Abstract
Although adipose derived stem cells (ADSCs) exert their therapeutic potential in ischemic stroke, the migration of ADSCs in injured area is not apparently observed after intravenous administration. ADSCs are an important source of exosomes which hold great promise as an endogenous drug delivery system for the treatment of cerebral ischemia given their ability to cross the blood-brain barrier. Here we investigated whether ADSCs-derived exosomes mediated miRNAs transfer and thus promoted neurological recovery after stroke. We first proved that miR-126 levels were reduced in patients’ plasma with acute ischemic stroke and in rat plasma and brain tissue after ischemia. To test the effect of exosomal miR-126, we employed overexpression and knock-down technologies to up-regulate or inhibit miR-126 level in ADSCs and thus acquired miR-126+ exosomes and miR-126- exosomes, respectively. Compared with control, systemic administration of ADSCs-derived exosomes significantly increased the expression of von Willebrand factor (an endothelia cell marker) and doublecortin (a neuroblasts marker) and improved functional recovery in stroke rats. ADSCs-derived exosomes also resulted in a decrease of neuron cell death and an increase of cell proliferation compared with control. Importantly, these outcomes were further enhanced with miR-126+ exosomes treatment and were significantly decreased with miR-126- exosomes treatment, compared to naïve exosomes treatment. MiR-126+ exosomes also inhibited microglial activation and the expression of inflammatory factors in vivo and in vitro. Our results suggest that intravenous administration of miR-126+ exosomes post stroke improves functional recovery, enhances neurogenesis, inhibits neuroinflammation, and represents a novel treatment for stroke.
Keywords: ADSCs, exosomes, miR-126, stroke, neurogenesis
Introduction
Ischemic stroke is one of the leading causes of physical disability and death worldwide [1]. Stem cell therapy has recently emerged as a promising new therapeutic approach for the treatment of stroke, including bone marrow-derived mesenchymal stem cells (BMSCs) [2], embryonic stem cells [3], endothelial progenitor cells and adipose-derived stem cells (ADSCs) [4]. Among these options from various stem cells, ADSCs are demonstrated to have the distinct advantages of abundancy, lower barrier to obtain invasiveness and avoiding the ethical issue due to sufficient quantity for autologous transplantation [4].
Exosomes are small membrane vesicles (30~100 nm) that are released from different types of cells in response to specific cellular stimulation [5]. They exhibit crucial messenger function in intercellular communication by delivering pre-wrapped cargoes like miRNAs, lncRNA and proteins to recipient cells [6]. miRNAs are short non-coding single-stranded RNAs that regulate cellular functions by acting at the post-transcriptional level [7]. The transfer of miRNAs mediated by exosomes has been continuously studied due to the complexity of functional regulations on the recipient cells. Xin et al reported that exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth [8]. Several studies reported that miR-124 and miR-145 can be delivered to neural progenitor cells through exosome-dependent procedure that changes the gene expression of recipient neural cells and thus promotes cortical neural progenitors to obtain neuronal identity [9,10]. Recently, the role of exosomes derived from miRNAs-modified ADSCs in different types of disease is gradually revealed [11-13]. Qu et al demonstrated that exosomes derived from miR-181-5p-modified ADSCs prevent liver fibrosis via autophagy activation [11]. Exosomes from miR-126-overexpressing ADSCs are therapeutic in relieving acute myocardial ischaemic injury [12].
Emerging studies have demonstrated miR-126 could be used as the probe to distinguish severe permanent ischemia from milder injury after transient ischemia [14-16]. Meanwhile, many studies have assessed miR-126 from blood and brain tissues of human stroke patients and animal stroke models [17-19]. However, only some finite reports revealed the elusive relationship between ADSCs and miR-126 in ischemia damage or treatment [12,20,21] and the underlying mechanisms of ADSCs-derived exosomal miR-126 deserved further investigation in ischemia injury.
Based on above findings, here we investigate whether tailored exosomes enriched with the miR-126 protects against cerebral ischemia injury in a rat model of MCAO, and determine the precise role of miRNA-126 in regulations on stroke in the context of ADSCs.
Materials and methods
Cell culture and oxygen-glucose deprivation
The use of human blood or tissue samples was approved by the institutional ethics review board of the First Affiliated Hospital of Wenzhou Medical University. Informed consent was obtained from all patients and healthy controls prior to study. Donors with malignancies, infectious or systemic diseases were not included in the present study. 13 male patients (Age: 57.3-69.4) with acute cerebral infarction and 17 normal control subjects (Age: 58.1-69.7) were enrolled in the study.
