Abstract
Nitidine chloride (NC) exhibits tumor suppressive function in a variety of human cancers. However, the molecular mechanism of NC-triggered anti-cancer activity has not been fully elucidated. In the present study, we aim to investigate the anti-tumor molecular basis of NC in prostate cancer cells. Multiple approaches including MTT, FACS, wound healing assay, Transwell invasion assay, Transfection, and Western blotting were performed. We found that NC inhibited cell growth and induced apoptosis in prostate cancer cells. Moreover, NC suppressed cell migration and invasion in prostate cancer cells. Notably, we found that NC decreased the expression of YAP oncoprotein in prostate cancer cells. Downregulation of YAP enhanced the anti-tumor function mediated by NC in prostate cancer cells. On the contrary, upregulation of YAP abrogated the anti-cancer activity of NC treatment in prostate cancer cells. Our findings indicate that NC could be useful as a YAP inhibitor for the treatment of prostate cancer cells.
Keywords: YAP, prostate cancer, nitidine chloride, hippo, growth
Introduction
Prostate cancer is one of common malignancy in males, which is the second leading cause of cancer death for men in America [1]. Due to PSA (prostate specific antigen) test screening, some prostate cancer patients were early diagnosed [2]. Several approaches including surgery, chemotherapy, and hormonal ablation therapy have been used in clinical treatments [3]. The prostate cancer patients with tumor metastasis and drug resistance often have poor survival, indicating that it is necessary to discover new drugs to treat prostate cancer for the better outcome.
Nitidine chloride (NC), which is a natural bioactive phytochemical alkaloid, was originally reported to have anti-fungal, anti-inflammatory, and anti-oxidant functions [4]. Subsequently, studies have shown that NC exhibited tumor suppressive functions in a variety of human cancers [5]. NC was reported to inhibit breast cancer cell migration and invasion through inactivation of c-Src/FAK associated signaling pathway [5]. NC suppressed the angiogenesis and cell growth of gastric cancer due to inhibition of STAT3 [6]. In hepatocellular carcinoma, NC suppressed cell growth via blocking the JAK1/STAT3 signaling pathway [7]. One study showed that NC inhibited cell proliferation and induced apoptosis via p53 upregulation in nasopharyngeal carcinoma cells [8]. NC inhibited renal cancer cell proliferation and metastasis and induced apoptosis through inhibition of Akt and ERK signaling pathways [9,10]. However, the function of NC in prostate cancer has not been reported, which is required to be explored.
In recent years, accumulating data showed that Hippo pathway plays a critical role in cancer development and progression. YAP and TAZ are two key molecules to regulate Hippo pathway in cancers. The C-terminal region of YAP/TAZ shares a phospho-degron motif when phosphorylated and bind to 14-3-3 protein, resulting in cytoplasmic sequestration for ubiquitylation and proteasome-mediated degradation [11]. YAP and its close paralog TAZ exert oncogenic activities in various cancers by cross-talking with pro- or anti-tumorigenic pathways such as Wnt/β-catenin, TGF-β (transforming growth factor beta), Notch and JAK-STAT3 (Janus kinase-signal transducer and activator of transcription 3) signaling and are deregulated by multiple factors including cell density/junction and microRNAs [12]. The oncogenic properties of YAP and TAZ depend on their interaction with other proteins in many cases with TEADs [13]. Indeed, genetic mutating amino acid residues critical for YAP-TEAD or TAZ-TEAD complex formation disrupts the interaction and abolishes the transforming ability of YAP and TAZ [14]. Since YAP is an oncoprotein, inhibition of YAP could be a promising strategy for cancer treatment. In the present investigation, we determine whether NC exerts its tumor inhibition function in prostate cancer. Importantly, we define whether NC could regulate YAP expression in prostate cancer cells. We found that NC inhibited cell growth, triggered cell apoptosis, suppressed cell migration and invasion via targeting YAP in prostate cancer cells. Thus, inhibition of YAP by NC could be helpful for treating prostate cancer.
Materials and methods
Reagents
MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) was bought from Sigma-Aldrich (St. Louis, MO, USA). Transwell inserts and Matrigel were bought from BD Biosciences. NC was purchased from Tauto Biotech Company (Shanghai, China). Lipofectamine 2000 reagent was obtained by Invitrogen (Waltham, MA USA). The YAP siRNA was bought from GenePharma Company (Shanghai, China). Annexin V-FITC/PI apoptosis assay kit was purchased from Beyotime Biotechnology (Shanghai, China). Anti-YAP and anti-tubulin antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
The human prostate cancer DU145 and PC-3 cells were purchased from ATCC Company (Manassas, VA, USA). Cells were grown in RPMI (Roswell park memorial institute)-1640 medium (Gibro Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. The cells were maintained in 5% CO2 culture incubator at 37°C.
MTT assay
Prostate cancer cells were cultured and seeded in 96-well plates for overnight. Cells were treated with NC or YAP siRNA or YAP cDNA or combinations for 72 hours. MTT assay was conducted as described previously [15].
Cell apoptosis assay
Prostate cancer cells were cultured in 6-well plates for overnight. Cells were transfected with YAP siRNA or YAP cDNA plasmid or combinations with NC treatment for 48 hours. Annexin V-FITC/PI (propidium iodide) assay was used to measure the cell apoptosis in prostate cancer cells after transfection or combinations with NC treatment as described before [15].
