Abstract
Hypoxia is a common cause of pulmonary vascular remodeling and endoplasmic reticulum stress (ERS). Upon ER stress, the unfolded protein response (UPR) which activates the IRE1α, PERK and ATF6 signaling pathways is activated to cope with ERS in mammalian cells; however, the role of the three UPR arms in pulmonary vascular remodeling has not been defined. The present study showed that GRP78, a marker of ERS, was upregulated in hypoxic pulmonary artery smooth muscle cells (PASMCs). Among the three arms of the UPR, the IRE1α pathway was noticeably upregulated in hypoxic PASMCs. An inhibitor of IRE1α/XBP1 pathway, 4u8c, inhibited hypoxia-induced cell proliferation and migration and increased cell apoptosis by downregulating PCNA and MMP9 and activating mitochondrial apoptosis by enhancing the expression of BAX, activating caspase-9 and caspase-3, and eventually cleaving PARP. Quercetin affects ERS in many cell types and was shown to relieve hypoxic pulmonary hypertension (HPH) in our previous study. We demonstrated that quercetin evoked excessive GRP78 expression in hypoxic PASMCs compared with hypoxia alone by evaluating the expression of GRP78. The expression of IRE1α and XBP1s, a cleavage form of XBP1u, was upregulated by quercetin in a dose-dependent manner. Pretreatment with 4u8c reversed the apoptosis-promoting effect of quercetin by inhibiting mitochondrial apoptosis. However, 4u8c amplified the effect of quercetin on proliferation and migration in hypoxic PASMCs. In conclusion, the study demonstrated that the IRE1α-XBP1 pathway is involved in the process of hypoxia-induced pulmonary vascular remodeling; 4u8c could restrain hypoxia-induced cell proliferation and migration and reverse the hypoxia-induced apoptosis arrest, while quercetin excited excessive ERS and the IRE1α pathway in hypoxic PASMCs and promoted apoptosis. Our data suggest that intervening the IRE1α-XBP1 pathway may be useful for hypoxia-induced pulmonary arterial hypertension therapy.
Keywords: Hypoxia, ERS, unfolded protein response, IRE1α, quercetin
Introduction
Pulmonary arterial hypertension (PAH) is a disease of the distal small pulmonary arteries, and its processes are influenced by genetic predisposition and diverse endogenous and exogenous stimuli [1]. Vascular proliferation and remodeling are the hallmarks of PAH pathogenesis. The process of pulmonary vascular remodeling involves all layers of the vessel wall. The increased proliferation, metastasis and resistance to apoptosis of pulmonary artery smooth muscle cells (PASMCs) play central roles in the diverse forms of PAH [2]. However, no effective targeted therapies exist to restrain and reverse pulmonary arterial remodeling.
Three proteins, protein kinase RNA (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α) and activating transcription factor 6 (ATF6), in endoplasmic reticulum membrane sense stresses such as an excess or limitation of nutrients, dysregulated calcium levels or redox homeostasis, inflammatory challenges, and hypoxia. When cells are activated by stress stimuli, misfolded or unfolded proteins accumulate in the endoplasmic reticulum (ER), a process known as endoplasmic reticulum stress (ERS), which evokes the unfolded protein response (UPR). The UPR is initially an adaptive response, but if unresolved, it could lead to cell death. Recent studies have shown that ERS plays an important role in the development of PAH. ATF6 signaling leads to PAH via disruption of the mitochondria-ER unit in vascular smooth muscle cells [3]. However, changes in the IRE1α and PERK branches of the UPR and their roles in PAH remain unclear. Knockdown of each UPR branch sensor activated other branches and promoted the proliferation of PASMCs stimulated by platelet-derived growth factor-BB [4]. Additionally, 4-phenylbutyric acid (4-PBA), a chemical chaperone, prevents and reverses pulmonary hypertension in mice and rats [3]. Salubrinal, a small molecule, can prevent and partially reverse well-established PAH and right ventricular remodeling [5]. However, the molecular mechanisms of the UPR-mediated pathogenesis of hypoxic pulmonary hypertension (HPH) are largely undefined. Understanding the role of UPR during hypoxia may provide new therapeutic targets in HPH.
Quercetin, a well-known natural flavonoid, exerts significant antioxidant, anti-inflammatory and anti-cancer effects [6]. Increasing evidence confirms that quercetin can modulate ERS, such as ERS provoked by calcium dynamic dysregulation in intestinal epithelial cells [7] and tunicamycin-induced ERS in endothelial cells [8]. Our previous studies demonstrated that quercetin could partially reverse hypoxia-induced PAH by inducing apoptosis and inhibiting the proliferation of PASMCs [9,10]. Whether or not quercetin can affect the proliferation and apoptosis of hypoxic PASMCs by modulating ERS is unknown. This process requires more study to provide evidence for quercetin in clinical applications.
Materials and methods
Ethics statement
All experiments were approved by the Huazhong University of Science and Technology Committee and the Tongji Medical College Ethics Committee, Tongji Hospital and were performed in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Reagents
Quercetin, and thapsigargin were purchased from Sigma (USA). The chemical 4u8c was purchased from Selleck. Polyclonal antibodies against PCNA, BAX, GRP78, ATF6, PARP, caspase-3, caspase-9 and β-Actin were from Proteintech Group (USA); αSMA was from Boster (China); anti-IRE1 and p-IRE1 antibodies were from Abcam; anti-PERK and p-PERK antibodies were from CST. Crystal violet was from Bioyear, and RNase and propidium iodide (PI) were from Promoter. The BCA protein assay reagent kit and enhanced chemiluminescent (ECL) plus reagent kit were obtained from Pierce (Pierce Biotech, USA).
