Abstract
Melittin (Mel), a major component of venom of honey bee (Apismellifera), has various biological effects. Recent researches have reported the anti-tumor activity of Mel in various human cancers, including hepatocellular carcinoma (HCC). In this study, we aimed to further discuss the role of Mel in HCC and investigate the correlation of autophagy with the effect of Mel in HCC cells. Methyl thiazolyl tetrazolium (MTT) assay and flow cytometry were used to detect the viability and apoptosis of HCC cells, respectively. To examine the changes of autophagy in HCC cells treated with Mel, transmission electronmicroscope (TEM) and immunofluorescence detection were adopted. Finally, we used western blot method to detect the changes of pivotal proteins in autophagy and mitochondrial apoptotic pathways. The results of MTT assay and flow cytometry revealed that Mel could suppress the cell viability and promote the apoptosis of HCC cells. Autophagy could be induced by the treatment with Mel in HCC cells. The inhibition of autophagy by chloroquine (CQ) contributed to the enhanced anti-tumor effect of Mel, but autophagy induction by RAPA decreased Mel effect in HCC cells. Mel was closely associated with the expression of proteins in mitochondrial apoptotic pathway. In summary, Mel could induce the autophagy of HCC cells, and the autophagy might offer protection against apoptosis in HCC. Mel might suppress the tumor through activating mitochondrial apoptotic pathway.
Keywords: Hepatocellular carcinoma cell, melittin, autophagy, apoptosis, chloroquine, rapamycin
Introduction
Hepatocellular carcinoma (HCC), a serious threat to human health, is the third leading cause of tumour-related deaths worldwide, resulting in about 700,000 deaths each year [1]. Despite advances in both interventional surgery and chemoradiotherapy, the five-year survival rates of HCC patients remain low, especially for those diagnosed with middle or late stages. Thus, it is urgent to find more effective anti-HCC drugs. During the past few decades, the traditional Chinese medicine (TCM) has received more and more attentions for its application value in managements of human malignancy. Modern researches demonstrate that melittin (Mel), one component of TCM bee venom, has a broad range of biological activities, such as inhibiting growth of multiple tumour cells [2,3], including HCC [4-7].
Autophagy means timely preventing the occurrence of cellular abnormalities such as tumourigenesis, and eliminating certain macromolecular substances (like old or damaged organelles and proteins that are mistakenly synthesized or folded) and small molecular substances including amino acids and fatty acids that can be recycled by cells [8,9]. Increasing evidences have illustrated the close relationships between autophagy and tumour development. Both autophagy inhibition and induction have been repeatedly discussed in tumour researches [10,11].
Chloroquine (CQ) has been extensively used for malaria treatment [12]. Moreover, it has been revealed to be able to inhibit autophagy through effectively blocking the combination of autophagosomes with lysosomes, which is the formation of autolysosomes. In addition to inhibiting autophagy, CQ has also been found to possess certain anti-tumor capacities [13,14]. As an anti-tumour polypeptide, Mel plays its role through activating the autophagy of tumour cells. Rapamycin (RAPA) is an activator of autophagy which is widely used in autophagy researches.
In our study, the anti-tumor action of Mel as well as the related mechanisms in tumor progression of HCC was investigated. In addition, we also found that Mel could induce the autophagy of HCC cells. Through the use of CQ and RAPA, the relationship between autophagy induced by Mel and its anti-tumour effect were studied in the present study.
Materials and methods
Materials
Mel (with a purity more than 97.06%) was synthesized by Shanghai ABBiochem Co., Ltd China, with amino acid sequence as GIGAVLKVLTTGLPALISWIKRKRQQ-NH2. The peptide was dissolved in phosphate buffer solution (PBS) with a stock concentration of 1 mg/mL, and then stored at -20°C. CQ and rapamycin (RAPA) were purchased from Selleck. The compound was dissolved in dimethylsulfoxide (DMSO) with a stock concentration of 50 mM, and then stored at -20°C. The final concentration of DMSO did not exceed 0.1% throughout the study. Fetal bovine serum (FBS) was purchased from Biowest (Shanghai, China) while Dulbecco’s modified Eagle’s medium (DMEM) and Roswell Park Memorial Institute-1640 (RPMI-1640) medium were purchased from Hyclone (Carlsbad, CA, USA). Trypan Blue was purchased from Shanghai Boguang biological technology co., Ltd. Annexin V-FITC (fluorescein isothiocyanate)/PI (propidium iodide) kit was purchased from BD Biosciences (NJ, USA). Antibodies of LC3, p62, Beclin 1 and cleaved caspase-3/9 (Asp175), and procaspase-3/9 were purchased from Cell Signaling Technology (CST, USA) except those specifically indicated. Plasmid of eGFP-LC3 was obtained from Addgene (NJ, USA).
Cell culture
Human HCC cell (HepG2) was purchased from Cell Bank of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). HepG2 cells were maintained in DMEM with 10% FBS. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Cell viability assay
Methyl thiazolyl tetrazolium (MTT) was used to detect the proliferation ability of hepatoma HepG2 cells at different concentrations of Mel for 24 h, 48 h and 72 h, respectively. Inverted phase contrast microscope was applied to observe the morphological changes of hepatoma HepG2 cells in different concentrations of Mel for 24 h.
