Abstract
Although losartan has inhibitory effects on acute kidney injury (AKI), the underlying molecular mechanisms have remained largely unclear. The expressional alteration of circular RNAs (circRNAs) was investigated in the present study to understand the therapeutic effects of losartan against AKI. AKI rat models were established by ischemia and reperfusion (I/R) treatment. Urea and creatinine levels were determined and histological features of kidney tissues examined following hematoxylin and eosin staining. Cell apoptosis was assessed by TUNEL. CircRNA profiles were obtained by RNA-Seq followed by Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses. Expression of circRNAs was validated by quantitative RT-PCR. I/R treatment induced an increase in plasma urea and creatinine levels, abnormal kidney tubular structure, and cell apoptosis in Sprague-Dawley (SD) rats, which were effectively inhibited by pre-treatment with losartan. Further RNA-Seq analysis revealed a wide range of differentially expressed circRNAs in I/R rat kidneys, which were reversed by losartan pre-treatment. GO and KEGG analyses revealed that the circRNAs are associated with various biological processes, including the PI3K-Akt signaling pathway. Specifically, circ-Dnmt3a, circ-Akt3, circ-Plekha7, and circ-Me1 were down-regulated in AKI rats and restored by losartan. The current study provides an overview of circRNAs expression profiles based on the inhibitory effects of losartan in ischemic AKI rats.
Keywords: Circular RNA, acute kidney injury, ischemia and reperfusion, losartan, PI3K-Akt signaling pathway
Introduction
Acute kidney injury (AKI) is a common clinical symptom often characterized by the abrupt loss of normal kidney functions, and which is clinically defined a significant increase in serum creatinine and a rapid reduction in urine output [1]. AKI could be induced by various conditions, including kidney ischemia, exposure to toxic substances, obstruction of urinary tracts, and severe inflammation [2,3]. AKI is associated with a high mortality, great economic, and social burdens, particularly in critically ill cases [4-6]. Numerous severe complications are also associated with AKI, such as metabolic acidosis, body fluid imbalance, uremia, and chronic kidney disease [4,7]. Due to the lack of an effective therapeutic reagent, pathogeny expelling, and renal replacement therapy remain the current major treatment strategies [1,8]. Notably, as an angiotensin II type 1 receptor (AT1) antagonist, losartan has been shown to exert renoprotection effects in ischemic AKI rats [9]. However, the underlying molecular mechanisms are yet to be understood.
Circular RNAs (circRNAs) are a recently identified group of endogenous RNA molecules that form covalently closed loops from precursor mRNA (pre-mRNA) by ligation of the 3’ and 5’ ends [10]. Different from the linear RNA molecule, the lack of 5’ caps and 3’ poly-A tails render resistance against exonuclease-mediated degradation and significantly higher stability in vivo in circRNAs [11]. Recent investigations have revealed that majority of circRNAs are derived from exon regions and are mainly distributed in the cytoplasm with high tissue specificity [12]. In addition, CircRNAs are associated with a wide range biological processes as microRNA (miRNAs) sponges, which directly bind to targeted miRNAs and suppress key gene expression-repressing roles of various miRNAs [13]. CircRNAs could also perform other biological functions independent of those associated with miRNAs, such as binding with RNA-binding proteins and regulating the expression of their parent genes in nuclei [14,15]. Consistent with their critical roles in regulating miRNA functions and gene expression, a large number of circRNAs are reportedly involved in pathogenesis of various human diseases such as retinal vascular dysfunction associated with diabetes mellitus [16], cancer development [17], vascular endothelial dysfunction [18], myocardial infarction [19], and mental sickness such as depression [20]. Such accumulating evidences suggest that circRNAs act as key regulators of biological and pathogenic processes.
CircRNAs are further associated with kidney function and renal diseases. One previous RNA-Seq study revealed that as many as 1664 circRNAs are significantly and highly expressed in human kidney tissues and 474 are unique circRNAs expressed in the kidney [21]. In addition, a recent genome-wide circRNA profiling study detected over 12,000 circRNAs expressed in the kidney tissues of a rat hypertension model, indicating the potential functions of circRNAs in renal disorders [22]. Similarly, the expression of 171 circRNAs were reported to be significantly elevated in renal biopsies of lupus nephritis patients compared with in normal kidney tissues collected from urology patients [23]. Renal circHLA-C were particularly highly expressed in lupus nephritis patients, and could bind with and suppress the expression of miR-150 during lupus nephritis pathogenesis [23]. The specific roles and molecular mechanisms of circRNAs in the regulation of kidney physiology and development of various renal diseases require further investigations.
Although large numbers of circRNAs have been identified in both human and animal kidney tissues, their expression profiles and potential roles during AKI development and its inhibition by losartan remain largely unknown. In this study, we performed a large-scale characterization of differentially expressed circRNAs in rat ischemic AKI model and treated with losartan. We reveal a group of circRNAs that are differentially expressed in AKI rats and restored in the presence of losartan. In particular, we investigated the expression of circ-Dnmt3a, circ-Akt3, circ-Plekha7, and circ-Me1 and predicted their circRNA-miRNA-mRNA interaction networks, which could be involved in the protective effects of losartan on AKI. Our findings may offer novel insights into the involvement of circRNAs in AKI progression and treatment.
Materials and methods
Animals and grouping
Sprague-Dawley (SD) rats aged between 6 and 8 weeks and weighing 400 to 700 g were purchased from Guangdong Medical Laboratory Animal Center and kept at the Forevergen laboratory animal center (Forevergen, GZ, China) for more than one week before the following experimental procedures. All experimental rats were raised at 22°C in standard breeding cages with a relative humidity of 55% and a 12:12 light-dark cycle, with free access to drinking water and standard food. The Laboratory Animal Ethics Committee of Guangdong Provincial People’s Hospital approved all experimental procedures using the SD rats and no fasting was carried out before the experiments. The SD rats were randomly divided into six groups, including a control group, a sham group, and the four other groups of rats were subjected to ischemia and reperfusion (I/R) treatment to induce ischemic AKI.
AKI model establishment
To induce AKI, the SD rats were subjected to I/R surgery as previously described with minor modifications [24]. Briefly, a skin-deep incision was made to open the retroperitoneal space after being anesthetized with isoflurane, and the vascular pedicles of both kidneys of each rat were then mobilized and bilateral renal arteries occluded using microvascular clamps for 45 min, followed with reperfusion by removal of microvascular clamps for 24, 48 and 72 h. The SD rats of the sham surgery group were subjected to similar experimental operations although without the occlusion of the renal artery. The control group was kept in normal conditions and not subjected to the experimental treatments. To evaluate of the efficacy of losartan, rats were pre-treated with 80 mg/kg losartan every day for seven days before model establishment. The rats were finally euthanized via cardiac incision under deep anesthesia using isoflurane and the kidneys collected for the following assays.
