Abstract
Whether programmed cell death 5 (PDCD5) is effective for tumor metastasis remains unclear. In this study, the expression of PDCD5 in 63 osteosarcoma (OS) tissues and two OS cell lines was analyzed. Then the relationship between PDCD5 expression and clinicopathological features of OS was studied. In addition, adhesion, wound healing, Transwell and Matrigel tube formation assays were used to explore the role of PDCD5 in OS cell adhesion, migration, invasion and angiogenesis. Western blotting was used to detect the protein expression of TGF-β1/Smad signaling pathway and epithelial-mesenchymal transition (EMT)-related markers. At the same time, key molecules involved in migration, invasion and EMT in tumor specimens were assessed by immunohistochemistry. The data showed that PDCD5 overexpression significantly attenuated OS cell adhesion, migration, invasion and angiogenesis. Furthermore, PDCD5 knockdown caused an opposite effect on these phenotypes in vitro. PDCD5 inhibited tumor metastasis by attenuating EMT in OS cells. PDCD5 knockdown enhanced the incidence of metastasis and EMT in OS cells. Furthermore, PDCD5 expression was reduced by transforming growth factor-β1 (TGF-β1) in a time-dependent manner, and TGF-β1-induced EMT was induced by PDCD5 knockdown. Inactivation of the TGF-β1/Smad signaling pathway was involved in the anti-tumor function of PDCD5 in OS. Furthermore, tumor progression in OS patients was associated with low expression of PDCD5, indicating a decrease in survival and a poor prognosis. Our results suggest that PDCD5 may attenuate EMT by inhibiting TGF-β1/Smad signaling pathway to inhibit OS metastasis and may be a potential adjuvant genetic therapy for OS.
Keywords: PDCD5, EMT, osteosarcoma, invasion, metastasis, TGF-β1
Introduction
Osteosarcoma is the most common malignant bone tumor, mainly occurring in children and adolescents. Although current treatment strategies include limb salvage surgery and neoadjuvant chemotherapy, the long-term disease-free survival rate of patients with local disease reaches 60%-75% [1]. However, patients with local recurrence and distant metastasis have a 5-year survival rate of only 27% to 55% of current treatments [2]. Moreover, there is no effective treatment available for metastatic patients, causing a poor prognosis, with a 5-year overall survival rate of less than 10% [3]. Therefore, there is an urgent need to elucidate the molecular mechanisms of OS cell metastasis, which will greatly promote the development of new therapeutic strategies for OS.
Tumor cell metastasis is a very complex process that is divided into several stages, including adhesion, migration, invasion, angiogenesis, and eventually spread in other parts of the body [4-6]. In this malignant process, tumor cells overexpress mesenchymal markers, including vimentin, N-cadherin, and fibronectin. At the same time, they lose expressions of epithelial markers, including E-cadherin and alpha-catenin, which result in epithelial-mesenchymal transition (EMT) and subsequent local/distant metastases. Some cytokines and growth factors such as TGF-β1, hepatocyte growth factor (HGF), epidermal growth factor (EGF), etc. can stimulate the development and progression of EMT. TGF-β1 is one of the strongest stimuli and is overexpressed in many types of human tumors, including OS [7]. However, the latent mechanism of the TGF-β1 signaling pathway in EMT has not been explored to a large extent, although EMT is thought to be involved in the metastasis process of OS [8,9].
The human PDCD5 gene is located on chromosome 19q12-q13 and consists of 6 exons and 5 introns. It was first used in 1999 as an up-regulated gene [10]. In the past decade, research on PDCD5 has made great progress, indicating the importance of PDCD5 in evolution and differentiation. It has been reported that PDCD5 expression is down-regulated in various tumor tissues, and up-regulation of PDCD5 can make different tumors significantly sensitive to chemotherapy. In addition, PDCD5 can interact with different molecules and play an important role in a variety of signaling pathways [11-13].
Our previous work has shown that PDCD5 was poorly expressed in OS and could inhibit the proliferation of OS cells, thereby promoting the sensitivity of chemotherapy to OS [14]. However, whether PDCD5 is involved in the invasion and metastasis of OS cells, and the related regulatory mechanisms remain unclear. Therefore, we examined the expression of PDCD5 in OS specimens and analyzed the correlation between its expression level and survival rate of OS patients. In addition, the negative impact of PDCD5 on invasion and migration was studied. Furthermore, since EMT plays an important role in the process associated with OS progression, the mechanism of action of PDCD5 on TGF-β1-induced EMT processes in OS cells was investigated.
Methods
Patients, specimens, and cell lines
The study was conducted in accordance with ethical standards and in accordance with the Helsinki Declaration and national and international guidelines, and was approved by the Ethics Committee of Peking University People’s Hospital. Informed consent was signed by each patient. From 2005 to 2010, 63 patients with OS tissue specimens were enrolled in the musculoskeletal tumor center of Peking University People’s Hospital for patients who did not require prior adjuvant chemotherapy or radiotherapy. All tissue specimens were clinically diagnosed by orthopedic oncologists and pathologists, routinely fixed with 10% formalin, and embedded in paraffin for immunohistochemical staining.
The human osteosarcoma cell line Saos-2 was obtained from Memorial Sloan-Kettering Cancer Center. Human OS cell line U2OS was kindly provided by Dr. Yi Guo (University of California, Irvine, USA), Human umbilical vascular endothelial cells, Human breast cancer cell line MCF-7 and human hepatocarcinoma cell line HepG2 were obtained from the ATCC (American Type Culture Collection, Manassas, VA, USA). All the cells were routinely maintained in RPMI medium 1640 containing 10% heat-inactivated fetal calf serum in 37°C humidified incubator with a mixture of 95% air and 5% CO2, and fed every 3-4 days with complete medium. Subcultrue was performed once the cells grew to confluence.
