Abstract
Background: Existence of acquired or intrinsic resistance to Temozolomide (TMD) remains a point of concern in treating glioblastoma (GBM). Here we established mechanism by which Phenethyl isothiocyanate (PEITC) reverses TMD resistance in T98G cell lines both in vitro and in vivo. Methods: For the study TMD-resistant cell lines were generated by stepwise exposing the parental cell lines (U87 and U373) to TMD. The 50% inhibitory concentration (IC50) values were established. MTT assay was done for cell survival studies, apoptosis assay by FITC Annexin V/PI staining, luciferase reporter assay for NF-κB transcription activity, cell colony survival and cell invasion assay, protein expression by western blot was done. For in vivo studies nude mouse model of GBM was established, TUNEL assay was done for apoptosis in tumor specimens. Results: We established that T98G, U87-R and U373-R showed higher NF-κB activity and exhibited higher IC50 of TMD with significantly increased MGMT expression compared to untreated cells. Next, we found that PEITC suppressed proliferation of resistant GBM cells, inhibited NF-κB activity, decreased expression of MGMT and reversed the resistance in U373-R, U87-R and T98G cells. Exposure to PEITC followed by sequential treatment of TMD produced synergistic effect. In U373-R grafted xenografts mouse model PEITC suppressed cell growth and enhanced cell death. Conclusion: Altogether, the present research established that combination of PEITC with TMD could enhance its clinical efficacy in resistant GBM by suppressing MGMT via inhibiting NF-κB activity.
Keywords: Temozolomide, glioblastoma, resistance, MGMT
Introduction
Glioblastoma multiforme (GBM) is graded to be one of the commonest types of Iry human brain tumor for malignancy having high rate of mortality. Even though treated with multimodality therapeutic approaches such as chemotherapy, surgery and radiation the survival time is less than 1 year [1]. Advancement in search of new molecules to halt GBM is the prime goal of research today. One of the major advancement that GBM therapy has undergone is by making use of DNA alkylating agents such as Temozolomide (TMD) [2]. TMD is a potent alkylating agent administered by oral route and is proved to have low toxicity, the molecule is reported to exert anticancer activity via interfering replication of DNA. Clinical studies have evidenced increased life span with quality in patients undergoing TMD therapy [3,4]. However, occurrence of resistance against TMD in treating GBM results in inadequate outcomes and also leads to dismal prognosis. The findings till now suggest involvement of O6-methylguanine-DNA methyl transferase (MGMT) for resistance of TMD against GBM cells. MGMT is key enzyme responsible for replication of DNA and growth of GBM cells [5,6].
MGMT is reported to act via targeting alkyl group by removing it from the DNA and converting them to an internal cysteine residue [7]. MGMT has been found to be the most important enzyme responsible for producing resistance in cancer cells against TMD [8]. Increased expression of MGMT in patients of GBM is reported to have poor prognosis compared to those having lower levels of MGMT. Studies in preclinical as well as in clinical phase have concluded MGMT as the responsible factor in decreasing TMD mediated death of GBM cells [9,10]. MGMT mediated methylation is considered to be an important predicating feature for clinical response to radiotherapy and associated survival in GBM [11,12].
Transcriptional factors such as NF-κB, AP-1, p53 and HIF-1α are reported to be involved in regulation of MGMT mediated transcription of cancerous cells [13-15]. NF-κB is a protein responsible for transcription by binding to a specific DNA sequence in a specific gene causing immunoregulation, growth, inflammation, cancer and apoptosis [16]. Upon activation, NF-κB can act as an oncogene in number of tumors promoting proliferation of tumor cells, inhibiting the activity of NF-κB could substantially increase the apoptotic activity of the alkylating agents [17].
In a study reported earlier involving HEK293 cells, NF-κB-p65 a subunit of NF-κB resulted in increased expression of MGMT, whereas NF-κB inhibitor abolishes the increased expression of MGMT. Reports have confirmed that MGMT plays a crucial role in resistance to alkylating agents via NF-κB pathway as MGMT has been found to be a target gene for NF-κB [14]. Hence, any inhibition to NF-κB-MGMT pathway could be helpful in overcoming TMD resistance in treating GBM.
Among the Isothiocyanate family Phenethyl isothiocyanate (PEITC) is one of the most important compound found in cruciferous vegetables, the compound is found to be effective in number of cancers such as breast, prostate, oral, ovarian and lung cancer [18]. The literatures have suggested number of mechanisms contributing to anti-tumor activity of PEITC which include detoxification enzymes [19], inhibition of cytochrome p450 enzymes, activation of death receptors 4 and 5 [20] and oxidative stress [21]. One of the study concluded anticancer activity via suppression of MAPK and NF-κB signaling pathway in gastric cancer cell lines [22].
Looking into the potential of PEITC in suppressing MGMT we postulated synergistic role of PEITC to TMD in TMD resistance GBM cell lines. In the present work, we evaluated the possible synergistic effect of PEITC in combination with TMD and hypothesized that PEITC could mediate anticancer effect of TMD partially via by NF-κB-dependent pathway decreasing expression of MGMT.
Methods
Reagents, chemicals and antibodies
Temozolomide (TMD) was procured from Santa Cruz Biotec, CA. The stock solution (100 mM) of TMD was prepared by dissolving in dimethyl sulphoxide (DMSO) (Sigma, USA). The stock of Phenethyl isothiocyanate (PEITC) (Sigma, USA) was prepared in DMSO to obtain stock solution of resultant concentration 100-mM. Both TMD and PEITC were diluted in fresh Dulbecco’s modified Eagle’s medium (DMEM) (Merck, USA) to obtain 100 μM solution of each. Care was taken in preparing TMD solution for concentration not exceeding more than 1% so as the cell growth remains unaffected. Antibodies such as MMP-2, MMP-9, p65, caspase-3 and Ki-67 against MGMT were procured from Abcam, USA.
Cell culture
For the study human GBM cells (T98G, U87MG, A172, and U251) were collected from American Type Culture Collection (Rockville, MD). The cell lines were cultured in DMEM medium along with 10% fetal bovine serum (FBS) in an incubator maintained at 37°C and 5% CO2 in humid conditions. The selected cells were transferred to medium free of serum 24 h before experiments. Temozolomide and PEITC were added to culture medium in predefined concentrations. Control group of cells were treated with DMSO diluted in Dulbecco’s modified Eagle’s medium at 100 μM.
