Abstract
Rho-kinase inhibitor Y27632, which is a factor in conditional reprogramming culture, induces airway progenitor clone formation. To investigate whether Y27632 enhances airway progenitor cells in nasal epithelium, primary cultures of HNECs transfected with human telomerase reverse transcriptase (hTERT-HNECs) were treated with Y27632. In TERT-HNECs treated with Y27632 for 5 days, upregulation of p63, gap junction molecules Cx26, Cx30, Cx43, cytochrome P450 enzymes CYP2C9, CYP2C18, CYP39A1, CYP4B1, CYP2G1P, CYP4Z1, and KLF families KLF10 and KLF11 were observed compared to the control. Downregulation of tight junction molecules claudin-4, -7, and -23 was observed. Circumfential submembrane F-actin was also induced. The functions of gap junctional intercellular communication (GJIC) and the epithelial barrier were upregulated. Knockdown of p63 by siRNAs of TAp63 or ΔNp63 inhibited Cx26, Cx43 and CYP2C18, and induced claudin-1, and -4. Knockdown of KLF11 prevented p63 expression and enhancement of the epithelial barrier function by Y27632. In nasal mucosal tissues from patients with allergic rhinitis (AR), localized alteration of p63, KLF11, RhoA, Cx30 and claudin-4 was observed. Treatment with Y27632 in long-term culture induced airway progenitor cells via KLF11 in p63-positive human nasal epithelium. Airway progenitor cells of nasal epithelium induced by Y27632 is important in understanding upper airway disease-specific characteristics.
Keywords: Human nasal epithelial cells, hTERT, gap junctions, tight junctions, CYP, p63, KLF11
Introduction
The airway epithelium of the human nasal mucosa interacts with various environmental agents and acts as a physical barrier that protects against inhaled substances and pathogens [1-3]. A defective epithelial barrier with decreased expression of tight junction proteins is found in patients with chronic rhinosinusitis (CRS) and nasal polyps (NPs) [4,5].
Rho-kinase inhibitor Y27632, which is a factor in conditional reprogramming culture, induces airway progenitor clone formation [6]. Y-27632-treatment alters expression of genes fundamental to the formation of the basal cell cytoskeleton, cell-cell junctions, and cell-extracellular matrix (ECM) interactions [6]. ROCK inhibition induces reorganization of apical F-actin and affects paracellular permeability but does not alter the distribution or detergent solubility of tight junction proteins [7].
The abundance of mRNAs of gap junction molecules connexin26 (Cx26), Cx30 and Cx43 is increased in CRS compared to normal mucosa [8]. Rho is involved in regulation of the assembly of Cx43 gap junctions, which is dependent on the formation of E-cadherin adherens junctions in corneal epithelium [9]. Y-27632 enhances gap junctional intercellular communication (GJIC) in NIH3T3 cells [10].
Transcriptional factor p63, which is a member of the p53 family and has two distinct isoforms, TAp63 and aNp63, plays an important role in the proliferation and differentiation of various epithelial basal cells [11]. Loss of ΔNp63 significantly reduces epithelial proliferation and increases E-cadherin expression in human airway epithelial cells (26). p63 and aNp63 are upregulated in the epithelium of chronic rhinosinusitis (CRS) and nasal polyps (NPs) [5,12]. p63 negatively regulates the epithelial tight junctional barrier of the nasal epithelium [5].
Human telomerase reverse transcriptase (hTERT)-transfected HNECs (hTERT-HNECs) can be used as a stable model for studying regulation of the nasal epithelial response [3,5,13]. Y27632 stabilizes telomere length during the course of long-term culture [14].
In the present study, when hTERT-HNECs were treated with Rho-kinase inhibitor Y27632 in long-term culture, Y27632 induced airway progenitor cells, indicated as changes of gap junctions, tight junctions, F-actin and cytochrome P450 enzymes in hTERT-HNECs. These changes induced by Y27632 were regulated via p63 and KLF11.
Materials and methods
Ethics statement
The protocol for human study was reviewed and approved by the ethics committee of the Sapporo Medical University School of Medicine. Written informed consent was obtained from each patient who participated in the investigation. All experiments were carried out in accordance with the approved guidelines and with the Declaration of Helsinki.
Antibodies and reagents
A mouse monoclonal anti-p63 (DAK-p63) antibody was obtained from Dako (Tokyo, Japan). Rabbit polyclonal anti-p63, anti-RhoA, anti-CYP2C18 antibodies and a mouse monoclonal anti-KLF11 (KLF5J027) antibody were obtained from Abcam (Cambridge, MA, USA). A rabbit polyclonal anti-p40 (aNp63) antibody was obtained from NICHIREI BIOSCIENCES INC. (Tokyo, Japan). A rabbit polyclonal anti-aNp63 antibody was obtained from BioLegend (Tokyo, Japan). Rabbit polyclonal anti-connexin (Cx)26, Cx30, anti-claudin (CLDN)-1, anti-CLDN-4, anti-CLDN-7, anti-occludin (OCLN), and anti-tricellulin (TRIC) antibodies as well as mouse monoclonal anti-Cx43 (3D8A5), anti-OCLN (OC-3F10), and anti-CLDN-4 (3E2C1) antibodies were from Zymed Laboratories (San Francisco, CA). A rabbit polyclonal anti-LSR antibody was obtained from Novus Biologicals (Littleton, CO, USA). A rabbit polyclonal anti-actin antibody was obtained from Sigma-Aldrich Inc. (St. Louis, MO). Alexa Fluor 488 (green)-conjugated anti-rabbit IgG, and Alexa Fluor 594 (red)-conjugated anti-mouse IgG antibodies and Axea Fluor 594 (red)-phalloidin were from Molecular Probes, Inc. (Eugene, OR). A Rho kinase inhibitor Y27632 was obtained from Sigma-Aldrich Inc. (St. Louis, MO). PKCα inhibitor Gö 6976 and p38 MAPK inhibitor SB203580 were purchased from Calbiochem-Novabiochem Corporation (San Diego, CA). HRP-conjugated polyclonal goat anti-rabbit IgG was from Dako A/S (Glostrup, Denmark). The ECL Western blotting system was from GE Healthcare UK, Ltd. (Buckinghamshire, UK).