Immunohistochemistry (IHC)
IHC was carried out as previously described [15]. Antibodies to PDCD5 (ab75430), phosphorylate (p)-Smad2 (ab53100), p-Smad3 (ab51177) and TGF-β1 (ab25121) were purchased from Abcam (Cambridge, MA). Two independent orthopaedic tumor pathologists (Peng Changliang and Lu Xinchang) blindly rated all parts and randomly selected 10 high power fields for each slide. The expression levels of each protein marker in tumor cells were then independently assessed. The percentage of positively stained cells was rated to 0 to 3: less than 5% of positively stained cells were grade 0, 5 to 25% were grade 1, 26 to 50% were grade 2, and more than 50% were grade 3. In addition, Stain intensity 0 to 2 was also graded: the weak intensity was 0, weak to medium intensity was 1, and medium to strong intensity was 2. Finally, we multiplied the percentage score of the intensity score. And the final score between 0 and 2 was defined as low expression, and the score higher than 2 was defined as high expression.
RNA extraction and real-time RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA using the M-MLV Reverse Transcriptase Kit (Promega, USA) according to the manufacturer’s instructions. PCR primers for PDCD5 and β-actin were as follows: PDCD5 forward: 5’-GTGATGCGGCCCAACAG-3’; reverse: 5’-ATCCAGAACTTGGGCTAAGATACTG-3’; and β-actin, forward: 5’-AGCGGGAAATCGTGCGTG-3’; To: 5’-CAGGGTACATGGTGGTGCC-3’ [14]. Real-time RT-PCR was then performed on an ABI PRISM 7300 Sequence Detection System (Applied Biosystems, Foster City, California, USA) using the SYBR Green Real-Time PCR Assay Kit (TAKARA, Otsu, Japan). The level of β-actin was used as an internal control. The expression fold change in expression was calculated using the 2-ΔΔCt method.
Vector construction and lentivirus infection
The lentiviral vector (LV-PDCD5) containing the PDCD5 DNA sequence, the lentiviral vector (LV-shPDCD5) containing the PDCD5 siRNA sequence, and the null scrambled shRNA (LV-Control) were constructed by Lanmeng (Langfang, China). The construct containing the PDCD5 and PDCD5 shRNA sequences was cloned into the pGCSIL-GFP (green fluorescent protein) vector (Lanmeng). Four target sequences were synthesized as follows: siPDCD5-1: 5’-TGGACTCTGATGAAGATGA-3’; siPDCD5-2: 5’-GGTATCAGAACAAGGTTTA-3’; siPDCD5-3: 5’-GTAATGGACTCTGATGAAG-3’. Then, Lentiviral production and infection were performed as previously reported [16]. Stable cell lines expressing PDCD5 or PDCD5 shRNA were selected for 15 days with 0.5 μg/ml puromycin. The expression of PDCD5 was detected and the strongest PDCD5 inhibitor was selected for the sequent research.
Western blot analysis
Standard Western blot analysis was performed as previously described [14]. Antibodies against PDCD5 (ab75430), vimentin (ab92547), E-cadherin (ab76055), TGF-β1 (ab25121) and VEGF (vascular endothelial growth factor, ab46154) were purchased from Abcam (Cambridge, MA). The following antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA): Smad2/3 (#3102), p-Smad2 (#3108), p-Smad3 (#9520), MMP-2 (matrix metalloproteinase-2, #4022) and MMP-9 (#3852). The Snail (sc-113766), Slug (sc-111905) and GAPDH (sc-47724) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Endothelial adhesion assay
Endothelial adhesion assays were performed as previously described [17]. First, HUVECs were harvested and washed twice with RPMI medium 1640 containing 10% heat-inactivated fetal calf serum and resuspended in the same medium at 2.5 × 105 cells/ml. 7.5 × 105 HUVECs were plated in 6-well plates. After incubation at 37 °C for 20 min, 3 × 104 tumor cells transfected with LV-PDCD5, LV-shPDCD5 or LV-Control were then delivered into the wells preincubated HUVEC cells for 30 min at 37°C. The wells were gently washed twice with PBS to remove non-adherent cells. For quantification, at least six random images are taken. Then, when the tumor cells adhered to the endothelial monolayer, we quantified and photographed using a fluorescence microscope system.
Wound healing assay
The migration ability of OS cells was measured by a wound healing assay. First, cells were seeded in a six-well plate at a cell density of 6 × 104/ml. After 24-hour culture, the cells were infected with LV-PDCD5, LV-shPDCD5 or LV-Control, and cultured until confluence for subsequent experiments. The wound was made by scratching the tip of a plastic pipette. The plate was washed twice with phosphate buffered saline (PBS) and then we removed cell debris and isolated cells. The cells were then incubated with the complete growth medium. When the cells migrated to the injured blank area, photographs were taken at 0 and 24 hours after the wound, respectively.
Cell migration and invasion assays
Cell migration assays were performed in a Transwell chamber with 8.0 um pore membrane as previously described [15]. Briefly, after 72 hours of infection with LV-PDCD5, LV-shPDCD5 or LV-Control, cells were harvested and resuspended in serum-free medium. The cells having a density of 5 × 104 cells/ml (200 μl) were then added to the upper chamber. 600 μl of medium containing 10% FBS was added to the lower chamber. The chamber was incubated at 37°C for 3 hours in a humidified atmosphere containing 5% CO2/95% air. Thereafter, the cells on the bottom surface were fixed in methanol and stained with 0.1% crystal violet, and countered in 5 randomly selected microscope fields per well, and the non-migrating cells on the upper surface were gently scraped off.
For the invasion assay, the same procedure as the migration assay described above was performed except that the upper chamber membrane was coated with 100 ul 200 μg/ml Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for 60 minutes at room temperature before the experiment.
