Abstract
Distraction osteogenesis (DO) is one of the most promising reconstructive methods for repairing large craniofacial defects or growth deficiencies through bone regeneration, but it is also a challenge because of an undesirably long process and its complications, which limit its application in clinical practice. The transplantation of mesenchymal stem cells (MSCs) is regarded as an innovative approach to accelerate bone regeneration. Dental pulp stem cells (DPSCs) have shown some advantages over other human adult MSCs, and DPSCs have been regarded as one of the most promising cell sources used in the endogenous tissue engineering. Furthermore, using stem cells modified by gene engineering in DO has been reported in previous studies. It has been shown that Sirtuin-1 (SIRT1) can directly regulate the differentiation of MSCs into osteoblasts. In this study, DPSCs expressing SIRT1 were prepared and their effects on the new bone formation were further investigated in rabbits with tibia. Rabbits were injected with the adenovirus (Adv)-SIRT1-green fluorescent protein (GFP)-transfected DPSCs (overexpression group, Group OE), Adv-GFP transfected DPSCs (negative control group, Group NC) or physiologic saline (control group, Groups CON) into the distraction gap. The new bone tissues in the distraction gap were harvested 8 weeks later, and subjected to by radiographic examination, micro-CT evaluation, and histological and mechanical testing. The better bone formation, the highest bone mineral density (BMD) and the highest bone mineral content (BMC) were observed in the OE group. In conclusion, SIRT1-modified DPSCs in DO was more effective to promote new bone formation during DO, which provides evidence for further investigation about the role of of SIRT1 in the DO.
Keywords: Distraction osteogenesis, dental pulp stem cells, SIRT1, bone formation
Introduction
Distraction osteogenesis (DO) is an endogenous process that promotes bone regeneration in both long bones and maxillofacial bones, and simultaneously, the soft tissues including muscles, nerves, skin and blood vessels are also elongated [1-3]. DO is also an orthopaedics surgery that provides a way to reconstruct the skeleton deformities in clinical practice, but the mechanism of DO has still not yet completely revealed. DO activates the endogenous tissue regeneration and new bone formation in the distraction gap. It has been reported that there are proliferation, differentiation, angiogenesis, ossification, and remodeling during DO in the enlarging gap [4-6]. These complicated processes have the risk of causing many uncontrolled complications (such as delayed union and nonunion enlarging gap), which limits its application in clinical application [7]. It is necessary to develop a safe and efficient way for the improvement of DO in clinical practice.
Dental pulp stem cells (DPSCs), a type of mesenchymal stem cells (MSCs), are multipotent cells which can differentiate into distinct specialized cell types, such as osteocytes, chondrocytes, and adipocytes [8-10]. Furthermore, it has been reported that DPSCs are more effective in proliferation and osteogenesis, and have lower immunogenicity than mesenchymal stem cells (MSCs) [11]. DPSCs originate from dental pulp tissues, and have become a kind of seed cells in regenerative medicine and tissue engineering because of their easier collection and higher osteogenic capacity as compared to MSCs [11]. Recently, it has been demonstrated that MSCs play a pivotal role during DO, which is regulated by series of signals such as proteins, drugs [12-14]. We have found that DPSCs have the osteogenic potential and can be used during DO [15]. Some studies have been conducted to investigate the effects of growth factors, hormonal proteins, miRNAs and some other biological factors on the DO [6,16,17], and findings reveal these factors can potentially enhance the osteogenesis and shorten the consolidation period after MSCs transplantation. In addition, injection of gene-modified DPSCs into the distraction gap can accelerate the distraction rate in a rabbit model [18].
Sirtuin 1 (SIRT1), an nicotinamide adenine dinucleotide (NAD) - dependent class III protein deacetylase, has been reported to involve in the regulation of various cellular processes, including bone homeostasis, gene expression, and metabolic pathways [19,20]. SIRT1 knockout mice show a reduction in bone mass characterized by increased marrow adipogenesis and decreased bone formation [21]. Moreover, SIRT1 is able to control the differentiation of MSCs into osteoblasts by up-regulating the expression of maker genes of osteogenesis and inhabiting adipogenic differentiation [22]. SIRT1 overexpression or activation enhances new bone formation characterized by the increased quality and quantity of bone regeneration [23]. These findings were confirmed in our previous study [24]. Thus, we hypothesized that the up-regulation of SIRT1 expression might promote bone regeneration during DO.
In this study, SIRT1 gene-modified DPSCs were prepared and injected into the tibia distraction gap, aiming to investigate the role of SIRT1 in the new bone formation after DPSCs transplantation during DO.
Materials and methods
Animals
Thirty-six mature (2.5-3.0 kg) male New Zealand white rabbits were used in this study. The study protocol was approved by the Animal Care Committee of Nantong University. All rabbits were anesthetized by intravenous injection of ketamine hydrochloride and xylazine at 20 mg/kg and 5 mg/kg body weight, respectively.