Animals
Adult male Sprague-Dawley rats were obtained from the Laboratory Animal Services Centre, Tongji Medical College. The chronic hypoxia-induced rats with pulmonary arterial hypertension were described previously [9-11].
Immunohistochemistry
All immunohistochemical experiments were performed on paraffin-embedded serial slides (4 mm) using a Quick kit (Biyuntian, China). The paraffin-embedded lung sections were stained with anti-IRE1 and p-IRE1 antibodies (Ab) (1:200 dilution). Negative controls were prepared by omitting the primary antibody.
Cell culture and experiments
PASMCs from SD rats weighing 100 to 120 g were cultured using a method from our previous study [9]. Hypoxia was achieved by placing cells in an airtight chamber (Billups-Rothberg) gassed with 5% CO2 and 90% or 92% or 94% N2 to achieve 5% O2 or 3% O2 or 1% O2 concentration. The normoxic control plates were placed in a CO2 incubator at 37°C.
Cell viability assay
Cells (approximately 3000 cells/well) were seeded into 96-well plates and treated with either vehicle control (DMSO) or increasing concentrations of 4u8c before exposure to hypoxia. After hypoxic exposure for 24 h, cell counting kit-8 (CCK-8) solution was added following the manufacturer’s instructions (Dojindo Laboratories, Tokyo, Japan).
Analysis of cell proliferation by CFSE dilution
Cells were harvested and diluted to 1 × 106 - 5 × 106 cells per ml in 1 ml of carboxyfluorescein diacetate succinimidyl ester (CFSE) stain solution (1x) and stained with an equal volume of CFSE stock solution (2x) for 10 min at 37°C. The staining was stopped by the addition of 10 ml of complete DMEM F12 medium, and the cells were washed twice with complete medium. Then, the cells were plated in 6-well plates at a density of 1 × 105 cells per well, and 1 × 105 cells were left alone as a positive control. After treatment for 48 h, cells were harvested, and excitation at 488 nm was measured using a flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with BD FACSDiva 6.1.
Cell apoptosis analyzed by Annexin V/PI assay
Cell apoptosis was quantified using an Annexin V/propidium iodide (PI) detection kit (Beyotime, Shanghai, China) and analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA) as described previously [12].
Cell migration assay
Transwell migration was performed in 24-well Transwell chambers with an 8 um pore size (Transwell; Corning). The migrated cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Quantification was performed by counting the number of cells in eight randomly chosen fields using a light microscope (Zeiss, Oberkochen, Germany).
Real-time PCR
Total RNA was extracted from PASMCs using Trizol reagent (Takara, Dalian, China). Assessment of cellular mRNA expression was performed using a reverse transcription polymerase chain reaction (RT-PCR) kit (Takara). Then, the products were electrophoresed on a 5% agarose gel; the size difference between the spliced and unspliced XBP1 is 26 nucleotides. The sequences of the primers are as follows: β-Actin, forward 5’-CGTAAAGACCTCTATGCCAACA-3’ and reverse 5’-CGGACTCATCGTACTCCTGCT-3’; XBP1, forward 5’-AGAGTCCAAGGGGAATGGAGT-3’ and reverse 5’-ATGTTCTGGGGAGGTGACAAC-3’.
Western blot studies
Cells treated with 4u8c, DMSO or quercetin under hypoxia were lysed in ice-cold RIPA lysis buffer. The lysates were centrifuged at 12,000 rpm for 10 min, and the supernatant was boiled with an addition of 5X loading buffer. Protein samples were electrophoresed in SDS-PAGE and transferred to a 0.22-µm pore size polyvinylidene fluoride (PVDF) membrane. Protein bands were detected using an enhanced chemiluminescence detection system (Bio-Rad, Hercules, USA). Densitometry of Western blots was quantified using ImageJ software.
Immunofluorescence
Cells that were seeded on glass cover slips were washed with PBS three times, fixed, permeabilized with 0.1% Triton X-100, blocked in 10% goat serum and incubated with antibodies against αSMA or GRP78 (1:200) overnight at 4°C. After incubating with a fluorescence-conjugated secondary antibody and DAPI, the slides were washed three times with PBS, covered with DABCO (Sigma-Aldrich) and observed under a fluorescence microscope (Olympus, Japan).
Statistical analysis
All experiments were repeated at least four times, and the data are presented as the mean ± SEM. Statistical analysis was performed in Graph Pad Prism (version 5.0), and statistical significance was determined using one-way analysis of variance (ANOVA). P < 0.05 was considered to be statistically significant.
