Abstract
Ovarian cancer (OC) is the leading cause of death from gynecological malignancy. Dual-specificity phosphatases (DUSPs) are proteins that are reported involved in carcinogenesis, but their roles in OC have not be extensively studied. Here, we found that DUSP5 is markedly down-regulated in OC tissues. We reanalyzed DUSP5 expression in OC using published microarray data from the Gene Expression Omnibus (GEO) database and found that patients with low DUSP5 expression have significantly shorter overall survival than those with high expression (P < 0.001). Down-regulation of DUSP5 in OC tissues was immunohistochemically confirmed in tissue microarrays containing 15 normal ovary tissue samples and 60 OC specimens. Functional studies suggest that DUSP5 silence facilitates cell proliferation, migration, and invasion of OC cells in vitro. DUSP5 over-expression inhibits cell proliferation but has no effect on OC cell migration or invasion. Mechanistically, silencing DUSP5 transcriptionally activates interleukin 33 (IL-33) expression and secretion. Blockage of IL-33 with a neutralizing anti-IL33 antibody attenuates the effect of DUSP5 silencing to promote cell proliferation, migration, and invasion. Moreover, recombinant IL-33 protein treatment dramatically promotes OC cell proliferation, migration, and invasion with DUSP5 over-expression. Our study provides proof of principle that DUSP5 down-regulation promotes proliferation, migration, and invasion of OC cells via activation of IL-33 signaling.
Keywords: Ovarian cancer, dual-specificity phosphatases 5 (DUSP5), interleukin 33 (IL-33), proliferation, migration, invasion
Introduction
Ovarian cancer (OC) is a major gynecologic malignancy; it is the sixth most prevalent cancer in the world, the fourth most common site of malignancy in females, and the seventh leading cause of cancer death in females [1]. OC is highly curable if caught early, but it is often asymptomatic in early stages. Approximately 80% patients with OC have a ~90% survival rate at early stages, but this decreases to 30% in those with stage III and IV disease [2,3]. Pelvic examination, ultrasonography, and CA125 detection are routine OC screening procedures, but their diagnostic values are limited. Although a variety of targeted drugs have been developed to treat OC, patient overall survival remains suboptimal [4]. It is therefore critical to identify the mechanisms of OC pathophysiology for use in diagnostic and clinical applications.
Dual-specificity phosphatase (DUSPs) dephosphorylate tyrosine and serine/threonine residues required to activate extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 mitogen-activated protein kinases (MAPKs) [5-7]. DUSPs are broadly classified into 3 groups based on their localization: group I (DUSPs 1, 2, 4 and 5) is nuclear, group II (DUSP 6, 7 and 9) is cytoplasmic, and group III (DUSPs 8, 10 and 16) exist in both the nucleus and cytoplasm. Functionally, individual DUSPs often prefer specific MAPK substrates: DUSPs 5, 6 and 7 are ERK selective, while DUSP9 prefers ERK over the p38 and JNK MAPKs [5]. DUSP5 is a nuclear ERK1/2-selective phosphatase induced by ERK signaling in mammalian cells [8,9]. Its expression is also stimulated by either heat shock or growth factor expression. Sustained inflammation caused by nuclear factor (NF)-κB activation in irradiated human arteries leads to DUSP5 over-expression [10]. Unlike other inducible mitogen-activated protein kinase phosphatases (MKPs) such as MKP-1/DUSP1, MKP-2/DUSP4 and PAC1/DUSP2, which interact with and inactivate both mitogen- and stress-activated MAPKs, DUSP5 selectively binds and inactivates ERK1 and ERK2 in vivo [8,9,11]. Furthermore, DUSP5 is a direct transcriptional target of the tumor suppressor p53 [12] and has tumor-suppressive functions in several types of cancer [7,8,10,13-16]. DUSP5 deficiency in the mouse epidermis increases H-Ras-driven papilloma formation in a carcinogen-induced model of skin cancer [8]. Consistent with this putative tumor suppressor role, DUSP5 levels are down-regulated in prostate and gastric cancers, where loss of its expression is associated with poor prognosis [14,15]. In gastric cancer, DUSP5 down-regulation correlates with promoter CpG island methylation, and its over-expression in gastric cancer cell lines reduced nuclear p-ERK levels and cell growth [15]. Low DUSP5 expression was also recently reported in colorectal cancer and was associated with worse outcome [10,16]. However, the role of DUSP5 in OC initiation and/or progression has not been reported.
In this study, we measured DUSP5 expression in clinical samples and investigated its effects on OC cell proliferation, invasion, and migration in vitro. Our findings demonstrate that DUSP5 is up-regulated in OC and exerts suppressive effects on tumor progression. Furthermore, DUSP5 knockdown transcriptionally elevated interleukin-33 (IL-33) expression and secretion, and altering IL-33 levels affected OC cell proliferation, invasion, and migration. The collective evidence suggests that DUSP5 suppresses OC by inhibiting IL-33 signaling and could serve as a target for developing new therapeutic strategies.