The cytotoxic activity in hepatoma HepG2 cells induced by diverse concentrations of Mel for different treatment periods was measured by MTT assay. The treatment time was respectively set as 24 h, 48 h and 72 h. Cells were placed in 96-well culture plates (5×103 cells/well). After incubation over night, cells were treated with different concentrations of Mel for indicated time. MTT (5 mg/mL) was dissolved in PBS, and filter was sterilized. Then, 20 μl prepared solution was added to each well and incubated for 4 h. The formed formazan crystals were dissolved in DMSO (150 μl/well). The absorbance was measured using an ELISA reader (Spectra Max Plus384, Molecular Devices, Sunnyvale, CA), with a test wave length of 570 nm and a reference wave length of 650 nm. Inhibitory rate was calculated using the following formula [15]: Inhibitory rate (%) = [1-(At/As)]×100%.
“At” and “As” denoted the absorbance of tested substances and solvent control (solvent without cells), respectively.
Flow-cytometric quantification of apoptosis
Annexin V-FIFC/PI double staining was used to detect the apoptosis rate of hepatoma HepG2 cells in different concentrations of Mel for 24 h. HepG2 cells were cultured in six-well plates under a humidified atmosphere with 5% CO2 at 37°C overnight, and then treated with various concentrations of Mel for 24 h. Cells were trypsinized and stained with Annexin V-FITC/PI, and measured by FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Annexin V+/PI-cells were considered as apoptotic cells.
Trypan blue-positive assay
Trypan blue staining was used to detect the cell death of hepatoma HepG2 cells in different concentrations of Mel for 24 h. The percentage of trypan blue-positive cells was counted using trypan blue (Bio-Rad 145-0021) in triplicate for each condition, with a minimum of 100 cells for each sample. For Sytox Green staining, cells were cultured in Lab-Tek chamber slides (Thermo Scientific), and Sytox Green (100 nM) (Invitrogen, S7020) was added in the medium 1 h after various treatments. After being washed with PBS for one time, Sytox Green-positive cells were visualized using a Zeiss Axioplan 2 microscope, and counted in triplicate for every condition, with a minimum of 100 cells for each sample.
Transmission electronmicroscope (TEM)
Human hepatoma HepG2 cells at logarithmic phase were digested for single-cell suspension, and then placed in 6-well culture plates. After cell adhesion with a fusion degree reaching 70%, they were divided into different groups for drug intervention. The collected cells were digested and then transferred into EP tubes. After centrifugation at 1000 rpm for 5 min, the supernatant was removed and stationary liquid was added at 4°C for the fixation of the remaining solution for 2-4 h. After embedding, sectioning, double-staining with 2% saturated uranyl acetate water solution and lead citrate for 15 min, respectively, and drying sections at room temperature overnight, TEM (TitanTM) was used for observing and photographing.
TEM was applied to observe the apoptotic and autophagic changes of HepG2 cells in 5 μg/mL Mel for 24 h.
Immunofluorescence detection
Immunofluorescence was used to assess the expression of Beclin 1 in HepG2 cells cultured with Mel. Single-cell suspension was obtained via digesting HepG2 cells at logarithmic phase. Sterile slides were put into 6-well plates in advance. After cell adhesion and reaching a fusion degree of 70%, they were grouped and then intervened with drugs for 24 h. The culture solution was washed with PBS, fixed with 4% paraformaldehyde for 30 min, and then washed with PBS two times. Then dehydration was performed with gradient ethanol. After antigen retrieval, Beclin 1 first antibody (diluted at 1:100) was added. Then the mixture was incubated at 4°C overnight. After the second antibody was added, the incubation was carried out at room temperature in dark for 1 h. The cell nucleus was counter-stained with DAPI. After incubation at room temperature in dark for 10 min, antifade mounting medium was added. Then, the samples were observed with an inverted fluorescence microscope, and photos were collected. The Image pro-plus 6.0 software was adopted for data analyses during immunofluorescence.
Transfection of small interfering RNA (siRNA) or pEGFP-LC3
Hepatoma HepG2 cells were transfected with pEGFP-LC3 plasmid to test the expression of LC3 using Confocal laser scanning microscope. siRNA for human Beclin 1 was purchased from CST. Cells were placed in 6-well plates for one night. Human hepatoma HepG2 cells at logarithmic phase were digested for single-cell suspension. The cells were handled to reach a density of 5×105, and inoculated into 6-well plates. After cell adhesion, the culture medium was replaced with serum-free and antibody-free RPMI-1640 culture medium. When the confluence reached 60-80%, plasmid transfection was performed in accordance with the steps listed on PEI Instructions. Within 4-6 hours after successful transfection, the culture solution was replaced with the culture medium containing FBS in preparation for induced autophagy later. The expression of green fluorescent protein was observed with a microscope under blue light channel.
Western blot analysis
Western blot was used to detect the expressions of LC3-I, LC3-II, Beclin 1 and p62 in hepatoma HepG2 cells cultured with different concentrations of Mel for 24 h and those with 5 μg/mL Mel for different duration. Whole cell lysates were prepared in a lysis buffer containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 150 mM NaCl, 0.02% NaN3, 100 mg/mL PMSF, 1 mM DTT, 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 1% Triton X-100. Total cell proteins and nuclear proteins were size-fractionated by SDS-PAGE, and electrotransferred to Immobilon-P membranes (Millipore, USA). Specific proteins were detected using enhanced chemiluminescence following the manufacturer’s instructions (Amersham Biosciences).