Results
Hypoxia triggered endoplasmic reticulum stress in PASMCs
PASMCs cultured by the tissue-sticking method at passage one were stained with αSMA (Figure 1A). The purity of PASMCs was greater than 90%, and passages two to five were used for further experiments. GRP78 protein expression, a marker of ERS, was significantly upregulated in 5% oxygen concentration compared with 1% and 3% oxygen concentrations (Figure 1B). Thus, we chose 5% oxygen concentration for the following hypoxia experiments. GRP78 expression in PASMCs exposed to hypoxia for different lengths was tested to investigate the changes in ERS. GRP78 protein expression was increased within 6 h of exposure to hypoxia, returned to basal levels after 12 h of exposure, and increased and peaked at 48 h (Figure 1C). The level of ERS triggered by hypoxia was also evaluated by immunofluorescence staining of GRP78 (Figure 1D).
Figure 1.
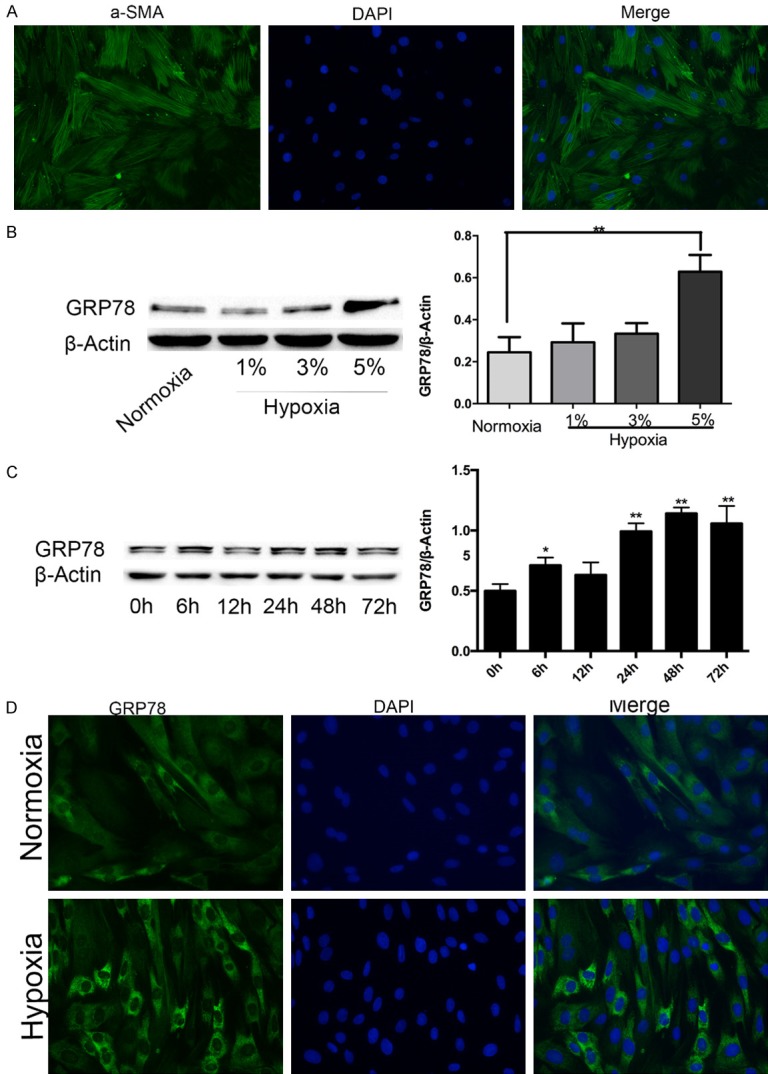
GRP78 is upregulated in hypoxic rat PASMCs. A. The purity of PASMCs was identified by αSMA immunofluorescence staining (magnification, × 200). B. GRP78 protein expression in PASMCs treated with different oxygen concentrations. C. GRP78 protein expression in PASMCs treated with the indicated times of hypoxia. D. Immunofluorescence staining of GRP78 visually verified ERS changes under hypoxia and normoxia. Data are shown as the mean ± SEM; n = 3. *P < 0.05, **P < 0.01. *compared with the normoxia group or 0 h time point; 0 h was considered to be a control point, which was compared with all of the other time points.
The IRE1 pathway is the main UPR pathway evoked by hypoxia
To evaluate UPR sensors activated by hypoxia, we evaluated the protein levels of PERK, IRE1α, ATF6. IRE1α and p-IRE1α were strongly upregulated with the extension of hypoxia time, especially after 48 h of hypoxia. PERK expression reached a peak after 48 h of hypoxia and returned to basal levels after 72 h of hypoxia. The protein level of p-PERK increased slightly after hypoxia treatment. ATF6 expression was increased after 6 h of hypoxia and peaked at 48 h of hypoxia, but the difference was not statistically significant (Figure 2A, 2B). The upregulation of p-IRE1α and IRE1α protein levels observed in PASMCs agreed with the results of pulmonary arteries stained by p-IRE1α and IRE1α antibodies between normal SD rats and rats treated with hypoxia for 4 weeks (Figure 2C).
Figure 2.