Matrigel tube formation assay
The procedure was carried out as described previously [15]. Briefly, cells were seeded in a 60 mm plate containing fresh 1% serum medium for 24 hours, and 2 ml of conditioned medium was harvested. Meanwhile, a 96-well plate was precoated with 50 μl of Matrigel (10 mg/ml; BD Biosciences) per well, and incubated at 37°C for 1 hour. Then, 5 × 104 HUVECs were suspended in conditioned medium of 100 μl of OS cells, and then gently added to the precoated 96-well plate. The plates were then incubated for 8 hours at 37°C and tube formation was assessed using a digital microscope system.
Statistical analysis
The GraphPad Prism software was used to execute experimental data. Kaplan-Meier analysis was used to assess the relationship between PDCD5 and patient survival. The relationship between PDCD5 and clinicopathological variables was analyzed using chi-square test. Log-rank test and χ2 were used to assess differences, Fisher’s exact probability and Student’s t-test were used for comparison between groups. Data were expressed as mean ± SD. P<0.05 was considered statistically significant.
Results
PDCD5 low expression correlated with progression and poor prognosis of OS patients
Table 1 shows the clinical and pathological features of 63 OS patients enrolled in this study. The PDCD5 expression of the OS samples was examined by immunohistochemistry. The definition criteria for the representative PDCD5 staining pattern in OS tissues are shown in Figure 1A. PDCD5 expression was stronger in 23 (36.51%) samples and weaker in the other 40 samples (63.49%). In subsequent studies, patients were therefore divided into PDCD5-low (weak expression; n=40) and PDCD5-high (strong expression; n=23) groups. Correspondingly, PDCD5 gene expression was significantly down-regulated in the two tested OS cell lines, human hepatoma cell line (HepG2) and human breast cancer cell line (MCF-7) (Figure 1B), which is consistent with previous reports [18,19].
Table 1.
Association of PDCD5 expression with the clinicopathological characteristics of OS
| Variables | Cases | Expression of PDCD5 | P-values | |
|---|---|---|---|---|
|
| ||||
| Low expression | High expression | |||
| Gender | 0.980 | |||
| Male | 33 | 21 | 12 | |
| Female | 30 | 19 | 11 | |
| Age | 0.384 | |||
| ≤20 | 40 | 27 | 13 | |
| >20 | 23 | 13 | 10 | |
| Tumor location | 0.797 | |||
| Femur | 26 | 15 | 11 | |
| Tibia | 19 | 12 | 7 | |
| Humerus | 10 | 7 | 3 | |
| Others | 8 | 6 | 2 | |
| Histological types | 0.984 | |||
| Osteoblastic | 33 | 21 | 12 | |
| Chondroblastic | 17 | 11 | 6 | |
| Others | 13 | 8 | 5 | |
| Enneking stage | 0.003 | |||
| I+II | 43 | 22 | 21 | |
| III | 20 | 18 | 2 | |
| Distant metastasis | 0.000 | |||
| No | 40 | 17 | 23 | |
| Yes | 23 | 23 | 0 | |
Figure 1.
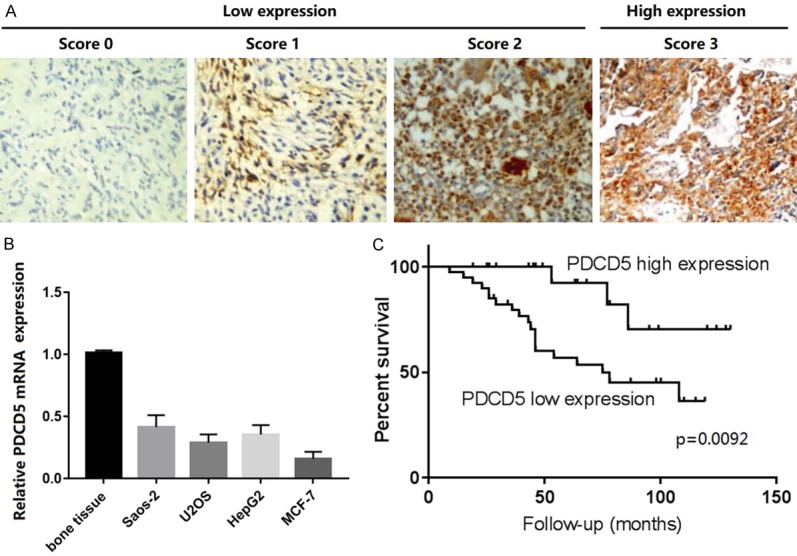
Strong PDCD5 expression in OS correlates with better patient overall survival. A. Scores indicating PDCD5 levels in representative OS tissues. Scale bar =100 μm. B. PDCD5 expression was determined by real-time RT-PCR on total RNA extracted from human Saos-2 and U2OS osteosarcoma cell lines, HepG2 hepatocarcinoma cell line, MCF-7 breast cancer cell line and normal bone tissues. C. Kaplan-Meier survival analysis of overall survival in all patients. The curves of overall survival according to PDCD5 expression level in 63 patients with osteosarcoma.
To assess the relationship between PDCD5 expression and OS prognosis, the correlation between high PDCD5 expression and OS clinicopathological features was analyzed. The results showed that PDCD5 expression was significantly associated with Enneking stage (P=0.003) and distant metastasis (P=0.000), whereas there was no significant correlation between PDCD5 expression and age (P=0.384), OS patients’ gender (P=0.980), tumor location (P=0.797), and histological type (P=0.984) (Table 1).
The comparison of the prognostic impact of PDCD5 on overall survival in OS patients with high PDCD5 protein and low PDCD5 protein expression indicated that patients with high PDCD5 levels had significantly higher 10-years survival rate than those with low PDCD5 levels according to the Kaplan-Meier curve evaluation (P=0.0092, log-rank test; Figure 1C). These results indicated that PDCD5 down-regulation was significantly associated with progression, metastasis, and adverse outcomes in OS patients.