Method and materials
Bioinformatics analyses
DUSP5 (NM_004419.3) expression patterns were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE49997.
Patients and tissue samples
For real-time polymerase chain reaction (RT-PCR) analysis, OC and normal adjacent specimens were collected from 15 patients who underwent resections between 2014 and 2017 at the First Affiliated Hospital of Soochow University. Samples were frozen in liquid nitrogen and kept at -80°C until use. This study was approved by the Ethical Committee of the First Affiliated Hospital of Soochow University. Written informed consent was provided by each participant prior to surgery.
The OC tissue-array HOva-Can090PT-01 was from Shanghai Outdo Biotech Co., Ltd. (Shanghai, China) was used for tissue-array assays. We processed samples from 75 OC patients (aged 17 to 86 years old, median age of 53). Finally, a total of 75 cases were included in the H-Score statistical analysis.
Cell lines and cell culture
Human OC cell lines SK-OV-3 and Caov-3 were obtained from the Chinese Academy of Sciences at the Shanghai Institutes for Biological Sciences. Both lines were maintained in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS) under standard culture conditions (5% CO2, 37°C).
Plasmids, siRNAs, and cell transfection
Human DUSP5 over-expressing vector (pLvx-DUSP5) and the control plasmid (pLVX-IRES-GFP) were purchased from Genelily Biotechnology Company (Shanghai, China). Small interference RNAs (siRNAs) specific to human DUSP5 were generated by GenePharma (Shanghai, China) to transiently silence expression. The transfection experiment was divided into the following treatments: si-NC (siRNA NC vector) si-DUSP5 (si-DUSP5 vector), oe-NC (over-expression NC vector) and oe-DUSP5 (DUSP5 over-expression vector). The above vectors were transfected with Lipofectamine® 3000 transfection reagent as previously reported. Forty-eight hours after transfection, the transfection efficiency of cells in each group was detected by reverse transcription quantitative PCR (RT-qPCR) and western blot analyses.
RT-PCR
RT-PCR was performed to measure the mRNA levels of DUSP5 and IL-33 in OC tissue or cells as described previously [17]. Briefly, total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) reagent, then purified RNA was reversely transcribed into cDNA using the RT reaction kit (Toyobo, Osaka, Japan). RT-PCR was conducted using the ABI vii7 system (Applied Biosystems, Foster City, CA, USA), and SYBR Green was used as a DNA-specific fluorescent dye. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as a housekeeping gene. Primers were synthesized as follows: human DUSP5 forward primer, 5’-GTGCCTACTGCACATTCCC-3’ and reverse primer, 5’-TCCCGAGAACCTACCCTGAG-3’; human IL-33 forward primer, 5’-GCTTCCCAAGAAAGGCATCG-3’ and reverse primer, 5’-TCTAGTCCCCAGTCATCGCA-3’; GAPDH forward, 5’-TGACTTCAACAGCGACACCCA-3’ and reverse: 5’-CACCCTGTTGCTGTAGCCAAA-3’. Relative gene expression was measured by the comparative CT method (ΔΔCT) where the fold enrichment was calculated as: 2-(ΔCT [sample]-ΔCT [calibrator]).
Western blot
Cells were collected, and proteins were extracted. Equal amounts of proteins from each sample were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blot analysis was performed according to established method with antibodies against DUSP5 and IL-33 (Abcam, Cambridge, UK). GAPDH was used as a loading control.
Cell viability assay
Cell growth was monitored with Cell-Counting Kit-8 (CCK8) assays (Dojindo Kumamoto, Japan) as previously reported [18]. Briefly, cells at a dilution of 5 × 103 per well were incubated in 96-well plates for 0, 24, 48, or 72 h, and CCK-8 was added into the well according to the manufacturer’s instructions. After 4 h of CCK-8 incubation, cell viability was measured by reading the optical density at a wavelength of 450 nm. All experiments were performed in triplicate.
Wound healing assay
Cell migration was assessed using wound scrape assays as previously reported [19]. Briefly, cells (5 × 104 per well) were maintained in 6-well plates. A small wound area was created using a 200-µL pipette tip when cells reached 90% confluence. Cells were then washed twice with phosphate-buffered saline and further incubated in serum-free DMEM at 37°C in a 5% CO2 incubator for 24 h. Photographs were acquired at the indicated times, and wound width was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Ten measurements were made at random intervals along the wound length, and data from three independent experiments were averaged and expressed as a percentage of the original width.
Invasion assay
Cells were plated into the upper Transwell chamber with 8-µm pores (BD Falcon, Franklin Lakes, NJ, USA) at a density of 5 × 104 per well. Chambers with Matrigel matrix for cell migration or invasion assays were inserted into 24-well plates containing 20% FBS. Cells in the chamber were cultivated without FBS. After 24 h, migrated or invasive cells adhering to the lower chamber surface were fixed and stained with 5% crystal violet. Cells were counted in five 20 × microscope fields per well.
Enzyme-linked immunosorbent assays (ELISAs)
The secreted human IL-33 was monitored by ELISA assays using human IL-33 ELISA kit (Proteintech, Chicago, IL, USA) as previously reported [13].
