Abstract
The dedifferentiation of vascular smooth muscle cells (VSMCs) is a key event in the pathogenesis of vascular remodeling-related disease. The present study aimed to investigate the effects of shexiangbaoxin (SXBX) pill, a traditional Chinese medicinal formula on VSMCs dedifferentiation and its potential mechanisms. High-fat diet (HFD) was introduced to lipoprotein receptor-deficient (LDLR-/-) mice to generate hyperhomocysteinemia (HHcy), and plasma Hcy and lipid levels were analyzed. The phenotype of VSMCs was assessed in mice with the treatment of low (45 mg/kg/d) or high (90 mg/kg/d) SXBX pill by measuring the contractile protein α-SMA, SM22α and synthetic proteins OPN using RT-qPCR, western blotting and immunofluorescence assay. In vitro, the proliferation, migration and dedifferentiation of VSMCs were measured by MTT, Edu incorporation, wound healing and western blotting assay. Small interfering RNA technology was used to examine the role of NLRP3 in the effects of SXBX pill on dedifferentiation. The results indicated that although SXBX pill had no influence on HFD-induced HHcy and hyperlipidaemia, it reversed HHcy-induced dedifferentiation of VSMCs in vivo. SXBX pill significantly inhibited proliferation, migration and dedifferentiation of Hcy-treated VSMCs. In addition, we found that Hcy activated NLRP3 inflammasomes in VSMCs and SXBX pill could attenuate NLRP3 inflammasomes activation. Moreover, subsequent analysis suggested that SXBX pill inhibited NLRP3 inflammasomes activation through regulation of ERK1/2 and p38 MAPK pathway. Knockdown of NLRP3 reversed the inhibitory effects of SXBX pill in VSMCs. In conclusion, SXBX pill inhibited Hcy-induced proliferation, migration and dedifferentiation of VSMCs by suppressing NLRP3 inflammasomes activation via of ERK/p38 MAPK pathway.
Keywords: SXBX pill, homocysteine, vascular smooth muscle cell, dedifferentiation, NLRP3 inflammasomes
Introduction
Hyperhomocysteinemia (HHcy) is a state in which too much homocysteine is present in the body and has been regarded as a risk factor for cardiovascular disease [1]. Our previous study reported that independently causes and promotes atherosclerosis in low-density lipoprotein receptor-deficient (LDLR-/-) mice [2]. Epidemiological studies have described HHcy could induce vascular remodeling and its possible mechanisms involve in homocysteine (Hcy)-mediated inflammation [3,4]. However, the mechanisms have not been well described. Vascular smooth muscle cells (VSMCs) are the major components of the vessel wall, and their hyperproliferation is an important risk factor for atherosclerosis [5]. Our previous study and other reports suggested homocysteine (Hcy) induces proliferation, migration and dedifferentiation of VSMCs [6,7], which serves as a major initiating factor for vascular remodeling in vascular diseases [8]. The phenotypic transformation from differentiated to dedifferentiated VSMCs is involved in the reduced expression of contractile proteins, such as alpha smooth muscle actin (α-SMA) and smooth muscle 22 alpha (SM22α), and increased expression of synthetic proteins (such as osteopontin, OPN) and inflammatory cytokines [9].
The NLRP3 (NLR family, pyrin domain containing 3) inflammasomes was discovered by researchers interested in the regulation of caspase-1. This enzyme processes the preforms of the cytokine interleukin-1β (IL-1β), a critical pro-inflammatory cytokines, whose purpose being to drive the inflammatory process and restore homeostasis [10]. Over the decades, a plenty of discoveries have been made suggesting a close interplay between NLRP3 and inflammatory diseases [11]. For instance, bone marrow deficiency in NLRP3 inflammasomes components markedly attenuates atherogenesis in LDLR-/- mice [12]. In addition, more importantly, Sun et al. found that NLRP3 inflammasomes activation contributes to the dedifferentiation of VSMCs and vascular remodeling in hypertension [13]. Above all indicated NLRP3 may be a target for inhibition of VSMCs dedifferentiation. However, it is unclear whether NLRP3 is involved in HHcy-induced VSMCs dedifferentiation and proliferation.
Shexiangbaoxin (SXBX) pill is a traditional Chinese medicinal compound, which has been widely used for treating coronary heart disease with angina pectoris [14]. SXBX pill could decrease myocardial collagen synthesis and deposition in left ventricle, thus to prevent and treat myocardial fibrosis [15]. In addition, SXBX pill reduces arterial levels of inflammation markers (C-reactive protein, fibrinogen, and D-dimer) in coronary syndrome subjects [16]. However, whether SXBX pill plays a role in Hcy-induced dedifferentiation of VSMCs remains unknown. Therefore, this study was carried out to investigate the effects of SXBX pill in Hcy-induced proliferation, migration and dedifferentiation of VSMCs, and the roles of NLRP3 in the action of the SXBX pill on VSMCs.
Materials and methods
Animal model and tissue preparation
A total of 40 low-density lipoprotein receptor-deficient (LDLR-/-) mice were obtained from Model Animal Research Center of Nanjing University (Nanjing, China). These mice were maintained on a 12-hour light-dark cycle in a pathogen-free environment at the animal research center of Shaoxing people’ Hospital. All experiments were performed on adult (6-8 weeks) male mice. After 1 week of acclimatization, at age 7 weeks, 30 mice were fed high fat diet (HFD) including standard rodent chow +10% fat +1.25% cholesterol +1% L-methionine [17]. They were randomly divided into three groups: HFD group, HFD + Low SXBX pill (45 mg/kg/d, obtained from Shanghai Hutchison Pharmaceuticals, China) group, HFD + High SXBX pill (90 mg/kg/d). SXBX pill was given at the beginning of the study. Another 10 LDLR-/- mice received normal chow diet were served as the control group. They were allowed free access to food and water. Experiments were approved by the Experimental Animal Care and Use Committee of Shaoxing people’ Hospital. After 14 weeks’ intervention, all mice were fasted overnight and sacrificed. Approximately 1 ml of blood was collected into EDTA tube, centrifuged at 3500 g for 10 minutes and plasma were retrieved and stored at -80°C. The aorta of the mice was fixed in 4% formaldehyde and embedded in paraffin. All procedures were conformed to the Guide for the Care and Use of Laboratory Animal published by the US National Institutes of Health (NIH publication, 8th edition, 2011).
Hcy and lipid analysis
Plasma total cholesterol and triglyceride levels were measured using enzymic method on Aeroset Aeroset fully automatic clinical biochemical analyzer (Abbott Labs, USA), as previously described [18]. Plasma Hcy levels were analyzed using a Hcy ELISA kit (Fanke Biological Technology Co., Ltd., Shanghai, China), according to the manufacturer’s instruction.