Plasma urea and creatinine measurement
After reperfusion for designated periods, about 2-ml blood samples were collected from each SD rat via the posterior orbital venous plexus. The blood samples were then centrifuged at 4,000 × g for 15 min and the serum samples were collected and used for the determination of urea nitrogen and creatinine concentrations using a Roche Cobas C111 analyzer (Roche, Switzerland). At least three biological repeats were performed for statistical comparisons of urea and creatinine abundance between different groups.
Histopathological evaluation and apoptosis analysis
For a histological analysis of rat kidney after model establishment, the kidneys were rinsed and fixed in 10% formalin for 24 h, embedded in paraffin, and sliced into serial 2-μm thick sections. Rat kidney sections were then mounted on slides, which were then subjected to hematoxylin and eosin staining. The injuries in rat kidneys were evaluated based on swelling, necrosis, and lysis of renal tubular epithelial cells under light microscopy, as well as renal interstitial congestion and inflammatory cells infiltration. The apoptosis of rat kidney cells was analyzed using the TUNEL method using a One Step TUNEL Apoptosis Assay Kit (#C1088; Beyotime, Shanghai, China) according to the manufacturer’s instructions. Kidney slides were stained with 50-μl TUNEL solution at 37°C for 1 h in the dark, and apoptotic cells were stained with green fluorescent dye and observed under fluorescence microscopy.
Circular RNA identification by RNA-Seq
Total RNA samples were extracted from kidney tissues of SD rats using TRIzol solution (Thermo Fishier Scientific; USA) according to manufacturer’s instructions. The integrity and size distribution of extracted RNAs samples were analyzed using an Agilent 2100 Bioanalyzer and agarose gel electrophoresis, as quality control. Subsequently, the rRNA constituents were obtained using a Qiagen RiboMinus Eukaryote Kit according to manufacturer’s instructions. The RNA library for RNA-Seq analysis was then constructed using the NEBNext® UltraTM II RNA Library Prep Kit for Illumina® (#E7770S; New England Biolabs) according to the manufacturer’s instructions, followed by RNA quantitation using an Agilent 2100 Bioanalyzer and RNA sequencing using the Hiseq 2000 system (Illumina, USA).
CircRNA annotation and quantitation
Subsequent bioinformatic analyses were conducted as previously described [25]. Briefly, clean reads from RNA-Seq analysis were aligned to the reference genome database using the Bowtie2 software (bowtie-bio.sourceforge.net/bowtie2). The back-splice algorithm was run to pick out the junctions of reads that had not been mapped to the reference genome database. Prediction and annotation of circular RNA candidates were completed using CIRI software. Differential expression of circRNAs between the sham and AKI group, and the AKI and AKI+losartan groups were determined by calculating the RPM values (Mapped backsplicing junction reads per million mapped reads), by normalizing to total read number. Differentially expressed circRNAs were defined by a fold change of higher than 2 and a P value < 0.05. The hierarchical clustering and volcano plot filtering analysis of circRNA expression were carried out in R (Version 1.0.8; cran.r-project.org/).
Target prediction, network analysis, and validation
The target miRNAs of differentially expressed circRNAs were predicted as previously described [25]. Gene ontology (GO) analyses were carried out using Database for Annotation, Visualization and Integrated Discovery to explore the biological processes, subcellular components, and molecular functions of target RNAs of the differentially expressed circRNAs. The Kyoto Encyclopedia of Genes and Genomes (KEGG) method was used to analyze the significantly enriched pathways linked to differentially expressed circRNAs (http://www.genome.jp/kegg/). The networks among circRNAs, miRNAs, and mRNAs were generated using Cytoscape (Version 3.2.1). The differential expression of four representative circRNAs was validated by quantitative RT-PCR using divergent primers (Table 1).
Table 1.
Sequences of primers used for quantitation of circRNA expression
| CircRNA ID | Primer sequences (5’-3’) | Product length (bp) |
|---|---|---|
| Circ-DNMT3a F | TGGCACGTTGGAAAAGGGAG | 86 |
| Circ-DNMT3a R | TGGGGGTGAGGAAAACACTT | |
| Circ-Akt3 F | TGGTTCGAGAGAAGGCAAGTG | 85 |
| Circ-Akt3 R | CTGTCCATTCTTCTCTTTGCGA | |
| Circ-Plekha7 F | TACCACCTTATGGCCTGGGA | 212 |
| Circ-Plekha7 F | GCATTCTTGACAGGCGTCAG | |
| Circ-Me1 F | CTGCCTTGGGGATTGCTCAT | 237 |
| Circ-Me1 F | CACAGACGCTGTTCCTTGAA |
Statistical analysis
All experiments were performed with at least three biological replicates. Data were analyzed in IBM SPSS 18.0 (IBM, NY, USA) and expressed as means ± SD. Differences in serum urea and creatinine levels and in the expression of circ-Dnmt3a, circ-Akt3, circ-Plekha7, and circ-Me1 between groups were analyzed using One-way Analysis of Variance followed by least significant difference tests. A P value < 0.05 was considered statistically significant.
Results
Establishment of AKI rats by I/R treatment
To explore the pathogenic mechanism of AKI and the therapeutic effects of losartan, the rat AKI models were established by I/R treatment. As illustrated in Figure 1, the plasma urea content in rats undergoing ischemia followed by reperfusion for 24, 48 and 72 h were greatly elevated in comparison to the control and the sham group (Figure 1A, n=5/group). In addition, the creatinine levels in the plasma of rats subjected to I/R were significantly higher than those of the control and sham groups (Figure 1A, n=5/group). The greatest increases in both plasma urea and creatinine levels in rats following ischemic treatment were observed in those followed by reperfusion for 24 h, and no significant differences in plasma urea and creatinine levels were observed between the control and the sham groups (Figure 1A, n=5/group). Our histological investigations revealed significantly swollen renal tubular epithelial cells with necrosis and cytolysis, as well as renal interstitial congestion and bleeding with inflammatory cell infiltration in the I/R groups compared with the control and sham groups (Figure 1B). In addition, the proportions of apoptotic cells in the kidney tissues of the I/R groups markedly increased compared with those of the control and sham groups (Figure 1C). The biochemical, histological, and cellular results demonstrated that the I/R treatment induced severe injuries in rat kidneys.
