Abstract
Transvaginal sonographic elastography can be used for the differentiation of malignant and benign lesions in different systems. Recent studies on strain elastography showed that different endometrial tissues demonstrated different stiffness characteristics. In this study, we aim to evaluate the diagnostic value of transvaginal sonographic elastography to distinguish endometrial cancer from benign masses. A prospective study was conducted on patients with suspected intracavitary uterine focal lesions, abnormal uterine bleeding for routine ultrasound examination of the pelvis. A total of 217 patients were included and underwent both conventional B-mode sonography and elastography. Endometrial lesions were categorized into 3 types for qualitative analysis and the strain ratio (SR) was measured for quantitative analysis. Pathological examinations were also conducted to establish final diagnosis. There were 104 patients diagnosed with endometrial cancer and 113 with benign lesions. All type 1 endometrial lesions were diagnosed as benign, while 95.8% of type 3 were malignant. For quantitative elastography results, the AUC for SR was 0.904 with 95% CI between 0.866 and 0.942. Using an ROC curve, the best cut-off SR value was found to be 3.02 and it could achieve a sensitivity of 81.7%, a specificity of 85%, a positive predictive value of 83.3% and a negative predictive value of 83.5%. Our results suggested that transvaginal sonographic elastography is a promising diagnostic tool for differentiating endometrial cancer from benign masses, and that SR has excellent diagnostic performance.
Keywords: Elastography, endometrial cancer, gynecology
Introduction
With bleeding being the major symptom, endometrial cancer is one of the most commonly seen gynecologic malignancies and poses increasing threat to postmenopausal women in developed countries [1]. The prognosis of the disease depends largely on patient age, histological type, and the presence and grade of invasion and metastases [2]. While the outcome of endometrial cancer is generally good with an overall 5-year survival rate in about 90%, if the patients are diagnosed at stage I. Those patients with myometrial invasion or cervical stroma invasion are usually with worse prognosis [3]. Thus accurate diagnosis and staging of the disease as well as proper selection of adjuvant therapy for endometrial cancer, are of great importance in reducing the locoregional recurrence rate [4].
Among all the methods used in clinical practice for diagnosis of endometrial pathologies, non-invasive preoperative diagnostic techniques are more favorable than other intra-operative examinations since healthcare resources can be optimized and surgery can be tailored to avoid unnecessary morbidity [5]. Imaging has always played an indispensable role in tumor staging, treatment planning, treatment response assessing and detecting recurrent disease. Due to wide availability, low cost and no radiation, transvaginal ultrasound is the most widely used imaging method to screen women with endometrial carcinoma [6]. However, the detection of lesions largely depends on the skills and clinical experience of the examiner. This makes conventional transvaginal ultrasound particularly prone to interobserver variation [7]. Thus, the application of a novel technique, which could both qualitatively and quantitatively evaluate the tissue characteristics, might enhance the diagnostic accuracy of the disease.
Elastography is an ultrasound technology first introduced in the 1990s and is known for being able to sensitively and non-invasively assess stiffness and the mechanical properties of tissues [8]. The stiffness of the tissue can be estimated either by evaluating the strain in the tissue under stress, or by imaging the shear waves and mechanical waves [9]. By using the quasi-static method elastography, a compression is applied to the tissue to induce a color-coded map of the strain and to visualize the stiffness of the examined areas. With changed elasticity in soft tissues, specific pathological processes can be detected by elastography, enabling the differentiation of solid tumors from normal tissues. Potential clinical applications, like assessing the severity of liver fibrosis and differentiating breast lesions [10], thyroid nodules and prostate abnormalities, are proposed by the guidelines published by the European Federation for Ultrasound in Medicine and Biology [11]. In obstetrics and gynecology, possible uses of elastography include prediction of preterm delivery [12,13] and successful labor induction [14,15]. A real-time elastographical study [16] showed high agreement with diagnosis related to magnetic resonance imaging for fibroids and adenomyosis. Considering that endometrial cancer might cause changes in tissue elasticity, elastography can provide extra insights into differentiating between endometrial malignancies and benign tumors. Thus, the aim of this study is to evaluate the diagnostic performance of elastography in differentiating endometrial cancer from benign masses. When available, the pathological results are used as the gold standard.