GeneChip analysis
Microarray slides were scanned using a 3D-GENE human Oligochip 25k. (TORAY, Tokyo, Japan) and microarray images were automatically analyzed using AROSTM, version 4.0 (Operon Biotechnologies, Tokyo, Japan).
Immunohistochemical analysis
Human nasal tissues were obtained from patients with each 12 hypertrophic rhinitis or chronic sinusitis who underwent inferior turbinectomy at Sapporo Medical University, the Sapporo Hospital of Hokkaido Railway Company, or the KKR Sapporo Medical Center Tonan Hospital. Informed consent was obtained from all patients and this study was approved by the ethics committees of the above institutions.
The tissues were embedded in paraffin after fixation with 10% formalin in PBS. Briefly, 5-μm-thick sections were dewaxed in xylene, rehydrated in ethanol, and heated with Vision BioSystems Bond Max using ER2 solution (Leica) in an autoclave for antigen retrieval. Endogenous peroxidase was blocked by incubation with 3% hydrogen peroxide in methanol for 10 min. The tissue sections were then washed twice with Tris-buffered saline (TBS) and preblocked with Block Ace for 1 h. After washing with TBS, the sections were incubated with anti-p63, anti-ΔNp63, anti-KLF-11, anti-RhoA, anti-Cx30 and anti-CLDN-4 antibodies (1:400) for 1 h. The sections were then washed three times in TBS and incubated with Vision BioSystems Bond Polymer Refine Detection kit DS9800. After three washes in TBS, a diamino-benzidine tetrahydrochloride working solution was applied. Finally, the sections were counterstained with hematoxylin.
Cell culture and treatments
The cultured HNECs were derived from mucosal tissues of each 6 patients with hypertrophic rhinitis or chronic sinusitis who underwent inferior turbinectomy at Sapporo Medical University, the Sapporo Hospital of Hokkaido Railway Company, or the KKR Sapporo Medical Center Tonan Hospital. Informed consent was obtained from all patients and this study was approved by the ethics committees of the above institutions.
The methods for primary culture of human nasal epithelial cells were as reported previously [15]. Some primary cultured HNECs were transfected with the catalytic component of telomerase, the human catalytic subunit of the telomerase reverse transcriptase (hTERT) gene, as described previously [15]. The cells were plated on 35-mm or 60-mm culture dishes (Corning Glass Works, Corning, NY), which were coated with rat tail collagen (500 μg of dried tendon/ml 0.1% acetic acid). They were then cultured in serum-free bronchial epithelial cell basal medium (BEBM, Lonza Walkersville, Inc.; Walkersville, MD) supplemented with bovine pituitary extract (1% v/v), 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 50 μg/ml gentamycin, 50 μg/ml amphotericin B, 0.1 ng/ml retinoic acid, 10 μg/ml transferrin, 6.5 μg/ml triiodothyronine, 0.5 μg/ml epinephrine, 0.5 ng/ml epidermal growth factor (Lonza Walkersville, Inc.), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) and incubated in a humidified, 5% CO2: 95% air incubator at 37°C. In this experiment, 2nd and 3rd passaged cells which maintained cellular function were used. Some cells cultured with or without FBS, were treated with 10 μM Y27632 (Rho kinase inhibitor).
siRNA experiment
For knockdown of human TAp63 and human ΔNp63, StealthTM Select RNAi against the genes was synthesized by Invitrogen (Carlsbad, CA). For knockdown of human KLF11, mission esiRNA targeting the gene was synthesized by Sigma-Aldrich Inc. (St. Louis, MO). The sequences were as follows: siRNA of TAp63 (sense: 5’-GGAAUGACUUCAACUUUGA-3’; antisense: 5’-UCAAAGUUGAAGUCAUUCC-3’), siRNA of aNp63 (sense: 5’-ACAAUGCCCAGACUCAAUU-3’; antisense: 5’-AAUUGAGUCUGGGCAUUGU-3’), and siRNA of KLF11 (EHU015881). hTERT-HNECs cultured at 24 h after plating were transfected with 100 nM siRNAs using LipofectamineTM RNAiMAX Reagent (Invitrogen) for 48 h. A scrambled siRNA sequence (BLOCK-iT Alexa Fluor fluorescent, Invitrogen) was employed as control siRNA.
Western blot analysis
The hTERT-transfected HNECs were scraped from a 60 mm dish containing 300 μl of buffer (1 mM NaHCO3 and 2 mM phenylmethylsulfonyl fluoride), collected in microcentrifuge tubes, and then sonicated for 10 s. The protein concentrations of the samples were determined using a BCA protein assay reagent kit (Pierce Chemical Co.; Rockford, IL). Aliquots of 15 μl of protein/lane for each sample were separated by electrophoresis in 5-20% SDS polyacrylamide gels (Wako, Osaka, Japan), and electrophoretically transferred to a nitrocellulose membrane (Immobilon; Millipore Co.; Bedford, UK). The membrane was saturated for 30 min at room temperature with blocking buffer (25 mM Tris, pH 8.0, 125 mM NaCl, 0.1% Tween 20, and 4% skim milk) and incubated with anti-RhoA, anti-p63, anti-aNp63, anti-Cx26, anti-Cx30, anti-Cx43, anti-CLDN-1, anti-CLDN-4, anti-CLDN-7, anti-OCLN, anti-TRIC, anti-LSR, anti-CYP2C18, anti-KLF11 and anti-actin antibodies (1:1000) at room temperature for 1 h. Then it was incubated with HRP-conjugated anti-mouse and anti-rabbit IgG antibodies at room temperature for 1 h. The immunoreactive bands were detected using an ECL Western blotting system.
Immunocytochemistry
hTERT-transfected HNECs grown in 35mm glass-coated wells (Iwaki, Chiba, Japan), were fixed with cold acetone and ethanol (1:1) at -20°C for 10 min. After rinsing in PBS, the cells were incubated with anti-p63, anti-RhoA, anti-Cx30, anti-CX43, and anti-KLF11 antibodies (1:100) overnight at 4°C. Alexa Fluor 488 (green)-conjugated anti-rabbit IgG and Alexa Fluor 592 (red)-conjugated anti-mouse IgG (Invitrogen) were used as secondary antibodies. The specimens were examined and photographed with an Olympus IX 71 inverted microscope (Olympus Co.; Tokyo, Japan) and a confocal laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany).
