Abstract
Berberine (BBR), derived from Huanglian (Coptis chinensis), is a traditional Chinese herbal medicine. In the current study, we investigated the effects of BBR in high glucose (HG) and hypoxia-induced apoptosis with normal rat renal tubular epithelial (NRK-52E) and human kidney proximal tubular cells (HK-2) and further explored the underlying molecular mechanism of hypoxia-inducible factor 1α (HIF-1α) in diabetic kidney disease (DKD). Apoptosis in NRK-52E and HK-2 cells induced by HG (30 mM)/hypoxia and anti-apoptosis with BBR pretreatment (30 μM) were analyzed by using the terminal uridine nick 3’ end labeling method. Activities of apoptotic proteins and anti-apoptotic factor at mRNA and protein levels were determined with real-time RT-PCR and Western blot. HIF-1α action in the apoptosis with BBR pretreatment or siRNA interfere was investigated with flow-cytometry and Western blot. Up-regulation of apoptotic proteins (Bax cytochrome C, caspase 9 and caspase 3) and down-regulation of anti-apoptotic factor Bcl-xL were accompanied with HG/Hypoxia-induced apoptosis in NRK-52E and HK-2 cells but all reversals were found after BBR pretreatment. Activity of HIF-1α was induced under HG/Hypoxia conditions and up-regulated with BBR pretreatment. Furthermore, knockdown of HIF-1α via siRNA significantly removed the anti-apoptosis effects of BBR, while the BBR-mediated HIF-1α activity was suppressed by the pharmacological inhibition of Akt. The present study thereby provided evidence that BBR protected renal tubular epithelial cells from hypoxia/HG-induced apoptosis through activation of HIF-1α in the PI3K/Akt signal pathway and suggested that BBR could be a potential drug in DKD.
Keywords: Apoptosis, berberine, diabetic kidney disease, hypoxia-inducible factor 1α, tubular epithelial cells, PI3K/Akt signal pathway
Introduction
Diabetic kidney disease (DKD) as a leading cause of end stage renal failure accounts for high mortality rates and disabilities in the patients with diabetes [1,2]. Experimental studies have reported that chronic hypoxia and tubulointerstitial injury are involved in the development and progression of DKD [3,4]. Clinical observation has demonstrated that DKD is closely associated with high levels of oxidative stress and alterations in kidney metabolism and oxygen handling and the functional alterations precede the structural changes of the kidney [5]. Furthermore, accumulating evidence has implicated that renal hypoxia in diabetes not only causes a reduction of oxygen delivery but also results in an increase of oxygen consumption [3,6]. Chronic renal hypoxia is a trigger for tubular epithelial cells to release a number of cytokines and growth factors, which may lead to phenotypic changes and apoptosis. Consequently, tubular atrophy or extracellular matrix (ECM) accumulation impairs tissue oxygenation and accelerates hypoxic injury. Therefore, the apoptosis of renal proximal tubular cells in hypoxic milieu in DN is a major factor causing renal functional and pathological changes [7,8].
Hypoxia-inducible factor 1 (HIF-1) is composed with α- and β-subunits and plays an important role in cellular oxygen homeostasis [9,10]. Under hypoxic conditions, the α-subunit (HIF-1α) accumulates and forms with the β-subunit as transcriptionally active heterodimers. HIF-1α is then transactivated and also translocated into the nuclei for binding to hypoxic responsive elements and up-regulates several hypoxia-responsive genes in oxidative phosphorylation, antioxidant defense angiogenesis and anaerobic metabolism [10]. In the kidneys, HIF-1α is expressed in tubular and glomerular epithelial cells in response to hypoxia [11,12] and plays a pivotal role in kidney injury in DKD [13,14]. In addition, oxidative stress and glucose transport alterations are found to be induced by high glucose in kidneys while intracellular ATP concentrations are decreased, which consequently result in HIF-1α stability and more HIF-1 production. Activation of HIF-1α may not only improve tubular transport efficiency related to reduced oxidative stress and tubulointerstitial damage but also prevent diabetes induced alteration in kidney oxygen metabolism by reducing tubular electrolyte load and mitochondrial leak respiration [14].
Berberine (BBR) has been used as a traditional Chinese herbal medicine because of its anti-inflammatory, anti-oxidant, anti-tumor and anti-cytotoxic effects [15-17]. In the recent years, several studies have implicated that BBR has anti-oxidative stress and anti-inflammation effects in diabetes [18-20]. Furthermore, BBR has the anti-oxidant properties, which may be effective for improving renal function based upon the studies with diabetic animal models and human kidney cells [16,21]. Furthermore, several studies have demonstrated that BBR exerts anti-apoptotic effects and attenuates DOX-induced cardiomyocyte apoptosis via inhibiting an increase of the AMP/ATP ratio and AMPK in the PI3K/Akt signaling pathways [22,23] but no study with renal tubular epithelial cells has been reported.
Taking together what we described above, we had a hypothesis that BBR might have the protective effects on hypoxia-induced apoptosis via the modulation of HIF-1α in DKD. To test this hypothesis, in the current study, we not only analyzed the effects of BBR on hypoxia/HG-induced apoptotic process in renal tubular epithelial cells but also investigated the activities of HIF-1α and the PI3K/Akt signaling pathways. Data would be useful for consideration of BBR as a potential drug in DKD.
Research design and methods
Cell culture
Normal rat kidney tubular epithelial cell line (NRK-52E) and human renal proximal tubular epithelial cell line (HK-2) were obtained from American Type Culture Collection (Rockville, MD, USA) and Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China), respectively and then cultured in Dulbecco’s modified Eagle’s medium (DMEM) or DMFM/F12 (Gibco Laboratories) containing 10% fetal bovine serum and 1% penicillin/streptomycin in 5% CO2 at 37°C. When the cultured cells were almost confluent state, they were transferred to serum-free DMEM or DMEM/F12 for 24 h to arrest and synchronize cell growth. The experimental condition of hypoxia was induced by exposure to 1% O2 and 5% CO2 balanced with nitrogen in a multi-gas incubator (APM-30D, ASTEC, Fukuoka, Japan). The concentration of high glucose used in the current study was previously described by our group [24]. There were four groups for tubular epithelial cell treatments. The first group is for control with normal medium under normoxia (21% O2); the second group is BBR sulfate treatment (30 μM) under normoxia (21% O2) for 24 h; the third group is combined hypoxia (1% O2) and HG (30 mM) conditions together and the final group is BBR treatment of hypoxia and HG i.e. 30 μM BBR sulfate pre-treatment for 24 h and then followed with HG (30 mM) incubation 24 h under hypoxic (1% O2) condition.