Western blot analysis
For western blot analysis the GBM cells were subjected to lyses at 4°C for 30 min in a solution of Tris buffer (50 mM, pH 8.0), sodium chloride (150 mM), EDTA (5 mM), Nonidet p-40 (1% v/v), aprotinin (20 g/mL), phenylmethylsulfonyl fluoride (1 mM) and leupeptin (25 g/mL). The protein extracts were determined using PierceTM SDS-PAGE Sample Prep Kit (Thermo ScientificTM), followed by transfer to a polyvinylidene difluoride (PVDF) membrane. The obtained fraction was then treated with Tris-HCl buffer (20 mM, pH 7.5), sodium chloride (500 mM), non-fat milk (5%) for 2 h at room temperature conditions followed by incubation with specific antibody (MMP-2, MMP-9, p65, caspase-3 and Ki-67) overnight at 4°C, the blots were imaged using Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore).
Cell survival studies (MTT assay)
The cell survival studies and 50% growth inhibitory concentration i.e IC50 of TMD or PEITC in both parental and TMD resistant cell lines was done by methyl-thiazolyl-tetrazolium (MTT) assay. Briefly the cells were subjected for culture in 96-well plates (Thermo Scientific, USA) at density of 5 × 103 cells/well consisting 10% fetal bovine serum (FBS), followed by incubation for 24 h. The culture medium was added up with TMD at concentrations ranging from 0, 500, 1000, 2000, 3000, 4000, 5000 and 6000 μM while PEITC was added at concentration ranging from 0, 10, 20, 30, 40 and 50 μM. After incubation of 48 h, MTT (Sigma Aldrich, USA) at 5 mg/ml dissolved in PBS was added in each well followed by incubation of 4 h at 37°C. The supernatants were aspirated carefully; the Formazan crystals formed during MTT assay were dissolved in DMSO followed by measurement of optical activity at 490 nm using a microplate reader (Bio-Rad, USA). The suppression in viability in various treatment groups, all the experiments were performed in triplicate.
Cell colony survival assay
For the assay each cell line i.e. U87-R, U373-R and T98G cells were plated in 94-mm petri dishes with each containing 10 ml of culture medium along with 10% FCS. The cells were allowed to attach overnight. The cells were then exposed to defined concentrations of PEITC i.e. 0, 10 or 20 μM, followed by colony formation assay as per the procedure described by Salloum et al. [23]. Presence of more than 50 or more cells was selected as criteria for counting surviving colonies after observing under microscope.
Cell invasion assay
The cancerous cells were exposed with different concentrations of PEITC (10, 20 and 30 μM. After receiving pretreatment for 8 h, TMD (270 μM) was added to cells. The cell invasion ability of cancerous glioma cells was evaluated after 48 h using Corning® Transwell® (Sigma-Aldrich, USA) following manufacturer’s instructions.
Cell apoptosis assay (FITC Annexin V/PI staining)
For studying the effect of PEITC on cell apoptosis, Annexin V assay was done with the help of FITC Annexin V Apoptosis Detection kit (Abcam, USA) for detection of apoptosis. Briefly, the cells previously cultured in 6-well plates for 24 h were treated with PEITC (10, 20 and 30 μM) followed by addition of TMD (270 μM) after 8 h, the cells were cultured for 48 h. The cells were collected and subjected to washing using cold PBS and incubated for 15 min along with fluorescein-conjugated Annexin V and propidium iodide followed by analysis using Attune Nxt Flow cytometer (Invitrogen, Thermo scientific, USA) equipped with AttuneTM NxT Software. Annexin V-positive cells were reported to be apoptotic, whereas the cells negative to Propidium Iodide (PI) and Annexin V were counted as normal. Extent of apoptosis was established by measuring activation of caspase-3/7 using Cell MeterTM Live Cell Caspase 3/7 Binding Assay Kit (AAT bioquest).
Luciferase reporter assay (NF-κB transcription)
The GBM Cells were subjected to seeding (1.5 × 104) in 24 well plates for 24 h. Luciferase reported plasmid (100 ng) containing NF-κB plasmid was transfected in GBM cell lines with the help of Lipofectamine 2000 reagent (Thermo Fisher Scientific, USA) as per the manufacturer’s instructions. The Luciferase activity was measured after 48 h of transfection with the help of Luciferase assay kit (Promega, WI). For study three independent experiments were done, all the results were represented as mean ± SD.
Nude mouse model of GBM
The animal studies were in accordance to animal ethical guidelines of China-Japan Union Hospital of Jilin University, the approval number of the study sanctioned was CJUHJU/DNS/AEC/17-18/078. For the study female mice aging 4-6 weeks mice were divided into 4 groups (6 in each group) (control, PEITC treated, TMD treated and combination of PEITC+TMD treated group). All the selected mice were inoculated with U373-R GBM cells by subcutaneous route in the right flank. The tumors were allowed to grow till the size reached near to 200 mm3, after this treatment regimen started with TMD 50 mg/kg/day in respective group for 5 days in a week for next 28 days, PEITC was injected by subcutaneous route (50 mg/kg/day) for 4 weeks. The 4th group of mice received combined treatment of TMD (50 mg/kg) along with PEITC (50 mg/kg) for 5 days in week for 28 days. The control group received isotonic saline (0.9% w/v). At the end of protocol, all the animals were sacrificed by CO2 asphyxiation method for recovering tumors and evaluating them further.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay
TUNEL assay was done by in vivo studies to assess apoptosis in tumor specimens of animal model using TUNEL assay kit (Thermo Fischer) opting manufacturer’s protocol.
Statistical analysis
All the data are presented as mean ± standard deviation of experimental values. The differences were established by t-test using Graph Pad software. Results with P-value < 0.05 or P < 0.01 were considered statistically significant.
Results
Expression of MGMT in different GBM cell lines
Expression of MGMT was assessed in various types of GBM cell lines (T98G, U87MG, A172 and SHG44 cells) selected for the study. From the experiment it was found that MGMT protein was highly expressed in T98G cells and was towards lower side or not expressed in U87MG, SHG44 and A172 cells (Figure 1). The results were similar to MGMT mRNA status which suggested higher expression in T98G cells. The results of IC50 suggested variable values of TMD in different cell lines, it was found that IC50 values were higher in T98G cells compared to other cell lines (Table 1) hence T98G cell lines were established to be TMD resistant and were chosen for further experiments.