Cell culture and treatment
Primary VSMCs were isolated from 4-weeks male Sprague-Dawley rats, and cultured in DMEM medium (Sigma, St. Louis, MO) with 10% FBS (Gibco, Grand Island, NY) at 37°C in a humidified atmosphere of 5% CO2. VSMCs of Passage 3-10 were used in the present study. The cells were treated with 500 μM of Hcy (H0159, Tokyo Chemical Industry Co., Ltd., Japan) for 48 h. SXBX pill (1 g) was dissolved in 20 mL sterile PBS with DMSO and stored at -20°C. VSMCs were treated with Low SXBX pill (0.1 g/L) and High SXBX pill (1 g/L). To knockdown of NLRP3 in VSMCs, cells were transfected with specific siRNA against NLRP3 (si-NLRP3), or negative control siRNA (si-NC, obtained from Shanghai GenePharma, Shanghai, China) using Lipofectamine 2000 Transfection Reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instruction. ERK1/2 inhibitors PD98059 (20 μmol/L, MedChemExpress) and p38 MAPK SB203580 (10 μmol/L, MedChemExpress) were used to study the pathways involved in the role of SXBX pill in VSMCs in this study.
Cell proliferation and migration assay
VSMCs were treated as indicated for 12, 24, 48, and 72 h, and an MTT assay was used to examine the cell proliferation of VSMCs. The medium was added to MTT (5 g/L) and the cells were then incubated for 4 h. Subsequently, 100 μl DMSO was added after the medium was removed. The plate was gently vibrated for 1 min to dissolve the precipitate. The microplate reader (3550; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to determine the absorbance at 570 nm. An Edu Cell Proliferation Assay Kit (Ribobio, Guangzhou, China) was used to detect cell proliferation according to the manufacturer’s instruction as described [19]. In brief, 5×105 cells were seeded in 24-well plates and forty-eight hours after treatment, cells were incubated with 50 µM Edu for 3 h before fixation, permeabilization and EdU staining. The nuclei were stained with DAPI (Sigma) at a concentration of 1 µg/mL for 5 min. The proportion of cells that incorporated Edu was determined by a Nikon Eclipse Ti-U fluorescence microscope (×400). For cell migration assay, approximately 106 of VSMCs were added into each well of 6-well plates. When cells were 80-90% confluent, a cell scratch spatula was used to manually scratch the monolayer after treatment. Plates were washed using PBS for three times to remove cellular debris, and cell migration was observed using an inverted microscope (Olympus Corp., Tokyo, Japan). Cells were then incubated at 37°C for 48 h, examined and photographed under the microscope. Each cell condition was assayed in triplicate.
Detection of caspase 1 activity and interleukin (IL)-1β levels
Caspase-1 activity was measured by Caspase 1 Activity Assay Kit (C1101, Beyotime, China). 50 ug of total cytosolic protein was incubated in a 96-well microtiter plate with 20 nmol acetyl-Tyr-Val-Ala-Asp p-nitroaniline (Ac-YVAD-pNA) overnight at 37°C. The absorbance values of p-nitroaniline (pNA) at 405 nm were tested using a microplate reader. The production of pNA in tested samples indicated the level of caspase-1 activation. IL-1β in cultured VSMCs supernatants were measured using commercial ELISA kits (Jiancheng, Nanjing, China), according to the manufacturer’s instruction.
Quantitative real-time PCR analysis
Total RNA was isolated with the Trizol (Invitrogen, USA) method, and cDNA was synthesized using reverse transcription kit PrimeScript RT reagent Kit (Takara, Otsu, Japan) from 1 µg of total RNA. Real-time PCR was performed with the cDNA samples and SYBR Premix Ex Taq kit (Takara) by using an ABI 7300 RT-PCR Detection System (Applied Biosystems, Foster, CA, USA). The qPCR conditions included one cycle of initial denaturation at 95°C for 5 min followed by 40 cycles at 95°C for 10 s; 60°C for 30 s; and 72°C for 15 s. All samples were amplified in triplicate. The relative changes in the number of transcripts in each sample were determined by normalizing with the β-actin RNA levels via the 2-ΔΔCt method. The sequences of the primers used were as follows: α-SMA forward 5’-GTGTTGCCCCTGAAGAGCAT-3’; reverse 5’-GCTGGGACATTGAAAGTCTCA-3’; Osteopontin forward 5’-CAAATACCCAGATGCTGTGGC-3’; reverse 5’-TCCTGGCTGTCCACATGGAC-3’; SM22α forward 5’-ACACGCTCAACGTCAGCAT-3’; reverse 5’-GCTCGTCCAGCTCTGGATAT-3’ and β-actin forward 5’-CGTTGACATCCGTAAAGACC-3’; reverse 5’-GGAGCCAGGGCAGTAATCT-3’.
Western blotting analysis
Aorta tissues or cells scraped from culture dishes were placed in RIPA lysis buffer (Beyotime). After homogenization on ice, the lysates were centrifuged at 11,000 g for 10 min and protein concentrations were determined by BCA method following the manufacturer’s protocol. Equal amounts of proteins were subjected to SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Millipore, MA, USA). Blots were incubated for 1 h at room temperature with 5% skimmed milk in TBST, and then incubated with primary antibodies for p-p38 (#4631), p38 (#9212), p-ERK (#4370), ERK (#4695), p-JNK (#9255), JNK (#9252, all obtained from Cell signaling technology, Danvers, MA, USA), NLRP3 (ab4207), procaspase-1 (ab179515), IL-1β (ab20478), α-SMA (ab7817), Osteopontin (ab8448), SM22α (ab14106, all obtained from Abcam, Cambridge, UK) at 4°C overnight. β-actin (bsm-33036M, Bioss antibodies, Woburn, MA, USA) were served as an internal reference protein. Subsequently, membranes were incubated in horseradish peroxidase-conjugated secondary antibodies (Beyotime). The blot was developed with an enhanced chemiluminescence reagent kit (Beyotime) and quantification of band intensity was carried out using Quantity One 5.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence analysis
Cells were grown in 8-well chamber slides (Millipore). After treatment, VSMCs were collected and fixed with 4% paraformaldehyde followed by permeabilization with 0.1% Triton X-100 for 15 min. Subsequently, 1% bovine serum albumin (BSA) in PBST was used to block unspecific regions for 30 min at room temperature. For double immunofluorescence staining, paraffin-embedded tissues were cut into 5 µm sections using a cryostat (Leica, Solms, Germany), deparaffinized, rehydrated, and then subjected to antigen retrieval. Tissue sections or cell slides were incubated with primary antibodies overnight at 4°C, followed by incubation with secondary antibodies for 1 hour at room temperature. The nuclei were counterstained for visualization with DAPI. The primary antibodies used were anti-α-SMA (ab7817), Osteopontin (ab8448), NLRP3 (ab4207), IL-1β (ab20478). The secondary antibodies used were Alexa Fluor 488-, Alexa Fluor 594-conjugated anti-immunoglobulin G (Abcam). Images were captured under a Nikon Eclipse Ti-U fluorescence microscope (×400).