Assessment of cell viability
Quantitative colorimetric assay with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was used for measuring the cell viability. The cells grown in 96-well plates were cultured with a density of 5×104. BBR treatments with the amount of 0, 10, 30 or 90 μg were performed for 6, 12, 24, 48, and 72 h, while 10 μl MTT (final concentration, 500 μg/mL) was added to the medium and the BBR treated cells were incubated at 37°C for 3 h. The reaction was ended up with 100 μl dimethyl sulfoxide (DMSO). MTT formazan product was measured according to the absorbance at 490 nm in a Sunrise RC microplate reader (TECAN, Switzerland).
Analysis of apoptosis
The apoptosis of NRK-52E and HK-2 cells was evaluated for differentiation between the survival and apoptotic cells with flow cytometry and the kit of annexin V-FITC and propidium iodide (PI) assay. All cells were the harvested with 0.25% trypsin and washed twice with cold PBS. After then, the cell pellets were suspended in 300 μl 1× binding buffer and incubated with staining solution (annexin V/PI, 1:2) in dark and room temperature for 30 min. Fuorescence-activated cell sorting (FACS) with FACSCaliburTM flow cytometer was used for the observation and analysis [25].
Terminal uridine nick 3’-end labelling
To detect apoptotic cells, the kit of terminal uridine nick 3’-end labelling (TUNEL) assay and epi-fluorescent microscope (Ernst Leitz, Inc., Rockleigh, NJ, USA) were used. The nuclei of cells were labelled with DAPI. The ratio of TUNEL-positive nuclei/DAPI-positive nuclei numbers from 10 randomly selected fields was used for determination of apoptosis. In each experiment, a total of 200 cells were counted.
Immunofluorescence staining
After washing once with cold PBS, the NRK-52E and HK-2 cells were fixed with 4% paraformaldehyde at 4°C for 30 min, and then incubated with 0.2% Triton X-100 for 10 min after washed with PBS. The cells were blocked by 5% bovine serum albumin (BSA) and incubated with a mouse monoclonal antibody against (1:200) HIF-1α overnight. The secondary antibody used for detection was Texas Red-conjugated donkey anti-goat (Texas Red-DAM) IgG. After rinsing with PBS, the sections were incubated with Texas Red-DAM (1:50) at RT for 2 h and examined with a confocal laser scanning microscope (CLSM, SP2, Leica, Germany).
Transient transfection with HIF-1α small interfering (si)RNA
HIF-1α siRNA was prepared according to the instruction from Invitrogen Life Technologies (Carlsbad, CA, USA). The cells at a density 2×105 cells/well were plated in 6-well plates and 2 ml DMEM or DMEM/F12 each. The siRNA sequences of forward and reverse oligos were 5’-GATCCGAGTTCACCTGAGCCTAATATTCAAGAGATATTAGGCTCAGGTGAACTCTTTTTTTCTAGAG-3’ and 5’-AATTCTCTAGAAAAAAAGAGTTCACCTGAGCCTAATATCTCTTGAATATTAGGCTCAGGTGAACTCG-3’. The target sequences of HIF-1α and control for siRNA were blasted using public Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence of control siRNA did not match any known human gene sequence. Lipofectamine 3000 was used for performing transient transfections. Knockdown of HIF-1α gene expression was analyzed and determined with Western blot. The transfection took 24 h and the cells were then cultured in medium containing different glucose concentrations and exposed to either normoxia (21% O2) or hypoxia (1% O2) conditions.
Real time RT-PCR
Total RNAs were extracted from NRK-52E and HK-2 cells using TRIzol reagent protocol (Invitrogen). According to the sequences of HIF-1α and β-actin genes, specific primers used for real time RT-PCR experiments were designed for over the exon-exon junction by using Primer Premier 5.0. The oligo primers (5’-GACTATAGCTCCGGAGAATGC-3’ and 5’-TCGTATCTGGTCAGCTATGG-3’) were synthesized by Takara Biotechnology and RT-PCR experiments were performed with Light Cycler 480 (Roche). Relative mRNA levels of the studied gene were normalized to the expression of β-actin by using the simplified comparative threshold cycle. Advanced Relative Quantification Software (Roche) were used for the relative gene expression data analysis.
Western blot
The protocols for preparation of cytosolic, mitochondrial and nuclear proteins and Western blot experiments were used as previously described [8]. Several primary antibodies, including anti-P-Akt (1:400), anti-caspase9 (1:400), anti-Bax (1:400), anti-Bcl-xL(1:400), anti-caspase3 (1:800), anti-Cyto C (1:800), anti-HIF-1α (1:400) and anti-β-actin (1:1000), were used. After washing with TBST, the secondary antibody was applied onto the membranes at 37°C for 30 min. The autoradiography was done with ECL reagent for HRP (60 s) and quantified with Quantity One Software (Bio-Rad Laboratories).
Statistical analysis
To perform statistical analysis, SPSS (Version 18 SPSS Inc. Chicago, Illinois, USA) was used. Data were expressed as mean ± SEM. Statistical significance was examined by using two-tailed paired or unpaired Student’s t test or among more than two groups by one-way analysis of variance (ANOVA). After ANOVA testing, the individual comparisons were made using Tukey’s multiple comparison test. P value < 0.05 was considered statistically significant.
Results
Berberine restored cell survival against hypoxia/HG injury
To assess whether BBR influenced hypoxia/HG-induced apoptosis in NRK-52E and HK-2 cells, the viability was detected with MTT analysis. The cell viability assay was then applied to select optimal concentrations. BBR sulfate at 30 μM was chosen accordingly (see Supplementary Figure 1A and 1B). The cells viability induced with hypoxia/HG was found to be significantly decreased compared with the control group (43 ± 3.6%, 41 ± 3.1%), while BBR treatment at 30 μM without hypoxia/HG resulted in a significant increase in cell survival and restoring cell survival (65.7 ± 6.3% and 63 ± 5.9%) (Supplementary Figure 1C and 1D).