Isolation, culture and treatment of DPSCs
The extracted normal human impacted third molars completely were collected from patients aged 18-28 years (n = 9) and the informed consent was obtained from each patient at the Dental Department of the Affiliated Hospital of Nantong University, which was approved by the Ethics Committee of the Affiliated Hospital of Nantong University (2016--077). All subjects had neither carious lesions nor any other oral infection. The pulp tissues were separated from the crown and root completely, and then incubated with digestive solution (3 mg/mL type I collagenase, 4 mg/mL dispase in 4 mL of phosphate-buffered saline (PBS), 100 U/mL penicillin, 100 μg/mL streptomycin) for 1 h at 37°C. Single-cell suspensions were obtained by passing the digested tissues through a 70-μm cell strainer (BD Falcon). Cell suspensions of the dental pulp were added into 25-cm2 culture dishes, followed by incubation in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in 5% CO2 atmosphere. The medium was refreshed once every 3 days. Cells were passaged at the ratio of 1:3 when the confluence reached 85% to 90%. Cells of the third passage were used in following experiments.
Construction of Adv-hSIRT1-GFP and Adv-GFP
Recombinant adenoviruses encoding human SIRT1 with green fluorescent protein (Adv-hSIRT1-GFP) and GFP alone (Adv-GFP) were constructed by direct cloning. The titers of the recombinant adenoviral vectors were as follows: Adv-hSIRT1-GFP, 5 × 109 plaque-forming units (PFUs)/mL and Adv-GFP, 2 × 1010 PFU/mL.
Transfection with adenovirus (Adv)-hSIRT1 and osteogenic differentiation
The recombinant adenoviruses were transduced into 293 cells and purified using standard cesium chloride banding techniques. DPSCs were prepared as described above and seeded in six-well culture plates (1.4 × 105 cells/well) on the day before transfection. DPSCs were transfected overnight with Adv-hSIRT1-GFP or Adv-GFP (multiplicity of infections [MOIs], XXX) at 37°C in α-minimum essential medium (MEM) control medium α-MEM. GFP expression was observed using fluorescence microscopy (Leica, Wetzlar, Germany) to determine the transfection efficiency of Adv-hSIRT1-GFP and Adv-GFP. At 96 h after transfection, cells were collected for RT-PCR and Western blotting to determine the expression of SIRT1. DPSCs were plated at a density of 2 × 104 cells/cm2 and cultured in proliferation medium supplemented with 0.1 mM dexamethasone, 10 mM β-glycerophosphate (Sigma) and 50 mg/mL ascorbic acid (Sigma). The differentiation of DPSCs was observed for 14 days. The degree of extracellular matrix calcification was estimated by Alizarin red S (Sigma) staining and Alkaline phosphatase (ALP, JianCheng, Nanjing, China) staining. In brief, DPSCs were fixed with 4% paraformaldehyde (PFA) for 1 h and washed with phosphate-buffered saline (PBS). Then, DPSCs were incubated with 2% Alizarin red S solution at 37°C for 2 h. Mineralization was quantified by extracting the stain using 100 mM cetylpyridinium chloride (Sigma-Aldrich) at room temperature for 2 h. The absorbance of the extracted Alizarin red S stain was measured at 570 nm. DPSCs were subjected to ALP staining using the ALP assay kit in accordance with the manufacturer’s instructions.
Determination of SIRT1 expression after transfection
Total cellular RNA was isolated from cells and then reversely transcribed using conventional protocols. PCR amplification was performed using the following primers: SIRT1: Forward 5’-GCAACATCTTATGATTGGCACA-3’, reverse 5’-AAATACCATCCCTTGACCTGAA-3’, GAPDH: Forward 5’-TCCATGACAACTTTGGTATCG-3’, reverse 5’-TGTAGCCAAATTCGTTGTCA-3’. All of the primer sequences were determined according to the gene sequences from GenBank. Each sample was analyzed in triplicate and GAPDH was used as a control. Cells were lysed in the buffer consisting of 50 mM Tris, 150 mM NaCl, 2% sodium dodecyl sulfate (SDS) and a protease inhibitor mixture. After centrifugation at 12,000 rpm for 12 min, protein concentrations were determined using the Bradford assay (BioRad). The resulting supernatant (50 mg of protein) was subjected to SDS-polyacrylamide gel electrophoresis (PAGE). The separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes at 350 mA for 2.5 h in a blotting apparatus (Bio-RAD, CA, USA). Membranes were blocked with 5% nonfat milk, incubated with primary antibodies (1:400) at 4°C overnight and subsequently treated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:1000) for 2 h at room temperature. Concomitantly, GAPDH served as a reference. The following primary antibodies were used: GAPDH (anti-rabbit, Santa Cruz), SIRT1 (anti-rabbit, Sigma).
Surgical procedure, postoperative care, and distraction
Each animal was placed in the dorsal position and a 2.0-cm vertical incision was made on the right hind leg to expose the tibia. Then, the periosteum was carefully elevated circumferentially. After a self-constructed external fixator had been applied to the tibia with four self-taping screws, a subperiosteal osteotomy was performed between the second and third screws using a fine wire saw at a level immediately below the area attached to the fibula. The periosteum, muscle, and skin were repositioned and closed with 3/0 silk sutures. The external fixator remained in place until necropsy. The animals were kept in separate cages with food and water under the standardized environmental condition with 12-h light/dark cycle, and allowed to move freely throughout the experimental period. Three days after osteotomy, the distraction was started at a rate of 2.0 mm per 24 h (twice per day and 1.0 mm per time) for 7 days. Upon completion of the distraction, all animals were randomly divided into three groups (n = 12): CON (control group or phosphate buffered saline group), NC (negative control or DPSCs infected with Adv-GFP), and OE (overexpression group or DPSCs infected with Adv-SIRT1-GFP). All rabbits were sacrificed at 8 weeks after the completion of lengthening, and the lengthened tibiae were harvested and processed for further examinations.