Wound healing assay
Prostate cancer cells were cultured in 6-well plate until cells reached almost confluent. The scratch wound was created by a pipette tip. Then, cells were treated YAP siRNA or YAP cDNA plasmid or combinations with NC treatment for 16 hours. Photographic images were taken at 0 h and 16 hours, respectively.
Transwell migration and invasion assay
The capacities of cell migration and invasion were measured in prostate cancer cells by Transwell assay. Cells were treated with NC or YAP cDNA or YAP siRNA or the combinations, and then cells were seeded into the upper chamber of inserts as described before [15]. The migrated and invaded cells were fixed and stained with Giemsa solution and then photographed by a microscope.
Western blotting analysis
To measure the expression of YAP in prostate cancer cells treated with NC or YAP cDNA or YAP siRNA or the combinations for 72 hours, Western blotting analysis was conducted. The treated cells were lysed and the proteins were separated on SDS-PAGE and immunoblotted with indicated antibodies as described previously [16]. Densitometric quantification of the protein blots was done by ImageJ software.
Transfection
Prostate cancer cells were seeded in 6-well plates for overnight, and then cells were transfected with YAP cDNA or YAP siRNA or empty vector as control by lipofectamine 2000 according to the manufacturer’s instructions [17].
Statistical analysis
The data were statistically analyzed to identify the differences between control group and treatment or transfection or combination groups by ANOVA using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA). P<0.05 was considered to have significance.
Results
NC suppresses prostate cancer cell growth
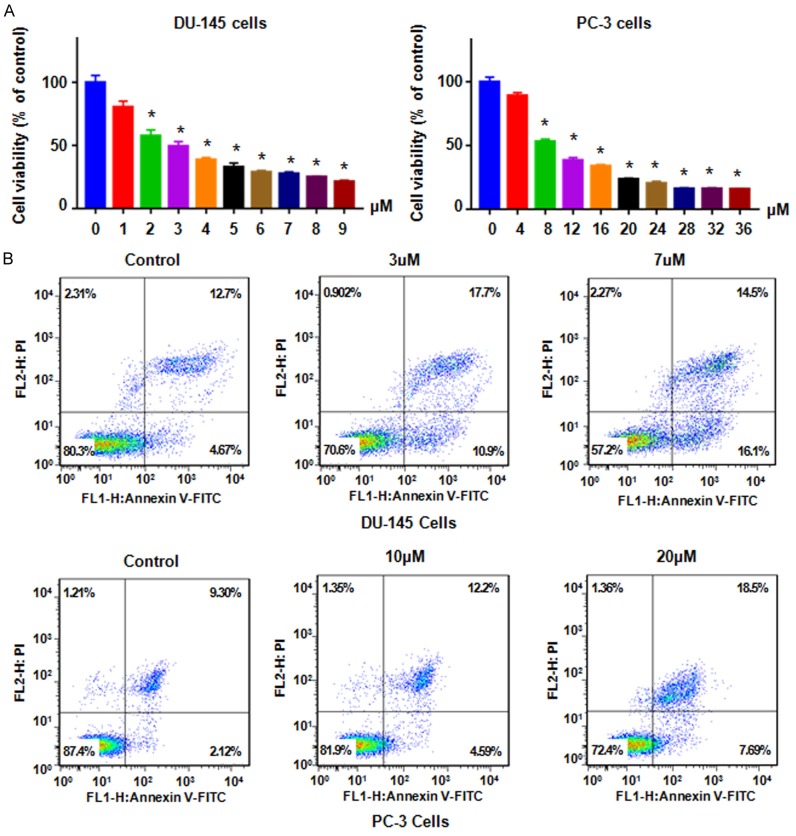
Several studies have shown that NC inhibited cell growth in various types of human cancers. However, the effects of NC in prostate cancer have remained elusive. Thus, we investigated whether NC inhibited growth of prostate cancer cells. We found that NC suppressed cell growth in a dose-dependent manner in DU145 and PC-3 cells (Figure 1A). Interestingly, 3.0 μM and 8.0 μM NC treatments for 72 h led to about 50% of cell growth inhibition in DU145 and PC-3 cancer cells, respectively (Figure 1A). This result indicated that DU145 and PC-3 cells have different sensitivities to NC treatment. Our MTT result revealed that NC inhibited cell growth in prostate cancer cells.
Figure 1.
NC inhibited prostate cancer cell proliferation and induced apoptosis. A. MTT was used to detect the cell viability in PC-3 and DU145 cells after treatment with NC for 72 h. *P<0.01 compared with control. B. Flow cytometry was used to measure the cell apoptosis in prostate cancer cells after NC treatment for 48 h.
NC enhances cell apoptosis in prostate cancer cells
NC has been reported to induce cell apoptosis in human cancer cells. Thus, we determined whether NC could induce cell apoptotic death in prostate cancer cells. Prostate cancer cells were treated by NC for 48 hours and then Annexin V-FITC/PI assay was used to measure the cell apoptosis. We found that NC induced cell apoptosis in a dose-dependent manner in both prostate cancer cell lines (Figure 1B). Clearly, 3.0 μM and 7 μM NC treatments resulted in the percentage of cell early apoptosis from 4.67% of control group to 10.9% and 16.1% in DU145 cells, respectively (Figure 1B). Similarly, NC treatment led to induction of apoptosis from 2.1% in control group to 4.6% and 7.7% in 10 μM and 20 μM treated groups in PC-3 cells, respectively (Figure 1B). NC exposure also slightly induced cell late apoptosis in both prostate cancer cells (Figure 1B). Therefore, NC enhanced cell apoptosis in prostate cancer cells.