Figure 1.
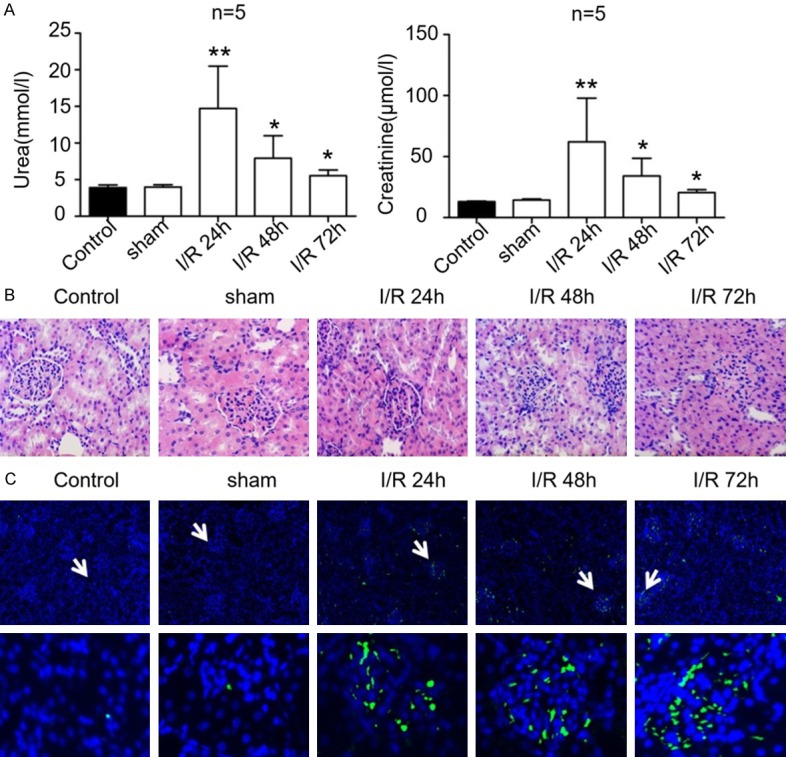
Acute kidney injury in rats induced by ischemia and reperfusion. A. The levels of urea (left) and creatinine (right) in plasma of rats that underwent ischemia and reperfusion treatment. The control group and the sham group were used as controls. B. Structural alteration of rat renal tubules induced by ischemia and reperfusion treatment. The histological properties were observed under a microscope following hematoxylin and eosin staining. C. Apoptosis of rat kidney cells during kidney injury induced by ischemia and reperfusion. Cell apoptosis was evaluated using the TUNEL method. I/R: ischemia and reperfusion. *, P < 0.05, **, P < 0.01.
Suppression of I/R-induced AKI by losartan
To confirm the inhibitory roles of losartan on the progression of AKI, the rats that were subjected to I/R for 24 h were pre-treated with losartan. We observed that the plasma urea levels in AKI rats were higher than those in the sham control, and the levels of plasma urea were higher than those in the AKI rats displayed in Figure 1. The plasma urea levels vary in different batches of animal models, potentially because AKI models are constructed independently so that parameters such as body weight and mental state may result in some differences. In the rats with losartan pre-treatment, the plasma urea levels markedly decreased in comparison with the rats in the AKI group without losartan pre-treatment, and were quite close to the levels in the control group (Figure 2A, n=5/group). Similarly, we observed that creatinine content in plasma of the rats pre-treated with losartan before I/R treatment were also greatly down-regulated, compared with the AKI group (Figure 2A, n=5/group). Abnormal alteration of rat renal tubular structures in AKI rats, including renal tubular epithelial cell swelling, renal interstitial bleeding, and inflammatory cell infiltration, were also greatly suppressed by pre-treatment with losartan (Figure 2B). In addition, compared with the AKI group, the number of apoptotic cells in rat kidney tissues pre-treated with losartan were also greatly decreased (Figure 2C). Our analysis showed that pre-treatment with losartan effectively reversed all the biochemical, cellular, and histological changes in rats induced by I/R treatment, which indicated that losartan is a potent inhibitor of ischemic AKI.
Figure 2.
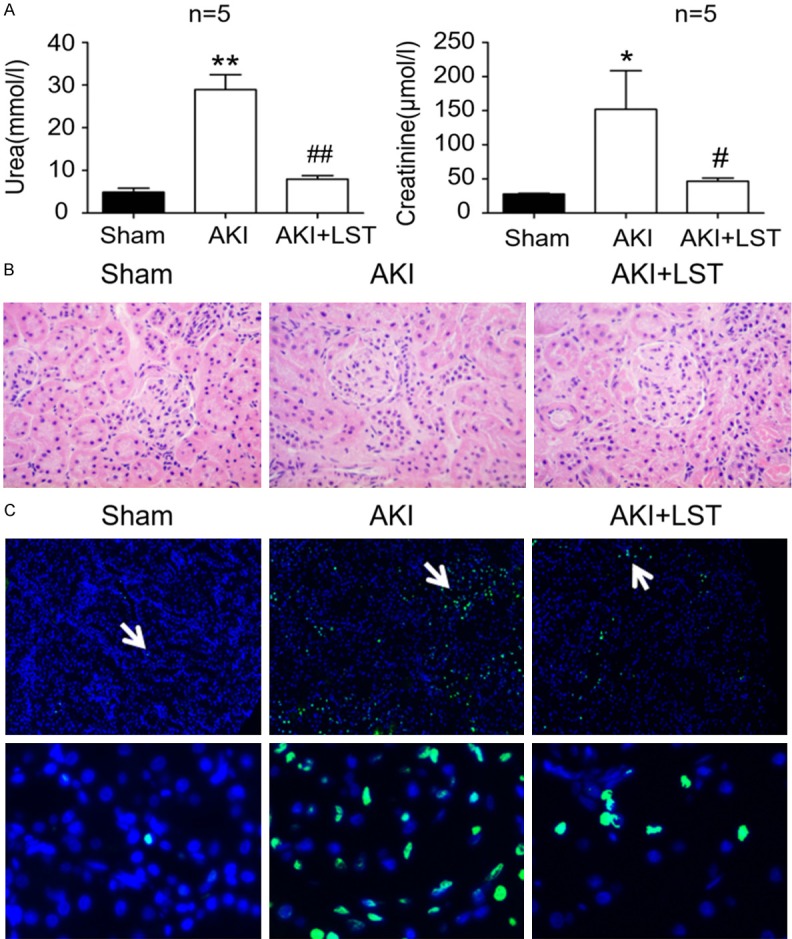
Inhibition of rat ischemic AKI by losartan. A. Urea (left) and creatinine (right) contents in AKI rats that were pre-treated with losartan. The sham group was used as the control, and reperfusion time was 24 h in the AKI group. B. Influence of losartan pre-treatment on structural alteration of rat renal tubules induced by ischemia and reperfusion treatment. The histological properties were observed under a microscope following hematoxylin and eosin staining staining. C. Proportions of apoptotic cells in losartan-treated rat kidney during kidney I/R-induced injury. Cell apoptosis was analyzed using the TUNEL method. AKI: acute kidney injury; LST: losartan; I/R: ischemia and reperfusion. *P < 0.05, **P < 0.01, compared with the sham group; #, P < 0.05, ##P, < 0.01, compared with the AKI group.