Effect of BBR on hypoxia/HG induced apoptosis
BBR’s anti-apoptotic effects were detected with TUNEL assay and flow-cytometric analysis. Figure 1 showed that hypoxia/HG led to a significant increase in the apoptotic population among the cells compared with control group. BBR-treatment resulted in a substantial decrease of hypoxia/HG-induced tubular epithelial cell apoptosis. The cell apoptosis was detected with Annexin V and PI double staining. Flow-cytometry assays allowed to examine a substantial increase of the apoptotic and dead cells treated with hypoxia/HG for 24 h, while BBR treatment significantly decreased the apoptotic and dead cells (Figure 2B). To further confirm the effect of BBR in hypoxia/HG-induced apoptosis, the experiments with Western blot was performed. In tubular epithelial cell exposed to hypoxia/HG for 24 h, Bcl-xL expression was decreased while the expressions of Bax, cytochrome C, cleaved caspase-3, and cleaved caspase-9 were increased, indicating that the apoptotic process was not return. Pretreated BBR at 30 μM partially rescued from Bcl-xL down-regulation, reduced up-regulation of apoptosis proteins (Bax, cytochrome C, cleaved caspase-3, and cleaved caspase-9) (Figure 2A and 2C-H), which were consistent with the previous studies and suggested that BBR exerted anti-apoptotic or cyto-protective effects [16,26].
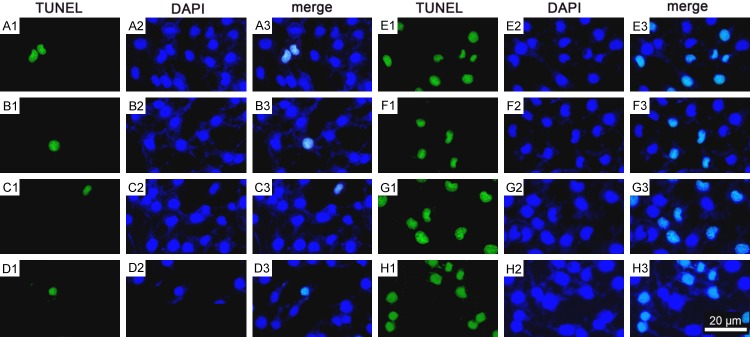
Figure 1.
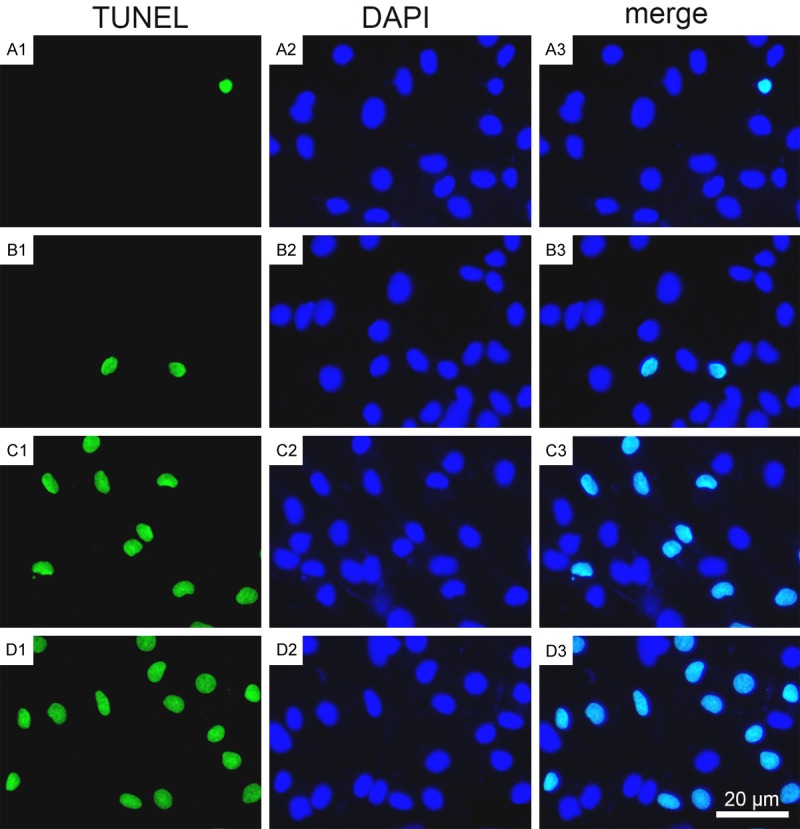
BBR decreased hypoxia/HG-induced apoptosis. The apoptosis was assessed by using TUNEL analysis as described in Materials and Methods. A1-A3. The control group; B1-B3. The BBR group; C1-C3. The hypoxia/HG/BBR group; D1-D3. The hypoxia/HG group. Results are representative of three experiments. Scale bars = 20 μm. The results in HK-2 cells are similar (Data not shown).
Figure 2.
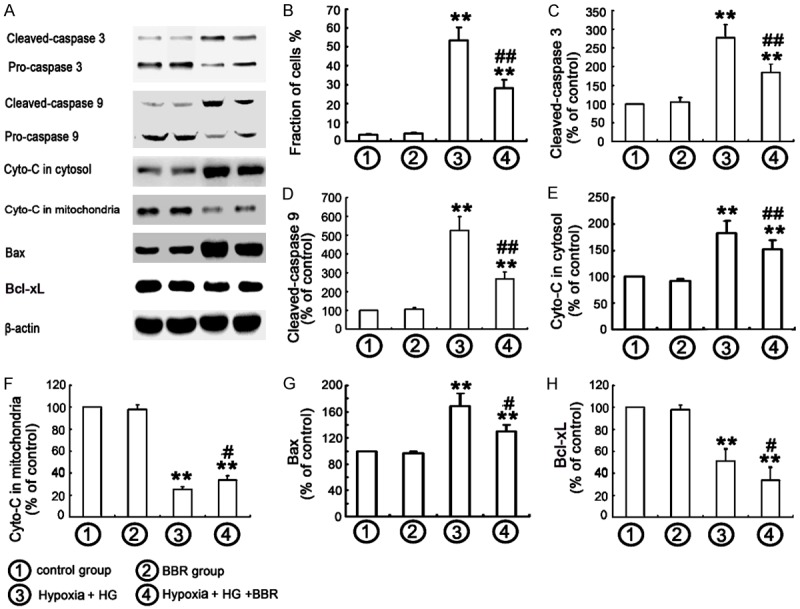
Effect of BBR on hypoxia/HG-induced apoptosis in NRK-52E cells. A. Western blots were performed with the antibodies indicated. Relative expression of apoptosis proteins was calculated and normalized to the loading control. B. The apoptosis was determined by flow cytometry, followed by Annexin V-PI double staining. The data were mean ± SEM (n = 6). (**P < 0.001, vs. control, ##P < 0.001, vs. hypoxia /HG). C-H. Corresponding protein levels were assessed using densitometry and expressed in relative intensities. All results were obtained from three independent experiments. (**P < 0.001, vs. control, ##P < 0.001, vs. hypoxia/HG, #P < 0.05, vs. hypoxia/HG).
