Abstract
Application of extracorporeal shockwave therapy (ESWT) to the subchondral bone of medial tibia condyle has shown chondroprotective effects of the knee with decreased cartilage degradation and improved subchondral bone remodeling in the osteoarthritis (OA) of rat knee. Recently, transplantation of ex vivo preparations of mesenchymal stem cells (MSCs) to animal or human joints with OA seems to induce therapeutically effective repair because of paracrine responses from host cells including progenitor cells residing within the synovium. This study compared ESWT, Wharton’s jelly mesenchymal stem cells (WJMSCs) and combination of ESWT and WJMSCs therapies for early OA of the rat knee. The results showed ESWT, WJMSCs and combination of therapies significantly improved early OA knee based on analysis of pathological findings, micro-CT and immunohistochemistry (IHC) stain. The combined therapy group increased the bone volume (61.755 ± 1.537), and trabecular thickness (0.215 ± 0.014; P < 0.01) as well as reduced synovitis (1.8 ± 0.37) more than ESWT or WJMSCs individually. However, there were no significant difference in combined ESWT and WJMSCS as shown in the expressions of IGF-1 and TGF-β1 and reduction of the TUNEL activity on OA knee. Furthermore, WJMSCs treatment significantly increased the expression of the type II collagen (22.62 ± 0.84; P < 0.001) when compared with ESWT (6.97 ± 0.54) and ESWT combined with WJMSCs (8.87 ± 0.31) in OA knee. In mechanistic factors analysis, the synergistic effect was observed by ESWT combined with WJMSCs in the expression of RUNX-2, SOX-9 and Collagen Xα1 on OA knee. Our results provided the innovative information of ESWT, and WJMSCs in the treatment of early osteoarthritis of the knee in rats.
Keywords: Shockwave therapy, osteoarthritis, Wharton’s jelly mesenchymal stem cell, articular cartilage, subchondral bone
Introduction
Osteoarthritis (OA) of the knee is a degenerative joint disorder manifested with pain, deformity and functional disability due to damage of the articular cartilage. Conservative treatment is recommended in symptomatic early stage osteoarthritis of the knee, and surgery including knee replacement for late stage disease or failure to conservative treatments. Currently available methods of conservative treatment include administration of non-steroidal anti-inflammatory drugs (NSAID), weight reduction, modification of activity levels for daily living, physical therapy, knee brace support, intra-articular injections of cortisone, hyaluronic acid and platelet rich plasma (PRP) etc. Some gained limited success, but none showed universal results.
Recent studies demonstrate that ESWT has chondroprotective effect on early OA changes of the knee and induces regression or retardation of OA changes in the knee on animal model [1,2]. ESWT showed a dose-related effect on different targets such as skin flap [3,4], tendon [5,6], tenocyte [7], plantar fasciitis [8], bone [9], osteoarthritis of the knee [10], hip necrosis [11] and cells [12]. ESWT is characterized as noninvasive with mostly painless, effective, convenient and safe procedure without the need of surgery or surgical risks. ESWT has the potential of replacing surgery in various musculoskeletal disorders. The complication rates are low and negligible. The mechanism of shockwave therapy on tissue regeneration remains unclear. It has been reported that ESWT improves tissue regeneration by anti-inflammation, neovascularization, bone remodeling and wound healing [13-17]. ESWT also showed the biological effects to induce specific growth factors, such as eNOS, PCNA, VEGF, BMP-2, and osteocalcin expressions during bone healing [2,14,18]. Extracorporeal shockwave technology has the great potential for translational medical research and development in new therapeutic methods.
Mesenchymal stem cells (MSCs) are progenitor cells of connective tissue cells, and can be isolated from many well-differentiating organs and other human sources including bone marrow, adipose tissue, skeletal muscle, umbilical cord blood and Wharton’s jelly (WJ) [19-21]. MSCs are demonstrated capablility of differentiating to osteogenic, adipogenic, and chondrogenic lineages in vitro and in vivo [22,23]. MSCs can differentiate into wide range of specialized cells of mesodermal origin such as bone cells, cartilage, fat, cardiomyocytes, muscle fibers, renal tubular cells, and break germ layer commitment as well as differentiate into cells of ectodermal origin [24]. MSCs treatment can be executed by direct replacement of injured tissue cells through paracrine effect on surrounding microenvironment, indirectly supporting revascularisation, anti-apoptosis, and modulating inflammatory response [25]. In addition, MSCs have become known as a capable tool for clinical and commercial applications of cell transplantation and cell therapy. Recently, researchers used intra-articular injection of mesenchymal stem cells (MSCs) for the repair of OA joint surface lesions through paracrine effects of MSCs [26-30]. The majority of the successful preclinical studies involved the use of autologous, culture-expanded bone marrow-derived mesenchymal stem cells (BMMSCs) or adipose-derived mesenchymal stem cells (ADMSCs) [27].