Differentially expressed circRNAs in AKI rats following pre-treatment with losartan
To explore the involvement of circRNAs in AKI progression and the inhibition of renal injury by losartan, the circRNA profiles in kidney tissues of the sham group, the AKI group, and the AKI+LST group (rats pre-treated with losartan and treated with I/R) were characterized by RNA-Seq (Figure 3). We observed that the lengths of identified circRNAs from the three groups of rat kidney tissues had similar distributions, which ranged from lower than 1000 bp and the highest distribution of circRNA length was less than 200 bp (Figure 3A). Also, we analyzed the properties of gene regions responsible for encoding the circular RNAs and observed that majority of circRNAs were encoded by the exon regions, followed by introns, 5’-UTR (untranslated region), 3’-UTR, intergenic regions, and the antisense sequences (Figure 3B). For instance, in the kidney tissues of rats with I/R-induced acute injury and pre-treated with losartan, 2697 circRNAs were encoded by the exon regions of encoding genes, 1578 circRNAs by the intron regions, 207 circRNAs by the 5’-UTR, 139 circRNAs by the 3’-UTR, 138 circRNAs by the intergenic regions, and 101 circRNAs were encoded by the antisense regions in the rat genome (Figure 3B). Moreover, the differentially expressed circRNAs were distributed in nearly all rat chromosomes, although more circRNAs were in certain chromosomes such as chromosome 1 and 10 (Figure 4). The widespread distribution of circRNA lengths and their genomic regions indicate that circRNAs might be generated by various mechanisms and might perform different biological functions.
Figure 3.
Differentially expressed circRNAs in rat kidney regulated by I/R and losartan pre-treatment. A. Frequency distribution of circRNA lengths in the sham group, the AKI group, and the AKI+LST group. X-axis represents the range of circRNA lengths, and the Y-axis represents the frequency of circRNAs with specific lengths. B. Distribution of circRNA-encoding regions on rat genome. The numbers of circRNAs encoded by the exon, intron, 5- and 3-UTR region, intergenic regions, and antisense regions were separately counted. C. Hierarchical clustering of differentially expressed circRNAs induced by I/R and losartan pre-treatment. Up- and down-regulated circRNAs are shown in red and green colors respectively in the heat map. D. Volcano plot analysis of the differential circRNA profiles between the sham and the AKI groups, and between the AKI and the LST groups. AKI: acute kidney injury; LST: losartan; I/R: ischemia and reperfusion.
Figure 4.
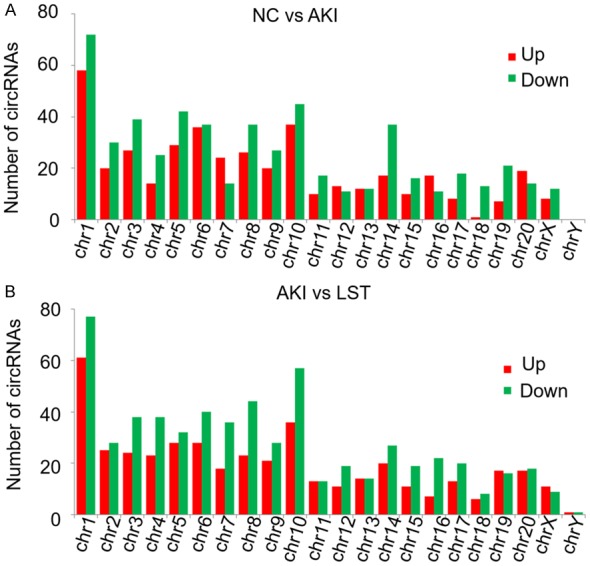
Genomic distribution of differentially expressed circRNAs. A. Numbers of differentially expressed circRNAs between the NC and the AKI groups distributed in each rat chromosome. Up- and down-regulated circRNAs were shown in red and green columns respectively. B. Numbers of differentially expressed circRNAs between the AKI and the LORSATAN groups distributed in rat chromosomes. NC: negative control (the sham group); AKI: acute kidney injury; chr: chromosome. LST: losartan.
We further demonstrated that a high number of circRNAs were differentially expressed in the sham group and the AKI group, which could also be regulated by pre-treatment with losartan (Figure 3C) (Table 2). As illustrated in Figure 3C, the expression of a large panel of circRNAs was significantly down-regulated in rat kidneys with I/R treatment, compared with the sham group. However, losartan pre-treatment resulted in a recovery of the expression levels of the circRNAs (Figure 3C). Conversely, numerous circRNAs were highly expressed in the AKI group compared with in the sham group, but were down-regulated following pre-treatment with losartan (Figure 3C). The volcano plots of circRNA expression among the sham group, the AKI group, and the AKI+LST groups exhibited similar distributions of differential circRNAs induced by I/R treatment and pre-treatment with losartan (Figure 3D). The marked changes in circRNA profiles following I/R treatment and losartan pre-treatment indicate that circRNA could play key roles in AKI development, and may mediate the suppression of kidney injury by losartan.
Table 2.