Measurement of gap junctional intercellular communication (GJIC)
Lucifer yellow (5% wt/vol, Sigma) was injected into individual cells through sharp microelectrodes (Sterile Femtotips II; Eppendorf, Hamburg, Germany). Photographs documenting the extent of dye coupling were taken 3 min after the end of the injection using an inverted fluorescence microscope equipped with an appropriate filter (Olympus, Tokyo, Japan).
Measurement of transepithelial electrical resistance (TEER)
hTERT-transfected HNECs were cultured to confluence in the inner chambers of 12-mm Transwell inserts with 0.4-µm pore-size filters (Corning Life Sciences). TER was measured using an EVOM voltmeter with an ENDOHM-12 (World Precision Instruments, Sarasota, FL) on a heating plate (Fine, Tokyo, Japan) adjusted to 37°C. The values were expressed in standard units of ohms per square centimeter and presented as the mean ± SD. For calculation, the resistance of blank filters was subtracted from that of filters covered with cells.
Data analysis
Signals were quantified using Scion Image Beta 4.02 Win (Scion Co.; Frederick, MA). Each set of results shown is representative of at least three separate experiments. Results are given as means ± SEM.
Results
Upregulation of p63, gap junction proteins, cytochrome P450 (CYP) enzymes, Kruppel-like factor (KLF) families and downregulation of tight junction proteins by treatment with Y27632 in hTERT-HNECs
To investigate the effects of Y27632 in hTERT-HNECs, we first performed GeneChip analysis of hTERT-HNECs treated with Y27632 for 5 days and selected gene probes that were regulated more or less than 2-fold compared to the controls and the Y27632-treatment (Table 1). In hTERT-HNECs treated with Y27632, upregulation of gap junction molecules Cx26, Cx30, Cx43, cytochrome P450 enzymes CYP2C9, CYP2C18, CYP39A1, CYP4B1, CYP2G1P, CYP4Z1, and KLF families KLF10 and KLF11 were observed compared to the control. Downregulation of tight junction molecules CLDN-4, -7, and -23 was also observed.
Table 1.
List of gene probes up- or downregulated in hTERT-HNEC treated with Y27632
| Gene name | ID | Gene Bank ID | Fold-change control vs Y27632 |
|---|---|---|---|
| Cx26 | opHsV0400000655 | XM011535049.1 | 2.92 |
| Cx30 | H200004912 | NM001110221.2 | 3.89 |
| Cx43 | H200005947 | NM000165.4 | 2.58 |
| CLDN4 | H300004950 | NM001305.4 | 0.19 |
| CLDN7 | H200017305 | NM001185023.1 | 0.44 |
| CYP2C9 | H300007405 | NM000771.3 | 3.30 |
| CYP2C18 | CHsGV10002772 | NM000772.2 | 6.68 |
| CYP4B1 | H200000168 | NR135003.1 | 2.28 |
| CYP2G1P | opHsV0400000206 | NR040249.1 | 2.79 |
| CYP4Z1 | opHsV04000002772 | NM178134.2 | 2.80 |
| KLF10 | opHsV04000004957 | NM001032282.3 | 2.28 |
| KLF10 | AHsV10002596 | NR103760.1 | 2.63 |
| KLF11 | CHsGV10003420 | NM003597.4 | 2.07 |
To confirm these changes, we performed Western blotting and immunocyotochemistry. In Western blotting, Y27632 induced expression of p63, Cx26, Cx30, Cx43 OCLN, TRIC, LSR and CYP2C18, while RhoA was decreased (Figure 1A, 1B). In phase contrasts images, cell volume was increased by treatment with Y27632 compared to control (Figure 2A). Cx30- and Cx43-positive spots were increased and expression of CLDN-4 and CLDN-7 was decreased at the membranes (Figure 2B). Submembranes circumfential F-actin was also induced (Figure 2B).
Figure 1.
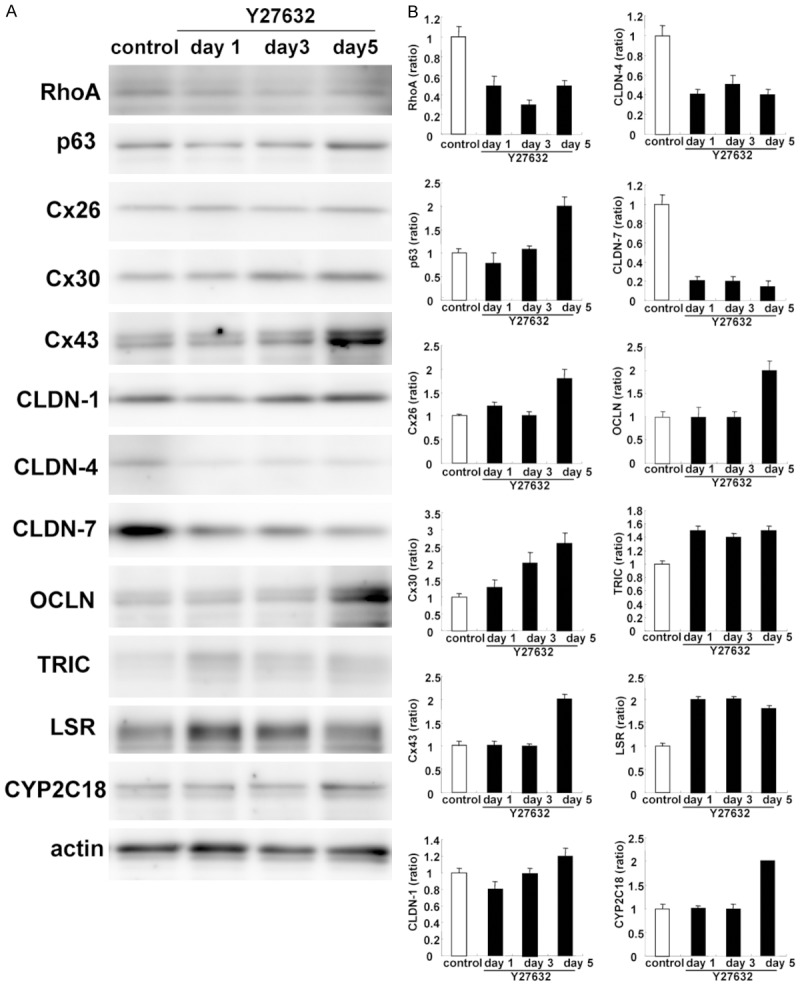
A. Western blotting for RhoA, p63, Cx26, Cx30, Cx43, claudin-1, -4, -7, OCLN, TRIC, LSR, and CYP2C18 in hTERT-HNECs treated with 10 μM Y27632 for 5 days. B. The corresponding expression levels are shown as bar graphs.