Figure 1.
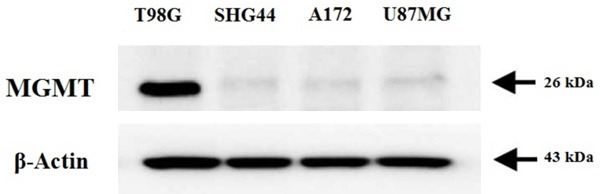
Expression of MGMT in GBM cell lines. The expression of MGMT is found to be positively expressed in T98G cells, whereas it is negatively expressed in other selected cell lines.
Table 1.
IC50 values (dose required for inhibiting 50% cell population) of various glioma cell lines exposed to TMD
| Cell lines | IC50 TMD (µM) |
|---|---|
| T98G | 3468.8 |
| U373 | 529.8 |
| LN229 | 987.4 |
| U87 | 766.4 |
| U251 | 891.4 |
Establishment of Temozolomide-resistant U373 and U87 GBM cell lines via exposing to TMD
In the present study Temozolomide-resistant U373 and U87 GBM cell lines were developed successfully by exposing the cells to increasing concentration of Temozolomide in a stepwise manner for 6 months. The two cell lines were found resistant to any TMD exposure further. The IC50 values of U373 and U87 were found to be 2120.1 versus 529.8 μM and 3242.10 versus 766.4 μM of TMD compared to their parental cell lines, the TMD resistant cell lines were nearly 4 times more resistant compared to their parental cell lines (Figure 2A). The resistant character against TMD is associated with activation of transcription factor NF-κB [24], hence we submitted both the resistant cell lines for measurement of NF-κB activity by transfecting cells with pNF-κB-luc reporter plasmids. The NF-κB transcription was activated significantly in both the TMD resistant cell lines compared to their parental (Figure 2B). Later in the study expression of MGMT was also evaluated in TMD-resistant cell lines by western blot studies suggesting increased expression of MGMT in both U373-R and U87-R cell lines compared to control (Figure 2C). The experiment hence concluded a relation between TMD-resistance and activity of NF-κB.
Figure 2.
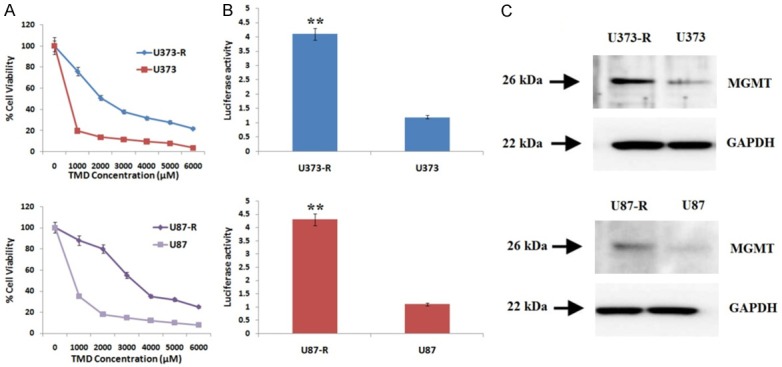
Temozolamide resistance in GBM cell lines (U373, U373-R, U87 and U87-R). A. Drug sensitivity assay for IC50 values of U373, U87 parental and resistance i.e U373-R and U87-R cell lines. B. NF-κB transcription activity in both U373-R and U87-R cell lines was significantly activated compared to their parental cell lines (**P < 0.01 compared to respective parental cell lines). C. Expression of MGMT in Temozolamide resistant cell lines showed increased expression in resistance cells.
PEITC inhibits colony formation and proliferation of TMD resistant cell lines
In the present investigation we proposed to evaluate inhibitory role of PEITC against TMD resistant cells. The antiproliferative effect of PEITC was evaluated by colony formation and MTT assay. The U373-R, T98G and U87-R cells were treated with 0, 10, 20, 30, 40 and 50 μM of PEITC for 48 h, number of viable cells were counted. The outcomes of MTT assay suggested growth inhibitory effect in dose dependent manner of PEITC on the selected cell lines (Figure 3A). The findings of colony formation assay suggested that cells treated with PEITC 10 and 20 μM for 48 h tend to form less colonies as compared to control (P < 0.01) (Figure 3B). Overall the results suggested inhibitory effect of PEITC on growth of TMD resistant cell lines.
Figure 3.
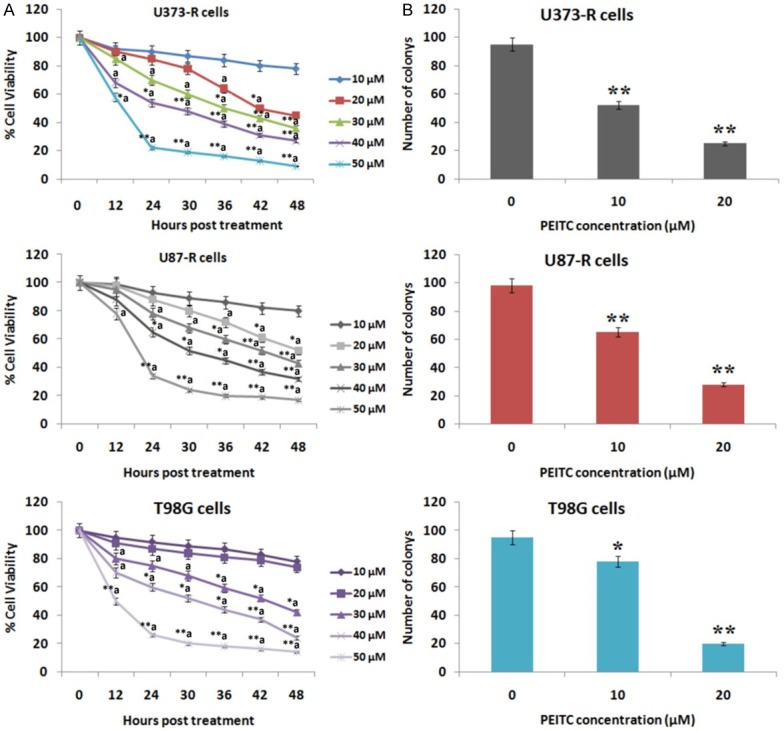
Anti proliferative effect of PEITC on Temozolamide resistant GBM cells. A. PEITC inhibits cell viability in T98G, U373-R and U87-R cell line in a dose dependent manner. (aP < 0.05, *aP < 0.01, **aP < 0.001 compared to dose of 10 μM). B. A significant decrease in number of colonies (**P < 0.01) for concentration 10 and 20 μM was observed for U373-R and U87-R, whereas in T98G cells, concentration of 20 μM resulted in significant decrease compared to control (**P < 0.01, *P < 0.05 compared to untreated cells).
