Abstract
The S-phase kinase associated protein 2 (Skp2), a member of the F-box protein family, regulates cell cycle progression and is highly expressed in pancreatic cancer (PC). Recently, we reported that arsenic trioxide (ATO) inhibited cell growth and invasion via downregulation of Skp2 in PC cells. Emerging evidence has revealed that Skp2 plays a crucial role in drug resistance in several kinds of cancers. Here, we determined whether ATO enhanced the sensitivity of PC cell lines to gemcitabine (GEM). We found that the combined treatment of ATO and GEM demonstrated strong antitumor effects in Patu8988 and Panc-1 PC cells. In addition, ATO potentiated the effects of GEM via downregulation of the Skp2 pathway in PC cells. Together, these findings suggested that Skp2 may be a promising therapeutic target to overcome resistance to GEM in PC.
Keywords: Arsenic trioxide, Skp2, drug resistance, gemcitabine, pancreatic cancer
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer-related death in the United States [1], and the morbidity and mortality of PC have also gradually increased in China in recent years [2]. The 5-year survival rate of patients with PC has remained relatively constant [3]. The poor prognosis results from delayed diagnosis, early metastasis, and resistance to many key chemotherapeutic agents [4]. The most common chemotherapeutic agent used to treat PC is gemcitabine (GEM), either in monotherapy or in combination with other chemotherapeutic agents [5]. Although the median survival of PC patients has slightly improved, the efficacy is still limited, and new therapies are urgently needed.
The F-box protein, S-phase kinase associated protein 2 (Skp2), is highly expressed and regulates cell cycle progression in PC [6]. It has been well-documented that Skp2 exerts its oncogenic effects through degradation of its ubiquitination targets, such as p21 [7], p27 [8], p57 [9], FOXO1 [10], and E-cadherin [11]. Consistent with these observations, Skp2 plays a key oncogenic role in cell growth, apoptosis, cell cycle progression, migration, invasion, metastasis, and angiogenesis [12]. Moreover, Skp2 is involved in drug resistance in several types of cancers [13-15].
Previous studies have shown that arsenic trioxide (ATO) has significant antitumor effects on several types of solid tumors [16-18]. ATO blocks the Skp2 signaling pathway in PC [19]. We hypothesized that ATO inhibited Skp2 expression to increase PC cell sensitivity to GEM. In the present study, we therefore investigated whether ATO and GEM have a synergistic anti-tumor effect in PC cells.
Materials and methods
Cell culture and experimental reagents
Human PC cell lines, Patu8988 and Panc-1, were obtained from the Chinese Academy of Sciences Cell Bank and were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 units/mL penicillin in standard cell culture conditions containing 5% CO2 at 37°C in a humidified atmosphere. Antibodies against Skp2, P57, P21, tubulin, and the secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ATO and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). ATO was dissolved in 1 mm NaOH to make a 1 mm stock solution, which was added directly to the media at different concentrations.
MTT assay
The Patu8988 and Panc-1 cells were seeded into 96-well culture plates for overnight incubation. The cells were then treated with either 3 µM ATO or 20 µM GEM, or a combination of both drugs for 48 h. At the end of the treatment period, 10 μL of MTT reagent [5 mg/mL in phosphate-buffered saline (PBS)] was added to each well. After 2 h of incubation, 100 µL of dimethyl sulfoxide was added to each well and further incubated for 10 min in the dark. The color intensity was measured using a SpectraMax M5 microplate fluorometer (Molecular Device, San Jose, CA, USA) at 490 nm.
The annexin V-fluorescein isothiocyanate (FITC) method for apoptosis analysis
An annexin V-FITC apoptosis detection kit (Biouniqure, China) was used to measure apoptotic cells. PC cells were treated with either 3 µM ATO or 20 µM GEM, or a combination of both drugs for 48 h. The cells were then collected via centrifugation and suspended in 500 μL of binding buffer. Then, 5 μL of annexin V-FITC and 5 μL of propidium iodide (PI) were added. All samples were kept in the dark for 15 min at room temperature. Finally, the stained cells were analyzed using a flow cytometer (BD Biosciences, San Jose, CA, USA).