Promoter activity assay
pGL3-Luciferase Reporter assays (Promega, Madison, WI, USA) were performed according to the manufacturer’s instructions to analyze IL-33 promoter activity. Briefly, the pGL3 luciferase reporter plasmid containing the promoter fragment of the IL-33 gene from nucleotides -2,500 to +50 was generated by Genelily. Prior to transfection, SK-OV-3 cells were cultured in 24-well plates for 24 h. Luciferase Assay Reagent II (LAR II) and Stop & Glo Reagent were added sequentially 48 h after transfection, and dual luciferase activities were measured.
Statistical analysis
Statistical analyses were completed using SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). Survival analysis was carried out using the Kaplan-Meier method, and log-rank tests were used to compare survival curves. Other data were assessed with two-tailed Student’s t-tests in the Prism 5.0 software package (GraphPad Software, Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
DUSP5 is down-regulated in OC tissues
We first examined DUSP5 expression in OC tissues and normal adjacent tissues and found that it was markedly down-regulated in cancerous tissues (Figure 1A). To further explore the relationship between DUSP5 levels and clinical outcomes, Kaplan-Meier survival analysis was performed using GEO dataset GSE8671. Patients with low DUSP5 expression had shorter overall survival (Figure 1B). We next immunohistochemically measured DUSP5 protein levels in human OC and normal adjacent tissues using a tissue microarray containing 60 OC cases and 15 normal tissue samples (Figure 1C). To objectively describe DUSP5 expression, the degree of immunohistochemical staining was quantified using the H score method (Figure 1C). All 15 normal tissue samples were positive for DUSP5 with a median H score of 79.5. Among the 60 OC samples, 42 samples showed weak or undetectable DUSP5 staining with < 5, 17 samples had modest staining with an H score between 5 and 30, and 1 sample had comparable staining to normal tissue. These results clearly indicate that DUSP5 expression is down-regulated in OC tissues.
Figure 1.
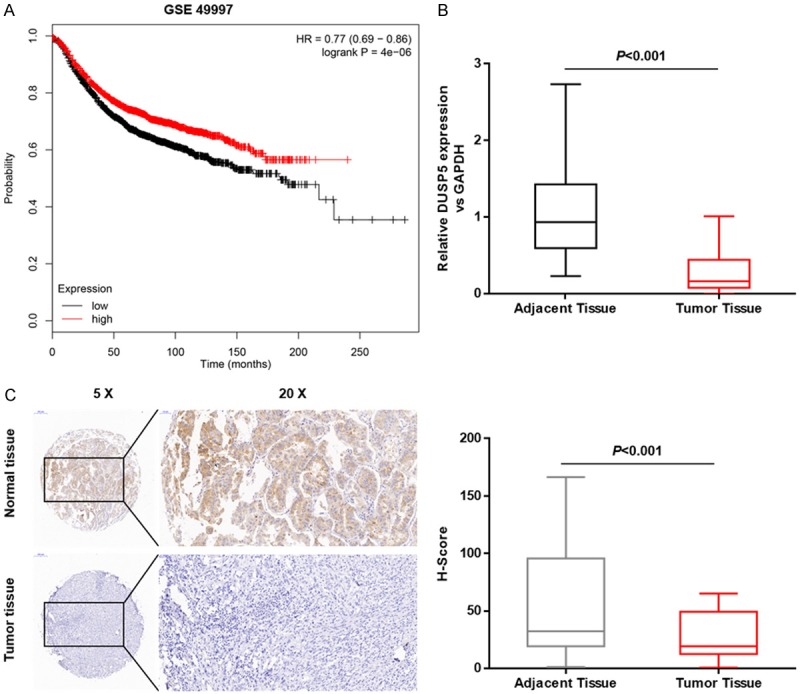
DUSP5 expression is down-regulated in OC tissues. A. Kaplan-Meier survival analysis of the association between RNF183 expression and overall survival in 194 patients. B. Relative DUSP5 mRNA levels were evaluated by real-time-PCR in 15 paired human OC tissues and adjacent normal tissues (control). C. An immunohistochemical tissue array was used to detect DUSP5 expression in human OC tissues and adjacent normal tissues (control). Positive DUSP5 staining was mainly observed in normal tissues. H-scores were used to analyze DUSP5 levels in 60 cases of OC and 15 noncancerous tissue samples. Data are presented as mean ± SEM, ***, P < 0.001.