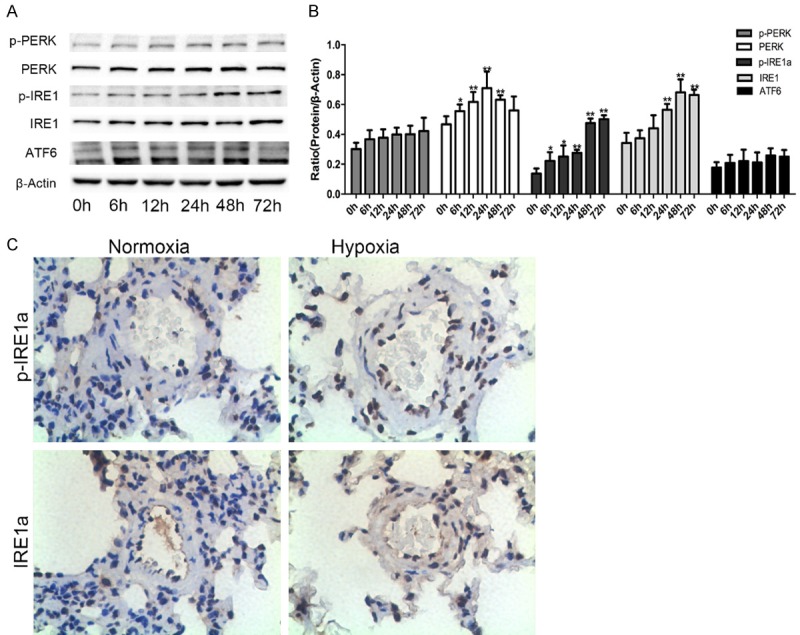
Changes in the three UPR sensors in hypoxic rat PASMCs. A, B. Representative Western blots for three UPR sensors in PASMCs exposed to hypoxia for different times, and densitometry of bands was analyzed by using ImageJ software. C. Lung tissue sections from normal and hypoxia-induced pulmonary artery hypertension rats were stained immunohistochemically for the UPR sensors: IRE1α and p-IRE1α (magnification, × 400). Data are shown as the mean ± SEM; n = 4. *P < 0.05, **P < 0.01 compared with 0 h.
Inhibition of the IRE1α pathway promoted PASMC apoptosis
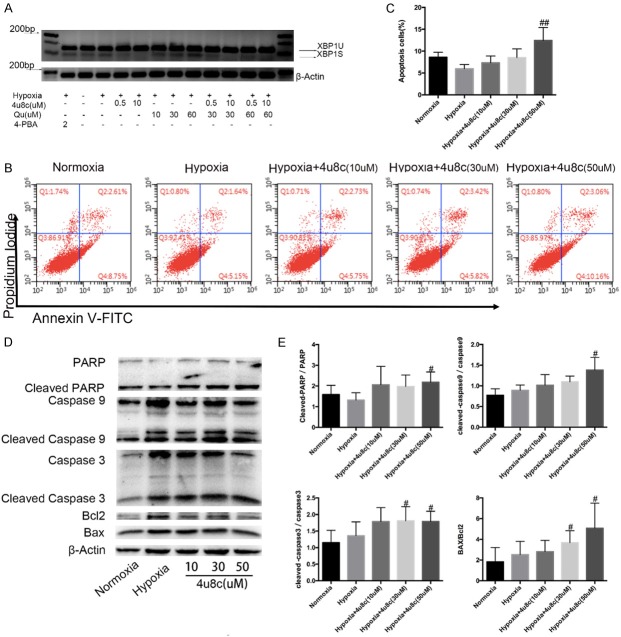
An IRE1α pathway inhibitor was used to evaluate its possible roles in PASMC biological behavior under hypoxia. The inhibitor, 8-formyl-7-hydroxy-4-methylcoumarin (4u8c), which blocks substrate access to the active site of IRE1α and selectively inactivates both XBP1 splicing and IRE1-mediated mRNA degradation [13,14], significantly inhibited hypoxia-induced splicing of XBP-1 mRNA at a low dose from 500 nM in PASMCs (Figure 3A). However, 4-PBA, which downregulates the expression of ATF6 [3], did not change XBP1 splicing obviously. The pro-apoptotic effect of 4u8c was determined by Annexin V/PI analysis, and 4u8c increased apoptosis in a dose-dependent manner (Figure 3B, 3C). The pro-apoptotic effect of 4u8c was further verified by Western blot analysis. The expression of mitochondrial apoptosis proteins cleaved caspase-3, caspase-9 and PARP were increased after 4u8c treatment (Figure 3D, 3E).
Figure 3.
Selective targeting of the IRE1α pathway by 4μ8c promoted PASMC apoptosis. A. XBP-1 splicing in hypoxic PASMCs treated with 4μ8c (XBP1U, unspliced form; XBP1S, spliced form). B, C. Annexin-V/PI assays were performed to assess cell apoptosis, and quantitation data of flow cytometry experiments are shown. D. Protein expression of mitochondrial apoptosis pathway members was evaluated by western blots. E. Quantification analysis of protein expression is shown. Data are shown as the mean ± SEM; n = 4. #P < 0.05, ##P < 0.01 compared with hypoxia-treated PASMCs.