NC retards migration and invasion of prostate cancer cells
Next, we investigated whether NC could affect migration of prostate cancer cells. Wound healing assay was performed to determine the cell migration in prostate cancer cells after NC treatment for 16 h. Our wound healing assay results showed that NC treatment inhibited cell migration in both DU145 and PC-3 cells (Figure 2A). To validate the inhibition of cell migration by NC, Transwell assay was used to measure the migration in prostate cancer cells treated with NC for 20 h. We observed that prostate cancer cell migration was retarded by NC treatment in both DU145 and PC-3 cells (Figure 2B). Furthermore, we found that invasiveness activity of prostate cancer cells was also inhibited by NC treatment (Figure 2B). Because NC did not inhibit the cell growth at 24 h (data not shown), the retardation of migration and invasion by NC treatment for 16-20 h is not due to cell growth inhibition of NC exposure. Taken together, NC inhibited cell migration and invasion in prostate cancer cells.
Figure 2.
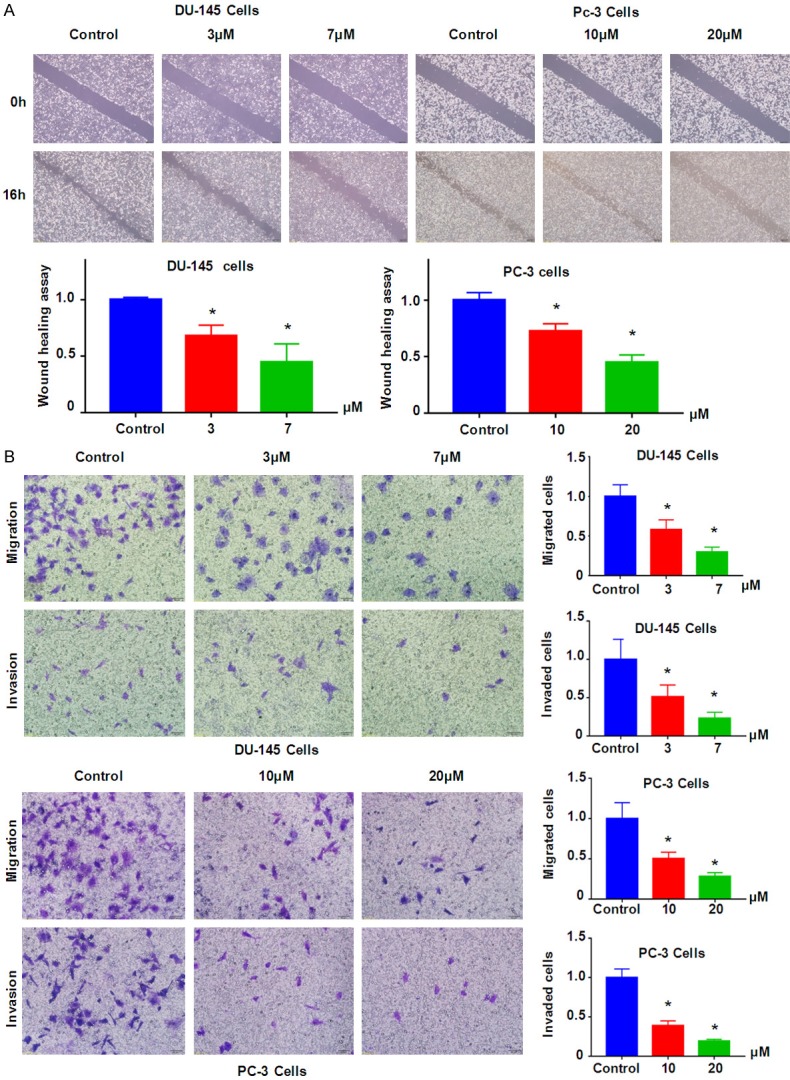
NC inhibited prostate cancer cell migration and invasion. A. Top panel: Wound healing assay was used to measure the cell motility in prostate cancer cells after NC treatment. Bottom panel: Quantitative results were illustrated for top panel. *P<0.01 compared with control. B. Left panel: Cell migration and invasion were measured using Transwell chambers assay in prostate cancer cells. Right panel: Quantitative results were illustrated for left panel. *P<0.01 compared with control.
NC downregulates YAP expression in prostate cancer cells
YAP plays an oncogenic role in prostate cancer cells. Thus, we tested whether NC could decrease the expression of YAP, leading to inhibition of prostate cancer cell growth and invasion. Western blotting analysis was applied for measuring the protein level of YAP in prostate cancer cells after NC treatment. Our results showed that NC treatment down-regulated the expression of YAP in DU145 and PC-3 cells (Figure 3A). This finding indicated that NC facilitated anti-tumor activity partly due to inhibition of YAP in prostate cancer cells.
Figure 3.