ESWT had demonstrated increased expressions of SDF-1, TGF-β and VEGF in injury tissue and these proteins induce MSCs or circulating endothelial progenitor cells (EPCs) to promote the healing of the damage tissue to regeneration or reconstruction [31]. Equine adipose tissue-derived MSCs are improved in cell differentiation and cell proliferation by ESWT and enhances the expression of Cx43 as well as activated almost 2 fold of Erk1/2 in vitro [32]. In the current study, the results showed the efficacy of ESWT, WJMSCs and combined ESWT and WJMSCs on early OA knee.
Methods
Animals
The animals were treated humanely according to the guidelines from the Care and Use of Laboratory Animals, published by the National Institute of Health. All animals were housed under standard conditions at 23 ± 1°C with a 12 hours light and dark cycle. The Center for Laboratory Animals at Chang Gung Memorial Hospital (CGMH) provided veterinary care to the animals. The study was subjected to the approval of the Institutional Animal Care and Use Committee (IACUC) guidelines for the use of animals by rules of 4 R’s (replacement, reduction, refinement and rehabilitation) at CGMH.
OA knee of rat model by anterior cruciate ligament transection and medial meniscectomy
The left knee was prepared for OA knee model by anterior cruciate ligament transection (ACLT) and medial meniscectomy (MM) [10,18]. After surgery, prophylactic antibiotic with ampicillin 25 mg/Kg was given for 3 days and a veterinarian cared for the animals. The wounds and the activities of the animals were observed daily before and after ESWT treatment until sacrifice.
ESWT application
The device of DUOLITH® SD1 ultra (Storz medical) was used to generate the focused shockwave. The shockwave was applied on the subchondral bone of the medial tibia of the left knee [1]. Each knee was treated with 800 impulses of shockwave at 0.25 mJ/mm2 energy flux density in a single dose. After ESWT, the animals were returned to the cage for observation.
Isolation of human Wharton’s jelly (WJ)-derived mesenchymal stem cells and cell phenotyping
The umbilical cord was collected and stored in a sterile specimen cup containing 0.9% normal saline at 4°C until processing after delivery. The umbilical cord was washed with sterile HBSS Solution (Gibco, USA) and cut into 2 to 3 cm long pieces using a sterile blade and the vessels of the umbilical artery, vein, and outlining membrane were dissociated from the umbilical cord on Dulbecco’s Modified Eagle Medium (DMEM)-LG basal medium (Gibco, USA). The umbilical cord was then cut into pieces smaller than 1 cm3 and culture on medium for 7 days at 37°C. After 7 days, the WJMSCs were cultured by the removal and replacement of half the medium every 3 days until the cells with approximately 80% confluence. The pieces of cord removed and collected to subculture or store for experiments.
The cellular morphology became homogenously spindle-shaped in cultures after 3 to 5 passages. The specific surface molecules of cells from the WJMSCs were identified by the flow cytometry. Cells were detached with 1 × Trypsin-EDTA in phosphate-buffered salinze (PBS) and incubated with the respective antibody conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) against the indicated markers: CD14, CD44, CD105, CD133 and CD166. Thereafter, the cells were analyzed by a flow cytometer (BD LSRII, USA).
Study design
In this experiment, thirty Sprague-Dawley rats were used. The animals were randomized into five groups: Sham group was the baseline control and receive sham surgery of the left knee without anterior cruciate ligament transection (ACLT) and medial meniscectomy (MM) or shockwave. OA group received ACLT+MM of left knee, but no ESWT, and was used as OA changes of the knee. OA+EWST group received ACLT+MM of the left knee, and 800 impulses of ESWT at 0.25 mJ/mm2. OA+WJMSCs group received ACLT+MM, and intra-articular injection of 1 × 106 WJMSCs which were suspended in 50 μL of 1 × PBS and its location was confirmed by an ultrasound Doppler scan [33,34]. OA+ESWT+WJMSCs group received ACLT+MM of the left knee, and intra-articular injection of 1 × 106 WJMSCs that were suspended in 100 μL of 1 × PBS and then after 30 min to apply 800 impulses of ESWT at 0.25 mJ/mm2. All animals were scarified at 12 weeks post-treatment. The evaluations included pathological and histopathological changes of the knee and micro-CT scan. The experiment design and flow chat were showed in Figure 1.
Figure 1.
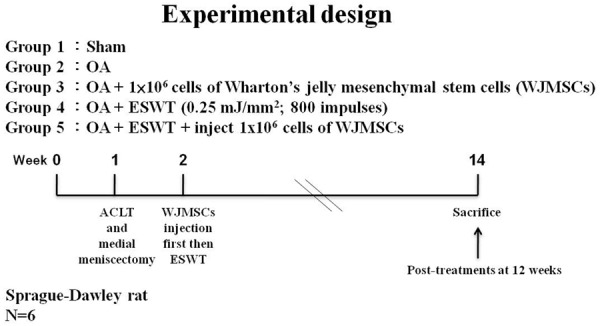
The study design. Graphic scheme depicted the study design of the experiment including knee surgery, shockwave application, WJMSCs injection, and sacrifice of animals. The six rats were used in the experiments.