Figure 2.
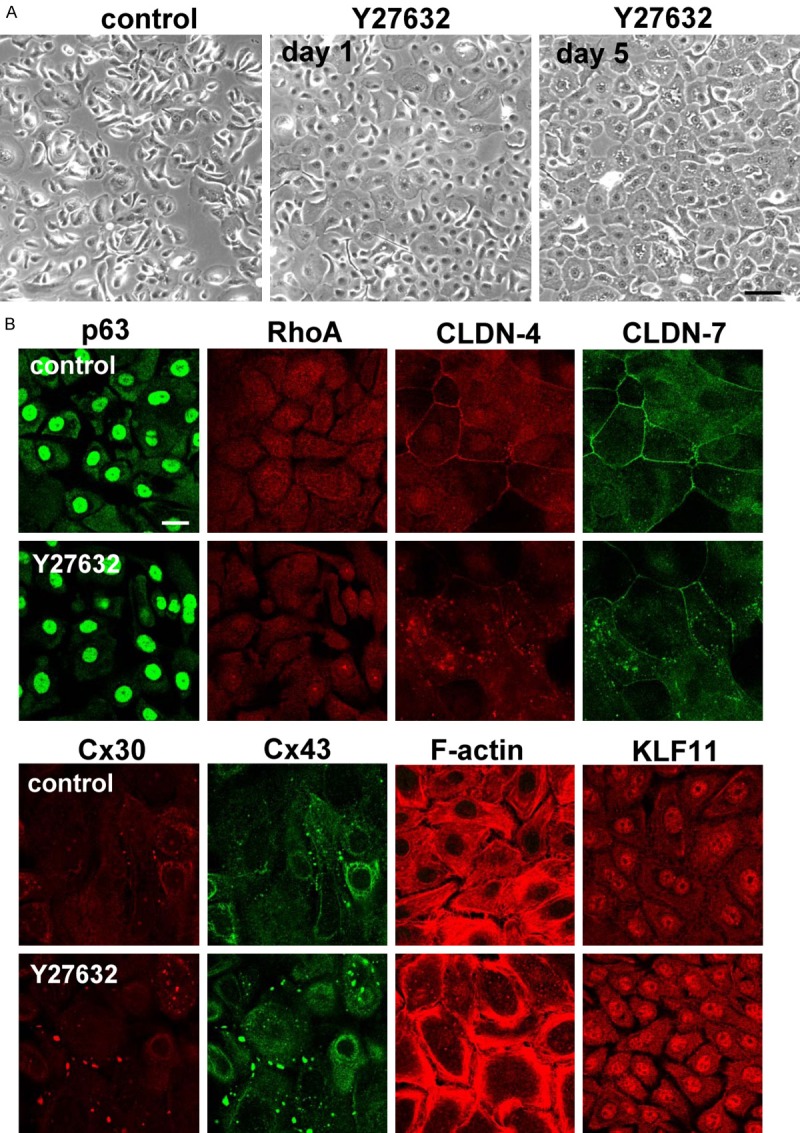
(A) Phase-contrast images and (B) images of immunocytochemical staining for p63, RhoA, CLDN-4, CLDN-7, Cx30, Cx43, F-actin and KLF11 in hTERT-HNECs treated with 10 μM Y27632 for 5 days. Bar: 20 μm.
Induction of GJIC and epithelial barrier by treatment with Y27632 in hTERT-HNECs
As an increase of gap junction molecules, a decrease of some tight junction molecules and induction of F-actin were induced by Y27632, we measured the functions of GJIC and the epithelial barrier. Treatment with Y27632 enhanced GJIC and epithelial barrier (Figure 3A, 3B).
Figure 3.
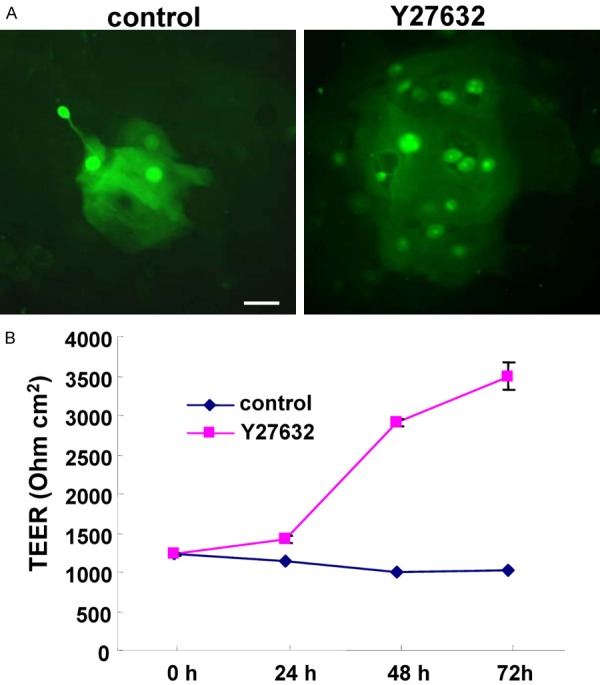
(A) GJIC shown by Lucifer yellow dye and (B) TEER values representing barrier function in hTERT-HNECs treated with 10 μM Y27632 for 3 days. Bar: 20 μm.