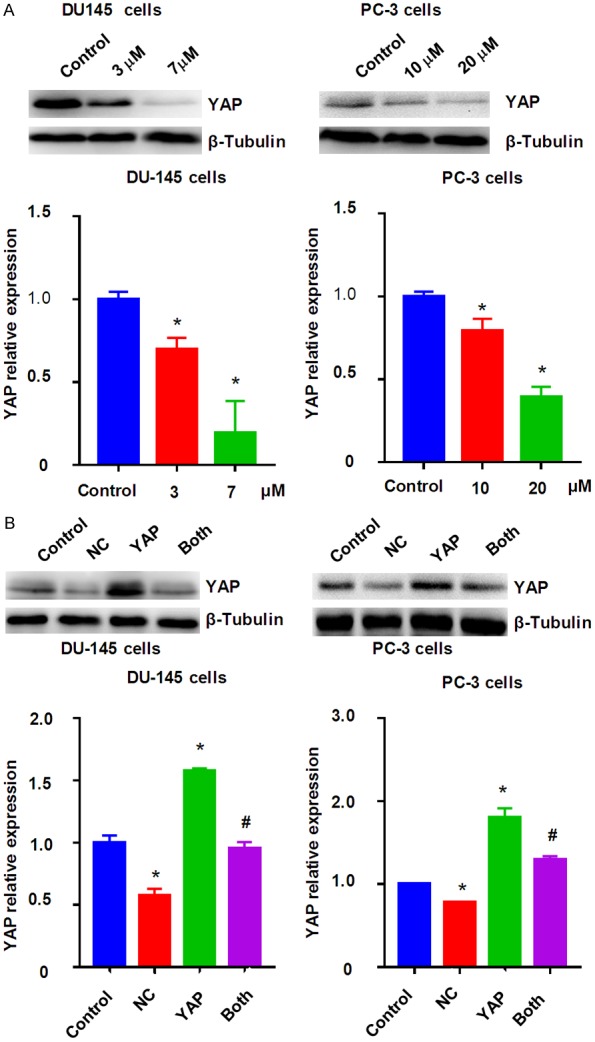
NC inhibited the expression of YAP in prostate cancer cells. A. Top panel: The expression of YAP was measured by Western blotting in prostate cancer cells after NC treatment. Bottom panel: Quantitative results were illustrated for top panel. *P<0.01 compared with control. B. Overexpression of YAP abolished NC-mediated YAP inhibition. Top panel: The expression of YAP was measured by Western blotting in prostate cancer cells after NC treatment or YAP cDNA transfection or combinations. Bottom panel: Quantitative results were illustrated for top panel. Control: pcDNA3.1; NC: 3 μM (DU145), 10 μM (PC-3) NC; YAP: YAP cDNA vector; Both: YAP cDNA+NC. *P<0.01, compared with control; #P<0.05, compared with NC treatment alone or YAP cDNA transfection alone.
Upregulation of YAP abolishes NC-mediated cell growth inhibition and apoptosis
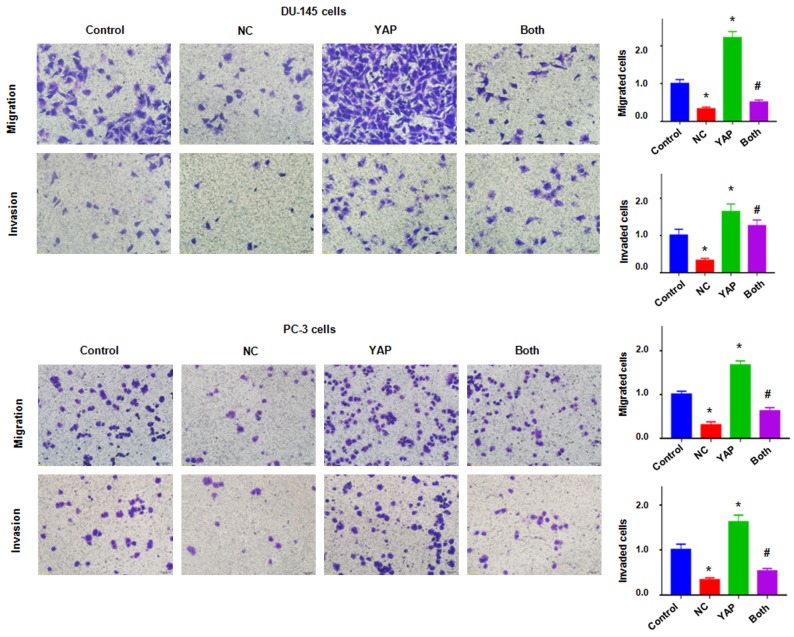
To characterize whether YAP is critically involved in NC-mediated tumor suppressive function, we performed the rescue experiment by overexpression of YAP in prostate cancer cells and treatment with NC. We found up-regulation of YAP in prostate cancer cells after YAP cDNA transfection (Figure 3B). Notably, YAP cDNA transfection rescued the suppression of YAP by NC treatment (Figure 3B). Our MTT assay showed that upregulation of YAP increased cell growth in both DU145 and PC-3 cells (Figure 4A). Importantly, upregulation of YAP rescued NC-mediated cell growth inhibition in prostate cancer cells (Figure 4A). We also found that overexpression of YAP inhibited cell apoptosis in prostate cancer cells (Figure 4B). Consistently, overexpression of YAP abolished NC-triggered cell apoptosis in prostate cancer cells (Figure 4B). These data indicated that YAP could play an essential role in NC-exerted anti-tumor activity in prostate cancer cells.
Figure 4.