Micro-CT scan
The harvested left knee was subjected to micro-CT scan (SkyScan, 1076, Kartuizersweg 3B 2550 Kontich, Belgium) analysis. The left knee was prepared and sized to fit the micro-CT for scanning. Examination of the trabecular bone included percentage trabecular bone volume fraction (BV/TV), and trabecular thickness were performed.
Histopathological examination
The articular cartilage and subchondral bone specimens were subjected to histopathological examination. The harvested specimens were fixed in 4% PBS-buffered formaldehyde at 37°C for 7 days, decalcified in 10% PBS-buffered EDTA at 37°C for 30 days. Decalcified specimens were fixed and subjected to paraffin wax embedding and dissection into 5 μm-thick sections. The specimens were stained with safranin-O stains. The degenerative changes of the articular cartilage were graded histologically using OARSI score.
Synovitis scoring
The tissue was stained with haematoxylin and eosin to evaluate synovitis score including thickening of the synovial lining, cellular hyperplasia and infiltration into joint cavity and synovium. Three features of chronic synovitis were described the scores ranks were defined as follow (1) 0 to 1 = no synovitis; (2) 2 to 4 = low-grade synovitis; (3) 5 to 9 = high-grade synovitis [35].
Immunohistochemical analysis
The articular cartilage and subchondral bone specimens were analyzed with immunohistochemical analysis by anti-human specific nuclei antigen antibody, anti-rat TGF-β1 (transforming growth factor β1), anti-rat IGF-1 (Insulin-like growth factor 1), anti-rat type II collagen, TUNEL activity, MMP-13, RUNX-2, SOX-9 and Collagen Xα1. The harvested specimens were fixed in 4% PBS-buffered formaldehyde for 48 hours and decalcified in PBS-buffered 10% EDTA solution. Decalcified tissues were embedded in paraffin wax. The specimens were cut longitudinally into 5 μm thick sections and transferred to poly-lysine-coated slides. Sections of the specimens were immunostained with specific reagents for anti-human specific nuclei antigen antibody, anti-rat TGF-β1, IGF-1 and type II collagen (Santa Cruz Biotechnology Inc, CA, USA), MMP-13, RUNX-2, SOX-9 (Abcam, Cambridge, UK), and Collagen Xα1 (Gene Tex, Inc., USA) to identify the location of WJMSCs, cartilage, and osteogenesis markers in bone remodeling and cartilage regeneration of rats. The immuno-reactivity in specimens were demonstrated using a horseradish peroxidase (HRP)-3’-, 3’-diaminobenzidine (DAB) cell and tissue staining kit (R & D Systems, Inc. Minneapolis, MN, USA). In Situ Cell Death Detection Kits (Roche Diagnostic, Mannheim, Germany) accomplished the TUNEL analysis following a manufacture instruction. TUNEL color stains were performed by using NBC/BCIP substrate (Sigma-Aldrich, St. Louis, MO, USA). The immuno-activities were quantified from five areas in three sections of the same specimen using a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Gottingen, Germany). All the images of each specimen were captured using a Cool CCD camera (SNAP-Pro c.f. Digital kit; Media Cybernetics, Silver Spring, MD, USA). Images were analyzed using an Image-Pro® Plus image-analysis software (Media Cybernetics, Sliver Spring, MD, USA). The percentage of each area was counted by immuno-labeled positive cells over the total cells as the results.
Statistical analysis
SPSS ver. 17.0 (SPSS Inc., USA) was used in statistical analysis. Calculated data was expressed as mean ± SD and One-way ANOVA with Tukey tests for post hoc (normal distribution) were used for group comparisons. Ranking data (non-normal distribution) was used Kruskal-Wallis test for comparisons of multiple groups. A statistical significance was set at P < 0.05, P < 0.01 and P < 0.001.
Results
Characterization of WJMSCs
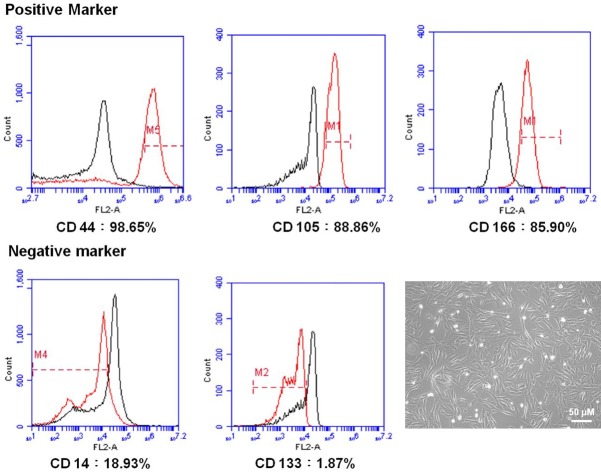
WJMSCs used in this experiment showed fibroblast-like morphology (Figure 2). Flow cytometric analysis demonstrated that the WJMSCs contained positive expressions of CD44 (98.65%), CD105 (88.86%), and CD166 (85.90%), and negative expressions of CD14 (18.93%), and CD133 (1.87%) (Figure 2).
Figure 2.