Materials and methods
Participants
This cross-sectional study was conducted at the Department of Obstetrics and Gynecology, from November 2015 to September 2018. The study protocol was approved by the local institutional review board and written consent was obtained from the subjects for this prospective study.
Subjects included women with suspected intracavitary uterine focal lesions, abnormal uterine bleeding and/or patients undergoing routine ultrasound examination of the pelvis. They were referred to the current study all subjects had to go through a thorough process which included an examination of their medical history, complete general check up including gynecological examination and preliminary transvaginal ultrasound. Subjects with intrauterine lesions confirmed through B-mode sonography were recruited, but those with uterine fibroids, adenomyosis, uterine anomalies and use of hormonal drugs within the past 3 months were excluded.
Experimental design
On the day of admission to the hospital, all subjects underwent a transvaginal sonographic elastography to assess the intrauterine lesion stiffness. Dilation and curettage (D&C) or hysteroscopy was conducted the following day and the lesions were sent for pathologic examination to confirm the final diagnosis (pathologists were blind to sonographic and elastographic findings).
Elastograms acquisition and analysis
All sonographic and elastographic examinations were performed by the same radiologists (Che and Wei) who have 10 years’ experience in gynecology. A digital sonography scanner (Voluson E8, GE Healthcare, Wisconsin, USA, equipped with a 4.0-9.0 MHz multifrequency transvaginal probe) to perform real-time tissue elastography was used for both B-mode ultrasound and elastography to assess the endometrial thickness and endometrial volume. During the procedure, patients were asked to breathe normally and the elastographic images of the uterus were generated by the breathing movements and arterial pulsation. The elasticity of the regions of interest (ROIs) was assessed by means of color-coded maps, with red as soft, yellow as medium soft, blue as medium hard and dark blue as hard. The percentages of the different colors of the total area were determined and lesions were classified qualitatively according to the dominant elastographic color pattern: type 1, predominantly non-blue; type 2, partly blue and partly non-blue and type 3, predominantly blue. ROIs were adjusted to include maximum homogeneous and thick tissue to avoid the ROI bias. On average, 3 (range 2-5) clips and 4 (range 3-6) static images were obtained for each subject. The strain ratio (SR) was obtained to evaluate the stiffness of the endometrium quantitatively. SR measurements were performed at least 3 times by two independent observers, based on different static images. The average SR was recorded as the final result.
Statistical analysis
For continuous variables, Kolmogorov-Smirnov Test was performed. For comparisons between endometrial cancer and different types of benign lesions, student’s T-test was used. In addition, receiver operating characteristics (ROC) curve was fitted and the area under the ROC curve (AUC) with 95% CI was determined to find the cut-off SR value to differentiate between endometrial cancer and benign masses. Sensitivity, specificity, positive predictive value and negative predictive value were calculated respectively. For all statistical analyses, level of significance was set at P < 0.05. SPSS 22.0 (SPSS, Chicago, IL) was used for all the statistical analyses.
Results
A total number of 217 females (mean age: 47.9 ± 8.3 years) with confirmed pathological results are included in the study (detailed demographics are summarized in Table 1). From 217 subjects, 104 subjects (32 pre-menopausal and 72 post-menopausal) were confirmed to have endometrial cancer (Figure 1), based on pathological findings. Out of the remaining 113 subjects, two types of benign endometrial lesions were found: endometrial polyps (65 subjects with mean age of 46.8 ± 7.3 years) as shown in Figure 2 and endometrial hyperplasia (48 subjects with mean age of 39.2 ± 4.3 years) as shown in Figure 3. Endometrial hyperplasia was confirmed after performing D&C in 31 patients and after hysterectomy in 17 patients. Considering that surgical removal is not recommended for benign lesions like endometrial polyps, all cases were monitored over time and patients were asked to go through routine ultrasound every 6 months.
Table 1.