Effect of BBR on expression of HIF-1α in tubular epithelial cells
To explore the mechanism of inhibition of apoptosis by BBR, the effects of BBR on HIF-1α activation in NRK-52E and HK-2 cells under hypoxia/HG conditions were examined. Data from Western blot analysis showed that the cells exposed to normal glucose levels did not change the distribution pattern of HIF-1α, which was consistent with the recent report [27]. Exposure to the hypoxia for 24 h resulted in a translocation of HIF-1α from cytoplasm into nuclei, indicating an activation of HIF-1α induced by hypoxia. Furthermore, a translocation of HIF-1α from cytoplasm into nuclei of NRK-52E cells induced by HG under hypoxic conditions was observed (Figure 3A1-C3). In addition, BBR treatment significantly enhanced the hypoxia/HG-induced HIF-1α nuclear translocation (Figure 3D1-D3), indicating that BBR activated HIF-1α expression under hypoxia/HG conditions (Figure 4A-F).
Figure 3.
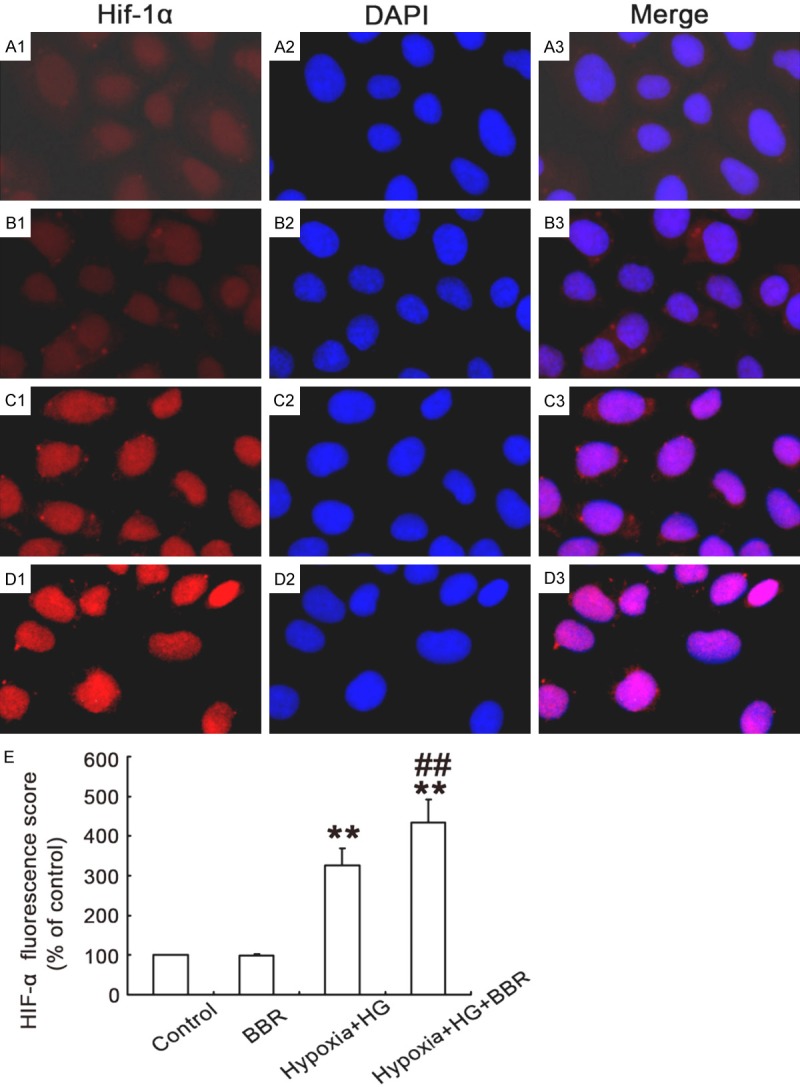
Influence of BBR on expression of HIF-1α in NRK-52E cells. NRK-52E cells were treated as indicated, and the HIF-1α protein was stained and observed under a fluorescence microscope as described in Materials and Methods. A1-A3. control group; B1-B3. BBR group; C1-C3. Hypoxia/HG group; D1-D3. Hypoxia/HG /BBR group. (Magnification, ×200). E. Relative HIF-1α fluorescaping score in NRK-52E cells. (**P < 0.001 vs. control, ##P < 0.001 vs. Hypoxia/HG). The results in HK-2 cells are similar (Data not shown).
Figure 4.
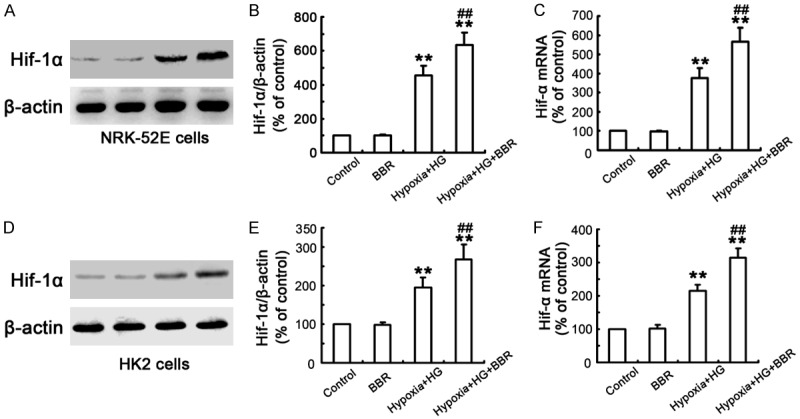
Influence of BBR on expression of HIF-1α in NRK-52E and HK-2 cells. A, D. Cells were treated as described above, and the expression of HIF-1α was detected by western blotting. B, E. Corresponding protein levels were assessed using densitometry and are expressed as relative intensities. Each value represents the mean ± SEM (n = 10) (**P < 0.001, vs. control, ##P < 0.001, vs. Hypoxia/HG). C, F. Transcript levels of HIF-1α gene was measured using real-time PCR analysis. Each value represents the mean ± SEM (n = 10) (**P < 0.001, vs. control, ##P < 0.001, vs. Hypoxia/HG).