Transfection of siPDCD5-1 reduced PDCD5 expression at protein level
Non-silencing siRNA control and the four candidate siRNAs (siPDCD5-1, -2, and -3) targeting PDCD5 were transfected into osteosarcoma cells. At 24 hours after transfection, the levels of PDCD5 protein in Saos-2 and U2OS cells transfected with siPDCD5-1 decreased significantly, as assessed by Western blotting (Figure 2). In contrast, siPDCD5-2, siPDCD5-3 and non-silent siRNA had no such effect. Therefore, siPDCD5-1 strongly and specifically inhibited the expression of PDCD5 protein in osteosarcoma cell lines.
Figure 2.
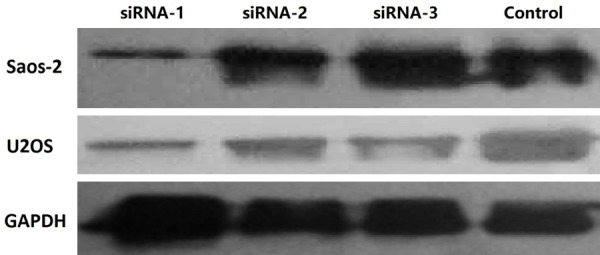
Transfection of siPDCD5-1 inhibits PDCD5 expression at protein level. Western blotting assessment on the effects of siPDCD5 on endogenous PDCD5 protein expression. Saos-2 and U2OS were transfected with non-silencing siRNA or siPDCD5s. At 24 hours post transfection, endogenous PDCD5 protein expression was measured by Western blotting. Significant decreased expression of endogenous PDCD5 was observed in siPDCD5-1 transfected cells, whereas in non-silencing siRNA transfected cells, endogenous PDCD5 shows no decrease. GAPDH serves as the internal control.
PDCD5 inhibited OS cell adhesion, metastasis and invasion in vitro
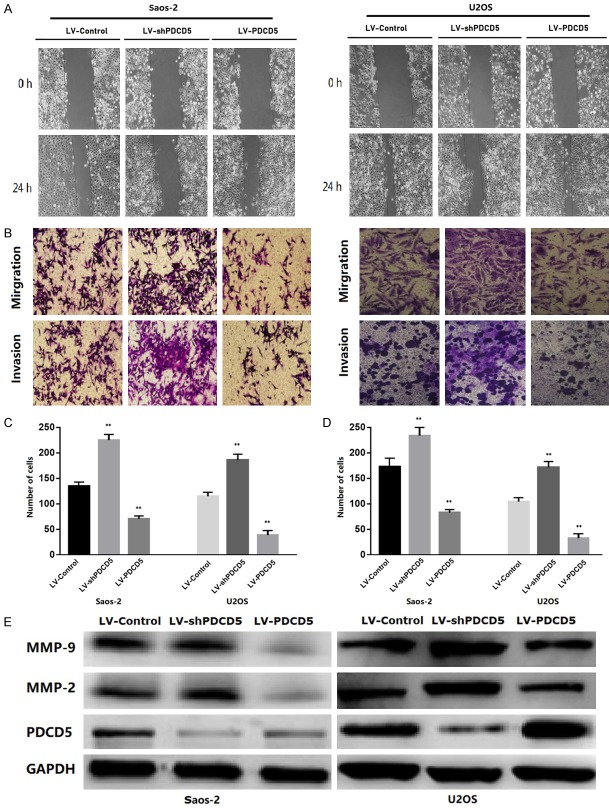
To examine whether PDCD5 affects OS cell adhesion, metastasis and invasion, lentiviral PDCD5 (LV-PDCD5) and PDCD5 shRNA (LV-shPDCD5) vectors were constructed. The in vitro migration and invasion ability of the transduced cells was then measured. Cell adhesion assays showed a significant decrease in cell adhesion capacity of LV-PDCD5 lentiviral cells compared to control lentiviral cells (Figure 3). In contrast, cells infected with LV-shPDCD5 showed enhanced adhesion (P<0.01) (Figure 3).
Figure 3.
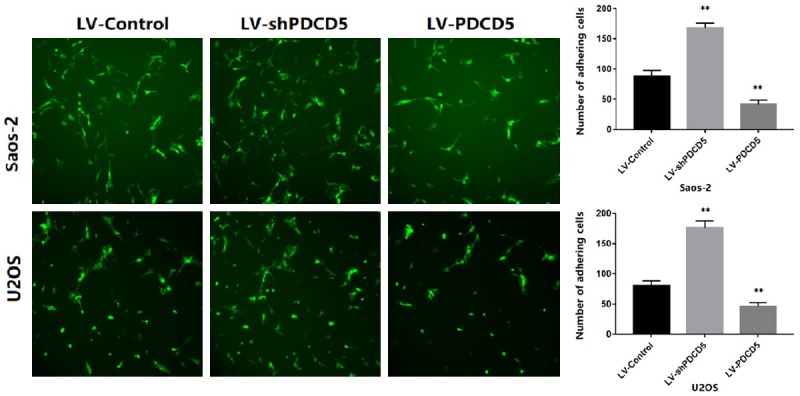
PDCD5 inhibits the OS cell adhesion. Representative images of GFP transfected tumor cells adhering to the endothelial monolayer (magnification × 100). The data (mean ± SD) are statistically significant of three separated experiments, P<0.01.
Wound healing and transwell migration assays were used to further analyze the effect of PDCD5 on OS cell migration, indicating that PDCD5 overexpressing OS cells exhibited significantly reduced cell migration (Figure 4A-D). In contrast, PDCD5 inhibition resulted in a significant increase in migration relative to control cells (Figure 4A-D). The effect of PDCD5 on tumor cell invasion in vitro was further examined by Matrigel-coated Transwell chamber. Corresponding to the results of the migration assay, PDCD5 overexpression inhibited cell invasion (Figure 4B-D), while PDCD5 inhibition was enhanced (Figure 4B-D).
Figure 4.