Top-20 differentially expressed circRNAs among the sham, AKI, and Losartan groups
| Chromosomal location | circRNA name | Gene Symbol | log2 Ratio (AKI/NC) | P value | Up/Down Regulation (AKI/NC) | log2 Ratio (LST/AKI) | P value | Up/Down Regulation (LST/AKI) |
|---|---|---|---|---|---|---|---|---|
| chr1:128978104|128978649 | circ-Igf1r | Igf1r | -2.52 | 0.0103948 | Down | 2.089 | 0.0435652 | Up |
| chr1:185495031|185495489 | circ-Plekha7 | Plekha7 | -1.826 | 0.00794926 | Down | 1.7 | 0.01157594 | Up |
| chr1:265396240|265401005 | circ-Fbxw4 | Fbxw4 | 2.109 | 0.045846 | Up | -1.956 | 0.0316308 | Down |
| chr10:56272370|56272459 | circ-Eif4a1 | Eif4a1 | 1.64 | 0.00281782 | Up | -1.612 | 0.001153238 | Down |
| chr10:91148261|91149247 | circ-Nmt1 | Nmt1 | 2.109 | 0.045846 | Up | -2.541 | 0.01200412 | Down |
| chr13:95193009|95196402 | circ-Akt3 | Akt3 | -2.763 | 4.09E-05 | Down | 2.332 | 0.000779938 | Up |
| chr16:20009431|20009965 | circ-Slc27a1 | Slc27a1 | 2.109 | 0.045846 | Up | -1.956 | 0.0316308 | Down |
| chr19:22373973|22382073 | circ-Itfg1 | Itfg1 | -1.131 | 0.0377542 | Down | 1.214 | 0.0173444 | Up |
| chr2:219090811|219097035 | circ-Vcam1 | Vcam1 | 1.602 | 0.01265634 | Up | -1.811 | 0.00263558 | Down |
| chr2:27326041|27333634 | circ-Polk | Polk | -1.139 | 0.0473424 | Down | 1.669 | 0.000763032 | Up |
| chr6:22362488|22376735 | circ-Memo1 | Memo1 | 2.055 | 0.01611016 | Up | -1.486 | 0.0313866 | Down |
| chr6:28272106|28272697 | circ-Dnmt3a | Dnmt3a | -1.302 | 0.009611 | Down | 1.17 | 0.0168323 | Up |
| chr6:91457048|91680319 | circ-Rn7sl1 | Rn7sl1 | 2.003 | 1.99E-141 | Up | -1.264 | 2.55E-86 | Down |
| chr7:64959567|64961215 | circ-Irak3 | Irak3 | 2.747 | 0.000233196 | Up | -1.763 | 0.00189136 | Down |
| chr8:130762665|130768360 | circ-Snrk | Snrk | 1.577 | 0.00254136 | Up | -1.271 | 0.00475414 | Down |
| chr8:131855074|131868157 | circ-Tcaim | Tcaim | -1.645 | 0.01092164 | Down | 1.504 | 0.01766768 | Up |
| chr8:132708288|132737659 | circ-Slc6a20 | Slc6a20 | -1.205 | 0.001832974 | Down | 1.447 | 3.65E-05 | Up |
| chr8:94283953|94292111 | circ-Me1 | Me1 | -1.508 | 0.00444308 | Down | 2.077 | 0.001335556 | Up |
| chr9:20596863|20599410 | circ-Tnfrsf21 | Tnfrsf21 | -1.679 | 5.20E-05 | Down | 1.031 | 0.0197837 | Up |
| chr9:65976760|65980632 | circ-Als2 | Als2 | -1.501 | 0.01426024 | Down | 1.951 | 0.000278664 | Up |
Functional annotation of differentially expressed circRNAs
To further evaluate the potential biological roles played by differentially expressed circRNAs during AKI and losartan-induced kidney protection, the target miRNAs and mRNAs of the significantly differentially expressed circRNAs were predicted using bioinformatic methods. A GO analysis revealed that the differentially expressed circRNAs were associated with various biological processes, including developmental processes, cellular component organization and biogenesis, localization, biological regulation, response to stimuli, signaling processes, positive regulation of biological processes, immune system processes, behavior, metabolism, cell proliferation, biological adhesion, cell aggregation, reproduction, locomotion, among other processes (Figure 5A). In addition, the differentially expressed circRNAs could also be associated with various molecular functions, such as catalytic activities, binding, antioxidant activities, and signaling transduction activities (Figure 5A). The differentially expressed circRNAs were also predicted to regulate the expression of proteins distributed in multiple subcellular components, including the protein-containing complex, membrane, supramolecular complex, cell junctions, membrane-enclosed lumen, synapse, extracellular region, and nucleoid (Figure 5A). The wide distribution of target genes’ biological processes, molecular functions, and subcellular components suggested that circRNAs perform key functions during the development of AKI and its suppression by losartan, which may be mediated by distinct molecular mechanisms.
Figure 5.
Functional annotation of differentially expressed circRNAs regulated by ischemia and losartan. A. Functional categorization of the gene targets of differentially expressed circRNAs among the sham group, the AKI group, and the AKI+LST group. The GO biological processes, molecular functions. and subcellular components with significantly enriched target genes of differentially expressed circRNAs were separately analyzed. B. Biological pathways associated with the gene targets of differentially expressed circRNAs among the sham group, the AKI group and the AKI+LST group. The biological pathways were analyzed using the KEGG method.
To provide more information on the molecular mechanisms underlying circRNA functioning in AKI pathogenesis and suppression by losartan, the biological pathways with significant enrichments of differentially expressed circRNAs were further analyzed using the KEGG method. We showed that the differentially expressed circRNAs could target genes associated with a number of biological pathways, including tuberculosis, toxoplasmosis, small cell lung cancer, Ras signaling pathway, proteoglycan in cancers, prostate cancer, PI3K-Akt signaling pathways, and neurotrophin signaling pathways, among others (Figure 5B). The revelation of the biological pathways, particularly several signaling pathways, targeted by the differentially expressed circRNAs indicated that the roles of circRNAs in AKI and its inhibition by losartan could be mediated by synergistic regulation of various biological processes and signaling pathways.
For a comprehensive view of the signaling pathways associated with AKI and losartan treatment, we drew an overall interaction network of the predicted miRNAs and functional genes associated with 24 differentially expressed circRNAs (Figure 6). The network involves 26 miRNAs and a large number of functional genes, which were associated with various biological processes and signaling pathways (Figure 6). The differentially expressed circRNAs and associated miRNAs were predicted to regulate the expression of numerous genes associated with PI3K-Akt signaling and related interactive pathways, such as PTEN (gene of phosphate and tension homology deleted on chromosome), FOXO3 (Forkhead Box O3), and SIRT1 (Sirtuin 1) (Figure 6). The significant enrichment of genes associated with the PI3K-Akt signaling, which were predicted to be targeted and regulated by differentially expressed circRNAs, suggested that circRNA-mediated PI3K-Akt signaling regulation performs key roles in the development of ischemic AKI and suppression of kidney injury by losartan (Figure 6).
