Abstract
Background: Skin wound healing is a challenging problem, especially in aging or diabetic patients, which becomes more difficult to heal, and easily results in considerable public health burden. The purpose of this study was to investigate the effects of metformin on wound healing and explore its underlying mechanism. Methods: Metformin was local topical application in rat skin defect models. Alterations in the wounded skin were observed, and angiogenesis in the wound also was analyzed by immunohistochemical staining. The markers associated with differentiation macrophage were analyzed by immunofluorescence staining. The roles of AMPK singling pathway and the relative protein of NLRP3 inflammasome in wound were also analyzed by western blotting. In addition, AMPK/mTOR/NLRP3 inflammasome signaling axis was investigated to further analyze the molecular mechanism of metformin treatment on inducing M2 macrophage polarization in vitro. Results: Out results showed that metformin improved wound healing and angiogenesis which was paralleled by M2 macrophage polarization. We also found that the level of relative proteins of NLRP3 inflammasome was markedly decreased after metformin treatment. Furthermore, blockage of AMPK or activation of mTOR abolished the effects of metformin treatment on depressing NLRP3 inflammasome activation, M2 polarization and improving wound healing. It suggested that the treatment effects of metformin on wound healing were through regulating AMPK/mTOR/NLRP3 inflammasome signaling axis. Conclusion: Metformin regulated AMPK/mTOR singling pathway to inhibit NLRP3 inflammasome activation, which boosted M2 macrophage polarization to accelerate the wound healing. These findings provided new insights into the molecular mechanism of metformin therapy and its therapeutic potential in wound healing.
Keywords: Wound healing, macrophage polarization, metformin, NLRP3, imflammasone
Introduction
Wound healing is a complex process involving overlapping stages of hemostasis, inflammation, tissue proliferation and remodeling. These stages are necessary for the development of normal wound healing [1-3]. If it could not be developed with a timely and orderly manner during the wound healing process, the wounds will turn into chronic, non-healing wounds that are a growing world health care issue. Macrophage was known as a critical role in in wound healing progress. Macrophages can polarize in two predominate phenotypes when it was upon activities. The two phenotypes of macrophages are the pro-inflammatory “classically” activated (M1 macrophage) and the “alternatively” activated (M2 macrophage). The M1 was mainly associated with inflammation, whereas M2 macrophage plays an important role in tissue repair and regeneration. Accumulating evidence suggested that M2 macrophages regulated revascularisation, fibroblast regeneration, myofibroblast differentiation and collagen production [4]. Non-functional or dysfunctional M2 macrophages are collected with chronic, non-healing wounds. Therefore, a timely and orderly M2 polarization is critical for successful wound healing.
It was reported that inflammasomes involves in the innate immune response and promotes inflammation [5]. NOD-like receptor protein 3 (NLRP3) inflammasome is the most common inflammasome, and consists of NOD-like receptor protein 3, apoptosis associated speck-like protein (ASC) and caspase-1. Emerging evidences indicate that NLRP3 inflammasome is a critical component element in the wound progress [6,7]. Down-regulating the NLRP3 inflammasome and cleaved IL-1β inhibited inflammation and improved wound healing [7,8]. Chiu and his colleagues indicated that suppressed the NLRP3 inflammasome activities via the activation of autophagy ameliorated burn wound progression and improved wound healing in a rat burn model [9]. Previous study also demonstrated that NLRP3 protein expression in macrophages was up-regulated by lipopolysaccharide (LPS) in M1 macrophages but not in M2 phenotype [10]. Besides, inhibition of the NLRP3 inflammasome converts lipopolysaccharide (LPS)-stimulated M1 macrophages toward an M2 phenotype [11]. Therefore, we propose that inducing M2 macrophage polarization through suppressing the NLRP3 inflammasome activities may be a new therapeutic target for improve the wound healing.
Metformin, a biguanide, is currently one of the most commonly medication for the therapy of type 2 diabetes [12,13]. Despite metformin was one of oldest prescribed hypoglycemic agents for patients with type 2 diabetes, few studies have investigated the potential for metformin to influence wound healing. Recently, Metformin was received increasing attention because of its anti-inflammatory properties [13-16]. Emerging evidences in the literature supported the novel hypothesis that metformin has immune-modulatory features [4,16]. Although metformin present a potential to ameliorate inflammation, whether metformin treatment can accelerate the wound healing and its effects on macrophage polarization regulation remains to be elucidated. In addition, whether metformin can depress NLRP3 inflammasome activation also requires further investigation.
Previous studies demonstrated that the pleotropic effects of metformin were associated with the activation of AMP-activated protein kinase (AMPK) [17,18]. Moreover, AMPK is known as the upstream of mTOR which play an important role in the regulation of inflammation. Recent studies demonstrated that metformin suppressed immune responses mainly through its direct effect on the cellular functions of various immune cell types by induction of AMPK and subsequent inhibition of mTOR [4,19]. Jing et al. [4] also found that metformin improved low-grade inflammation in obesity and modulated macrophage polarization to M2 phenotype via the activation of AMPK [4]. Therefore, we hypothesized that metformin can improve the wound healing by inducing the M2 macrophage polarization which involved in AMPK/mTOR/NLRP3 inflammasome signaling pathway.
The purpose of this study was to investigate the effect of metformin treatment on wound healing and explore its underlying mechanism. In this study, we found metformin treatment can accelerate wound healing which was paralleled by inducing the M2 macrophage polarization in rat skin defect models. Our data also indicated that NLRP3 inflammasome was depressed after metformin treatment. We further illustrated the AMPK/mTOR/NLRP3 inflammasome signaling pathway involved in therapeutic effect of metformin on wound healing. This study supported the translation of metformin as a therapeutic drug for accelerating wound healing.
Materials and methods
Animals
Sprague-Dawley rats (10 weeks old, weight 200-250 g) were obtained from department of laboratory animals of central south university. The rats were housed in the animal care center of central south university and provided with free access to food and water. Rats were kept at a regulated temperature (21±3°C) and humidity (55±5%), and a 12 h light/dark cycle. All procedures involving animals were approved by the central south university committee on laboratory animals.
Excisional wounding and treatment
Wounds were created in the rat dorsal skin under sterile conditions as it has been described elsewhere [5,8]. Briefly, animals were anesthetized with pentobarbital sodium (50 mg/kg; Boster, Wuhan, China) through peritoneal injection (i.p.). The dorsal skin was picked up at the midline and punched with a sterile biopsy punch (1 cm in diameter; Miltex, USA), and generating two wounds on each side of the midline. Wounds were treated with the metformin or AMPK inhibitor (compound C) as indicated; treatment was initiated 3 days post-injury to allow the initial inflammatory response to proceed normally. Metformin (2 mM; solarbio, China) and Compound C (10 μM) [17] were applied topically to wounds every other day in F-127 pluronic gel (50 mL of a 25% gel in saline) [20]. Controls were treated with saline vehicle-loaded gel. For animal wounds, healing was assessed on day 10 post injury. The image J software (Media Cybernetics) was used to measure the wound areas. Changes in wound areas were presented as the percentage of initial (day 0) wound area.