Characterization of WJMSCs and analysis of cell surface makers on WJMSCs. The cells were labeled with indicated markers as following: the CD44 (98.65%), CD105 (88.85%), and CD166 (85.90%) for the positive markers as well as CD14 (18.93%), and CD133 (1.87%) for the negative markers. The cultured WJMSCs showed fibroblast-like morphology.
The injection of WJMSCs into articular cavity by ultrasound guidance
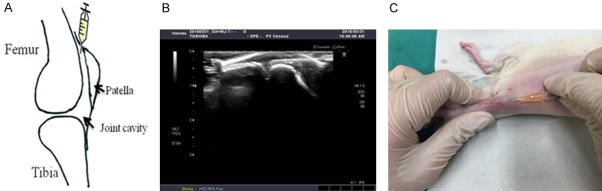
In the experiment, we defined the location for intra-articular injection of 1 × 106 WJMSCs into the rat knee. The ultrasound equipment was used to confirm the location for intra-articular injection of WJMSCs. The best location for injection was the upper position of the patella (Figure 3A). The needle of injector was put into the knee smoothly by use of ultrasound guidance (Figure 3B). Then WJMSCs were smoothly injected into the knee joint cavity (Figure 3B and 3C). After 30 min of WJMSCs injection, ESWT was applied to OA rat knee.
Figure 3.
The injection of WJMSCs. A. The knee sketch outlined the locations of WJMSCs injection in the rats knee. B. The image showed that ultrasound machine guide the localizations of WJMSCs for injection. C. The WJMSCs were injected in the knee of rats using above technique.
ESWT, WJMSCs and ESWT combined with WJMSCs significantly improved the damage of articular cartilage in early OA knee
ESWT, WJMSCs and ESWT combined with WJMSCs showed protection of the damaged articular cartilage in early treatment of OA rat knee. We also observed the changes of articular cartilage of osteoarthritis with OARSI score by safranin O stain in sham (0 ± 0; P < 0.001), OA+ESWT (6.6 ± 0.33; P < 0.001), OA+WJMSCs (4.9 ± 0.83; P < 0.001) and OA+ESWT+WJMSCs (6.0 ± 0.32; P < 0.001) by compared with OA (13.2 ± 0.73), respectively (Figure 4). The levels of protective effect on articular cartilage were not significant difference among ESWT, WJMSCs and ESWT+WJMSCs on OA knee. In the experiment, we surveyed the WJMSCs by using anti-human specific nuclei antigen antibody for IHC stain, but there were no signal detected at 12 week post-treatment.
Figure 4.
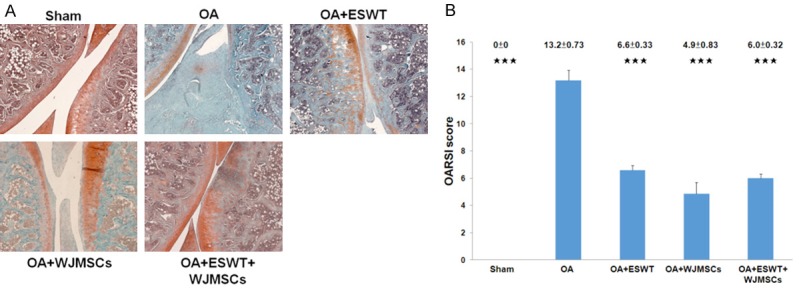
The pathological changes of the rat knee after treatments. A. The microphotographs of the knee showed cartilage degradation in the different groups. The microphotographs of cartilage and subchondral bone were shown the changes of cartilage in OA group, OA+ESWT, OA+WJMSCs and OA+ESWT+WJMSCs groups (n = 6). The field of the view was 50 × magnification. B. The bar chart illustrated the results of OARSI score. ***P < 0.001 as compared with OA group.
ESWT combined with WJMSCs improve the bone volume and trabecular thickness on OA knee
The results of micro-CT scan were summarized in Figure 5. The bone volume (BV/TV) and trabecular thickness decreased in the proximal tibia in OA (55.208 ± 0.999 and 0.162 ± 0.005) as compared with Sham (62.383 ± 0.692; P < 0.001 and 0.173 ± 0.004; P = 0.06). ESWT, WJMSCs and ESWT combined with WJMSCs significantly improved the bone volume in OA+ESWT (59.767 ± 0.88; P < 0.01), OA+WJMSCs (60.155 ± 1.049; P < 0.01) and OA+ESWT+WJMSCs (61.755 ± 1.537; P < 0.01) compared with OA (55.208 ± 0.999). In trabecular thickness, the results showed more effective in the improvement of subchondral plate thickness in OA+WJMSCs (0.196 ± 0.005; P < 0.05) and OA+ESWT+WJMSCs (0.215 ± 0.014; P < 0.05) than OA+ESWT (0.175 ± 0.002). Finally, ESWT combined with WJMSCs had a synergistic effect for bone regeneration in the treatment of early OA knee.
Figure 5.