Patient demographics and histopathological diagnosis (n = 217)
| Characteristics | Benign (n = 113) | Malignant (n = 104) | P value |
|---|---|---|---|
| Age (years) | 43.9 ± 6.1 | 56.2 ± 5.4 | < 0.001** |
| Weight (kg) | 71.2 ± 10.4 | 73.5 ± 9.8 | 0.882 |
| Height (cm) | 159.2 ± 5.3 | 158.1 ± 5.7 | 0.724 |
| Body mass index (kg/m2) | 32.7 ± 1.4 | 34.2 ± 1.8 | 0.01* |
| Final diagnosis; n (%) | |||
| Endometrial cancer | - | 104 (47.9) | - |
| Endometrial hyperplasia | 48 (22.1) | - | - |
| Endometrial polyps | 65 (30.0) | - | - |
| Endometrial thickness (mm) | 7.2 ± 5.1 | 19.8 ± 8.3 | < 0.001** |
| Endometrial volume (cm3) | 3.3 ± 2.6 | 7.4 ± 3.4 | < 0.001** |
| Strain ratio | 2.8 ± 1.3 | 4.5 ± 2.3 | < 0.001** |
p < 0.05;
p < 0.001.
Figure 1.
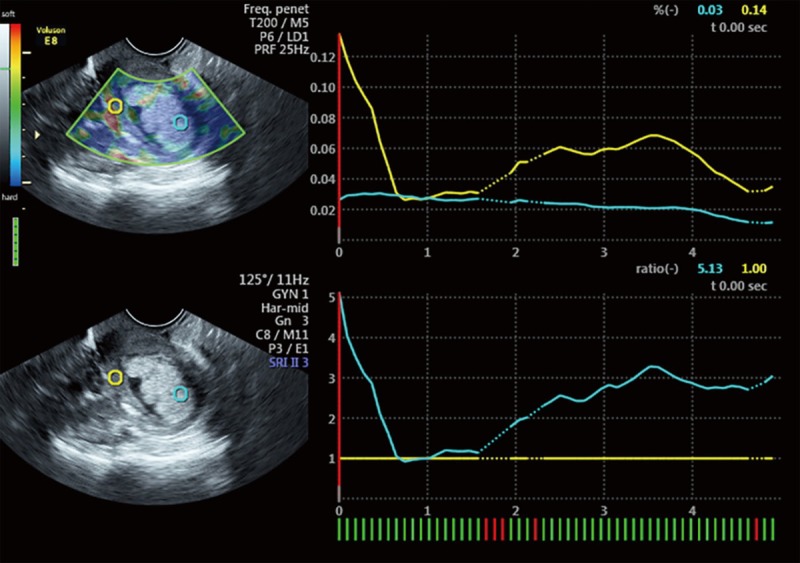
Transvaginal sonographic elastography image of a 52-year-old woman with thickened endometrium and confirmed to be endometrial carcinoma. The right side shows the two images obtained by transvaginal sonographic transducer. The upper shows the elastography mode while the lower shows the routine B-mode sonography. The two circles represent the regions of interest (ROI) which were used for the calculation of strain ratio (SR). Green circle (reference ROI) is placed on the endometrium and the yellow circle on the myometrium. The right lower side shows the line graph obtained from the ROIs and indicates the SR of endometrial tissue versus myometrial tissue. This lesion is predominantly blue thus was qualitatively categorized as type 3 with SR value of 5.13.
Figure 2.
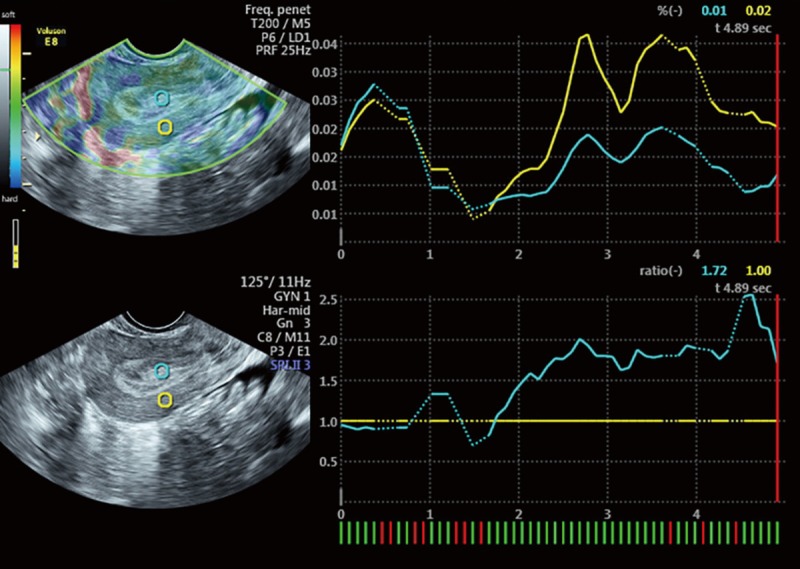
Transvaginal sonographic elastography image of a 45-year-old woman with thickened endometrium and confirmed to be endometrial polyps. This lesion is predominantly non-blue thus was qualitatively categorized as type 1 with SR value of 1.72.