Histology and Immunohistochemical analysis
Skin tissues samples were fixed in a 4% paraformaldehyde solution and then dehydrated using graded ethanol, following by embedding in paraffin and sectioned at 5 μm thick, and then stained with hematoxylin/eosin (H&E) using standard histology protocols. The samples were used to evaluate microvessel density (MVD). For the immunohistochemical analysis, the sections were incubated at 4°C overnight with Rabbit anti-CD31 (1:50 dilutions, Abcam) at 4°C. Rinse 3 × 5 min TBS 0.025% Triton with gentle agitation at the next day. After washing, the sections were incubated with an enzyme-conjugated secondary antibody (Abcam) for 1 h at room temperature. Slides were then rinsed in PBS and were incubated with HRP-conjugated secondary antibody and counterstained with hematoxylin. Flap tissues were imaged at × 20 magnifications. Images were calculated with Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA) for integral absorbance quantitation of CD31-positive blood vessels counting.
Cell culture and drug treatment
The RAW264.7 macrophage cell lines were purchase from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in 4.5% glucose DMEM supplemented with 10% FBS, 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. For experiments, the cells were seeded at 3 × 105 cells/ml and treated with 100 ng/mL LPS for 6 hours as it have been described in the previously paper. The non-adherent cells were removed with PBS. Adherent cells were further culture with fresh medium containing intervention condition for an additional 24 h. The RAW264.7 cells were randomly divided into the following four groups: (1) control group (LPS-stimulated cells); (2) metformin (2 mM) [4,21] group (LPS-stimulated cells treated with metformin); (3) metformin intervention+compound C (10 μM) group [22] (LPS-stimulated cells treated with metformin and compound C); (4) metformin intervention+MHY1485 (10 μM) [23] group (LPS-stimulated cells treated with metformin and MHY1485). After the treatment, the cells were collected and analyzed as described below.
Immunofluorescence staining
After fixation with 4% paraformaldehyde and punched with 0.3% Triton X-100, cutaneous excised tissues or RAW264.7 cells were blocked with 5% normal BSA, and then incubated with the desired primary antibodies against the M1 marker CD86 (anti-Rat for the cells 1:100, Abcam; anti-mouse for the tissue sections 1:100, immunnoway Biotechnology Company) and the M2 marker CD206 (anti-Rabbit 1:100; Proteintech) overnight at 4°C, following by Alexa Fluor-conjugated IgG secondary antibodies (1:100; multiscience) for 90 min at room temperature. After washing three times for 5 mines, the nucleus was stained with DAPI (Invitrogen, Carlsbad, CA) for 15 mins. The staining slides were observed by fluorescent Nikon Ti-E inverted microscope (Nikon, Kobe, Japan). For quantification, 5 different fields from each sample preparation were randomly selected, and then the CD86, CD206 and DAPI areas were analyzed using Image J software (Media Cybernetics).
Western blotting analysis
Cells or frozen tissue sample were lysed in ice-cold lysis buffer which includes 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5% deoxycholate,1% NP-40, 0.1% SDS, 10 mM NaF, 10 mM Na2P2O7, 10 mg/ml leupeptin, 1 mg/ml aprotinin, 1 mM sodium vanadate, and 1 mM PMSF. Those samples were incubated for 20 minutes at 4°C, following centrifuged at 12,000 rpm, for 20 min at 4°C. The supernatants were collected for Western blotting. A BCA protein kit (Bio-rad USA) was used to measure the protein concentration. The lysates were loaded onto SDS-PAGE and transferred to PVDF membrane (Bio-Rad). They were subsequently transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% non-fat milk in TBS with 0.1% Tween 20 for 1.5 hour at room temperature, and then were incubated with primary antibody overnight at 4°C according to the manufacturer’s recommendations. The membranes were washed with TBS for 5 min three times at next day, and then incubated incubation with horseradish peroxidase-coupled secondary antibody. Signals were visualized using the ChemiDicTM XRS+Imaging System (Bio-Rad). Quantification analysis was performed using Image lab software.
Elisa
Interleukin-1β, IL-10, VEGF levels in cell culture supernatants were determined by ELISA, according to manufacturer’s instructions (Abcam, USA). The levels of IL-1β, IL-10, and VEGF were calculated with reference to standard curves of purified recombinant IL-1β, IL-10, VEGF at various dilutions.
qRT-PCR analysis
Total RNA was isolated from sample using Trizol Reagent (Invitrogen) according to the manufactures protocols. cDNA was synthesized by a Revert Aid first-strand cDNA synthesis kit (Fermentas, Life Sciences, Canada) from total RNA. and these cDNA products were then amplified using Real-time qPCR with SYBR-Green RT-PCR kit (Bioteke, Beijing, China). β-actin served as the reference, relative-expression levels of RNA were quantified using (2-ΔΔCT) methods. The primers sequences of the Real-time qPCR used in animal experiments are listed in Table S1. RNA samples from cultured cells were reverse-transcribed into cDNA using the SuperScript III kit (Invitrogen, USA), and these cDNA products were then amplified using qPCR. The primers sequences of the Real-time qPCR used in cell experiments are listed in Table S2.
Statistical analysis
Statistical significance was performed using the GraphPad Prism 6.0 soft tool (GraphPad Software, La Jolla, CA, USA) and SPSS 19.0 statistical software. All values are presented as the mean ± SEM in the paper. Student’s t test was utilized to evaluate the statistical significance different between two experimental groups. When multiple groups were compared, data were statistical evaluated by using one-way analysis of variance (ANOVA) and Dunnett’s post hoc test. P values <0.05 were optioned to consider statistically significant.
Results
Metformin accelerated wound healing and angiogenesis
To demonstrate the effects of treatment with metformin on wound healing, alterations in the wounded skin were observed on alternate days until day 10. The dates showed that metformin treatment presented a markedly improvement in wound healing compared with the control group (P<0.05). Furthermore, wounds treated with the AMPK inhibitor compound C reversed this effect (Figure 1A, 1B). In order to further understand the role of metformin therapy in wound, the number of angiogenesis, indicated by CD31 staining of the tissue which harvested from the wound area, was observed. The capillary formation was significantly increased in metformin treatment group (P<0.05) compared with the control group. Capillary formation in compound c pretreatment was significantly decreased compared with the treatment with metformin group (P<0.05) (Figure 1C, 1D). The relative mRNA expression of VEGF was significant higher in the metformin treatment group compare with in the control group, and interfered with AMPK inhibitor compound c can reverse the effect of Metformin (Figure 1E). Altogether the results indicated that metformin treatment significantly accelerated wound healing.