ADSCs were isolated as previously described [22,23]. Briefly, subcutaneous adipose tissue was separated from the inguinal region of rats, and digested for 60 minutes at 37°C with equal volumes of dispase (final concentration 10 U/ml, BD, CA, USA) and collagenase (final concentration 66.7 U/ml, Sigma-Aldrich, MO, USA). The top lipid layer was removed after centrifugation at 200 g for 10 minutes. The pellet was re-suspended in DMEM supplemented with 10% FBS (Gibco, CA, USA) and filtered through 40 μm nylon cell strainer (BD Falcon, NJ, USA) after lysing the red blood cells. The isolated cells were then plated and expanded in MesenCult-XF culture medium (Stem Cell Technologies, Vancouver, Canada) supplemented with 1% (v/v) penicillin/streptomycin (Gibco) and 2 mM L-glutamine (Gibco). ADSCs culture was maintained at sub-confluent levels (<80% confluency) at 37°C with 5% atmosphere.
The mouse BV2 microglial cells were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10% FBS and 1% penicillin as well as streptomycin (Gibco). The oxygen-glucose deprivation (OGD) was initiated by exposure of BV2 cells to DMEM without serum or glucose in a humidified atmosphere of 95% nitrogen and 5% CO2 for 6 hours.
Exosomes preparation
ADSCs grown to approximate 80% confluence were washed with PBS thrice and cultured with exosome-depleted FBS-contained medium (Sigma-Aldrich). After 48 h incubation, the medium was collected, and filtered through a 0.22 μm filter (BD Falcon). Exosomes in culture medium were extracted using the ExoQuickTM Exosome Precipitation Solution (System Biosciences, CA, USA). Briefly, the ExoQuickTM Exosome Precipitation Solution was added to the culture medium and refrigerated overnight. The sample was centrifuged for 30 min at 1500 RPM and then at 3000 RPM for 5 min at 4°C. Exosome pellet was re-suspended in 200 μl of cell medium.
MiR-126+ exosomes and miR-126- exosomes
MiR-126 (GCGUAAUAAUGAGUGCCAUGCU) and 2’-O-methyl modified miR-126 inhibitor (AGCA-UGGCACUCAUUAUUACGC) were purchased from Genepharm (Shanghai, China) and transfected to ADSCs cells using LipofectamineTM 3000 (Invitrogen, CA, USA) according to the manufacturer. ADSCs or ADSCs treated with miR-126 or miR-126 inhibitor were used to prepare exosomes, miR-126+ exosomes and miR-126- exosomes, respectively.
Rat MCAO model
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Wenzhou Medical University. Male SD rats (weight, 280±10 g) were obtained from the Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China), and maintained in a pathogen-free facility and routinely monitored. Rats were used to induce focal ischemia by occluding the MCA using the intraluminal technique as previously described [24,25]. Briefly, SD rats were anesthetized by ketamine intraperitoneally. Atropine was subcutaneously injected with exposure of the CCA (common carotid artery), ICA (internal carotid artery) and ECA (external carotid artery) by surgery. The ECA was coagulated and inserted into the ICA through the ECA to occlude the MCA. Two hours later, the suture was withdrawn to allow MCA perfusion. The regional cerebral blood flow was observed to verify the occurrence of ischemia by MCAO, using laser Doppler flowmetry (PeriFlux System 5000, Sweden). The sham control rats underwent the same procedures except the occlusion of the MCA. The temperature was maintained at 37.0°C using a heating pad (Malvern, UK) and the rats were kept on it until the closure of the skin incision.
Behavioral tests
For functional recovery evaluation, a Foot-fault test and a modified neurologic severity score (mNSS) were carried out at the indicated time by an investigator masked to the treatments as previously described [26,27].
ELISA assay for TNF-α and IL-1β
The ELISA assay was carried out using ELISA kit (ADL, San Diego, USA) according to instructions. To quantify TNF-α and IL-1β protein in brain tissues, 96 well plates were coated with antibodies specific for rat, with the addition of the supernatants of brain tissue homogenates (1:20 dilution). After the reaction between enzyme and substrate, the absorbances of samples were measured at 450 nm with a microplate reader. Standard curves were applied using diluted standard solutions to provide the comparison for the calculation of rat TNF-α and IL-1β in the samples. All the procedures were repeated for at least three times.