DUSP5 suppresses OC cell proliferation, migration, and invasion ability
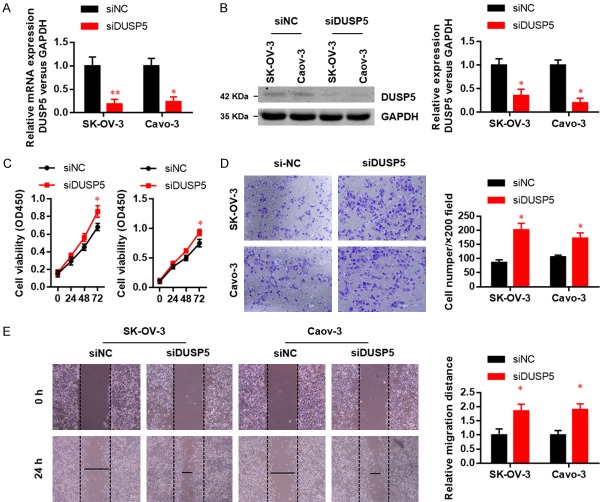
Unlimited cell proliferation, migration, and invasiveness are hallmarks of tumor malignancy. We therefore explored the role of DUSP5 in OC progression using gain- and loss-of-function approaches. We silenced DUSP5 expression in SK-OV-3 and Caov3 cells and confirmed the knockdown efficiency by real-time PCR (Figure 2A) and western blot (Figure 2B). DUSP5 knockdown accelerated SK-OV-3 and Caov3 cell proliferation (Figure 2C). Subsequently, we examined the function of DUSP5 silence on OC cell motility. In wound healing assays and invasion assays, DUSP5 knockdown significantly promoted the migration (Figure 2D) and invasion (Figure 2E) abilities of both SK-OV-3 and Caov3 cells.
Figure 2.
Silenced of DUSP5 promotes the proliferation, migration and invasion ability in OC cells. DUSP5 knockdown efficiencies in two OC cell lines were examined by real-time PCR (A) and western blots (B). (C) Effects of DUSP5 silencing on SK-OV-3 and Caov-3 cell proliferation were monitored with CCK8 assays. (D) Effects of DUSP5 silencing on SK-OV-3 and Caov-3 cell migration were assessed using wound healing assays. (E) Effects of DUSP5 silencing on SK-OV-3 and Caov-3 cell invasion were monitored by Transwell invasion assays. Data are presented as mean ± SEM, *, P < 0.05, **, P < 0.01.
We next investigated whether DUSP5 over-expression affects cell proliferation, migration, or invasiveness. Over-expression efficiency was confirmed by real-time PCR (Figure 3A) and western blot (Figure 3B). As expected, DUSP5 over-expression impaired the proliferation of both cell lines in CCK8 assays (Figure 3C). However, DUSP5 over-expression did not affect migration (Figure 3D) or invasion (Figure 3E) in either cell line. Collectively, these data suggest that DUSP5 suppresses OC cell proliferation, migration, and invasion.
Figure 3.
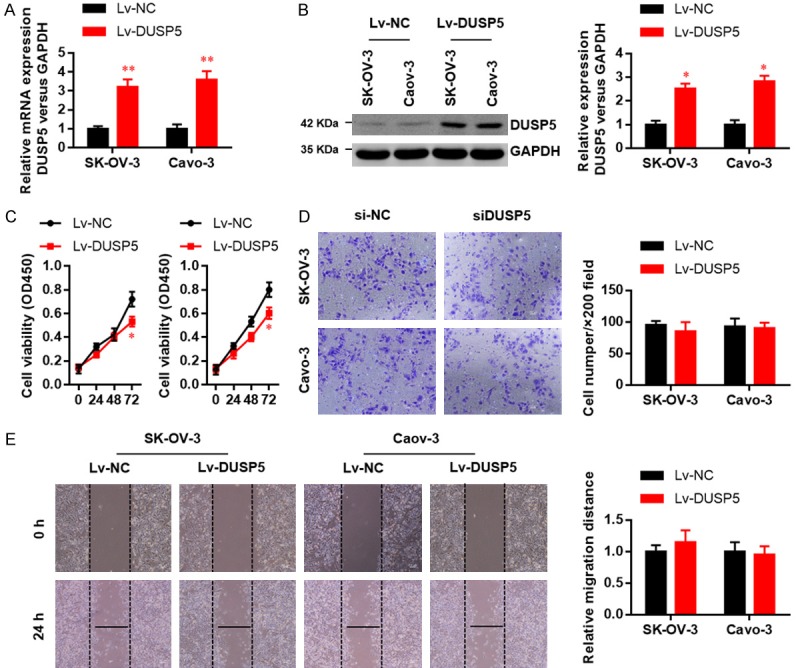
DUSP5 over-expression suppresses the proliferation, migration and invasion ability in OC cells. DUSP5 over-expression efficiencies in two OC cell lines were examined by real-time PCR (A) and western blots (B). (C) Effects of DUSP5 over-expression on SK-OV-3 and Caov-3 cell proliferation were monitored with CCK8 assays. (D) Effects of DUSP5 over-expression on SK-OV-3 and Caov-3 cell migration were assessed using wound healing assays. (E) Effects of DUSP5 over-expression on SK-OV-3 and Caov-3 cell invasion were monitored by Transwell invasion assays. Data are presented as mean ± SEM, *, P < 0.05, **, P < 0.01.