Figure 1.
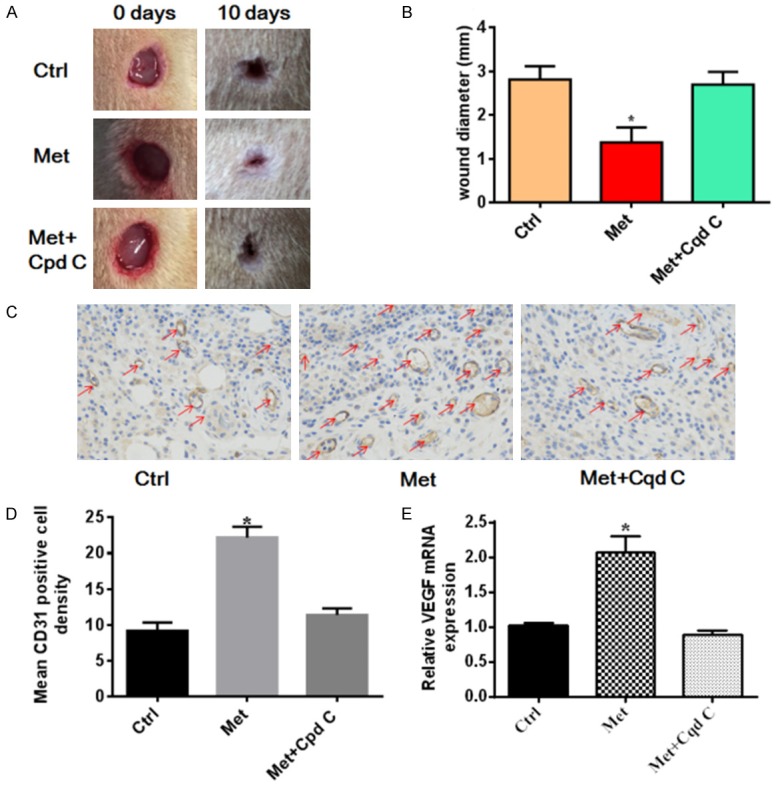
Metformin treatment accelerates wound healing in a rat model. A dorsal skin wound was created via a 1 cm circular punch biopsy and digital images of the wound were captured until day 10. A. Representative images of wound healing. B. Quantitative analysis of wound diameter. Metformin therapy accelerated wound healing compared with control group, and interfered with AMPK inhibitor compound C can reverse the effect of Metformin. *P<0.05 vs. Con or Cpd C group. C. Representative images of CD31 staining on day 7 and quantitative analysis. Metformin therapy enhances wound angiogenesis in the rat model, and Cpd C reversed the effect of Metformin. Red arrows indicate CD31-positive capillaries (magnification × 400). The HE staining results of mean vessels density was available in Figure S1. D. Quantitative analysis of capillaries in each field demonstrated that wound capillaries in metformin-treated group were increased on days 7 compared with the untreated rats, and interfered with AMPK inhibitor compound C can reverse the effect of Metformin. The number of CD31 positive vessels in the control group was 9.200±1.158, in the the metformin treatment group was 22.20±1.497 and 11.40±0.9274 in the Metformin+Compound C group. E. The relative mRNA expression of VEGF was significant higher in the metformin treatment group compare with in the control group, and interfered with AMPK inhibitor compound C can reverse the effect of Metformin. Values are expressed as the mean ± SEM; *P<0.05 vs. Con or Cpd C group. Ctrl, control; Met, metformin; Cpd C, compound C.
Metformin induced the M2 macrophage polarization in the wound
Wound healing is known to require the participation of macrophages, previous studies displayed that inducing M2 macrophage polarization can improve angiogenesis and accelerates wound healing [1-3]. To demonstrate whether metformin treatment induces the M2 macrophage polarization in the wound healing process, the molecular marker CD86 (M1) and CD206 (M2) was used to identify the subpopulation of macrophage by Immunofluorescence staining. Immunofluorescence staining results indicated that the density of M2 macrophages was significantly increased, whilst M1 macrophages were markedly reduced in the metformin treatment group on the day 7 (P<0.05). By contrast, tissues obtained from rats treated with compound c revealed reversal results (P<0.05) (Figure 2A, 2B). We also have measured the concentration of the anti-inflammatory cytokines (IL-10) and proinflammatory cytokines (IL-1β) by using the qRT-PCR. The results showed that the relative mRNA expression of IL-10 was significant higher in the metformin treatment group compare with in the control group, in contrast, the relative mRNA expression of IL-1β was markedly decreased in the metformin treatment group, and interfered with AMPK inhibitor compound C can reverse the effect of Metformin (Figure 2C). All of these results indicated that metformin treatment is an effective therapeutic strategy for improving the wound healing through AMPK protein regulates the M2 macrophage polarization.
Figure 2.
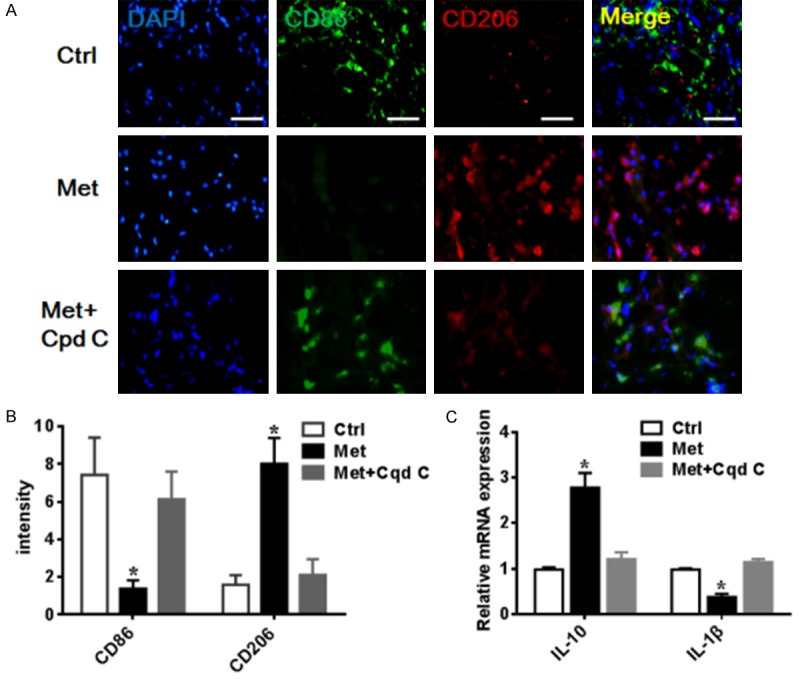
Metformin improved wound healing involving in the M2 macrophage polarization. A. Representative images of immunofluorescence analysis of macrophage phenotype. Wound immunostained for the M1 marker (CD86 green) and M2 marker (CD206 Red); scale bar 50 μm. B. Quantitative analysis of the M2 macrophage positive cell. C. Representative images of the relative mRNA expression of IL-10 and IL-1β. IL-10 was significant higher in the metformin treatment group, while IL-1β significantly decreased with metformin treatment, and interfered with AMPK inhibitor compound C can reverse the effect of Metformin. Data were presented as the mean ± SEM. *P<0.05 vs. Con or Cpd C group.