PEITC decreases the expression of MGMT in TMD resistant glioblastoma cells
Experiment for IC50 value was done in order to choose a correct dose for studying in vitro effects of PEITC on the three selected GBM cell lines. The IC50 values of PEITC for T98G, U373-R and U87-R was 50.4, 50.1 and 56.4 μM respectively the results are presented in Figure 4. The concentrations selected for further experiments were less than the IC50 values. For analyzing whether PEITC would enhance the sensitivity of TMD resistant glioblastoma cell lines by decreasing the levels of MGMT via inhibiting NF-κB, the effect of PEITC on NF-κB transcription activity was examined. Transfection of T98 was done with NF-κB reporter plasmids. The transfected cells were exposed to various concentrations of PEITC (Figure 5A) for different time intervals (3 h and 6 h). The outcomes of study suggested significant attenuation of transcriptional activity of NF-κB with increasing dose. The Luciferase activity significantly decreased with increasing concentration of PEITC, more significantly with increased exposure time. Previously a study has been reported suggesting MGMT as a target gene for NF-κB [14]. On western blot analysis, decreased expression of MGMT was observed with increasing concentration of PEITC in Temozolomide resistant GBM cell lines (Figure 5B).
Figure 4.
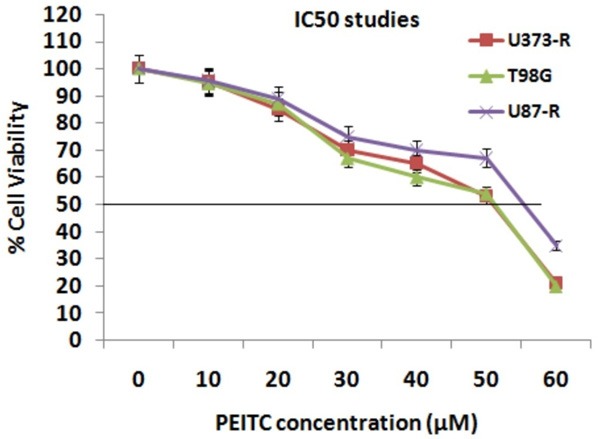
Results of IC50 values for PEITC for T98G, U373-R and U87-R cell lines were 50.4, 50.1 and 56.4 μM respectively.
Figure 5.
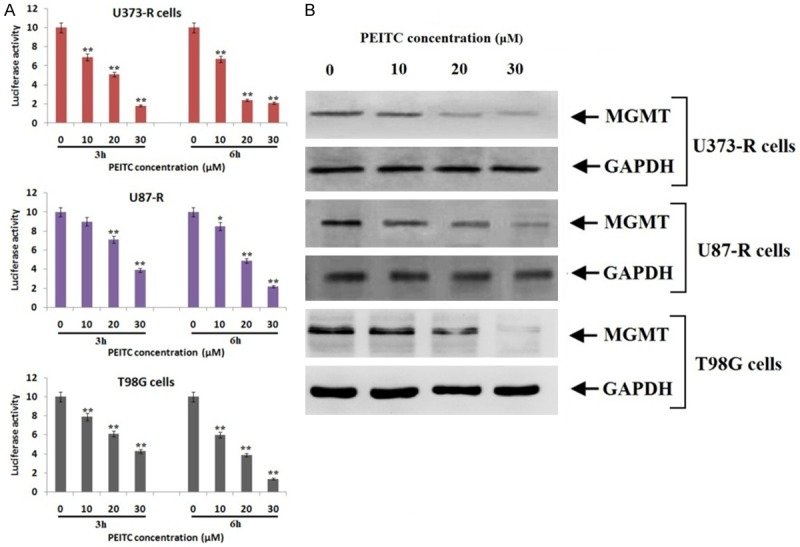
PEITC inhibits the levels of MGMT via NF-κB pathway in all the three TMD resistant cell lines. A. Luciferase assay showed that treatment of PEITC significantly decreased NF-κB transcriptional activity. B. The treatment of PEITC suppressed levels of MGMT in all the three resistant cell lines with increasing concentrations.
PEITC enhances cytotoxicity of TMD and reverses the resistance in glioblastoma cells in vitro
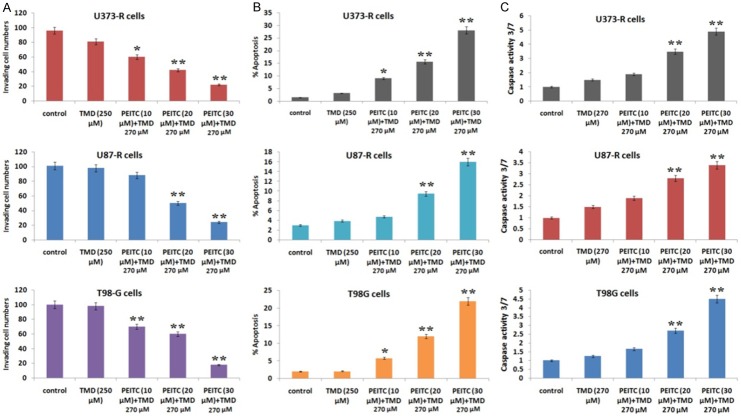
To fix a dose of Temozolomide which would evidence no growth inhibitory effect on TMD resistant cell lines was selected by exposing different doses of TMD, a dose 270 μM was finalized which resulted in no growth inhibitory effect. In order to analyze synergistic role of PEITC in enhancing cytotoxicity of TMD, various dose response model were created such as nonlinear regression of a sigmoid model and combination index (CI) approach. Initially the cells (U373-R, T98G and U87-R) were simultaneously treated with TMD and each selected concentration of PEITC, the results suggested an antagonistic effect (Cl > 1). However, the effect was synergistic when the exposure pattern was reversed (Cl < 1) i.e. sequential treatment beginning with PEITC first at different concentrations for 8 h and then followed by TMD. The exposure pattern resulted in high values of dose reduction index (DRI) indicating that doses of TMD could be reduced (Table 2). The TMD resistant cells were exposed to PEITC (8 h) first and then followed by TMD for further experiments. Further, Transwell Matrigel invasion assay was done to establish the synergistic effects of PEITC and TMD on cell invasive ability of U373-R, T98G and U87-R cells. The results clearly indicated in sufficiency of TMD alone in inhibiting cell invasion; however the U373-R, T98G and U87-R cells which received pretreatment of PEITC at different concentrations combined with TMD showed significant reduction in cell invasion capacity (Figure 6A). Further study was done to mark the effect of PEITC on TMD-induced apoptosis, it was observed that TMD alone do not inhibited cell invasion but the on receiving pretreatment of PEITC at different concentrations combined with TMD caused decreased cell invasive capacity (Figure 6A).