Western blotting
Total protein was extracted with homogenization in lysis buffer and centrifuged at 12,000 rpm for 15 min. Bicinchoninic acid (BCA) assay was used to determine protein concentrations. Equal amount of proteins (60 µg) were electrophoresed through a 12% SDS-polyacrylamide gel, and then electro-transferred to PVDF membranes (Invitrogen). The blots were incubated 2 h at room temperature with the indicated antibodies (caspase-3: #9662, Cell Signaling Technology, Boston, MA, 1:1000; β-actin, #4967, CST, 1:2000), and subsequently detected using the secondary antibody (1:2000; Cell Signaling Technology, Boston, MA).
Immunofluorescence staining
The immunofluorescence staining in brain tissues was performed as previously described [28]. Specific primary antibodies against von Willebrand factor (1:50, ab9378, Abcam), doublecortin (1:100, ab216566, Abcam) or NeuN (1:100, ab190195, Abcam) and Iba1 (1:500, ab50765, Abcam) were used to mark the section at 4°C overnight. Secondary antibody for immunofluorescence was anti-rat Alexa Fluor 488 (1:1000, Invitrogen) and then counterstained with DAPI (ATOM, USA). The samples were observed and analyzed by the LEICA TCS SPE microscope (Leica, Germany) and LEICA software LAS AF, respectively. And the positive cells were statistically counted and plotted.
Cell activity assay
BV2 cell activity was determined using Cell Counting Kit-8, according to manufacturer’s protocol (Dojindo Laboratories, Janpan).
Neural cell death in the infarct area was determined by TUNEL staining assay using TdT-FragELTM DNA Fragmentation Detection Kit (Sigma-Aldrich). Cell counts was conducted on the brain tissues from each rat (n=5 per group) with positive cells in 10 different microscopic fields based on their nuclear morphology and color.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from cells or tissues using Trizol reagent (Invitrogen) following the manufacturer’s instructions. Reverse transcription was performed using Prime Script TM Master Mix (Takara, Japan) and designed primers. qPCR was performed using 2 × SYBR Green Mix (Vazyme Biotech, China) on ABI PRISM 7300HT Sequence Detection System (Applied Biosystems, USA). The primer information was shown in Table S1.
Statistical analysis
Data analysis was performed using the SPSS software version 16. Each experiment was carried out at least in triplicates and data were presented as mean ± SD. Two-tailed student’s t-test was performed to analyze the difference between two groups. One-way ANOVA followed by the Scheffé test was carried out to comparison more than 2 groups. A value of P<0.05 was considered statistically significant.
Results
MiR-126 was significantly reduced in patients with ischemic stroke and MCAO rats
Several studies reported that miR-126 was highly expressed in endothelial cells (ECs) and endothelial progenitor cells (EPCs), enhanced cell proliferation and migration leading to the vascular integrity and angiogenesis [20]. However, in some ischemia-associated human diseases such as stroke and coronary artery disease, miR-126 was downregulated in various kinds of cell types. The analysis from public databases GSE110993 and GSE97532 demonstrated that miR-126 was significantly decreased in ischemic stroke (Figure 1A and 1B). In the present study, the role of miR-126 in regulating functional recovery after stroke was investigated. qPCR analysis showed that the expression levels of miR-126 in plasma at 4 h, 12 h and 24 h (Figure 1C) and brain tissues at 24 h in MCAO rats (Figure 1D) were significantly decreased compared to the sham. The similar results were confirmed in clinical data of stroke patients. Figure 1E showed that the miR-126 level in the plasma of stroke patients was significantly lower than that of the normal control volunteers. Correlation between barthel index and miR-126 level showed a positive trend (Figure 1F, R2=0.4825, P=0.034), indicating that the patients with higher miR-126 level in plasma have better activities of daily living.
Figure 1.

MiR-126 was significantly reduced in patients with ischemic stroke and MCAO rats. A, B. The public database GSE110993 and GSE97532 was used to analyze the expression level of miR-126. *P<0.05 vs sham group. C. Rats were subjected to MCAO (middle cerebral artery occlusion), and then the miR-126 level in the plasma of rat MCAO was assessed at 4 h, 12 h and 24 h. Relative miR-126 level in plasma was decreased in a time-dependent manner. *P<0.05 vs sham group. D. Rats were subjected to MCAO, and then the miR-126 level in the brain tissues of rat MCAO was assessed at 24 h. *P<0.05 vs sham group. E. The relative level of miR-126 in plasma of stroke patients (n=13) and normal control (n=17) was assessed using qPCR. **P<0.01. F. The association of barthel index and miR-126 level in plasma of stroke patients (R2=0.4825, P=0.034). The miR-126 level was positively associated with barthel index.