Figure 3.
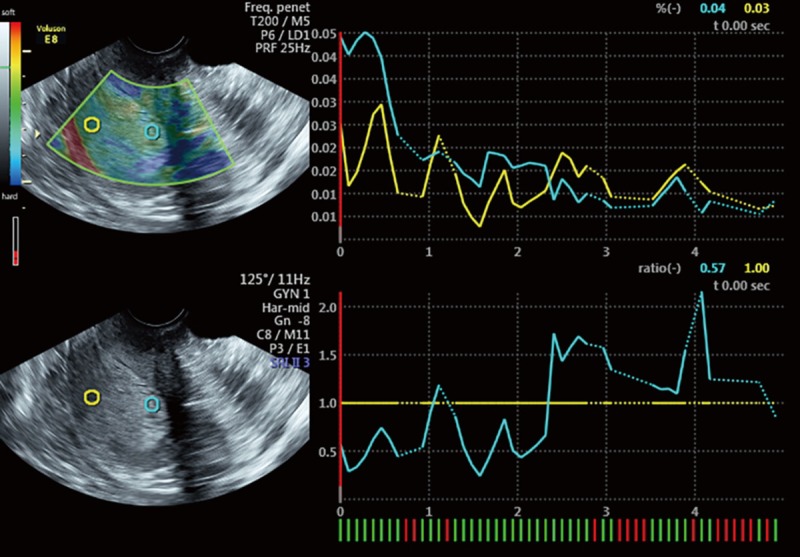
Transvaginal sonographic elastography image of a 37-year-old woman with thickened endometrium and confirmed to be endometrial hyperplasia. This lesion is predominantly non-blue thus is qualitatively categorized as type 1 with SR value of 0.57.
Patients with endometrial cancer were significantly older than those with benign endometrial lesions, with a significantly higher BMI (P = 0.01). Moreover, based on the endometrial thickness and endometrial volume measured by traditional B-mode sonography, patients with endometrial cancer showed a significantly higher value for both measurements.
With interpreting the results, Table 2 shows the distribution of endometrial lesions according to qualitative elastography and final diagnosis. All type 1 endometrial lesions were diagnosed as benign, while 95.8% of type 3 were found to be malignant. Table 2 shows the SR values of benign and malignant lesions, results obtained from a quantitative assessment of elastography. Patients with endometrial cancer had a significantly higher SR value (P < 0.001) than patients with endometrial benign lesions. The ROC curve is shown in Figure 4 and the AUC for SR is 0.904 with 95% CI between 0.866 and 0.942. Using an ROC curve, the best cut-off SR value is 3.02 with a sensitivity of 81.7%, specificity of 85%, positive predictive value of 83.3% and negative predictive value of 83.5%.
Table 2.
Qualitative and quantitative elastography results of endometrial lesions
| Elastographic features | Benign (n = 113) | Malignant (n = 104) | P value |
|---|---|---|---|
| Type 1 | 74 (65.5) | 0 (0) | < 0.001** |
| Type 2 | 35 (31.0) | 13 (12.5) | < 0.001** |
| Type 3 | 4 (3.5) | 91 (87.5) | < 0.001** |
| Strain ratio | 2.2 ± 0.8 | 4.1 ± 1.3 | < 0.001** |
p < 0.001.