Total cell lysis, nuclear and mitochondria extracts were prepared according to the manufacturer’s instructions. Western blot analysis was performed as previously described. Briefly, the proteins were separated by SDS-PAGE and electrophoretically transferred onto polyvinylidene fluoride membranes (Millipore). Antibodies against Bcl-2, cytochrome c, caspase-3, cleaved caspase-3, caspase-9 and cleaved caspase-9 were used as primary antibodies. HRP-conjugated goat anti-rabbit IgG was used as the secondary antibody. Bound immuno-complexes were detected using ChemiDOC XRS system (BioRad Laboratories, Hercules, CA).
Statistical analysis
All experiments were performed at least three times unless otherwise stated. The results were analyzed using one-way ANOVA with Tukey multiple comparison test. The data were shown as the mean ± SD. Differences with P<0.05 were considered statistically significant.
Results
Mel inhibits HCC cell growth and induces cell apoptosis in vitro
In this study, we firstly used MTT method to measure the effect of Mel on the viability of human HCC cells (HepG2). The IC50 (50% inhibition of cell viability) values for Mel in HepG2 cells were shown in Table 1. As demonstrated in Figure 1A, Mel could inhibit HCC cell proliferation, and such inhibitory effect was dependent on Mel concentration and treatment time.
Table 1.
The IC50 values of Mel in human HCC cell lines
| Human HCC cell lines | IC50 values (μg/mL) | ||
|---|---|---|---|
|
| |||
| 24 h | 48 h | 72 h | |
| HepG2 | 5.942±0.334 | 5.651±0.323 | 4.877±0.334 |
Data are expressed as the mean ± SD according to the dose-response curves from at least three independent experiments.
Figure 1.
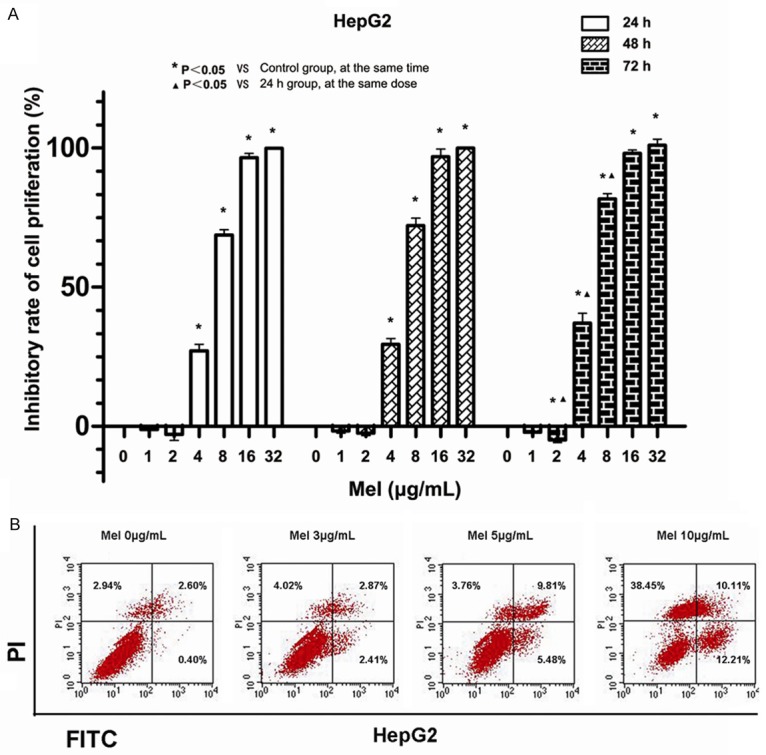
Effect of Mel on the proliferation and apoptosis of HepG2 cells. A. The cells were treated with different concentrations of Mel for 24, 48 and 72 h, respectively. The cell viability measured by MTT indicated that Mel could inhibit cell proliferation, and the inhibition was increased along with the levels of Mel and treatment time (all P<0.05). B. Mel induces the apoptosis of HepG2 cells, and the induction was promoted with increasing concentration of Mel.
HepG2 cells were treated using Mel with gradient concentration of 0, 3, 5, and 10 μg/mL for 24 h to detect the impact of Mel on apoptosis. Annexin V-FITC/PI double-staining apoptosis detection kit and flow cytometry were used to detect the apoptosis degree. As reflected in Figure 1B, the left bottom demonstrated the proportion of normal viable cells, the right bottom displayed the proportion of cells in early apoptosis, the upper right showed the proportion of cells in late apoptosis and necrocytosis, and the upper left exhibited the proportion of cell debris and a few necrotic cells. The results of flow cytometry suggested that the proportions of apoptotic and necrotic cells were increased in the Mel group, and the proportion increased with increasing Mel dose (Figure 1B).
Mel induces autophagy in HCC cell lines in vitro
In the presents study, the correlation of Mel with autophagy was examined in HCC cells. Through the application of TEM, the formation of autophagosome in HepG2 cells was observed after the intervention with an effective dose of Mel (5 μg/mL) for 24 h. As shown in Figure 2A, the number of autophagosomes (pointed by the black arrows) was higher in Mel group than that in the control group.
Figure 2.