MiR-126+ exosomes enhanced functional recovery after stroke
It was reported that miR-126-overexpressed EPCs (Endothelial progenitor cells) contribute to decrease infarct volume and promote functional recovery in cerebral ischemic damage, but our unpublished data showed that intravenous injection of miR-126 did not change the infarct volume and regulate functional recovery in stroke rats model. Although intravenous injection of ADSCs is functional, it cannot pass the blood-brain barrier to the injury area after stroke. ADSCs are an important source of exosomes which play an important regulatory role in stroke [13,29], and can pass through the blood-brain barrier to delivery miRNAs to the injury area [13]. Thus we focused on effect of exosomes derived from miR-126-modified ADSCs in vitro and in vivo. As expected, the miR-126 level in plasma exosomes was significantly decreased in stroke patients when compared to the normal control volunteers (Figure 2A). To test the effect of exosomal miR-126, we employed overexpression and knock-down technologies to up-regulate or inhibit miR-126 level in ADSCs and thus acquired ADSCs-derived miR-126+ exosomes and miR-126- exosomes respectively. Figure 2B showed that miR-126 was highly expressed in miR-126+ exosomes while there was decreased miR-126 level in miR-126- exosomes when compared to the exosomes control. The sensorimotor performance of the stroke severity was then evaluated by assaying foot-fault and modified neurological severity score (mNSS). Rats subjected to MCAO, and then received PBS control, exosomes, miR-126+ exosomes or miR-126- exosomes intravenously at 2 h after MCAO. The Foot-fault test (Figure 2C) and mNSS (Figure 2D) were carried out prior to the treatment after MCAO, at day 1, 3, 7 and 14 after MCAO. Compared with PBS control, exosomes treatment significantly improved functional recovery at day 3 after MCAO. miR-126+ exosomes further improved functional recovery, whereas miR-126- exosomes impaired functional recovery compared to exosomes at day 7 and day 14 after MCAO (Figure 2C and 2D). These results demonstrated that increased miR-126 expression in ADSCs-derived exosomes enhances functional recovery after stroke.
Figure 2.
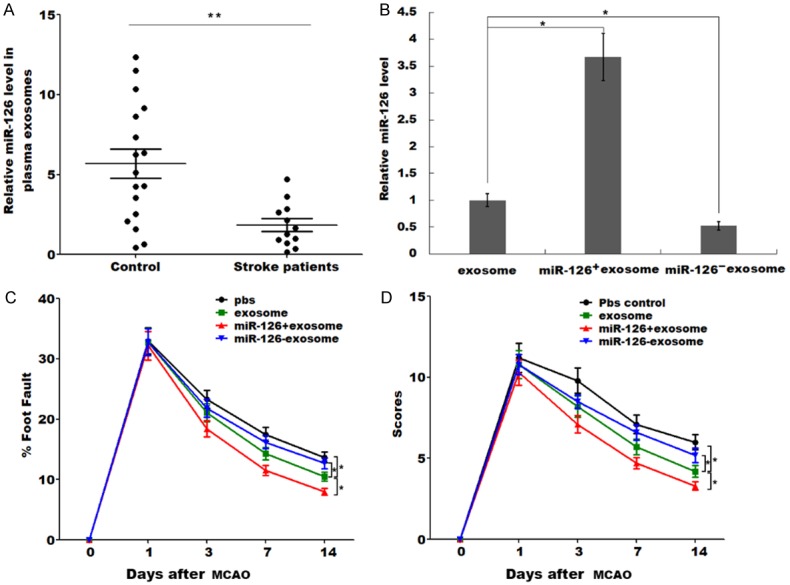
MiR-126+ exosomes enhanced functional recovery after stroke. (A) Exosomes were isolated from plasma of stroke patients (n=12) and normal controls (n=17), and the miR-126 level was assessed using qPCR. **P<0.01. (B) Overexpression and knock-down technologies was employed to up-regulate or inhibit miR-126 level in ADSCs and thus acquired ADSCs-derived miR-126+ exosomes and miR-126- exosomes respectively. The miR-126 level in exosomes, miR-126+ exosomes and miR-126- exosomes was was assessed using qPCR. *P<0.05. (C) Rats were subjected to MCAO and treated via intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome, and then the foot fault test (C) and modified neurological severity scores (mNSS) of rat behavior (D) was carried out at 1, 3, 7 and 14 days after MCAO. *P<0.05.