Inhibition of the IRE1α branch inhibited the migration and proliferation of PASMCs under hypoxia
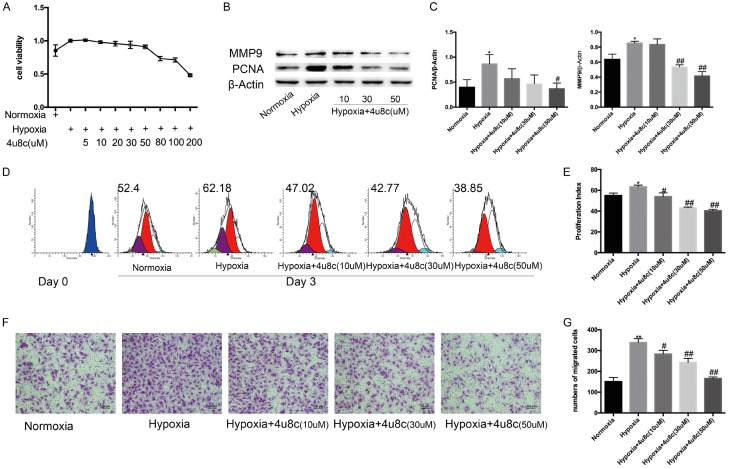
CCK-8 tests were performed to determine PASMC viability after exposure to different concentrations of 4u8c. Under hypoxia conditions, 4u8c significantly inhibited cell viability concentration-dependently, especially 50 mM concentration (Figure 4A). PCNA expression was downregulated significantly by 4u8c (Figure 4B, 4C). The results of CFSE staining indicated that 4u8c also inhibited PASMC proliferation in dose-dependent manner (Figure 4D, 4E). Transwell assays were performed to evaluate the role of 4u8c on PASMCs migration, and the results indicated that 4u8c significantly inhibited the migration of hypoxia-treated PASMCs (Figure 4F, 4G), and the migration-related protein MMP9 was downregulated by 4u8c (Figure 4B, 4C).
Figure 4.
PASMC proliferation and migration were inhibited by 4u8c. A. CCK8 tests were performed to measure cell viability after 48 h of treatment, and OD values were analyzed. B, C. Expression of proteins related to proliferation and migration was evaluated. D, E. CFSE dilution analysis of PASMCs after CFSE labeling. F. Crystal violet staining of PASMCs in a transwell assay (original × 100, scale bars: 100 µm). G. The number of migrated cells was counted and analyzed. Data are shown as the mean ± SEM; n = 3. *P < 0.05, **P < 0.01 compared with the normoxia group; #P < 0.05, ##P < 0.01 compared with the hypoxia group.
Quercetin evoked excessive ERS of PASMCs compared with the hypoxic group and mainly activated the IRE1α pathway among the three UPR pathways
In ovarian cancer, quercetin elicited obvious endoplasmic reticulum stress (ERS) and activated all three branches of UPR, and quercetin pretreatment potentiates the antitumor effects of platinum drugs in ovarian cancer [15]. Thus, we explored the role of quercetin in ERS in hypoxic PASMCs. By exposing PASMCs to different doses (10 mM, 30 mM, and 60 mM) of quercetin, the GRP78 and UPR pathway proteins were analyzed. The results of immunofluorescence staining of GRP78 demonstrated that quercetin upregulated GRP78 expression in a dose-dependent manner compared with hypoxia alone in PASMCs (Figure 5A). Western blotting was performed to test the expression of GRP78, PERK, ATF6, and IRE1α. We found that quercetin significantly upregulated the protein expression of GRP78 and IRE1α; however, quercetin decreased p-eIF2α in a dose dependent manner (Figure 5B, 5C). From the results of agarose gel electrophoresis (Figure 3A), we found quercetin could also inhibit the cleavage of XBP1 in a dose-dependent manner.
Figure 5.
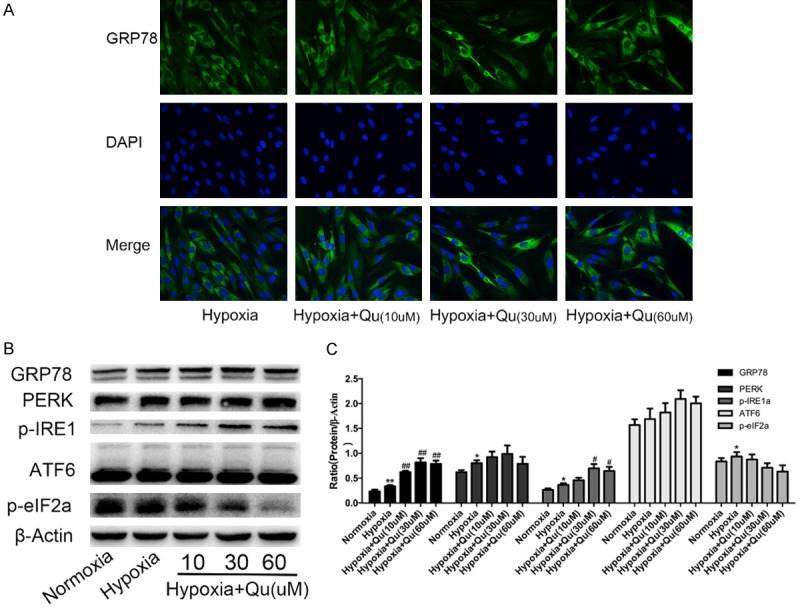
Quercetin elicited obvious ERS and selectively activated the IRE1α branch in hypoxic rat PASMCs. A. Immunofluorescence staining was performed to analyze the expression of GRP78 after quercetin treatment. B, C. The levels of UPR sensors were quantified by Western blots in PASMCs treated with quercetin and hypoxia for 48 h, the representative bands and protein quantifications are shown. Qu, quercetin. Data are shown as the mean ± SEM; n = 4. *P < 0.05, **P < 0.01 compared with normoxia group; #P < 0.05, ##P < 0.01 compared with hypoxia.