Statistical analysis
Data from individual experiments were represented as mean ± standard deviation. Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Prism Software, San Diego, USA). Comparisons between groups were made by one-way ANOVA analysis, followed with Tukey’s post hoc analysis. A P value <0.05 was considered statistically significant.
Results
SXBX pill inhibits HHcy-induced dedifferentiation of VSMCs in vivo
Firstly, we analyzed plasma Hcy and lipid levels of experimental mice. Plasma total cholesterol, triglycerides and LDL-C levels were significantly higher in mice fed with HFD compared to the control group. However, treatment with SXBX pill had no influence on plasma lipids. Similarly, plasma Hcy levels were obviously increased in the HFD group compared with the control group. However, dietary SXBX pill had no significant effect on Hcy levels in mice fed with HFD (Table 1). These data indicate that SXBX pill had no effect on HFD-induced hyperlipidaemia or hyperhomocysteinemia on LDLR-/- mice. Due to the critical role of VSMCs dedifferentiation in vascular remodeling, we subsequently investigated the effects of SXBX pill on hyperhomocysteinemia-related vascular remodeling in the aorta of mice. Immunofluorescence double staining experiments showed that α-SMA was highly expressed in the medial layers of the aorta in the control group and decreased in the HFD group. However, SXBX pill treatment abolished HFD-induced reduction in α-SMA expression. However, OPN expression was rarely detected in medial VSMCs in the control groups and its expression was upregulated in the aorta of mice received HFD. Whereas SXBX pill treatment markedly decreased OPN expression (Figure 1A). To quantitatively investigate the effects of SXBX pill on the changes of markers of VSMCs dedifferentiation, both RT-qPCR and western blotting confirmed that HFD induced downregulation of α-SMA and SM22α expression, and induced upregulation of OPN expression. In contrast, treatment with low or high SXBX pill significantly abolished HFD-induced alteration of above markers (Figure 1B and 1C). Collectively, above results demonstrated that SXBX pill could inhibit HFD-related VSMCs dedifferentiation in vivo.
Table 1.
Plasma biochemical values of different animal group (mean ± SD, n = 10)
| Parameter | Control | HFD | HFD + Low SXBX pill | HFD + High SXBX pill |
|---|---|---|---|---|
| TC (mg/dl) | 97.39±3.74 | 210.67±16.87* | 217.62±26.75 | 201.43±21.56 |
| Triglyeride (mg/dl) | 35.66±4.87 | 157.46±22.42* | 153.08±19.17 | 143.94±22.95 |
| LDL-C (mg/dl) | 19.97±2.03 | 62.17±3.95* | 64.31±6.84 | 58.38±6.92 |
| Hcy (μmol/l) | 1.96±0.09 | 12.58±1.76* | 11.68±1.29 | 10.86±1.91 |
TC, Total cholesterol, LDL-C, low density lipoprotein cholesterol. Results were presented as mean ± SD, n = 10.
P<0.05 vs. control group.
Figure 1.
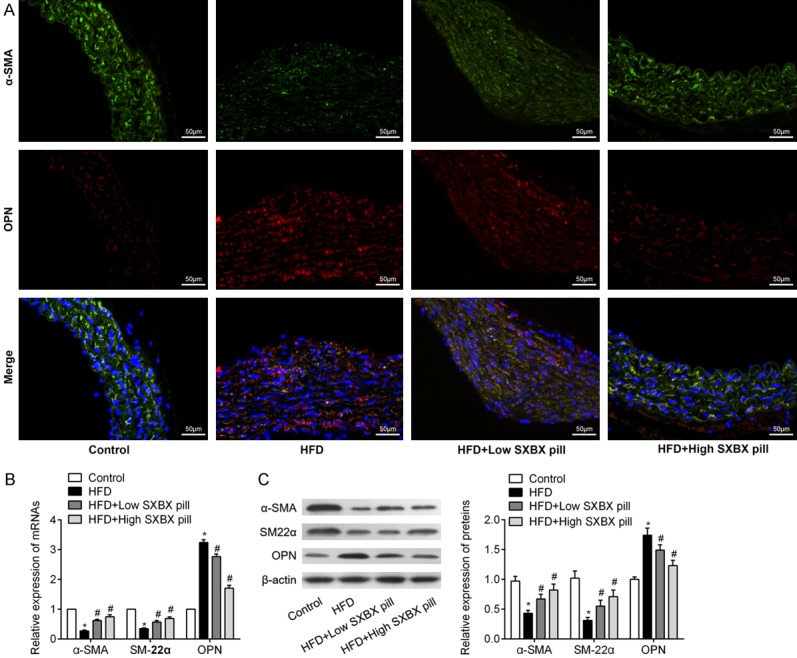
SXBX pill inhibits high fat diet mediated dedifferentiation of VSMCs in vivo. A. Immunofluorescence double staining showing the overlap of SMA (green) and OPN (red) in aortic of LDLR-/- mice continuously received normal chow diet (control), high fat diet (HFD), HFD + Low SXBX pill (45 mg/kg/d) and HFD + High SXBX pill (90 mg/kg/d) for 14 weeks. Nuclei were stained by DAPI (blue). Bar = 50 μm. B. Relative mRNA levels of SMA, SM22α and OPN in the aorta of LDLR-/- mice (n = 10). C. The expression levels of contractile protein α-SMA, SM22α and synthetic proteins OPN in the aortic of mice from above four groups (n = 10). *P<0.05 vs. control group; #P<0.05 vs. Hcy group.
SXBX pill inhibits Hcy-induced proliferation, migration and dedifferentiation of VSMCs in vitro
Subsequently, we explored the role of SXBX pill on the proliferation, migration and dedifferentiation of VSMCs stimulated by Hcy. As shown in Figure 2A, SXBX pill inhibited Hcy-induced VSMCs proliferation in a dose-dependent manner, as detected by MTT assay. Edu incorporation assay also showed that a significant increase in EdU positive cells was detected in Hcy-treated VSMCs and the increased percentage in EdU positive cells was significantly attenuated with the treatment of low or high SXBX pill (Figure 2B). In addition, the results of wound healing assay illustrated that SXBX pill suppressed Hcy-enhanced cell migration ability in a dose-dependent manner (Figure 2C). Moreover, consistent with the results in vivo, western blotting analysis revealed a significant downregulation of α-SMA and SM22α, and an upregulation of OPN in Hcy group comparable to the control group. SXBX pill could obviously increase α-SMA and SM22α expression and decrease OPN expression in a dose-dependent manner in Hcy-stimulated VSMCs (Figure 2D). Taken together, these data indicated that SXBX pill inhibited Hcy-induced proliferation, migration and dedifferentiation of VSMCs.