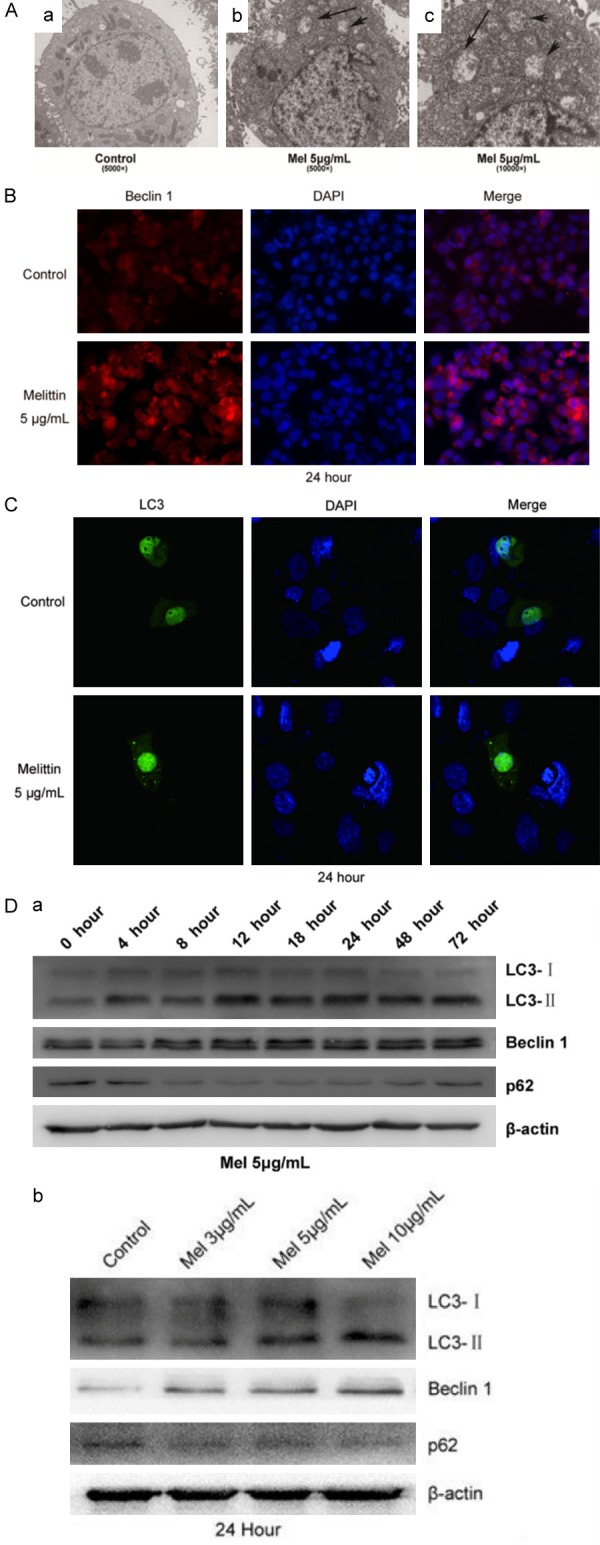
Effect of Mel on autophagy in HCC cells. A. The number of autophagosomes (pointed by the black arrows) was higher in Mel group than in the control group. B. Beclin 1 expression in HCC cells was increased after treated with Mel (5 μg/mL) for 24 h. C. LC3 expression was elevated in HCC cells after treated with Mel (5 μg/mL) for 24 h. D. Effect of Mel on cell autophagic-associated proteins. The rates of LC3-II/LC3-I to Beclin 1 were increased with time lapse and increasing dose of Mel in intervention. The degradation product p62 was decreased with the time lapse and increasing dose of Mel in intervention.
In addition to the autophagosomes, the expression of Beclin 1 protein was also measured using immunofluorescence technique. As shown in Figure 2B, the expression of Beclin 1 was significantly elevated in HCC cells treated with 5 μg/mL Mel for 24 h when compared with the control group.
To further comprehensively know about the condition of autophagy, the HepG2 cells were transfected with p-EGFP-LC3 plasmid. After the intervention with Mel, we used a laser scanning confocal microscope to observe the expression of green fluorescence protein GFP-LC3 in each group. The cell nucleus was marked with DAPI, and LC3 was marked with green fluorescence. After intervention with Mel (5 μg/mL) for 24 h, the expression of LC3 in HepG2 cells was increased when compared with the control groups (Figure 2C).
Finally, the expression of autophagy markers LC3-I, LC3-II, Beclin 1 and p62 in different periods after intervention with different concentrations of Mel in HepG2 cells were detected using western blot technology. The results shown in Figure 2D revealed that the rates of LC3-II/LC3-I to Beclin 1 increased with time lapsing in a manner dependent on the dose of Mel in intervention. As the degradation product of autophagy, p62 was decreased along with the time and dose of Mel intervention.
Influence of CQ on the anti-tumour effect of Mel in human HCC cells
CQ is one of the classic inhibitors of autophagy, which can block the formation of autolysosome [16]. To clarify the above mentioned questions, we pre-treated the HCC cells with CQ for 4 h, and then compared the anti-tumour effect of Mel in HCC cells.