Wound healing assay
A wound healing assay was conducted to examine cell migration ability. Patu8988 and Panc-1 cells were seeded into a 6-well plate at a concentration of 2 × 106 cells per well. The wound was generated in cells at 90%-95% confluency by scratching the surface of the plates with a sterile pipette tip. The cells were then incubated in either 3 µM ATO or 20 µM GEM, or a combination of both drugs for 20 h. The wound healing images were photographed at 0 h and 20 h using an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Transwell invasion assay
The invasive capacity of PC cells was determined using Transwell filters (8-μm pore size; Corning, Corning, NY, USA) with Matrigel (BD Biosciences). Briefly, cells in serum-free media were transferred to the upper chamber in either 3 µM ATO or 20 µM GEM, or a combination of both drugs. Then, 0.5 mg of culture medium with 10% FBS was added into each bottom chamber with either ATO or GEM, or a combination of both drugs. After incubation for 20 h, the cells in the upper chamber were removed (the upper surfaces of the Transwell chambers were scraped with cotton swabs), and the invaded cells were fixed and stained using the Wright’s-Giemsa stain set (Jiancheng Scientific, China). The stained cells were photographed and counted under a light microscope in six randomly-selected fields.
Protein extraction and western blotting
For protein extraction, cells were harvested and lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA). The protein concentrations were measured using a BCA protein assay kit. Proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the gels were transferred onto nitrocellulose membranes. The membranes were blocked with 4% nonfat dried milk or bovine serum albumin in 1 × PBS containing 0.1% Tween-20 (PBS-T) and incubated overnight at 4°C with the appropriate primary antibody. The membranes were then washed three times with PBS-T, and subsequently incubated with the secondary antibody for 1 h at room temperature. The protein bands were detected using an enhanced chemiluminescence detection system.
Data analysis
Data were expressed as the mean ± standard deviation (SD) for the absolute values or as the percentage of controls, as indicated in the vertical axis legends of the figures. The statistical significance of differential findings between experimental groups and control groups was evaluated by ANOVA using GraphPad StatMate software (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
ATO potentiates the cytotoxicity of GEM in PC cells
Previously, we reported that ATO inhibited cell growth in Patu8988 and Panc-1 cells [19]. To further investigate whether ATO enhanced the sensitivity of PC cells to GEM, we used the MTT assay to evaluate viability of treated Patu8988 and Panc-1 cells. PC cells were simultaneously treated with either each drug alone or a combination of both drugs for 48 h. We found that the combined treatment of 3 µM ATO and 20 µM GEM caused more significant growth inhibition than 3 µM ATO or 20 µM GEM alone in PC cells (Figure 1). These findings suggested that a combination of ATO and GEM significantly increased the sensitivity of PC cells to GEM.
Figure 1.
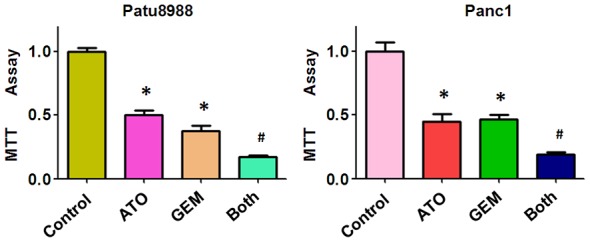
The antitumor effect of combined treatment with ATO and GEM. Pancreatic cancer cells were treated with either 3 µM arsenic trioxide (ATO) or 20 µM GEM, or co-treated with 3 µM ATO and 20 µM gemcitabine (GEM) for 48 h, and the number of viable cells was determined using the MTT assay. Vertical bars indicate the means ± SD of three independent experiments. Both: ATO plus GEM. *P < 0.05 compared with the control; #P < 0.05 compared with ATO alone or GEM alone.