Figure 2.
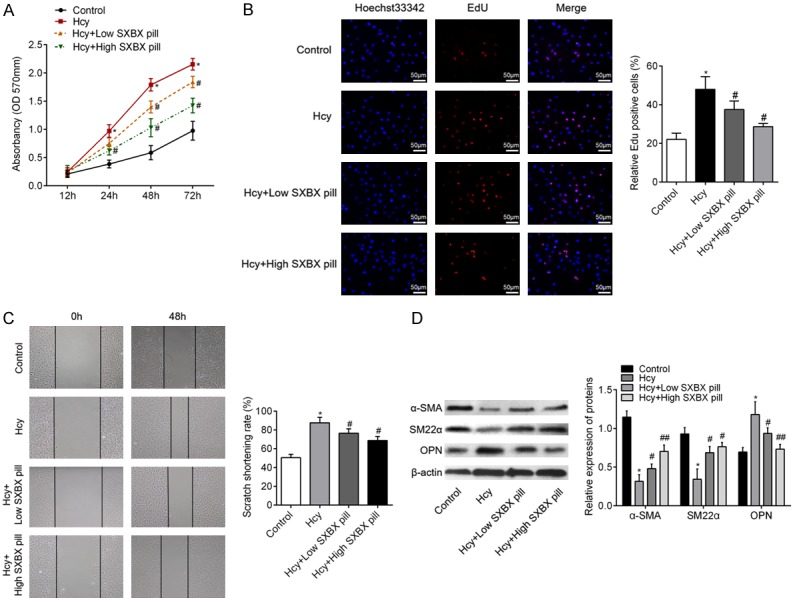
SXBX pill inhibits proliferation, migration and dedifferentiation of VSMCs. A. After incubation with 500 μM Hcy with or without SXBX pill treatment for various time points, VSMC proliferation was examined by MTT assay. B. DNA synthesis in VSMCs under Hcy with or without SXBX pill for 48 h was determined with EdU incorporation assay. Blue fluorescence (Hoechst 33342) shows cell nuclei and red fluorescence (Edu) stands for cells with DNA synthesis. Bar = 50 μm. C. Representative pictures of VSMCs migration through scratch-wound. Bar graphs showing the scratch shortening rate, which was determined by width at 48 h divided the width at 0 h. D. Western blot was employed to quantitate the expression levels of contractile protein α-SMA, SM22α and synthetic proteins OPN in VSMCs in different groups. Bar graph showing the relative protein level of α-SMA, SM22α and OPN. *P<0.05 vs. control group; #P<0.05 vs. Hcy group; ##P<0.05 vs. Hcy + Low SXBX pill group.
SXBX pill attenuates Hcy activated NLRP3 inflammasomes in VSMCs
Recent studies have shown that the NLRP3 was involved in the dedifferentiation of VSMCs, we thus further investigate whether SXBX pill inhibits VSMCs dedifferentiation through regulation of NLRP3 inflammasomes activation. As shown in Figure 3A and 3B, the levels of caspase 1 and IL-1β were significantly higher in Hcy groups when compared with the control group. However, SXBX pill markedly reduced Hcy-induced caspase 1 and IL-1β release in VSMCs. Subsequent western blotting analysis demonstrated that Hcy treatment dramatically increased the expression of NLRP3, ASC, procaspase-1 and mature IL-1β protein, indicating activation of NLRP3 inflammasomes in VSMCs. Importantly, SXBX pill substantially suppressed Hcy-induced upregulation of NLRP3, and ASC, procaspase-1 and mature IL-1β (Figure 3C-G). Immunofluorescence staining in Figure 3H showed that SXBX pill significantly abolished Hcy-induced NLRP3 and ASC upregulation in the cytoplasm of VSMCs. These results indicated the activation of NLRP3 inflammasomes induced by Hcy was blocked by SXBX pill.
Figure 3.
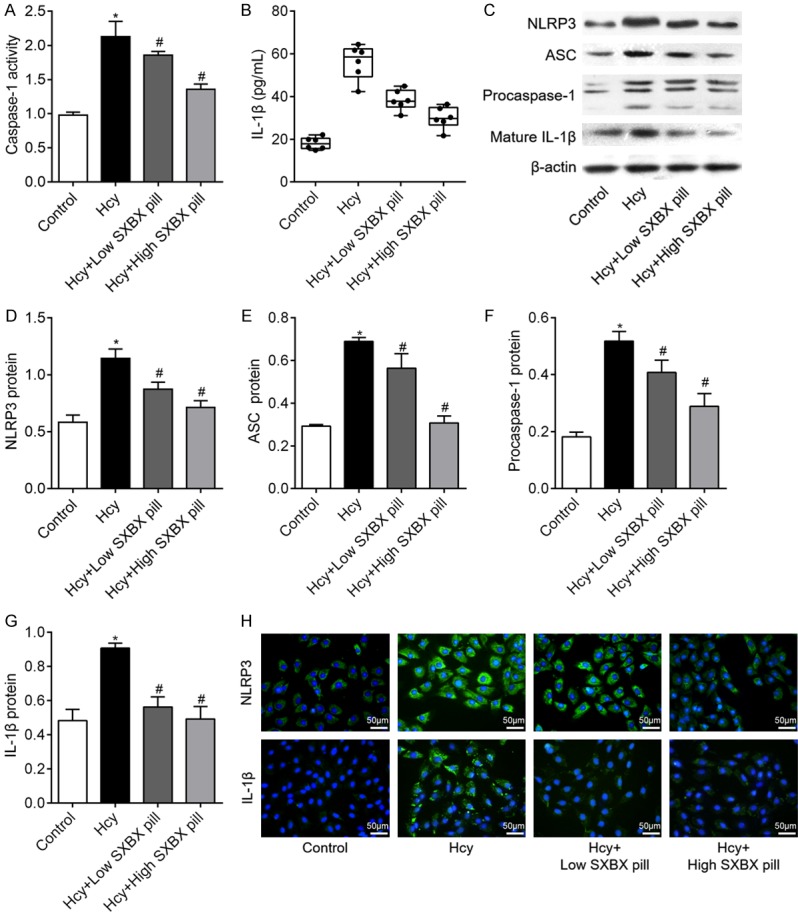
SXBX pill inhibits the Hcy activated NLRP3 inflammasomes in VSMCs. A. The activity of caspase-1 was examined using caspase-1 activity assay kit. B. release of IL-1β in the supernatant of cell cultures with or without Hcy stimulation was evaluated by enzyme-linked immunosorbent assay (ELISA); n = 6. C-G. VSMCs were treated with 500 μM Hcy for 24 h and western blotting was used to investigate the effects of SXBX pill on Hcy-activated NLRP3 inflammasomes. H. Immunofluorescence photomicrographs of VSMCs treated with 500 μM Hcy for 24 h followed by the treatment with low or high SXBX pill. Bar = 50 μm. *P<0.05 vs. control group; #P<0.05 vs. Hcy group.
