Abstract
Objectives: The cellular mechanisms of calcific aortic valve (AV) disease and optimal medications for its treatment are poorly elucidated. Glycogen synthase kinase (GSK)-3β and non-canonical wingless-related integration site (Wnt) signaling play crucial roles in regulating the pathogenesis of valvular interstitial cell (VIC) calcification. Histone acetylation was found to regulate VIC calcification. However, whether histone deacetylases (HDACs) modulate the pathophysiology of AV calcification is unclear. Different HDAC isoforms have dissimilar cardiovascular effects. We hypothesized that distinctive HDAC inhibitors modulate runt-related transcription factor 2 (RUNX2) in aortic VICs through the regulation of Wnt signaling. Methods: Western blotting, real-time polymerase chain reaction, and proliferation assay were used to analyze osteogenesis marker expression, Wnt signaling, bone morphogenetic protein (BMP) signaling, and proliferation in porcine VICs treated with osteogenic (OST) medium alone or in combination with HDAC inhibitors. Results: VICs treated with OST medium for 5 days exhibited higher RUNX2 and GSK-3β expression levels than did control cells. A class I HDAC inhibitor (MS-275 at 1 μM) reduced the RUNX2 mRNA and protein expression levels and alkaline phosphatase activity and downregulated non-canonical Wnt/GSK-3β signaling, canonical Wnt/β-catenin signaling, and BMP signaling. By contrast, a combined class IIa (MC1568) and IIb HDAC (tubacin) inhibitor (0.1 μM) increased RUNX2 expression. MS-275, MC1568, and tubacin reduced VIC proliferation; however, the extent of reduction differed. MS-275 reduced RUNX2 and osteocalcin expression in VICs treated with OST medium for an extended period (14 days). Conclusions: MS-275 critically regulates RUNX2 transactivation in VICs through both canonical and non-canonical Wnt signaling pathways.
Keywords: Calcific aortic valvular disease, RUNX2, MS-275, glycogen synthase kinase 3β, β-catenin, Wnt
Introduction
Calcific aortic valve (AV) disease (CAVD) is the most common valvular heart disease in adults. However, the cellular mechanisms of CAVD and optimal medications for preventing or reversing CAVD progression have yet to be established. Epigenetic regulation substantially modulates cell calcification. Our previous study revealed that C646-induced suppression of p300 activity reduces valvular interstitial cell (VIC) calcification and downregulates alkaline phosphatase (ALP) [1]. However, the role of histone deacetylases (HDACs) in VIC calcification is unclear.
Runt-related transcription factor 2 (RUNX2) is the principal transcriptional regulator of osteoblast differentiation through four notable pathways and plays an essential role in VIC mineralization [2]. The wingless-related integration site (Wnt)/β-catenin pathway and bone morphogenetic protein 2/4 (BMP-2/-4) signaling pathway activate RUNX2. The Notch and transforming growth factor-β (TGF-β) signaling pathways inhibit calcification. Wnt proteins regulate cardiovascular development, morphogenesis, cardiac valve formation, inflammation, bone metabolism, angiogenesis, and matrix regulation. The canonical Wnt signaling pathway that activates gene transcription is involved in the development of multiple diseases [3]. Increasing evidence suggests that in humans and animals, the Wnt signaling pathway is activated by a calcified AV [4-7]. Calcified AVs profoundly express canonical low-density lipoprotein receptor-related protein 5 (LRP5)/Wnt3 signaling [4]. Increased serum levels of secreted Wnt antagonists in patients with symptomatic aortic stenosis were also noted [8]. By contrast, the non-canonical Wnt signaling pathway has the potential to activate the Ras homolog gene family member A (RhoA), Jun N-terminal kinase, or calcium signaling pathway. RhoA and receptor tyrosine kinase-like orphan receptor 2 (ROR2) were demonstrated to upregulate RUNX2 [9]. Moreover, non-canonical Wnt signaling could activate the RhoA/Rho-associated protein kinase (ROCK) pathway and induce cell differentiation into the osteoblastic or chondrogenic lineage.
GSK-3β plays multifaceted roles in both canonical and non-canonical Wnt signaling. GSK-3 can phosphorylate and stabilize LRP5/6 to promote canonical Wnt/β-catenin signaling. Furthermore, GSK-3 increases the destruction of β-catenin by forming a destruction complex [10-13]. Albanese et al. proved that a GSK-3β inhibitor could considerably reduce mineralization induced through non-canonical Wnt signaling [14].
HDAC inhibitors increase RUNX2 expression in osteoblasts [15]. However, the role of HDACs in RUNX2 of VICs is unclear. Many HDACs are involved in the function of RUNX2 in the transcription of other osteogenesis-associated genes, implying that HDACs may also critically regulate RUNX2 in VICs. Because different HDAC isoforms have dissimilar effects on cardiac pathophysiology, we hypothesized that distinctive HDAC inhibitors modulate RUNX2 in aortic VICs through the regulation of Wnt signaling.