Upregulation of RhoA, and tight junction proteins and downregulation of gap junction proteins, and cytochrome P450 (CYP) enzymes by tranfection with siRNAs of TAp63 and ΔNp63 in hTERT-HNECs
To investigate whether Y27632 functioned via p63 in HNECs, hTERT-HNECs were transfected with siRNAs of TAp63 and aNp63. When p63 was downregulated by siRNAs of TAp63 and aNp63 in Western blotting, upregulation of RhoA, CLDN-1, and CLDN-4, and downregulation of Cx26, Cx30, TRIC, and CYP2C18 were observed (Figure 4A, 4B). The immunocytochemical results showed that Cx30- and Cx43-positive spots were decreased and expression of CLDN-4 was increased at the membranes (Figure 5).
Figure 4.
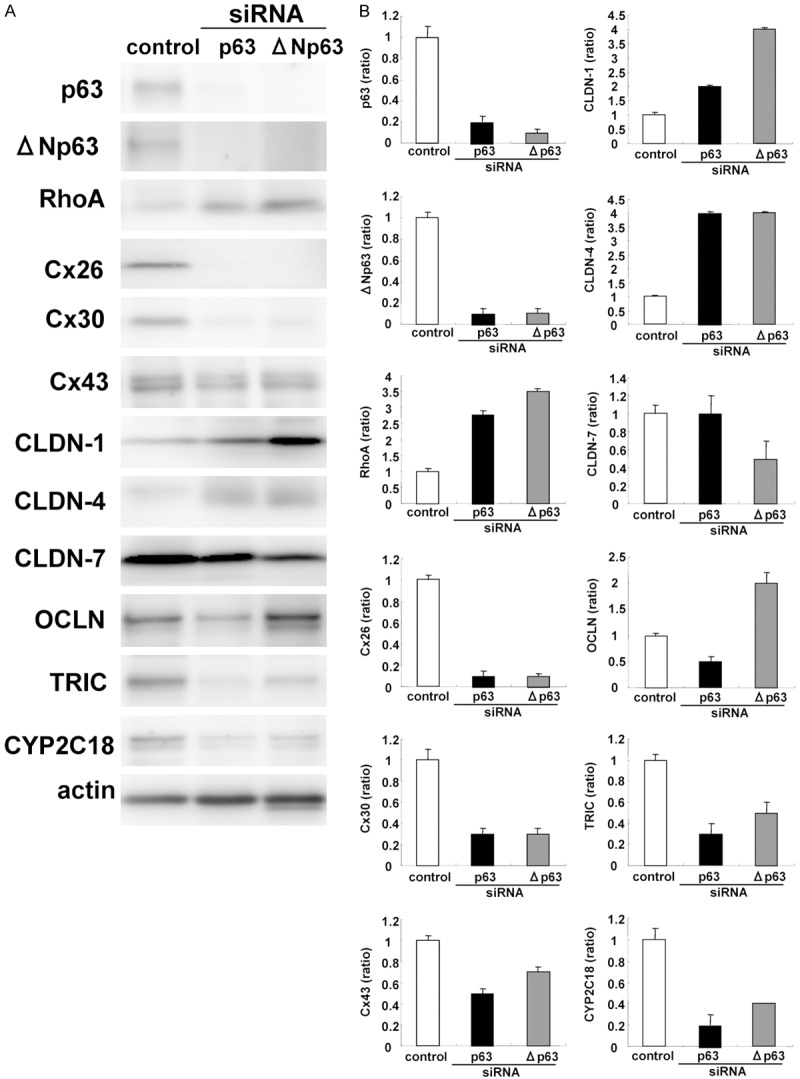
A. Western blotting for p63, ΔNp63, RhoA, Cx26, Cx30, Cx43, CLDN-1, -4, -7, OCLN, TRIC and CYP2C18 in hTERT-HNECs transfected with siRNAs of p63 and ΔNp63. B. The corresponding expression levels are shown as bar graphs.
Figure 5.
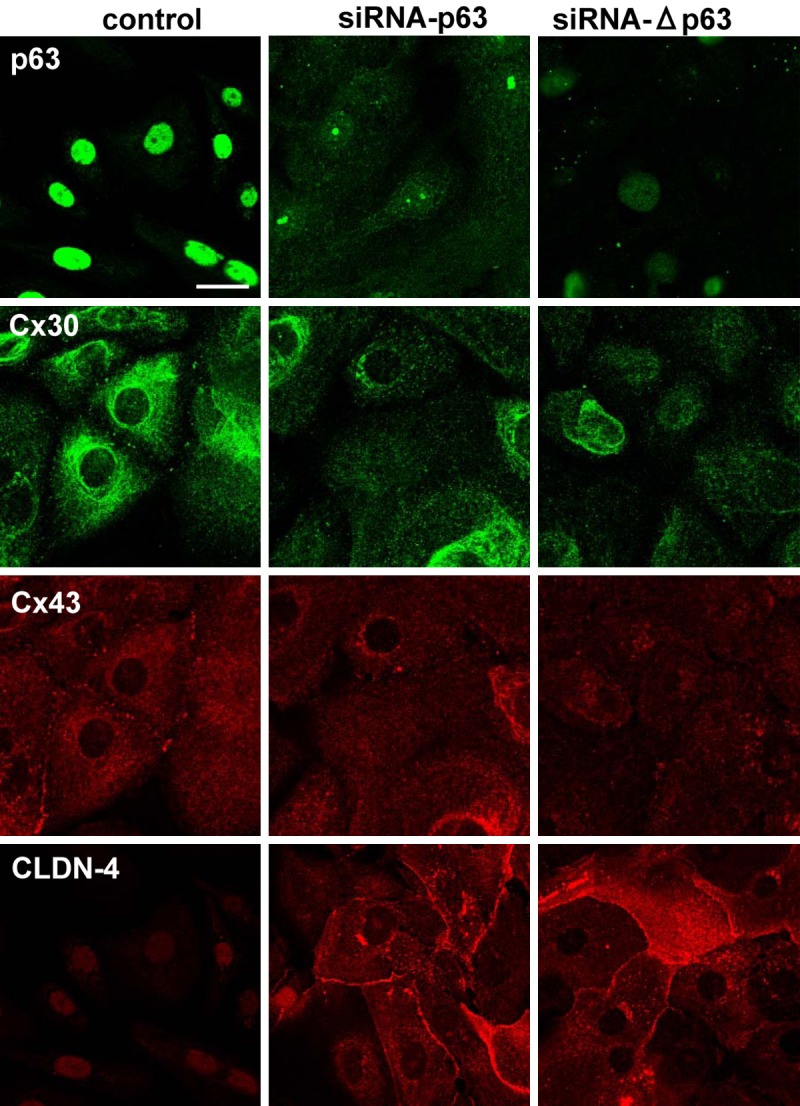
Images of immunocytochemical staining for p63, Cx30, Cx43, and CLDN-4 in hTERT-HNECs transfected with siRNAs of p63 and ΔNp63. Bar: 20 μm.