Metformin suppressed NLRP3 inflammasome in the wound via activating the AMPK
Previously studies indicated that NLRP3 inflammasome was associated with macrophage plasticity, suppressing the NLRP3 inflammasome activation can facilitate the M1 macrophage to M2 macrophage [24,25]. Despite accumulating evidences presented that metformin can modulate the inflammation [4,15], few studies presented the effects of metformin on the NLRP3 inflammasome. To test whether metformin regulated the NLRP3 inflammasome complex activation for macrophage polarization in the skin wound, the levels of p-AMPK, AMPK, NLRP3, IL-1β and caspase-1 were analyzed by western blotting. The results indicated that the NLRP3, IL-1β, and caspase-1 levels were markedly decreased and the p-AMPK was increased in the metformin therapy group compared with in the control group, whereas AMPK inhibitor compound C disturbed the influence of Metformin (P<0.05) (Figure 3). These findings presented that the therapy effect of Metformin on inducing M2 macrophage polarization is related to suppress NLRP3 inflammasome.
Figure 3.
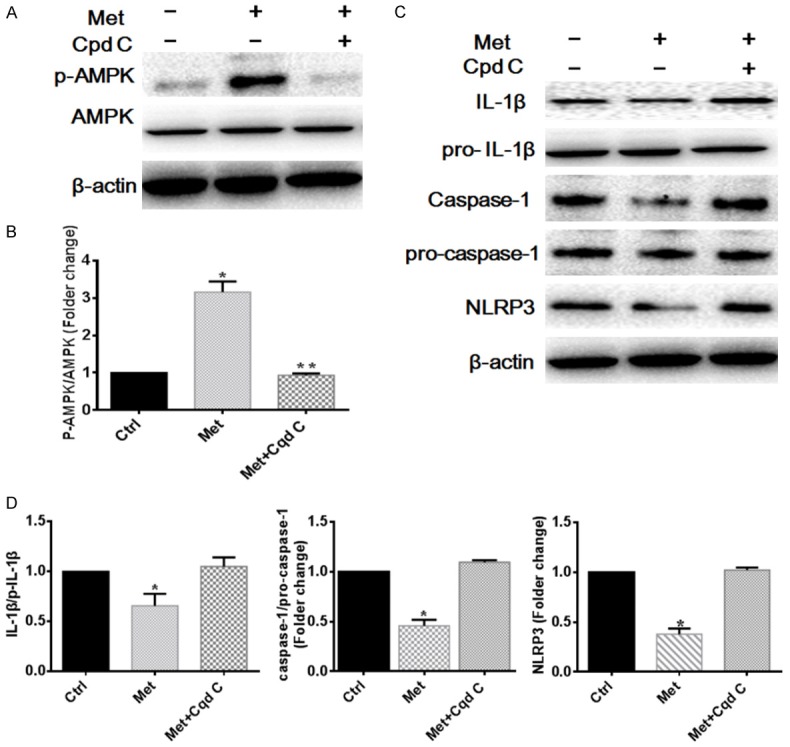
Metformin attenuates NLRP3 inflammasome activation in the wound via activating the AMPK. A, B. Representative images and quantitation of the western blot showing the protein expression of p-AMPK, AMPK. The original images are available in Figure S2. C, D. Representative images and quantitation of the western blot showing the protein expression of NLRP3, Pro-IL-1β, IL-1β, Pro-caspase-1, caspase-1. The original images are available in Figure S2. Data are presented as the mean ± SEM. *P<0.05 vs. Con or Cpd C group, **P<0.01 vs. Cpd C group. Ctrl, control; Met, metformin; Cpd C, compound C.
Metformin converted inflammatory macrophage cells to M2 polarization via modulating AMPK/mTOR signaling pathway
To further demonstrate the effect of metformin on macrophage cells polarization, we also investigated the therapy effect of metformin in vitro. RAW246.75 cells were activated by LPS and treatment with metformin. Immunofluorescence staining was performed to display the subpopulation of macrophages. Immunofluorescence staining results indicated that the density and distribution of M2 macrophages was significantly increased in the metformin treatment group (P<0.05). However, the AMPK inhibitor compound c prevented the effects of metformin on RAW246.75 cells (P<0.05) (Figure 4A). It is reported that metformin inhibited mTOR by activating AMPK [13]. To further determine whether metformin accelerated M2 macrophage polarization via regulating AMPK/mTOR signaling pathway, western blot were performed to investigate the level of p-AMPK, AMPK, p-mTOR, mTOR. As the results shown, metformin significantly increased the ratio of p-AMPK/AMPK (P<0.05), and significantly suppressed activation of mTOR (P<0.05). By contrast, the ratio of p-AMPK/AMPK and p-mTOR/mTOR in cells co-treated with metformin and compound C, indicating that metformin inhibited mTOR by activating AMPK in LPS-induced RAW264.7 cells (Figure 4B-D). To confirm that metformin induces the M2 macrophage polarization by suppressing mTOR signaling pathway, mTOR activator MHY1485 was further added to metformin treatment cells. Immunofluorescence staining results also indicated that mTOR activator MHY1485 can also disturbed the effect of metformin treatment in the macrophage culture (P<0.05) (Figure 5). Taken together, these data demonstrate that metformin regulated the macrophage polarization and facilitated the differentiation of macrophages to M2 via regulating the AMPK/mTOR signaling pathway.
Figure 4.