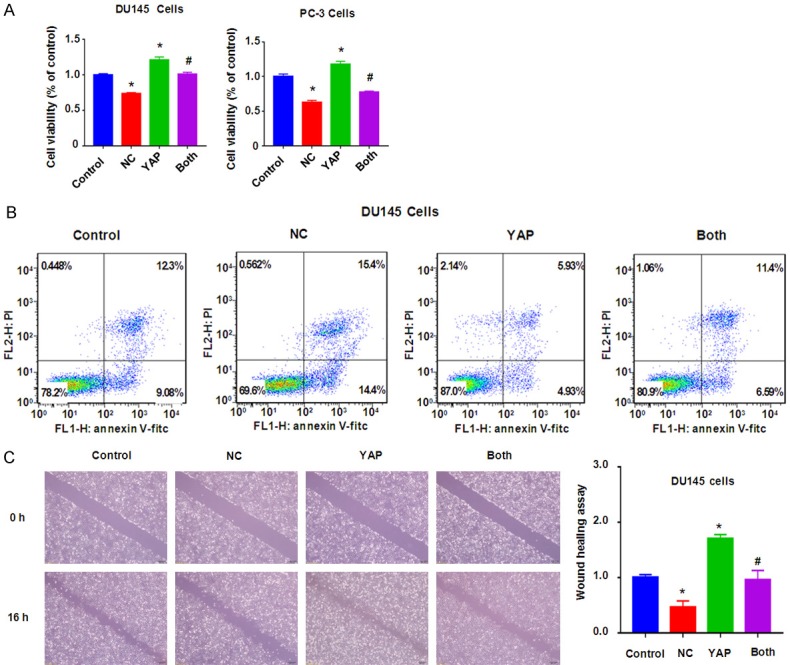
Overexpression of YAP abrogated NC-mediated cell growth inhibition, apoptosis, and migration retardation. A. MTT assay was used to measure the cell growth in prostate cancer cells after NC treatment or YAP cDNA transfection or combinations. B. Flow cytometry was used to measure the cell apoptosis in DU145 cells after NC treatment or YAP cDNA transfection or combinations for 48 h. C. Left panel: Wound healing assay was used to measure the cell motility in DU145 cells after NC treatment or YAP cDNA transfection or combinations. Right panel: Quantitative results were illustrated for left panel. Control: pcDNA3.1; NC: 3 μM (DU145), 10 μM (PC-3) NC; YAP: YAP cDNA vector; Both: YAP cDNA+NC. *P<0.01, compared with control; #P<0.05, compared with NC treatment alone or YAP cDNA transfection alone.
Upregulation of YAP rescues NC-triggered inhibition of cell migration and invasion
To further confirm whether YAP is involved in NC-induced inhibition of migration and invasion in prostate cancer cells, Transwell assay and wound healing assay were used to test the cell motility activity in DU145 and PC-3 cells after overexpression of YAP in combination with NC treatment. We found that upregulation of YAP remarkably increased cell migration and invasion in both prostate cancer cell lines (Figures 4C and 5). Overexpression of YAP rescued NC-induced suppression of migration and invasion in prostate cancer cells (Figure 5). These results clearly suggested that YAP is critically in NC-mediated cell motility retardation.
Figure 5.
Overexpression of YAP abrogated NC-mediated inhibition of migration and invasion. Left panel: Cell migration and invasion were measured using Transwell chambers assay in prostate cancer cells after NC treatment or YAP cDNA transfection or combinations. Right panel: Quantitative results were illustrated for left panel. Control: pcDNA3.1; NC: 3 μM (DU145), 10 μM (PC-3) NC; YAP: YAP cDNA vector; Both: YAP cDNA+NC. *P<0.01, compared with control; #P<0.05, compared with NC treatment alone or YAP cDNA transfection alone.
Downregulation of YAP enhances NC-mediated tumor suppressive activities
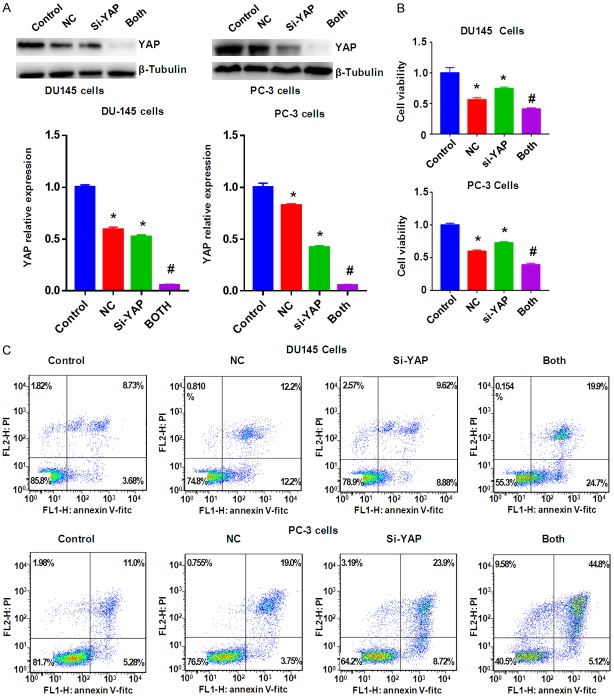
To deeper define the role of YAP in NC-mediated anti-cancer activity, YAP siRNA transfection was applied for downregulation of YAP in prostate cancer cells. Our Western blotting results showed that YAP expression was significantly downregulated by YAP siRNA transfection in prostate cancer cells (Figure 6). Our MTT assay data showed that inhibition of YAP by its siRNA suppressed cell growth in prostate cancer cells (Figure 6B). Moreover, down-regulation of YAP enhanced cell growth suppression by NC treatment in prostate cancer cells (Figure 6B). We also found that down-regulation of YAP induced apoptosis in two prostate cancer cell lines (Figure 6C). YAP downregulation enhanced NC-induced apoptosis in prostate cancer cells (Figure 6C). Our Transwell assay data supported that down-regulation of YAP suppressed cell migration and invasion in prostate cancer cells (Figure 7). In keeping with this, YAP down-regulation promoted NC-mediated inhibition of cell migration and invasive activity (Figure 7). Altogether, these data indicated that YAP plays a critical role in NC-exerted anti-tumor activity in prostate cancer cells.