Gene therapy
DPSCs transfected with Adv-hSIRT1-GFP were harvested 2 days after transfection. They were washed with PBS and diluted in normal saline into 1 × 107 cells/mL. On the last day of the distraction period, 1 mL of DPSCs suspension was injected directly into the distraction gap in Group OE. Similarly, animals in Group NC received an injection with 1 × 107 DPSCs transfected with Adv-GFP, and animals in Group CON were injected with 1.0 mL of normal saline. The animals were held in rigid fixation until they were sacrificed.
Radiographic and dual-energy X-ray absorptiometry (DXA) examinations
The lengthened tibiae were harvested after sacrifice, then lateral radiographs of the specimens were obtained, and the bone mineral density (BMD) was determined. Bone mineral content (BMC) was detected by DXA (Lunar iDXATM, GE Healthcare, USA). BMD and BMC of the region of interest (ROI) were measured for each distracted region. Both were also measured in the controlateral intact tibia as a normal control. All examinations were performed three times by an operator blind to the experiment.
Micro-CT evaluation
The samples were collected 5 mm outside the distracted regions, then placed in a custom jig with water, and scanned with a micro-CT 80 scanner (Scanco Medical, Bassersdorf, Switzerland) in an axial direction parallel to the long axis of the tibiae. Nearly 1000 images with a resolution of 2048 × 2048 pixels and with an isotropic voxel size of 10 p.m were obtained. The system was set to 70 kV, 114 mA, and an integration time of 500, which allows accurate analysis of the osteogenesis of the distracted regions. The volume of interest (VOI) was selected as the distracted gap extending to a total of 500 slices. The following micro-architecture parameters were assessed in VOI images: bone volume to total volume ratio (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N). BV/TV indicates the portion of mineralized tissue, and Tb.Th, Tb.Sp, and Tb.N reflect the thickness, organization, and number of trabeculae. After above examinations, six samples were randomly selected from each group and processed for histological examination. Remaining six samples were prepared for mechanical testing.
Histological examination
Samples were fixed in 4% paraformaldehyde for 24 h, and then decalcified in 14.5% ethylenediaminetetraacetic acid buffer (Ph = 7.2) at room temperature for 6 weeks. The specimens were sectioned longitudinally along the long axial, embedded in paraffin, and then cut with a microtome (Leica, German) into 5-μm sections for hematoxylin eosin (H&E) staining.
Mechanical testing
The samples were stored at -20°C until the day of mechanical testing. The three-point bending strength was measured until failure between the distraction regions with a support span of 5 mm, using an electronic universal material test machine (Instron 5566, Instron, Norwood, MA, USA) with a 1-kN load cell under displacement control (5 mm/min). The load-displacement curve was recorded during the downward compression and the ultimate load at failure (N; maximum force that the specimen sustained) was calculated. The controlateral intact tibia was also measured as a normal control.
Statistical analysis
All the data as expressed as mean ± standard deviation (SDs). One-way analysis of variance (ANOVA) and Student-Newman Keuls (SNK) test were employed to compare the differences between groups using SPSS 19.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Evaluation of transfected cells
The expression of GFP in DPSCs was evaluated by observation under a fluorescence microscope. After 24-h transfection, the proportion of positive cells was approximately 100% (Figure 1A). At the end of the distraction (at 7 days), fibro-tissues had filled in the distracted gap. A large amount of green fluorescence was seen in Group OE and Group NC (Figure 1B), but in Group CON, little green fluorescence was observed. The expression of SIRT1 in Group OE was significantly higher than in Group NC and Group CON (Figure 1C). The mRNA level, RT-PCR also showed the SIRT1 expression in Adv-SIRT1-GFP (Group OE) was much higher than in Adv-GFP group (Group NC) and control group (Group CON) (Figure 1D). More calcium accumulation after Adv-SIRT1-GFP transfected DPSCs injection was shown by Alizarin red S staining (Figure 2A) (*P < 0.05 vs XXXXX). Similarly, more ALP positive cells were observed after injection of DPSCs transfected with Adv-SIRT1-GFP (90 - 93 ± 3.2%) than after injection with DPSCs transfected with Adv2-GFP (73 - 75 ± 2.4%) at 14 days (Figure 2B) (*P < 0.05).
Figure 1.