ATO enhances apoptotic cell death induced by GEM
To further assess the effect of ATO and GEM on apoptosis in PC cells, we performed the cell apoptosis assay using annexin V/PI staining. We used flow cytometry to investigate the extent of apoptosis in cells treated with either ATO or GEM, or a combination of both drugs. We found that both ATO and GEM treatment individually led to increased apoptosis rates in PC cells (Figure 2). The percentage of apoptotic cells was increased in Patu8988 cells (10.93% vs. 1.84% in control cells) and Panc-1 cells (6.97% vs. 1.36% in control cells) when treated with ATO (Figure 2). The percentages of apoptotic cells also increased in Patu8988 cells (5.73% vs. 1.84% in control cells) and in Panc-1 cells (11.94% vs. 1.36% in control cells) when treated with GEM (Figure 2). Furthermore, there was a marked increase in the rate of apoptosis in cells treated with both ATO and GEM compared with those treated with ATO or GEM alone (Patu8988 cells: 18.03% vs. 1.84% in control; Panc-1 cells: 21.55% vs. 1.36% in control) [Figure 2]. Together, our findings suggested that ATO synergistically acted with GEM to enhance apoptotic cell death in PC.
Figure 2.
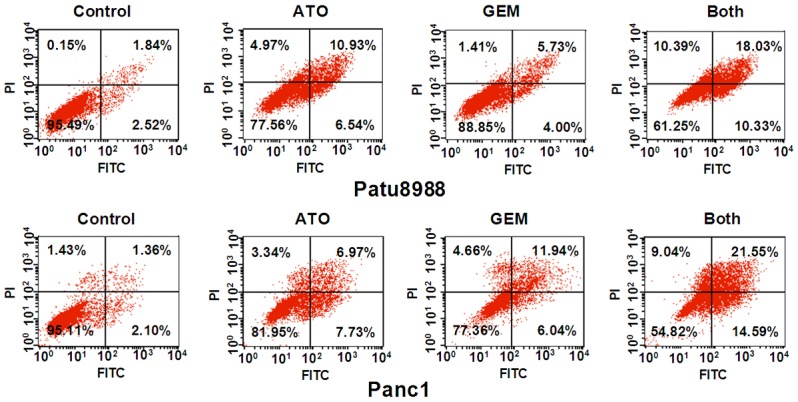
Arsenic trioxide (ATO) enhances gemcitabine (GEM)-induced apoptotic cell death. Patu8988 and Panc-1 cells were treated either with 3 µM ATO or 20 µM GEM, or a combination of both drugs for 48 h. Apoptotic cells were detected by annexin V/PI staining as described in the Materials and Methods. Both: ATO plus GEM.
ATO and GEM reduce cell migration in PC cells
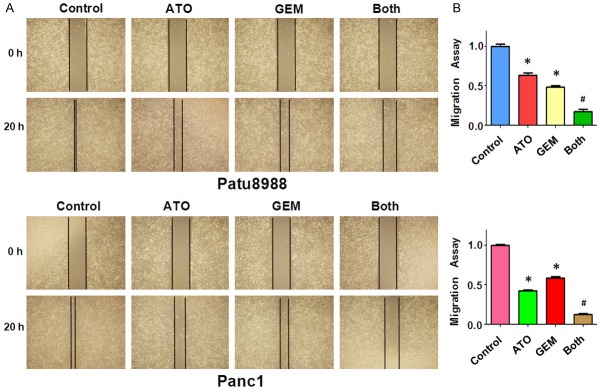
In order to examine whether ATO and GEM had an additive effect in preventing migration of Patu8988 and Panc-1 PC cells, we conducted wound-healing assays in cells treated with ATO or GEM, or a combination of both drugs. We found that the wound closure rate was significantly decreased in cells treated with ATO or GEM compared with that in control cells (Figure 3). However, cells treated with both ATO and GEM showed a remarkable decrease in wound closure rate compared with cells treated with either ATO or GEM (Figure 3). Together, these results indicated that ATO and GEM additively inhibited the migration of PC cells.
Figure 3.
The effect of arsenic trioxide (ATO) and gemcitabine (GEM) on cell migration. (A) Cell migration was detected using a wound-healing assay in Patu8988 and Panc-1 cells after treatment with either 3 µM ATO or 20 µM GEM, or a combination of the two drugs for 20 h. (B) Quantitative results are shown in (A). Both: ATO plus GEM. *P < 0.05 compared with the control; #P < 0.05 compared with ATO alone or GEM alone.