Figure 6.
circRNA-miRNA-mRNA interaction networks based on differentially expressed cirRNAs. A comprehensive view of the interaction network containing 24 differentially expressed circRNAs and their predicted associating miRNAs and targeted functional genes. Differentially expressed circRNAs are shown as yellow ellipses, predicted interacting miRNAs as green diamonds, and target genes as red irregular quadrilaterals. Predicted direct targeting and associations are indicated using connections with black solid lines.
Validation of circRNA expressional alterations
To validate the expression alteration of circRNAs induced by I/R and losartan treatments in rat kidney tissues, the expression levels of four representative circRNAs (circ-Dnmt3a, circ-Akt3, circ-Plekha7, and circ-Me1), which were in the top 20 most significantly differential expressed circRNAs list and are predicted to be associated with PI3K-Akt signaling, were further determined by quantitative RT-PCR. We demonstrated that the expression of circ-Dnmt3a greatly decreased in rats that were subjected I/R treatment. However, pre-treatment with losartan significantly reversed the decrease in circ-Dnmt3a expression in AKI rat kidney tissues (Figure 7A). Similarly, the expression levels of circ-Akt3, circ-Plekha7, and circ-Me1 in rat kidney tissues were considerably decreased by I/R treatment (Figure 7B-D). Nevertheless, pre-treatment with losartan effectively increased the expression of the three circRNAs compared with the AKI group (Figure 7B-D). The quantitative RT-PCR evidence confirms the result obtained from RNA-Seq that the four circRNAs decreased in the AKI rats and their expression increased in the presence of losartan, indicating that the circRNAs could play critical roles in ischemic AKI.
Figure 7.

Expression of representative circRNAs regulated by ischemia and losartan. The expression levels of circ-Dnmt3a (A), circ-Akt3 (B), circ-Plekha7 (C), and circ-Me1 (D) in kidney tissues of rats treated with ischemia and reperfusion, and the influence of losartan pre-treatment on their expression were determined using quantitative RT-PCR. The sham group of rats was the control and b-actin mRNA was used as the internal standard for quantitation. AKI: acute kidney injury; LST: losartan; DMNT3a: DNA methyltransferase-3a; AKT3: protein kinase B; ME1: cytosolic malic enzyme; *, P < 0.05; ** and ***, P < 0.01.
Discussion
Losartan is a widely applied AT1 antagonist in the treatment of a wide range of diseases including AKI [9,26-28]. However, the underlying molecular mechanisms of the effects of losartan on AKI remain largely unknown. Animal AKI models established by I/R treatment have been widely used in the study of disease basis and treatment development due to their relative ease of operation, short time requirements, and low costs [29,30]. In the present study, SD rats subjected to I/R treatment were used as AKI models to investigate the therapeutic effects of losartan on the progression of ischemic AKI. We verified that losartan has positive effects on AKI apoptosis. Based on the RNA-Seq data, the expression of a group of circRNAs increased and some circRNAs decreased. Among the circRNAs, quantitative RT-PCR results verified four circRNAs, circ-Dnmt3a, circ-Akt3, circ-Plekha7, and circ-Me1, whose expression decreased in AKI rats and increased in the losartan pre-treated AKI rats. In addition, based on a KEGG analysis and circRNA-miRNA-mRNA interaction networks, we have highlighted that the PI3K-Akt signaling pathway plays a critical role in the protective effect of losartan. We demonstrate in the present study that pre-treatment with losartan greatly inhibits the progression of kidney injury in ischemic AKI rat models, which further highlights the potential of losartan as a novel drug in the management of AKI and other renal diseases.
CircRNAs functions have key roles in gene expression regulation and biological processes, mainly through the regulation of miRNA functions and target gene expression [10,13]. Based on the expression and roles of circRNAs in renal diseases [21-23], we hypothesize that circRNAs could also play critical roles in the inhibition of ischemic AKI. To test this, circRNA profile alteration in AKI rats pre-treated with losartan was comprehensively analyzed using RNA-Seq method. We have identified a large number circRNAs differentially expressed in rat kidney following pre-treatment with losartan. The large number of differentially expressed circRNAs in AKI rats, particularly in those treated with losartan, indicated that circRNAs might be critical mediators of the therapeutic effects of losartan in AKI treatment. Considering the application of losartan in the treatment of other conditions such as aberrant blood pressure and diabetic kidney disease [31], the regulation of circRNA expression could be key mechanisms underlying treatment of such disorders by losartan. Apart from losartan, the family of angiotensin II receptor antagonists, also known as angiotensin II receptor blockers, consists of a number of other members such as valsartan [32], which has been demonstrated to play protective role in AKI [33]. Therefore, an understanding of generally and specifically regulated circRNAs between AKI and other renal diseases following treatment with losartan and other ARB members could offer additional novel insights into the pathogenesis of the renal disorders and therapeutic mechanisms in ARB members.
Among the AKI associated circRNAs, we verified the expression of circ-Dnmt3a, circ-Akt3, circ-Plekha7, and circ-Me1, which decreased in the AKI rats and were restored in kidney tissues by losartan pre-treatment. To date, no functions of the four circRNAs have been identified. Only the expression of circ-Akt3 has been observed in lymphoid cells, suggesting its involvement in immunity [34]. Considering infiltration of T lymphocytes is one of the character of AKI [35], downregulation of circ-Akt3 in the AKI model may be associated with immunity responses and inflammatory injury events. Although little is known about their functions, the roles of their linear counterparts have been documented. Pleckstrin homology domain containing family A member 7 (Plekha7) is a blood pressure associated gene. Genetic variations of plPlekha7 are associated with hypertension, which is reported to be an AKI risk factor [36-38]. DNMT3a is a de novo methyltransferase that functions in the generation of 5-methylcytosine, which may result in transcriptional repression. In addition, knockout of DNMT3a in proximal tubules results in the suppression of renal fibroblast activation and ameliorates renal fibrosis [39]. Cytoplasmic malic enzyme 1 (ME1) is a multifunctional protein that can generate pyruvate and ultimately NADPH used in lipogenesis. It plays a critical role in multiple diseases and may be involved in ischemia/reperfusion injury [40-42]. Since circRNAs were transcripted from pre-mRNA, the expression of the circRNAs may decrease the expression levels of linear mRNAs [12,43-45]. We propose that decreases in the four circRNAs observed in the current study could influence their linear counterparts’ expression and impair AKI associated functions while the effect of losartan pre-treatment on AKI may partially benefit from the restoration of circRNA functions.