Materials and methods
All AVs used in this study were sourced from a commercial slaughterhouse (Yahsen Frozen Foods, Taoyuan, Taiwan). Our study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Taipei Medical University (IACUC, LAC-2018-0245). The slaughterhouse follows the Humane Slaughter Act guidelines and the Animal Protection Act for the care and slaughter of swine.
Isolation and culture of VICs
AV leaflets excised from 6-month-old pig hearts were obtained from the mentioned commercial slaughterhouse (Yahsen Frozen Foods) and were shipped in ice-cold saline within 3 h after slaughter. The AVs were digested with collagenase I (250 U/mL, Sigma, St. Louis, MO, USA) for 30 min with gentle shaking at 37°C to remove endothelial cells; they were then subjected to a second digestion with collagenase I (250 U/mL) for 12 h. The isolated VICs were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium containing 10% heat-inactivated fetal bovine serum, 2 mmol/L L-glutamine, and an antibiotic/antimycotic (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere of 5% CO2. VICs from passages 3 to 5 were used in subsequent experiments. The VICs were seeded in six-well plates at a density of 2 × 104 cells/cm2 and treated with indicated chemicals for 5 days. The culture medium and chemicals were changed daily.
Induction of osteogenesis of aortic VICs
Aortic VICs at 80% confluence were cultured in osteogenic (OST) medium with DMEM/F12 complete medium containing 10 nM dexamethasone, 10 mM β-glycerophosphate, and 50 mg/mL ascorbic acid for 5 days with or without the following HDAC inhibitors: MS-275 (a relative HDAC-1 inhibitor, IC50 = 0.3 μM, EPS002, Sigma-Aldrich), MC1568 (a selective class IIa HDAC inhibitor for HDAC-4 and HDAC-6, IC50 = 220 nM, Cat. 4077, Tocris Bioscience), tubacin (a relative HDAC-6 inhibitor, IC50 = 4 nM, S2239, Selleck), MPT0E014 (a pan-HDAC inhibitor; IC50 for HDAC-1 = 12 nM, IC50 for HDAC-2 = 4 nM, and IC50 for HDAC-6 = 1 nM; synthesized in-house), BIO (a GSK-3 inhibitor, IC50 = 5 nM, Cat. 361550, Merk Millipore), and IWR-1-endo (a β-catenin inhibitor, IC50 = 180 nM, sc-295215, Santa Cruz).
Proliferation assay
VIC proliferation was measured using a commercial MTS kit (CellTiter 96® Aqueous, Promega, Madison, WI, USA). Cells were cultured in a 96-well culture plate at a density of 10,000 cells/well. After 5 days of culture, cell growth was analyzed by adding the MTS reagent 4 h before spectrophotometric analysis at 490 nm.
ALP assay
ALP activity was measured using an ALP assay kit (ab83371, Abcam) according to the manufacturer’s instructions. In brief, VICs treated with OST medium with or without MS-275 (1 µM) for 5 days were harvested and resuspended in 100 μL of assay buffer. A nonfluorescent 4-methylumbelliferyl phosphate disodium salt substrate was converted to an equal amount of fluorescent 4-methylumbelliferone in the presence of ALP. After a reaction time of 30 min, the stop solution was added, after which the fluorometric reading (Ex/Em = 360 nm/440 nm) was recorded. Each sample was assayed in duplicate and normalized to the total protein amount.
Western blot analysis
VICs were lysed in protein extraction reagent (Thermo Scientific, Waltham, MA, USA) with a protease inhibitor cocktail (Sigma). The proteins were separated through gradient sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and electrophoretically transferred to an equilibrated polyvinylidene difluoride membrane (Amersham Biosciences, Buckinghamshire, UK). Blots were probed with primary antibodies against the myofibroblast marker alpha-smooth muscle actin (α-SMA, ab7817, Abcam, 1:5000), BMP-2/-4 (sc-6267, Santa Cruz, 1:1000), GSK-3β (ab73173, Abcam, 1:1000; 27C10, Cell Signaling, 1:1000), β-catenin (ab6302, Abcam, 1:4000), osteocalcin (AB10911, Millipore, 1:500), p-SMAD 1/5/8 (sc-12353, Santa Cruz, 1:1000), and RUNX2 (sc-10758, Santa Cruz, 1:1000), then probed with secondary antibodies conjugated with horseradish peroxidase. Bound antibodies were detected using an enhanced chemiluminescence detection system (Merck Millipore, Billerica, MA, USA) and analyzed using Image-Pro Plus software. The targeted bands were normalized to β-actin (ab6276, Abcam, 1:10,000) to confirm equal protein loading.
Alizarin red S staining
Alizarin Red S (ARS) staining was used for measuring calcium deposition. VICs were fixed with 4% paraformaldehyde for 15 min and then rinsed and incubated with 2% ARS solution (pH 4.2) for 30 min. Excessive dye was removed by washing with distilled water. Images of the stained cells were acquired using an Olympus CKX41 inverted phase-contrast microscope (Tokyo, Japan). For semiquantitative analysis, the ARS stain area was measured using ImageJ software (NIH; http://rsb.info.nih.gov/ij/) at 40 × magnification.