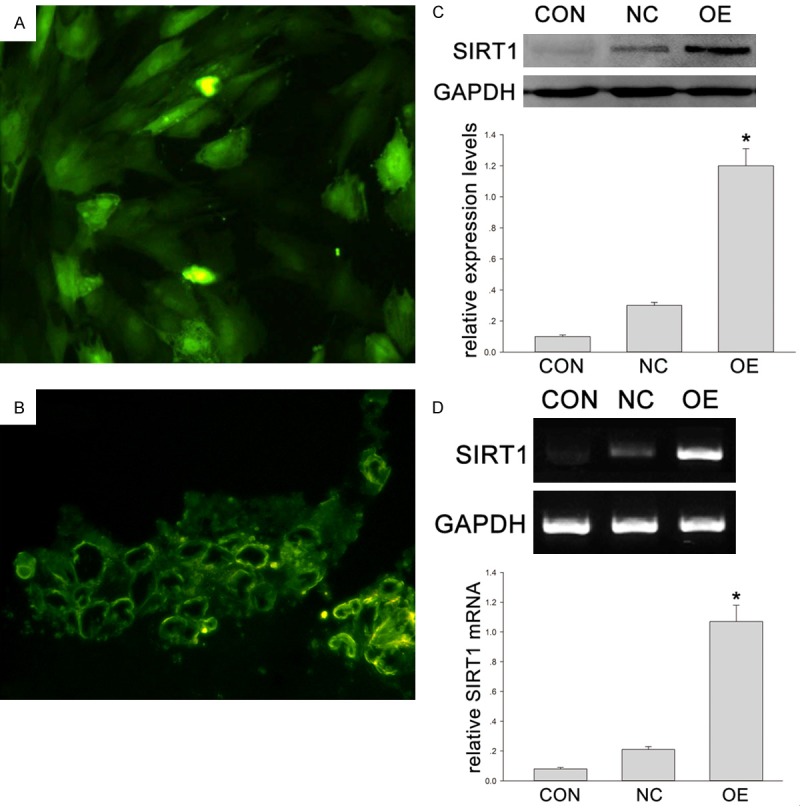
A. The green fluorescence of DPSCs after 3-day transfection under a fluorescence microscope. B. A large amount of green fluorescence was observed under a microscope. C, D. Animals were divided into three groups: CON (control group or phosphate buffered saline group), NC (negative control or DPSCs transfected with Adv-GFP) and OE (overexpression group or DPSCs transfected with Adv-Runx2-GFP). C. The protein expression of SIRT1 in DPSCs transfected by adenovirus vector containing human SIRT1 gene (Western blotting). GAPDH served as a control. The optical density of SIRT1 was normalized to that of GAPDH at each time point. *P < 0.05. D. SIRT1 mRNA expression in DPSCs transfected by adenovirus vector containing human SIRT1 gene using (RT-PCR). GAPDH served as a control. Quantification of RT-PCR products. The quantity of amplified product was analyzed by an image analyzer. *P < 0.05.
Figure 2.
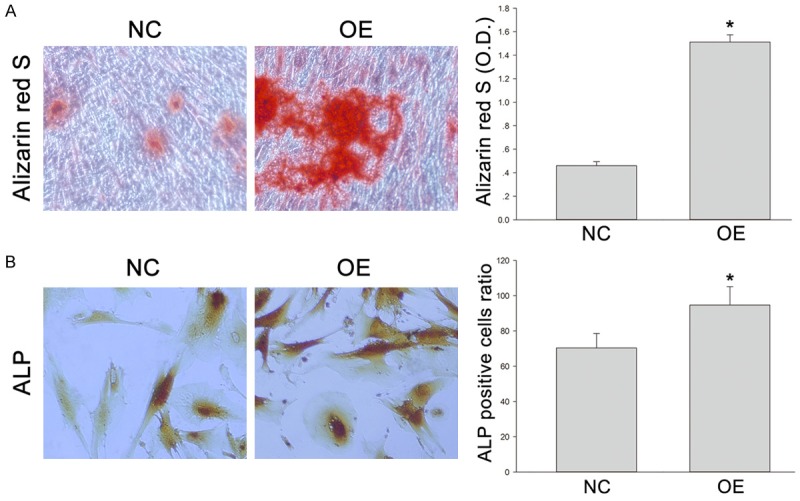
(A, B) DPSCs in Group NC and Group OE were cultured in osteogenic differentiation medium for 14 days, and then stained with Alizarin red S (A) or ALP (B). Quantification of Alizarin red S positive deposits and the ratio of ALP positive cells were described in right. *P < 0.05.
Clinical observation
Generally, the experiment animals well tolerated the distraction surgery. The whole distraction process was stabe and the lengthened distraction gaps maintained. At the pre-designed time point, the samples were harvested for histological and radiological examinations. Results showed the newly formed bone in Group OE seemed to be more mature than in Group NC and Group CON.
Histological observation
All samples in three groups were observed under a light microscopy after H&E staining. In Group CON, the newly formed trabeculae were sparse, and focal defects were seen in the distraction gap (Figure 3A). In Group NC, the newly formed trabeculae in the distraction gap were thin and partial trabeculae bridged discontinuously (Figure 3B). In Group OE, the newly formed trabeculae in the distraction gap were thicker than in Group CON. More mature and regular trabecular bone was observed in Group OE (Figure 3C).
Figure 3.
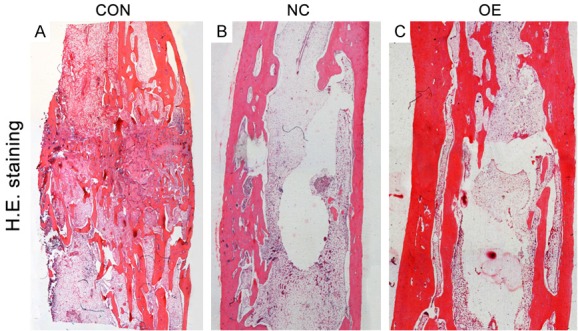
(A-C) All samples from Group CON (A), Group NC (B) and Group OE (C) after 8-week consolidation were observed under a light microscope after H&E staining. The newly formed cortex in Group NC and Group OE was more continuously than in Group CON. In Group NC, the newly formed trabeculae in the distraction gap were thin, and partial trabeculae bridged discontinuously. More mature and regular trabecular bone were seen in Group OE.