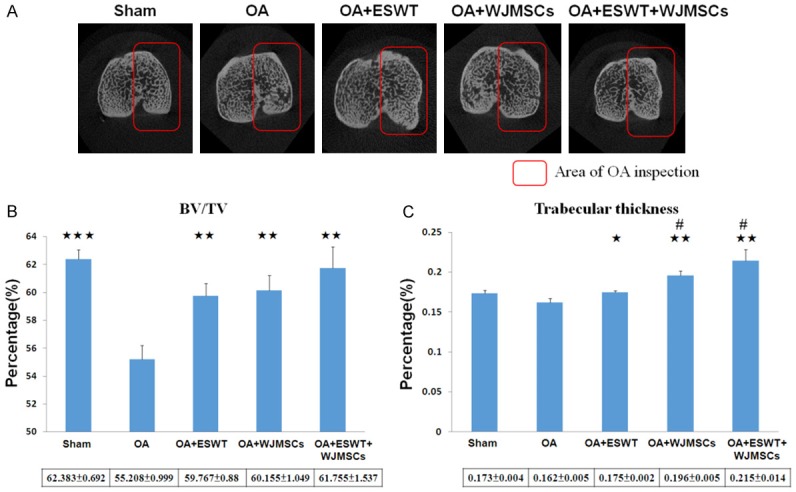
Micro-CT analysis in the proximal tibia after treatments. (A) The photomicrographs of the knee were shown in the transverse plane. The medial compartment of subchondral bone from each group was marked in red box. (B) The graphic illustrated the percentage of the trabecular bone volume fraction (BV/TV), and (C) trabecular thickness compared with OA groups. *P < 0.05, **P < 0.01, ***P < 0.001 comparing to OA group. #P < 0.05 was OA+WJMSCs and OA+ESWT+WJMSCs groups comparing to OA+ESWT groups. All rats were n = 6.
ESWT combined with WJMSCs significantly improved synovitis of OA knee
Synovitis of synovial membrane was measured after treatment (Figure 6). The mononuclear cells and neutrophils were infiltrated to the synovial membrane in OA knee. Lining cell layers were observed in composed large cells to form the irregular layered tissue (Figure 6A). ESWT (2.6 ± 0.40; P < 0.05), WJMSCs (2.4 ± 0.24; P < 0.05) and ESWT combined with WJMSCs (1.8 ± 0.37; P < 0.01) were significant improved the synovitis by comparing with OA (4.2 ± 0.58) (Figure 6B). Among the treatment groups, ESWT plus WJMSCs group was better than ESWT and WJMSCs alone on synovitis of OA knee, but no statistical significance was noticed among the groups.
Figure 6.
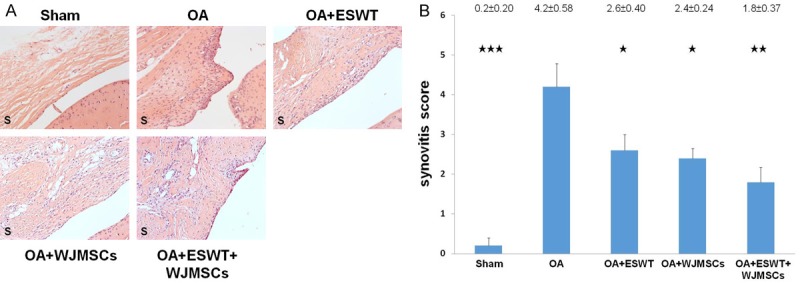
Evaluation of the histological changes in the membrane of OA knee before and after treatments. A. The sections of synovium were observed by HE stain at magnifications of 50 ×. B. The synovitis scores were calculated for each group in rat knee and all groups compared with OA group after treatment. *P < 0.05, **P < 0.01, ***P < 0.001. All rats were n = 6.
The effect of ESWT, WJMSCs and combination of both therapies for regulation the specific molecular factors on OA knee
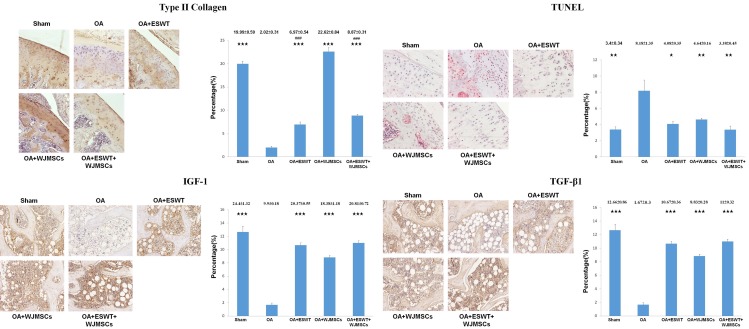
Immunohistochemical analysis of IGF-1, TGF-β1, type II collagen and TUNEL activity in subchondral bone and articular cartilage in Sham, OA, OA+ESWT, OA+WJMSCs and OA+ESWT+WJMSCs were shown in Figure 7. The expression of type II collagen, IGF-1, TGF-β1 increased and TUNEL activity decreased in OA+ESWT, OA+WJMSCs and OA+ESWT+WJMSCs than OA. ESWT+WJMSCs showed no advantage to improve the changes of specific factors (IGF-1, TGF-β1 and TUNEL activity) by comparing with ESWT and WJMSCs alone. It was noticed that the expression of type II collagen after WJMSCs (22.62 ± 0.84) treatment was almost three fold higher than ESWT (6.97 ± 0.54; P < 0.001) and ESWT+WJMSCs (8.87 ± 0.31; P < 0.001) in OA knee.