SXBX pill suppresses Hcy activated MAPKs pathways in VSMCs
It is well-known that the MAPKs activation is related to the proliferation and migration of VSMC. To reveal the potential mechanisms underlying the action of SXBX pill on VSMCs under Hcy treatment, we detected the activation of MAPKs pathways in Hcy-treated cells. VSMCs were cultured with or without SXBX pill for 24 h and then treated with Hcy (500 μM) for 10 min. Intriguingly, we found that phosphorylation of p38, JNK and ERK1/2 was increased after stimulated with Hcy. However, SXBX pill significantly inhibited p38 and ERK1/2 but not JNK phosphorylation compared with Hcy group (Figure 4A-C). Thus, it was believed that SXBX pill suppressed p38, ERK1/2/MAPK signaling pathway in Hcy stimulated VSMCs.
Figure 4.
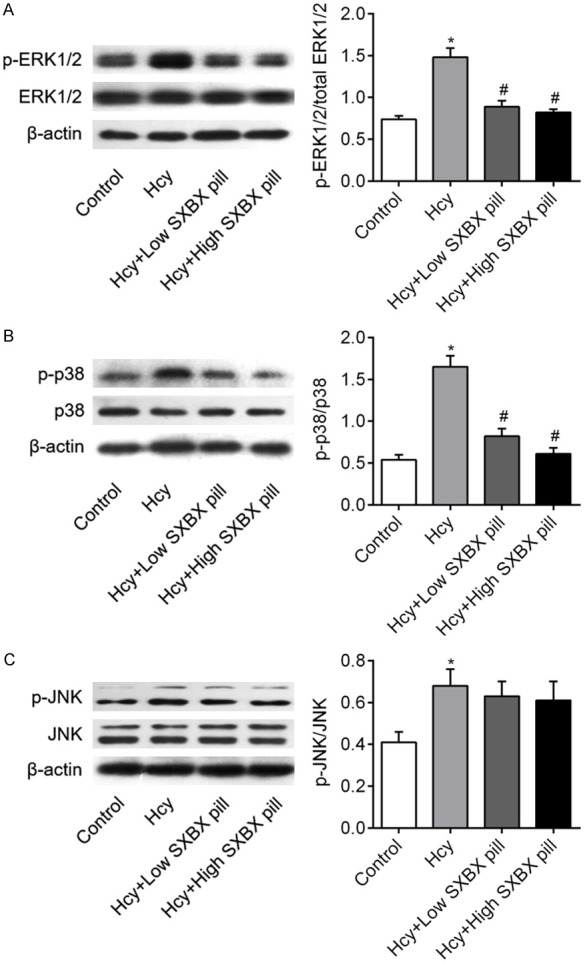
Effect of SXBX pill on Hcy-induced phosphorylation of ERK1/2, p38 MAPK, and JNK. Phosphorylation of (A) ERK1/2, (B) p38 MAPK, and (C) JNK was detected by western blotting using specific antibodies. Total ERK1/2, p38 MAPK, and JNK proteins were used as internal controls. Quantification of band intensities from three independent experiments was determined by densitometry. Data was described as means ± SD from three independent experiments. *P<0.05 vs. control group; #P<0.05 vs. Hcy group.
SXBX pill inhibits NLRP3 inflammasomes activation through regulation of ERK1/2 and p38 MAPK pathway
To assess how SXBX pill regulates the activation of NLRP3 inflammasomes in Hcy-induced VSMCs were infected with NLRP3 si-RNAs to knock down NLRP3 gene Blocking efficiency of NLRP3 was detected by protein expression of NLRP3 (Figure 5A). Subsequently, cells with or without NLRP3 silencing were pretreated with ERK1/2 inhibitor (20 μmol/L PD98059) and p38 p38 MAPK inhibitor SB203580 (10 μmol/L) for 30 min before Hcy administration. As shown in Figure 5B-D, PD98059 significantly inhibited ERK1/2 phosphorylation and SB203580 inhibited p38 phosphorylation. However, knockdown of NLRP3 had no influence on Hcy-induced MAPKs activation. The result in Figure 5E suggested that Hcy-induced NLRP3, ASC, procaspase-1 and IL-1β protein expressions were significantly inhibited in NLRP3 silenced VSMCs as compared with the Hcy + si-NC group. More importantly, consistent with SXBX pill, PD98059 and SB203580 obviously attenuated the Hcy-induced activation of NLRP3 inflammasomes, including the protein expressions of NLRP3 (Figure 5F), ASC (Figure 5G), procaspase-1 (Figure 5H) and mature IL-1β (Figure 5I). Taken together, above results indicated that SXBX pill inhibits Hcy-induced NLRP3 inflammasomes activation via suppressing ERK1/2 and p38 MAPK pathway.
Figure 5.