Radiological findings, BMD and BMC
The distraction was displayed in the lateral radiograph (Figure 4A). At 0 (Figure 4Aa) and 8 weeks (Figure 4Ab-d), the focal defects were large, and tiny trabeculae could be found in the DO gaps of Group CON (Figure 4B). In the distraction gap of Group NC, the disordered trabeculae were observed, and the newly formed trabeculae showed insufficiently robust (Figure 4C). The newly regenerated bone in Group OE displayed more mature and regular trabeculae as compared to other groups (Figure 4Ad). The BMC and BMD of regenerate regions were detected by DXA at the end of distraction. BMC and BMD in Group OE were significantly higher than in Group NC and Group CON (Figure 4B, 4C) (*, #P < 0.05).
Figure 4.
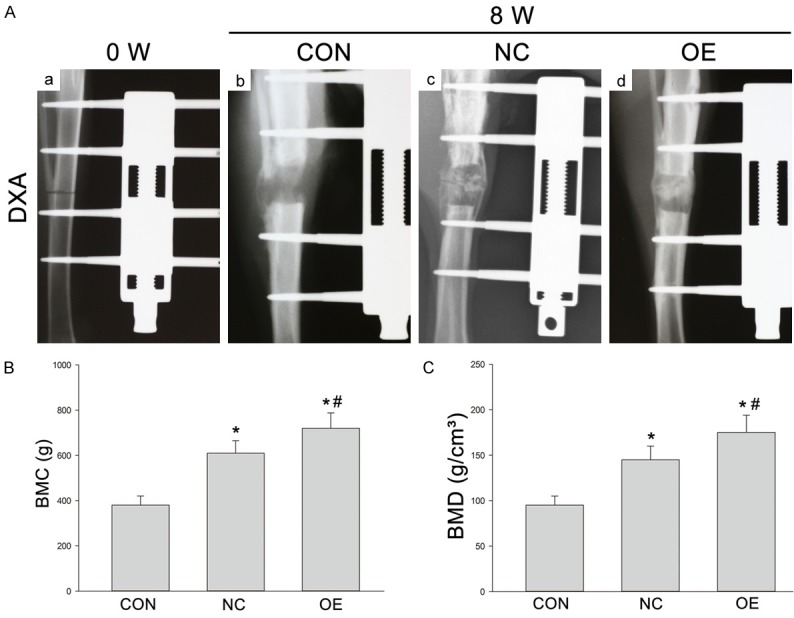
(A) X-ray examination of new bone formation in three groups after 8-week consolidation (A: b-d). Lateral radiographs of the distracted tibia before the distraction (0 week) (a). The newly regenerated bone in Group OE showed more mature and regular trabeculae as compared to other two groups. (B, C) BMC (g) and BMD (g/cm2) of the regenerated bone in Group CON, Group NC and Group OE after 8-week consolidation as measured by DXA. *P < 0.05 vs Group CON; #P < 0.05 vs Group NC.
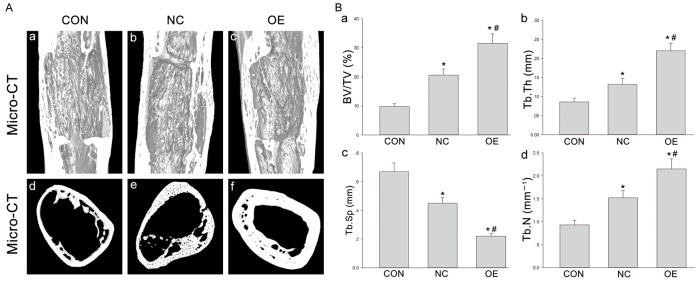
Micro-CT
High-resolution 3D images from micro-CT were employed to display the microstructure of the regenerated gap in different groups. The cortical bone in Group NC was thinner than in Group OE, and the bone trabeculae in Group NC were incompact and immature (Figure 5A). Both longitudinal reconstruction and transversal section were built. The parameters of bone regeneration assessed by micro-CT were significantly different among three groups. The samples in Group CON had the lowest BV/TV (9.29% ± 0.103), Tb.Th (0.087 ± 0.011 mm), and Tb.N (0.89 ± 0.08 mm) as well as the highest Tb.Sp (0.68 ± 0.12 mm), which were significantly different from those in other groups (Figure 5B) (*P < 0.05). The transfected DPSCs groups, both NC and OE, exhibited a significant effect in bone regeneration. Specially, the newly formed trabeculae in Group OE were the most mature in both morphology and texture, with the highest BV/TV, Tb.Th and Tb.N as well as the lowest Tb.Sp as compared to other two groups (Figure 5B) (*P < 0.05).
Figure 5.
A. The 3D micro-CT images of the distal femur distraction gap in Group CON (a, d), Group NC (b, e) and Group OE (c, f); longitudinal reconstruction (upper) and transversal section (lower) were shown. B. Parameters of the micro-structure of distraction gaps in all groups. *P < 0.05 vs Group CON; #P < 0.05 vs Group NC.
Mechanical testing
At the end of the distraction, the three-point bending test were used to test the peak load of the lengthened tibia. The loading of the samples in Group NC and Group OE had increased by 49% and 118% as compared to Group CON, respectively (Figure 6) (*P < 0.05, respectively). The peak load was also significantly different between Group NC and Group OE (*P < 0.05).
Figure 6.