Figure 4.
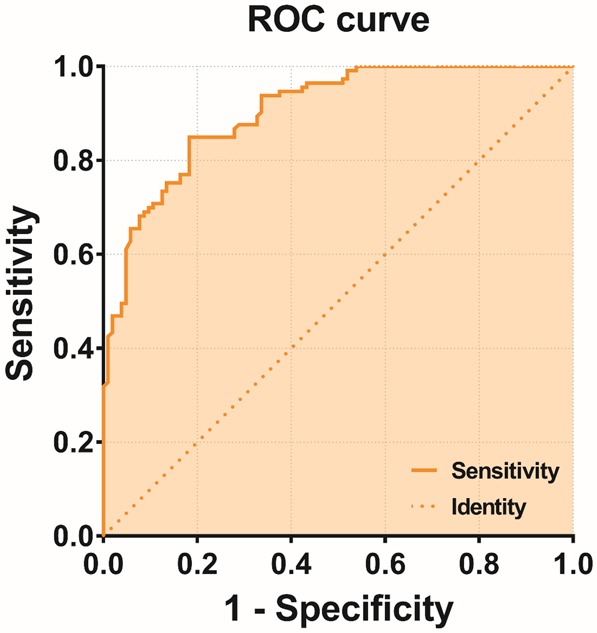
Receiver operating characteristic curve (ROC) for elastography strain ratio in differentiating endometrial cancer from benign endometrial lesions.
Discussion
To the best of our knowledge, this is the first large-population study conducted with subjects of Chinese ethnicity, to evaluate the efficacy of strain elastography for differentiating endometrial cancer from other benign endometrial masses. Our study demonstrated that elastography is a sensitive tool, complementary to traditional B-mode sonography in detecting endometrial malignancies. By using strain elastography, its derived SR value demonstrated excellent performance in differentiating endometrial cancer from benign lesions. Thus, elastography is applicable to clinical practices and can provide extra diagnostic accuracy in daily work.
When endometrial lesions are suspected, D&C and pathological examinations are usually administered by clinicians to secure a definite diagnosis [17,18]. While cytology, biopsy and D&C results are the universal gold standards for establishing accurate diagnosis, these procedures are invasive and sometimes bring serious complications for patients with vaginal or cervical stenosis [19,20]. A significant number of patients do not go through any pathological testing. Thus, there are no treatment plans suggested or treatment received after the medical examination, and this leads to a waste of medical resources. Thankfully, as a safe, accessible and less expensive imaging method, sonography has become the first-line diagnostic tool to identify the cause of clinical gynecological problems and avoid unnecessary procedures.
According to studies [21,22] in the past, ultrasound measurement of endometrial thickness was commonly used to triage patients with abnormal uterine bleeding. In spite of the high sensitivity involved with diagnosing endometrial cancer, measuring endometrial thickness also has a well-defined false-negative rate. For instance, Naftalin [23] reported two cases of endometrial cancer that were found to have a thin, normal endometrium detected after a traditional B-mode ultrasound. This suggested that cancer may also originate from foci of adenomyosis within the myometrium and has a normal appearance of the endometrium. Thus, measuring endometrial thickness is not enough and may not detect all cases of endometrial cancer. Thankfully, as an extension of conventional sonography, elastography integrated with conventional scanning is more powerful than any single one of them. In clinical practices like assessment of fibrosis in chronic liver diseases, thyroid nodules detection, assessment of gastrointestinal tract contractility, classification of benign and malignant lymph nodes, and screening of prostate abnormalities, different forms of elastography showed great potential [11]. Further, in previous gynecological studies, the diagnostic value of elastography in uterine disorders was also investigated. For instance, Lu [24] observed that the performance of elastography in identifying cervical lesions that are likely to be malignant was positive, and that SR yielded the best results in differential diagnosis of benign and malignant cervical lesions than other elastic scores. When studying different endometrial pathologies, Krzysztof [25] found that atrophic and normal endometrial tissues were the softest among all pathologies, followed by endometrial polyps and hypertrophy. Our study found that like other tumorous tissues that are usually harder than the healthy tissue, endometrial cancer demonstrated highest SR than other benign pathologies. Similar results were shown in a recent study [26] that although elastography was not capable of distinguishing different benign endometrial lesions, like endometrial polyps and endometrial hyperplasia, it could sensitively detect endometrial pathologies with thickened endometrium at an early stage. When distinguishing fibroids and adenomyosis, elastographical results were in full agreement with those of magnetic resonance imaging [16].