RNA isolation and real-time polymerase chain reaction
Messenger RNA (mRNA) expression was analyzed through quantitative polymerase chain reaction executed using an ABI Prism 7300 system (Applied Biosystems, Foster City, CA, USA) and SYBR Green (Applied Biosystems). For RUNX2 amplification, 5’-GGACGAGGCAAGAGTTTCAC-3’ forward and 5’-GTGGATTAAAAGGACTTGGTGC-3’ reverse primer sequences were used. In addition, 5’-ATAACACCGCTTCTGCGTCA-3’ forward and 5’-AACGTGACCAGTGTTGCTGA-3’ reverse primers were used for GSK-3β amplification. Finally, 5’-ACACTCACTCTTCTACCTTTG-3’ forward and 5’-CAAATTCATTGTCGTACCAG-3’ reverse primers were used for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) amplification. Relative transcript levels were estimated on the basis of cycle threshold values and normalized to the corresponding GAPDH cycle threshold values.
Statistical analysis
Cells subjected to various treatments were compared through one-way repeated-measures analysis of variance and Fisher’s least significant difference post hoc test. Systat Sigmaplot 12.0 software (San Jose, CA, USA) was used to perform all data analyses. Quantitative data are expressed as the mean ± standard error of the mean (SEM) of at least two or three independent measurements with five replicates.
Results
Effects of HDAC inhibitors on RUNX2 expression and porcine VIC proliferation
OST medium-treated VICs had higher RUNX2 protein levels than did the control cells (Figure 1). VICs treated with OST medium combined with MS-275 (1 μM) had a lower RUNX2 protein level (Figure 1A) than did those treated with OST medium alone. In addition, VICs treated with OST medium combined with MC1568 (0.1 μM, Figure 1B) or tubacin (0.1 μM, Figure 1C) had a higher RUNX2 expression level than did the control cells. Treatment with OST medium combined with MPT0E014 (0.1 μM, Figure 1D) also attenuated RUNX2 transactivation. As illustrated in Figure 1E, VICs treated with OST medium combined with MS-275, MC1568, or tubacin exhibited lower levels of proliferation than did those treated with OST medium alone. However, MS-275 induced a greater reduction in VIC proliferation than did MC1568 and tubacin.
Figure 1.
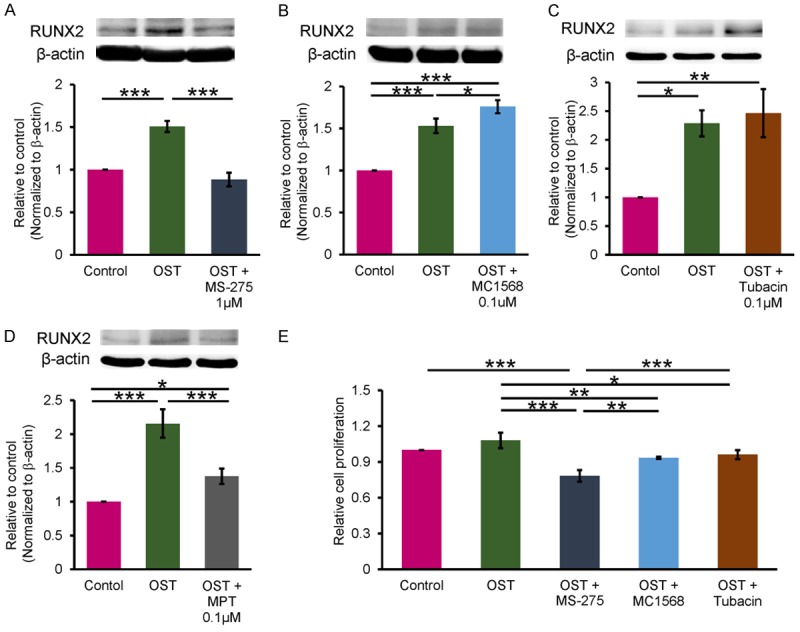
Effects of various classes of HDAC inhibitors on RUNX2 protein expression and cell proliferation in porcine VICs. A-D. Western blot analysis for different HDAC inhibitors on RUNX2 protein expression in OST medium-treated VICs for 5 days (n = 5). β-actin was used as an internal control. E. MTS cell proliferation assay for different HDAC inhibitors in OST medium-treated VICs for 5 days (n = 6). Band intensities were quantified using Image-Pro Plus software. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Effects of MS-275 on osteogenesis-associated signaling and transcription of RUNX2 and GSK-3β
OST medium-treated VICs exhibited higher GSK-3β and β-catenin expression levels than did the control cells (Figure 2A). VICs treated with OST medium combined with MS-275 (1 μM) showed significantly lower GSK-3β and β-catenin expression levels than did VICs treated with OST alone (Figure 2A). Moreover, compared with the control cells, the OST medium-treated VICs had a higher p-SMAD1/5/8 expression, which was attenuated by MS-275 (Figure 2B). The osteocalcin and α-SMA levels were not significantly changed in the OST medium-treated VICs compared with the control cells (Figure 2C and 2D). However, the VICs treated with OST medium combined with MS-275 had lower α-SMA protein expression levels than did the control cells and VICs treated with OST medium alone. Furthermore, the VICs treated with OST medium had higher ALP activity than did the control cells and VICs treated with OST medium combined with MS-275 (Figure 2E). As presented in Figure 2F, compared with the other cells, the OST medium-treated VICs had higher RUNX2 and GSK-3β transcription levels, which were significantly attenuated by MS-275. Additionally, MS-275 significantly reversed the effects of OST medium on cell aggregation and calcium deposition (Figure 3).