KLF11-kockdown prevents upregulation of p63 and epithelial barrier by treatment with Y27632 in hTERT-HNECs
Treatment with Y27632 induced KLF11mRNA (Table 1). To investigate whether Y27632 functioned via KLF11 in HNECs, hTERT-HNECs were transfected with siRNAs ofKLF11. Knockdown of KLF11 by the siRNA prevented upregulation of p63 by Y27632 in Western blotting (Figure 6A). Furthermore, KLF11-knockdown inhibited the upregulation of the epithelial barrier induced by Y27632 (Figure 6B). The immunocytochemical results showed that KLF11-knockdown prevented the upregulation of Cx30- and Cx43-positive spots and downregulation of CLDN-4 induced by Y27632 (Figure 7).
Figure 6.
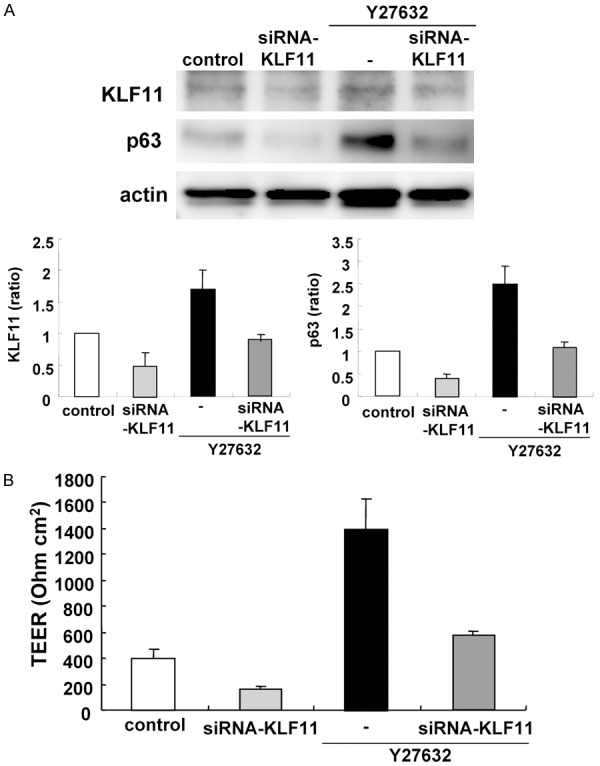
A. Western blotting for KLF11 and p63 in hTERT-HNECs treated with 10 μM Y27632 for 5 days after transfection with siRNAs of p63 and ΔNp63 for 2 days. The corresponding expression levels are shown as bar graphs. B. TEER values representing barrier function in hTERT-HNECs treated with 10 μM Y27632 for 3 days after transfection with siRNAs of p63 and ΔNp63 for 2 days.
Figure 7.
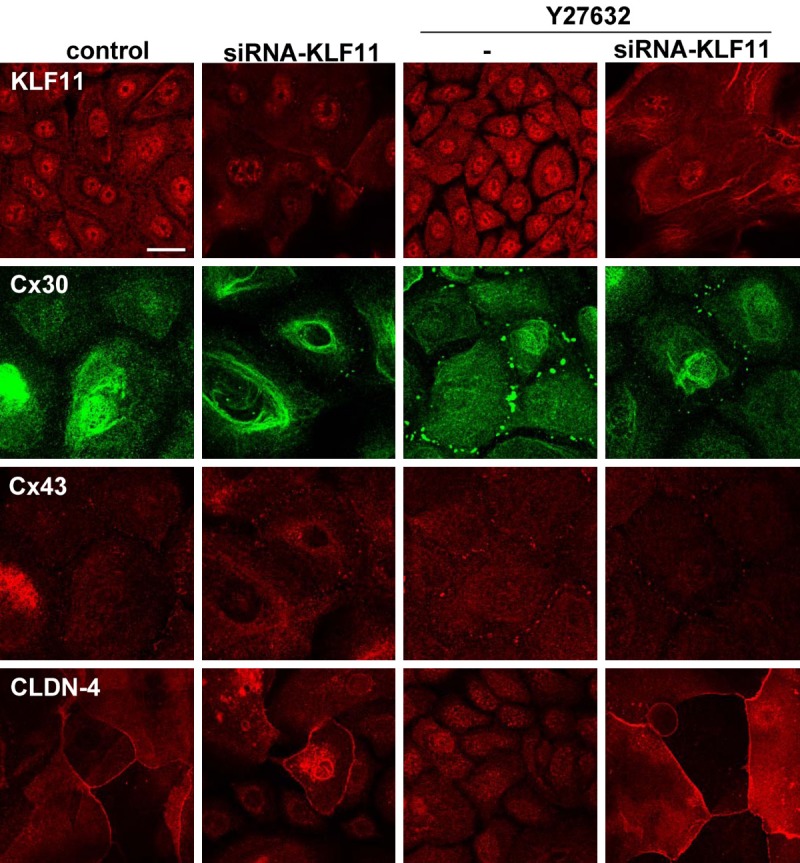
Images of immunocytochemical staining for KLF11, Cx30, Cx43, and CLDN-4 in hTERT-HNECs hTERT-HNECs treated with 10 μM Y27632 for 5 days after transfection with siRNAs of p63 and ΔNp63 for 2 days. Bar: 20 μm.