Anti-apoptosis effects of BBR were mediated by activation of HIF signaling
To understand whether BBR protected the cells against HG-induced apoptosis through HIF-1α pathway, the apoptosis was investigated by flow-cytometry and Western blot. Firstly, siRNA knockdown experiments of the HIF-1α gene at various time-points were conducted with siRNA-HIF-1α transfection and the expression of HIF-1α at protein levels was detected by using Western blot (Figure 5A and 5B). Secondly, the cells were divided into six groups, including 1) the control with normal medium and normoxia; 2) scrambled group, subjected to scrambled siRNA transfection; 3) HIF-1α siRNA group, subjected to HIF-1α siRNA transfection; 4) Hypoxia/HG group, treated with HG and under hypoxia for 24 h; 5) Hypoxia/HG/BBR group, pretreated with BBR, followed with HG treatment under hypoxia for 24 h; 6) Hypoxia/HG/BBR/HIF-1α siRNA group, the cells with 24 h post-transfection were pretreated with BBR for 24 h, followed with HG treatment under hypoxia for 24 h. The results revealed that BBR pretreatment partially prevented hypoxia/HG-induced apoptosis, and were confirmed with flow-cytometry (Figures 5C and S2B). In addition, BBR treatment decreased the expression of apoptosis proteins (Bax, cytochrome C, caspases-9, and caspases-3) and increased Bcl-xL levels in NRK-52E and HK-2 cells under hypoxia/HG conditions (Figures 5D-I and S2A, S2C-G). Moreover, siRNA knockdown of HIF-1α removed the BBR-induced anti-apoptosis effects (Figures 5D-I and S2A, S2C-G).
Figure 5.
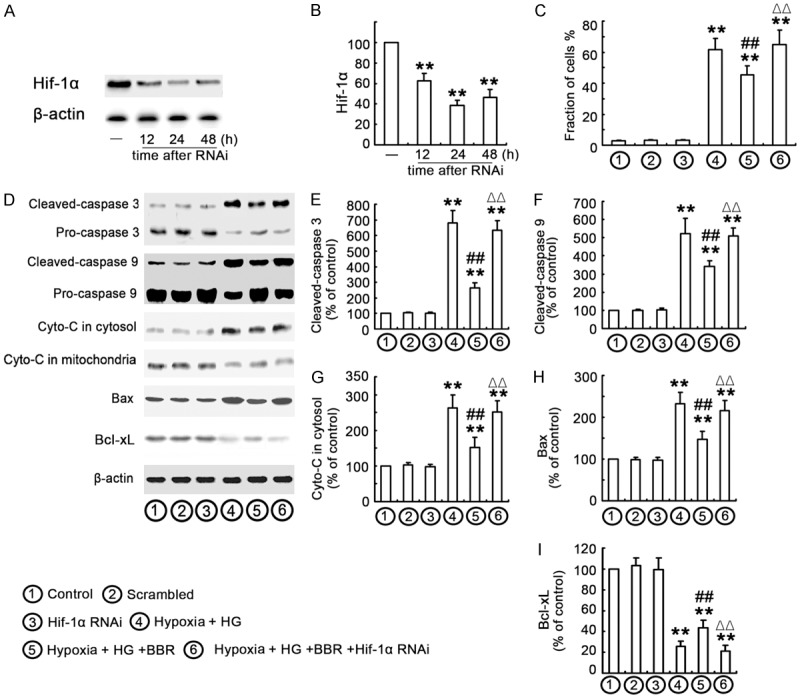
Anti-apoptosis effects of BBR are mediated by activation of HIF signaling. A, B. NRK-52E cells were transfected with HIF-1α-siRNA and Western blot analysis was performed with an antibody against HIF-1α was performed at various time-points following transfection (12, 24 and 48 h). Relative HIF-1α expression levels were calculated and normalized to the loading control. Corresponding protein levels were assessed using densitometry and expressed in relative intensities. All results were obtained from three independent experiments. Values were expressed as the mean ± SEM (n = 6; **P < 0.001, vs. control). The results in HK-2 cells are similar (data not shown). C. Cells were treated as above indicated and the apoptosis was determined by flow cytometry, followed by Annexin V-PI double staining. The data were mean ± SE (n = 6). (**P < 0.001 vs. control. ## P < 0.001 vs. hypoxia/HG. ΔΔP < 0.001 vs. hypoxia/HG/BBR). D-I. Corresponding protein levels were assessed using densitometry and expressed in relative intensities. All results were obtained from three independent experiments. Each value represents the mean ± SEM (n = 10) (**P < 0.001 vs. control. #P < 0.05 vs. hypoxia/HG. ##P < 0.001 vs. hypoxia/HG. ΔΔP < 0.001 vs. hypoxia/HG/BBR).
PI3K/Akt signaling pathway in relation with HIF-1α activation
A previous study has demonstrated that the PI3K/Akt signaling pathway plays an important role in the regulation of HIF-1α expression [28]. We thus investigated the effects of BBR in relation of HIF-1α activity and the PI3K/Akt signaling pathway. The cells were divided into 8 groups, including 1) the control group: normal medium under normoxia; 2) BBR group: BBR sulfate pre-treatment (30 μM) under normoxia; 3) LY294002 group (an inhibitor of PI3K, 10 μM for 1 h); 4) Wortmannin (Wort) group (an inhibitor of PI3K, 5 μM for 1 h); 5) Hypoxia/HG group (30 mM HG treatment under hypoxic condition); 6) Hypoxia/HG/BBR group (30 μM BBR sulfate pre-treatment followed by 30 mM HG incubation under hypoxic condition); 7) Hypoxia/HG/BBR/LY294002 group (30 μM BBR and 10 μM LY294002 sulfate pre-treatment followed by 30 mM HG incubation under hypoxic conditions); and 8) Hypoxia/HG/BBR/ Wort group (30 μM BBR and 5 μM Wort sulfate pre-treatment followed by 30 mM HG incubation under hypoxic (1% O2) conditions as previously reported [29]). As shown in Figure 6A, 6B, 6E and 6F, Akt phosphorylation was induced in the cells treated with HG/hypoxia. The increase in Akt phosphorylation was accompanied by up-regulation of HIF-1α in HG/hypoxia-treated NRK-52E and HK-2 cells. Compared with the control group, the pretreatment of NRK-52E and HK-2 cells with 30 μM BBR significantly increased the expression of phospho-Akt and HIF-1α under HG/hypoxia condition. In addition, both PI3K inhibitors LY294002 and Wort were used to investigate whether BBR activated and/or inhibited the stabilization of HIF-1α. HG/hypoxia-induced apoptosis was found to be caused by modulation of the PI3K/Akt pathway. The expression of phosphorylated Akt was reduced by LY294002 and Wort treatment, respectively, which led further reduction of HIF-1α (Figure 6A-C, 6E-G). Furthermore, the apoptosis of NRK-52E and HK-2 cells was detected with flow-cytometry or TUNEL (Figure 7). The results showed that hypoxia/HG-induced apoptosis was significantly increased and BBR treatment might effectively protect cells against HG/hypoxia-induced cell apoptosis (Figures 7 and S2D and S2H). When the cells were pretreated with both BBR and LY294002 or Wort, however, the protective effect of BBR was abolished (Figures 6D, 6H and 7). The results of TUNEL in HK-2 cells were similar with what observed in NRK-52E cells as described above. Taking together, these data implicated that BBR opposed cell survival. This action might be through HIF-1α and PI3K/Akt signal pathways and consequently protected the NRK-52E and HK-2 cells from HG/hypoxia-induced apoptotic process.