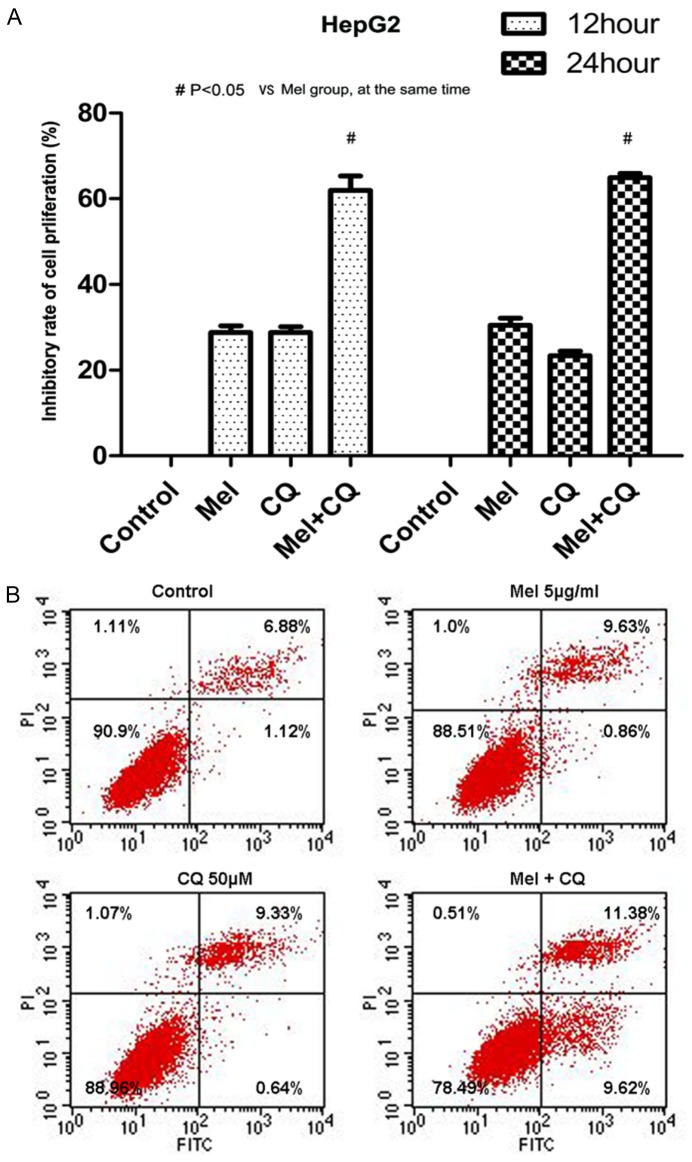
We pre-treated human hepatoma HepG2 cells using CQ and Mel alone or together for 12 h and 24 h, respectively, and then the cell proliferation was detected. As shown in Figure 3A, CQ had certain inhibitory effects on the proliferation of HepG2 cells. The differences in proliferation inhibitory effects on HepG2 cells between the two time points of 12 h and 24 h were not statistically significant. At both points of 12 h and 24 h, the inhibitory rates of Mel+CQ group was apparently higher than those of Mel group, and the differences were statistically significant (P<0.05). The combined use of CQ and Mel might enhance the inhibitory effects induced by Mel in HepG2 cells.
Figure 3.
The anti-tumor effect of Mel was promoted by CQ in HCC cells. A. The inhibitory effect of Mel against cell proliferation was promoted by CQ (P<0.05). B. The effects of Mel on cell apoptosis was enhanced by the application of CQ.
Similarly, 50 μM CQ was used alone or together with 5 μg/mL Mel for 24 h. And AV/PI double-staining was used to detect the apoptosis of HepG2 cells in different groups. As shown in Figure 3B, CQ+Mel had stronger apoptosis-inducing effect on HepG2 cells than Mel alone, and the proportion of apoptotic cells was also higher (P<0.05).
Influence of RAPA on the anti-tumour effect of Mel in human HCC cells
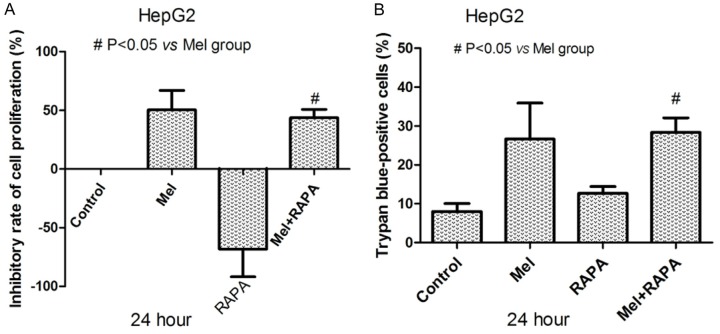
MTT assay indicated that RAPA could promote the proliferation of human HCC cells and reduce the inhibitory effect of Mel on the proliferation of HepG2 cells (P<0.05, Figure 4A). The results from trypan blue dye exclusion technique suggested that RAPA could reduce the apoptosis-inducing effect of Mel in human hepG2 cells (P<0.05, Figure 4B).
Figure 4.
Influence of RAPA on anti-tumor effects induced by Mel in HCC cells. A. The promoting effect of RAPA on cell proliferation was suppressed by Mel (P<0.05). B. The number of death cells resulting from Mel was decreased by using RAPA (P<0.05).