DUSP5 down-regulation transcriptionally enhances IL-33 expression and secretion in OC cells
The suppressive functions of DUSP5 in OC cell growth, migration, and invasion suggest that it has a regulatory role in oncogenesis. DUSP5 is reportedly a novel negative regulator of IL-33-dependent eosinophil function and survival [20]. Importantly, IL-33 was reported to predict poor prognosis and promote OC cell growth and metastasis [21]. We therefore performed real-time PCR to evaluate the influence of DUSP5 on IL-33 expression and secretion. DUSP5 knockdown increased IL-33 transcription (Figure 4A). ELISA (Figure 4B) results suggested that DUSP5 knockdown markedly increased IL-33 secretion in the culture medium of SK-OV-3 and Caov3 cells. Western blot (Figure 4C) results further confirmed that DUSP5 knockdown markedly increased the level of pro-IL-33 and mature IL-33 in SK-OV-3 and Caov3 cells. To elucidate whether IL-33 is transcriptionally induced following DUSP5 down-regulation, we performed luciferase assays using a wild-type IL-33 promoter (pGL3-2.5k-luc). As shown in Figure 4D, DUSP5 silencing activated IL-33-Luc but not the vector control group, suggesting that DUSP5 transcriptionally regulates IL-33 expression.
Figure 4.
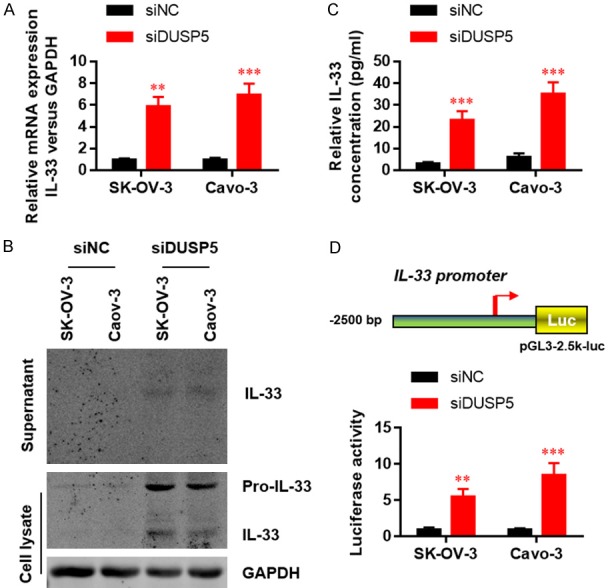
IL-33 expression and secretion are elevated in DUSP5 silenced OC cells. (A) Relative IL-33 mRNA levels were evaluated by real-time-PCR in OC cells with or without DUSP5 silencing. IL-33 protein levels in culture medium were evaluated by western blot (B) and ELISA (C). (D) IL-33 promoter activity was determined by dual-luciferase assays. Data are presented as mean ± SEM; *, P < 0.05, **, P < 0.01, ***, P < 0.001.
The anti-oncogenic function of DUSP5 is dependent on IL-33 signaling
Based on the above results, we decided to determine whether targeting IL-33 signaling could attenuate the anti-oncogenic function of DUSP5. SK-OV-3 and Caov3 cells were treated with IL-33 neutralizing antibody (anti IL-33), which inhibited the effects of DUSP5 silencing on cell proliferation (Figure 5A), migration (Figure 5B), and invasion (Figure 5C). Conversely, treatment with recombinant human IL-33 protein rescued cell proliferation inhibition induced by DUSP5 over-expression (Figure 6A). Moreover, migration (Figure 6B), and invasion (Figure 6C) abilities were further enhanced by adding recombinant IL-33 protein to the culture medium of SK-OV-3 and Caov3 cells with or without DUSP5 over-expression, suggesting an oncogenic role of IL-33. Taken together, these findings suggest that the anti-oncogenic function of DUSP5 is largely dependent on transcriptional inhibition of IL-33 expression.
Figure 5.
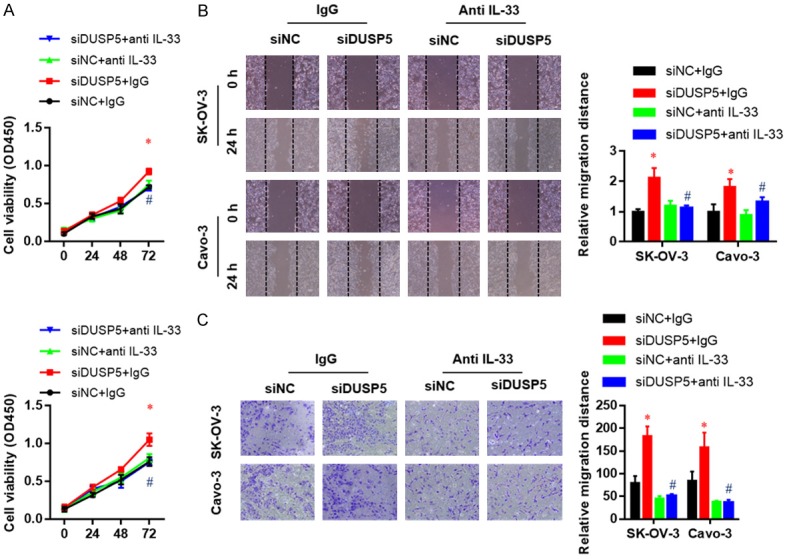
IL-33 neutralizing abrogates the effect of DUSP5 silence on the proliferation, migration, and invasion ability of SK-OV-3 and Caov-3 cells. Effects of an IL-33 neutralizing antibody on the proliferation (A), migration (B), and invasion (C) ability of DUSP5-silenced SK-OV-3 and Caov-3 cells as measured by CCK8, wound healing, and Transwell invasion assays, respectively. Data are presented as mean ± SEM; *, P < 0.05 versus corresponding si-NC group, #, P < 0.05, ##, P < 0.01 versus corresponding IgG group.