In the past, applications of elastography which were used to distinguish endometrial pathologies, the tissue elasticity was only assessed quantitatively by measuring the SR in ROIs. To our knowledge, we are the first to characterize the endometrial tissue elasticity both qualitatively and quantitatively. For qualitative analysis, endometrial lesions were categorized into 3 types, based on the dominant elastographic color patterns. This method was used in other studies and demonstrated its accuracy in predicting benign and malignant lymph nodes [27,28]. Owing to subjective interpretations, quantitative method to assess SR is developed to improve diagnostic accuracy and has shown efficacy in benign/malignant differentiation in previous studies [29,30]. Mahmoud [31] found that by using the SR value of 7.2 as the cut-off value, elastography achieved the sensitivity and specificity of 92.3% and 100% respectively in differentiating endometrial carcinoma and endometrial hyperplasia. Similarly, when distinguishing endometrial carcinoma from atypical endometrial hyperplasia, Metin [32] observed a sensitivity of 92.9% and specificity of 71.9% with SR value of 1.05. In the current study, we included both endometrial hyperplasia and endometrial polyps in benign endometrial masses group. Although we were not able to achieve the high sensitivity and specificity described in Mahmoud’s study, the heterogeneity of tissue characteristics due to the inclusion of more pathological types makes our analysis more comprehensive in nature. By using the cut-off SR of 3.02, our method could distinguish endometrial cancer from two commonly seen benign endometrial masses and achieve the sensitivity of 81.7% and the specificity of 85%, despite the very large patient population we have. Thus our study can be considered superior to past studies in some ways.
However, there are also several limitations to this study. Firstly, this is a single centered study with only 2 radiologists as operators and observers. Thus, the inter-observer reliability of current method is not tested. Nevertheless, based on past quality rating studies, the inter- and intra-rater reliability of elastography are high and reached ICC = 0.84-0.95 and ICC = 0.77-0.94 respectively [33]. For examinations conducted on different body sites, the interrater reliability is high for 15 out of 17 body sites with ICC = 0.613-0.916 [34]. Moderate or poor interrater reliability has only been observed on body site like fingers, which were all hard tissues [34]. This issue will be addressed in a future study of ours. Secondly, we did not further stage the patients with endometrial cancer to investigate the possible relations between SR and malignancy staging. This would be a very interesting topic to include in the future studies. Thirdly, we did not compare the diagnostic performance of elastography with conventional B-mode sonography. Although we believe the combination of two methods offers more diagnostic value than either one alone, it would still be meaningful to study this topic. Lastly, all the patients included in the current study were selected based on B-mode findings and this may yield potential selection bias.
Conclusion
In conclusion, transvaginal sonographic elastography is a promising diagnostic tool, complementary to conventional sonography for differentiating between malignant and benign endometrial lesions. Both qualitative and quantitative methods could be applied to improve the diagnostic performance of elastography, and SR has demonstrated good diagnostic performance.
Acknowledgements
The study was supported by the Science and Technology Foundation of Ministry of Education in Heilongjiang Province (grant number 12541500).
Disclosure of conflict of interest
None.