Impact of crosstalking between autophagy and apoptosis on the effect of Mel in human hepatoma HepG2 cells
CQ, RAPA, and Mel were used alone or together in hepG2 cells for 24 h, and western-blot was used to detect the protein expression. When Mel was used to induce the autophagy of human HCC cells, the expression of Beclin 1 showed distinct increase. In addition, Beclin 1 played a role in the cross-talking between autophagy and apoptosis through a complex system formed by Bcl-2. Bcl-2 is an anti-apoptosis protein that can bind with over-expressed Beclin 1 and reduce the expression of the latter. The Cyto-C/caspase-9 proteins were crucial members in mitochondrial apoptotic pathway which were modulated by Bcl-2, so they were also examined in this study.
As shown in Figure 5A, Mel could induce the autophagy of hepG2 cells, up-regulate Beclin 1 protein expression, down-regulate the autophagy degradation substrate p62, and inhibit the expression of anti-apoptosis protein Bcl-2. When Mel was used in combination with the autophagy inhibitor CQ, it possessed weaker down-regulating effect on Bcl-2, promoted the Cyto-C expression, activated the expressions of downstream caspase 9 and caspase 3, and increased cell apoptosis. However, opposite effects were observed when the autophagy inducer RAPA was used together with Mel. Figure 5B showed that when the essential autophagy protein Beclin 1 was silenced, p62 was decreased, demonstrating inhibited autophagy. Meanwhile, silenced Beclin 1 could also enhance the down-regulating effect of Mel on Bel-2, promote Cyto-C expression, activate downstream caspase 9 and caspase 3 expressions, and enhance cell apoptosis, showing a similar impact as that of CQ.
Figure 5.
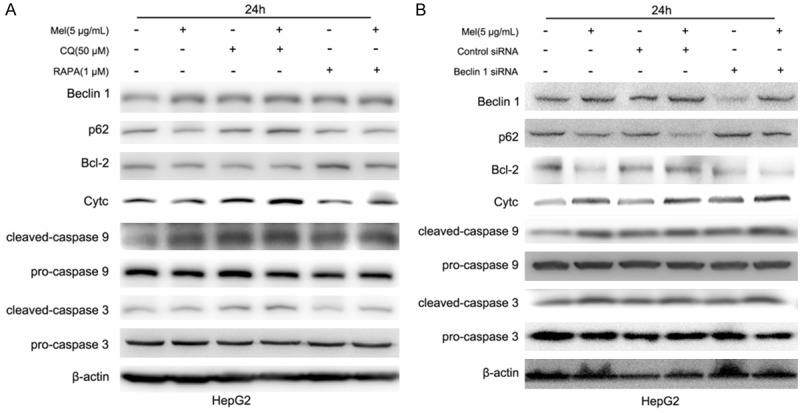
Expression changes of crucial proteins in HCC cells treated with Mel. A. Impact on the expression of proteins related to human hepatoma cells, Beclin 1, p62, Bcl-2, Cyto-C, caspase 9 and caspase 3 when Mel, CQ, and RAPA were used alone and together. B. The influence of silenced Beclin 1 on the role of Mel in regulating the expressions of Beclin 1, p62, Bcl-2, Cyto-C, caspase 9 and caspase 3.
Discussion
It is reported that autophagy has close relationship with cancer development [10,17,18]. Thus, drugs that can regulate autophagy may be involved in tumour progression [19,20]. Mel has a wide spectrum of killing effects against multiple types of cells. Previous studies have found that Mel exerts inhibitory effects on proliferation of multiple tumour cells, including hepatoma cells [5,6,11,17,20]. In this study, we firstly used MTT method to measure the effects of Mel on the viability of human HCC cells (HepG2). The results indicated that Mel could inhibit HCC cell proliferation. Therefore, Mel might be associated with HCC development. In the present study, we aimed to further explore the mechanism of anti-tumour effect of Mel in human HCC cells.
In this research, the correlation of Mel with autophagy was examined in HCC cells. Our study demonstrated that Mel could induce autophagy of human hepatoma cells. However, the autophagy has dual functions in human malignancy [17]. The role of Mel-induced autophagy in HCC cells remained unclear. Thus, CQ and RAPA were applied to investigate the effects of Mel-induced autophagy in HCC cells.
CQ is one of the classic inhibitors of autophagy, which can inhibit autophagy via blocking the formation of autolysosome [16]. To clarify the role of autophagy in HCC cells, we pre-treated the HCC cells with CQ for 4 h. Studies indicated that Mel could inhibit viability, and induce the autophagy of human HCC cells. However, the anti-HCC effect of Mel might be enhanced when autophagy was inhibited by the application of CQ. RAPA represents a commonly used inducer of autophagy since it could provoke autophagy through mTOR-mediated pathway [21]. When HCC cells were co-cultured with RAPA and Mel, the viability of the cells were significantly enhanced, compared with Mel-treatment alone. All the results demonstrated that Mel-induced autophagy was a cellular protection approach in Mel-mediated apoptosis in HCC.