ATO and GEM reduce cell invasion in PC cells
To further confirm the effect of ATO and GEM on cell motility, we measured the cell-invasion ability of PC cells treated with either ATO or GEM, or a combination of both drugs, using a Matrigel invasion chamber assay. We found that both ATO and GEM individually retarded cell invasion (Figure 4). Furthermore, cells treated with both ATO and GEM showed an increase in the inhibition of invasion rate compared with cells treated with ATO or GEM alone (Figure 4). Together, these data indicated that ATO and GEM additively inhibited cell invasion in PC cells.
Figure 4.
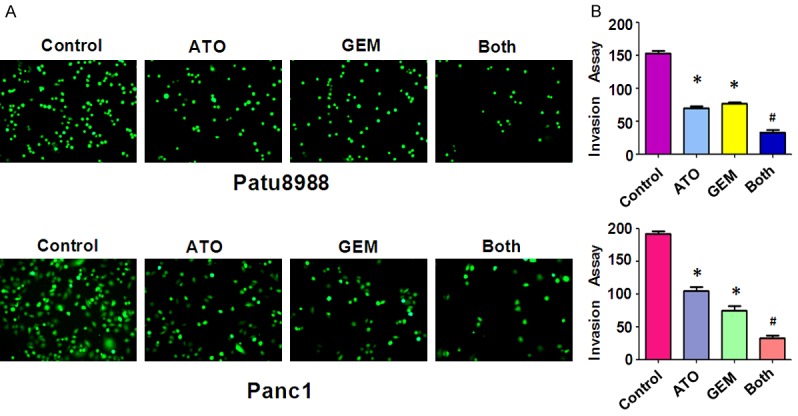
The effect of arsenic trioxide (ATO) and gemcitabine (GEM) on pancreatic cancer cell invasion. (A) Cell invasion was measured using a Transwell assay of Patu8988 and Panc-1 cells after treatment with either 3 µM ATO or 20 µM GEM, or a combination of the two drugs for 20 h. The stained cells were counted using a light microscope in six randomly-selected fields, and the numbers of invaded cells were determined. The number of cells was normalized against the number of cells in the corresponding control cells. (B) The quantitative results are illustrated in (A). *P < 0.05 compared with the control; #P < 0.05 compared with ATO alone or GEM alone. Both: ATO plus GEM.
ATO and GEM decrease Skp2 expression
Skp2 has been shown to be a potential ATO target [20]. It has been reported that Skp2 is involved in chemosensitivity in prostate cancer cells [21]. The proteins, p57 and p21, have been identified as downstream substrates of Skp2 [9,22]. Therefore, we used western blotting to investigate the protein expression levels of Skp2, p57, and p21 in PC cells treated with either ATO or GEM alone, or a combination of both drugs. We found that the combined treatment of ATO and GEM caused downregulation of Skp2 and upregulation of p57 and p21 to a greater degree compared with single drug treatment (Figure 5).
Figure 5.
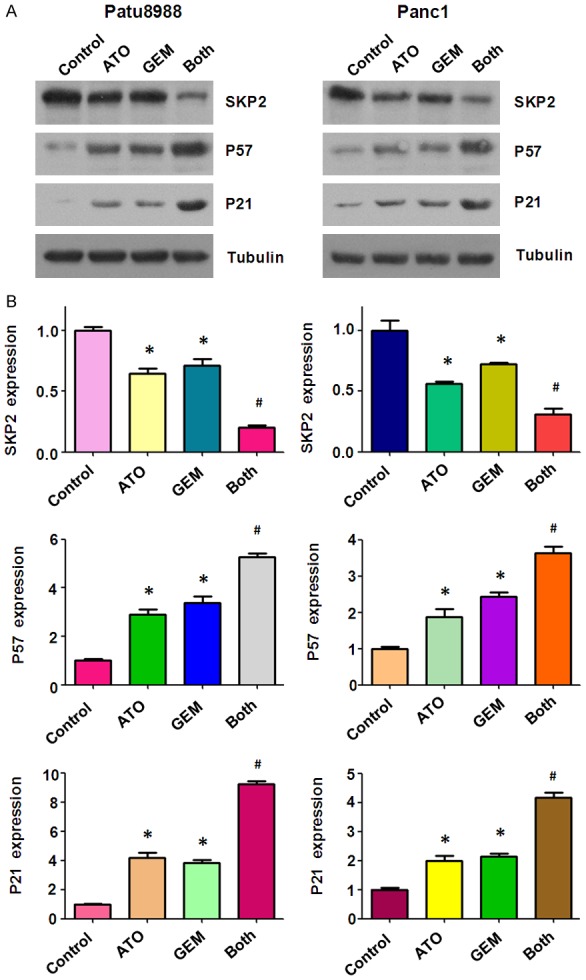
The effect of arsenic trioxide (ATO) and gemcitabine (GEM) on the expression of Skp2. (A) The expression of Skp2, and its downstream proteins, p21 and p57, were measured by western blotting of Patu8988 and Panc-1 cells treated with either 3 µM ATO or 20 µM GEM, or a combination of the two drugs for 48 h. (B) The quantitative results are illustrated in (A). *P < 0.05 compared with the control; #P < 0.05 compared with ATO alone or GEM alone. Both: ATO plus GEM.