MiR-126+ exosomes promoted neurogenesis and vasculogenesis after ischemic stroke
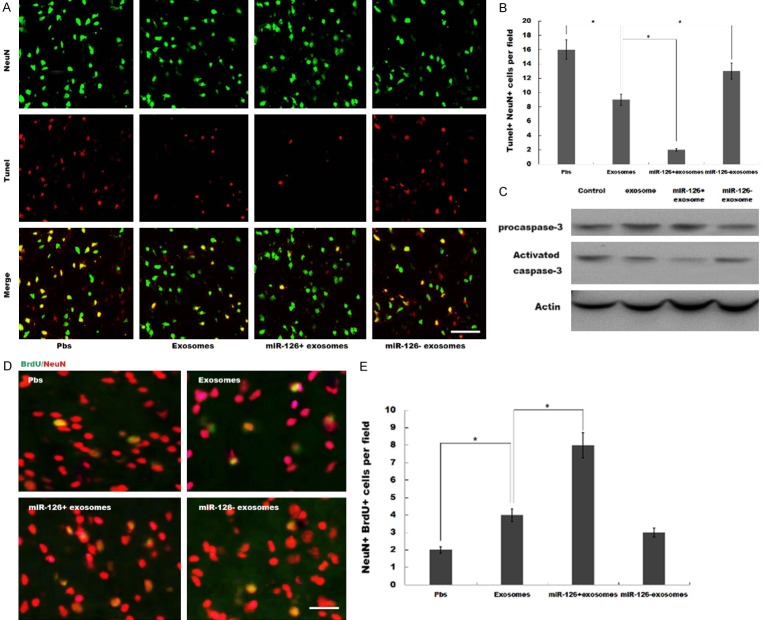
The expression of vWF, a marker of endothelial cells of cerebral blood vessels, was significantly upregulated in peri-infarct zone after exosomes treatment compared to PBS control. miR-126+ exosomes further increased vWF expression in peri-infarct zone, whereas miR-126- exosomes suppressed vWF expression compared to exosomes (Figure 3A and 3B). The expression of doublecortin, an indication of migrating neuroblasts, was increased in peri-infarct zone after exosomes treatment compared to PBS control. miR-126+ exosomes further increased doublecortin expression, whereas miR-126- exosomes suppressed doublecortin expression compared to exosomes (Figure 3C and 3D). Moreover, miR-126+ exosomes decreased terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive neurons compared with exosomes (Figure 4A and 4B). The result was further verified by western blot analysis, showing that activated caspase-3 was decreased by the pretreatment with miR-126+ exosomes (Figure 4C). Additionally, immunofluorescence analysis revealed a notably increased number of BrdU-positive neurons (Figure 4D and 4E) by the pretreatment with miR-126+ exosomes. These data showed a miR-126+ exosomes-induced enhancement in neurogenesis and vasculogenesis after ischemic stroke.
Figure 3.

MiR-126+ exosomes promoted neurogenesis and vasculogenesis after ischemic stroke. (A and B) Rats were subjected to MCAO and treated via intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome, and thenthe expression of vWFin peri-infarct zone was assessed using immunofluorescence staining (A) and the vWF+ cells were counted (B). *P<0.05. (C and D) Rats were subjected to MCAO and treated via intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome, and then the expression of DCX in peri-infarct zone was assessed using immunofluorescence staining (C) and the DCX+ cells were counted (D). *P<0.05.
Figure 4.
MiR-126+ exosomes repressed neuron apoptosis and promoted cell proliferation. (A) Neuronal apoptosis in peri-infarct zone was detected by TUNEL assay after MCAO and intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome. (B) The Tunel+NeuN+ cells per field was statistically analyzed and plotted. *P<0.05. (C) Activated caspase-3 and procaspase-3 were detected by western blotting after MCAO and intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome. (D, E) Rats were subjected to MCAO and treated via intravenous injection of BrdU and PBS, exosome, miR-126+ exosome or miR-126- exosome, and then the NeuN+BrdU+ cells in peri-infarct zone was assessed using immunofluorescence staining (D) and were counted (E). *P<0.05.