Figure 2.
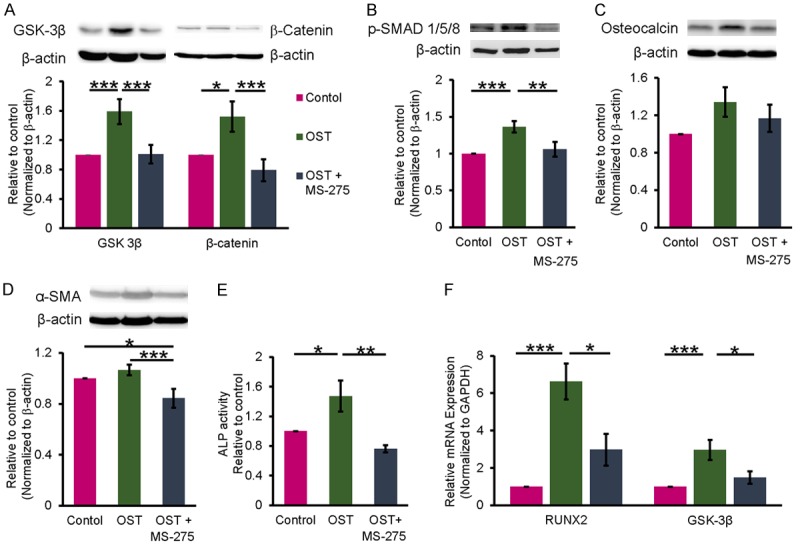
Effect of a class I HDAC inhibitor (MS-275) on osteogenesis-related signaling of porcine VICs. A-D. The representative immunoblots of Wnt signaling proteins of GSK-3β and β-catenin, p-SMAD1/5/8, osteocalcin, and α-SMA in OST medium treated VICs with or without MS-275 (1 μM) for 5 days (n = 7). β-actin was used as an internal control. Band intensities were quantified using Image-Pro Plus software. E. Alkaline phosphatase (ALP) activity was measured by using an ALP assay kit, which detected fluorescence of 4-methylumbelliferone by alkaline phosphatase assay (n = 4). F. Real-time PCR analysis for mRNA expressions of RUNX2 and GSK-3β in OST medium treated VICs with or without MS-275 (1 μM) for 5 days (n = 6). GADPH was used as an internal control. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Figure 3.
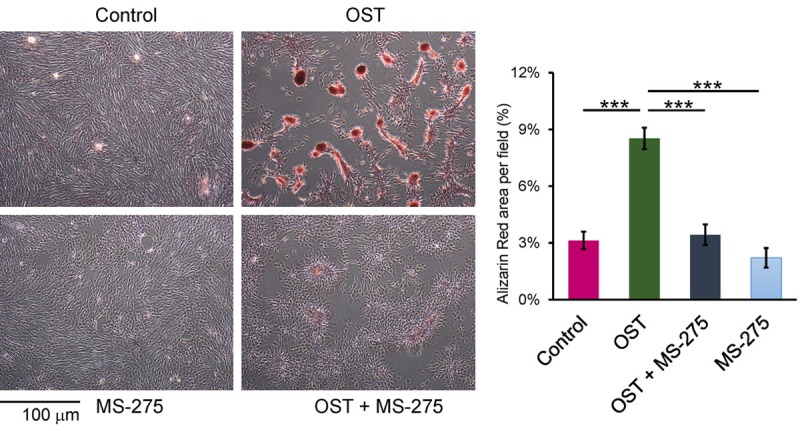
Alizarin red S staining for calcification measurement. MS-275 significantly reduced OST-medium-induced cell aggregation and calcium deposition. The images were photographed using an inverted phase contrast microscope with original magnification × 40, and the stained area was quantified using ImageJ software. The average ratio of the red color in the images are presented as the mean ± SEM of alizarin red area per field (n = 4), ***P < 0.005.
Effects of GSK-3β inhibitor and β-catenin inhibitor on RUNX2, GSK-3β, and p-SMAD protein expression
VICs treated with a combination of OST medium and BIO (1 μM), a GSK-3 inhibitor, exhibited lower RUNX2 and GSK-3β expression levels than did those treated with OST medium alone (Figure 4A and 4B). BIO also significantly attenuated OST medium-upregulated p-SMAD1/5/8 in the VICs (Figure 4C).