The PI3K-Akt signaling pathway was shown in the present study to be involved in kidney injury inhibition by losartan. The protein kinase B (Akt) regulates numerous biological processes, including glucose uptake, angiogenesis, and cell survival, and is associated with pathogenic processes in multiple human diseases such as ischemia, cancer, and diabetic kidney disease [46]. More critically, the activation of Akt-related signaling pathways reportedly exerts protective functions in AKI induced by ischemia and reperfusion [47]. The regulation of Akt activation and signaling have also been shown to be involved in the suppression of AKI by other reagents such as tempol and lipoic acid [48,49]. In the present study, we confirmed that circ-Aktin addition three other circRNAs, was regulated by losartan in rats with induced AKI. They were predicted to target PI3K-Akt signaling pathway factors, which further indicated the roles of PI3K-Akt signaling in AKI pathogenesis. In addition, our data shed some light on AKI pathogenesis mechanisms, which are mediated by circRNAs. Further exploration of the function of major signaling components during AKI pathogenesis and circRNAs could facilitate a better understanding of the pathology of AKI and the therapeutic mechanism of losartan.
In summary, we established a rat ischemic AKI model and revealed the protective effects of losartan against AKI development. A large number of circRNAs were differentially expressed in AKI rat kidneys, which was considerably reversed by pre-treatment with losartan, indicating the involvement of circRNAs in protective effects of losartan against AKI. Future work characterizing the functions of circRNAs in AKI could offer novel insights on the pathogenic mechanisms of AKI and the inhibitory effects of losartan on ischemic AKI, in addition to providing basic data to facilitate the clinical application of circRNAs in AKI treatment.
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (81671963 to CC), the Science and Technology Planning Project of Guangdong Province (Key Program) (2014B020212023 to CC), the National Science and Technology Support Program (2015BAI12B07 to CC), and the Science and Technology Planning Project of Guangdong Province (2016A020215129 to YD). We wish to thank all the administrators and technicians for their dedication in the study.
Disclosure of conflict of interest
None.
References
- 1.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 2.Gong Y, Zhang F, Ding F, Gu Y. Elderly patients with acute kidney injury (AKI): clinical features and risk factors for mortality. Arch Gerontol Geriatr. 2012;54:e47–51. doi: 10.1016/j.archger.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, Costa LW, Zavascki AP. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother. 2015;70:1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Chi R, Chen S, Ye H, Yuan J, Wang L, Zhai Y, Gao L, Zhang D, Hu L, Lv B, Long Y, Sun C, Yang X, Zou X, Chen C. Evaluation of clinically available renal biomarkers in critically ill adults: a prospective multicenter observational study. Crit Care. 2017;21:46. doi: 10.1186/s13054-017-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y, Yuan J, Chi R, Ye H, Zhou D, Wang S, Mai C, Nie Z, Wang L, Zhai Y, Gao L, Zhang D, Hu L, Deng Y, Chen C. The incidence, risk factors and outcomes of postoperative acute kidney injury in neurosurgical critically ill patients. Sci Rep. 2017;7:4245. doi: 10.1038/s41598-017-04627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J, Wang Y, Geng X, Chen R, Xu X, Zhang X, Lin J, Teng J, Ding X. Metabolic acidosis as a risk factor for the development of acute kidney injury and hospital mortality. Exp Ther Med. 2017;13:2362–2374. doi: 10.3892/etm.2017.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segev G, Langston C, Takada K, Kass PH, Cowgill LD. Validation of a clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. J Vet Intern Med. 2016;30:803–807. doi: 10.1111/jvim.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molinas SM, Cortes-Gonzalez C, Gonzalez-Bobadilla Y, Monasterolo LA, Cruz C, Elias MM, Bobadilla NA, Trumper L. Effects of losartan pretreatment in an experimental model of ischemic acute kidney injury. Nephron Exp Nephrol. 2009;112:e10–19. doi: 10.1159/000210574. [DOI] [PubMed] [Google Scholar]
- 10.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 11.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. CircRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 14.Bolisetty MT, Graveley BR. Circuitous route to transcription regulation. Mol Cell. 2013;51:705–706. doi: 10.1016/j.molcel.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, Zhang YY, Liu B, Zhang SJ, Wang JJ, Zhang SH, Wu JH, Zhao C, Yan B. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136:1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Yao MD, Li CP, Shan K, Yang H, Wang JJ, Liu B, Li XM, Yao J, Jiang Q, Yan B. Silencing of circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics. 2017;7:2863–2877. doi: 10.7150/thno.19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salgado-Somoza A, Zhang L, Vausort M, Devaux Y. The circular RNA MICRA for risk stratification after myocardial infarction. Int J Cardiol Heart Vasc. 2017;17:33–36. doi: 10.1016/j.ijcha.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang G, Ma Y, An T, Pan Y, Mo F, Zhao D, Liu Y, Miao JN, Gu YJ, Wang Y, Gao SH. Relationships of circular RNA with diabetes and depression. Sci Rep. 2017;7:7285. doi: 10.1038/s41598-017-07931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu T, Wu J, Han P, Zhao Z, Song X. Circular RNA expression profiles and features in human tissues: a study using RNA-seq data. BMC Genomics. 2017;18:680. doi: 10.1186/s12864-017-4029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng X, Joe B. Circular RNAs in rat models of cardiovascular and renal diseases. Physiol Genomics. 2017;49:484–490. doi: 10.1152/physiolgenomics.00064.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luan J, Jiao C, Kong W, Fu J, Qu W, Chen Y, Zhu X, Zeng Y, Guo G, Qi H, Yao L, Pi J, Wang L, Zhou H. CircHLA-C plays an important role in lupus nephritis by sponging miR-150. Mol Ther Nucleic Acids. 2018;10:245–253. doi: 10.1016/j.omtn.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bienholz A, Petrat F, Wenzel P, Ickerott P, Weinberg JM, Witzke O, Kribben A, de Groot H, Feldkamp T. Adverse effects of alpha-ketoglutarate/malate in a rat model of acute kidney injury. Am J Physiol Renal Physiol. 