MiR-126+ exosomes inhibited microglial activation and inflammatory response induced by ischemic stroke
Post-ischemic inflammation is an primary step in the progression of brain ischemia-reperfusion injury [30], and microglia are the primary cellular source of increased proinflammatory cytokine levels in the brain [31]. To further unveil the potential function of miR-126+ exosomes in treating MCAO rats, we analyzed the activation of microglial cell by Iba1 immunofluorescence. Figure 5A showed that microglial activation was significantly repressed after treatment with exosomes, and miR-126+ exosomes further suppressed microglial activation compared to exosomes. To verify the effects of miR-126+ exosomes on the secretion of TNF-α and IL-1β, ELISA assay was performed to measure the protein expression of TNF-α and IL-1β in brain tissues. As shown in Figure 5B and 5C, miR-126+ exosomes significantly inhibited TNF-α and IL-1β production, whereas miR-126- exosomes results in an increased TNF-α and IL-1β production compared to naïve exosomes. These data suggest that exosomal miR-126 plays an important role in exosomes-regulated neuroinflammation in MCAO rats.
Figure 5.

MiR-126+ exosomes inhibited microglial activation and inflammatory response induced by ischemic stroke. (A) Rats were subjected to MCAO and treated via intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome, and then the Iba1+ cells in peri-infarct zone was assessed using immunofluorescence staining. (B, C) Rats were subjected to MCAO and treated via intravenous injection of PBS, exosome, miR-126+ exosome or miR-126- exosome, and thenTNF-α level (B) as well as IL-1β level (C) in brain tissues was assessed using ELISA. *P<0.05.
The role of miR-126+ exosomes in regulating microglia activation in vitro was further investigated by Cell Counting Kit-8 assay. Figure 6A showed that miR-126+ exosomes significantly inhibited cell activity of BV2 microglial cells, whereas miR-126- exosomes results in an increased BV2 cell activity compared to naïve exosomes.
Figure 6.
MiR-126+ exosomes repressed OGD-induced secretion of inflammatory factors. (A) BV2 microglia was treated with PBS control, exosome, miR-126+ exosome or miR-126- exosome, and then cell activity was assessed using Cell Counting Kit-8 kit. *P<0.05. (B, C) BV2 microglia was treated with PBS control, exosome, miR-126+ exosome or miR-126- exosome, and then the mRNA levels of TNF-α (B) and IL-1β (C) was assessed using qPCR. *P<0.05.
To further verify the effects of miR-126+ exosomes on the secretion of TNF-α and IL-1β in vitro, oxygen and glucose deprivation (OGD) cellular models were constructed and then BV2 cells were treated with exosomes, miR-126+ exosomes or miR-126- exosomes. Figure 6B and 6C showed that exosomes treatment attenuated OGD-induced production of TNF-α and IL-1β. More important, miR-126+ exosomes further inhibited the secretion of TNF-α and IL-1β, whereas miR-126- exosomes results in an increased secretion of TNF-α and IL-1β compared to naïve exosomes. There results suggest that exosomes from miRNA-126-modified ADSCs contribute to functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation.
Discussion
In this study, we investigated the possible role of tailored exosomes enriched with miR-126 in protecting against cerebral ischemia injury, and determine the precise role of miRNA-126 in regulating stroke in the context of ADSCs. The current data demonstrate that (i) miR-126 was significantly reduced in patients with ischemic stroke and MCAO rats, (ii) miR-126+ exosomes promoted neurogenesis and vasculogenesis after ischemic stroke, (iii) miR-126+ exosomes inhibited microglial activation and inflammatory response induced by ischemic stroke, (iv) miR-126+ exosomes enhanced functional recovery after stroke. These results reveal a potential role of exosomal miR-126 in regulating neurogenesis and neuroinflammation, and may provide a novel opportunity to treat ischemic stroke.