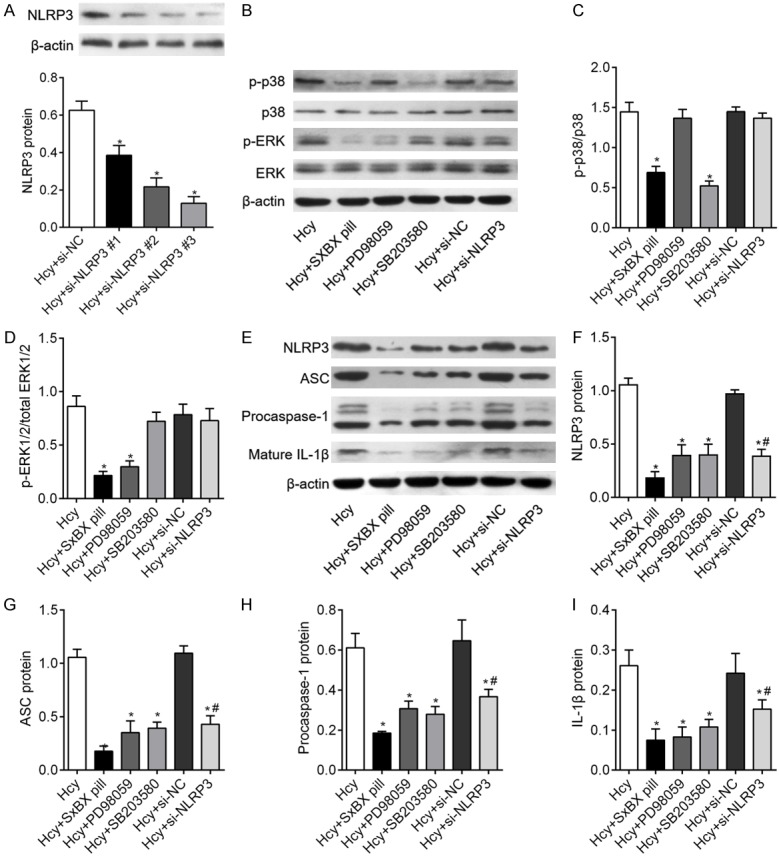
Activation of ERK and p38 mitogen-activated protein kinase (MAPK) is required for Hcy-mediated activation of NLRP3 inflammasomes. (A) Specific siRNAs target NLRP3 were used to knockdown of NLRP3 and the efficiency was validated by detection the NLRP3 protein expression. (B) VSMCs were treated with 500 μM Hcy for 5 min and western blotting was used to access the effects of ERK1/2 inhibitors PD98059 (20 μmol/L), p38 MAPK SB203580 (10 μmol/L) and NLRP3 silencing on Hcy-induced ERK (C) and p38 (D) MAPKs phosphorylation. (E) The activation of NLRP3 inflammasomes was investigated in Hcy-treated VSMCs pretreatment with PD98059, SB203580 and si-NLRP3 for 24 h and relative expression of NLRP3 (F), ASC (G), procasepase-1 (H) and IL-1β (I) were quantified from three independent experiments. *P<0.05 vs. control group; #P<0.05 vs. Hcy group; *P<0.05 vs. control group; %P<0.05 vs. Hcy + si-NC group.
Knockdown of NLRP3 reverse the inhibitory effects of SXBX pill in VSMCs
Using Edu assay and wound healing assay, we found that knockdown of NLRP3 markedly attenuated Hcy-promoted cell proliferation and migration in VSMCs (Figure 6A-D). As expected, inhibition of ERK1/2 and p38 MAPK pathway also inhibited Hcy-induced cell proliferation and migration (Figure 6A-D). Then, we found that NLRP3 gene deletion significantly inhibited the Hcy-induced downregulation of contractile proteins α-SMA and SM22α, and upregulation of synthetic protein OPN in VSMCs. And ERK1/2 and p38 MAPK pathway inhibition achieved similar results to NLRP3 silencing in terms of VMSC dedifferentiation (Figure 6E-H). These results confirm that NLRP3 plays an important role in regulating VSMC dedifferentiation.
Figure 6.
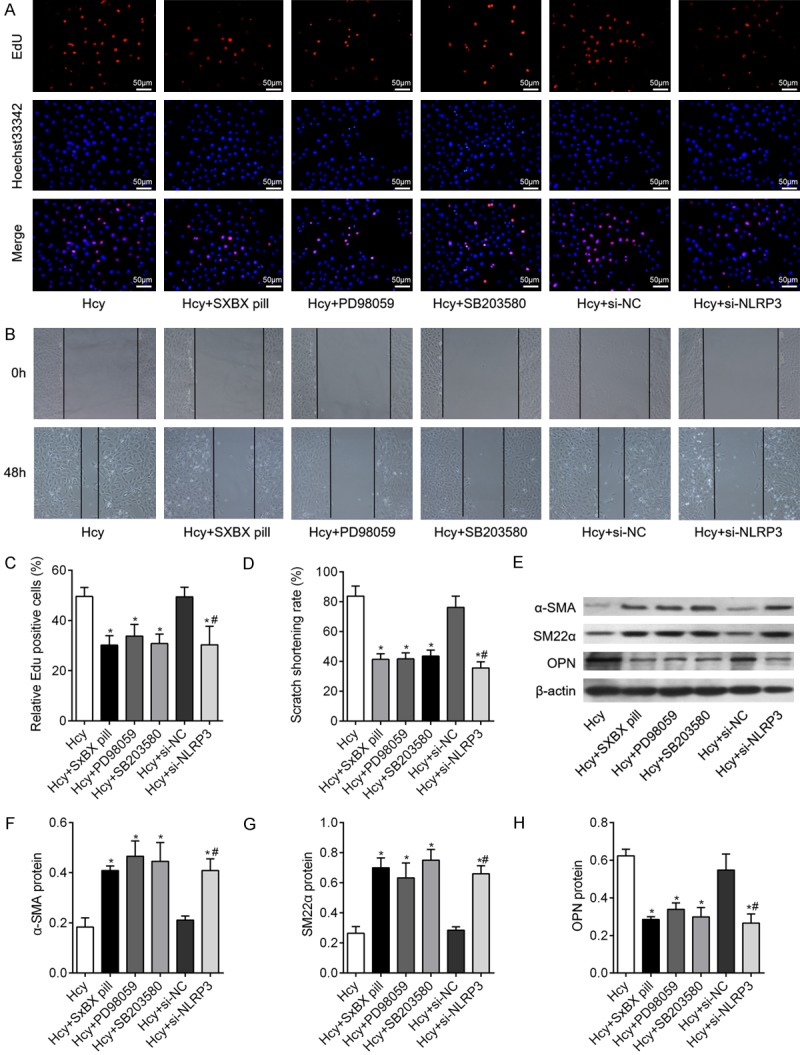
NLRP3 silencing inhibits proliferation, migration and dedifferentiation of VSMCs. (A) Edu incorporation assay was used to determine the proliferation of VSMCs in different group, as indicated. Bar = 50 μm. (B) wound healing assay was performed to investigate the migration of VSMCs under different simulation. Bar graphs showed the quantification of (C) Edu positive rates and (D) scratch shorting rate of VSMCs in different group. (E, F) The markers of VSMCs dedifferentiation, including α-SMA, SM22α and OPN were measured by western blot in VSMCs following different treatment, as indicated. Relative expression of α-SMA (F), SM22α (G), and OPN (H) were quantified from three independent experiments. *P<0.05 vs. Hcy group; #P<0.05 vs. Hcy + si-NC group.