Table 2.
Effect of median doses for studying the effect of PEITC treatment at various doses along with Temozolamide
| U373-R cells | |||||||
|
| |||||||
| Sequential addition of TMD (270 µM) | Concomitant addition of TMD (270 µM) | ||||||
|
| |||||||
| PEITC (µM) | fa | CI | DRI | PEITC (µM) | fa | CI | DRI |
|
| |||||||
| 10 | 0.28 | 0.92 | 2.55 | 10 | 0.03 | 14.2 | 0.32 |
| 20 | 0.52 | 0.80 | 6.40 | 20 | 0.04 | 4.52 | 0.21 |
| 30 | 0.78 | 0.52 | 7.10 | 30 | 0.18 | 1.45 | 0.09 |
|
| |||||||
| U87-R cells | |||||||
|
| |||||||
| Sequential addition of TMD (270 µM) | Concomitant addition of TMD (270 µM) | ||||||
|
| |||||||
| PEITC (µM) | fa | CI | DRI | PEITC (µM) | fa | CI | DRI |
|
| |||||||
| 10 | 0.36 | 0.71 | 3.95 | 10 | 0.02 | 8.45 | 0.88 |
| 20 | 0.42 | 0.60 | 6.10 | 20 | 0.03 | 6.10 | 1.11 |
| 30 | 0.66 | 0.20 | 12.4 | 30 | 0.05 | 3.16 | 1.35 |
|
| |||||||
| T98G cells | |||||||
|
| |||||||
| Sequential addition of TMD (270 µM) | Concomitant addition of TMD (270 µM) | ||||||
|
| |||||||
| PEITC (µM) | fa | CI | DRI | PEITC (µM) | fa | CI | DRI |
|
| |||||||
| 10 | 0.40 | 0.60 | 3.10 | 10 | 0.01 | 14.2 | 0.18 |
| 20 | 0.64 | 0.41 | 5.41 | 20 | 0.02 | 8.40 | 0.51 |
| 30 | 0.78 | 0.17 | 16.4 | 30 | 0.15 | 4.10 | 0.89 |
fa, fraction affected, CI, Combination index, DRI, Dose reducing index.
Figure 6.
Treatment of PEITC reversed the resistance against Temozolomide. A. The invasive capacity reduced significantly in cells receiving pretreatment of PEITC at 10 and 20 μM combined with TMD 270 μM. B. The outcomes of FACS analysis showed that PEITC enhanced the TMD mediated apoptosis significantly (P < 0.01) at dose of 20 μM combined with 270 μM of TMD. C. The caspase 3/7 activity increased significantly in all the three cell lines exposed to PEITC at 10 and 20 μM combined with TMD 270 μM.
On investigating the effect of PEITC on apoptosis inducing capacity of TMD, it was observed that TMD alone was ineffective in causing apoptosis compared to control, however the resistant cells on receiving pretreatment of PEITC followed by TMD caused significant rise in apoptotic mediated death at lower dose of PEITC. The outcomes suggested a synergistic role of PEITC pretreatment and TMD in causing apoptosis (Figure 6B). The results indicating synergism were supported by elevated caspase-3/7 activity (Figure 6C). All the results collectively supported pretreatment of PEITC in reversing the extent of TMD resistance in glioma cells.
PEITC enhances sensitivity towards TMD in animal models (in vivo)
We investigated the potentiating effect of PEITC in combination with TMD by xenograft growth assay in glioma xenograft nude mouse models. We found that TMD alone was ineffective in suppressing growth of tumors induced by U373-R cell lines (P > 0.05). However a significant suppression in tumor growth was observed by PEITC alone as compared to control (P < 0.05), combination of PEITC along with TMD suppressed growth of tumors more significantly compared to control (P < 0.01). The tumor mass reduced significantly in group treated with combination compared to control (P < 0.01) and respective individual treatments (Figure 7A), proving effectiveness of combining PEITC along with TMD against TMD-resistant glioma xenografts animal model. To confirm this further, TUNEL and western blot analysis was done. The expression of NF-κB p65 and MGMT decreased in group of animals receiving combined treatment of PEITC and TMD. The levels of MMP2/9 and Ki-67 decreased and expression of caspase-3 was found to be increased in animals receiving combined treatment as compared to their individual treatments (Figure 7A), the results were parallel to results of in vitro studies. The results of TUNEL assay confirmed the synergistic effect of combination in vivo (Figure 7C).
Figure 7.