There are three apoptotic signaling pathways, namely endoplasmic reticulum signaling pathway, death receptor signaling pathway and mitochondrial signaling pathway [22]. Mel exhibits significant pro-death effects on cancer cells, leading to cell apoptosis and necrosis [2]. In the death receptor signaling pathway, Mel can up-regulate the expression of Fas [4,7] which participates in the activation of caspase 3 and the induction of cell apoptosis [23]. Moreover, mitochondrion is another important target for the anti-tumor effect of Mel [7]. In Candida albicans, Lee et al. [24] recently found that Mel was involved in the mitochondria-and caspase-dependent apoptotic pathway which was mediated by Cyto-C release. In cancer researches, Mel was found to be able to induce the expression of mitochondrial membrane antigen 7A6 [7] and regulate the expression of the mitochondrial membrane protein Bcl-2. The mitochondrial apoptotic pathway involves opening mitochondrial permeability transition pore (MPTP) [25,26]. Apoptotic factors, such as Cyto-C and apoptosis inducing factor (AIF), are released from mitochondria, that ultimately induces the caspase cascade and results in cell death. Some studies on Mel have focused on the regulation of proteins in Bcl-2 family, including Bcl-2, Bcl-XL, and Bid (in the anti-apoptotic Bcl-2 family). Bcl-2 is activated after dissociating from NF-κB, and then inhibits reactive oxygen species (ROS) production and opens MPTP [27]. Bcl-2 can also be activated by signal transducer and activator of transcription 3 (STAT3) which is considered as an oncogene in human cancers [26-28]. Mel displays a significant inhibitory impact on STAT3 [29]. Activated STAT3 upregulates various anti-apoptotic genes (Bcl2x), cyclin regulatory genes (cyclins D1/D2), and angiogenic proteins like vascular endothelial growth factor (VEGF). The NF-κB signaling pathway is involved in the anti-tumor properties of Mel. Through binding to different genes, NF-κB could possess either cancer promoting or anti-tumor effect [30,31]. After activation, NF-κB predominantly interacts with TNF-α, and affects multiple inflammatory factors. It could also activates the expression of Bax and other pro-apoptotic mitochondrial proteins, resulting in apoptosis. Activated NF-κB promotes the transcription of anti-apoptotic genes, such as Bcl-2. Bcl-2 interrupts TNF-α-induced caspase activation, and thus inhibits apoptosis [32]. Cytoskeletal proteins are degraded, and then necrosis occurs.
A major non-mTOR dependent signal pathway in the autophagy is the beclin 1-Hvps34 pathway. Some studies have shown that Mst1 (mammalian Ste20-like kinase 1) could phosphorylate the Thr108 of the N-terminal of Beclin 1-BH3 structural domain, enhance the interaction between Beclin l and Bcl-2 or Bcl-xL hydrophobic groove α3 spiral, stabilize Beclin l homodimer, and decrease the combination between Atg14L and Beclin 1. Decreased activity of Beclin 1-P13K-Atg14L complex lipid kinase can inhibit autophagy [33]. The death-related protein kinase DAPK1 can dissociate Beclin 1 from Bcl-2 and/or Bcl-xL through phosphorylating Beclin 1 protein, and then form Beclin 1-P13K-Atgl4L-Vps34 complex lipid kinase with Atgl4L, thus initiating the formation of autophagosomes [34,35]. In the present study, we found that Mel regulated autophagy and apoptosis in a cross-talking manner through the cross-talk protein Bcl-2. When autophagy was inhibited, Mel could further downregulate Bcl-2 expression, promote the release of Cyto-C and activate the mitochondrial apoptotic pathway, thus exerting a more powerful role in inducing cell apoptosis. Our study had provided a new insight into the relationship between Mel and autophagy in HCC cells.
Conclusions
In conclusion, to our best knowledge, we demonstrated for the first time that Mel could induce autophagy in human hepatoma cells, but the induced autophagy might be a cellular protective approach for Mel-mediated cell apoptosis in HCC. Mel might possess anti-tumor effects in HCC through mitochondrial apoptotic pathway.
Acknowledgements
This work was supported in part by National Natural Science Foundation of China 30772877 (to BL) and Grant from Natural Science Foundation of Shanghai 15ZR1412800 (to FF), 07ZR14138 (to BL) and 12ZR1437400 (to LW).
Disclosure of conflict of interest
None.