Figure 6.
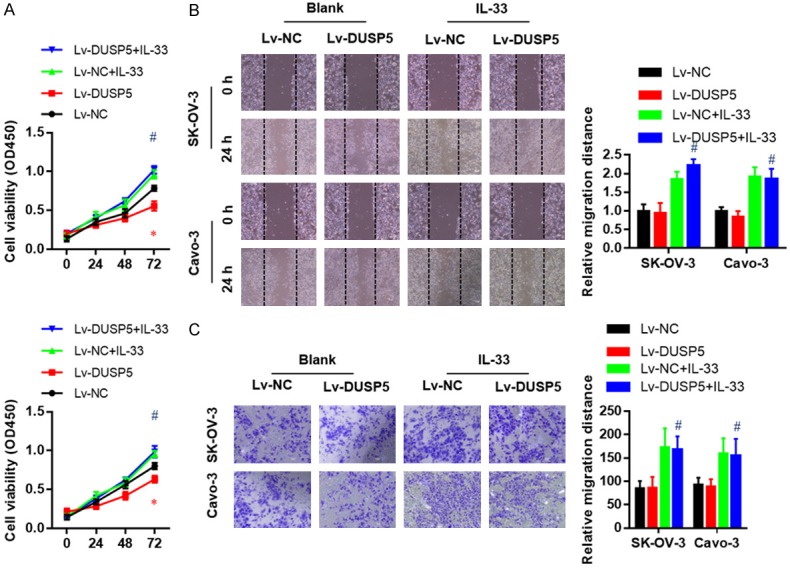
Exogenous IL-33 treatment extremely promoted the proliferation, migration, and invasion ability of both wildtype and DUSP5 over-expressed SK-OV-3 and Caov-3 cells. Effects of exogenous IL-33 treatment on the proliferation (A), migration (B), and invasion (C) ability of DUSP5 over-expressed SK-OV-3 and Caov-3 cells as measured by CCK8, wound healing, and Transwell invasion assays, respectively. Data are presented as mean ± SEM; *, P < 0.05 versus corresponding Lv-NC group, #, P < 0.05, ##, P < 0.01 versus corresponding blank group.
Discussion
Ras/ERK signaling is implicated in the development and progression of most human cancers [22]. ERK activation also increases the expression of MKPs that inactivate ERK [23]. Given that DUSP5 is unique in its targeting of classical ERK1/2, it acts as a negative feedback regulator of nuclear Ras/ERK signaling [8,9]. Although DUSP5 has tumor-suppressive functions in several types of cancer, its role in OC remains unclear. In this study, we initially demonstrated that the DUSP5 is a potent tumor suppressor in OC. We provided evidence that DUSP5 is aberrantly down-regulated in OC tissues. Patients with low DUSP5 expression have shorter overall survival, indicating that DUSP5 could serve as an independent prognostic factor in OC. DUSP5 silence promotes proliferation, migration and invasion of OC cells, whereas over-expression increased proliferation, indicating that DUSP5 functions as a tumor suppressor in the pathogenesis of OC. We uncovered a key function of DUSP5 in suppressing IL-33 expression and secretion, and these actions are likely responsible for tumor suppression.
IL-33 is a member of the IL-1 family of cytokines that is released by necrotic epithelial cells or activated innate immune cells [24,25]. This cytokine plays important roles in immune-mediated conditions such as infection, allergy, and autoimmune diseases [26,27]. Recent studies revealed its potential function in modulating anti-tumor immunity and tumor growth in different organs [28-31]. A previous study indicated that up-regulation of IL-33 expression in epithelial OC (EOC) leads to tumor development and progression [13]. Furthermore, the IL-33/ST2 axis is closely associated with poor prognosis in EOC patients, and it promotes OC growth and metastasis by regulating ERK and JNK signaling [20]. These results further suggest crosstalk between DUSP5S/ERK and IL-33/ST2 signaling. In this study, we found that DUSP5 transcriptionally inhibits IL-33 expression. We also confirmed that DUSP5 decreases IL-33 levels in the culture medium of OC cells. In accordance with previous reports that IL-33 contributes to each step of progression including proliferation, migration, and invasion, we found that IL-33 neutralization blocks DUSP5’s tumor-suppressive activity. Moreover, recombinant IL-33 protein treatment dramatically enhances OC cell proliferation, migration, and invasion ability with or without DUSP5 over-expression, suggesting an oncogenic role of IL-33.