PDCD5 was associated with the invasive and metastatic potential of OS. A. Representative micrographs of the motility of PDCD5-overexpressing or PDCD5-silencing cells in the wound healing assay at 0 h and 24 h compared to vector control cells. B-D. Representative micrographs and quantification of the invasiveness of PDCD5-overexpressing or PDCD5-silencing cells in the transwell migration and invasion assays compared to vector control cells. E. PDCD5 decreased the expression of MMP-2 and MMP-9 in OS cells. Error bars represent mean ± SD from three independent experiments, **P<0.01.
Furthermore, in LV-PDCD5 lentiviral cells, the expression of MMP-2 and MMP-9 was significantly reduced in tumor invasion and migration. In contrast, when OS cells were infected with LV-shPDCD5, the results were reversed (Figure 4E). These results indicated that PDCD5 could inhibit adhesion, metastasis and invasion of OS cells.
PDCD5 inhibited HUVEC tube formation and expression of VEGF in OS cells
HUVECs were cultured on Matrigel-coated plates in conditioned medium to investigate the effect of PDCD5 on neovascularization. After 8 hours of incubation, cells infected with LV-PDCD5 lentivirus showed significantly reduced HUVEC tube formation relative to control lentiviral cells (P<0.01) (Figure 5). However, cells infected with LV-shPDCD5 showed the opposite effect (Figure 5). VEGF was the most potent angiogenic factor in tumor angiogenesis [20]. VEGF protein level assay showed a significant increase in VEGF expression in LV-shPDCD5 lentiviral cells, whereas VEGF expression was significantly reduced in LV-PDCD5 lentiviral cells (Figure 5). Therefore, it can be concluded that PDCD5 can significantly inhibit angiogenesis of OS cells.
Figure 5.
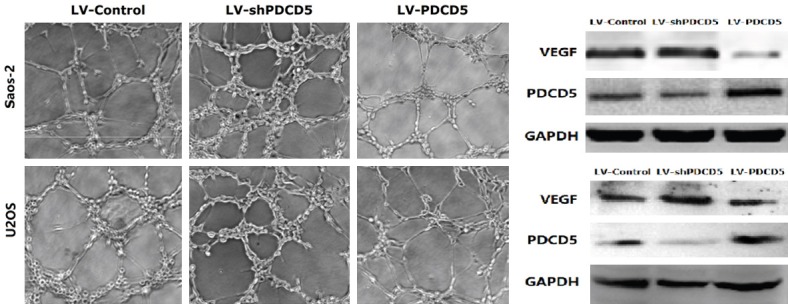
PDCD5 inhibits HUVECs tube formation and downregulates the expression of VEGF of OS cells. Representative phase-contrast images of the tubes that formed (magnification × 100). PDCD5 suppressed the expression of VEGF protein.
PDCD5 reduced EMT of OS cells in vitro
EMT enables the tumor cells to gain invasive properties and metastatic growth characteristics. During the process of EMT, the tumor cells would mislay the expression of cellular adhesion proteins and gain expression of mesenchymal markers [21,29]. To determine if PDCD5 reduced EMT, the expression of EMT markers was assessed. The results showed that PDCD5 overexpression increased the level of epithelial markers (E-cadherin) in both OS cell lines and decreased the level of mesenchymal markers (vimentin) (Figure 6A). In contrast, silencing PDCD5 reduced the level of epithelial markers and increased the level of mesenchymal markers (Figure 6A). Similar results in real-time RT-PCR analysis of these genes was obtained (Figure 6B). Together, these findings indicate that PDCD5 can reduce EMT in OS cells.
Figure 6.
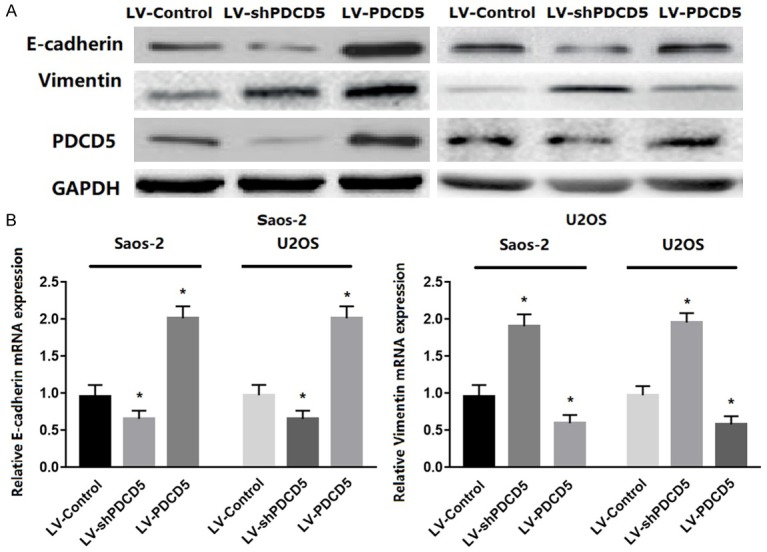
PDCD5 regulated EMT in vitro. A. Western blot analysis of E-cadherin and vimentin proteins in PDCD5-overexpressing or PDCD5-silencing cells. B. Representative micrographs of E-cadherin and vimentin mRNA expressions in PDCD5-overexpressing or PDCD5-silencing cells as determined by real-time RT-PCR. Bars represent the mean ± SD of three independent experiments, *P<0.01.
PDCD5 was involved in TGF-β1 reduced EMT
For the most potent EMT inducer TGF-β, the effect of PDCD5 on EMT of OS cells was evaluated. TGF-β1 reduced PDCD5 expression in a time-dependent manner. High expression of mesenchymal marker vimentin and low expression of E-cadherin were accompanied by this decrease (Figure 7A). To further investigate the effect of PDCD5 on TGF-β1 reduced EMT and cell invasiveness, LV-shPDCD5 and LV-controls were transfected into Saos-2 and U2OS cells. The results showed that the expression of the epithelial marker was decreased and the expression of the mesenchymal marker was increased after 48 hours of LV-shPDCD5 infection with TGF-β1 (Figure 7B). Furthermore, silencing PDCD5 increased the invasive ability of tumor cells according to the above changes in EMT (Figure 7C). These results indicate that PDCD5 plays an important role in EMT in TGF-β1 reduction in OS.