Figure 6.
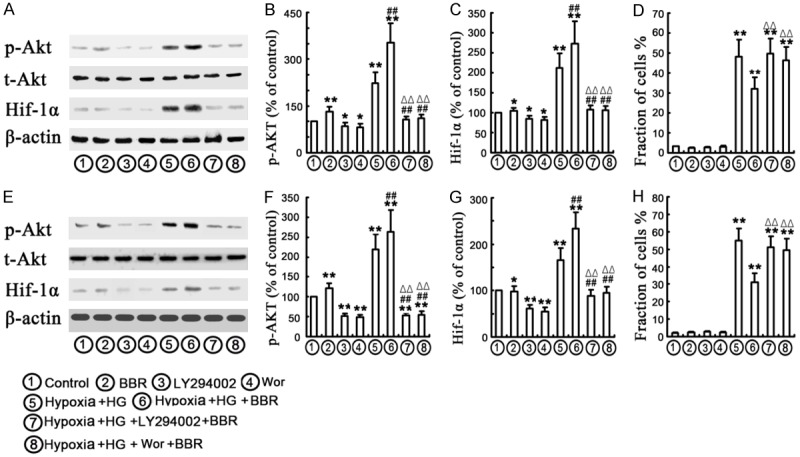
PI3K /Akt signaling pathway may contribute to expression of HIF-1α. The cells were incubated with as indicated. A-C, E-G. NRK-52E and HK-2 cells were divided into 8 groups as mentioned above, corresponding protein levels of t-Akt, p-Akt, HIF-1α were assessed using densitometry and expressed in relative intensities. β-actin was used as loading control. All results were obtained from three independent experiments. Values are expressed as the mean ± SEM. (**P < 0.001, vs. control, *P < 0.05, vs. control, ##P < 0.001, vs. hypoxia/HG. ΔΔP < 0.001, vs. hypoxia/HG/BBR). D, H. Apoptosis was assessed by flow cytometry, followed by Annexin V/PI double staining. Each value represented mean ± SEM (n = 5). (**P < 0.001, vs. control, ##P < 0.001, vs. hypoxia/HG. ΔΔP < 0.001, vs. hypoxia/HG/BBR).
Figure 7.
BBR-mediated activation of HIF-1α and PI3K/Akt signaling pathway protecting cells from hypoxia/HG induced apoptosis. A1-F3. The apoptosis was assessed by using TUNEL analysis as described in Materials and Methods. A1-A3. The control group; B1-B3. The BBR group; C1-C3. LY294002 group; D1-D3. Wortmannin (Wort) group; E1-E3. The hypoxia/HG group; F1-F3. The hypoxia/HG/BBR group; G1-G3. Hypoxia/HG/BBR/LY294002 group; H1-H3. Hypoxia/HG/BBR/Wort group. Results were representative of three experiments. Scale bars = 20 μm.
Discussion
We have conducted a series of experiments to test our hypothesis whether BBR has the protective effects on hypoxia/HG-induced apoptosis in renal tubular epithelial cells. Apoptosis in renal tubular epithelial cells is a major feature of DKD and is triggered in two pathways i.e. the extrinsic pathway stimulated by activation of plasma membrane death receptor and the intrinsic mitochondrial pathway [30]. Both pathways are followed by the increase of mitochondrial membrane permeability, which causes the release of cytochrome C and apoptosis inducing factor (AIF) from mitochondria into the cytosol and activation of among of caspase family that leading to apoptosis [31]. Bax exists in equilibrium between cytosolic and mitochondria-associated forms and shifts toward the latter when activated with mitochondrial stress stimulus to induce cell death [32]. Activated Bax accumulates on mitochondria and oligomerizes and permeabilizes the mitochondrial outer membrane. Bcl-xL, as an important anti-apoptosis factor, prevents accumulation of Bax to the mitochondria and oligomerization and permeabilization of the outer mitochondrial membrane [32,33]. Furthermore, one study in cultured human renal tubular epithelial cells has previously showed that BBR exerted anti-apoptotic effect against H/R injury through inhibiting endoplasmic reticulum stress and mitochondrial-dependent pathways [15]. Another study has recently demonstrated that BBR suppresses DOX-induced cardiomyocyte apoptosis via protecting mitochondria, reducing the increased ratio of AMP to ATP and inhibiting AMPK phosphorylation as well as elevating Bcl-2 expression in the early stage of DOX treatment [23]. To test whether BBR protects from apoptosis, in the current study, we have focused on the investigation of intrinsic mitochondrial pathway and analyzed the key molecules as described above. Our findings concerning the effects of BBR treatment mainly include: 1) inhibited hypoxia/HG-induced apoptosis; 2) the decrease of mitochondrial membrane permeability; 3) increased Bcl-xL expression while decreased the expression of apoptosis-related proteins (Bax, cytochrome C, caspases-9, and caspases-3). All these results indicated that BBR protected renal tubular epithelial cells from hypoxia/HG-induced cell apoptosis through the mitochondria-mediated pathway.