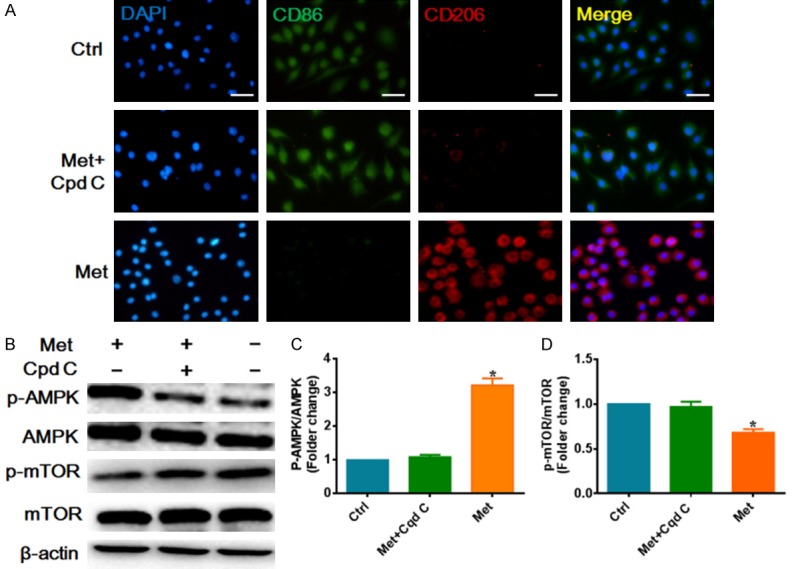
Metformin converted inflammatory macrophage cells to M2 polarization via modulating AMPK/mTOR signaling pathway. A. Representative images of immunofluorescence analysis of macrophage phenotype in vitro, CD86 (M1 green) or CD206 (M2 red) were analysis. Scale bar 50 μm. B. Representative images of the western blot showing the protein expression of p-AMPK, AMPK, p-mTOR, mTOR. C. Relative quantitation of p-AMPK, AMPK. D. Relative quantitation of p-mTOR, mTOR. The original images are available in Figure S3. Data are presented as the mean ± SEM. *P<0.05 vs. Ctrl or Cpd C group. Ctrl, control; Met, metformin; Cpd C, compound C.
Figure 5.
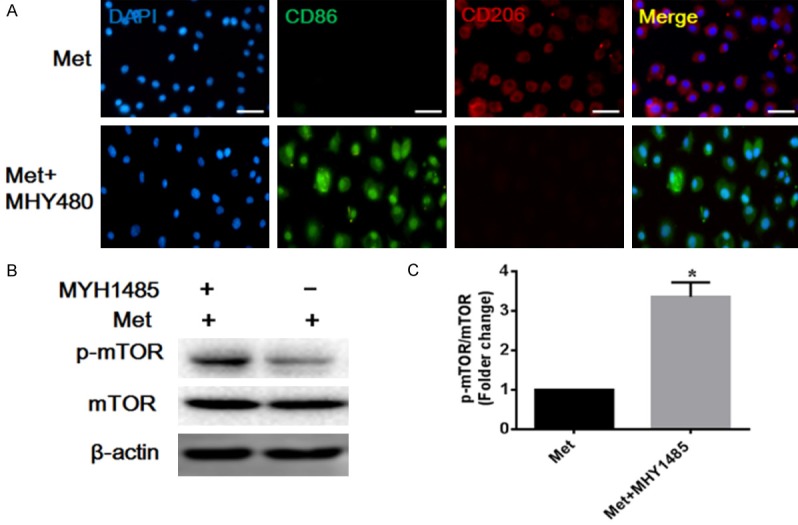
Metformin directs M2 macrophages polarization involved in mTOR signaling pathway. mTOR activator MHY1485 can disturb the therapy effect of metformin. A. Representative images of immunofluorescence analysis of macrophage phenotype in vitro. Scale bar 50 μm. B, C. Representative images and quantitation of the western blot showing the protein expression of p-mTOR, mTOR. The original images are available in Figure S4A. Data are presented as the mean ± SEM, *P<0.05 vs. Met group.
Metformin promotes M2 macrophage polarization via modulating AMPK/mTOR signaling pathway to inhibit NLRP3 inflammasome in vitro
In order to further analyze the molecular mechanism of metformin inducing M2 macrophage polarization, AMPK/mTOR/NLRP3 inflammasome signaling axis was investigated. Recent studies also demonstrated that mTOR was associated with NLRP3 inflammasome [26]. To test whether metformin modulate NLRP3 inflammasome complex activation through AMPK/mTOR signaling pathway, metformin, compound C and MHY1485 were utilized to treat LPS-simulated RAW264.7 cells. Western blot was performed to investigate the level of NLRP3 and caspase-1 (P<0.05). Western blot analyses revealed that the levels of NLRP3 and caspase-1 were markedly decreased in metformin treatment group, whereas compound C and MHY1485 treatment group prevented the effects of metformin on NLRP3 inflammasome (Figure 6A, 6B). The relative mRNA expression of NLRP3, IL-1β and caspase-1 also significantly decreased in the metformin treatment group, and interfered with compound C or MHY1485 can reverse the effect of Metformin (Figure 6C-E). We also have measured the concentration of the anti-inflammatory cytokines (IL-10) and proinflammatory cytokines (IL-1β) in the supernatant of cell culture medium. The EILSA results displayed that after metformin treatment, macrophage dramatically produced more anti-inflammatory cytokines (IL-10), and less proinflammatory cytokines (IL-1β) (P<0.05) (Figure 6F, 6G). We found that the concentration of VEGF also was significant increase in the metformin treatment group (P<0.05). Treatment of compound c or MHY1485 had exactly the reverse effect (Figure 6H). The results indicated that Metformin mediated NLRP3 inflammasome activation is associated with upregulation of AMPK and downregulation of mTOR signaling, demonstrating that metformin induces M2 macrophage polarization via modulating AMPK/mTOR signaling pathway to inhibit NLRP3 inflammasome. All of these results indicated that metformin mediate the modulation of M2 macrophage polarization through AMPK/mTOR/NLRP3 inflammasome regulatory signaling.
Figure 6.