References
- 1.Haldorsen IS, Salvesen HB. What is the best preoperative imaging for endometrial cancer? Curr Oncol Rep. 2016;18:25. doi: 10.1007/s11912-016-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–43. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 4.Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–34. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell D, Baum R. Standard imaging techniques for neuroendocrine tumors. Endocrinol Metab Clin North. 2011;40:153–62. ix. doi: 10.1016/j.ecl.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Epstein E, Fischerova D, Valentin L, Testa AC, Franchi D, Sladkevicius P, Frühauf F, Lindqvist PG, Mascilini F, Fruscio R, Haak LA, Opolskiene G, Pascual MA, Alcazar JL, Chiappa V, Guerriero S, Carlson JW, Van Holsbeke C, Giuseppe Leone FP, De Moor B, Bourne T, van Calster B, Installe A, Timmerman D, Verbakel JY, Van den Bosch T. Ultrasound characteristics of endometrial cancer as defined by international endometrial tumor analysis (IETA) consensus nomenclature: prospective multicenter study. Ultrasound Obstet Gynecol. 2018;51:818–828. doi: 10.1002/uog.18909. [DOI] [PubMed] [Google Scholar]
- 7.Chan FY, Chau MT, Pun TC, Lam C, Ngan HY, Leong L, Wong RL. Limitations of transvaginal sonography and color Doppler imaging in the differentiation of endometrial carcinoma from benign lesions. J Ultrasound Med. 1994;13:623–628. doi: 10.7863/jum.1994.13.8.623. [DOI] [PubMed] [Google Scholar]
- 8.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487–495. doi: 10.1016/j.diii.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Carlsen J, Ewertsen C, Sletting S, Vejborg I, Schäfer F, Cosgrove D, Bachmann Nielsen M. Ultrasound elastography in breast cancer diagnosis. Ultraschall Med. 2015;36:550–62. doi: 10.1055/s-0035-1553293. quiz 563-5. [DOI] [PubMed] [Google Scholar]
- 11.Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja O, Klauser A, Sporea I, Calliada F, Cantisani V, D’Onofrio M, Drakonaki E, Fink M, Friedrich-Rust M, Fromageau J, Havre R, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F, Dietrich C. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. part 2: clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 12.Wozniak S, Czuczwar P, Szkodziak P, Milart P, Wozniakowska E, Paszkowski T. Elastography in predicting preterm delivery in asymptomatic, low-risk women: a prospective observational study. BMC Pregnancy Childbirth. 2014;14:238. doi: 10.1186/1471-2393-14-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woźniak S, Czuczwar P, Szkodziak P, Wrona W, Paszkowski T. Elastography for predicting preterm delivery in patients with short cervical length at 18-22 weeks of gestation: a prospective observational study. Ginekol Pol. 2015;86:442–7. doi: 10.17772/gp/2401. [DOI] [PubMed] [Google Scholar]
- 14.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HS, Sohn IS, Kwon HS. Imaging analysis of cervical elastography for prediction of successful induction of labor at term. J Ultrasound Med. 2013;32:937–946. doi: 10.7863/ultra.32.6.937. [DOI] [PubMed] [Google Scholar]
- 16.Stoelinga B, Hehenkamp WJ, Brölmann HA, Huirne JA. Real-time elastography for assessment of uterine disorders. Ultrasound Obstet Gynecol. 2014;43:218–226. doi: 10.1002/uog.12519. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein SR, Nachtigall M, Snyder JR, Nachtigall L. Endometrial assessment by vaginal ultrasonography before endometrial sampling in patients with postmenopausal bleeding. Am J Obstet Gynecol. 1990;163:119–23. doi: 10.1016/s0002-9378(11)90683-8. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda H, Kawabata M, Yamamoto K, Inoue T, Umesaki N. Prospective study to compare endometrial cytology and transvaginal ultrasonography for identification of endometrial malignancies. Gynecol Oncol. 1997;65:383–386. doi: 10.1006/gyno.1997.4699. [DOI] [PubMed] [Google Scholar]