Expression patterns of p63, aNp63, KLF11, RhoA, Cx30 and CLDN-4 in the nasal epithelium of allergic rhinitis (AR)
We performed immunohistochemical analysis for p63, aNp63, KLF11, RhoA, Cx30 and CLDN-4 in the normal nasal epithelium and that of AR. The immunohistochemical results showed that expression of p63, aNp63, KLF11, Cx30 and RhoA was upregulated, and CLDN-4 expression was downregulated in the nasal epithelium of AR (Figure 8).
Figure 8.
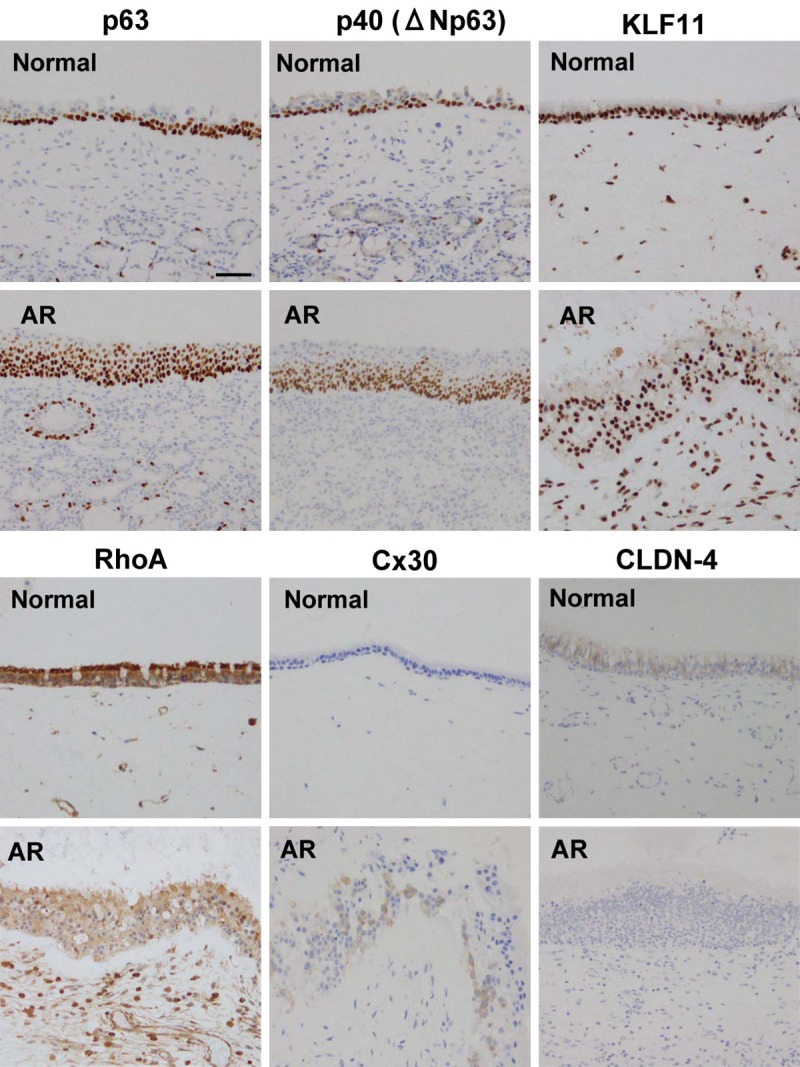
Images of immunohistochemical staining of p63, p40 (ΔNp63), KLF11, RhoA, Cx30, CLDN-4 in normal nasal mucosal tissues and those from patients with allergic rhinitis (AR). Bar: 50 μm.
Discussion
In this study, we first found that Rho-kinase inhibitor Y27632 alone induced airway progenitor cells of hTERT-human nasal epithelium in vitro. Y27632 and feeder cells induce the conditional reprogramming of various epithelial cells (CRC) [16]. Y27632 induces airway progenitor clone formation [6]. It also leads led to the selective expansion of the numbers of basal epithelial stem cells during the critical early stages of culture [17]. On the other hand, it is possible to immortalize primary human adult cells with exogenous human telomerase reverse transcriptase (hTERT). Some cells immortalized with hTERT alone have disrupted differentiation. In the present study, we used hTERT-human nasal epithelial cells that were not immortalized [15]. Y27632 stabilizes the telomere length during the course of long-term culture [14]. In the present study, Y27632 alone without feeder cells induced airway progenitor cells indicated as changes of gap junctions, tight junctions, F-actin and cytochrome P450 enzymes in hTERT-HNECs. These results suggested that treatment with Y27632 in long-term culture might affect hTERT expression in hTERT-HNECs.
p63 contributes to the formation and maintenance of differentiated pseudostratified bronchial epithelium [18]. p63 and aNp63 are upregulated in the epithelium of chronic rhinosinusitis (CRS) and nasal polyps (NPs) [5,12]. In hTERT-HNECs, which mostly p63-positive nuclei, knockdown of p63 by siRNAs of TAp63 or aNp63 induces expression of CLDN-1 and -4 with an increase of Sp1 activity and enhances the barrier and fence functions [5]. p63 negatively regulates nasal epithelial tight junction proteins and their functions in hTERT-HNECs [5]. In the present study, treatment with Y27632 in long-term culture, induced p63 in hTERT-HNECs. Furthermore, with the knockdown of p63 by siRNAs of TAp63 or aNp63, upregulation of RhoA, CLDN-1, CLDN-4 and downregulation of Cx26, Cx30, TRIC, and CYP2C18 were observed. It was thought that Y27632 induced airway progenitor cells via p63.
Kruppel-like factor-11 (KLF11) constitutes a family of transcription factors involved in the regulation of diverse metabolic networks [19. 20]. However, the role of KLF11 remains unknown in airway progenitor cells. In the present study, Y27632 induced KLF11 mRNA and the knockdown of KLF11 by the siRNA prevented the upregulation of p63 and the epithelial barrier induced by Y27632. It is possible that KLF11 induced by Y27632 might enhance airway progenitor cells via p63.