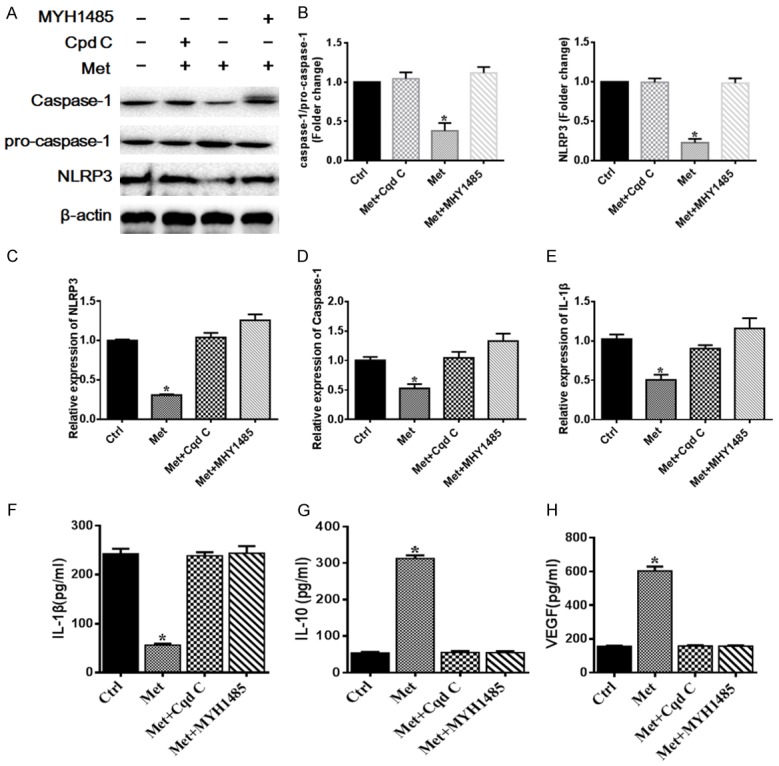
Metformin treatment facilitates M2 macrophages polarization via regulating AMPK/mTOR/NLRP3 inflammasome signaling axis. A, B. Representative images and quantitation analysis of western blot showing the protein expression of NLRP3, Pro-caspase-1 and caspase-1. The original images are available in Figure S4B. C-E. The relative mRNA expression of NLRP3, IL-1β and caspase-1 significantly decreased in the metformin treatment group, and interfered with compound C or MHY1485 can reverse the effect of Metformin. F-H. Pro-inflammatory cytokines (IL-1β), anti-inflammatory cytokines (IL-10) and VEGF levels were measured using ELISA kit. The expression level of IL-1β in the control, the metformin, the metformin+compound C and metformin+MHY1485 groups was 242.7±10.23, 56.33±2.917, 238.5±7.478 and 243.8±14.52, respectively. The expression level of IL-10 in the control, the metformin, the metformin+compound C and metformin+MHY1485 groups was 53.83±2.960, 312.5±8.543, 55.33±3.667 and 54.83±3.902, respectively. The expression level of VEGF in the control, the metformin, the metformin+compound C and metformin+MHY1485 groups was 155.3±3.190, 602.8±26.40, 157.7±4.014 and 156.5±4.410, respectively. Data are presented as the mean ± SEM. *P<0.05 vs. Ctrl or Cpd C or MHY1485 group. Ctrl, control; Met, metformin; Cpd C, compound C.
Discussion
Metformin is a synthetic derivative of guanidine, extracted from the extracts of Galega officinalis, represents a worldwide milestone in treatment of patients with type 2 diabetes [27,28]. Recently, emerging evidences demonstrated that metformin inhibited the expression of pro-inflammatory cytokines, protected against oxidative damage and direct macrophage polarization in vitro and in vivo [16,17,29]. Zhao and his colleague [17] discovered that local administration of metformin accelerated wound healing with improved epidermis, hair follicles, and collagen deposition in young rodents. Despite the potential for metformin to ameliorate inflammation and direct macrophage have been descripted in the previous literature, However, correlations among wound healing, macrophage polarization, NLRP3 inflammasome, and metformin remain unknown. In the present study, we investigated the treatment effect of metformin on wound healing and explore its underlying mechanism. Those results dates clearly demonstrated that metformin treatment is an effective therapeutic strategy on improving the wound healing through inhibition of NLRP3 inflammasome activation to direct macrophage polarization. All of these results indicated that metformin provides new therapeutic potential in the treatment of wound healing.
Macrophages are a heterogeneous population of cells that may undergo classical M1 or alternative M2 activation in response to various signals. Previous studies demonstrated that M1 macrophage polarization plays an important role in the early stage of wound healing, which contributes to an increased and sustained inflammatory response by producing high levels of pro-inflammatory cytokines, as well as ROS [1-3]. M2 macrophage polarization has been indicated that it was associated with resolution of tissue repair processes. Accumulating evidences indicated that M2 macrophages regulate re-vascularisation, fibroblast regeneration and myofibroblast differentiation and collagen production in the wound healing processes [2,3]. Previous studies also showed that reduced M2 macrophage levels lead to a reduction in growth factor levels (such as TGF-β, insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF)) which regulate the proliferative stage of repair in the diabetic wounds healing process [30-32]. Moreover, high levels of pro-inflammatory cytokines and mediators, such as TNF, IL-1β, IL-17 and iNOS, contributed to the non-healing phenotype [33,34]. Since M2 macrophage cells have been observed to improve wound repairs, it has been proposed that increasing M2 macrophage numbers in the wound might accelerate wound closure. As shown in our present study, metformin treatment promotes alternative M2 macrophage polarization in vivo and in vitro. Therefore, metformin therapy may be a therapeutic drug for accelerating wound healing through directs macrophage polarization.
To analyze the molecular mechanism of metformin inducing M2 macrophage polarization during the wound healing process, we have further investigated AMPK/mTOR/NLRP3 inflammasome signaling axis. In the present study, we found that the metformin treatment suppressed expression of NLRP3, IL-1β, and caspase-1 in vivo and vitro, whereas presented M2 macrophage polarization in metformin treatment group, Furthermore, we also found that p-AMPK inhibitor compound c prevented the effects of metformin on NLRP3 inflammasome activities. NLRP3 inflammasome is a multi-protein complex, including NLRP3, ASC and caspase-1, which control caspase-1 activity and the release of inflammation cytokine IL-1β and IL-18 in the innate immune system. Inflammasome signaling and downstream cytokine responses mediated by the inflammasome have been found to involve in wound healing process [35]. Recently studies also strongly suggest that blockade of the NLRP3 inflammasome is one of the key contributors to accelerate wounds healing in diabetic mice [36]. It is reported that NLRP3 inflammasome expression in macrophages was up-regulated by LPS in M1 macrophages but not in M2. Besides, inhibits NLRP3 inflammasomes activation promotes macrophage polarization toward an M2 phenotype [11,37]. Previous studies have described that NLRP3 inflammasome activation may contribute to the delay wound healing in the diabetic patients, whereas the negative regulation of NLRP3 inflammasomes was a potential therapeutic target for improving the wound healing. Importantly, suppressing inflammasome activity in wounds of mice using topical application of pharmacological inhibitors improved healing of these wounds, induced a switch from pro-inflammatory (M1 macrophage) to healing-associated macrophage (M2 macrophage) phenotypes, and increased levels of pro-healing growth factors [20]. In accordance with previous studies, we also provided evidence supporting that metformin treatment is an effective therapeutic strategy on inducing the M2 Macrophage polarization through inhibition of NLRP3 inflammasome activation.
Metformin as an AMPK activator has long been used to therapy diabetic hyperglycemia, which substantially impairs wound healing. However, controversial results exist regarding whether AMPK activation by metformin improves healing of diabetic wounds [38,39]. Recent studies demonstrated that metformin suppresses immune responses mainly through its direct effect on the cellular functions of various immune cell types by induction of AMPK and subsequent inhibition of mTOR [4,19]. Recent study also identified NLRP3 as a binding partner of mTOR, and the inhibition of mTOR/NLRP3 signaling pathway ameliorates intestinal inflammation [26]. As shown in our present study, metformin treatment promotes alternative M2 macrophage polarization by inducing the activation of AMPK. Activating and phosphorylating AMPK can suppress mTOR/NLRP3 inflammasome signaling pathway activation. Therefore, we supposed that the effect of metformin on wound healing might be related to the alteration of macrophage phenotype via regulating AMPK/mTOR/NLRP3 inflammasome signaling axis (Figure 7).