Figure 7.
Immunohistochemical analysis of the specific molecular factors in the treatments on OA knee. The immunohistochemical stains (left) and quantification (right) showed the expressions of the type II collagen, TUNEL activity, IGF-1 and TGF-β1 after treatments on OA knee. The type II collagen and TUNEL activity were measured in the articular cartilage as well as IGF-1 and TGF-β1 were observed in the subchondral bone of the knee. The field of view was 100 × magnification. *P < 0.05, **P < 0.01, ***P < 0.001 comparing to OA group. The ###P < 0.001 comparing to OA+WJMSCs group. All rats were n = 6.
The synergistic effect of OA+WJMSCs+ESWT in the expression of MMP-13, RUNX-2, SOX-9 and Collagen Xα1 on OA knee
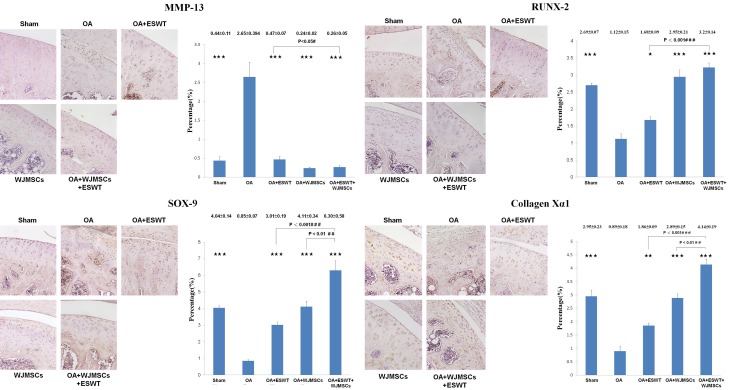
The key factors in chondrogenesis were measured by immunohistochemical analysis including MMP-13, RUNX-2, SOX-9 and Collagen Xα1 in the OA knee treatments (Figure 8). The expression of MMP-13 was significantly reduced in OA+ESWT, OA+WJMSCs and OA+ESWT+WJMSCs groups compared with OA group (P < 0.001). Further, the expressions of RUNX-2, SOX-9 and Collagen Xα1 were statistically increased in OA+ESWT, OA+WJMSCs and OA+ESWT+WJMSCs groups by compared with OA group. Among the treatment groups, the ESWT combined with WJMSCs has the synergistic effect in the expression of RUNX-2, SOX-9 and Collagen Xα1.
Figure 8.
The expression of the factors in the chondrogenesis after treatments on OA knee. The immunohistochemical stains (left) and quantification (right) showed the levels of the MMP-13, RUNX-2, SOX-9 and Collagen Xα1 after treatments on OA knee. The field of view was 100 × magnification. *P < 0.05, **P < 0.01, ***P < 0.001 comparing to OA group. The #P < 0.05, ##P < 0.01 and ###P < 0.001 comparing to OA+ESWT group. All rats were n = 6.
Discussion
In the current study, we found that the treatment of the rat OA knee with ESWT, WJMSCs and combined therapy resulted in up-regulation of cartilage specific protein of type II collagen and growth factors including IGF-1 and TGF-β1 and reduced activity of TUNEL expression in articular cartilage and inflammation of synovial membrane.
Bone marrow-derived mesenchymal stem cells (BMMSCs) were studied and documented in the field of stem cell studies. However, BMMSCs have limitation in cell numbers and require expansion in vitro with the risk of loss of stemness properties, inducing artifactual chromosomal changes and contamination [22,36]. ADMSCs from adipose tissue recently emerged as another resource of MSCs but it requires tissue collection to gain MSCs [37,38]. In this study, WJMSCs singularly and combined with ESWT on OA knee were utilized. WJMSCs are extra-embryonic tissue-derived MSCs with immune privilege, broader multipotent differentiation plasticity, and faster proliferation than adult MSCs [39,40]. Moreover, WJMSCs could be isolated and used without ethical problem, because extra-embryonic tissues are normally discarded after birth [39,41]. WJMSCs enhance chondrogenic differentiation more than BMMSCs in nanofibrous scaffolds and two-stage culture medium environment [42]. WJMSCs are effective in preventing OA changes of the knee, including synovitis that is a major target and modulation in matrix-degrading enzymes in animal studies [43]. There are many clinical trials to initiate and observe the delivery of MSCs by an intra-articular injection into the knee but, no optimal dose and vehicle are reported [26]. The process of treatment for OA knee are complicated factors, and the long-term results of MSCs for the treatment of OA knee are not completely available [44-46].