Discussion
The present study originally identified that (1) Hcy stimulation activated NLRP3 inflammasomes in VSMCs through regulation of ERK1/2 and p38 MAPK pathways; (2) SXBX pill inhibited proliferation, migration and differentiated VSMC phenotype concurrent with the suppression of NLRP3 inflammasomes via inactivating ERK1/2 and p38 MAPK pathways, highlighting a critical role for NLRP3 in the dedifferentiation of VSMCs.
Hcy is a non-protein-coding a-amino acid that is synthesized from dietary protein-derived methionine [20]. High-fat diet (HFD) containing methionine-mediated HHcy can cause and accelerate atherosclerotic lesion in mice [2,21] and HHcy is an effective inducer for VSMCs phenotypic change [22]. Therefore, in the present study, HFD was introduced to produce the dedifferentiation of VSMCs in vivo. The dedifferentiation of VSMCs is required for vascular formation and maturation during embryogenesis and vascular remodeling [23]. Within mature blood vessels, VSMCs rarely proliferate or migrate. Rather, VSMCs mainly perform contractile functions and express specific contractile markers, including a-SMA and SM22a. They would undergo phenotypic modulation in response to injury, which is characterized by decreased contractile marker expression, increased synthetic marker (such as OPN) and increased proliferation and migration [23,24]. We report that SXBX pill could effectively inhibit HHcy and Hcy mediated VSMCs dedifferentiation in vivo and in vitro, as confirmed by reversing Hcy-induced decreased a-SMA and SM22a and increased OPN expression. In addition, SXBX pill suppressed Hcy-activated VSMCs via inhibiting proliferation and migration. Although SXBX pill had no influence on HFD induced hyperlipidemia in LDLR-/- mice, our data indicated that SXBX pill may be served as an effective agent for inhibiting Hcy-induced dedifferentiation of VSMCs. We therefore further investigate the potential mechanism through which SXBX pill influences the activity of VSMCs.
Considering Hcy triggers vascular inflammation and atherosclerosis, we focused on NLRP3, which has been identified as the cellular machinery responsible for activation of inflammatory processes [25], to investigate whether it was involved in the action of SXBX pill on VSMCs. We showed that Hcy significantly stimulated NLRP3 inflammasomes activation in VSMCs, as confirmed by increased the production of caspase-1 and IL-1β. In support of our results, Wang et al. identified that HHcy-induced NLRP3 inflammasomes activation contributes to recruitment of macrophages in aorta [26]. These data provide the first evidence that SXBX pill could inhibit Hcy-activated NLRP3 inflammasomes in VSMCs.
We then carried out experiments to further explore how SXBX pill suppress NLRP3 inflammasomes. As previously described, MAPKs pathways are key signaling molecules that regulate cellular behavior in response to environmental stresses [27], and ERK1/2 and p38 MAPK pathways were found to be implicated in the proliferation and migration of VSMCs [28]. We also noticed that activation of MAPKs pathways in response to Hcy was confirmed by a variety of studies [29,30]. In this study, we found that SXBX pill treatment significantly inhibited Hcy-induced ERK1/2 and p38 MAPK pathway, but had no influence on Hcy stimulated JNK signaling pathway in VSMCs. Moreover, recent studies suggested that p38 MAPK was identified as a novel regulator of NLRP3 inflammasomes activation in macrophages [31]. On the basis of the above data, we hypothesize that in VSMCs, Hcy may induce phosphorylation of ERK1/2 and p38, leading to further activation of NLRP3 inflammasomes, and caused phenotypic change of VSMCs. Therefore, specific inhibitors were used to determine whether MAPKs signaling in VSMCs would contribute to NLRP3 inflammasomes activation and phenotypic transformation.
As expected, after inhibition of ERK1/2 and p38 signaling pathway using PD98058 and SB203580, our results demonstrate that Hcy-induced activation of NLRP3 inflammasomes is attenuated by ERK1/2 and p38 inhibitor. Consistent with the previous study [10], we confirmed that knockdown of NLRP3 effectively inhibited HHcy-induced NLRP3 inflammasomes activation. Moreover, we showed that inhibition of ERK1/2 and p38 signaling pathway, as well as NLRP3 silencing all suppressed Hcy-induced proliferation, migration and dedifferentiation of VSMCs. These all suggested Hcy may activate NLRP3 inflammasomes through regulation ERK1/2 and p38 signaling pathway. However, some studies also found that knockout of NLRP3 gene inhibited chronic unpredictable mild stress-induced activation of the p38 and JNK MAPK pathway [32]. In addition, Cao et al. identified that Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing NLRP3 inflammasomes activation and MAPK pathway [33]. Similarly, Huang et al. found that corylin attenuated the activation of both MAPK pathway and NLRP3 inflammasomes pathways in lipopolysaccharide-activated murine microglial cells [34]. However, whether MAPK pathway could regulate NLRP3 inflammasomes was not investigated in their studies. We showed that MAPK pathway inhibition caused suppressed NLRP3 inflammasomes activation. Further study should be needed to investigate if there exert some regulators between MAPKs pathway and NLRP3 inflammasomes.
In conclusion, our novel findings indicated that SXBX pill prevented the Hcy-induced NLRP3 inflammasomes activation through the suppression of ERK1/2 and p38 MAPK signaling, thus to inhibit cell proliferation, migration and dedifferentiation. Our data indicated that SXBX pill can exert therapeutic effects in vascular diseases involving in the dedifferentiation of VSMCs.
Acknowledgements
This work was supported by grants from the Plan Project of Zhejiang Provincial Department of Health, China (Grants No. 2016RCA027) and the Science and Technology Plan Project of Shaoxing city (No. 2018C30021).
Disclosure of conflict of interest
None.