Figure 4.
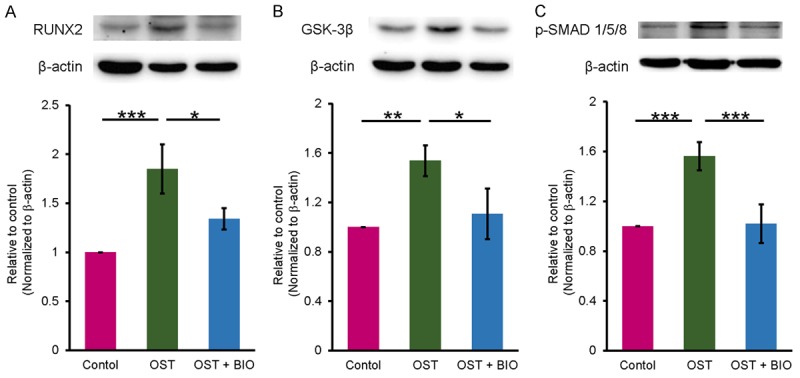
Effects of a GSK-3β inhibitor (BIO) on Wnt and BMP-2 signaling of VICs. BIO at 1 μM attenuated the upregulation of RUNX2 (A), GSK-3β (B), and p-SMAD1/5/8 (C) in OST medium treated VICs. Protein expression was analyzed using Western blot. β-actin was used as an internal control. Data are presented as the mean ± SEM (n = 7). *P < 0.05, **P < 0.01, ***P < 0.005.
We compared the effects of BIO with and without MS-275 on RUNX2 and GSK-3β expression in OST medium-treated VICs (Figure 5A and 5B). BIO combined with MS-275 engendered lower RUNX2 and GSK-3β expression levels than did BIO alone (Figure 5A and 5B), implying that MS-275 might reduce RUNX2 through a mechanism other than GSK-3β inhibition. VICs treated with a combination of a β-catenin Inhibitor (IWR-1-endo, 10 µM) and OST had less RUNX2 and GSK-3β than OST medium-treated VICs (Figure 5C and 5D), further confirming that the inhibition of β-catenin may contribute-at least in part-to the effects of MS-275 on RUNX2 levels.
Figure 5.
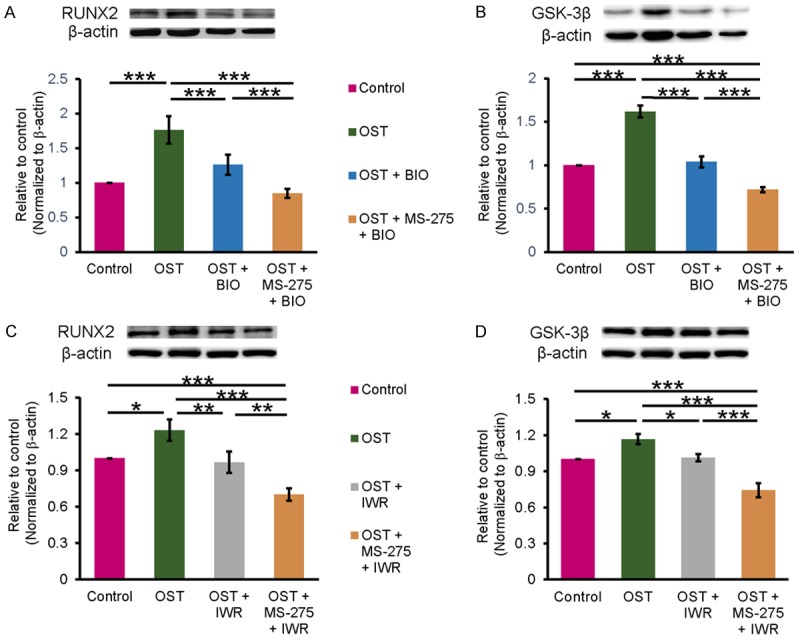
GSK-3β and β-catenin are involved in MS-275 induced RUNX2 downregulation. The osteogenic medium-treated VICs received MS-275 (1 μM) combined with BIO (GSK-3β inhibitor) or IWR (β-catenin inhibitor) for 5 days, then the cells were harvested for Western blotting. (A and B) show that MS-275 combined with BIO had larger protein attenuation of RUNX2 and GSK-3β than BIO alone (n = 6). (C and D) show that MS-275 combined with IWR had larger protein attenuation of RUNX2 and GSK-3β than IWR alone. β-actin was used as an internal control. Band intensities were quantified using Image-Pro Plus software. Data are presented as the mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ***P < 0.005.
Effects of MS-275 on canonical and non-canonical Wnt signaling in a 14-day calcification model
We studied the effect of MS-275 on VIC mineralization in a relatively long-term model. MS-275 reduced RUNX2, β-catenin, and GSK-3β expression levels in VICs treated with OST medium for 14 days. Additionally, MS-275 significantly attenuated the upregulation of osteocalcin, a late osteoblastic marker, after a 14-day culture (Figure 6).
Figure 6.