ESWT has been shown effectiveness on OA disease associated with many growth factors and cytokines for tissue repair [47,48]. However, the time for tissues repair after ESWT is still unknown. In addition, shortening the time interval to achieve tissue regeneration after ESWT is uncertain. In the study, combination of ESWT and WJMSCs with is the best approach in OA knee. Prior studies reported that ESWT and MSCs can induce expressions of growth factors and cytokines to promote cell proliferation and osteogenesis [26,48,49]. However, the synergistic effects of the combined therapy remain unclear and further investigations are needed. In the current study, we had shown that ESWT, WJMSCs and combined therapy improved the OA knee in a rat model. In addition, the synergistic effects of ESWT and WJMSCs in the improvement of bone volume and trabecular thickness (Figure 5) as well as in the expressions of RUNX-2, SOX-9 and Collagen Xα1 which were important factors in the chondrogenesis (Figure 8). However, no significant difference was found in articular cartilage repair, synovitis score and the expression of growth factors, such as IGF-1 and TGF-β1 (Figures 4, 6 and 7). No synergistic effects of combined therapy might be due to the expression levels of cytokines and growth factors or other unknown factors. Additional experiments are needed to elucidate the phenomenon. In OA knee, WJMSCs significantly improved the expression of type II collagen more than OA+ESWT or OA+ESWT+WJMSCs groups (Figure 7). The expression of type II collagen of WJMSCs might be modulated by ESWT in combined therapy on OA knee.
The study has several limitations as followings. The results of the study are obtained from small animals that may differ from the larger animal model or human clinical trial. Furthermore, the dose of shockwave were based on previous animal studies and reference for cell-based therapies [1,33,34]. These may not be optimal dose for human clinical trial. The expression of growth factors and cytokines after ESWT and WJMSCs in small animals may not be as accurate as human projects.
In conclusion, combined ESWT and WJMSCs is more effective in early knee OA in rats. These finding elucidated the insights for innovation strategy for combined ESWT with WJMSCs in the treatment of OA knees.
Acknowledgements
We are grateful to the Department of Medical Research, Kaohsiung Chang Gung Memorial Hospital for the supporting of this project. The funding sources are from Chang Gung Medical Foundation (CMRPG8E1231 and CLRPG8E0131).
Disclosure of conflict of interest
None.
References
- 1.Wang CJ, Weng LH, Ko JY, Wang JW, Chen JM, Sun YC, Yang YJ. Extracorporeal shockwave shows regression of osteoarthritis of the knee in rats. J Surg Res. 2011;171:601–608. doi: 10.1016/j.jss.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 2.Wang CJ, Sun YC, Wong T, Hsu SL, Chou WY, Chang HW. Extracorporeal shockwave therapy shows time-dependent chondroprotective effects in osteoarthritis of the knee in rats. J Surg Res. 2012;178:196–205. doi: 10.1016/j.jss.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Kamelger F, Oehlbauer M, Piza-Katzer H, Meirer R. Extracorporeal shock wave treatment in ischemic tissues: what is the appropriate number of shock wave impulses? J Reconstr Microsurg. 2010;26:117–121. doi: 10.1055/s-0029-1243296. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Yan X, Wang C, Tang T, Chai Y. The dose-effect relationship in extracorporeal shock wave therapy: the optimal parameter for extracorporeal shock wave therapy. J Surg Res. 2014;186:484–492. doi: 10.1016/j.jss.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Rompe JD, Kirkpatrick CJ, Kullmer K, Schwitalle M, Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis. A sonographic and histological study. J Bone Joint Surg Br. 1998;80:546–552. doi: 10.1302/0301-620x.80b3.8434. [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Cheng B, Grimmer-Somers K. The midterm effectiveness of extracorporeal shockwave therapy in the management of chronic calcific shoulder tendinitis. J Shoulder Elbow Surg. 2011;20:845–854. doi: 10.1016/j.jse.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Han SH, Lee JW, Guyton GP, Parks BG, Courneya JP, Schon LC. J.Leonard Goldner Award 2008. Effect of extracorporeal shock wave therapy on cultured tenocytes. Foot Ankle Int. 2009;30:93–98. doi: 10.3113/FAI-2009-0093. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Kang JH, Kim JY, Kim JH, Yoon SR, Jung KI. Dose-related effect of extracorporeal shock wave therapy for plantar fasciitis. Ann Rehabil Med. 2013;37:379. doi: 10.5535/arm.2013.37.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH. Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone. 2004;34:225–230. doi: 10.1016/j.bone.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Wang CJ, Hsu SL, Weng LH, Sun YC, Wang FS. Extracorporeal shockwave therapy shows a number of treatment related chondroprotective effect in osteoarthritis of the knee in rats. BMC Musculoskelet Disord. 2013;14:44. doi: 10.1186/1471-2474-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CJ, Huang CC, Yip HK, Yang YJ. Dosage effects of extracorporeal shockwave therapy in early hip necrosis. Int J Surg. 2016;35:179–186. doi: 10.1016/j.ijsu.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Martini L, Fini M, Giavaresi G, Torricelli P, de Pretto M, Rimondini L, Giardino R. Primary osteoblasts response to shock wave therapy using different parameters. Artif Cells Blood Substit Immobil Biotechnol. 2003;31:449–466. doi: 10.1081/bio-120025415. [DOI] [PubMed] [Google Scholar]
