalling. J Cell Mol Med. 2017;21:1315–1328. doi: 10.1111/jcmm.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller-Deile J, Schiffer M. Podocyte directed therapy of nephrotic syndrome-can we bring the inside out? Pediatr Nephrol. 2016;31:393–405. doi: 10.1007/s00467-015-3116-4. [DOI] [PubMed] [Google Scholar]
- 47.Flaquer M, Romagnani P, Cruzado JM. Growth factors and renal regeneration. Nefrologia. 2010;30:385–393. doi: 10.3265/Nefrologia.pre2010.Jun.10463. [DOI] [PubMed] [Google Scholar]
- 48.Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol. 2006;291:F422–F428. doi: 10.1152/ajprenal.00448.2005. [DOI] [PubMed] [Google Scholar]
- 49.Mathieson PW. Podocyte actin in health, disease and treatment. Nephrol Dial Transplant. 2010;25:1772–1773. doi: 10.1093/ndt/gfq121. [DOI] [PubMed] [Google Scholar]
- 50.Neal CR. Podocytes… What’s under yours? (Podocytes and foot processes and how they change in nephropathy) Front Endocrinol. 2015;6:9. doi: 10.3389/fendo.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frankel LB, Lund AH. MicroRNA regulation of autophagy. Carcinogenesis. 2012;33:2018–2025. doi: 10.1093/carcin/bgs266. [DOI] [PubMed] [Google Scholar]
- 52.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6:8474. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan PC, Chen CC, Chen YC, Chang YS, Chu PH. MicroRNAs in acute kidney injury. Hum Genomics. 2016;10:29. doi: 10.1186/s40246-016-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M, Toi M, Kon Y. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One. 2014;9:e110383. doi: 10.1371/journal.pone.0110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koga K, Yokoi H, Mori K, Kasahara M, Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, Toda N, Saleem MA, Sugawara A, Nakao K, Yanagita M, Mukoyama M. MicroRNA-26a inhibits TGF-β-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia. 2015;58:2169–2180. doi: 10.1007/s00125-015-3642-4. [DOI] [PubMed] [Google Scholar]
- 56.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med. 2013;19:481. doi: 10.1038/nm.3142. [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Yin H, Hao S, Wang L, Gao J, Tan X, Yang Z. miR-200 family promotes podocyte differentiation through repression of RSAD2. Sci Rep. 2016;6:27105. doi: 10.1038/srep27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srivastava SP, Koya D, Kanasaki K. MicroRNAs in kidney fibrosis and diabetic nephropathy: roles on EMT and EndMT. Biomed Res Int. 2013;2013:125469. doi: 10.1155/2013/125469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kietzmann L, Guhr SS, Meyer TN, Ni L, Sachs M, Panzer U, Stahl RA, Saleem MA, Kerjaschki D, Gebeshuber CA, Meyer-Schwesinger C. MicroRNA-193a regulates the transdifferentiation of human parietal epithelial cells toward a podocyte phenotype. J Am Soc Nephrol. 2015;26:1389–1401. doi: 10.1681/ASN.2014020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY, Chuang PC, Huang YT, Wang SY, Wu SL, Chen YS. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol. 2014;25:1698–1709. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu M, Azeloglu EU, Ron A, Tran-Ba KH, Calizo RC, Tavassoly I, Bhattacharya S, Jayaraman G, Chen Y, Rabinovich V. A biomimetic gelatin-based platform elicits a pro-differentiation effect on podocytes through mechanotransduction. Sci Rep. 2017;7:43934. doi: 10.1038/srep43934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreidberg JA. Podocyte differentiation and glomerulogenesis. J Am Soc Nephrol. 2003;14:806–814. doi: 10.1097/01.asn.0000054887.42550.14. [DOI] [PubMed] [Google Scholar]
- 64.Hartleben B, Widmeier E, Wanner N, Schmidts M, Kim ST, Schneider L, Mayer B, Kerjaschki D, Miner JH, Walz G. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One. 2012;7:e36705. doi: 10.1371/journal.pone.0036705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