The ADSC-based therapy has developed as a promising approach for treating stroke due to its ability to reduce neuronal injury, limit proinflammatory immune responses and promote neuronal repair [32]. ADSC transplantation is capable of enhancing the migratory ability in the infarct core and border zone, attenuating neurological deficits and reducing the lesion volume of cerebral infarction [33,34]. ADSCs could also directly differentiate into endothelial cells, promote angiogenesis, and thus enhance recovery after ischemia injury by secreting IL-6 [35]. However, the clinical use of ADSC has been limited as a result of the potential risk of tumorigenicity and low survival rate of implanted cells [36]. Previous studies have also stated that ADSCs were not able to reach the infarct area after intravenous administration but somehow with the complete functional recovery observed [37].
Given that ADSCs are an important source of exosomes which hold great promise as an endogenous drug delivery system for the treatment of cerebral ischemia, ADSCs might play protective role in cerebral ischemia through secreting exosomes. Exosomes secreted by ADSCs (ADSCs-Exos) are reported to act as the ‘courier’ that responsible for miRNA transfer to the lesion site in stoke [13], and exosomes can lead the path for ADSCs to fulfill its regulatory role in functional recovery after stroke by applying specific miRNA modifications. Yang et al demonstrated that ADSCs-Exos promote the mobility and angiogenesis of brain microvascular endothelial cells after oxygen-glucose deprivation [33]. Exosomes play regulatory roles by delivering pre-wrapped cargoes like miRNAs, lncRNA and proteins to recipient cells [6]. Yang et al reported that exosomes modified with rabies virus glycoprotein fused to exosomal protein lysosome-associated membrane glycoprotein 2b, could efficiently deliver miR-124 to the infarct site, and tailored exosomes enriched with miR-124 could promote cortical neural progenitors to obtain neuronal identity and protect against ischemic injury [10]. miR-30d-5p-enhanced ADSC-derived exosomes prevent cerebral injury by inhibiting autophagy-mediated microglial polarization to M1 [13].
Although intravenous injection of miR-126 did not change the infarct volume and regulate functional recovery in stroke rats model (data not shown), miR-126 was significantly reduced in patients with ischemic stroke and MCAO rats. Based on the above analysis, we speculated whether miR-126 plays regulatory role in cerebral ischemic injury through the mediation of exosomes, and investigated whether ADSCs-Exos mediated miR-126 transfer and thus promoted neurological recovery after stroke. Current data demonstrated that systemic administration of ADSCs-Exos significantly improves functional recovery, enhances neurogenesis, inhibits neuroinflammation, and represents a novel treatment for stroke. More important, these outcomes are further enhanced with miR-126+ exosomes treatment and significantly decreased with miR-126- exosomes treatment, compared to naïve exosomes treatment. These results suggest tnat exosomes can be utilized therapeutically for the targeted delivery of gene drugs to the brain, thus having great potential for clinical applications.
Given that ADSCs are unlikely to pass the blood-brain barrier and the low survival rate of implanted cells, our result proposed a potent approach to increase the efficiency of ADSC-related treatment for ischemic stroke. Taken together, this study suggests an interesting prospect to target ADSCs-derived miR-126+ exosomes as a potent therapy for ischemic stroke.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81774109), Zhejiang Provincial Department of Education (Y201839270), and Wenzhou Science and Technology Plan Project (Y20180508, Y20180496, Y20170164, Y20170023).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Barr TL, Simpkins JW. Ischemic stroke: a consequence of a diseased immune system? Aging Dis. 2014;5:292–293. doi: 10.14336/AD.2014.0500292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Cheng G, Sheu C, Tseng GF, Wang T, Huang Y. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat. 2008;213:249–258. doi: 10.1111/j.1469-7580.2008.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SU, De Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 4.Leu S, Lin Y, Yuen C, Yen C, Kao Y, Sun C, Yip H. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med. 2010;8:63. doi: 10.1186/1479-5876-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes H. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 7.Lee RC, Feinbaum RL, Ambros VR. The C. elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 8.Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. 2014;23:2851–2861. doi: 10.1089/scd.2014.0146. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–2502. doi: 10.1111/jcmm.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from miR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44:2105–2116. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from miR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Du Y, Esposito E, Liu Y, Guo S, Wang X, Lo EH, Xing C, Ji X. Effects of focal cerebral ischemia on exosomal versus serum miR126. Transl Stroke Res. 2015;6:478–484. doi: 10.1007/s12975-015-0429-3. [DOI] [PubMed] [Google Scholar]