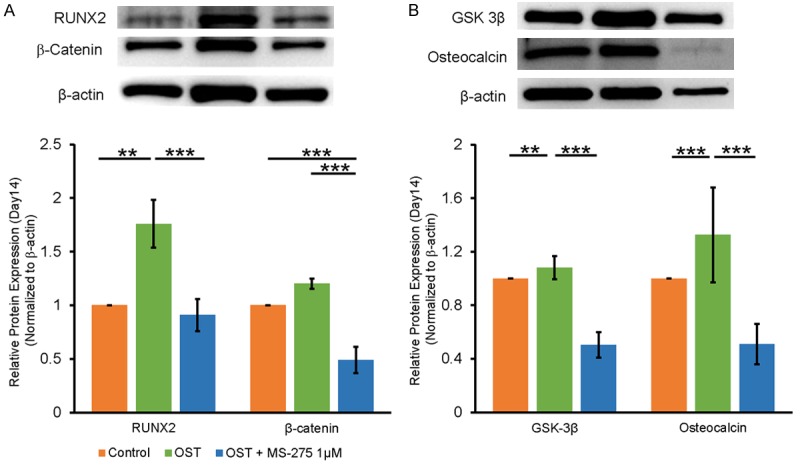
MS-275 attenuated canonical and non-canonical Wnt signaling in a 14-day calcification model. Porcine VICs were cultured in osteogenic induction medium with or without MS-275 (1 μM) for 14 days, then cells were harvested for Western blotting. MS-275 attenuated the upregulation of RUNX2 and β-catenin (A), GSK-3β and osteocalcin (B) in OST medium-treated VICs. β-actin was used as an internal control. Band intensities were quantified using Image-Pro Plus software. Data are presented as the mean ± SEM (n = 6). **P < 0.01, ***P < 0.005.
Discussion
This study elucidates, for the first time, the role of HDACs in the regulation of RUNX2 expression in OST medium-treated VICs. The HDAC I inhibitor MS-275 (1 μM), but not the class IIa HDAC inhibitor MC1568 or class IIb HDAC inhibitor tubacin (0.1 μM), attenuated the effects of OST medium. A combined class I and class IIb HDAC inhibitor (MPT0E014 at 0.1 μM) attenuated RUNX2 transactivation. These results indicate the pivotal role of HDAC I in RUNX2 expression. MS-275 simultaneously repressed GSK-3β, β-catenin, and p-SMAD1/5/8 expression. Because of its interactions with canonical and non-canonical Wnt signaling, MS-275 in combination with BIO or IWR exerted greater attenuation than did BIO or IWR alone.
Canonical β-catenin signaling was upregulated 3.5-4-fold in calcified tricuspid AVs compared with normal AVs [4]. Albanese et al. found that non-canonical Wnt-5a, Wnt-5b, and Wnt-11 were highly expressed in stenotic AVs, particularly in bicuspid AVs [14]. The non-canonical Wnt-5a group (Wnt-4, Wnt-5a, and Wnt-11) combined with ROR2 prevents canonical-Wnt-induced GSK-3 inactivation [16-18]. The GSK-3β inhibitor CHIR99021 was demonstrated to inhibit human aortic VIC mineralization [14]. Yu et al. incubated human aortic VICs with 50 mg/mL Lp(a) for 48 h and found that GSK-3α/β and β-catenin translocation were simultaneously upregulated [19]. They proved that GSK-3α/β inhibition by CHIR99021 significantly reduced ALP activity and calcium deposition.
Non-canonical Wnt signaling seemed to play a pivotal role in our OST VIC model. GSK-3β mRNA and protein expression levels were increased in OST medium-treated VICs, further confirming that non-canonical Wnt signaling contributes to RUNX2 upregulation. MS-275 inhibited the mRNA transcription of GSK-3β. We used BIO-a highly potent, selective, reversible, and ATP-competitive inhibitor of GSK-3α/β-to clarify the effect of GSK-3β inhibition [20]. Kellar et al. used BIO to elevate β-catenin levels artificially and found that it drastically reduced subsequent mineralization [21]. Similarly, we discovered that BIO could reduce RUNX2 expression levels.
When MS-275 was added to the treatment medium, both non-canonical Wnt/GSK-3β and canonical Wnt/β-catenin were repressed, implying that MS-275 could attenuate the transactivation of RUNX2 through both canonical Wnt signaling and non-canonical Wnt signaling. The role of canonical β-catenin signaling in VIC mineralization has been established. In a previous study, GSK-3β and β-catenin protein and mRNA levels significantly increased in porcine VICs after stimulation with oxidized low-density lipoprotein [22]. This finding implies that both types of Wnt signaling contribute to VIC mineralization. Research has shown that IWR-1-endo-a potent inhibitor of Wnt signaling that operates through proteasomal degradation of β-catenin through the stabilization of the destruction complex-significantly reduces RUNX2 expression without interfering with GSK-3β activity or expression [3,23-26]. Similarly, Li et al. proved that IWR-1-endo inhibited OST differentiation [27]. We found that the inhibition of canonical Wnt signaling reduced RUNX2 expression. MS-275 combined with BIO or IWR suppressed canonical and non-canonical Wnt signaling; this suppressive effect may have contributed to a greater reduction in RUNX2 expression than did the suppressive effect engendered by BIO or IWR alone. Moreover, in VICs treated with OST medium for 14 days, MS-275 attenuated RUNX2 and osteocalcin upregulation, suggesting the interventional potential of the HDAC I inhibitor in calcified AVs.