Discussion
The use of GEM remains the most widely used therapy for PC [23]. However, a substantial fraction of patients achieve little clinical benefit due to intrinsic or acquired chemoresistance [24]. However, there are many possibilities to improve the efficacy of GEM by using various combinations of other drugs or novel targeted therapeutics. In the present study, we investigated the antitumor effect of the combined treatment of ATO and GEM in PC cells, and found that ATO and GEM had a synergistic antitumor effect via decreased Skp2 expression in PC cells.
In our previously study, we reported that ATO inhibited cell growth and invasion via downregulation of Skp2 in PC cells [19]. Skp2 is thought to play a pivotal oncogenic role in PC [25]. Furthermore, Skp2 is involved in drug resistance in cancer cells. For example, a study has reported that Skp2 was closely involved in the resistance of osteosarcoma cells to methotrexate, and in the induction of epithelial-mesenchymal transition properties [26]. Moreover, Huang et al. reported that Skp2 positively regulated MAD2 expression and that inhibition of Skp2 sensitized human lung cancer cells to paclitaxel treatment [15]. Consistent with these findings, we observed that ATO potentiated the effects of GEM via the Skp2 pathway in Panc-1 and Patu8988 PC cells.
We believe that Skp2 may be a promising therapeutic target to overcome PC cell resistance to GEM. Further studies will be needed to demonstrate the molecular mechanism of Skp2 as a novel potential therapeutic target when used in combination with GEM in PC. In conclusion, our study shows that ATO significantly enhanced the antitumor effects of GEM in PC cells. Our findings may provide a novel strategy for the use of this drug combination in the treatment of PC patients.
Acknowledgements
This work was supported by a grant from the Mianyang Science & Technology and Intellectual Property Office (16S-03).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988–8003. doi: 10.3748/wjg.v21.i26.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz AR, Chakravarthy D, Gong J, Halff GA, Ghosh R, Kumar AP. Pancreatic cancer: current status and challenges. Curr Pharmacol Rep. 2017;3:396–408. doi: 10.1007/s40495-017-0112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu X, Shen X, Qu J, Straubinger RM, Jusko WJ. Proteomic analysis of combined gemcitabine and birinapant in pancreatic cancer cells. Front Pharmacol. 2018;9:84. doi: 10.3389/fphar.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong HQ, Hong YB, Kim JS, Lee HS, Yi YW, Kim YJ, Wang A, Zhao W, Cho CH, Seong YS, Bae I. Inhibition of checkpoint kinase 2 (CHK2) enhances sensitivity of pancreatic adenocarcinoma cells to gemcitabine. J Cell Mol Med. 2013;17:1261–1270. doi: 10.1111/jcmm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuler S, Diersch S, Hamacher R, Schmid RM, Saur D, Schneider G. SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. Int J Oncol. 2011;38:219–225. [PubMed] [Google Scholar]
- 7.Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 9.Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama KI. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, Wang Z, Gygi SP, Nakayama K, Teruya-Feldstein J, Toker A, Haigis MC, Pandolfi PP, Wei W. Acetylation-dependent regulation of Skp2 function. Cell. 2012;150:179–193. doi: 10.1016/j.cell.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Chan CH, Gao Y, Lin HK. Novel roles of Skp2 E3 ligase in cellular senescence, cancer progression, and metastasis. Chin J Cancer. 