Figure 6.
Downregulation of YAP enhanced NC-mediated YAP inhibition and cell growth inhibition and apoptosis. A. Top panel: The expression of YAP was measured by Western blotting in prostate cancer cells after NC treatment or YAP siRNA transfection or combinations. Bottom panel: Quantitative results were illustrated for top panel. B. MTT assay was used to measure the cell growth in prostate cancer cells after NC treatment or YAP siRNA transfection or combinations. C. Flow cytometry was used to measure the cell apoptosis in prostate cancer cells after NC treatment or YAP siRNA transfection or combinations for 48 h. Control: siRNA control; NC: 3 μM (DU145), 10 μM (PC-3) NC; si-YAP: YAP siRNA; Both: YAP siRNA+NC. *P<0.01, compared with control; #P<0.05, compared with NC treatment alone or YAP siRNA transfection alone.
Figure 7.
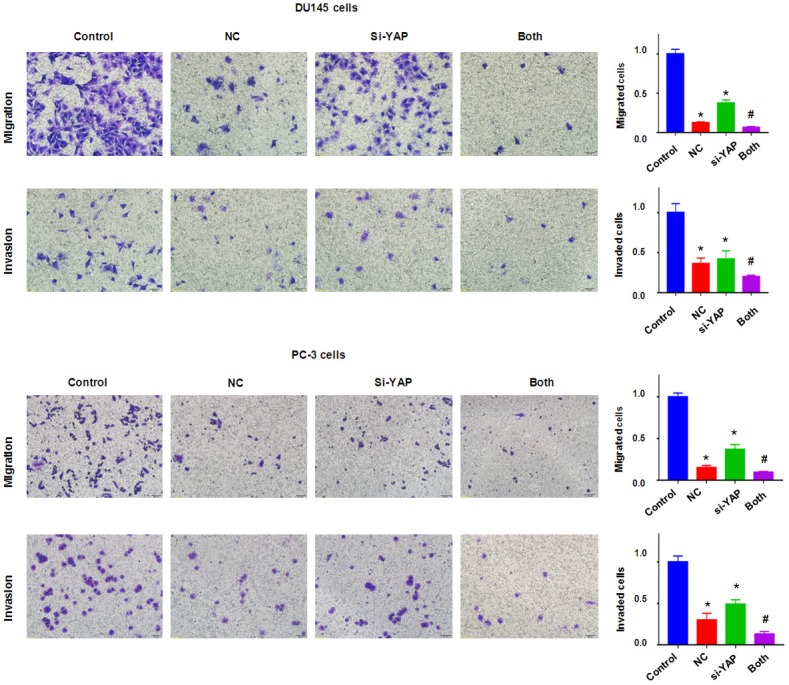
Downregulation of YAP enhanced NC-mediated inhibition of migration and invasion. Left panel: Cell migration and invasion were measured using Transwell chambers assay in prostate cancer cells after NC treatment or YAP siRNA transfection or combinations. Right panel: Quantitative results were illustrated for left panel. Control: siRNA control; NC: 3 μM (DU145), 10 μM (PC-3) NC; si-YAP: YAP siRNA; Both: YAP siRNA+NC. *P<0.01, compared with control; #P<0.05, compared with NC treatment alone or YAP siRNA transfection alone.
Discussion
Emerging evidence has demonstrated that YAP plays oncogenic role in prostate cancer. For example, it has been reported that enhanced expression of YAP can transform immortalized prostate epithelial cells and promoted migration and invasion in prostate cancer cells [18]. Moreover, YAP knockdown reduced the rates of migration and invasion in prostate cancer cells. Upregulation and hyperactivation of YAP is observed in castration-resistant prostate tumors [18]. In line with this, another study identified that YAP was closely associated with castration-resistant prostate cancer, and inhibition of YAP reduced proliferation and induced apoptosis in prostate cancer PC-3 cells [19]. Similarly, YAP knockdown inhibited proliferation and induced apoptosis in prostate cancer DU145 cells [20]. Several studies indirectly showed the oncogenic role of YAP in prostate cancer. For instance, Wnt signaling promoted cell growth through upregulation of YAP in prostate cancer cells [21]. Angiomotin regulated prostate cancer cell growth through YAP pathway [22]. Consistently, expression of ntrin-1 by hypoxia contributed to the migration and invasion by regulation of YAP activity in prostate carcinoma [23]. These reports suggest that inhibition of YAP is a potential approach for the treatment of prostate cancer.