Different classes of HDAC inhibitors exerted various effects on VIC osteogenesis. Because class II HDACs may have neutral or precipitating effects on VIC calcification, pan-HDAC inhibition may have deleterious effects on VICs. We found that MPT0E014 reduced RUNX2 in OST medium-treated VICs. Similarly, valproic acid, a combined class I and II HDAC inhibitor and a GSK-3β inhibitor [28], reduced calcification induced by high phosphate levels (3 mmol/L) [29]. However, MPT0E014 had weaker inhibitory effects on RUNX2 than did MS-275, which may have been caused by the counterbalancing effects of the combined class I and II HDAC inhibitor on RUNX2 regulation.
The decrease in BMP/p-SMAD1/5/8 expression may have been induced by the attenuation of canonical Wnt signaling. The mechanism governing the osteoblastic differentiation and maintenance of skeletal homeostasis is a crosstalk between the BMP and Wnt pathways. Wnt signaling was proven to be an upstream regulator of BMP signaling [30]. Wnt/β-catenin directly regulates BMP-2 transcription by interacting with the BMP-2 promoter. Tamura and Nemoto also suggested the crosstalk between the canonical Wnt and BMP-2 signaling pathways [31]. In the current study, OST medium-treated VICs had higher p-SMAD1/5/8 expression levels than did the other cells, suggesting that BMP signaling has a role in VIC calcification. We discovered that BIO-induced GSK-3β inhibition was associated with BMP attenuation in OST medium-treated VICs.
In the 5-day culture model, we did not observe OST medium-induced upregulation of osteocalcin or α-SMA. Similarly, Monzack et al. demonstrated that treating porcine aortic VICs with mineralization medium increased ALP expression and reduced α-SMA expression but had no effect on osteocalcin expression [32]. An extended period of culture (14 days) with OST medium significantly increased VIC mineralization, which was attenuated by MS-275.
RUNX2 was shown to play a role in cell proliferation [33]. Another study demonstrated that HDAC inhibitors might reduce cardiac fibrosis [34]. In the current study, MS-275 considerably reduced VIC proliferation. These findings suggest that MS-275 may reduce VIC activity through the reduction-at least in part-of RUNX2 expression. Figure 7 presents a summary of the effects of the HDAC I inhibitor (MS-275) on VICs. Through both GSK-3β and β-catenin inhibition, MS-275 reduced the expression of RUX2 at the transcription level.
Figure 7.

Schematic illustration of the proposed mechanisms for the inhibitory effects of MS-275 on osteogenesis in VICs. MS-275 regulates RUNX2 expressions via canonical and non-canonical Wnt/β-catenin signaling pathway in VICs. MS-275 works as a coordinated inhibitor of GSK-3β and β-catenin, which results in the down-regulation of RUNX2 gene transcription and protein expression in OST medium-treated VICs. This effect may lead to osteogenesis inhibition in VICs.
Study limitations
This study has some limitations. Although CAVD commonly develops over a long period, in our model, VIC calcification was induced by the applied OST medium over a relatively short period. Moreover, the studied VICs were isolated from healthy pigs, and none of the study findings were validated in a more physiological context. In addition, whether, and to what extent, the canonical or non-canonical Wnt signaling pathways are involved in human or even experimental CAVD should be further explored.
Conclusions
A GSK-3β inhibitor reduced RUNX2 expression in OST medium-treated VICs. Additionally, a class I (but not class II) HDAC inhibitor reduced RUNX2 expression in OST medium-treated VICs through the canonical and non-canonical Wnt signaling pathways.