Figure 7.
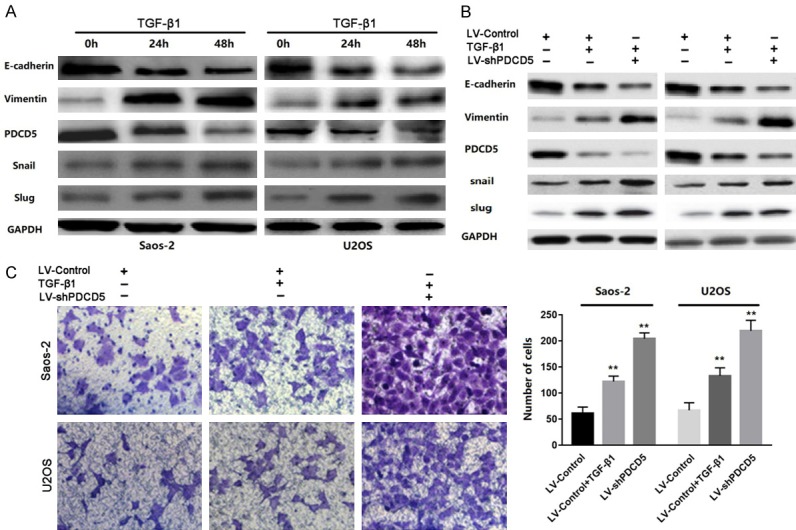
PDCD5 participates in TGF-β1-induced EMT. A. Western blot of PDCD5, vimentin, E-cadherin, Snail and slug in the indicated cells in response to treatment with 10 ng/mL TGF-β1 for 0, 24, and 48 h. B. Twenty-four hours post-transfection of LV-Control or LV-shPDCD5 lentivirus, the cells were treated with TGF-β1 (2 ng/ml) for an additional 48 h. The expressions of PDCD5, E-cadherin, vimentin, Snail and slug were detected by Western blot. C. Representative images and data of a transwell invasion assay for Saos-2 and U2OS cells. TGF-β1 stimulation significantly increased the invasiveness of both tumor cell lines compared with that in the absence of TGF-β1 (LV-Control), and LV-shPDCD5 lentivirus cells enhanced the effect of TGF-β1. Each bar represents the mean ± SD, **P<0.01. All images are representative of three independent experiments with similar findings.
PDCD5 inactivated TGF-β1 signaling pathway
TGF-β1/Smad signaling plays a key role in the invasion and migration of many cancer cells. Therefore, the TGF-β1/Smad signaling pathway was examined to investigate the mechanism by which PDCD5 inhibits OS cell migration and invasion. As shown in Figure 7, expression of TGF-β1 was significantly reduced in PDCD5 overexpressing cells and increased in PDCD5 silencing cells. We all know that activated TGF-β1 can induce phosphorylation of downstream targets Smad2 and Smad3. When Smad4 forms a heterooligomer complex with these phosphorylated Smads and translocates to the nucleus to regulate mRNA transcription, thereby modulating the biological effects of TGF-β1, such as cell growth, invasion and migration [22]. As expected, PDCD5 overexpression was significantly reduced, but silencing PDCD5 induced phosphorylation of smad2 and smad3 (Figure 8), suggesting that PDCD5 contributes to the regulation of the TGF-β1/Smad signaling pathway. Our data indicate that PDCD5 can inhibit the invasion of OS cells through the inactivation of the TGF-β1/Smad signaling pathway.
Figure 8.
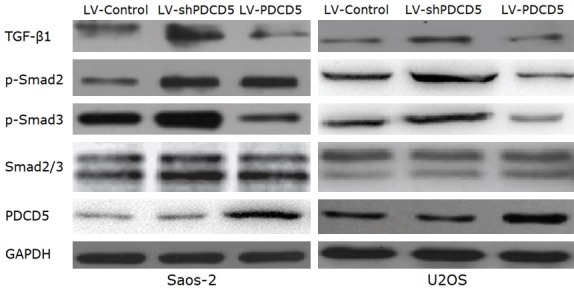
PDCD5 deactivates the TGF-β1/Smad signaling pathway. Western blot analysis of TGF-β1, p-Smad2, p-Smad3, and total Smad2/3 in PDCD5-overexpressing or PDCD5-silencing cells.
Discussion
Our previous study reported that programmed cell death 5 (PDCD5) acted as a tumor suppressor gene by inhibiting cell proliferation and inducing apoptosis in osteosarcoma (OS) cells [14]. Here, we revealed a significant association between PDCD5 expression in depression and Enneking stage, distant metastasis. Similar to endometrioid endometrial cancer and chondrosarcoma [23,24], patients with lower PDCD5 expression levels have shorter overall survival than patients with higher PDCD5 expression levels, suggesting that PDCD5 may be an independent prognostic factor for OS patients. More importantly, down-regulation of PDCD5 can increase adhesion, migration and invasion of OS cells by inducing EMT and activating TGF-β1/Smad signaling pathway, and is significantly associated with OS progression.