Emerging evidence has demonstrated that NC facilitated anti-cancer activity in various human cancers. Sun et al. reported that NC induced cell growth inhibition and cell cycle arrest in breast cancer cells, which is associated with upregulation of p53 and p21 [24]. This group further found that NC upregulated Bax, cleaved caspase-9 and caspase-3, and cleaved PARP, but downregulated Bcl-2 in breast cancer cells [24]. Interestingly, NC in combination with doxorubicin caused more degree of cell growth inhibition of breast cancer and ovarian cancer cells [24,25]. Kin et al. found that NC treatment led to significant decrease tumor growth via inactivation of STAT3, ERK, and SHH pathways, inhibition of Bcl-2, Cyclin D1, CDK4, VEGF-A and VEGFR2 in hepatic cancer growth [26]. Another study further confirmed the effect of NC on hepatocellular carcinoma cells through regulation of Bcl-2, Bax, p53, and p21 [27]. NC inhibited cell proliferation and induced apoptosis through suppression of ERK signaling pathway in colorectal cancer cells [28]. Moreover, NC retarded cell migration and invasion via inhibition of MMP-2/9 production in ovarian cancer cells [29]. Strikingly, NC suppressed EMT (epithelial-to-mesenchymal transition) via regulation of Akt/GSK-3b/Snail signaling pathway in osteosarcoma cells [30]. Similarly, NC inhibited EMT and cancer stem cells-like properties by controlling hedgehog pathway in breast cancer cells [31]. Recently, NC targeted PI3K/Akt/mTOR pathway and inhibited the malignant behavior in glioblastoma cells [32]. Currently, NC was found to inhibit the expression of Skp2 in ovarian cancer cells [33]. In the present study, our results showed that NC inhibited cell growth, induced apoptosis, and suppressed migration and invasion in prostate cancer cells. This is the first time to show the effects of NC in prostate cancer cells.
Due to YAP as an important oncoprotein, inhibition of YAP could provide the benefit for cancer patients. Several inhibitors of YAP have been discovered. Verteporfin, a photosensitizer initially designed for cancer treatment, has been considered as an inhibitor of the YAP [34]. Porphyrin- and dipyrrin-related derivatives have been identified as the inhibitors to directly target YAP [35]. Curcumin was reported to inhibit the expression of YAP in bladder cancer cells and pancreatic cancer cells [36,37]. Here, we identified NC as one new inhibitor of YAP in prostate cancer cells. Deeper exploration is essential to investigate whether NC could exhibit anti-tumor activity in mouse model via inhibition of YAP expression in prostate cancer.
Acknowledgements
We thank our colleague for their critical comments. This work was supported by National Students’ platform for innovation and entrepreneurship training program 201810367020.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, Ward EM. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–2061. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Zhang WD, Liu RH, Zhang C, Shen YH, Li HL, Liang MJ, Xu XK. Benzophenanthridine alkaloids from zanthoxylum nitidum (Roxb. ) DC, and their analgesic and anti-inflammatory activities. Chem Biodivers. 2006;3:990–995. doi: 10.1002/cbdv.200690108. [DOI] [PubMed] [Google Scholar]
- 5.Pan X, Han H, Wang L, Yang L, Li R, Li Z, Liu J, Zhao Q, Qian M, Liu M, Du B. Nitidine chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway. Cancer Lett. 2011;313:181–191. doi: 10.1016/j.canlet.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Wang J, Lin L, He L, Wu Y, Zhang L, Yi Z, Chen Y, Pang X, Liu M. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. 2012;11:277–287. doi: 10.1158/1535-7163.MCT-11-0648. [DOI] [PubMed] [Google Scholar]
- 7.Liao J, Xu T, Zheng JX, Lin JM, Cai QY, Yu DB, Peng J. Nitidine chloride inhibits hepatocellular carcinoma cell growth in vivo through the suppression of the JAK1/STAT3 signaling pathway. Int J Mol Med. 2013;32:79–84. doi: 10.3892/ijmm.2013.1358. [DOI] [PubMed] [Google Scholar]
- 8.Kang M, Ou H, Wang R, Liu W, Tang A. The effect of nitidine chloride on the proliferation and apoptosis of nasopharyngeal carcinoma cells. J BUON. 2014;19:130–136. [PubMed] [Google Scholar]
- 9.Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W, Shi B, Xu Z, Liu Z. Nitidine chloride inhibits renal cancer cell metastasis via suppressing AKT signaling pathway. Food Chem Toxicol. 2013;60:246–251. doi: 10.1016/j.fct.2013.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W, Xu Z, Liu Z. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food Chem Toxicol. 2014;66:210–216. doi: 10.1016/j.fct.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 11.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 12.Kang W, Cheng AS, Yu J, To KF. Emerging role of hippo pathway in gastric and other gastrointestinal cancers. World J Gastroenterol. 2016;22:1279–1288. doi: 10.3748/wjg.v22.i3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Cao T, Huang H, Lian C, Yang Y, Wang Z, Ma J, Xia J. Arsenic trioxide inhibits cell growth and motility via up-regulation of let-7a in breast cancer cells. Cell Cycle. 2017;16:2396–2403. doi: 10.1080/15384101.2017.1387699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Zeng F, Ma C, Pang H, Fang B, Lian C, Yin B, Zhang X, Wang Z, Xia J. Synergistic reversal effect of epithelial-to-mesenchymal transition by miR-223 inhibitor and genistein in gemcitabine-resistant pancreatic cancer cells. Am J Cancer Res. 2016;6:1384–1395. [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget. 2016;7:69770–69782. doi: 10.18632/oncotarget.