In summary, our results indicate that DUSP5 down-regulation contributes to malignancy by activating IL-33 expression and secretion in OC cells. Enhancing DUSP5 expression could be an effective treatment for patients with OC with elevated IL-33 levels.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant number: 81772773, 81672560, 81302275), Major scientific and technological project of Changzhou municipal health and Family Planning Commission (grant number: ZD201716), Science and Technology Development Project of Nanjing Medical University (grant number: 2017NJMUZD043), and Natural Sciences Project of Zhejiang Education Department (grant number: LY19H160044).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81:17–38. doi: 10.1007/s00280-017-3501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527:S217. doi: 10.1038/527S217a. [DOI] [PubMed] [Google Scholar]
- 4.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ, Kassahn KS, Newell F, Quinn MC, Kazakoff S, Quek K, Wilhelm-Benartzi C, Curry E, Leong HS Australian Ovarian Cancer Study Group. Hamilton A, Mileshkin L, Au-Yeung G, Kennedy C, Hung J, Chiew YE, Harnett P, Friedlander M, Quinn M, Pyman J, Cordner S, O’Brien P, Leditschke J, Young G, Strachan K, Waring P, Azar W, Mitchell C, Traficante N, Hendley J, Thorne H, Shackleton M, Miller DK, Arnau GM, Tothill RW, Holloway TP, Semple T, Harliwong I, Nourse C, Nourbakhsh E, Manning S, Idrisoglu S, Bruxner TJ, Christ AN, Poudel B, Holmes O, Anderson M, Leonard C, Lonie A, Hall N, Wood S, Taylor DF, Xu Q, Fink JL, Waddell N, Drapkin R, Stronach E, Gabra H, Brown R, Jewell A, Nagaraj SH, Markham E, Wilson PJ, Ellul J, McNally O, Doyle MA, Vedururu R, Stewart C, Lengyel E, Pearson JV, Waddell N, deFazio A, Grimmond SM, Bowtell DD. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 5.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 6.Buiga P, Elson A, Tabernero L, Schwartz JM. Regulation of dual specificity phosphatases in breast cancer during initial treatment with Herceptin: a Boolean model analysis. BMC Syst Biol. 2018;12:11. doi: 10.1186/s12918-018-0534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu J, Lee DH. Dual-specificity phosphatase 18 modulates the SUMOylation and aggregation of Ataxin-1. Biochem Biophys Res Commun. 2018;502:389–396. doi: 10.1016/j.bbrc.2018.05.178. [DOI] [PubMed] [Google Scholar]
- 8.Rushworth LK, Kidger AM, Delavaine L, Stewart G, van Schelven S, Davidson J, Bryant CJ, Caddye E, East P, Caunt CJ, Keyse SM. Dual-specificity phosphatase 5 regulates nuclear ERK activity and suppresses skin cancer by inhibiting mutant Harvey-Ras (HRasQ61L)-driven SerpinB2 expression. Proc Natl Acad Sci U S A. 2014;111:18267–18272. doi: 10.1073/pnas.1420159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidger AM, Rushworth LK, Stellzig J, Davidson J, Bryant CJ, Bayley C, Caddye E, Rogers T, Keyse SM, Caunt CJ. Dual-specificity phosphatase 5 controls the localized inhibition, propagation, and transforming potential of ERK signaling. Proc Natl Acad Sci U S A. 2017;114:E317–E326. doi: 10.1073/pnas.1614684114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Togel L, Nightingale R, Wu R, Chueh AC, Al-Obaidi S, Luk I, Davalos-Salas M, Chionh F, Murone C, Buchanan DD, Chatterton Z, Sieber OM, Arango D, Tebbutt NC, Williams D, Dhillon AS, Mariadason JM. DUSP5 is methylated in CIMP-high colorectal cancer but is not a major regulator of intestinal cell proliferation and tumorigenesis. Sci Rep. 2018;8:1767. doi: 10.1038/s41598-018-20176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutty RG, Talipov MR, Bongard RD, Lipinski RAJ, Sweeney NL, Sem DS, Rathore R, Ramchandran R. Dual specificity phosphatase 5-substrate interaction: a mechanistic perspective. Compr Physiol. 2017;7:1449–1461. doi: 10.1002/cphy.c170007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Zheng H, Mu W, He Z, Yang B, Ji Y, Hui L. DUSP16 ablation arrests the cell cycle and induces cellular senescence. FEBS J. 2015;282:4580–4594. doi: 10.1111/febs.13518. [DOI] [PubMed] [Google Scholar]
- 13.Saied EM, El-Etreby NM. The role and prognostic value of inducible nitric oxide synthase (iNOS) and interleukin-33 (IL-33) in serous and mucinous epithelial ovarian tumours. Ann Diagn Pathol. 2017;27:62–68. doi: 10.1016/j.anndiagpath.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Cai C, Chen JY, Han ZD, He HC, Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, Zou J, Liang YX, Dai QS, Jiang FN, Zhong WD. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int J Clin Exp Med. 2015;8:4186–4194. [PMC free article] [PubMed] [Google Scholar]