Inhibition of the IRE1α pathway partially alleviated the apoptosis-promoting activity of quercetin
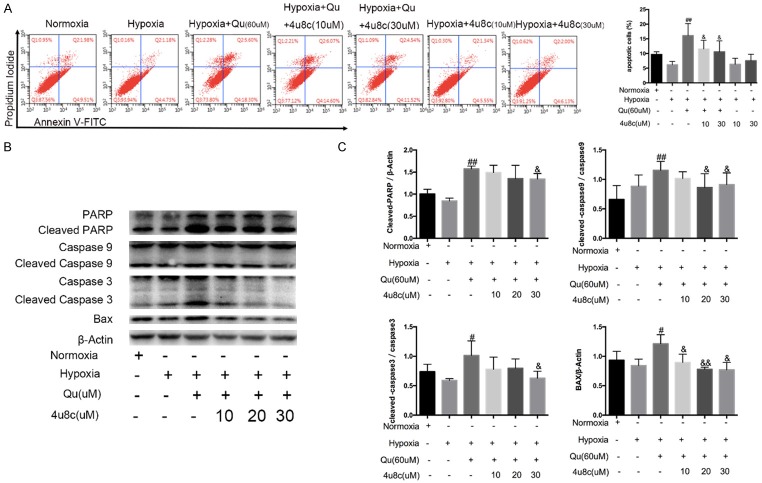
As demonstrated by our previous data [10], quercetin reversed the excessive proliferation and apoptotic resistance of PASMCs under hypoxia. In our study, quercetin highly evoked the IRE1α pathway under hypoxia. The IRE1α pathway may be responsible for the quercetin-mediated reversal of hypoxia-induced apoptosis resistance, proliferation and migration promotion. To verify this hypothesis, we added 4u8c 30 min before quercetin treatment. After pretreatment with 4u8c, the apoptosis promotion of quercetin was partially antagonized (Figure 6A). The results of apoptosis-related proteins verified that IRE1α pathway participates in the process of apoptosis promotion of quercetin (Figure 6B, 6C).
Figure 6.
The IRE1α pathway participated in apoptosis promoting effect of quercetin. A. Annexin-V/PI assays were performed to assess cell apoptosis, and analyses of early and late phase apoptosis are shown. B, C. Protein expression of mitochondrial apoptosis was valuated, and quantification analysis was shown. Qu, quercetin. Data are shown as the mean ± SEM; n = 4. #P < 0.05, ##P < 0.01 compared with hypoxia-treated PASMCs, &P < 0.05, &&P < 0.01 compared with quercetin-treated PASMCs.
Inhibiting the IRE1α pathway played additive roles on the proliferation and migration inhibition of quercetin
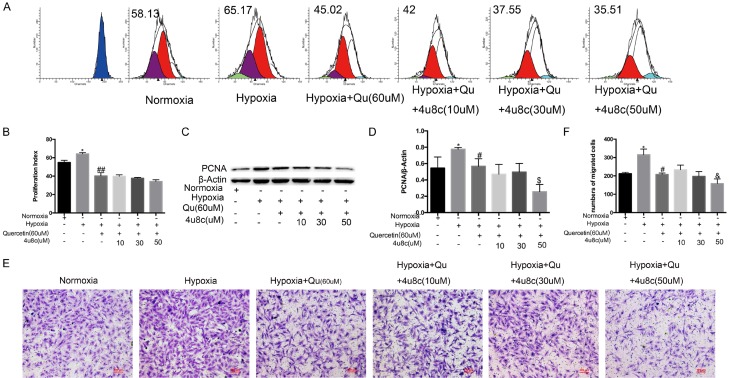
CFSE staining indicated that quercetin inhibited the proliferation of PASMCs in hypoxia, and 4u8c (50 mM) did not alleviate the proliferation inhibition of quercetin but increased quercetin-induced apoptosis (Figure 7A, 7B). The expression of PCNA demonstrated that 4u8c had an additive effect on the proliferation inhibition of quercetin (Figure 7C, 7D). Transwell assays were performed to evaluate the effect of 4u8c on migration repression of quercetin. The results indicated that 4u8c (mM) slightly alleviated migration inhibition of quercetin in a hypoxic environment, but the effect was slight; 4u8c (50 mM) strongly enhanced the migration inhibition of quercetin (Figure 7E, 7F).
Figure 7.
Quercetin and 4u8c have an additive effect on the proliferation and migration of hypoxic PASMCs. A, B. CFSE dilution analysis of PASMCs was performed by pretreating with 4u8c for 24 h, and quercetin was added for another 24 h. C, D. Protein expression related to proliferation was evaluated. E, F. Transwell assays were performed by adding 1 × 104 cells per transwell chamber, pretreating cells with different doses of 4u8c for 12 h and adding quercetin (60 mM) for another 12 h in the lower chamber. The number of migrated cells was counted and analyzed. Qu, quercetin. Data are expressed as the mean ± SEM; n = 4. *P < 0.05 compared with the normoxia group, #P < 0.05, ##P < 0.01 compared with hypoxia-treated PASMCs, &P < 0.05, &&P < 0.01 compared with quercetin-treated PASMCs.