2012;303:F56–63. doi: 10.1152/ajprenal.00070.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Guo J, Chen Y, Chang C, Xu C. Comprehensive circRNA expression profile and selection of key circRNAs during priming phase of rat liver regeneration. BMC Genomics. 2017;18:80. doi: 10.1186/s12864-016-3476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukutomi M, Hoshide S, Eguchi K, Watanabe T, Shimada K, Kario K. Differential effects of strict blood pressure lowering by losartan/hydrochlorothiazide combination therapy and high-dose amlodipine monotherapy on microalbuminuria: the ALPHABET study. J Am Soc Hypertens. 2012;6:73–82. doi: 10.1016/j.jash.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Hamada T, Ichida K, Hosoyamada M, Mizuta E, Yanagihara K, Sonoyama K, Sugihara S, Igawa O, Hosoya T, Ohtahara A, Shigamasa C, Yamamoto Y, Ninomiya H, Hisatome I. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients. Am J Hypertens. 2008;21:1157–1162. doi: 10.1038/ajh.2008.245. [DOI] [PubMed] [Google Scholar]
- 28.Cheng SY, Chou YH, Liao FL, Lin CC, Chang FC, Liu CH, Huang TM, Lai CF, Lin YF, Wu VC, Chu TS, Wu MS, Lin SL. Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep. 2016;6:34265. doi: 10.1038/srep34265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunugi S, Shimizu A, Kuwahara N, Du X, Takahashi M, Terasaki Y, Fujita E, Mii A, Nagasaka S, Akimoto T, Masuda Y, Fukuda Y. Inhibition of matrix metalloproteinases reduces ischemia-reperfusion acute kidney injury. Lab Invest. 2011;91:170–180. doi: 10.1038/labinvest.2010.174. [DOI] [PubMed] [Google Scholar]
- 30.Sohotnik R, Nativ O, Abbasi A, Awad H, Frajewicki V, Bishara B, Sukhotnik I, Armaly Z, Aronson D, Heyman SN, Nativ O, Abassi Z. Phosphodiesterase-5 inhibition attenuates early renal ischemia-reperfusion-induced acute kidney injury: assessment by quantitative measurement of urinary NGAL and KIM-1. Am J Physiol Renal Physiol. 2013;304:F1099–1104. doi: 10.1152/ajprenal.00649.2012. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Peng W, Zhang X, Qiao H, Wang L, Xu Z, Wu C. The association of ACE gene polymorphism with diabetic kidney disease and renoprotective efficacy of valsartan. J Renin Angiotensin Aldosterone Syst. 2016;17 doi: 10.1177/1470320316666749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, Powers B, Samsa GP, Gray RN. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148:16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Peng PA, Ma Y, Liu XL, Yu Y, Jia S, Xu XH, Wu SJ, Zhou YJ. Valsartan protects against contrast-induced acute kidney injury in rats by inhibiting endoplasmic reticulum stress-induced apoptosis. Curr Vasc Pharmacol. 2017;15:174–183. doi: 10.2174/1570161114666161025100656. [DOI] [PubMed] [Google Scholar]
- 34.Nicolet BP, Engels S, Aglialoro F, van den Akker E, von Lindern M, Wolkers MC. Circular RNA expression in human hematopoietic cells is widespread and cell-type specific. Nucleic Acids Res. 2018;46:8168–8180. doi: 10.1093/nar/gky721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y, Lai X, Chen B, Xu Y, Huang B, Chen Z, Zhu S, Yao J, Jiang Q, Huang H, Wen J, Chen G. Genetic variations in CYP17A1, CACNB2 and PLEKHA7 are associated with blood pressure and/or hypertension in she ethnic minority of China. Atherosclerosis. 2011;219:709–714. doi: 10.1016/j.atherosclerosis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Hong KW, Jin HS, Lim JE, Kim S, Go MJ, Oh B. Recapitulation of two genomewide association studies on blood pressure and essential hypertension in the Korean population. J Hum Genet. 2010;55:336–341. doi: 10.1038/jhg.2010.31. [DOI] [PubMed] [Google Scholar]
- 38.James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, de Jong P, Gansevoort RT, Levey AS, Warnock DG, Sarnak MJ. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66:602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo CY. DNA methylation regulation in acute kidney injury. https://augusta.openrepository.com/augusta/handle/10675.2/621803.
- 40.Nakashima C, Yamamoto K, Fujiwara-Tani R, Luo Y, Matsushima S, Fujii K, Ohmori H, Sasahira T, Sasaki T, Kitadai Y, Kirita T, Kuniyasu H. Expression of cytosolic malic enzyme (ME1) is associated with disease progression in human oral squamous cell carcinoma. Cancer Sci. 2018;109:2036–2045. doi: 10.1111/cas.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Dwairi A, Pabona JM, Simmen RC, Simmen FA. Cytosolic malic enzyme 1 (ME1) mediates high fat diet-induced adiposity, endocrine profile, and gastrointestinal tract proliferation-associated biomarkers in male mice. PLoS One. 2012;7:e46716. doi: 10.1371/journal.pone.0046716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tica J, Bradbury EJ, Didangelos A. Combined transcriptomics, proteomics and bioinformatics identify drug targets in spinal cord injury. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19051461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly S, Greenman C, Cook PR, Papantonis A. Exon skipping is correlated with exon circularization. J Mol Biol. 2015;427:2414–2417. doi: 10.1016/j.jmb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Huang G, Li S, Yang N, Zou Y, Zheng D, Xiao T. Recent progress in circular RNAs in human cancers. Cancer Lett. 2017;404:8–18. doi: 10.1016/j.canlet.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Chao CW, Chan DC, Kuo A, Leder P. The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol Med. 1998;4:614–628. [PMC free article] [PubMed] [Google Scholar]
- 46.Heljic M, Brazil DP. Protein kinase B/Akt regulation in diabetic kidney disease. Front Biosci (Schol Ed) 2011;3:98–104. doi: 10.2741/s135. [DOI] [PubMed] [Google Scholar]
- 47.Park SW, Chen SW, Kim M, Brown KM, D’Agati VD, Lee HT. Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther. 2010;333:736–747. doi: 10.1124/jpet.110.166884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, McCullough PA. Lipoic acid in the prevention of acute kidney injury. Nephron. 2016;134:133–140. doi: 10.1159/000448666. [DOI] [PubMed] [Google Scholar]
- 49.Zhang G, Wang Q, Zhou Q, Wang R, Xu M, Wang H, Wang L, Wilcox CS, Liu R, Lai EY. Protective effect of tempol on acute kidney injury through PI3K/Akt/Nrf2 signaling pathway. Kidney Blood Press Res. 2016;41:129–138. doi: 10.1159/000443414. [DOI] [PMC free article] [PubMed] [Google Scholar]