2012;31:169–177. doi: 10.5732/cjc.011.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Chen Y, Saha MN, Chen J, Evans K, Qiu L, Reece D, Chen GA, Chang H. Targeting phospho-MARCKS overcomes drug-resistance and induces antitumor activity in preclinical models of multiple myeloma. Leukemia. 2015;29:715–726. doi: 10.1038/leu.2014.255. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X, Chen S, Chen C, Wang Z. Acquisition of epithelial-mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br J Cancer. 2014;110:1958–1967. doi: 10.1038/bjc.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang T, Yang L, Wang G, Ding G, Peng B, Wen Y, Wang Z. Inhibition of Skp2 sensitizes lung cancer cells to paclitaxel. Onco Targets Ther. 2017;10:439–446. doi: 10.2147/OTT.S125789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan X, Li T, Han X, Ren J, Chen P, Li H, Gong S. The antitumor effect of arsenic trioxide on hepatocellular carcinoma is enhanced by andrographolide. Oncotarget. 2017;8:90905–90915. doi: 10.18632/oncotarget.18677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao X, Ren T, Huang Y, Wang S, Zhang F, Liu K, Zheng B, Guo W. Induction of the mesenchymal to epithelial transition by demethylation-activated microRNA-125b is involved in the anti-migration/invasion effects of arsenic trioxide on human chondrosarcoma. J Exp Clin Cancer Res. 2016;35:129. doi: 10.1186/s13046-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Zhang H, Yang L, Jiang H, Guo S, Li Y, Tao S. Construction of a metabolomics profile of arsenic trioxide effect in gastric carcinoma cell line SGC7901. Acta Biochim Biophys Sin (Shanghai) 2016;48:474–481. doi: 10.1093/abbs/gmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao JK, Wang LX, Long B, Ye XT, Su JN, Yin XY, Zhou XX, Wang ZW. Arsenic trioxide inhibits cell growth and invasion via down-regulation of Skp2 in pancreatic cancer cells. Asian Pac J Cancer Prev. 2015;16:3805–3810. doi: 10.7314/apjcp.2015.16.9.3805. [DOI] [PubMed] [Google Scholar]
- 20.Lam SK, Li YY, Zheng CY, Ho JC. Downregulation of thymidylate synthase and E2F1 by arsenic trioxide in mesothelioma. Int J Oncol. 2015;46:113–122. doi: 10.3892/ijo.2014.2716. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Lu Y, Wang L, Mizokami A, Keller ET, Zhang J, Fu J. Skp2 is associated with paclitaxel resistance in prostate cancer cells. Oncol Rep. 2016;36:559–566. doi: 10.3892/or.2016.4809. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa K, Kotake Y, Kitagawa M. Ubiquitin-mediated control of oncogene and tumor suppressor gene products. Cancer Sci. 2009;100:1374–1381. doi: 10.1111/j.1349-7006.2009.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 24.Miyake M, Fujimoto K, Anai S, Ohnishi S, Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, Okajima E, Tanaka N, Hirao Y. Inhibition of heme oxygenase-1 enhances the cytotoxic effect of gemcitabine in urothelial cancer cells. Anticancer Res. 2010;30:2145–2152. [PubMed] [Google Scholar]
- 25.Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X, Zhou X, Wang Z. Rottlerin exhibits anti-cancer effect through inactivation of S phase kinase-associated protein 2 in pancreatic cancer cells. Am J Cancer Res. 2016;6:2178–2191. [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Wang C, Cui Y, Han X, Zhou Y, Bai J, Li R. S-phase kinase-associated protein 2 is involved in epithelial-mesenchymal transition in methotrexate-resistant osteosarcoma cells. Int J Oncol. 2018;52:1841–1852. doi: 10.3892/ijo.2018.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]