Figure 7.
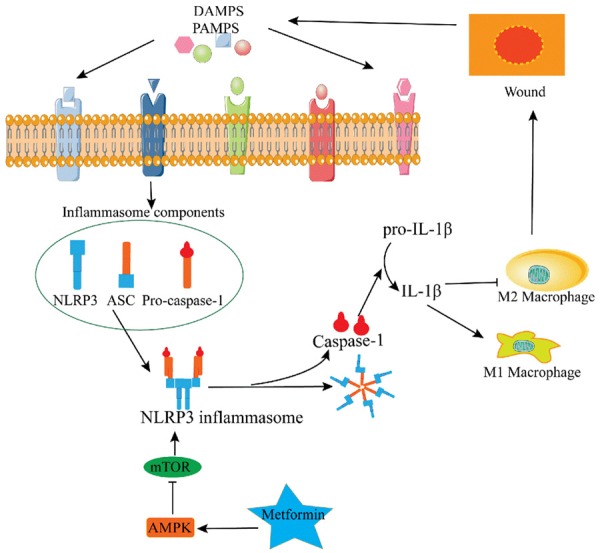
After the skin was injured to form a wound, the alarm signal has bound and activated the NLRP3 inflammasome by damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). The activation of NLRP3 inflammasome sustained a pro-inflammatory macrophage phenotype (M1) and inhibited upregulation of the pro-healing macrophage phenotype (M2). However, our studies results showed that metformin regulated AMPK/mTOR/NLRP3 inflammasome signaling axis to promote the M2 macrophages polarization which will accelerate the wound healing.
In our current study, we also found that angiogenesis was markedly increased in the metformin treatment group, and the levels of VEGF also presented up regulation in the metformin treat-LPS stimulated RAW264.7 cells. In addition, we found that the expression of IL-1β markedly decreased and the expression of IL-10 increased in the metformin treatment group. Previous studies also found that locally applied metformin improved vascularization of the wound beds, which were attributed to stimulation of adenosine monophosphate-activated protein kinase (AMPK) pathway, the key mediator of wound healing [40]. Interesting, some authors also found that AMPK pathway was inhibited in aged skin, thus, it easily impaired vasculature regeneration and reduced wound healing ability for the old patients [17]. Weinheimer-Haus et al. [41] reported that angiogenesis and levels of the pro-angiogenic growth factor VEGF were further reduced in IL-1β treated wounds, suggesting that IL-1β has a negative effect on angiogenesis. IL-1β is a very important marker for M1 macrophage, and it also is known as the downstream molecular of NLRP3 inflammasome. Bitto and his colleague [36] showed that the diabetic mice exhibited increased activation of NLRP3 inflammasome and a reduced expression of VEGF during wound healing progress. Blocking activation of the NLRP3 inflammasome decreased the levels of pro-inflammatory molecules and improved the impaired healing pattern [36]. Taken together, these data demonstrate that metformin facilitated the differentiation of macrophages into M2 subtype which involved in the AMPK/mTOR/NLRP3 inflammasome signaling pathway, and then the M2 macrophage improve the wound healing through releasing VEGF.
In conclusion, this study detected the effects of metformin treatment on wound healing, NLRP3 inflammasome activations, and macrophage polarization after the skin injury. Our results revealed that metformin regulates AMPK/mTOR singling pathway to inhibit NLRP3 inflammasome activation which boosted M2 macrophage polarization to accelerate the wound healing. These findings provide new insights into the molecular mechanism of metformin therapy and its therapeutic potential in the treatment of wound healing.
Acknowledgements
This publication was supported by a grant from the National Natural Science Foundation of China (81472104). This study was also funded by the Natural Science foundation of HuNan Province (2018JJ2640).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ. 2017;62:353–364. doi: 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair Regen. 2016;24:613–629. doi: 10.1111/wrr.12444. [DOI] [PubMed] [Google Scholar]
- 4.Jing Y, Wu F, Li D, Yang L, Li Q, Li R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol Cell Endocrinol. 2018;461:256–264. doi: 10.1016/j.mce.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Kanbe A, Sakai H, Seishima M. Activation of NLRP3 signalling accelerates skin wound healing. Exp Dermatol. 2018;27:80–86. doi: 10.1111/exd.13441. [DOI] [PubMed] [Google Scholar]
- 6.Bian F, Xiao Y, Zaheer M, Volpe EA, Pflugfelder SC, Li DQ, de Paiva CS. Inhibition of NLRP3 inflammasome pathway by butyrate improves corneal wound healing in corneal alkali burn. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao M, Li L, Li C, Liu L, Yu Y, Ma L. 3,4-methylenedioxy-beta-Nitrostyrene ameliorates experimental burn wound progression by inhibiting the NLRP3 inflammasome activation. Plast Reconstr Surg. 2016;137:566e–575e. doi: 10.1097/01.prs.0000479972.06934.83. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Zhao J, Zhang J, Mei J, Shao M, Pan Y, Yang W, Jiang Y, Liu F, Jia W. Heparan sulfate inhibits inflammation and improves wound healing by downregulating the NLR family pyrin domain containing 3 (NLRP3) inflammasome in diabetic rats. J Diabetes. 2018;10:556–563. doi: 10.1111/1753-0407.12630. [DOI] [PubMed] [Google Scholar]
- 9.Chiu HW, Chen CH, Chang JN, Chen CH, Hsu YH. Far-infrared promotes burn wound healing by suppressing NLRP3 inflammasome caused by enhanced autophagy. J Mol Med (Berl) 2016;94:809–819. doi: 10.1007/s00109-016-1389-0. [DOI] [PubMed] [Google Scholar]
- 10.Awad F, Assrawi E, Jumeau C, Georgin-Lavialle S, Cobret L, Duquesnoy P, Piterboth W, Thomas L, Stankovic-Stojanovic K, Louvrier C, Giurgea I, Grateau G, Amselem S, Karabina SA. Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation. PLoS One. 2017;12:e0175336. doi: 10.1371/journal.pone.0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang BC, Li Z, Xu W, Xiang CH, Ma YF. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am J Transl Res. 2018;10:265–273. [PMC free article] [PubMed] [Google Scholar]
- 12.Han X, Tao Y, Deng Y, Yu J, Sun Y, Jiang G. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol Med Rep. 2017;16:8691–8698. doi: 10.3892/mmr.2017.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ursini F, Russo E, Pellino G, D’Angelo S, Chiaravalloti A, De Sarro G, Manfredini R, De Giorgio R. Metformin and autoimmunity: a “new deal” of an old drug. Front Immunol. 2018;9:1236. doi: 10.3389/fimmu.2018.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai M, Uchiba M, Komura H, Mizuochi Y, Harada N, Okajima K. Metformin, an antidiabetic agent, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. J Pharmacol Exp Ther. 2010;334:206–213. doi: 10.1124/jpet.109.164970. [DOI] [PubMed] [Google Scholar]