Several studies have demonstrated that HIF-1α plays an important role in the regulation of hypoxia-induced apoptosis [34,35]. The role of HIF-1α on the epithelial cells is multi-factorial but overall it promotes cell survival by mediating cellular adaptation to hypoxia [10]. The activation of HIF by cobalt may ameliorate the CsA-induced nephropathy by inhibiting apoptosis, inflammation and fibrosis in renal tubular cells [35]. These reports suggest that HIF-1α is a potential therapeutic target in order to mitigate hypoxia-induced tissue injury including DKD [9,10], because hyperfiltration triggers a vicious circle between increasing oxygen delivery and increasing oxygen consumption that leads to more needs for oxygen supply [9,36,37]. In the current study, the NRK-52E and HK-2 cells, which were exposed to high glucose, responded to hypoxia with increased HIF-1α expression, while down-regulation of HIF-1-α using HIF-1α-siRNA significantly decreased the anti-apoptosis effect of BBR as evidenced by increasing the level of apoptosis-related protein (such as Bax, caspases-3, and caspases-9). Data indicated that the renal tubular epithelial cells treated with BBR resulted in activated a protective HIF-1α to adapt to the stressful conditions and protected tubular epithelial cells from apoptosis.
The PI3K/Akt pathway links extracellular survival signals with the apoptosis-related pathway and plays a critical role in cell survival by blocking apoptotic pathways [22,38]. Akt as a key modulation factor in PI3K/Akt pathway, was able to induce HIF-1α transcriptional activity, lead to HIF-1α translocation to the nucleus, and triggering the signaling cascade for survival [39]. A previous study reported that BBR reduces ischemia/ reperfusion-induced myocardial apoptosis against myocardial ischemic/reperfusion injury and improves cardiac functional recovery following reperfusion via activating AMPK and PI3K/Akt signaling in diabetic rats [22]. One of our previous study demonstrated that β-elemene could increase the expression of HIF-1α through ROS and PI3K/Akt/mTor signaling pathway that partially prevents human osteosarcoma cells from undergoing apoptosis [40]. Recently, we have demonstrated that anti-oxidative drugs protect from HG-induced cells apoptosis and activate survival signals through the PI3K/Akt and Nrf2 signaling pathways in tubular epithelial cells [41]. In the current study, we found that the PI3K/Akt signaling pathway was promoted the expression of HIF-1α protein, while the BBR-mediated HIF-1α activation and anti-apoptosis capacity was inhibited by a PI3K inhibitor. The results thus suggested that BBR activated survival signals in tubular epithelial cells at least partly through the PI3K/Akt and HIF-1α signaling pathways.
Conclusions
The current study provides novel evidence that BBR has a protection on tubular epithelial cells from HG/hypoxia-induced apoptosis. This protective effect of BBR is most likely functioned through activation of HIF-1α in the PI3K/Akt signaling pathway. Thereby, we suggest that BBR may be a potential drug for DKD.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81670670) and Postdoctoral Science Foundation of China (2014MM551144) and the Liaoning Province Science and Technology Plan Project (201602405).
Disclosure of conflict of interest
None.
Abbreviations
- BBR
Berberine
- BSA
bovine serum albumin
- DKD
diabetic kidney disease
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- HG
high glucose
- HIF-1α
hypoxia-inducible factor 1α
- HK-2
human kidney proximal tubular cells
- NRK-52E
normal rat renal tubular epithelial cells
- TUNEL
terminal uridine nick 3’-end labelling
Supporting Information
References
- 1.Williamson JR, Tilton RG, Chang K, Kilo C. Basement membrane abnormalities in diabetes mellitus: relationship to clinical microangiopathy. Diabetes Metab Rev. 1988;4:339–70. doi: 10.1002/dmr.5610040404. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert RE, Cox A, Wu LL, Allen TJ, Hulthen UL, Jerums G, Cooper ME. Expression of transforming growth factor-beta1 and type IV collagen in the renaltubulointerstitium in experimental diabetes: effects of ACE inhibition. Diabetes. 1998;47:414–22. doi: 10.2337/diabetes.47.3.414. [DOI] [PubMed] [Google Scholar]
- 3.Ries M, Basseau F, Tyndal B, Jones R, Deminière C, Catargi B, Combe C, Moonen CW, Grenier N. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003;17:104–13. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 4.Palm F, Hansell P, Ronquist G, Waldenström A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia. 2004;47:1223–1231. doi: 10.1007/s00125-004-1434-3. [DOI] [PubMed] [Google Scholar]
- 5.Palm F. Intrarenal oxygen in diabetes and a possible link to diabetic nephropathy. Clin Exp Pharmacol Physiol. 2006;33:997–1001. doi: 10.1111/j.1440-1681.2006.04473.x. [DOI] [PubMed] [Google Scholar]
- 6.Persson MF, Franzén S, Catrina SB, Dallner G, Hansell P, Brismar K, Palm F. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia. 2012;55:1535–43. doi: 10.1007/s00125-012-2469-5. [DOI] [PubMed] [Google Scholar]
- 7.Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1 alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem. 2003;278:40296–304. doi: 10.1074/jbc.M302560200. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Kim YA, Yokozawa T. Pycnogenol modulates apoptosis by suppressing oxidative stress and inflammation in highglucose-treated renal tubular cells. Food Chem Toxicol. 2011;49:2196–201. doi: 10.1016/j.fct.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Eckardt KU, Bernhardt W, Willam C, Wiesener M. Hypoxia-inducible transcription factors and their role in renal disease. Semin Nephrol. 2007;27:363–72. doi: 10.1016/j.semnephrol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Gu HF, Zheng X, Abu Seman N, Gu T, Botusan IR, Sunkari VG, Lokman EF, Brismar K, Catrina SB. Impact of the hypoxia-inducible factor-1 α (HIF1A) Pro582Ser polymorphism on diabetesnephropathy. Diabetes Care. 2013;36:415–21. doi: 10.2337/dc12-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13:1721–32. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberger C, Griethe W, Gruber G, Wiesener M, Frei U, Bachmann S, Eckardt KU. Cellular responses to hypoxia after renal segmental infarction. Kidney Int. 2003;64:874–86. doi: 10.1046/j.1523-1755.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 13.Pagé EL, Robitaille GA, Pouysségur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–9. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 14.Nordquist L, Friederich-Persson M, Fasching A, Liss P, Shoji K, Nangaku M, Hansell P, Palm F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–38. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu W, Sheng M, Xu R, Yu J, Cui K, Tong J, Shi L, Ren H, Du H. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury viainhibiting endoplasmic reticulum and mitochondrial stress pathways. J Transl Med. 2013;11:24. doi: 10.1186/1479-5876-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domitrović R, Cvijanović O, Pernjak-Pugel E, Skoda M, Mikelić L, Crnčević-Orlić Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Hsu YY, Tseng YT, Lo YC. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol Appl Pharmacol. 2013;272:787–96. doi: 10.1016/j.taap.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, Xu S, Peng J, Xie X, Huang H. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: involvement of NF-κB signaling pathway. Mol Cell Endocrinol. 2011;331:34–40. doi: 10.1016/j.mce.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Shan CY, Yang JH, Kong Y, Wang XY, Zheng MY, Xu YG, Wang Y, Ren HZ, Chang BC, Chen LM. Alteration of the intestinal barrier and GLP2 secretion in Berberine-treated type 2 diabetic rats. J Endocrinol. 2013;218:255–62. doi: 10.1530/JOE-13-0184. [DOI] [PubMed] [Google Scholar]
- 20.Qiu YY, Tang LQ, Wei W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol Cell Endocrinol. 2017;443:89–105. doi: 10.1016/j.mce.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Moghaddam HK, Baluchnejadmojarad T, Roghani M, Khaksari M, Norouzi P, Ahooie M, Mahboobi F. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induceddiabetic rats. Mol Neurobiol. 2014;49:820–6. doi: 10.1007/s12035-013-8559-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Li G, Geng F, Zhang Z, Li J, Yang M, Dong L, Gao F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19:946–57. doi: 10.1007/s10495-014-0977-0. [DOI] [PubMed] [Google Scholar]
- 23.Lv X, Yu X, Wang Y, Wang F, Li H, Wang Y, Lu D, Qi R, Wang H. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrialdysfunction and increasing Bcl-2 expression. PLoS One. 2012;7:e47351. doi: 10.1371/journal.pone.0047351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Zhao Y, Chu Q, Wang ZY, Li H, Chi ZH. Zinc modulates high glucose-induced apoptosis by suppressing oxidative stress in renal tubularepithelial cells. Biol Trace Elem Res. 2014;158:259–67. doi: 10.1007/s12011-014-9922-x. [DOI] [PubMed] [Google Scholar]
- 25.Yu R, Zhang ZQ, Wang B, Jiang HX, Cheng L, Shen LM. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014;14:49. doi: 10.1186/1475-2867-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadeghnia HR, Kolangikhah M, Asadpour E, Forouzanfar F, Hosseinzadeh H. Berberine protects against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cells. Iran J Basic Med Sci. 2017;20:594–603. doi: 10.22038/IJBMS.2017.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Liang D, Fan J, Lian X, Zhao Y, Wang X, Chi ZH, Zhang P. Zinc attenuates tubulointerstitial fibrosis in diabetic nephropathy via inhibition of HIF through PI-3K signaling. Biol Trace Elem Res. 2016;173:372–83. doi: 10.1007/s12011-016-0661-z. [DOI] [PubMed] [Google Scholar]
- 28.Mahfouz N, Tahtouh R, Alaaeddine N, El Hajj J, Sarkis R, Hachem R, Raad I, Hilal G. Gastrointestinal cancer cells treatment with bevacizumab activates a VEGF autoregulatorymechanism involving telomerase catalytic subunit hTERT via PI3K-AKT, HIF-1α and VEGF receptors. PLoS One. 2017;12:e0179202. doi: 10.1371/journal.pone.0179202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Liang D, Guo B, Deng W, Chi ZH, Cai Y, Wang L, Ma J. Zinc transporter 5 and zinc transporter 7 induced by high glucose protects peritoneal mesothelialcells from undergoing apoptosis. Cell Signal. 2013;25:999–1010. doi: 10.1016/j.cellsig.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Krijnen PA, Simsek S, Niessen HW. Apoptosis in diabetes. Apoptosis. 2009;14:1387–8. doi: 10.1007/s10495-009-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eastman A. Apoptosis: a product of programmed and unprogrammed cell death. Toxicol Appl Pharmacol. 1993;121:160–4. doi: 10.1006/taap.1993.1141. [DOI] [PubMed] [Google Scholar]
- 32.Burlaka I, Nilsson LM, Scott L, Holtbäck U, Eklöf AC, Fogo AB, Brismar H, Aperia A. Prevention of apoptosis averts glomerular tubular disconnection and podocyte loss in proteinuric kidney disease. Kidney Int. 2016;90:135–48. doi: 10.1016/j.kint.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burlaka I, Liu XL, Rebetz J, Arvidsson I, Yang L, Brismar H, Karpman D, Aperia A. Ouabain protects against Shiga toxin-triggered apoptosis by reversing the imbalance between Bax and Bcl-xL. J Am Soc Nephrol. 2013;24:1413–23. doi: 10.1681/ASN.2012101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao MX, Wang X, Jiao KL. MicroRNA-17-5p mediates hypoxia-induced autophagy and inhibits apoptosis by targeting signal transducer and activator of transcription 3 in vascular smooth muscle cells. Exp Ther Med. 2017;13:935–941. doi: 10.3892/etm.2017.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh SW, Ahn JM, Lee YM, Kim S, Chin HJ, Chae DW, Na KY. Activation of hypoxia-inducible factor by cobalt is associated with the attenuation of tissue injury and apoptosis in cyclosporine-induced nephropathy. Tohoku J Exp Med. 2012;226:197–206. doi: 10.1620/tjem.226.197. [DOI] [PubMed] [Google Scholar]
- 36.Biju MP, Akai Y, Shrimanker N, Haase VH. Protection of HIF-1-deficient primary renal tubular epithelial cells from hypoxia-induced cell deathis glucose dependent. Am J Physiol Renal Physiol. 2005;289:F1217–26. doi: 10.1152/ajprenal.00233.2005. [DOI] [PubMed] [Google Scholar]
- 37.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, Makino Y, Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60:981–92. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Wang H, Shen R, Fang J, Yang Y, Dai W, Zhu Y, Zhou M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronalapoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am J Transl Res. 2018;10:1887–1899. [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–307. doi: 10.1038/labinvest.3700328. [DOI] [PubMed] [Google Scholar]
- 40.Liang D, Yang M, Guo B, Yang L, Cao J, Zhang X. HIF-1α induced by β-elemene protects human osteosarcoma cells from undergoing apoptosis. J Cancer Res Clin Oncol. 2012;138:1865–77. doi: 10.1007/s00432-012-1256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Liang D, Lian X, Jiang Y, He H, Liang W, Zhao Y, Chi ZH. Berberine activates Nrf2 nuclear translocation and inhibits apoptosis induced by high glucose in renal tubular epithelial cells through a phosphatidylinositol 3-kinase/Akt-dependent mechanism. Apoptosis. 2016;21:721–36. doi: 10.1007/s10495-016-1234-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.