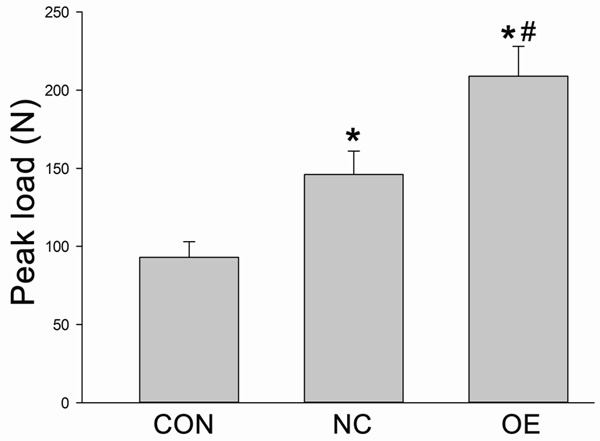
The three-point bending test was used to test the peak load of the lengthened tibia. *P < 0.05 vs Group CON; #P < 0.05 vs Group NC.
Discussion
In this study, the SIRT1 was successfully introduced into DPSCs via adenovirus vector, and the increased calcium accumulation was observed in DPSCs transfected with SIRT1 as compared to negative controls in vitro. These DPSCs were transplanted into tibia DO of rabbits in the following experiments, and the bone regeneration was found to be accelerated significantly in both quantity and quality. These results indicate that SIRT1 may facilitate the osteogenic effect of DPSCs, and DPSCs transfected with SIRT1 may have potential clinical application value.
MSCs are used as seedl cells in tissue engineering which has been proven in many studies, and MSCs combined with scaffolds or modified by gene engineering have also been employed in some studies [5,25]. It has been shown that human MSCs (hMSCs) treated with rat peripheral blood lymphocyte fail to induce significant lymphocyte proliferation because of hMSCs’ low immunogenicity [14]. DPSCs are a group of MSCs and have been identified as a source of multiple potentiality cells in vitro and in vivo. They can differentiate into various specific cell types and promote the regeneration of some tissues, including bone, cartilage, and other tissues with high proliferative capability, low immune status preserved after differentiation, and the use of these cells has no ethical problems [26-28]. In this study, calcium accumulation in DPSCs transfected with SIRT1 was significantly higher rather than in control group after osteogenic differentiation in vitro. Furthermore, ALP is widely used to test the mineralized matrices produced by osteoblast-like cells in early differentiation [29]. Our results indicated that SIRT1 increased the ALP activity significantly. These demonstrate the SIRT1 plays a key role in the calcium accumulation and may benefit the osteogenic differentiation of DPSCs.
DO is a tissue engineering technique which has been widely applied in bone regeneration for skeletal deformities and bone defects [30], and it has been clinically used in some patients with ankylosis of the temporomandibular joint (TMJ), Robin Sequence (RS), obstructive sleep apnea-hypopnea syndrome (OSAHS) and other deformities. The elongation after DO may result in more stable as compared to SSRO and the inverted “L” osteotomy, because the process of elongation is slow, and the attached soft tissues can rebuild gradually. The therapeutic efficacy can be evaluated effectively in terms of the appearance, improvements of maximum interincisal opening (MIO) and some other functions. However, some clinical trials and laboratory experiments indicate that the long treatment period should be shorten and the risk for fibrous nonunion under some unknown circumstances should be controlled [31]. Otherwise, the wide application of DO will be prevented. Some special microenvironments in the distraction zone, including oxidative stress, inflammatory microenvironment, some proteins, miRNAs and other kinds of molecules, may influence this process [32-34]. To reduce the adverse effects and complications, a short latency period and a rapid distraction rate are needed. Some molecules have been found to significantly promote the osteogenesis of MSCs in vitro and mineralization in vivo during DO. Therefore, it seems to be reasonable to supplement DPSCs modified with SIRT1 to promote the bone regeneration into the distraction zone.
In our previous study, results showed SIRT1 could promote the osteogenic differentiation of DPSCs in vitro in both osteogenic medium and inflammatory microenvironment [24]. SIRT1 has the ability to deacetylate many transcription factors in the nucleus such as β-catenin, p53, NF-κB and STAT3, and then actives the classical osteogenesis related pathways [35-38]. On the contrary, SIRT1 knockout mice exhibit reduced bone formation and increased marrow adipogenesis [21]. Importantly, SIRT1 can suppress inflammatory responses and senescence through inducing the deacetylation of some transcription factors and influence some anti-inflammatory or anti-senescence pathways, which promotes the differentiation of MSCs [10,39-41]. In the present study, SIRT1 accelerated the osteogenic differentiation of DPSCs in vitro. On the basis of findings from histological examination, radiological examination, micro-CT and mechanical testing, SIRT1-transfected DPSCs significantly promoted the bone regeneration after DO as compared to empty vectors transfected DPSCs or the control group, indicating that SIRT1 may enhance bone regeneration of DO in vivo.
In summary, the SIRT1 modified DPSCs are successfully established, and these DPSCs are more effective to enhance the osteogenic differentiation in a tibia DO model of rabbits after injection into the distraction zone as compared to control group. These findings suggest that SIRT1-modified DPSCs can promote new bone formation during DO and have the potential for future clinical application.
Acknowledgements
This work was supported by the “Top Six Types of Talents” Financial Assistance of Jiangsu Province Grant (No. 2013-WSW-048).
Disclosure of conflict of interest
None.