- 15.Yang Q, Yuan H, Chen M, Qu J, Wang H, Yu B, Chen J, Sun S, Tang X, Ren W. Metformin ameliorates the progression of atherosclerosis via suppressing macrophage infiltration and inflammatory responses in rabbits. Life Sci. 2018;198:56–64. doi: 10.1016/j.lfs.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Schuiveling M, Vazirpanah N, Radstake T, Zimmermann M, Broen JC. Metformin, a new era for an old drug in the treatment of immune mediated disease? Curr Drug Targets. 2018;19:945–959. doi: 10.2174/1389450118666170613081730. [DOI] [PubMed] [Google Scholar]
- 17.Zhao P, Sui BD, Liu N, Lv YJ, Zheng CX, Lu YB, Huang WT, Zhou CH, Chen J, Pang DL, Fei DD, Xuan K, Hu CH, Jin Y. Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell. 2017;16:1083–1093. doi: 10.1111/acel.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Shen L, Gao P, Li G, He Y, Wang M, Zhou H, Yuan H, Jin X, Wu X. Effect of AMPK signal pathway on pathogenesis of abdominal aortic aneurysms. Oncotarget. 2017;8:92827–92840. doi: 10.18632/oncotarget.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Y, Sun X, Jiang L, Hu L, Kong H, Han Y, Qian C, Song C, Qian Y, Liu W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflammation. 2016;13:294. doi: 10.1186/s12974-016-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103–1114. doi: 10.2337/db13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD, Kim KR, Cheon HG. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014;289:23246–23255. doi: 10.1074/jbc.M114.577908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunduz D, Klewer M, Bauer P, Tanislav C, Sedding D, Rohrbach S, Schulz R, Aslam M. Compound C inhibits in vitro angiogenesis and ameliorates thrombin-induced endothelial barrier failure. Eur J Pharmacol. 2015;768:165–172. doi: 10.1016/j.ejphar.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Park YJ, Park JY, Jeong HO, Kim DH, Ha YM, Kim JM, Song YM, Heo HS, Yu BP, Chun P, Moon HR, Chung HY. Inhibitory effect of mTOR activator MHY1485 on autophagy: suppression of lysosomal fusion. PLoS One. 2012;7:e43418. doi: 10.1371/journal.pone.0043418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders HJ, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J, Marschner JA, Honarpisheh M, Shi C, Jordan J, Muller L, Burzlaff N, Bauerle T, Mulay SR. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 2018;93:656–669. doi: 10.1016/j.kint.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Shin TH, Kim HS, Kang TW, Lee BC, Lee HY, Kim YJ, Shin JH, Seo Y, Won Choi S, Lee S, Shin K, Seo KW, Kang KS. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7:e2524. doi: 10.1038/cddis.2016.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosin-Roger J, Simmen S, Melhem H, Atrott K, Frey-Wagner I, Hausmann M, de Valliere C, Spalinger MR, Spielmann P, Wenger RH, Zeitz J, Vavricka SR, Rogler G, Ruiz PA. Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun. 2017;8:98. doi: 10.1038/s41467-017-00213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia C, Liang S, He Z, Zhu X, Chen R, Chen J. Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur J Pharmacol. 2018;830:59–67. doi: 10.1016/j.ejphar.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Serrao CR, Bastos MF, Cruz DF, de Souza Malta F, Vallim PC, Duarte PM. Role of metformin in reversing the negative impact of hyperglycemia on bone healing around implants inserted in type 2 diabetic rats. Int J Oral Maxillofac Implants. 2017;32:547–554. doi: 10.11607/jomi.5754. [DOI] [PubMed] [Google Scholar]
- 29.El-Bahy AAZ, Aboulmagd YM, Zaki M. Diabetex: a novel approach for diabetic wound healing. Life Sci. 2018;207:332–339. doi: 10.1016/j.lfs.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leal EC, Carvalho E, Tellechea A, Kafanas A, Tecilazich F, Kearney C, Kuchibhotla S, Auster ME, Kokkotou E, Mooney DJ, LoGerfo FW, Pradhan-Nabzdyk L, Veves A. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185:1638–1648. doi: 10.1016/j.ajpath.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo H, Amano H, Shichiri M, Majima M. Suppressed recruitment of alternatively activated macrophages reduces TGF-beta1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomed Pharmacother. 2015;70:317–325. doi: 10.1016/j.biopha.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Yuan R, Geng S, Chen K, Diao N, Chu HW, Li L. Low-grade inflammatory polarization of monocytes impairs wound healing. J Pathol. 2016;238:571–583. doi: 10.1002/path.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, Koh TJ. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J Pathol. 2015;236:433–444. doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Artlett CM. Inflammasomes in wound healing and fibrosis. J Pathol. 2013;229:157–167. doi: 10.1002/path.4116. [DOI] [PubMed] [Google Scholar]
- 36.Bitto A, Altavilla D, Pizzino G, Irrera N, Pallio G, Colonna MR, Squadrito F. Inhibition of inflammasome activation improves the impaired pattern of healing in genetically diabetic mice. Br J Pharmacol. 2014;171:2300–2307. doi: 10.1111/bph.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha C, Gomes C. Exploring new inflammatory biomarkers and pathways during LPS-induced M1 polarization. Mediators Inflamm. 2016;2016:6986175. doi: 10.1155/2016/6986175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochoa-Gonzalez F, Cervantes-Villagrana AR, Fernandez-Ruiz JC, Nava-Ramirez HS, Hernandez-Correa AC, Enciso-Moreno JA, Castaneda-Delgado JE. Metformin induces cell cycle arrest, reduced proliferation, wound healing impairment in vivo and is associated to clinical outcomes in diabetic foot ulcer patients. PLoS One. 2016;11:e0150900. doi: 10.1371/journal.pone.0150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baraka AM, Deif MM. Role of activation of 5’-adenosine monophosphate-activated protein kinase in gastric ulcer healing in diabetic rats. Pharmacology. 2011;88:275–283. doi: 10.1159/000331879. [DOI] [PubMed] [Google Scholar]
- 40.Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: actions beyond metabolic regulation. J Mol Cell Cardiol. 2016;91:188–200. doi: 10.1016/j.yjmcc.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One. 2015;10:e0119106. doi: 10.1371/journal.pone.0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