- 19.Van Dongen H, De Kroon C, Jacobi C, Trimbos J, Jansen F. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2007;114:664–675. doi: 10.1111/j.1471-0528.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi M, Matsuzaki K, Uehara H, Yoshida S, Nishitani H, Shimazu H. Pathologies of the uterine endometrial cavity: usual and unusual manifestations and pitfalls on magnetic resonance imaging. Eur Radiol. 2005;15:2244–2255. doi: 10.1007/s00330-005-2814-x. [DOI] [PubMed] [Google Scholar]
- 21.Schramm A, Ebner F, Bauer E, Janni W, Friebe-Hoffmann U, Pellegrino M, De Gregorio N, Friedl TWP. Value of endometrial thickness assessed by transvaginal ultrasound for the prediction of endometrial cancer in patients with postmenopausal bleeding. Arch Gynecol Obstet. 2017;296:319–326. doi: 10.1007/s00404-017-4439-0. [DOI] [PubMed] [Google Scholar]
- 22.Visser NC, Sparidaens EM, van den Brink JW, Breijer MC, Boss EA, Veersema S, Siebers AG, Bulten J, Pijnenborg JM, Bekkers RL. Long-term risk of endometrial cancer following postmenopausal bleeding and reassuring endometrial biopsy. Acta Obstet Gynecol Scand. 2016;95:1418–1424. doi: 10.1111/aogs.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naftalin J, Nunes N, Hoo W, Arora R, Jurkovic D. Endometrial cancer and ultrasound: why measuring endometrial thickness is sometimes not enough. Ultrasound Obstet Gynecol. 2012;39:106–109. doi: 10.1002/uog.9062. [DOI] [PubMed] [Google Scholar]
- 24.Lu R, Xiao Y, Liu M, Shi D. Ultrasound elastography in the differential diagnosis of benign and malignant cervical lesions. J Ultrasound Med. 2014;33:667–671. doi: 10.7863/ultra.33.4.667. [DOI] [PubMed] [Google Scholar]
- 25.Preis K, Zielinska K, Swiatkowska-Freund M, Wydra D, Kobierski J. The role of elastography in the differential diagnosis of endometrial pathologies, preliminary. Ginekol Pol. 2011;82:494–497. [PubMed] [Google Scholar]
- 26.Gultekin IB, Imamoglu GI, Turgal M, Gultekin S, Öcal FD, Alkan A, Kucukozkan T. Elastosonographic evaluation of patients with a sonographic finding of thickened endometrium. Eur J Obstet Gynecol Reprod Biol. 2016;198:105–109. doi: 10.1016/j.ejogrb.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Izumo T, Sasada S, Chavez C, Matsumoto Y, Tsuchida T. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol. 2014;44:956–962. doi: 10.1093/jjco/hyu105. [DOI] [PubMed] [Google Scholar]
- 28.Korrungruang P, Boonsarngsuk V. Diagnostic value of endobronchial ultrasound elastography for the differentiation of benign and malignant intrathoracic lymph nodes. Respirology. 2017;22:972–977. doi: 10.1111/resp.12979. [DOI] [PubMed] [Google Scholar]
- 29.He HY, Huang M, Zhu J, Ma H, Lyu XD. Endobronchial ultrasound elastography for diagnosing mediastinal and hilar lymph nodes. Chin Med J (Engl) 2015;128:2720. doi: 10.4103/0366-6999.167296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozman A, Marc Malovrh M, Adamic K, Subic T, Kovac V, Flezar M. Endobronchial ultrasound elastography strain ratio for mediastinal lymph node diagnosis. Radiol Oncol. 2015;49:334–340. doi: 10.1515/raon-2015-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdel Latif M, Shady M, Nabil H, Mesbah Y. Trans-vaginal sono-elastography in the differentiation of endometrial hyperplasia and endometrial carcinoma. Egypt J Radiol Nucl Med. 2016;47:1123–1131. [Google Scholar]
- 32.Metin MR, Aydın H, Ünal Ö, Akçay Y, Duymuş M, Türkyılmaz E, Avcu S. Differentiation between endometrial carcinoma and atypical endometrial hyperplasia with transvaginal sonographic elastography. Diagn Interv Imaging. 2016;97:425–431. doi: 10.1016/j.diii.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Hou Y, Zhu Q, Liu H, Jiang Y, Wang L, Xu D, Li M, Zeng X, Zhang F. A preliminary study of acoustic radiation force impulse quantification for the assessment of skin in diffuse cutaneous systemic sclerosis. J Rheumatol. 2015;42:449–455. doi: 10.3899/jrheum.140873. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Bhatia K, Chu W, He L, Leung S, Ahuja A. Shear wave elastography - a new quantitative assessment of post-irradiation neck fibrosis. Ultraschall Med. 2015;36:348–54. doi: 10.1055/s-0034-1366364. [DOI] [PubMed] [Google Scholar]