Depressed PDCD5 expression contributes to enhance aggressive behavior of OS cells, and overexpression of PDCD5 reduces in vitro adhesion, angiogenesis, migration and invasion, while silencing enhances these malignant behaviors. In other words, there is a significant negative correlation between the expression of PDCD5 and the metastasis and invasion of OS. Tumor metastasis is a complex multi-stage process. In this process, tumor cells will express a variety of different properties, including altered adhesion, increased motility, invasiveness, and angiogenic capacity to achieve distant metastasis [25]. At the same time, the degradation of stromal extracellular matrix (ECM) is a critical step in tumor invasion and metastasis. Dozens of studies have shown that MMP-2 and MMP-9 play key roles in tumor invasion and metastasis [26], including OS [27]. In addition, VEGF is the most potent tumor angiogenic factor involved in the invasion and metastasis of tumor cells. It can stimulate the spread of many human cancers. In fact, VEGF expression is frequently upregulated in many tumors, including OS [28]. In our study, we found that expression of MMP-2, MMP-9 and VEGF increased in PDCD5 silenced cells and decreased in PDCD5 overexpressing cells, suggesting that PDCD5 can at least partially reduce metastasis and invasion by modulating expression of MMP-2, MMP-9 and VEGF.
Previous studies have shown that tumor cells acquire invasive ability mainly through EMT, a process in which the tumor cells mislay the expression of cellular adhesion proteins and gain expression of mesenchymal markers, which play a key role in the development and progression, invasion and migration of various human tumors, including OS [29]. Our results suggest that down-regulation of PDCD5 can increase adhesion, migration and invasion, accompanied by increased expression of the mesenchymal marker vimentin and decreased expression of the epithelial marker Ecadherin. Conversely, enhancing PDCD5 has the opposite effect. These results indicate that down-regulation of PDCD5 plays a key role in obtaining cell motility and invasiveness of OS cells by inducing EMT.
TGF-β1 is a multifunctional growth factor that affects many functions of various cellular processes, such as cell differentiation, proliferation, angiogenesis, invasion, and metastasis. Dozens of reports have confirmed that TGF-β1 is up-regulated and over-expressed in a variety of human malignancies including OS [30]. TGF-β1 not only inhibits tumors, but also promotes tumors, depending on the stage of tumor progression [31]. TGF-β1 signaling inhibits tumor proliferation by inducing cell cycle arrest and apoptosis in the early stages of tumorigenesis. At the same time, in the advanced stage of cancer, it promotes tumor invasion and metastasis through EMT [32]. In this present study, we found that TGF-β1-induced EMT in OS cells was associated with significant downregulation of PDCD5 expression. Related changes in OS cells included a significant decrease in E-cadherin expression, increased expression of vimentin, and enhanced invasiveness. These data indicate that PDCD5 acts as an inhibitor of TGF-β1-induced EMT in OS.
In order to better explore the molecular mechanism of PDCD5 down-regulating OS cell metastasis, the TGF-β/Smad signaling pathway was further studied. TGF-β1 binds to the TGF-β1 type I receptor, which is a transmembrane protein with a Ser/Thr kinase domain, then phosphorylates Smad2 and Smad3, regulates the expression of downstream target genes, and promotes EMT and metastasis of tumor cells [33]. In this study, we found that levels of phosphorylated smad2 and phosphorylated smad3 were reduced in PDCD5 overexpressing cells and increased in PDCD5 silenced cells. Thus, the effect of PDCD5 on reducing EMT and promoting OS cell invasion is at least partially mediated by inactivating TGF-β1/Smad signaling.
In conclusion, PDCD5 was found to be under-expressed in OS cell lines and tissues, which was associated with poor clinical prognosis and OS aggressive progression. In addition, PDCD5 downregulated invasion and metastasis by modulating the TGF-β1/Smad signaling pathway and inhibiting the EMT in OS. Thus, PDCD5 might be helpful in the understanding of the mechanisms of metastasis and invasion in osteosarcoma, and exploring PDCD5 based adjuvant genetic therapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No.81202124 and 81301727) and Beijing Municipal Administration of Hospitals’ Youth Program (QML20160302).
Disclosure of conflict of interest
None.
References
- 1.Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr. 2016;28:26–33. doi: 10.1097/MOP.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18:39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 3.Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16:543–556. doi: 10.1586/14737140.2016.1168697. [DOI] [PubMed] [Google Scholar]
- 4.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 5.Gemoll T, Epping F, Heinrich L, Fritzsche B, Roblick UJ, Szymczak S, Hartwig S, Depping R, Bruch HP, Thorns C, Lehr S, Paech A, Habermann JK. Increased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignancies. Oncotarget. 2015;6:16517–16526. doi: 10.18632/oncotarget.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantel K, Otte M. Occult micrometastasis: enrichment, identification and characterization of single disseminated tumour cells. Semin Cancer Biol. 2001;11:327–337. doi: 10.1006/scbi.2001.0388. [DOI] [PubMed] [Google Scholar]
- 7.Yang RS, Wu CT, Lin KH, Hong RL, Liu TK, Lin KS. Relation between histological intensity of transforming growth factor-beta isoforms in human osteosarcoma and the rate of lung metastasis. Tohoku J Exp Med. 1998;184:133–42. doi: 10.1620/tjem.184.133. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Liang Z, Gao K, Li H, Zhao G, Wang S, Fang J. MicroRNA-128 inhibits EMT of human osteosarcoma cells by directly targeting integrin alpha2. Tumour Biol. 2016;37:7951–7957. doi: 10.1007/s13277-015-4696-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Xue GB. Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem Biophys Res Commun. 2018;495:27–34. doi: 10.1016/j.bbrc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G, Tang J, Ma D. TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun. 1999;254:203–210. doi: 10.1006/bbrc.1998.9893. [DOI] [PubMed] [Google Scholar]