- 15.Shin SH, Park SY, Kang GH. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am J Pathol. 2013;182:1275–1285. doi: 10.1016/j.ajpath.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Yan X, Liu L, Li H, Huang L, Yin M, Pan C, Qin H, Jin Z. Dual specificity phosphatase 5 is a novel prognostic indicator for patients with advanced colorectal cancer. Am J Cancer Res. 2016;6:2323–2333. [PMC free article] [PubMed] [Google Scholar]
- 17.Geng R, Tan X, Wu J, Pan Z, Yi M, Shi W, Liu R, Yao C, Wang G, Lin J, Qiu L, Huang W, Chen S. RNF183 promotes proliferation and metastasis of colorectal cancer cells via activation of NF-kappaB-IL-8 axis. Cell Death Dis. 2017;8:e2994. doi: 10.1038/cddis.2017.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Yang B, She Y, Ye Y. The lncRNA TP73-AS1 promotes ovarian cancer cell proliferation and metastasis via modulation of MMP2 and MMP9. J Cell Biochem. 2018;119:7790–7799. doi: 10.1002/jcb.27158. [DOI] [PubMed] [Google Scholar]
- 19.Eoh KJ, Lee SH, Kim HJ, Lee JY, Kim S, Kim SW, Kim YT, Nam EJ. MicroRNA-630 inhibitor sensitizes chemoresistant ovarian cancer to chemotherapy by enhancing apoptosis. Biochem Biophys Res Commun. 2018;497:513–520. doi: 10.1016/j.bbrc.2018.02.062. [DOI] [PubMed] [Google Scholar]
- 20.Holmes DA, Yeh JH, Yan D, Xu M, Chan AC. Dusp5 negatively regulates IL-33-mediated eosinophil survival and function. EMBO J. 2015;34:218–235. doi: 10.15252/embj.201489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong X, Barbour M, Hou K, Gao C, Cao S, Zheng J, Zhao Y, Mu R, Jiang HR. Interleukin-33 predicts poor prognosis and promotes ovarian cancer cell growth and metastasis through regulating ERK and JNK signaling pathways. Mol Oncol. 2016;10:113–125. doi: 10.1016/j.molonc.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TH, Yoo JY, Kim HI, Gilbert J, Ku BJ, Li J, Mills GB, Broaddus RR, Lydon JP, Lim JM, Yoon HG, Jeong JW. Mig-6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer Res. 2014;74:7371–7382. doi: 10.1158/0008-5472.CAN-14-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67:11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- 24.Dalmas E, Lehmann FM, Dror E, Wueest S, Thienel C, Borsigova M, Stawiski M, Traunecker E, Lucchini FC, Dapito DH, Kallert SM, Guigas B, Pattou F, Kerr-Conte J, Maechler P, Girard JP, Konrad D, Wolfrum C, Boni-Schnetzler M, Finke D, Donath MY. Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity. 2017;47:928–942. e927. doi: 10.1016/j.immuni.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 26.Ndaw VS, Abebayehu D, Spence AJ, Paez PA, Kolawole EM, Taruselli MT, Caslin HL, Chumanevich AP, Paranjape A, Baker B, Barnstein BO, Haque TT, Kiwanuka KN, Oskeritzian CA, Ryan JJ. TGF-beta1 suppresses IL-33-induced mast cell function. J Immunol. 2017;199:866–873. doi: 10.4049/jimmunol.1601983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto SM, Subbannayya Y, Rex DAB, Raju R, Chatterjee O, Advani J, Radhakrishnan A, Keshava Prasad TS, Wani MR, Pandey A. A network map of IL-33 signaling pathway. J Cell Commun Signal. 2018;12:615–624. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akimoto M, Takenaga K. Role of the IL-33/ST2L axis in colorectal cancer progression. Cell Immunol. 2018 doi: 10.1016/j.cellimm.2017.12.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Eissmann MF, Dijkstra C, Wouters MA, Baloyan D, Mouradov D, Nguyen PM, Davalos-Salas M, Putoczki TL, Sieber OM, Mariadason JM, Ernst M, Masson F. Interleukin 33 signaling restrains sporadic colon cancer in an interferon-gamma-dependent manner. Cancer Immunol Res. 2018;6:409–421. doi: 10.1158/2326-6066.CIR-17-0218. [DOI] [PubMed] [Google Scholar]
- 30.Wu CW, Wu YG, Cheng C, Hong ZD, Shi ZM, Lin SQ, Li J, He XY, Zhu AY. Interleukin-33 predicts poor prognosis and promotes renal cell carcinoma cell growth through its receptor ST2 and the JNK signaling pathway. Cell Physiol Biochem. 2018;47:191–200. doi: 10.1159/000489766. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y, Xu L, Ramadan M, Brown J, Turnquist HR, Billiar TR. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: a reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. doi: 10.1371/journal.pmed.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]