Discussion
In the current study, we found that hypoxia activated ERS in PASMCs, and among the three UPR pathways, the IRE1α pathway was significantly upregulated in hypoxia. An inhibitor of the IRE1α pathway, 4u8c, promoted apoptosis and repressed cell proliferation and migration of PASMCs. Combined with a study of quercetin, we found that quercetin activated excessive ERS in a dose-dependent manner compared with the hypoxia group and selectively evoked the IRE1α pathway among the three UPR pathways. Quercetin induced the apoptosis of PASMCs, and the effect could be partially reversed by 4u8c. However, 4u8c had an additive effects on the inhibition of proliferation and migration of quercetin. The study suggested that inhibition of the IRE1α pathway may be a method to relieve HPH, but this hypothesis needs further verification using an animal experiment. The IRE1α pathway also played a role in quercetin-induced apoptosis, and this finding provided more support for the use of quercetin in clinical tests for HPH in future.
Altered changes in the environment, such as inflammation, nutrient deprivation or hypoxia, leads to the accumulation of unfolded or misfolded proteins in the ER lumen, known as ERS, which activates UPR. UPR activation can promote cell survival [3] or cell death [16] if the ERS is chronic or severe. In our study, we demonstrated that hypoxia promoted PASMC proliferation and inhibited apoptosis by activating ERS.
Several studies have suggested that UPR sensors may respond differently to various forms of stimuli. An early report suggested that under certain conditions, ATF6 may be activated first before IRE1α and PERK [17]. Furthermore, a systematic analysis of UPR signaling demonstrated that UPR sensors actually have distinct sensitivities to specific inducers of ERS [18]. The ATF6 pathway was reported to participate in the process of PAH [3], but it did not illuminate the role of the other two pathways in PAH. Chronic stimulation of platelet-derived growth factor-BB demonstrated that the knockdown of each UPR branch sensor activated other branches and promoted proliferation of PASMCs [4], but these phenomena were not demonstrated in hypoxia. In our study, we discovered that hypoxia selectivity upregulated the protein level of IRE1α and p-IRE1α, and their upregulation was obvious after long-term exposure to hypoxia. We next verified the role of the IRE1α pathway in hypoxia-induced proliferation, migration promotion and apoptosis inhibition of PASMCs. The inhibitor of IRE1α pathway 4u8c promoted apoptosis and inhibited the proliferation of PASMCs under hypoxia in a dose-dependent manner. The effect of 4u8c on hypoxic PASMCs suggested that the IRE1α pathway may participate in the process of hypoxic pulmonary vascular remodeling, and inhibiting the IRE1α pathway may be a novel strategy for HPH treatment.
Quercetin, which exists in nearly all plants, has been studied as promising chemopreventative agent in monocrotaline-induced [19] and hypoxia-induced [10] rat PAH models. An increasing number of studies demonstrated that quercetin can affect ERS [20] and plays a role in enhancing [15] or alleviating ERS [8] depending on different stimuli. We verified that quercetin evoked excessive ERS in a dose-dependent manner and alternatively upregulated IRE1α and p-IRE1α expression compared with hypoxia group in PASMCs. Quercetin promoted hypoxia-induced splicing of XBP1 mRNA in a dose-dependent manner, and 4u8c pretreatment counteracted the effect of quercetin on the splicing of XBP1 mRNA.
The IRE1α pathway was upregulated distinctly in response to quercetin treatment. Whether or not the IRE1α pathway participates in the process of quercetin-mediated changes in biological behaviors of PASMCs is unknown. In our study, we verified that 4u8c pretreatment alleviated the apoptosis promotion of quercetin. This finding suggested that the IRE1α pathway may participate in the process of quercetin alleviating PAH, but this hypothesis needs more study. The inhibitory effect of quercetin on migration and proliferation was enhanced by the pretreatment of PASMCs with 4u8c.
The IRE1α pathway has a pro-survival or pro-death role depending on the stimulus, such as hypoxia or quercetin in hypoxic PASMCs. ERS was reported to act as a double-edged sword in deciding cell fate [21], and the IRE1α pathway functions similarly [22]. The pro-death or pro-survival function of the IRE1α pathway may depend on the stimulus and its intensity [23]. IRE1α controls the initiation of several downstream signaling pathways in addition to processing XBP1 mRNA. The cytosolic domain of activated IRE1α recruits the adaptor protein TNFR-associated factor 2 (TRAF2), which then activates the apoptosis signal-regulating kinase 1 (ASK1) and JNK pathway [24]. The RNase activity of IRE1α has recently been linked to a process referred to as regulated IRE1-dependent decay of mRNAs (RIDD). RIDD was described to mediate the rapid decay of ER-localized mRNAs [25]. RIDD has pro-survival or pro-apoptotic roles depending on different stimuli [26,27]. Other reports indicate that IRE1α may also initiate the activation of stress pathways downstream of p38, ERK [28], and NF-kB [29]. However, the possible impact of these downstream UPR signaling branches in the context of ERS is still not well defined. In our study, hypoxia may be a moderate stress, and ERS and the IRE1α pathway contribute to a pro-survival role. However, quercetin may be a strong stress that evokes intense stress and induces cell death.