In CRS and NPs, disruption of the epithelial barrier is observed with decreased expression of tight junction proteins [4,5]. Gap junction molecules are increased in CRS [8]. In the nasal epithelium of AR, expression of p63, aNp63, KLF11, Cx30 and RhoA was upregulated and CLDN-4 expression was downregulated. In hTERT-HNECs, treatment with Y27632 enhanced GJIC with an increase of gap junction molecules and the epithelial barrier, and decreases of tight junction molecules and induction of F-actin. Airway progenitor clone formation by Y27632 in long-term culture in part indicated the phenomenon of AR in vitro.
Conclusions
In conclusion, Y27632 in long-term culture induces airway progenitor cells via p63 and KLF11 in hTERT-human nasal epithelial cells. Airway progenitor cells of nasal epithelium induced by Y27632 is important in understanding airway disease-specific characteristics such as AR.
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labour and Welfare of Japan.
Disclosure of conflict of interest
None.
References
- 1.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima T, Go M, Takano K, Kurose M, Ohkuni T, Koizumi J, Kamekura R, Ogasawara N, Masaki T, Fuchimoto J, Obata K, Hirakawa S, Nomura K, Keira T, Miyata R, Fujii N, Tsutsumi H, Himi T, Sawada N. Regulation of tight junctions in upper airway epithelium. Biomed Res Int. 2013;2013:947072. doi: 10.1155/2013/947072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130:1087–1096. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko Y, Kohno T, Kakuki T, Takano K, Ogasawara N, Miyata R, Kikuchi S, Konno T, Ohkuni T, Yajima R, Kakiuchi A, Yokota SI, Himi T, Kojima T. The role of transcriptional factor p63 in regulation of epithelial barrier and ciliogenesis of human nasal epithelial cells. Sci Rep. 2017;7:10935. doi: 10.1038/s41598-017-11481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, Nichols DP, Seibold MA. Airway progenitor clone formation is enhanced by Y-27632-dependent changes in the transcriptome. Am J Resprir Cell Mol Biol. 2016;55:323–336. doi: 10.1165/rcmb.2015-0274MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology. 2001;121:566–579. doi: 10.1053/gast.2001.27060. [DOI] [PubMed] [Google Scholar]
- 8.Kim R, Chang G, Hu R, Phillips A, Douglas R. Connexin gap junction channels and chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:611–617. doi: 10.1002/alr.21717. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SC, Stone C, Tkach L, SundarRaj N. Rho and Rho-kinase (ROCK) signaling in adherens and gap junction assembly in corneal epithelium. Invest Ophthalmol Vis Sci. 2001;43:978–986. [PubMed] [Google Scholar]
- 10.Hampson L, He XT, Oliver AW, Hadfield JA, Kemp T, Butler J, McGown A, Kitchener HC, Hampson IN. Analogues of Y27632 increase gap junction communication and suppress the formation of transformed NIH3T3 colonies. Br J Cancer. 2009;101:829–839. doi: 10.1038/sj.bjc.6605208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candi E, Terrinoni A, Rufini A, Chikh A, Lena AM, Suzuki Y, Sayan BS, Knight RA, Melino G. p63 is upstream of IKKα in epidermal development. J Cell Sci. 2006;119:4617–4622. doi: 10.1242/jcs.03265. [DOI] [PubMed] [Google Scholar]
- 12.Hupin C, Gohy S, Bouzin C, Lecocq M, Polette M, Pilette C. Features of mesenchymal transition in the airway epithelium from chronic rhinosinusitis. Allergy. 2014;69:1540–1549. doi: 10.1111/all.12503. [DOI] [PubMed] [Google Scholar]
- 13.Miyata R, Nomura K, Kakuki T, Takano K, Kohno T, Konno T, Sawada N, Himi T, Kojima T. Irsogladine maleate regulates gap junctional intercellular communication-dependent epithelial barrier in human nasal epithelial cells. J Membr Biol. 2015;248:327–336. doi: 10.1007/s00232-015-9774-0. [DOI] [PubMed] [Google Scholar]
- 14.Uzawa K, Kasamatsu A, Saito T, Takahara T, Minakawa Y, Koike K, Yamatoji M, Nakashima D, Higo M, Sakamoto Y, Shiiba M, Tanzawa H. Long-term culture of human odontoma-derived cells with a Rho kinase inhibitor. Exp Cell Res. 2016;347:232–240. doi: 10.1016/j.yexcr.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Kurose M, Kojima T, Koizumi J, Kamekura R, Ninomiya T, Murata M, Ichimiya S, Osanai M, Chiba H, Himi T, Sawada N. Induction of claudins in passaged hTERT-transfected human nasal epithelial cells with an extended life span. Cell Tissue Res. 2007;330:63–74. doi: 10.1007/s00441-007-0453-z. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. Rock inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gowers KHC, Hynds RE, Thakrar RM, Carroll B, Birchall MA, Janes SM. Optimized isolation and expansion of human airway epithelial basal cells from endobronchial biopsy samples. J Tissue Eng Regen Med. 2018;12:e313–e317. doi: 10.1002/term.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arason AJ, Jonsdottir HR, Halldorsson S, Benediktsdottir BE, Bergthorsson JT, Ingthorsson S, Baldursson O, Sinha S, Gudjonsson T, Magnusson MK. deltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS One. 2014;9:e88683. doi: 10.1371/journal.pone.0088683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomberk G, Grzenda A, Mathison A, Escande C, Zhang JS, Calvo E, Miller LJ, Iovanna J, Chini EN, Fernandez-Zapico ME, Urrutia R. Krüppel-like factor 11 regulates the expression of metabolic genes via an evolutionarily conserved protein interaction domain functionally disrupted in maturity onset diabetes of the young. J Biol Chem. 2013;288:17745–17758. doi: 10.1074/jbc.M112.434670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Chen Q, Yang M, Zhu B, Cui Y, Xue Y, Gong N, Cui A, Wang M, Shen L, Zhang S, Fang F, Chang Y. Mouse KLF11 regulates hepatic lipid metabolism. J Hepatol. 2013;58:763–770. doi: 10.1016/j.jhep.2012.11.024. [DOI] [PubMed] [Google Scholar]