- 11.Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Kallioniemi OP, Wilfond B, Borg A, Trent J, Raffeld M, Yakhini Z, Ben-Dor A, Dougherty E, Kononen J, Bubendorf L, Fehrle W, Pittaluga S, Gruvberger S, Loman N, Johannsson O, Olsson H, Sauter G. Gene-expression profiles in hereditary breast cancer. N Engl J Med. 2001;344:539–548. doi: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 12.Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J, Liu F, Huang QH, Cheng ZH, Li NG, Du JJ, Hu W, Shen KT, Lu G, Fu G, Zhong M, Xu SH, Gu WY, Huang W, Zhao XT, Hu GX, Gu JR, Chen Z, Han ZG. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci U S A. 2001;98:15089–15094. doi: 10.1073/pnas.241522398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YH, Zhao M, Li WM, Lu YY, Chen YY, Kang B, Lu YY. Expression of programmed cell death 5 gene involves in regulation of apoptosis in gastric tumor cells. Apoptosis. 2006;11:993–1001. doi: 10.1007/s10495-006-6714-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Peng C, Ruan G, Zhou J, Li Y, Hai Y. Adenovirus-delivered PDCD5 counteracts adriamycin resistance of osteosarcoma cells through enhancing apoptosis and inhibiting Pgp. Int J Clin Exp Med. 2014;7:5429–5436. [PMC free article] [PubMed] [Google Scholar]
- 15.Peng C, Zhao H, Song Y, Chen W, Wang X, Liu X, Zhang C, Zhao J, Li J, Cheng G, Wu D, Gao C, Wang X. SHCBP1 promotes synovial sarcoma cell metastasis via targeting TGF-beta1/Smad signaling pathway and is associated with poor prognosis. J Exp Clin Cancer Res. 2017;36:141. doi: 10.1186/s13046-017-0616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LN, Wang Y, Ma DL, Chen YY. Short interfering RNA against the PDCD5 attenuates cell apoptosis and caspase-3 activity induced by Bax overexpression. Apoptosis. 2006;11:101–111. doi: 10.1007/s10495-005-3134-y. [DOI] [PubMed] [Google Scholar]
- 17.Ji T, Guo Y, Kim K, McQueen P, Ghaffar S, Christ A, Lin C, Eskander R, Zi X, Hoang BH. Neuropilin-2 expression is inhibited by secreted Wnt antagonists and its down-regulation is associated with reduced tumor growth and metastasis in osteosarcoma. Mol Cancer. 2015;14:86. doi: 10.1186/s12943-015-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu DZ, Cheng Y, He H, Liu HY, Liu YF. The fate of Kruppel-like factor 9-positive hepatic carcinoma cells may be determined by the programmed cell death protein 5. Int J Oncol. 2014;44:153–160. doi: 10.3892/ijo.2013.2147. [DOI] [PubMed] [Google Scholar]
- 19.Jiao J, Chen G, Huang M. [Effect of rhTFAR19 protein on cell cycle and apoptosis of MCF-7 induced by gamma-ray] . Zhonghua Zhong Liu Za Zhi. 2000;22:102–104. [PubMed] [Google Scholar]
- 20.Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G, Fu S, Zhang Y, Feng K, Feng Y. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur J Cancer. 2014;50:2336–2350. doi: 10.1016/j.ejca.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, Mauviel A. TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res. 2011;71:5606–5610. doi: 10.1158/0008-5472.CAN-11-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao M, Gao W, Wang Z, Liu Y, Li Y, Wei C, Sun Y, Guo C, Zhang L, Wei Z, Wang X. The reduced PDCD5 protein is correlated with the degree of tumor differentiation in endometrioid endometrial carcinoma. Springerplus. 2016;5:988. doi: 10.1186/s40064-016-2698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarea M, Mohammadian Bajgiran A, Sedaghati F, Hatami N, Taheriazam A, Yahaghi E, Shakeri M. Diagnostic investigations of DKK-1 and PDCD5 expression levels as independent prognostic markers of human chondrosarcoma. IUBMB Life. 2016;68:597–601. doi: 10.1002/iub.1519. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Li F, Zhao K, Yao J, Cheng Y, Zhao L, Li Z, Lu N, Guo Q. LFG-500 inhibits the invasion of cancer cells via down-regulation of PI3K/AKT/NF-kappaB signaling pathway. PLoS One. 2014;9:e91332. doi: 10.1371/journal.pone.0091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Che YL, Luo SJ, Li G, Cheng M, Gao YM, Li XM, Dai JM, He H, Wang J, Peng HJ, Zhang Y, Li WY, Wang H, Liu B, Linghu H. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett. 2015;359:241–249. doi: 10.1016/j.canlet.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Su Y, Wan D, Song W. Dryofragin inhibits the migration and invasion of human osteosarcoma U2OS cells by suppressing MMP-2/9 and elevating TIMP-1/2 through PI3K/AKT and p38 MAPK signaling pathways. Anticancer Drugs. 2016;27:660–668. doi: 10.1097/CAD.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 28.Cortini M, Avnet S, Baldini N. Mesenchymal stroma: role in osteosarcoma progression. Cancer Lett. 2017;405:90–99. doi: 10.1016/j.canlet.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Yang G, Yuan J, Li K. EMT transcription factors: implication in osteosarcoma. Med Oncol. 2013;30:697. doi: 10.1007/s12032-013-0697-2. [DOI] [PubMed] [Google Scholar]
- 30.Lamora A, Talbot J, Mullard M, Brounais-Le Royer B, Redini F, Verrecchia F. TGF-beta signaling in bone remodeling and osteosarcoma progression. J Clin Med. 2016;5 doi: 10.3390/jcm5110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massague J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YY, Peck K, Chang YL, Pan SH, Cheng YF, Lin JC, Yang RB, Hong TM, Yang PC. SCUBE3 is an endogenous TGF-beta receptor ligand and regulates the epithelial-mesenchymal transition in lung cancer. Oncogene. 2011;30:3682–3693. doi: 10.1038/onc.2011.85. [DOI] [PubMed] [Google Scholar]
- 33.Xu M, He J, Li J, Feng W, Zhou H, Wei H, Zhou M, Lu Y, Peng W, Du F, Gong A. Methyl-CpG-binding domain 3 inhibits epithelial-mesenchymal transition in pancreatic cancer cells via TGF-beta/Smad signalling. Br J Cancer. 2017;116:91–99. doi: 10.1038/bjc.2016.397. [DOI] [PMC free article] [PubMed] [Google Scholar]