- 15.Xiang Y, Guo J, Peng YF, Tan T, Huang HT, Luo HC, Wei YS. Association of miR-21, miR-126 and miR-605 gene polymorphisms with ischemic stroke risk. Oncotarget. 2017;8:95755–95763. doi: 10.18632/oncotarget.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, Yu P, Chopp M. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res. 2014;5:711–718. doi: 10.1007/s12975-014-0364-8. [DOI] [PubMed] [Google Scholar]
- 18.Bai X, Luo J, Zhang X, Han J, Wang Z, Miao J, Bai Y. MicroRNA-126 reduces blood-retina barrier breakdown via the regulation of VCAM-1 and BCL2L11 in ischemic retinopathy. Ophthalmic Res. 2017;57:173–185. doi: 10.1159/000454716. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Tao Y, Huang Q. Effect and mechanism of miR-126 in myocardial ischemia reperfusion. Genet Mol Res. 2015;14:18990–18998. doi: 10.4238/2015.December.29.6. [DOI] [PubMed] [Google Scholar]
- 20.Pan Q, Zheng J, Du D, Liao X, Ma C, Yang Y, Chen Y, Zhong W, Ma X. MicroRNA-126 priming enhances functions of endothelial progenitor cells under physiological and hypoxic conditions and their therapeutic efficacy in cerebral ischemic damage. Stem Cells Int. 2018;2018:2912347. doi: 10.1155/2018/2912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prochazka V, Jurcikova J, Vitkova K, Pavliska L, Porubova L, Lassak O, Buszman P, Fernandez CA, Jaluvka F, Spackova I, Lochman I, Prochazka M, Janikova M, Tauber Z, Frankova J, Lachnit M, Hiles MC, Johnstone BH. The role of miR-126 in critical limb ischemia treatment using adipose-derived stem cell therapeutic factor concentrate and extracellular matrix microparticles. Med Sci Monit. 2018;24:511–522. doi: 10.12659/MSM.905442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda K, Sowa Y, Nishino K, Itoh K, Fushiki S. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015;74:728–736. doi: 10.1097/SAP.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 24.Hata R, Mies G, Wiessner C, Fritze K, Hesselbarth D, Brinker G, Hossmann K. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab. 1998;18:367–375. doi: 10.1097/00004647-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Li X, Matei N, McBride D, Tang J, Yan M, Zhang JH. Ezetimibe, a NPC1L1 inhibitor, attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats. Exp Neurol. 2018;307:12–23. doi: 10.1016/j.expneurol.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 28.Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation. 2011;8:54. doi: 10.1186/1742-2094-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691:87–93. doi: 10.1016/j.brainres.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Shichita T, Sakaguchi R, Suzuki M, Yoshimura A. Post-ischemic inflammation in the brain. Front Immunol. 2012;3:132. doi: 10.3389/fimmu.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Han K, Chen J, Wang C, Dong Y, Yu M, Bai R, Huang C, Hou L. Vascular endothelial growth factor is neuroprotective against ischemic brain injury by inhibiting scavenger receptor A expression on microglia. J Neurochem. 2017;142:700–709. doi: 10.1111/jnc.14108. [DOI] [PubMed] [Google Scholar]
- 32.Zhao K, Li R, Gu C, Liu L, Jia Y, Guo X, Zhang W, Pei C, Tian L, Li B. Intravenous administration of adipose-derived stem cell protein extracts improves neurological deficits in a rat model of stroke. Stem Cells Int. 2017;2017:1–11. doi: 10.1155/2017/2153629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Cai Y, Zhang Y, Liu J, Xu Z. Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through MicroRNA-181b/TRPM7 axis. J Mol Neurosci. 2018;65:74–83. doi: 10.1007/s12031-018-1071-9. [DOI] [PubMed] [Google Scholar]
- 34.Du G, Liu Y, Dang M, Zhu G, Su R, Fan Y, Tan Z, Wang LX, Fang J. Comparison of administration routes for adipose-derived stem cells in the treatment of middle cerebral artery occlusion in rats. Acta Histochem. 2014;116:1075–1084. doi: 10.1016/j.acthis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Pu CM, Liu CW, Liang CJ, Yen YH, Chen SH, Jiang-Shieh YF, Chien CL, Chen YC, Chen YL. Adipose-derived stem cells protect skin flaps against ischemia/reperfusion injury via IL-6 expression. J Invest Dermatol. 2017;137:1353–1362. doi: 10.1016/j.jid.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:468748. doi: 10.1155/2014/468748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–685. doi: 10.3109/14653249.2010.549122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.