- 13.Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, Yang LC. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003;21:984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang CJ, Wang FS, Yang KD. Biological effects of extracorporeal shockwave in bone healing: a study in rabbits. Arch Orthop Trauma Surg. 2008;128:879–884. doi: 10.1007/s00402-008-0663-1. [DOI] [PubMed] [Google Scholar]
- 15.Frairia R, Berta L. Biological effects of extracorporeal shock waves on fibroblasts. Muscles Ligaments Tendons J. 2011;1:138–147. [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo YR, Wang CT, Wang FS, Chiang YC, Wang CJ. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regen. 2009;17:522–530. doi: 10.1111/j.1524-475X.2009.00504.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Yang YJ, Huang CC. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol Int. 2011;31:871–877. doi: 10.1007/s00296-010-1384-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang CJ, Huang CY, Hsu SL, Chen JH, Cheng JH. Extracorporeal shockwave therapy in osteoporotic osteoarthritis of the knee in rats: an experiment in animals. Arthritis Res Ther. 2014;16:R139. doi: 10.1186/ar4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 20.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 23.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Zhang M, Zhang Y, Li W, Yang B. Mesenchymal stem cells derived from Wharton jelly of the human umbilical cord ameliorate damage to human endometrial stromal cells. Fertil Steril. 2011;96:1029–1036. doi: 10.1016/j.fertnstert.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Kalaszczynska I, Ferdyn K. Wharton’s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int. 2015;2015:430847. doi: 10.1155/2015/430847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nature reviews. Nat Rev Rheumatol. 2013;9:584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Cao W. Treatment of osteoarthritis with mesenchymal stem cells. Sci China Life Sci. 2014;57:586–595. doi: 10.1007/s11427-014-4673-7. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 29.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 30.Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 2014;23:505–512. doi: 10.3727/096368914X678328. [DOI] [PubMed] [Google Scholar]
- 31.Sun CK, Shao PL, Wang CJ, Yip HK. Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: a review. Am J Transl Res. 2011;3:259–268. [PMC free article] [PubMed] [Google Scholar]
- 32.Raabe O, Shell K, Goessl A, Crispens C, Delhasse Y, Eva A, Scheiner-Bobis G, Wenisch S, Arnhold S. Effect of extracorporeal shock wave on proliferation and differentiation of equine adipose tissue-derived mesenchymal stem cells in vitro. Am J Stem Cells. 2013;2:62–73. [PMC free article] [PubMed] [Google Scholar]
- 33.Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23:610–617. doi: 10.1016/j.arthro.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 34.El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20:523–544. doi: 10.1089/ten.TEB.2013.0664. [DOI] [PubMed] [Google Scholar]
- 35.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, Haupl T. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 36.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 37.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren Y, Wu H, Zhou X, Wen J, Jin M, Cang M, Guo X, Wang Q, Liu D, Ma Y. Isolation, expansion, and differentiation of goat adipose-derived stem cells. Res Vet Sci. 2012;93:404–411. doi: 10.1016/j.rvsc.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med. 2008;12:730–742. doi: 10.1111/j.1582-4934.2008.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med. 2009;4:423–433. doi: 10.2217/rme.09.12. [DOI] [PubMed] [Google Scholar]
- 41.Sibov TT, Severino P, Marti LC, Pavon LF, Oliveira DM, Tobo PR, Campos AH, Paes AT, Amaro E Jr, L FG, Moreira-Filho CA. Mesenchymal stem cells from umbilical cord blood: parameters for isolation, characterization and adipogenic differentiation. Cytotechnology. 2012;64:511–521. doi: 10.1007/s10616-012-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fong CY, Subramanian A, Gauthaman K, Venugopal J, Biswas A, Ramakrishna S, Bongso A. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. 2012;8:195–209. doi: 10.1007/s12015-011-9289-8. [DOI] [PubMed] [Google Scholar]
- 43.Saulnier N, Viguier E, Perrier-Groult E, Chenu C, Pillet E, Roger T, Maddens S, Boulocher C. Intra-articular administration of xenogeneic neonatal mesenchymal stromal cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 2015;23:122–33. doi: 10.1016/j.joca.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner S, Robinson B, Hanson R. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2011;6:368–378. doi: 10.2174/157488811797904371. [DOI] [PubMed] [Google Scholar]
- 45.Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 46.Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1:237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitz C, Császár NBM, Milz S, Schieker M, Maffulli N, Rompe JD, Furia JP. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br Med Bull. 2015;116:115–38. doi: 10.1093/bmb/ldv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romeo P, Lavanga V, Pagani D, Sansone V. Extracorporeal shock wave therapy in musculoskeletal disorders: a review. Med Princ Pract. 2014;23:7–13. doi: 10.1159/000355472. [DOI] [PMC free article] [PubMed] [Google Scholar]