Acknowledgements
This study was supported by grants from Wan Fang Hospital, Taipei Medical University (104-wf-phd-02, 105-wf-phd-01, 106-wf-eva-08, 107-wf-eva-02, and 107TMU-WFH-01-1). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- α-SMA
alpha-smooth muscle actin
- ALP
alkaline phosphatase
- ARS
Alizarin Red S
- AV
aortic valve
- BMP
bone morphogenetic protein
- CAVD
calcific aortic valve disease
- Dvl
Dishevelled
- Fzd
Frizzled
- GSK-3
glycogen synthase kinase 3
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- LRP
low-density lipoprotein receptor-related protein
- RhoA
Ras homolog gene family, member A
- ROCK
Rho-associated protein kinase
- ROR2
receptor tyrosine kinase-like orphan receptor 2
- RUNX2
runt-related transcription factor 2
- TGF
transforming growth factor
- VIC
valvular interstitial cell
- Wnt
wingless-related integration site
References
- 1.Li SJ, Kao YH, Chung CC, Chen WY, Cheng WL, Chen YJ. Activated p300 acetyltransferase activity modulates aortic valvular calcification with osteogenic transdifferentiation and downregulation of Klotho. Int J Cardiol. 2017;232:271–279. doi: 10.1016/j.ijcard.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016;22:95–106. doi: 10.12659/MSMBR.901142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajamannan NM. The role of Lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the ApoE-/-/Lrp5-/- mice. J Cell Biochem. 2011;112:2987–2991. doi: 10.1002/jcb.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajamannan NM. The role of Lrp5/6 in cardiac valve disease: LDL-density-pressure theory. J Cell Biochem. 2011;112:2222–2229. doi: 10.1002/jcb.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askevold ET, Gullestad L, Aakhus S, Ranheim T, Tonnessen T, Solberg OG, Aukrust P, Ueland T. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc. 2012;1:e002261. doi: 10.1161/JAHA.112.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jho E, Lomvardas S, Costantini F. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266:28–35. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 13.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 14.Albanese I, Yu B, Al-Kindi H, Barratt B, Ott L, Al-Refai M, de Varennes B, Shum-Tim D, Cerruti M, Gourgas O, Rheaume E, Tardif JC, Schwertani A. Role of noncanonical Wnt signaling pathway in human aortic valve calcification. Arterioscler Thromb Vasc Biol. 2017;37:543–552. doi: 10.1161/ATVBAHA.116.308394. [DOI] [PubMed] [Google Scholar]
- 15.Dudakovic A, Evans JM, Li Y, Middha S, McGee-Lawrence ME, van Wijnen AJ, Westendorf JJ. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. J Biol Chem. 2013;288:28783–28791. doi: 10.1074/jbc.M113.489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279:24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–1223. doi: 10.1111/j.1365-2443.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 18.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 19.Yu B, Hafiane A, Thanassoulis G, Ott L, Filwood N, Cerruti M, Gourgas O, Shum-Tim D, Al Kindi H, de Varennes B, Alsheikh-Ali A, Genest J, Schwertani A. Lipoprotein(a) induces human aortic valve interstitial cell calcification. JACC Basic Transl Sci. 2017;2:358–371. doi: 10.1016/j.jacbts.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Keller KC, Ding H, Tieu R, Sparks NR, Ehnes DD, Zur Nieden NI. Wnt5a supports osteogenic lineage decisions in embryonic stem cells. Stem Cells Dev. 2016;25:1020–1032. doi: 10.1089/scd.2015.0367. [DOI] [PubMed] [Google Scholar]
- 22.Gu GJ, Chen T, Zhou HM, Sun KX, Li J. Role of Wnt/beta-catenin signaling pathway in the mechanism of calcification of aortic valve. J Huazhong Univ Sci Technolog Med Sci. 2014;34:33–36. doi: 10.1007/s11596-014-1228-x. [DOI] [PubMed] [Google Scholar]
- 23.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Ma Z, Hsieh JC, Fan CW, Chen B, Longgood JC, Williams NS, Amatruda JF, Lum L, Chen C. Structure-activity relationship studies of small-molecule inhibitors of Wnt response. Bioorg Med Chem Lett. 2009;19:3825–3827. doi: 10.1016/j.bmcl.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 27.Li G, Liu J, Wang Y, Yang K, Zhao M, Xiao Y, Wen X, Liu L. LNGFR targets the Wnt/beta-catenin pathway and promotes the osteogenic differentiation in rat ectomesenchymal stem cells. Sci Rep. 2017;7:11021. doi: 10.1038/s41598-017-11555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, Kong W, Zhu WG, Xu MJ, Wang X. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013;83:1042–1051. doi: 10.1038/ki.2012.482. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW, Zhao M. Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013;52:145–156. doi: 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura M, Nemoto E. Role of the Wnt signaling molecules in the tooth. Jpn Dent Sci Rev. 2016;52:75–83. doi: 10.1016/j.jdsr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monzack EL, Masters KS. Can valvular interstitial cells become true osteoblasts? A side-by-side comparison. J Heart Valve Dis. 2011;20:449–463. [PMC free article] [PubMed] [Google Scholar]
- 33.Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, Meloche J, Pflieger A, Breuils-Bonnet S, Nadeau V, Paradis R, Tremblay E, Girerd B, Hautefort A, Montani D, Fadel E, Dorfmuller P, Humbert M, Perros F, Paulin R, Provencher S, Bonnet S. Role for runt-related transcription factor 2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:1273–1285. doi: 10.1164/rccm.201512-2380OC. [DOI] [PubMed] [Google Scholar]
- 34.Nural-Guvener HF, Zakharova L, Nimlos J, Popovic S, Mastroeni D, Gaballa MA. HDAC class I inhibitor, Mocetinostat, reverses cardiac fibrosis in heart failure and diminishes CD90+ cardiac myofibroblast activation. Fibrogenesis Tissue Repair. 2014;7:10. doi: 10.1186/1755-1536-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