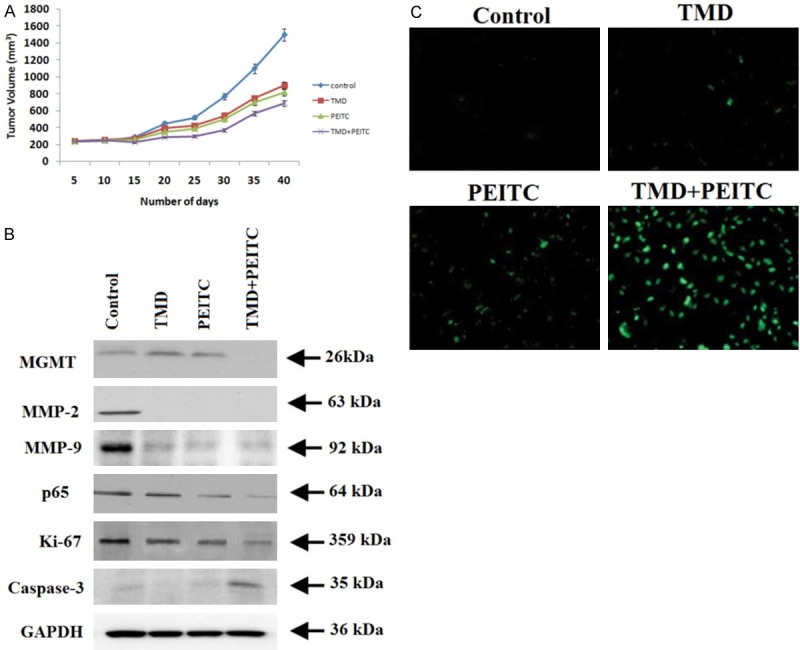
The in vivo studies using GBM tumor mouse model confirmed PEITC increased the sensitivity against Temozolamide. A. The tumor volume decreased significantly after 25 days of treatment compared to control (P < 0.01). B. The combined treatment of PEITC with TMD decreased the expression of MGMT, MMP-2, MMP-9, p65 and Ki-67 whereas showed expression of caspase 3. C. The TUNEL assay demonstrated increase in number of TUNEL positive cells indicating xenograft apoptosis.
Discussion
Pre-existing chemo resistance is one of the important factors responsible for selecting an aggressive therapeutic course in treating GBM leading to poor success rate. TMD is used as a first line agent in treating patients diagnosed with GBM, resistance to TMD in majority of cases has become an matter of concern in treating GBM [25,26]. Numbers of studies have emerged with agents contributing in enhancing the sensitivity of GBM towards TMD [27,28].
GBM is found to be involved with different types of tumors having variable response to different chemo agents due cell lines having specific genetic makes. Numbers of pathways are discovered to be involved in resistance mechanism of GBM [29,30]. Activity of Nuclear factor-κB (NF-κB) is regarded to be as a prominent factor involved in transcription of MGMT which is independent of MGMT methylation and is over-expressed as response to DNA damage, amplification of tumor, invasion and chemo resistance [31]. As previously reported, over expression of NF-κB leads to tumor growth and also is linked with resistance towards chemotherapy involving alkylating agents [32]. Numbers of compounds having NF-κB inhibitory effect are in development, such as Triptolide, Dehydroxymethylepoxyquinomicin and Smac Mimetic which are found to inhibit NF-κB activity and increase the chemo-sensitivity of GBM cell lines towards TMD [33-35]. The evidences in all of these studies suggest inhibition of NF-κB activity can lead to enhanced sensitivity of GBM cells towards alkylating agents such as TMD and the approach can successfully be employed in treating cases of acquired or induced chemo resistance.
Despite these, concluding studies regarding role of NF-κB, development of potential therapeutic agents for improving efficacy of TMD are still under investigational process for achieving a needed clinical response. Recently a report showing combination of TMD with natural bioactive compound against GBM is presented [36]. Therefore, the current study was undertaken to study the chemosensitizing role of PEITC in TMD resistant GBM cell lines. Here we evaluated three GBM cell lines i.e T98G, U87-R and U373-R (TMD resistance cell lines) and found that all of them showed differential expression of MGMT, we also found that T98G showed significantly higher IC50 values of TMD compared to other cell lines. In our study we successfully developed TMD resistant GBM cell model by exposing the cell lines with increasing concentration of Temozolomide for six months.
Phenethyl isothiocyanate (PEITC) is a member of isothiocyanate family; a bioactive potential compound found in cruciferous vegetables, it is reported to be effective against number of cancers such as breast, prostate, oral, ovarian and lung [18]. Several mechanisms have been evidenced for anticancer activity of PEITC such as induction of apoptosis, cell cycle arrest, formation of reactive oxygen species and modulation of NF-κB signaling pathway [37-41]. PEITC causes apoptotic cell death of Gefitinib-resistance NCl-H460 human cancer cell lines in vitro by acting on mitochondria-dependent pathway [43,44].
NF-κB signaling pathway has been identified to be one of the cascades responsible for the anticancer effect of PEITC, [42] also the resistant character against TMD in Gliomas is associated with activation of transcription factor NF-κB [24]. In the present study we provide evidence that PEITC could exert anticancer activity against TMD resistant GBM cell lines and could enhance the sensitivity towards TMD by down-regulating the expression of MGMT via NF-κB pathway. We demonstrated that treatment of PEITC inhibited the proliferation of TMD resistance cell lines. Next, we evidenced the sensitizing effect of PEITC in TMD resistant cells by blocking the NF-κB pathway and then we focused our study on the synergistic effect of PEITC along with TMD in inhibiting invasiveness, proliferation and inducing apoptosis in both in vitro and in vivo models. We reported pre-treatment of PEITC prior to exposing the cells to TMD as a key feature of our study, establishing the synergistic role of PEITC when combined with TMD. We also found sequential treatment of PEITC was required for enhancing the sensitivity of cells towards TMD.
For creating TMD resistance cell lines we subjected them to increasing concentration of Temozolomide in a stepwise manner for 6 months. The cell lines showed elevated levels of MGMT, which may be an important feature associated with long time exposure to TMD. These outcomes will definitely contribute in studies involving TMD associated resistance both in pre-clinical and clinical phase.
The novelty of the current research holds with the finding that pre-treatment of PEITC to GBM cell lines enhances the sensitivity towards TMD by NF-κB pathway in TMD resistant cell lines. Our reports propose that long-run treatment of TMD may cause in development of TMD resistance and can lead to malignant phenotypes in GBM cells. We establish PEITC as a potential chemo-sensitizing agent which may turn TMD resistance in glioma cells. Our findings could successfully be applied into clinical phase for dealing with gliomas showing resistance. Overall the findings suggest PEITC may be a molecule of potential clinical significance for enhancing the sensitivity of glioma cells showing TMD resistance.
Conclusion
The outcomes of in vitro and in vivo experiments suggested potential role of PEITC against Temozolomide resistant GBM cell lines. Pretreatment of PEITC enhanced the sensitivity of TMD resistant cell lines towards TMD by inhibiting the activity of NF-κB and then decreasing the expression of MGMT to reverse the chemo resistance in U373-R, T98G and U87-R cell lines in vitro. The outcomes of in vivo studies in chemo resistant xenograft mouse model were parallel to in vitro findings. All the findings suggested PEITC could be a potential molecule in treating chemo resistant glioblastoma.