References
- 1.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo HY, Xu FK, Lv HT, Liu LB, Ji Z, Zhai XY, Tang WL, Chi JF. Hyperhomocysteinemia independently causes and promotes atherosclerosis in LDL receptor-deficient mice. J Geriatr Cardiol. 2014;11:74–78. doi: 10.3969/j.issn.1671-5411.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y, Shang MS, Dong JZ, Ma CS. Homocysteine in non-valvular atrial fibrillation: Role and clinical implications. Clin Chim Acta. 2017;475:85–90. doi: 10.1016/j.cca.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Familtseva A, Chaturvedi P, Kalani A, Jeremic N, Metreveli N, Kunkel GH, Tyagi SC. Toll-like receptor 4 mutation suppresses hyperhomocysteinemia-induced hypertension. Am J Physiol Cell Physiol. 2016;311:C596–c606. doi: 10.1152/ajpcell.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng L, Liu L, Zhou C, Pan S, Zhai X, Jiang C, Guo Y, Ji Z, Chi J, Peng F, Guo H. Polyphenols and polypeptides in chinese rice wine inhibit homocysteine-induced proliferation and migration of vascular smooth muscle cells. J Cardiovasc Pharmacol. 2016;67:482–490. doi: 10.1097/FJC.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 7.Ma SC, Zhang HP, Jiao Y, Wang YH, Zhang H, Yang XL, Yang AN, Jiang YD. Homocysteine-induced proliferation of vascular smooth muscle cells occurs via PTEN hypermethylation and is mitigated by Resveratrol. Mol Med Rep. 2018;17:5312–5319. doi: 10.3892/mmr.2018.8471. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Yang M, Ju D, Jiang H, Zheng JP, Xu Z, Li L. Disruption of SM22 promotes inflammation after artery injury via nuclear factor kappaB activation. Circ Res. 2010;106:1351–1362. doi: 10.1161/CIRCRESAHA.109.213900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi N, Chen SY. Mechanisms simultaneously regulate smooth muscle proliferation and differentiation. J Biomed Res. 2014;28:40–46. doi: 10.7555/JBR.28.20130130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haneklaus M, O’Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 11.Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T, Rouis M. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, Kang YM, Zhu GQ. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8:e3074. doi: 10.1038/cddis.2017.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banlong HE, Guo H, Yang G, Chen Y. Clinical observation of Shexiangbaoxin pills in treating 72 cases of coronary heart disease angina pectoris. China Modern Medicine. 2009 [Google Scholar]
- 15.Wu DJ, Hong HS, Jiang Q. Effect of shexiang baoxin pill in alleviating myocardial fibrosis in spontaneous hypertensive rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:350–353. [PubMed] [Google Scholar]
- 16.Hong YD, Wu H, Zhao P. Effects of shexiang baoxin pill on carotid atheromatous plaque. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:780–783. [PubMed] [Google Scholar]
- 17.Guo H, Liu L, Shi Y, Sun A, Xu F, Chi J, Huang D. Chinese yellow wine and red wine inhibit matrix metalloproteinase-2 and improve atherosclerotic plaque in LDL receptor knockout mice. Cardiovasc Ther. 2010;28:161–168. doi: 10.1111/j.1755-5922.2009.00132.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo H, Xu F, Sun A, Liu L, Shi Y. Heparin inhibits the production of matrix metalloproteinase-2 and improves atherosclerosis in LDL receptor-deficient mice. Coron Artery Dis. 2010;21:39–45. doi: 10.1097/MCA.0b013e328333f53b. [DOI] [PubMed] [Google Scholar]
- 19.Su J, Liang H, Yao W, Wang N, Zhang S, Yan X, Feng H, Pang W, Wang Y, Wang X, Fu Z, Liu Y, Zhao C, Zhang J, Zhang CY, Zen K, Chen X, Wang Y. MiR-143 and MiR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer. PLoS One. 2014;9:e114420. doi: 10.1371/journal.pone.0114420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai WK, Kan MY. Homocysteine-induced endothelial dysfunction. Ann Nutr Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 21.Dai J, Li W, Chang L, Zhang Z, Tang C, Wang N, Zhu Y, Wang X. Role of redox factor-1 in hyperhomocysteinemia-accelerated atherosclerosis. Free Radic Biol Med. 2006;41:1566–1577. doi: 10.1016/j.freeradbiomed.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Ni T, Zhang J, Meng L, Gao F, Pan S, Luo H, Xu F, Ru G, Chi J, Guo H. Knockdown of Herp alleviates hyperhomocysteinemia mediated atherosclerosis through the inhibition of vascular smooth muscle cell phenotype switching. Int J Cardiol. 2018;269:242–249. doi: 10.1016/j.ijcard.2018.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YN, Xie BD, Sun L, Chen W, Jiang SL, Liu W, Bian F, Tian H, Li RK. Phenotypic switching of vascular smooth muscle cells in the ‘normal region’ of aorta from atherosclerosis patients is regulated by miR-145. J Cell Mol Med. 2016;20:1049–1061. doi: 10.1111/jcmm.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng F, Xing S, Gong Z, Mu W, Xing Q. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm. 2014;2014:507208. doi: 10.1155/2014/507208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Wang Y, Mu N, Lou X, Li W, Chen Y, Fan D, Tan H. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Invest. 2017;97:922–934. doi: 10.1038/labinvest.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisk M, Gajendragadkar PR, Maki-Petaja KM, Wilkinson IB, Cheriyan J. Therapeutic potential of p38 MAP kinase inhibition in the management of cardiovascular disease. Am J Cardiovasc Drugs. 2014;14:155–165. doi: 10.1007/s40256-014-0063-6. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Huang X, Huang K, Gui C, Huang Q, Wei B. Ligustrazine attenuates the platelet-derived growth factor-BB-induced proliferation and migration of vascular smooth muscle cells by interrupting extracellular signal-regulated kinase and P38 mitogen-activated protein kinase pathways. Mol Med Rep. 2015;12:705–711. doi: 10.3892/mmr.2015.3383. [DOI] [PubMed] [Google Scholar]
- 29.Ingram AJ, Krepinsky JC, James L, Austin RC, Tang D, Salapatek AM, Thai K, Scholey JW. Activation of mesangial cell MAPK in response to homocysteine. Kidney Int. 2004;66:733–745. doi: 10.1111/j.1523-1755.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- 30.Poddar R, Paul S. Novel crosstalk between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor-mediated neuronal cell death. J Neurochem. 2013;124:558–570. doi: 10.1111/jnc.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajamaki K, Mayranpaa MI, Risco A, Tuimala J, Nurmi K, Cuenda A, Eklund KK, Oorni K, Kovanen PT. p38delta MAPK: a novel regulator of NLRP3 inflammasome activation with increased expression in coronary atherogenesis. Arterioscler Thromb Vasc Biol. 2016;36:1937–1946. doi: 10.1161/ATVBAHA.115.307312. [DOI] [PubMed] [Google Scholar]
- 32.Su WJ, Zhang Y, Chen Y, Gong H, Lian YJ, Peng W, Liu YZ, Wang YX, You ZL, Feng SJ, Zong Y, Lu GC, Jiang CL. NLRP3 gene knockout blocks NF-kappaB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res. 2017;322:1–8. doi: 10.1016/j.bbr.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin M, Ma X, Zhou K, Qi J, Yu B, Kou J. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 inflammasome activation and the MAPK pathway. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang MY, Tu CE, Wang SC, Hung YL, Su CC, Fang SH, Chen CS, Liu PL, Cheng WC, Huang YW, Li CY. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC Complement Altern Med. 2018;18:221. doi: 10.1186/s12906-018-2287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