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM, Fu K, Datta K, Palermo N, Chen Y, Dong J. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015;35:1350–1362. doi: 10.1128/MCB.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng X, Li WB, Wang DL, Chen KH, Cao JJ, Luo Z, He J, Li MC, Liu WJ, Yu C. YAP is closely correlated with castration-resistant prostate cancer, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol Med Rep. 2015;12:4867–4876. doi: 10.3892/mmr.2015.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin X, Zhao W, Zhou P, Niu T. YAP knockdown inhibits proliferation and induces apoptosis of human prostate cancer DU145 cells. Mol Med Rep. 2018;17:3783–3788. doi: 10.3892/mmr.2017.8352. [DOI] [PubMed] [Google Scholar]
- 21.Seo WI, Park S, Gwak J, Ju BG, Chung JI, Kang PM, Oh S. Wnt signaling promotes androgen-independent prostate cancer cell proliferation through up-regulation of the hippo pathway effector YAP. Biochem Biophys Res Commun. 2017;486:1034–1039. doi: 10.1016/j.bbrc.2017.03.158. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Xu Y, Zhong W, Zhao J, Chen M, Zhang L, Li L, Wang M. Total DNA input is a crucial determinant of the sensitivity of plasma cell-free DNA EGFR mutation detection using droplet digital PCR. Oncotarget. 2017;8:5861–5873. doi: 10.18632/oncotarget.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Chen Q, Luo Q. Expression of netrin-1 by hypoxia contributes to the invasion and migration of prostate carcinoma cells by regulating YAP activity. Exp Cell Res. 2016;349:302–309. doi: 10.1016/j.yexcr.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Sun M, Zhang N, Wang X, Cai C, Cun J, Li Y, Lv S, Yang Q. Nitidine chloride induces apoptosis, cell cycle arrest, and synergistic cytotoxicity with doxorubicin in breast cancer cells. Tumour Biol. 2014;35:10201–10212. doi: 10.1007/s13277-014-2327-9. [DOI] [PubMed] [Google Scholar]
- 25.Ding F, Liu T, Yu N, Li S, Zhang X, Zheng G, Lv C, Mou K, Xu J, Li B, Wang S, Song H. Nitidine chloride inhibits proliferation, induces apoptosis via the Akt pathway and exhibits a synergistic effect with doxorubicin in ovarian cancer cells. Mol Med Rep. 2016;14:2853–2859. doi: 10.3892/mmr.2016.5577. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Shen A, Chen H, Liao J, Xu T, Liu L, Peng J. Nitidine chloride inhibits hepatic cancer growth via modulation of multiple signaling pathways. BMC Cancer. 2014;14:729. doi: 10.1186/1471-2407-14-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ou X, Lu Y, Liao L, Li D, Liu L, Liu H, Xu H. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2. Oncol Rep. 2015;33:1264–1274. doi: 10.3892/or.2014.3688. [DOI] [PubMed] [Google Scholar]
- 28.Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G, Zhang Y, Sui M, Liu H, Jiang L. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway. Mol Med Rep. 2016;13:2536–2542. doi: 10.3892/mmr.2016.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Lin L, Chen Y, Liu T, Liu R, Wang Z, Mou K, Xu J, Li B, Song H. Nitidine chloride inhibits ovarian cancer cell migration and invasion by suppressing MMP-2/9 production via the ERK signaling pathway. Mol Med Rep. 2016;13:3161–3168. doi: 10.3892/mmr.2016.4929. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Z, Guo Y, Yang Y, Kan J, Dai S, Helian M, Li B, Xu J, Liu C. Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3beta/Snail signaling pathway. Oncol Rep. 2016;36:1023–1029. doi: 10.3892/or.2016.4846. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Zhang N, Wang X, Li Y, Qi W, Zhang H, Li Z, Yang Q. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci. 2016;6:44. doi: 10.1186/s13578-016-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Wang J, Qi Q, Huang B, Chen A, Li X. Nitidine chloride inhibits the malignant behavior of human glioblastoma cells by targeting the PI3K/AKT/mTOR signaling pathway. Oncol Rep. 2016;36:2160–2168. doi: 10.3892/or.2016.4998. [DOI] [PubMed] [Google Scholar]
- 33.Mou H, Guo P, Li X, Zhang C, Jiang J, Wang L, Wang Q, Yuan Z. Nitidine chloride inhibited the expression of S phase kinase-associated protein 2 in ovarian cancer cells. Cell Cycle. 2017;16:1366–1375. doi: 10.1080/15384101.2017.1327490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong J, Sun X, Li J. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108:478–487. doi: 10.1111/cas.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibault F, Bailly F, Corvaisier M, Coevoet M, Huet G, Melnyk P, Cotelle P. Molecular features of the YAP inhibitor verteporfin: synthesis of hexasubstituted dipyrrins as potential inhibitors of YAP/TAZ, the downstream effectors of the hippo pathway. ChemMedChem. 2017;12:954–961. doi: 10.1002/cmdc.201700063. [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K, Wang K, Wang X, Chang LS, He D, Guo P. Curcumin promotes KLF5 proteasome degradation through downregulating YAP/TAZ in bladder cancer cells. Int J Mol Sci. 2014;15:15173–15187. doi: 10.3390/ijms150915173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Su J, Feng S, Wang L, Yin X, Yan J, Wang Z. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget. 2016;7:79076–79088. doi: 10.18632/oncotarget.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]