Acknowledgements
We express thanks to the staff and management Department of Neurosurgery, China-Japan Union Hospital of Jilin University, China, for creating necessary facilities for the work. The funds come out of the authors own private finances.
Disclosure of conflict of interest
None.
Abbreviations
- GBM
Glioblastoma
- TMD
Temozolamide
- PEITC
Phenethyl isothiocyanate
- MGMT
O6-methylguanine-DNA methyl transferase
- MAPK
Mitogen-activated protein kinase
- RT-PCR
Reverse transcription-polymerase chain reaction
- MTT
methyl-thiazolyl-tetrazolium
- TUNEL
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
References
- 1.Fuller GN. The WHO classification of tumours of the central nervous system. 4th edition. Arch Pathol Lab Med. 2008;132:906. doi: 10.5858/2008-132-906-TWCOTO. [DOI] [PubMed] [Google Scholar]
- 2.Yeom SY, Nam DH, Park C. RRAD promotes EGFRmediated STAT3 activation and induces temozolomide resistance of malignant glioblastoma. Mol Cancer Ther. 2014;13:3049–3061. doi: 10.1158/1535-7163.MCT-14-0244. [DOI] [PubMed] [Google Scholar]
- 3.Loftus JC, Dhruv H, Tuncali S, Kloss J, Yang Z, Schumacher CA, Cao B, Williams BO, Eschbacher JM, Ross JT. TROY (TNFRSF19) promotes glioblastoma survival signaling and therapeutic resistance. Mol Cancer Res. 2013;11:865–874. doi: 10.1158/1541-7786.MCR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliva CR, Nozell SE, Diers A, McClugage SG 3rd, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie GY, Landar A. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. J Biol Chem. 2010;285:39759–39767. doi: 10.1074/jbc.M110.147504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross AH, Wheelhouse RT, Sakaria JN. Evaluation of novel imidazotetrazine analogues designed to overcome temozolomide resistance and glioblastoma regrowth. Mol Cancer Ther. 2014;14:111–119. doi: 10.1158/1535-7163.MCT-14-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park I, Mukherjee J, Ito M, Chaumeil MM, Jalbert LE, Gaensler K, Ronen SM, Nelson SJ, Pieper RO. Changes in pyruvate metabolism detected by magnetic resonance imaging are linked to DNA damage and serve as a sensor of temozolomide response in glioblastoma cells. Cancer Res. 2014;74:7115–7124. doi: 10.1158/0008-5472.CAN-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalley S, Chalmers AJ, Morley SJ. mTOR inhibition and levels of the DNA repair protein MGMT in T98G glioblastoma cells. Mol Cancer. 2014;13:144. doi: 10.1186/1476-4598-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta SK, Mladek AC, Carlson BL, Boakye-Agyeman F, Bakken KK, Kizilbash SH, Schroeder MA, Reid J, Sarkaria JN. Discordant in vitro and in vivo chemopotentiating effects of the PARP inhibitor veliparib in temozolomidesensitive versus-resistant glioblastoma multiforme xenografts. Clin Cancer Res. 2014;20:3730–3741. doi: 10.1158/1078-0432.CCR-13-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder MA, Decker PA, Anderson SK, Giannini C, Wu W. Efficacy of protracted temozolomide dosing is limited in MGMT unmethylated GBM xenograft models. Neuro Oncol. 2015;15:735–746. doi: 10.1093/neuonc/not010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melguizo C, Prados J, González B, Ortiz R, Concha A, Alvarez PJ, Madeddu R, Perazzoli G, Oliver JA, López R. MGMT promoter methylation status and MGMT and CD133 immunohistochemical expression as prognostic markers in glioblastoma patients treated with temozolomide plus radiotherapy. J Transl Med. 2012;10:250. doi: 10.1186/1479-5876-10-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boniface R, Figarella-Branger D, Karayan-Tapon L, Quillien V. DGKI methylation status modulates the prognostic value of MGMT in glioblastoma patients treated with combined radiochemotherapy with temozolomide. PLoS One. 2014;9:e104455. doi: 10.1371/journal.pone.0104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen SA, Stechishin OD, Luchman HA, Lun XQ, Senger DL, Robbins SM, Cairncross JG, Weiss S. Novel MSH6 mutations in treatment-naïve glioblastoma and anaplastic oligodendroglioma contribute to temozolomide resistance independently of MGMT promoter methylation. Clin Cancer Res. 2014;20:4894–4903. doi: 10.1158/1078-0432.CCR-13-1856. [DOI] [PubMed] [Google Scholar]
- 13.Lee KE. Immunohistochemical assessment of O (6)-methylguanine-DNA methyltransferase (MGMT) and its relationship with p53 expression in endometrial cancers. J Cancer Prev. 2013;18:351–354. doi: 10.15430/JCP.2013.18.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavon I, Fuchs D, Zrihan D, Efroni G, Zelikovitch B, Fellig Y, Siegal T. N ovel m echanism w hereby n uclear f actor kappaB mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007;67:8952–8959. doi: 10.1158/0008-5472.CAN-06-3820. [DOI] [PubMed] [Google Scholar]
- 15.Persano L, Pistollato F, Rampazzo E, Della Puppa A, Abbadi S, Frasson C, Volpin F, Indraccolo S, Scienza R, Basso G. BMP2 sensitizes glioblastoma stem-like cells to temozolomide by affecting HIF-1α stability and MGMT expression. Cell Death Dis. 2012;3:e412. doi: 10.1038/cddis.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawat N, Alhamdani A, McAdam E, Cronin J, Eltahir Z, Lewis P, Griffiths P, Baxter JN, Jenkins GJ. Curcumin abrogates bile-induced NF-κB activity and DNA damage in vitro and suppresses NF-κB activity whilst promoting apoptosis in vivo, suggesting chemopreventative potential in Barrett’s oesophagus. Clin Transl Oncol. 2012;14:302–311. doi: 10.1007/s12094-012-0799-x. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wei S, Wang J, Fang Q, Chai Q. Phenethyl isothiocyanate inhibits growth of human chronic myeloid leukemia K562 cells via reactive oxygen species generation and caspases. Mol Med Rep. 2014;10:543–549. doi: 10.3892/mmr.2014.2167. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Huong le D, Shim JH, Choi KH, Shin JA, Choi ES, Kim HS, Lee SJ, Kim SJ, Cho NP, Cho SD. Effect of β-phenylethyl isothiocyanate from cruciferous vegetables on growth inhibition and apoptosis of cervical cancer cells through the induction of death receptors 4 and 5. J Agric Food Chem. 2011;59:8124–8131. doi: 10.1021/jf2006358. [DOI] [PubMed] [Google Scholar]