References
- 1.Au JS, Frenette CT. Erratum: management of hepatocellular carcinoma: current status and future directions. Gut Liver. 2015;9:811. doi: 10.5009/gnl15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajski G, Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Orsolic N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31:173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoodzadeh A, Zarrinnahad H, Bagheri KP, Moradia A, Shahbazzadeh D. First report on the isolation of melittin from Iranian honey bee venom and evaluation of its toxicity on gastric cancer AGS cells. J Chin Med Assoc. 2015;78:574–583. doi: 10.1016/j.jcma.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Kong GM, Tao WH, Diao YL, Fang PH, Wang JJ, Bo P, Qian F. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J Gastroenterol. 2016;22:3186–3195. doi: 10.3748/wjg.v22.i11.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong YJ, Choi Y, Shin JM, Cho HJ, Kang JH, Park KK, Choe JY, Bae YS, Han SM, Kim CH, Chang HW, Chang YC. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem Toxicol. 2014;68:218–225. doi: 10.1016/j.fct.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Qu L, Jiang M, Li Z, Pu F, Gong L, Sun L, Gong R, Ji G, Si J. Inhibitory effect of biosynthetic nanoscale peptide melittin on hepatocellular carcinoma, driven by survivin promoter. J Biomed Nanotechnol. 2014;10:695–706. doi: 10.1166/jbn.2014.1830. [DOI] [PubMed] [Google Scholar]
- 8.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 9.Ryter SW, Choi AM. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015;4:215–225. doi: 10.1016/j.redox.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SF, Wang XY, Fu ZQ, Peng QH, Zhang JY, Ye F, Fu YF, Zhou CY, Lu WG, Cheng XD, Xie X. TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy. 2015;11:225–238. doi: 10.1080/15548627.2014.998931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–678. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waqar T, Khushdil A, Haque K. Efficacy of Chloroquine as a first line agent in the treatment of uncomplicated malaria due to plasmodium vivax in children and treatment practices in Pakistan: a Pilot study. J Pak Med Assoc. 2016;66:30–33. [PubMed] [Google Scholar]
- 13.Sasaki K, Tsuno NH, Sunami E, Tsurita G, Kawai K, Okaji Y, Nishikawa T, Shuno Y, Hongo K, Hiyoshi M, Kaneko M, Kitayama J, Takahashi K, Nagawa H. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. doi: 10.1186/1471-2407-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Shi X, Li Y, Fan J, Zeng X, Xian Z, Sun Y, Wang S, Song P, Zhao S, Hu H, Ju D. Blocking autophagy enhanced cytotoxicity induced by recombinant human arginase in triple-negative breast cancer cells. Cell Death Dis. 2014;5:e1563. doi: 10.1038/cddis.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z, Yao Y. The influence of di-acetylation of the hydroxyl groups on the anti-tumor-proliferation activity of lutein and zeaxanthin. Asia Pac J Clin Nutr. 2007;1:447–452. [PubMed] [Google Scholar]
- 16.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarzynska JM. The importance of autophagy regulation in breast cancer development and treatment. Biomed Res Int. 2014;2014:710345. doi: 10.1155/2014/710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorzalczany Y, Gilad Y, Amihai D, Hammel I, Sagi-Eisenberg R, Merimsky O. Combining an EGFR directed tyrosine kinase inhibitor with autophagy-inducing drugs: a beneficial strategy to combat non-small cell lung cancer. Cancer Lett. 2011;310:207–215. doi: 10.1016/j.canlet.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Qin W, Li C, Zheng W, Guo Q, Zhang Y, Kang M, Zhang B, Yang B, Li B, Yang H, Wu Y. Inhibition of autophagy promotes metastasis and glycolysis by inducing ROS in gastric cancer cells. Oncotarget. 2015;6:39835–39854. doi: 10.18632/oncotarget.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian CY, Wang KL, Fang FF, Gu W, Huang F, Wang FZ, Li B, Wang LN. Triple-controlled oncolytic adenovirus expressing melittin to exert inhibitory efficacy on hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:10403–10411. [PMC free article] [PubMed] [Google Scholar]
- 21.Urbanek T, Kuczmik W, Basta-Kaim A, Gabryel B. Rapamycin induces of protective autophagy in vascular endothelial cells exposed to oxygen-glucose deprivation. Brain Res. 2014;1553:1–11. doi: 10.1016/j.brainres.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann KC, Green DR. How cells die: apoptosis pathways. J Allergy Clin Immunol. 2001;108:S99–103. doi: 10.1067/mai.2001.117819. [DOI] [PubMed] [Google Scholar]
- 23.Punsawad C, Viriyavejakul P, Setthapramote C, Palipoch S. Enhanced expression of Fas and FasL modulates apoptosis in the lungs of severe P. falciparum malaria patients with pulmonary edema. Int J Clin Exp Pathol. 2015;8:10002–10013. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Lee DG. Melittin triggers apoptosis in Candida albicans through the reactive oxygen species-mediated mitochondria/caspase-dependent pathway. FEMS Microbiol Lett. 2014;355:36–42. doi: 10.1111/1574-6968.12450. [DOI] [PubMed] [Google Scholar]
- 25.Bonora M, Pinton P. The mitochondrial permeability transition pore and cancer: molecular mechanisms involved in cell death. Front Oncol. 2014;4:302. doi: 10.3389/fonc.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li D, Liu M, Tao TQ, Song DD, Liu XH, Shi DZ. Panax quinquefolium saponin attenuates cardiomyocyte apoptosis and opening of the mitochondrial permeability transition pore in a rat model of ischemia/reperfusion. Cell Physiol Biochem. 2014;34:1413–1426. doi: 10.1159/000366347. [DOI] [PubMed] [Google Scholar]
- 27.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Qu Z, Yan S, Sun F, Whitsett JA, Shapiro SD, Xiao G. Differential roles of STAT3 in the initiation and growth of lung cancer. Oncogene. 2015;34:3804–3814. doi: 10.1038/onc.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 31.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer. 2012;12:121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Wu W, Huang W, Hu G, Yuan W, Li W. NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm Res. 2013;62:387–397. doi: 10.1007/s00011-013-0590-7. [DOI] [PubMed] [Google Scholar]
- 33.Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J. Mst1 inhibits autophagy by promoting the interaction between Beclin 1 and Bcl-2. Nat Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg-Lerner A, Kimchi A. PKD is a kinase of Vps34 that mediates ROS-induced autophagy downstream of DAPk. Cell Death Differ. 2012;19:788–797. doi: 10.1038/cdd.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin-Salomon V, Bialik S, Kimchi A. DAP-kinase and autophagy. Apoptosis. 2014;19:346–356. doi: 10.1007/s10495-013-0918-3. [DOI] [PubMed] [Google Scholar]