References
- 1.Lesensky J, Prince DE. Distraction osteogenesis reconstruction of large segmental bone defects after primary tumor resection: pitfalls and benefits. Eur J Orthop Surg Traumatol. 2017;27:715–727. doi: 10.1007/s00590-017-1998-5. [DOI] [PubMed] [Google Scholar]
- 2.Lou S, Lv H, Li Z, Tang P, Wang Y. Effect of low-intensity pulsed ultrasound on distraction osteogenesis: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018;13:205. doi: 10.1186/s13018-018-0907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossini G, Vinci B, Rizzo R, Pinho TM, Deregibus A. Mandibular distraction osteogenesis: a systematic review of stability and the effects on hard and soft tissues. Int J Oral Maxillofac Surg. 2016;45:1438–1444. doi: 10.1016/j.ijom.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Hvid I, Horn J, Huhnstock S, Steen H. The biology of bone lengthening. J Child Orthop. 2016;10:487–492. doi: 10.1007/s11832-016-0780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma G, Zhao JL, Mao M, Chen J, Dong ZW, Liu YP. Scaffold-based delivery of bone marrow mesenchymal stem cell sheet fragments enhances new bone formation in vivo. J Oral Maxillofac Surg. 2017;75:92–104. doi: 10.1016/j.joms.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Chen Y, Liu Y, Zhang J, Kang Q, Ho K, Chai Y, Li G. Effect of SDF-1/Cxcr4 signaling antagonist AMD3100 on bone mineralization in distraction osteogenesis. Calcif Tissue Int. 2017;100:641–652. doi: 10.1007/s00223-017-0249-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhao K, Wang F, Huang W, Wu Y. Clinical outcomes of vertical distraction osteogenesis for dental implantation: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33:549–564. doi: 10.11607/jomi.6140. [DOI] [PubMed] [Google Scholar]
- 8.Mangano C, De Rosa A, Desiderio V, d’Aquino R, Piattelli A, De Francesco F, Tirino V, Mangano F, Papaccio G. The osteoblastic differentiation of dental pulp stem cells and bone formation on different titanium surface textures. Biomaterials. 2010;31:3543–3551. doi: 10.1016/j.biomaterials.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Spath L, Rotilio V, Alessandrini M, Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro F, Filippini A, Papaccio G. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med. 2010;14:1635–1644. doi: 10.1111/j.1582-4934.2009.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Chen K, Wan X, Wang F, Guo Z, Mo Z. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem Biophys Res Commun. 2017;484:871–877. doi: 10.1016/j.bbrc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Ching HS, Luddin N, Rahman IA, Ponnuraj KT. Expression of odontogenic and osteogenic markers in dpscs and shed: a review. Curr Stem Cell Res Ther. 2017;12:71–79. doi: 10.2174/1574888x11666160815095733. [DOI] [PubMed] [Google Scholar]
- 12.Du Z, Wang L, Zhao Y, Cao J, Wang T, Liu P, Zhang Y, Yang X, Cheng X, Liu B, Lei D. Sympathetic denervation-induced MSC mobilization in distraction osteogenesis associates with inhibition of MSC migration and osteogenesis by norepinephrine/adrb3. PLoS One. 2014;9:e105976. doi: 10.1371/journal.pone.0105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Hadidi YN, El Kassaby M, El Fatah Ahmed SA, Khamis NS. Effect of mesenchymal stem cell application on the distracted bone microstructure: an experimental study. J Oral Maxillofac Surg. 2016;74:1463.e1–1463.e11. doi: 10.1016/j.joms.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Wang B, Sun Y, Wu T, Liu Y, Zhang J, Lee WY, Pan X, Chai Y, Li G. Human fetal mesenchymal stem cell secretome enhances bone consolidation in distraction osteogenesis. Stem Cell Res Ther. 2016;7:134. doi: 10.1186/s13287-016-0392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng G, Zhang J, Feng X, Wu S, Huang D, Hu J, Zhu S, Song D. Runx2 modified dental pulp stem cells (DPSCs) enhance new bone formation during rapid distraction osteogenesis (DO) Differentiation. 2016;92:195–203. doi: 10.1016/j.diff.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Sun YX, Zhang JF, Xu J, Xu LL, Wu TY, Wang B, Pan XH, Li G. MicroRNA-144-3p inhibits bone formation in distraction osteogenesis through targeting Connexin 43. Oncotarget. 2017;8:89913–89922. doi: 10.18632/oncotarget.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu B, Wang L, Yang X, Mao M, Ye C, Liu P, Yang Z, Yang X, Lei D, Zhang C. Norepinephrine inhibits mesenchymal stem cell chemotaxis migration by increasing stromal cell-derived factor-1 secretion by vascular endothelial cells via NE/abrd3/JNK pathway. Exp Cell Res. 2016;349:214–220. doi: 10.1016/j.yexcr.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang WB, Zheng LW, Chua DT, Cheung LK. Treatment of irradiated mandibles with mesenchymal stem cells transfected with bone morphogenetic protein 2/7. J Oral Maxillofac Surg. 2012;70:1711–1716. doi: 10.1016/j.joms.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Rai E, Sharma S, Kaul S, Jain K, Matharoo K, Bhanwer AS, Bamezai RN. The interactive effect of SIRT1 promoter region polymorphism on type 2 diabetes susceptibility in the North Indian population. PLoS One. 2012;7:e48621. doi: 10.1371/journal.pone.0048621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun. 2012;427:191–196. doi: 10.1016/j.bbrc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D’Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152:4514–4524. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 22.Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML, Yen ML. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res. 2011;26:2552–2563. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- 23.Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin. EMBO Mol Med. 2013;5:430–440. doi: 10.1002/emmm.201201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng G, Zheng K, Song D, Xu K, Huang D, Zhang Y, Cao P, Shen S, Zhang J, Feng X, Zhang D. SIRT1 was involved in TNF-alpha-promoted osteogenic differentiation of human DPSCs through Wnt/beta-catenin signal. In Vitro Cell Dev Biol Anim. 2016;52:1001–1011. doi: 10.1007/s11626-016-0070-9. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Tee BC, Kennedy KS, Kennedy PM, Kim DG, Mallery SR, Fields HW. Scaffold-based delivery of autologous mesenchymal stem cells for mandibular distraction osteogenesis: preliminary studies in a porcine model. PLoS One. 2013;8:e74672. doi: 10.1371/journal.pone.0074672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Alimonte I, Mastrangelo F, Giuliani P, Pierdomenico L, Marchisio M, Zuccarini M, Di Iorio P, Quaresima R, Caciagli F, Ciccarelli R. Osteogenic differentiation of mesenchymal stromal cells: a comparative analysis between human subcutaneous adipose tissue and dental pulp. Stem Cells Dev. 2017;26:843–855. doi: 10.1089/scd.2016.0190. [DOI] [PubMed] [Google Scholar]
- 27.Paino F, La Noce M, Giuliani A, De Rosa A, Mazzoni S, Laino L, Amler E, Papaccio G, Desiderio V, Tirino V. Human DPSCs fabricate vascularized woven bone tissue: a new tool in bone tissue engineering. Clin Sci (Lond) 2017;131:699–713. doi: 10.1042/CS20170047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabatabaei FS, Torshabi M. In vitro proliferation and osteogenic differentiation of endometrial stem cells and dental pulp stem cells. Cell Tissue Bank. 2017;18:239–247. doi: 10.1007/s10561-017-9620-y. [DOI] [PubMed] [Google Scholar]
- 29.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 30.Savoldi F, Tsoi JKH, Paganelli C, Matinlinna JP. Biomechanical behaviour of craniofacial sutures during distraction: an evaluation all over the entire craniofacial skeleton. Dent Mater. 2017;33:e290–e300. doi: 10.1016/j.dental.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Lai QG, Yuan KF, Xu X, Li DR, Li GJ, Wei FL, Yang ZJ, Luo SL, Tang XP, Li S. Transcription factor osterix modified bone marrow mesenchymal stem cells enhance callus formation during distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:412–419. doi: 10.1016/j.tripleo.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Baj A, Trapella G, Lauritano D, Candotto V, Mancini GE, Gianni AB. An overview on bone reconstruction of atrophic maxilla: success parameters and critical issues. J Biol Regul Homeost Agents. 2016;30:209–215. [PubMed] [Google Scholar]
- 33.Santinoni CD, Oliveira HF, Batista VE, Lemos CA, Verri FR. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: a systematic review. J Photochem Photobiol B. 2017;169:83–89. doi: 10.1016/j.jphotobiol.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Xu J, Xu L, Zhang J, Chan K, Pan X, Li G. MiR-503 promotes bone formation in distraction osteogenesis through suppressing Smurf1 expression. Sci Rep. 2017;7:409. doi: 10.1038/s41598-017-00466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenwitheesuk A, Park S, Wongchitrat P, Tocharus J, Mukda S, Shimokawa I, Govitrapong P. Comparing the effects of melatonin with caloric restriction in the hippocampus of aging mice: involvement of Sirtuin1 and the FOXOs pathway. Neurochem Res. 2018;43:144–152. doi: 10.1007/s11064-017-2369-7. [DOI] [PubMed] [Google Scholar]
- 36.Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Won SS, Jeon SJ, Choi BJ, Jeong W, Kim SJ. Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells. Stem Cell Res Ther. 2017;8:181. doi: 10.1186/s13287-017-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thirupathi A, de Souza CT. Multi-regulatory network of ROS: the interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J Physiol Biochem. 2017;73:487–494. doi: 10.1007/s13105-017-0576-y. [DOI] [PubMed] [Google Scholar]
- 38.Zainabadi K, Liu CJ, Guarente L. SIRT1 is a positive regulator of the master osteoblast transcription factor, RUNX2. PLoS One. 2017;12:e0178520. doi: 10.1371/journal.pone.0178520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Li M, Yan J, Liu T, Pan G, Yang H, Pei M, He F. Alcohol induces cellular senescence and impairs osteogenic potential in bone marrow-derived mesenchymal stem cells. Alcohol Alcohol. 2017;52:289–297. doi: 10.1093/alcalc/agx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Y, Wang Y, Du L, Xu C, Cao J, Fan T, Liu J, Su X, Fan S, Liu Q, Fan F. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int J Mol Sci. 2013;14:14105–14118. doi: 10.3390/ijms140714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Liu Y, Zong C, Yu Q, Yang X, Liang L, Ye F, Nong L, Jia Y, Lu Y, Han Z. Mesenchymal stem cells with Sirt1 overexpression suppress breast tumor growth via chemokine-dependent natural killer cells recruitment. Sci Rep. 2016;6:35998. doi: 10.1038/srep35998. [DOI] [PMC free article] [PubMed] [Google Scholar]