- 21.Trachootham D, Zhou Y, Zhang H. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Yang MD, Lai KC, Lai TY, Hsu SC, Kuo CL, Yu CS, Lin ML, Yang JS, Kuo HM, Wu SH, Chung JG. Phenethyl isothiocyanate inhibits migration and invasion of human gastric cancer AGS cells through suppressing MAPK and NF-kappaB signal pathways. Anticancer Res. 2010;30:2135–2143. [PubMed] [Google Scholar]
- 23.Salloum RM, Jaskowiak NT, Mauceri HJ, Seetharam S, Beckett MA, Koons AM, Hari DM, Gupta VK, Reimer C, Kalluri R, Posner MC, Hellman S, Kufe DW, Weichselbaum RR. NM-3, an isocoumarin, increases the antitumor effects of radiotherapy without toxicity. Cancer Res. 2000;60:6958–6963. [PubMed] [Google Scholar]
- 24.Caporali S, Levati L, Graziani G, Muzi A, Atzori MG, Bonmassar E, Palmieri G, Ascierto PA, D’Atri S. NF-κB is activated in response to temozolomide in an AKT-dependent manner and confers protection against the growth suppressive effect of the drug. J Transl Med. 2012;10:252. doi: 10.1186/1479-5876-10-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Tang D, Zhang J, Wu J, Wang L, Dong J. The temozolomide derivative 2T-P400 inhibits glioma growth via administration route of intravenous injection. J Neurooncol. 2014;116:25–30. doi: 10.1007/s11060-013-1255-7. [DOI] [PubMed] [Google Scholar]
- 26.Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J. NABTT CNS consortium: survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the united states. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee NP, Day PJ, Lui WM, Fung CF, Leung GK. Inhibition of prolyl 4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide resistance in malignant glioma via the endoplasmic reticulum stress response (ERSR) pathways. Neuro Oncol. 2013;15:562–577. doi: 10.1093/neuonc/not005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller M, Gorlia T, Cairncross JG, van den Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, MacDonald DR, Forsyth P. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77:1156–1164. doi: 10.1212/WNL.0b013e31822f02e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Han EK, Anderson M, Shi Y, Semizarov D, Wang G, McGonigal T, Roberts L, Lasko L, Palma J. Acquired resistance to combination treatment with Temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. Mol Cancer Res. 2009;7:1686–1692. doi: 10.1158/1541-7786.MCR-09-0299. [DOI] [PubMed] [Google Scholar]
- 30.Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B. Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine a-induced cell death. Oncogene. 2013;32:1518–1529. doi: 10.1038/onc.2012.174. [DOI] [PubMed] [Google Scholar]
- 31.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 32.Lavon I, Fuchs D, Zrihan D, Efroni G, Zelikovitch B, Fellig Y, Siegal T. Novel mechanism whereby nuclear factor kappaB mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007;67:8952–8959. doi: 10.1158/0008-5472.CAN-06-3820. [DOI] [PubMed] [Google Scholar]
- 33.Sai K, Li WY, Chen YS, Wang J, Guan S, Yang QY, Guo CC, Mou YG, Li WP, Chen ZP. Triptolide synergistically enhances temozolomide-induced apoptosis and potentiates inhibition of NF-κB signaling in glioma initiating cells. Am J Chin Med. 2014;42:485–503. doi: 10.1142/S0192415X14500323. [DOI] [PubMed] [Google Scholar]
- 34.Brassesco MS, Roberto GM, Morales AG, Oliveira JC, Delsin LE, Pezuk JA, Valera ET, Carlotti CG Jr, Rego EM, de Oliveira HF. Inhibition of NF-κB by dehydroxymethylepoxyquinomicin suppresses invasion and synergistically potentiates temozolomide and γ-radiation cytotoxicity in glioblastoma cells. Chemother Res Pract. 2013;2013:593020. doi: 10.1155/2013/593020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner L, Marschall V, Karl S, Cristofanon S, Zobel K, Deshayes K, Vucic D, Debatin KM, Fulda S. Smac mimetic sensitizes glioblastoma cells to Temozolomide-induced apoptosis in a RIP1- and NF-κB-dependent manner. Oncogene. 2013;32:988–997. doi: 10.1038/onc.2012.108. [DOI] [PubMed] [Google Scholar]
- 36.Turrini E, Ferruzzi L, Fimognari C. Natural compounds to overcome cancer chemoresistance: Toxicological and clinical issues. Expert Opin Drug Metab Toxicol. 2014;10:1677–1690. doi: 10.1517/17425255.2014.972933. [DOI] [PubMed] [Google Scholar]
- 37.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Chen YR, Wang W, Kong AN, Tan TH. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 39.Yang YM, Conaway CC, Chiao JW, Wang CX, Amin S, Whysner J, Dai W, Reinhardt J, Chung FL. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 40.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Talalay P. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 42.Prawan A, Saw CL, Khor TO, Keum Y, Yu S, Hu L, Kong A. Anti-NF-κB and anti-inflammatory activities of synthetic isothiocyanates: effect of chemical structures and cellular signaling. Chem Biol Interact. 2009;179:202–211. doi: 10.1016/j.cbi.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsia T, Huang Y, Jiang Y, Chen H, Cheng Z, Hsiao Y. Phenethyl isothiocyanate induces apoptotic cell death through the mitochondria-dependent pathway in gefitinib-resistant NCI-H460 human lung cancer cells in vitro . Anticancer Res. 2018;38:2137–2147. doi: 10.21873/anticanres.12454. [DOI] [PubMed] [Google Scholar]
- 44.Dewani AP, Bakal RL, Kokate PK, Chandewar AV, Patra S. Development of a single ion pair HPLC method for analysis of terbinafine, ofloxacin, ornidazole, clobetasol and two preservatives in a cream formulation: application to in-vitro drug release in topical simulated media-phosphate buffer through rat skin. J AOAC Int. 2015;98:913–920. doi: 10.5740/jaoacint.14-189. [DOI] [PubMed] [Google Scholar]