In conclusion, our results indicate that the IRE1α pathway participates in the process of hypoxic pulmonary vascular remodeling and provides for quercetin as a potentially effective drug for alleviating clinical PAH by affecting the IRE1α pathway.
Acknowledgements
This work was supported by grants from the National Key Research and Development Programs of China (No. 2016YFC1304500, 2016YFC0903600) and National Natural Science Foundation of China (No. 81670048).
Disclosure of conflict of interest
None.
References
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dromparis P, Paulin R, Stenson TH, Haromy A, Sutendra G, Michelakis ED. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation. 2013;127:115–125. doi: 10.1161/CIRCULATIONAHA.112.133413. [DOI] [PubMed] [Google Scholar]
- 4.Koyama M, Furuhashi M, Ishimura S, Mita T, Fuseya T, Okazaki Y, Yoshida H, Tsuchihashi K, Miura T. Reduction of endoplasmic reticulum stress by 4-phenylbutyric acid prevents the development of hypoxia-induced pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2014;306:H1314–1323. doi: 10.1152/ajpheart.00869.2013. [DOI] [PubMed] [Google Scholar]
- 5.He YY, Liu CL, Li X, Li RJ, Wang LL, He KL. Salubrinal attenuates right ventricular hypertrophy and dysfunction in hypoxic pulmonary hypertension of rats. Vascul Pharmacol. 2016;87:190–198. doi: 10.1016/j.vph.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Kelly GS. Quercetin. Monograph. Altern Med Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- 7.Natsume Y, Ito S, Satsu H, Shimizu M. Protective effect of quercetin on ER stress caused by calcium dynamics dysregulation in intestinal epithelial cells. Toxicology. 2009;258:164–175. doi: 10.1016/j.tox.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Suganya N, Bhakkiyalakshmi E, Suriyanarayanan S, Paulmurugan R, Ramkumar KM. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014;47:231–240. doi: 10.1111/cpr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Cao X, Guo P, Li X, Shang H, Liu J, Xie M, Xu Y, Liu X. Quercetin induces autophagy via FOXO1-dependent pathways and autophagy suppression enhances quercetin-induced apoptosis in PASMCs in hypoxia. Free Radic Biol Med. 2016;103:165–176. doi: 10.1016/j.freeradbiomed.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Cao X, Liu X, Li X, Xu Y, Liu J, Shi J. Quercetin reverses experimental pulmonary arterial hypertension by modulating the TrkA pathway. Exp Cell Res. 2015;339:122–134. doi: 10.1016/j.yexcr.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Li X, He Y, Xu Y, Huang X, Liu J, Xie M, Liu X. KLF5 mediates vascular remodeling via HIF-1alpha in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;310:L299–310. doi: 10.1152/ajplung.00189.2015. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Liu X, Xu Y, Liu J, Xie M, Ni W, Chen S. KLF5 promotes hypoxia-induced survival and inhibits apoptosis in non-small cell lung cancer cells via HIF-1alpha. Int J Oncol. 2014;45:1507–1514. doi: 10.3892/ijo.2014.2544. [DOI] [PubMed] [Google Scholar]
- 13.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, Harding HP. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A. 2012;109:E869–878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Q, Zheng Z, Chang L, Zhao YS, Tan C, Dandekar A, Zhang Z, Lin Z, Gui M, Li X, Zhang T, Kong Q, Li H, Chen S, Chen A, Kaufman RJ, Yang WL, Lin HK, Zhang D, Perlman H, Thorp E, Zhang K, Fang D. Toll-like receptor-mediated IRE1α activation as a therapeutic target for inflammatory arthritis. EMBO J. 2013;32:2477–90. doi: 10.1038/emboj.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Liu Y, Liao J, Gong C, Sun C, Zhou X, Wei X, Zhang T, Gao Q, Ma D, Chen G. Quercetin induces endoplasmic reticulum stress to enhance cDDP cytotoxicity in ovarian cancer: involvement of STAT3 signaling. FEBS J. 2015;282:1111–1125. doi: 10.1111/febs.13206. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003;4:265–271. doi: 10.1016/s1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 18.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell. 2006;17:3095–3107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales-Cano D, Menendez C, Moreno E, Moral-Sanz J, Barreira B, Galindo P, Pandolfi R, Jimenez R, Moreno L, Cogolludo A, Duarte J, Perez-Vizcaino F. The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS One. 2014;9:e114492. doi: 10.1371/journal.pone.0114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y, Li J, Gao C, Xu Y, Li Y, Yu X, Wang J, Liu L, Yao P. Hepatoprotective effect of quercetin on endoplasmic reticulum stress and inflammation after intense exercise in mice through phosphoinositide 3-Kinase and nuclear factor-kappa B. Oxid Med Cell Longev. 2016;2016:8696587. doi: 10.1155/2016/8696587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 22.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91:1219–1243. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 24.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 26.Logue SE, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- 27.Lu M, Lawrence DA, Marsters S, Acosta-Alvear D, Kimmig P, Mendez AS, Paton AW, Paton JC, Walter P, Ashkenazi A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345:98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen DT, Kebache S, Fazel A, Wong HN, Jenna S, Emadali A, Lee EH, Bergeron JJ, Kaufman RJ, Larose L, Chevet E. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell. 2004;15:4248–4260. doi: 10.1091/mbc.E03-11-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]